Abstract
Throughout history, many medical milestones have been achieved to prevent and treat human diseases. Man’s early conception of illness was naturally holistic or integrative. However, scientific knowledge was atomized into quantitative and qualitative research. In the field of medicine, the main trade-off was the creation of many medical specialties that commonly treat patients in advanced stages of disease. However, now that we are immersed in the post-genomic era, how should we reevaluate medicine? Genomic medicine has evoked a medical paradigm shift based on the plausibility to predict the genetic susceptibility to disease. Additionally, the development of chronic diseases should be viewed as a continuum of interactions between the individual’s genetic make-up and environmental factors such as diet, physical activity, and emotions. Thus, personalized medicine is aimed at preventing or reversing clinical symptoms, and providing a better quality of life by integrating the genetic, environmental and cultural factors of diseases. Whether using genomic medicine in the field of gastroenterology is a new approach or a new medical specialty remains an open question. To address this issue, it will require the mutual work of educational and governmental authorities with public health professionals, with the goal of translating genomic medicine into better health policies.
Keywords: Chronic diseases, Nutritional genomics, Obesity, Liver disease, Gastrointestinal diseases
Core tip: New knowledge is growing on how genes are involved in the physiopathology of liver and gastrointestinal diseases and how environmental factors, such as diet, physical activity, and emotions modulate the onset and progression of chronic disease. In the era of genomic medicine, gastroenterologists and hepatologists should be determined to integrate genetic, environmental and cultural factors into medical practice to prevent or reverse medical symptoms.
INTRODUCTION
Throughout past and present history, humans have sought cures for their illnesses based on whatever they believed was the cause of the malady in question[1,2]. In the ancient world, Greek medicine was influenced by Hippocrates’ theorem, which proposed that diseases arose in response to a natural imbalance of four basic humors and that treatment could be accomplished by means of balancing the opposites[1,3]. Among the world’s oldest medical techniques is the traditional Chinese Medicine method of using acupuncture to unblock the body’s energy channels or meridians to restore health[1,4]. The practitioners of Indian Ayurvedic medicine promote the use of herbal compounds and special diets based on their conceptions about what causes health and disease[1,3,5]. During the Middle Ages in Europe diseases were perceived as a consequence of people’s sins; in this case, treatment consisted of prayer and herbalism. The practice of alchemy also arose during this time, which became a protoscience of medicine that later produced a broad range of contributions to chemistry and the physical sciences.
Although Hippocrates is considered the “Father of Medicine”, it wasn’t until the introduction of the scientific method, inspired by Rene Descartes’ “Discourse on Method,” that medicine began its next stage of development[6]. Major progress began when the previously concealed dissection of the human body gave rise to the subject of anatomy, which later enabled the improvement of surgical procedures[7]. The development of the microscope fostered the transition from the macroscopic world to the microscopic scale, impacting two fields of knowledge: the study of human tissues and cells and the study of microorganisms. These two areas of study led to the fields of cell biology and bacteriology (and, later on, virology), respectively, and inspired Louis Pasteur’s germ theory of disease, in addition to the development of antiseptics/antibiotics and vaccines[8].
However, it was not until the twentieth century that scientific medicine emerged. The development of biochemistry and physiology significantly advanced the understanding of many metabolic processes and laid the foundation for the fields of pathophysiology and pharmacology. These discoveries were the starting point of the current paradigm that exists in scientific-based medicine, which relies on either surgical intervention or drug therapy to treat disease. Additionally, the discovery of the double-stranded DNA molecule in the 1950’s by Watson and Crick[9] was a significant step forward in the field of molecular biology. Decoding the DNA of the prokaryotic bacterial cell led to the “one gene, one ribosome, one protein” hypothesis[10]. In the early 1980’s, recombinant DNA techniques based on the combined use of the PBR422 plasmid and restriction enzymes[11] enabled the cloning of other biologically relevant DNA sequences and marked the beginning of the pre-genomic era. The genomic era began nearly 20 years later with the sequencing of the entire human genome in the year 2000[12,13].
What’s next in medicine? We began this editorial with a brief overview of the world’s history of medicine to elicit only one question: How should these medical milestones be reevaluated, especially now that we are immersed in the post-genomic era?
PARADIGM SHIFT IN MEDICINE, OR “BACK TO THE FUTURE”
Interestingly, the 2000-year-old Biblical verse that asks “Do you not know that your bodies are temples of the Holy Spirit, who is in you, whom you have received from God?” (Corinthians 6:19) is the ancient predecessor of the expression “Orandum est ut mens sana in corpore sano sit”, a phrase which has been ascribed to the Roman poet Juvenal, author of the famous Satires. From this famous Latin aphorism emerges the medical proverb “Body, Mind and Spirit”, which describes the ideal triad necessary for maintaining or restoring health (Figure 1). However, following the onset of the Descartian method, only the measurable corporal phenomenon that could be proven rationally was considered to be science. Thus, quantitative studies became used to reinforce the paradigm of providing medical treatment by either surgery or prescription drugs[2,14]. Unfortunately, the formal study of how spirituality and emotions affect health fell outside of the scientific context of that time, only to be taken up years later by religious leaders or professionals in the fields of behavioral and social sciences[2,14,15].
Figure 1.
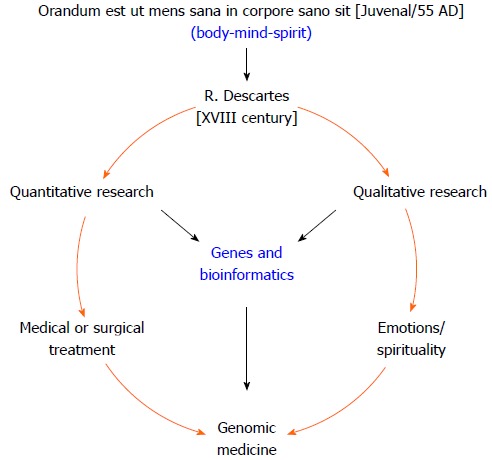
Esquematic representation of genomic medicine: a new paradigm shift or “back to the future”.
Interestingly, modern day genomic-era scientists have been faced with an unexpected paradox. They realized that the massive human DNA blueprint making up the “book of life” was insufficient to understanding how the human body works[16], i.e., what makes us get sick and how can we heal. However, there is now a consensus that additional environmental factors have been interacting with the human genome for millennia, thus contributing to man’s evolution. Some of these factors include diet, physical activity, and emotions/spirituality[17]. Overall, the advent of genomic medicine has evoked a medical paradigm shift caused by the effects that the evolving and dynamic interactions between the human genome and the environment have on the processes of the human body[18]. This shift in medical thinking is providing new knowledge into what makes people either prone or resistant to chronic diseases and is laying the framework needed to establish strategies for disease prevention and the development of new therapeutic interventions[19]. Thus, genomic medicine, with the aid of bioinformatics and biotechnology, should enable greater understanding of the medical aspects of human disease[20]. Such understanding may be achieved by studying the complex interaction between genes, the environment and culture with the goals of preventing illness and treating diseases[21].
What is making us sick and how should we manage illness?
The major public health obstacles of any given country or region are the diseases that cause the highest rates of mortality. In Latin America, as well as in several other developed countries, non-communicable chronic diseases are the foremost illnesses that affect people[22,23]. Most of these illnesses are caused by the ongoing obesity epidemic, and include cardiovascular disease, diabetes, cirrhosis, and cancer, as well as alcoholism. Obese patients commonly seek medical attention only once the clinical complications of obesity have become manifest, e.g., insulin resistance or type 2 diabetes, dyslipidemia, impaired distal circulation, nonalcoholic steatohepatitis (NASH) or vascular brain disease, among others. Furthermore, chronic illnesses frequently occur with co-morbidities. For example, alcoholism is frequently combined with some degree of liver damage due to NASH or viral hepatitis. Subsequently, this creates a costly burden for both the patient and the health care system because medicine is increasingly comprised of specialists that only treat one facet of a disease. For example, in the case listed above, an endocrinologist will control the metabolic problem, an infectious disease specialist will treat the viral infection, a hepatologist or gastroenterologist will treat the liver damage, and a cardiologist will treat any vascular problems. Similarly, in alternative medical fields such as medical molecular biology or the behavioral and social sciences, health professionals approach patients with chronic disease from their respective points of view (Figure 2).
Figure 2.
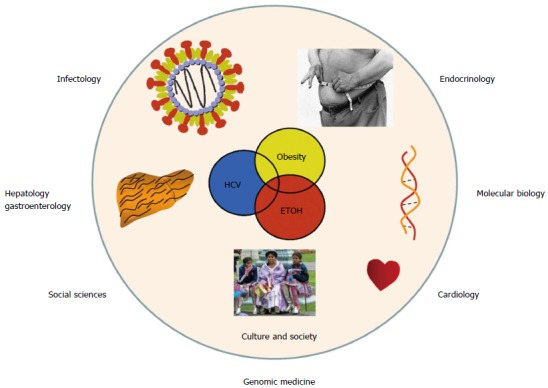
From medical specialties to genomic medicine. The same patient usually requires consultation by different medical specialists. However, from a genomic medicine viewpoint, modern medicine should be integrative to prevent or halt chronic disease at an early stage of development.
For this reason, the paradigm shift toward genomic medicine should focus more on disease prevention and less on treating patients at advanced stages of disease[24]. Genomic medicine can empower both physicians and their patients to more effectively prevent chronic disease, particularly if we come to understand the key genome-environmental interactions that affect our health. Furthermore, because these interactions regularly occur, chronic diseases should be looked upon as a continuum of bodily (unhealthy) changes that occur over the course of a lifetime. For example, obesity-related chronic diseases may advance through four stages of development: induction, progression, clinical complications, and death[25] (Figure 3). The induction phase begins in young adults between approximately 18 to 25 years of age. During this period of life the balance between caloric intake and expenditure can be lost as a once active person stops regularly exercising and becomes less physically active. As he or she enters into a sedentary lifestyle, which is associated with increased caloric intake, metabolic abnormalities may become manifest. First comes dyslipidemia and weight gain, followed by a gradual increase in blood glucose, which in turn raises serum insulin. Hyperglycemia and hyperinsulinemia develop next, followed by insulin resistance and, finally, overt type 2 diabetes mellitus. However, a period of 20 to 30 years may have elapsed between the start of induction and the advent of overt disease, and many patients present with additional medical complications by the time of diagnosis. For this reason, if we are to advance to old age in better health, we must ideally avoid the induction and progression stages of chronic disease (Figure 3). Thus, if a patient is either only gradually progressing through or just starting at an early stage of disease the best approach is to try to halt or reverse the physiopathological process, whereas in cases of patients that are in advanced stages of disease the goal of treatment is the provision of a better quality of life.
Figure 3.
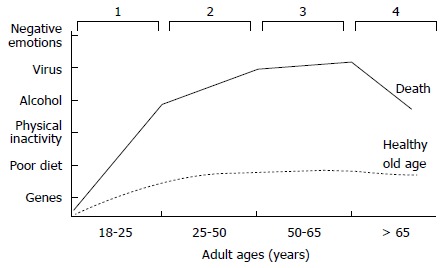
Four stages of the continuum of most chronic diseases. 1: Induction; 2: Disease progression; 3: Clinical complications; 4: Death. An individual with several risk factors may die at an earlier age (DARK LINE), in contrast, with an individual who has a healthier lifestyle (DASHED LINE).
However, co-morbidities are often associated with the same periods of the induction and progression stages of illness. For example, among the Mexican population the consumption of alcohol starts at adolescence[26]. The period between the initiation of drinking and the development of alcohol dependence and addiction is usually 25 to 30 years[26]. In the case of viral hepatitis and HIV, sexual promiscuity plays a predominant role as one of the major risk factors, as well as the exposure to viral hepatitis by contact with contaminated biological fluids[25,27]. As discussed above, 25 to 30 years may pass between the onset of infection and the onset of liver damage and cirrhosis. In the case of NASH it has been observed that liver damage may be detectable as early as ten years after a patient initially becomes overweight or obese. The result is that an individual who becomes overweight or obese may become addicted to alcohol, infected with a hepatotropic virus and/or may be susceptible to developing NASH[28]. Furthermore, patients suffering from obesity or type 2 diabetes may also have cardiovascular disease (Figure 4).
Figure 4.
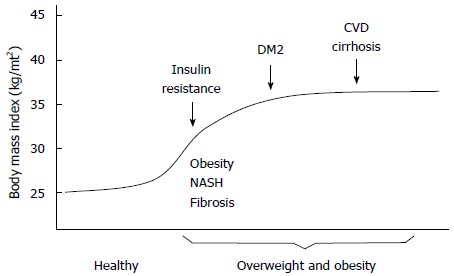
Overweight and obesity influence the onset of insulin resistance syndrome, a primary stage towards the development of type 2 diabetes mellitus, cardiovascular disease, nonalcoholic steatohepatitis fibrosis and cirrhosis. DM2: Type 2 diabetes mellitus; CVD: Cardiovascular disease; NASH: Nonalcoholic steatohepatitis.
However, most physicians know from experience that not every obese patient will progress to type 2 diabetes. Likewise, not all those who consume excessive alcohol will develop cirrhosis because some patients are more prone than others to developing liver damage[29]. Furthermore, NASH generally only manifests in obese patients who are genetically susceptible to the disease. In the case of viral hepatitis, not every infected patient will progress to cirrhosis and hepatocellular carcinoma[30]. Therefore, the variance in a patient’s susceptibility to developing a chronic disease seems to involve two important factors: genes and the environment.
GENETIC FACTORS
The genetic factors that modify the expression of genes comprise a broad range of structural and functional variations of the DNA molecule. The history of clinical genetics dates back to the time when chromosomal disorders, such as abnormalities in the structure or number of chromosomes, were associated with a particular syndrome. In general, these disorders are accompanied by congenital malformation, growth retardation, and short stature. Severe chromosomal abnormalities that arise in utero usually lead to miscarriage or the premature termination of gestation[31].
Further advances have spurred the investigation of pathologies that are associated with innate errors of metabolism, which can be identified by biochemical and genetic testing. Although the genetic defect may be diagnosed during the first few years of life, offspring born with these metabolic disorders are generally healthy at birth. These and other single-gene (monogenic) pathologies are denoted as Mendelian inheritance disorders and generally result from de novo or inherited mutations that are transmitted to offspring as dominant, recessive or X-linked traits[32]. For example, in the case of obesity-related disorders, a single structural change in one gene may lead to rare genetic diseases such as Prader-Willi, Fraser-Jequier-Chen, Alström or Bardet-Biedl syndromes[33]. In contrast, complex diseases (e.g., cardiovascular disease, cancers, type 2 diabetes, cirrhosis, obesity) are associated with multiple risk factors, i.e., the risk of disease arises from more than one gene (polygenic) interacting with different environmental factors[32]. By this definition, common obesity can be considered a polygenic disease because of its association with single nucleotide polymorphisms (SNPs) that encode allelic forms of proteins that act in lipid and carbohydrate metabolism, the regulation of food appetite and the circadian cycle[34] (Figure 5). However, not all SNPs and environmental factors are alike in all individuals, which results in variability in the worldwide distribution of SNPs and non-genomic risk factors within the global population[35,36].
Figure 5.
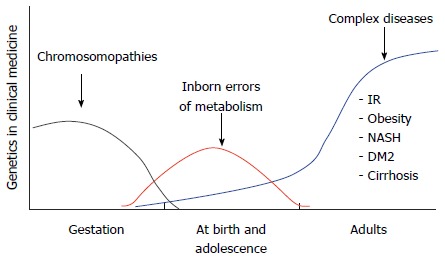
Impact of molecular biology in medicine: From cytogenetics to the study of complex diseases. DM2: Type 2 diabetes mellitus; IR: Insulin resistance; NASH: Nonalcoholic steatohepatitis.
With the advent of the Human Genome Project came an onslaught of novel medical technology that was based on the newly available human genetic data[37]. A current goal of genetic and genomic research is the identification of panels of SNPs that can be associated with complex common diseases, to better understand which populations are at risk of developing specific health conditions (e.g., colorectal cancer)[38]. Additionally, next generation sequencing technologies have advanced from whole-genome to whole-exome sequencing (WGS/WES) to better identify DNA variants that are associated with disease[39]. Likewise, genome-wide association studies analyze the relationship between the distribution of SNPs and disease outcomes among distinct human populations worldwide[40]. Given these advanced technologies, several genomic catalogs currently serve as complementary resources to the Online Mendelian Inheritance in Man database (http://www.omim.org), including the Human HapMap Project, the 1000 Genome Project, the Human Gene Mutation Database, and, most recently, the reference genes of the Human Gut Microbiome Project and Human Epigenome Project[41,42]. Furthermore, current research is focused on integrating these different genomic datasets to aid in the discovery of associations between genetic variants, expression quantitative trait loci (eQTLs), and disease phenotypes[43]. Altogether, these findings have had great impact on the practice of medicine and will continue to do so as we better refine disease prevention and treatment strategies based on the genome/environmental interactions that occur on the level of each individual, hence the term “personalized medicine”[44].
ENVIRONMENTAL FACTORS
Among numerous environmental factors, diet, physical activity, and emotional state may all serve to modulate man’s genetic susceptibility to chronic disease.
Diet
The ingestion of unhealthy food is one of the main factors associated with chronic disease. As discussed above, these diseases often begin at a young age, as an individual begins to become overweight, and then progress in response to obesity. Over the past 30 years, people in modern societies worldwide have acquired unhealthy lifestyle habits such as spending increasing amounts of time engaging in activities requiring very little energy expenditure and engaging in less exercise than before[45]. Additionally, the majority of obese patients have a high intake of calories in conjunction with reduced energy expenditure. If one considers obesity to be a mismatched disease[17] it is easy to understand how young people with unhealthy food habits gradually gain weight over their lifetimes, eventually leading to the development of specific dyslipidemias.
Dyslipidemia may be a primary or secondary lipid disorder; the former is gene-related and the latter is associated with the eating habits of an individual or a population[46]. Dyslipidemia that presents in the form of hypertriglyceridemia represents a critical risk factor for the development of insulin resistance and liver fibrosis[47], whereas hypercholesterolemia is associated with cardiovascular disease[48]. Furthermore, these altered metabolic states are associated with glucotoxicity and lipotoxicity in target organs, in addition to systematic inflammation that further deteriorates bodily health[49].
Global rates of obesity have risen dramatically, and Mexico has one of the world’s highest proportions of overweight and obese adults[50]. Genetically, Mexicans are both ApoE2 and ApoE4 carriers according to the population’s ancestral background[51]. The former has been associated with hypertriglyceridemia and the latter has been associated with hypercholesterolemia. Unhealthy Mexican diets are also hepatopathogenic because they consist of large quantities of simple carbohydrates and saturated animal fats and are low in fiber, vitamins and minerals[52,53]. The chronic consumption of this type of diet in conjunction with the genetic makeup of the population offers an explanation for the high prevalence of hypertriglyceridemia, hypercholesterolemia, and mixed dyslipidemia within Mexico, each of which are in turn risk factors for further metabolic complications[54].
With regard to the link between diet and inflammation, research into how the gut microbiome impacts health and disease has become a rapidly growing field[55,56]. Among the multiple causes of obesity and obesity-associated chronic diseases is the shift in dietary preferences from traditional/ethnic staple foods to more processed/industrial foodstuffs, a change which has altered the natural microbiome[50]. These foods have a high energy density and lack the natural and vital ingredients that act as antioxidants, which limits their capacity to inhibit inflammation and potentially prevent liver fibrosis. Furthermore, this so-called Western diet has been associated with an increased rate of food allergies[57], Crohn’s disease[58], ulcerative colitis[59], gastritis and gastroesophageal reflux disease[60]. Many of these conditions can be avoided if we focus on the relationship between health and the microbiota[61,62].
Physical activity
All activity generates some degree of caloric expenditure; thus any person that is not resting in a bed (sleeping) is undergoing some caloric expenditure through physical activity. However, routine physical activity must be differentiated from exercise, which is defined as a physical activity that is performed systematically in a timely manner[63]. Engaging in physical activity is another important factor toward maintaining good general health. There was once a myth among patients and physicians that those suffering from viral hepatitis just needed to rest and eat plenty of sweets. Currently, patients with NASH can reverse liver damage during the early stages of fibrosis by ensuring that they are of healthy weight, or alternatively by losing 10% of their original body weight over the course of six months[64]. This may be achieved by instructing the patient to increase physical activity or formally exercise, in addition to adopting a low-calorie diet. It is important to note that it is unlikely that the patient will succeed in losing weight if the indicated diet is not prescribed together with physical activity or exercise[65].
Regular exercise and/or increased physical activity may improve insulin sensitivity and cause significant metabolic changes in the lipid and glucose profiles of patients with initial or on-going chronic disease[66]. Additionally, physical activity can counteract low metabolic rates, improve the cardiovascular system, and promote a faster rate of weight loss than diet alone, in addition to promoting a feeling of well-being and alleviating psychological stress[66].
Emotions
Studies of the impact of emotions and spirituality on physical health make up a qualitative field of medical research that has been almost completely disregarded by health professionals[2,14]. Spiritual issues are mainly attended to by religious leaders, while altered states of emotional and mental health are the purview of psychologists and psychiatrists. However, with recent advances in molecular biology and the development of genomic medicine we can now begin to link genes to emotions. For instance, several polymorphisms may influence the response of an individual to the environment and thus influence an individual’s response to different emotions. One such example is the Val158Met polymorphism of the catechol-O-methyltransferase gene, which metabolizes dopamine and norepinephrine. The Met158 polymorphism encodes an unstable enzyme that increases the levels of dopamine in the prefrontal cortex generating a “worrier” personality type whereas the Val158 allele can generate a “warrior” personality type[67]. Individuals with the “warrior” Val158 allele perform worse on memory tests and at work, and in terms of executive cognition. Furthermore, individuals with the “warrior” Val158 allele tend to have stronger emotions, reduced pain thresholds and an increased resistance to stress[67].
Overall, the worldwide adoption of healthier lifestyle habits has proven to be a difficult undertaking. Many factors are involved in the lack of compliance in following medical indications on how to regain health. However, with regard to obesity, as is the case with any chronic disease, health professionals might be better at motivating more patients if they personally act to acquire healthier lifestyle habits, thus leading by example.
FINAL CONSIDERATIONS
From bench to bedside
Genomic medicine was once believed to be a promise of science fiction that would never progress into an actual practice; however, numerous medical disciplines have recently found applications to using this medical approach. For instance, individualized medicine has arisen in the clinical practices of gastroenterology and hepatology[68]. Several SNPs have been proposed that are believed to influence whether an alcoholic patient is either resistant or sensitive to liver damage[26]. Additionally, genes that control alcohol metabolism, genes that control addiction, and, more recently, genes which influence an individual to favor the taste of alcohol are all currently under investigation[26,69,70]. Several genes have been identified that influence the susceptibility of an individual to liver damage, which may explain why not all patients who are overweight or obese will develop NASH. Additionally, there are various genes that have been proposed to serve as genetic markers to help identify patients that may develop liver damage faster and more severely than others, who appear to tolerate liver damage for long periods of time[29,71]. In the first group the presence of hepatocellular carcinoma is usually very rare[30], whereas in the second group it is more prevalent. Finally, there are genes that have been found to be associated with sustained viral responses in patients being treated with antiviral therapy for viral hepatitis C[72].
Additionally, because many chronic diseases are related to nutrition, nutritional genomics has provided a new scope for the dietary management of these diseases[73]. As in the paradigm of personalized medicine, practitioners of nutritional genomics recommend a diet plan based on an individual’s genetic profile, in addition to the principles of conventional diet therapy and lifestyle modifications[50]. Furthermore, differences in the food preferences and types of food consumed between different populations are also considered to have evolved in response to gene-nutrient interactions[50]. Currently, nutritional genomic research is unraveling the evolutionary aspects that have influenced the selection of genes that cause modern-day chronic diseases[50,73-75]. As shown in Figure 6, this integrated approach of genomic medicine provides a new medical paradigm for disease prevention and treatment.
Figure 6.
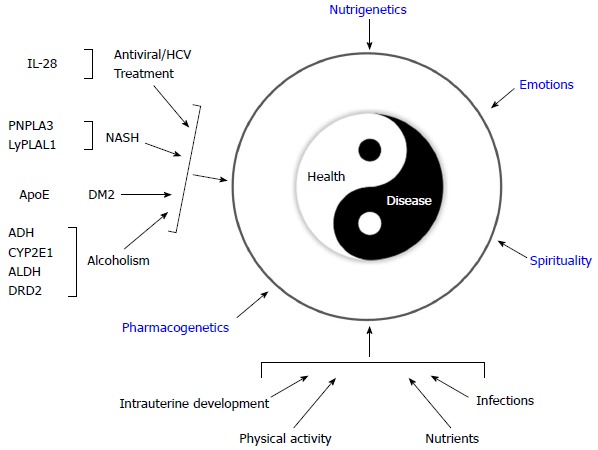
Genomic medicine: Interaction between specific polymorphisms with clinical implications and different environmental factors. Disease-related gene polymorphisms: IL-28: Interleukin 28B; PNPLA3: Patatin-like phospholipase domain-containing protein 3; LyPLAL1: lysophospholipase-like 1; ApoE: Apolipoprotein E; ADH: Alcohol dehydrogenase; CYP2E1: Cytochrome P450 2E1; ALDH: Aldehyde dehydrogenase; DRD2: Dopamine receptor D2. NASH: Nonalcoholic steatohepatitis; DM2: Type 2 diabetes mellitus.
Perspectives
Any scientific knowledge that causes a paradigm shift should eventually modify university curriculums. All professionals in the field of health science should strive to stay updated in their field, and genomic medicine is at present a reality. Thus, changes in curricula for medical and other health sciences students, residents and health professionals are only the beginning[76,77]. Furthermore, issues regarding the ethical, legal and social implications of human genomics in many fields of medicine, including gastroenterology, remain unresolved and controversial[38]. Although pre-test genetic counseling is formally handled by clinical geneticists, the delivery of genetic results by gastroenterologists and hepatologists may present a challenge that requires the acquisition of new training skills.
Therefore, whether using genomic medicine in the field of gastroenterology is a new approach or a new medical specialty remains an open question. Any decision made with regard to that matter will require the mutual work of educational and governmental authorities with public health professionals, with the goal of translating the knowledge generated in the field of genomic medicine (gene discovery and gene-environmental associations) into better health policies.
Footnotes
Conflict-of-interest statement: There is no conflict of interest in this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 28, 2015
First decision: March 10, 2015
Article in press: May 4, 2015
P- Reviewer: El-Nezami H, Elpek GO, Sanal MG, Liu ZW S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Ma S
References
- 1.Subbarayappa BV. The roots of ancient medicine: an historical outline. J Biosci. 2001;26:135–143. doi: 10.1007/BF02703637. [DOI] [PubMed] [Google Scholar]
- 2.Panduro A. Evolución de la medicina científica: dogmas, mitos y realidades. In: Panduro A, Ed , editors. Biología molecular en la clínica. 2da Ed. México, D. F: McGraw Hill; 2012. pp. 85–90. [Google Scholar]
- 3.Ventegodt S, Thegler S, Andreasen T, Struve F, Jacobsen S, Torp M, Aegedius H, Enevoldsen L, Merrick J. A review and integrative analysis of ancient holistic character medicine systems. ScientificWorldJournal. 2007;7:1821–1831. doi: 10.1100/tsw.2007.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao JJ, Kapur R. Acupuncture in primary care. Prim Care. 2010;37:105–117. doi: 10.1016/j.pop.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhruva A, Hecht FM, Miaskowski C, Kaptchuk TJ, Bodeker G, Abrams D, Lad V, Adler SR. Correlating traditional Ayurvedic and modern medical perspectives on cancer: results of a qualitative study. J Altern Complement Med. 2014;20:364–370. doi: 10.1089/acm.2013.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Descartes R. Discourse on method and meditations on first philosophy. 4th Ed. Indianapolis: Hackett Publishing Company; 1999. pp. 1–128. [Google Scholar]
- 7.González Hernández A, Domínguez Rodríguez MV, Fabre Pi O, Cubero González A. [Descartes’ influence on the development of the anatomoclinical method] Neurologia. 2010;25:374–377. [PubMed] [Google Scholar]
- 8.Looking back on the millennium in medicine. N Engl J Med. 2000;342:42–49. doi: 10.1056/NEJM200001063420108. [DOI] [PubMed] [Google Scholar]
- 9.Watson JD, Crick FH. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953;171:964–967. doi: 10.1038/171964b0. [DOI] [PubMed] [Google Scholar]
- 10.Portin P. Historical development of the concept of the gene. J Med Philos. 2002;27:257–286. doi: 10.1076/jmep.27.3.257.2980. [DOI] [PubMed] [Google Scholar]
- 11.Balbás P, Soberón X, Merino E, Zurita M, Lomeli H, Valle F, Flores N, Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50:3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- 12.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigó R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 13.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 14.Panduro A, Zepeda-Carrillo EA. De los genes a las emociones y a la espiritualidad. In: Panduro A, Ed , editors. Biología molecular en la clínica. 2da Ed. México, D. F: McGraw Hill; 2012. pp. 121–127. [Google Scholar]
- 15.Mabry PL, Olster DH, Morgan GD, Abrams DB. Interdisciplinarity and systems science to improve population health: a view from the NIH Office of Behavioral and Social Sciences Research. Am J Prev Med. 2008;35:S211–S224. doi: 10.1016/j.amepre.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldhous P. Genomics. Beyond the book of life. Nature. 2000;408:894–896. doi: 10.1038/35050235. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DE. The story of the human body: Evolution, health and disease. New York: Random House; 2013. pp. 1–480. [PubMed] [Google Scholar]
- 18.Loscalzo J, Kohane I, Barabasi AL. Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol. 2007;3:124. doi: 10.1038/msb4100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, Wilson R, Bick D, Bottinger EP, Brilliant MH, Eng C, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15:258–267. doi: 10.1038/gim.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bio*Medical Informatics and Genomic Medicine: Research and Training. J Biomed Inform. 2007;40:1–4. doi: 10.1016/j.jbi.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louie B, Mork P, Martin-Sanchez F, Halevy A, Tarczy-Hornoch P. Data integration and genomic medicine. J Biomed Inform. 2007;40:5–16. doi: 10.1016/j.jbi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Webber L, Kilpi F, Marsh T, Rtveladze K, Brown M, McPherson K. High rates of obesity and non-communicable diseases predicted across Latin America. PLoS One. 2012;7:e39589. doi: 10.1371/journal.pone.0039589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barquera S, Campos-Nonato I, Aguilar-Salinas C, Lopez-Ridaura R, Arredondo A, Rivera-Dommarco J. Diabetes in Mexico: cost and management of diabetes and its complications and challenges for health policy. Global Health. 2013;9:3. doi: 10.1186/1744-8603-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazaridis KN, McAllister TM, Babovic-Vuksanovic D, Beck SA, Borad MJ, Bryce AH, Chanan-Khan AA, Ferber MJ, Fonseca R, Johnson KJ, et al. Implementing individualized medicine into the medical practice. Am J Med Genet C Semin Med Genet. 2014;166C:15–23. doi: 10.1002/ajmg.c.31387. [DOI] [PubMed] [Google Scholar]
- 25.Panduro A, Maldonado-Gonzalez M, Fierro NA, Roman S. Distribution of HBV genotypes F and H in Mexico and Central America. Antivir Ther. 2013;18:475–484. doi: 10.3851/IMP2605. [DOI] [PubMed] [Google Scholar]
- 26.Roman S, Zepeda-Carrillo EA, Moreno-Luna LE, Panduro A. Alcoholism and liver disease in Mexico: genetic and environmental factors. World J Gastroenterol. 2013;19:7972–7982. doi: 10.3748/wjg.v19.i44.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman S, Panduro A, Aguilar-Gutierrez Y, Maldonado M, Vazquez-Vandyck M, Martinez-Lopez E, Ruiz-Madrigal B, Hernandez-Nazara Z. A low steady HBsAg seroprevalence is associated with a low incidence of HBV-related liver cirrhosis and hepatocellular carcinoma in Mexico: a systematic review. Hepatol Int. 2009;3:343–355. doi: 10.1007/s12072-008-9115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman S, Jose-Abrego A, Fierro NA, Escobedo-Melendez G, Ojeda-Granados C, Martinez-Lopez E, Panduro A. Hepatitis B virus infection in Latin America: a genomic medicine approach. World J Gastroenterol. 2014;20:7181–7196. doi: 10.3748/wjg.v20.i23.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández-Nazará ZH, Ruiz-Madrigal B, Martínez-López E, Roman S, Panduro A. Association of the epsilon 2 allele of APOE gene to hypertriglyceridemia and to early-onset alcoholic cirrhosis. Alcohol Clin Exp Res. 2008;32:559–566. doi: 10.1111/j.1530-0277.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 30.Roman S, Fierro NA, Moreno-Luna LE, Panduro A. Hepatitis B virus genotype H and environmental factors associated to the low prevalence of hepatocellular carcinoma in Mexico. J Cancer Ther. 2013;4:367–376. [Google Scholar]
- 31.van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 2012;1822:1951–1959. doi: 10.1016/j.bbadis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Genetic Alliance, the New England Public Health Genetics Education Collaborative. Understanding Genetics: A New England Guide for Patients and Health Professionals. Washington (DC): Genetic Alliance: 2010. Available from: http://www.ncbi.nlm.nih.gov/books/NBK132168/ [PubMed] [Google Scholar]
- 33.National Center for Advancing Translational Sciences. Office of Rare Diseases Research. Available from: http://rarediseases.info.nih.gov/ [DOI] [PMC free article] [PubMed]
- 34.Loos RJ. Recent progress in the genetics of common obesity. Br J Clin Pharmacol. 2009;68:811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Zolezzi I, Hidalgo-Miranda A, Estrada-Gil J, Fernandez-Lopez JC, Uribe-Figueroa L, Contreras A, Balam-Ortiz E, del Bosque-Plata L, Velazquez-Fernandez D, Lara C, et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc Natl Acad Sci USA. 2009;106:8611–8616. doi: 10.1073/pnas.0903045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentley DR. The Human Genome Project--an overview. Med Res Rev. 2000;20:189–196. doi: 10.1002/(sici)1098-1128(200005)20:3<189::aid-med2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 37.Wilson BJ, Nicholls SG. The Human Genome Project, and recent advances in personalized genomics. Risk Manag Healthc Policy. 2015;8:9–20. doi: 10.2147/RMHP.S58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008;322:881–888. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van El CG, Cornel MC, Borry P, Hastings RJ, Fellmann F, Hodgson SV, Howard HC, Cambon-Thomsen A, Knoppers BM, Meijers-Heijboer H, et al. Whole-genome sequencing in health care: recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2013;21:580–584. doi: 10.1038/ejhg.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manry J, Quintana-Murci L. A genome-wide perspective of human diversity and its implications in infectious disease. Cold Spring Harb Perspect Med. 2013;3:a012450. doi: 10.1101/cshperspect.a012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naidoo N, Pawitan Y, Soong R, Cooper DN, Ku CS. Human genetics and genomics a decade after the release of the draft sequence of the human genome. Hum Genomics. 2011;5:577–622. doi: 10.1186/1479-7364-5-6-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, Rodriguez-Mueller B, Zucker J, Thiagarajan M, Henrissat B, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput Biol. 2012;8:e1002358. doi: 10.1371/journal.pcbi.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabakchiev B, Silverberg MS. Expression quantitative trait loci analysis identifies associations between genotype and gene expression in human intestine. Gastroenterology. 2013;144:1488–1496, 1496.e1-1496.e3. doi: 10.1053/j.gastro.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epstein CJ. Medical genetics in the genomic medicine of the 21st century. Am J Hum Genet. 2006;79:434–438. doi: 10.1086/507610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 46.Pejic RN, Lee DT. Hypertriglyceridemia. J Am Board Fam Med. 2006;19:310–316. doi: 10.3122/jabfm.19.3.310. [DOI] [PubMed] [Google Scholar]
- 47.Berlanga A, Guiu-Jurado E, Porras JA, Auguet T. Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol. 2014;7:221–239. doi: 10.2147/CEG.S62831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee S, Kahali D, Banerjee A, Brilakis ES. LDL cholesterol: should guidelines include targets? Expert Rev Cardiovasc Ther. 2014;12:285–290. doi: 10.1586/14779072.2014.874284. [DOI] [PubMed] [Google Scholar]
- 49.Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roman S, Ojeda-Granados C, Ramos-Lopez O, Panduro A. Genome-based nutrition: an intervention strategy for the prevention and treatment of obesity and nonalcoholic steatohepatitis. World J Gastroenterol. 2015;21:3449–3461. doi: 10.3748/wjg.v21.i12.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aceves D, Ruiz B, Nuño P, Roman S, Zepeda E, Panduro A. Heterogeneity of apolipoprotein E polymorphism in different Mexican populations. Hum Biol. 2006;78:65–75. doi: 10.1353/hub.2006.0021. [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Lopez O, Román S, Ojeda-Granados C, Sepúlveda-Villegas M, Martínez-López E, Torres-Valadez R, Trujillo-Trujillo E, Panduro A. Patrón de ingesta alimentaria y actividad física en pacientes hepatópatas en el Occidente de México. Rev Endocrinol Nutr. 2013;21:7–15. [Google Scholar]
- 53.Aguilar-Salinas CA, Olaiz G, Valles V, Torres JM, Gómez Pérez FJ, Rull JA, Rojas R, Franco A, Sepulveda J. High prevalence of low HDL cholesterol concentrations and mixed hyperlipidemia in a Mexican nationwide survey. J Lipid Res. 2001;42:1298–1307. [PubMed] [Google Scholar]
- 54.Mathus-Vliegen L, Toouli J, Fried M, Khan AG, Garisch J, Hunt R, Fedail S, Štimac D, Lemair T, Krabshuis J, et al. World Gastroenterology Organisation global guidelines on obesity. J Clin Gastroenterol. 2012;46:555–561. doi: 10.1097/MCG.0b013e318259bd04. [DOI] [PubMed] [Google Scholar]
- 55.Konkel L. The environment within: exploring the role of the gut microbiome in health and disease. Environ Health Perspect. 2013;121:A276–A281. doi: 10.1289/ehp.121-a276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YK. Effects of diet on gut microbiota profile and the implications for health and disease. Biosci Microbiota Food Health. 2013;32:1–12. doi: 10.12938/bmfh.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Legatzki A, Rösler B, von Mutius E. Microbiome diversity and asthma and allergy risk. Curr Allergy Asthma Rep. 2014;14:466. doi: 10.1007/s11882-014-0466-0. [DOI] [PubMed] [Google Scholar]
- 58.Stasi C, Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohn’s disease. Dig Dis. 2008;26:156–166. doi: 10.1159/000116774. [DOI] [PubMed] [Google Scholar]
- 59.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 60.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 62.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 63.Luís Griera J, María Manzanares J, Barbany M, Contreras J, Amigó P, Salas-Salvadó J. Physical activity, energy balance and obesity. Public Health Nutr. 2007;10:1194–1199. doi: 10.1017/S1368980007000705. [DOI] [PubMed] [Google Scholar]
- 64.Glass LM, Dickson RC, Anderson JC, Suriawinata AA, Putra J, Berk BS, Toor A. Total body weight loss of ≥ 10 % is associated with improved hepatic fibrosis in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:1024–1030. doi: 10.1007/s10620-014-3380-3. [DOI] [PubMed] [Google Scholar]
- 65.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 66.Vázquez-Vandyck M, Roman S, Vázquez JL, Huacuja L, Khalsa G, Troyo-Sanromán R, Panduro A. Effect of Breathwalk on body composition, metabolic and mood state in chronic hepatitis C patients with insulin resistance syndrome. World J Gastroenterol. 2007;13:6213–6218. doi: 10.3748/wjg.v13.i46.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein DJ, Newman TK, Savitz J, Ramesar R. Warriors versus worriers: the role of COMT gene variants. CNS Spectr. 2006;11:745–748. doi: 10.1017/s1092852900014863. [DOI] [PubMed] [Google Scholar]
- 68.Lazaridis KN, Juran BD. American Gastroenterological Association future trends committee report: the application of genomic and proteomic technologies to digestive disease diagnosis and treatment and their likely impact on gastroenterology clinical practice. Gastroenterology. 2005;129:1720–1752. doi: 10.1053/j.gastro.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 69.Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramos-Lopez O, Roman S, Martinez-Lopez E, Gonzalez-Aldaco K, Ojeda-Granados C, Sepulveda-Villegas M, Panduro A. Association of a novel TAS2R38 haplotype with alcohol intake among Mexican-Mestizo population. Annals Hepatol. 2015:In press. [PubMed] [Google Scholar]
- 71.Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 2015;21:711–725. doi: 10.3748/wjg.v21.i3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fierro NA, Gonzalez-Aldaco K, Torres-Valadez R, Martinez-Lopez E, Roman S, Panduro A. Immunologic, metabolic and genetic factors in hepatitis C virus infection. World J Gastroenterol. 2014;20:3443–3456. doi: 10.3748/wjg.v20.i13.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibney MJ, Walsh MC. The future direction of personalised nutrition: my diet, my phenotype, my genes. Proc Nutr Soc. 2013;72:219–225. doi: 10.1017/S0029665112003436. [DOI] [PubMed] [Google Scholar]
- 74.Minich DM, Bland JS. Personalized lifestyle medicine: relevance for nutrition and lifestyle recommendations. ScientificWorldJournal. 2013;2013:129841. doi: 10.1155/2013/129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fenech M, El-Sohemy A, Cahill L, Ferguson LR, French TA, Tai ES, Milner J, Koh WP, Xie L, Zucker M, et al. Nutrigenetics and nutrigenomics: viewpoints on the current status and applications in nutrition research and practice. J Nutrigenet Nutrigenomics. 2011;4:69–89. doi: 10.1159/000327772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panduro A, Roman S, Escobedo G. Medicina genómica y hepatologíca. Investigación en Salud. 2006;8:105–111. [Google Scholar]
- 77.Román-Maldonado SM, Panduro A. Medicina genómica: Curriculum y formación de recursos humanos en salud. Investigación en Salud. 2005;8:98–104. [Google Scholar]


