Abstract
Liver cancer, a large proportion of which is hepatocellular carcinoma (HCC), is diagnosed in more than 700000 people each year worldwide. Liver cancer is particularly prevalent in Asia, Sub-Saharan Africa and the South Pacific, where hepatitis B and hepatitis C infection rates are very high. However, due to resistance to chemotherapy, patients with intermediate and advanced-stage disease cannot benefit from this treatment. Clusterin, which is overexpressed in many different cancers, is a stress-induced cytoprotective protein that confers treatment resistance. Custirsen (OGX-011) is a novel 2’-methoxyethyl modified phosphorothioate antisense oligonucleotide that targets secretory clusterin protein expression and is currently in clinical trials for patients with different cancers. In recent years, a number of different clinical trials have been performed, and two phase III clinical trials of custirsen evaluating combinations with chemotherapy in patients with metastatic castration-resistant prostate cancer and metastatic non-small cell lung cancer are currently in progress. The aims of this review are to summarize the current state of research on clusterin, predict future research directions and analyze the potential of the clinical application of custirsen in HCC.
Keywords: Hepatocellular carcinoma, Clusterin, Custirsen, Chemotherapy, Antisense oligonucleotides
Core tip: Resistance of liver cancer cells to chemotherapy continues to be a major clinical obstacle to extending the survival rate of patients with hepatocellular carcinoma (HCC). Custirsen, targeting secretory clusterin protein expression, is currently in clinical trials for patients with different cancers and considered to play a role in chemosensitization. This review is to summarize the current state of research on clusterin, to predict future research directions and to analyze the potential of the clinical application of custirsen in HCC.
INTRODUCTION
Liver cancer is the sixth most common neoplasm and the second most frequent cause of cancer-related death worldwide, and hepatocellular carcinoma (HCC) accounts for 70%-90% of the total liver cancer burden[1-3]. HCC is often associated with liver cirrhosis resulting from major chronic liver diseases including chronic hepatitis B and C virus infections, alcoholic liver disease and non-alcoholic steatohepatitis. Despite improved diagnostic techniques and increased screening of high-risk patients in developed countries, only 20%-30% of patients with HCC are diagnosed in early stages and have the opportunity to receive potentially curative treatments (surgical resection, transplantation or local ablation). Another 20% of patients have multifocal intra-hepatic tumors. For these patients, trans-arterial chemoembolization (TACE) has been considered a standard treatment strategy. The remaining half of patients present with advanced disease and must receive systemic therapy without surgical treatment[4,5].
Decreasing tolerance to chemotherapy is the key to improving the curative effects of systemic therapy for HCC. Clusterin is a stress-induced cytoprotective chaperone that confers treatment resistance and is overexpressed in response to cellular stress across a number of cancers, including standard cancer treatments, such as hormone therapy, radiation therapy and chemotherapy. Custirsen (OGX-011) is a novel phosphorothioate antisense oligonucleotide (ASO) compound that targets expression of the secretory clusterin protein (sCLU)[6,7]. This review aims to summarize important data and review research progress about the role of novel targeted agents in enhancing chemotherapy sensitivity and then indicate the potential research direction in HCC.
CLUSTERIN GENE STRUCTURE AND FUNCTION
The Clusterin (CLU) gene is a single-copy gene, located on chromosome 8p21-p12, where it is organized into nine exons, eight introns and a 5’-untranslated region, and it encodes three different transcriptional isoforms in humans [isoform 1, NM_001831, mRNA (GenBank); isoform 2, NR_038335, non-coding RNA (GenBank); and isoform 3, NR_045494, non-coding RNA (GenBank)][8]. The latest data from GenBank showed that isoform 1 encodes the functional protein and isoforms 2 and 3 represent non-coding RNAs due to the presence of an upstream ORF that is predicted to interfere with translation of the longest ORF. In humans, there are two proteins encoded by this gene: secretory CLU protein (sCLU) (75-80 kDa) and nuclear CLU protein (nCLU) (55 kDa)[9,10]. The CLU gene encodes for an mRNA of 2877 bp, which translates to a 449-amino acid polypeptide. The first 22 amino acids of the secretory glycoprotein (75-80 kDa) represent a classical hydrophobic leader signal sequence, and these acids are translated into a leader polypeptide that targets the protein to the endoplasmic reticulum (ER). There, the 22-mer peptide is removed through proteolytic processing, producing a 50-kDa precursor, which is further processed to a 60-kDa form in the ER. As the protein is transported to the Golgi complex, it is glycosylated and further proteolytically cleaved into two 40-kDa subunits, α- and β-chains. Then, the two subunits are assembled into a heterodimeric complex, which is linked by five disulfide bonds[9,11]. The nuclear CLU protein (55 kDa) is synthesized from a truncated, alternatively spliced nuclear clusterin mRNA, which lacks exon II (containing the first AUG and encoding the ER-targeting secretion signal sequence). Exons I and III are spliced together, placing the second in-frame AUG in exon III as the first available translation start site. Translation of the nuclear clusterin mRNA produces a about 49-kDa pre-nuclear clusterin (pnCLU) protein, which localizes in the cytoplasm and remains dormant, presumably by hiding the nuclear localization site (NSL) within the C-terminal portion of the protein. Following whole-body ionizing radiation (IR) or cell damage, the pnCLU protein can be post-translationally modified to generate a mature about 55-kDa pro-apoptotic protein form (nCLU), which has N-and C-terminal coiled-coil domains[10,12]. Multiple reports have shown that the sCLU protein is cytoprotective and anti-apoptotic, whereas the nCLU protein is pro-apoptotic[10,13]. Overexpression of sCLU in human prostate cancer cells results in treatment resistance and cytoprotection against TNF, androgen withdrawal, and a variety of cytotoxic agents, such as paclitaxel, gemcitabine, cisplatin and docetaxel, whereas depletion of the sCLU protein using siRNA increases sensitivity to treatment[14-18]. Similar sCLU properties have been observed in chemoresistance to various chemotherapeutic agents found in many other tumor types, including breast, lung, kidney and pancreas cancer cells[13,17,19-22]. Based on these results, targeting sCLU to sensitize cancer cells to chemotherapy has become an attractive new strategy for cancer treatment.
CUSTIRSEN (OGX-011) IN CLINICAL TRIALS
Antisense oligonucleotide (ASO) therapy is a method for inhibiting specifically targeted genes. ASOs are short synthetic analogues of natural nucleic acids, complementary to mRNA regions of a targeted gene that induce selective degradation of the mRNA or inhibit translation of the selected mRNA into protein[23]. Custirsen is a second-generation 2’-MOE-modified phosphorothioate ASO, which is a 21-nucleoside complement to the clusterin gene exon II mRNA translation initiation site with one CpG motif. It is considered to be a potent inhibitor of sCLU expression in vitro, in vivo and in human clinical trials, with no apparent effect on the expression of nCLU[20]. Hence, custirsen plays the role of chemosensitization by influencing the anti-apoptotic protein sCLU instead of the pro-apoptotic protein nCLU. In preclinical studies, custirsen significantly increased the treatment effects of hormone therapy and chemotherapy in multiple tumors, including non-small cell lung, prostate, breast, bladder and kidney cancers[9,13,18]. Thus far, there have been three phase I and six phase II clinical trials, which have been completed with a total of 390 patients enrolled (101 patients in phase I and 289 patients in phase II)[24-29].
Phase I clinical trials
Twenty-five patients with localized prostate carcinoma and high-risk features (the first-in-humans phase I trial), were treated with custirsen (40 mg, 80 mg, 160 mg, 320 mg, 640 mg) by 2-h intravenous infusion on days 1, 3, 5, 8, 15, 22, and 29 for one cycle. Then, radical prostatectomy was performed within 7 d of the last custirsen dose. In this trial, one subject was designated to determine the relationship between the plasma and prostate tissue concentration of custirsen and clusterin expression in prostate tissues and lymph nodes following dose-dependent custirsen treatments. Custirsen treatment in all of the patients was well tolerated and produced a custirsen distribution in the prostate tissues, with a dose-dependent decrease in clusterin expression and an associated increase in the apoptotic index. Furthermore, an effective biologic dose of 640 mg was established for phase II trials, based on its ability to suppress clusterin mRNA expression by > 90%[24]. In the subsequent phase I trial[25], 40 patients with different malignancy types, including prostate, ovary, renal, non-small cell lung, bladder and breast cancers, were enrolled in two groups with treatments of escalating doses of custirsen (40 mg-640 mg) with weekly docetaxel (30 mg/m2, intravenous) or docetaxel (75 mg/m2, intravenous) every 3 wk. In this study, custirsen at a biologically active dose was shown to be viable when combined with standard doses of docetaxel. More importantly, although the incidence of gastrointestinal toxicity was higher for higher dose levels of custirsen, the overall toxicity of the combination was mild or moderate. Furthermore, the incidence and quality of these toxicities were considered consistent with those of single-agent docetaxel, and there was no significant worsening with dose escalation. In addition, a consistent decrease in serum clusterin level was observed at the 640 mg dose, which has been recommended as the biologically effective dose for subsequent phase II trials. The third trial, registered in Clinical Trials.-Gov., was an open-label, one-arm, one-sequence crossover drug-drug interaction study in advanced solid tumor subjects whose aim was to evaluate the potential effects of custirsen on the pharmacokinetics of paclitaxel. This trial started on December 9, 2011, and was completed in October 2013. The results of this trial are being analyzed, and thus for, no original articles have been published in PubMed (Table 1).
Table 1.
Summary of the phase I clinical trials of custirsen
| Clinical trial | Notes | Drugs | Patients, n | Phase |
| OGX-011-01[24] | Dose escalation, single-center study in patients receiving hormone therapy and custirsen prior to radical prostatectomy | Buserelin, custirsen sodium, flutamide | 25 | I |
| Hormone therapy and OGX-011 before radical prostatectomy in treating patients with prostate cancer (NCT00054106) | ||||
| OGX-011-2[25] | Dose escalation, multi-center, study with custirsen in combination with docetaxel in patients with CRPC, NSCLC, breast, ovarian, bladder or renal cancer | Custirsen sodium, docetaxel | 40 | I |
| OGX-011 and docetaxel in treating patients with metastatic or locally recurrent solid tumors (NCT 00471432) | ||||
| OGX-011-10 | Open-label, one arm, one-sequence crossover drug-drug interaction study in advanced solid tumors subjects to evaluate the potential effect of custirsen on the pharmacokinetics of paclitaxel | Custirsen, paclitaxel and carboplatin | 36 | I |
| A clinical study in cancer patients to investigate the potential impact of custirsen on the blood levels of the chemotherapeutic drug, paclitaxel, when given together as part of a treatment regimen (NCT01497470) |
Phase II clinical trials
Six phase II clinical trials of custirsen were performed to further evaluate the safety and efficacy of custirsen in combination with various cancer therapies: three prostate cancer trials[26,27]; one lung cancer trial[28]; and one breast cancer trial[29]. In the breast cancer trial, five of fifteen patients had confirmed partial responses for an overall response rate of 33%, which was one of the primary objectives of this trial. The median duration of stable disease was 9.3 mo, and the toxic effects were similar to those with single-agent docetaxel. Serum clusterin levels as mean percentage changes (relative to baseline) decreased to 23.3%, 25.9% and 32.1% on days 8, 15, and 22, respectively, yet there was no relationship between the response rate and the serum clusterin[29]. Subsequently, in lung cancer trial, the results were confirmed by Laskin et al[28] In this trial, custirsen was administered for patients with NSCLC. Tumor response to custirsen in combination with gemcitabine/platinum occurred in 25 (31%) of 81 patients, and the 1- and 2-year survival rates were 54% and 30%, respectively. The toxicity of the combination was not obviously different from what has been reported for gemcitabine/platinum combinations. Serum CLU levels were significantly decreased after custirsen treatment by day 1 of cycle 2 or 3, with 52 of the 55 patients exhibiting a reduction in CLU during treatment compared with their baseline levels (Table 2).
Table 2.
Summary of the phase II clinical trials of custirsen
| Clinical trial | Notes | Drugs | Patients, n |
| OGX-011-3[26] Docetaxel and prednisone with or without OGX-011 in treating patients with recurrent or metastatic prostate cancer that did not respond to previous hormone therapy (NCT00258388) | Randomized, two-arm, open label, multi-center study in patients who received docetaxel/prednisone with/without custirsen | Custirsen sodium: 640 mg iv for 2 h-Cycle 1: days 7, 5, 3, 1, 8, 15 (4 wk cycle) Docetaxel: 75 mg/m2 iv for 1 h-day 1 every 3 wk (3 wk cycles) Prednisone: 5 mg PO bid | 82 |
| 0GX-011-04 Hormone ablation therapy in patients with localized prostate cancer | A single-arm, open-label, single center study to assess the effects of combined hormone ablation therapy and weekly treatment with custirsen prior to surgical removal of the prostate gland | LHRH agonist (hormone ablation) was given with weekly custirsen treatment for 12 wk and prostatectomy performed within 14 d of the last dose of custirsen | 24 |
| OGX-011-05[28] A study of OGX-011/Gemcitabine/Platinum-based regimen in stage IIIB/IV non-small cell lung cancer (NCT00138658) | An open-label trial to assess the safety and anti-tumor effect of OGX-011 when given to patients in combination with GEM and CIS/CARB | Custirsen sodium: 640 mg iv for 2 h Cycle 1: days 7, 5, 3 and days 1, 8, 15 of every 21-d cycle GEM: days 1 and 8 Cisplatin/Carboplatin: day 1 6 cycle 2 | 81 |
| OGX-011-06[29] OGX-011 and docetaxel in treating women with locally advanced or metastatic breast cancer (NCT00258375) | To study how well giving OGX-011 together with docetaxel works in treating women with locally advanced or metastatic breast cancer | Custirsen sodium: 640 mg iv for 2 h on days 7, 5, 3, 1, 8 and 15 of cycle 1 and on days 1, 8, 15 on the other cycle Docetaxel: 75 mg/m2 iv for 1 h on days 1 and 8 | 15 |
| OGX-011-07[27] Evaluation of safety and feasibility of OGX-011 in combination with second line chemotherapy in patients with HRPC (NCT00327340) | A multicenter, open-label, randomized study evaluating the safety and feasibility of custirsen in combination with second line chemotherapy in patients with HRPC | Prednisone: 5 mg twice daily through completion of the final treatment cycle. Custirsen: 640 mg iv for 2 h on days 9 to 1 of every cycle Mitoxantrone: 12 mg/m2, iv on day 1. Docetaxel: 75 mg/m2, iv on day 1 | 42 |
| Assessing the effects of combined therapy with androgen ablation and OGX-011 given prior to radical prostatectomy on pathologic complete response rates in men with localized prostate cancer and high risk features (NCT00138918) | An open-label, non-blinded, phase II clinical, tissue pharmacokinetic and pharmacodynamics study of weekly OGX-011 and neoadjuvant hormone therapy prior to radical prostatectomy in patients with localized prostate carcinoma and high risk features | Neoadjuvant hormone therapy: buserelin 9.9 mg subcutaneously × 1 injection with flutamide 250 mg orally tid for the first 4 wk OGX-011: 640 mg by intravenous infusion over 2 h on days 1, 3, and 5 | 45 |
Then subsequently, a total of 82 patients with metastatic CRPC were enrolled in a multicenter trial to evaluate the clinical and biologic activity of custirsen in combination with standard docetaxel and prednisone treatment[26]. Median serum clusterin decreased by 26% in the custirsen plus docetaxel/prednisone arm (arm A, 41 patients) and increased by 0.9% in the docetaxel/prednisone only arm (arm B, 41 patients). A PSA decrease of ≥ 50 was observed in 23 patients in arm A and 22 patients in arm B. The results from this trial also showed that the median PFS time and the median OS estimate were 7.3 mo and 23.8 mo in arm A and 6.1 mo and 16.9 mo in arm B, respectively. This trial demonstrated that clusterin is a useful therapeutic target, and it supported the conclusion of improved survival outcomes for patients treated with docetaxel and custirsen, so further evaluation of the combination (phase III trial) is warranted. In a recent phase II trial of custirsen, male patients were randomized to receive docetaxel/mitoxantrone in combination with custirsen to evaluate the safety and efficacy of two second-line treatments for mCRPC[27]. Twenty patients were treated with docetaxel plus custirsen (arm A), and twenty-two patients were treated with mitoxantrone plus custirsen (arm B). The overall survival rate and median time to pain progression were 15.8 mo and 10.0 mo in arm A and 11.5 mo and 5.2 mo in arm B, respectively. PSA decreases of 90% or more, 50% or more, and 30% or more occurred in 4, 8 and 11 patients, respectively, in arm A, and PSA decreases of 50% or more and 30% or more in 6 and 7 patients, respectively, in arm B. This trial provided the evidence that combining custirsen with chemotherapy is feasible in patients with progressive metastatic CRPC following first-line docetaxel therapy and that custirsen treatment significantly reduces the mean CLU level during treatment, compared with baseline. For the first time, this trial identified a correlation between serum levels and survival that supported further evaluation of serum CLU, which is being evaluated in phase III studies of custirsen as a predictive biomarker. Another trial was designed to assess the effects of combined hormone ablation therapy and weekly treatment with custirsen prior to surgery in patients with localized prostate cancer. Twenty-four patients were enrolled in this trial and were treated with an LHRH agonist in combination with weekly custirsen treatment for 12 wk, with prostatectomy performed within 14 d of the last dose of custirsen. Decreased expression of clusterin and an increase in the apoptotic index of prostate tumor cells were observed, although neoadjuvant treatment was not associated with pathologic complete response.
Ongoing phase III clinical trials
Currently, there are two randomized, global, phase III trials of custirsen in combination with chemotherapy agents compared with chemotherapy agents alone. The AFFINITY trial (NCT01578655) aims to compare cabazitaxel/prednisone alone to a combination with custirsen for second-line chemotherapy in prostate cancer. This trial is a randomized, open-label, multicenter, international trial that was designed to confirm that adding custirsen to cabazitaxel/prednisone treatment can slow tumor progression and enhance survival outcomes compared to standard cabazitaxel/prednisone treatment in men with metastatic CRPC. A total of approximately 630 patients will be randomized in equal numbers to the two arms. The estimated study completion date of this trial is December 2015. Another phase III trial, known as ENSPIRIT (NCT01630733), has been designed to compare the overall survival of patients randomized to receiving custirsen in combination with docetaxel (arm A) to that of patients randomized to receive docetaxel alone (arm B). Eleven hundred patients with advanced or metastatic NSCLC who have failed platinum-based therapy will be required for this trial. This trial started in September 2012, and it is estimated to conclude in July 2017 (Table 3).
Table 3.
Summary of the phase III clinical trials of custirsen
| Phase III trial | Treatment and Arms | Patients, n | Completion Date |
| ENSPIRIT A multinational, randomized, open-label study of custirsen in patients with advanced or metastatic non-small cell lung cancer (NCT01630733) | Arm A: Custirsen Docetaxel Custirsen: Three loading doses of custirsen 640 mg iv over 2 h administered in 5 to 9 d prior to day 1 of cycle 1, then custirsen 640 mg iv weekly every 21-d cycle Docetaxel: 75 mg/m2 iv over 1 h on day 1 of every 21-d cycle Arm B: Docetaxel Docetaxel: 75 mg/m2 iv over 1 h on day 1 of every 21-d cycle until disease progression, unacceptable toxicity, withdrawal of consent or protocol specified parameters to stop treatment | 1100 | July, 2017 |
| AFFINITY | Drugs: cabazitaxel, prednisone, custirsen sodium | 630 | December, 2015 |
| Comparison of cabazitaxel/prednisone alone or in combination with custirsen for second line chemotherapy in prostate cancer (NCT01578655) | Following 2 loading doses of custirsen (640 mg iv), cabazitaxel (25 mg/2 iv) is administered on a 3-wk cycle with weekly custirsen (640 mg iv) and daily prednisone (10 mg PO) until disease progression, unacceptable toxicity, or completion of 10 cycles Drugs: cabazitaxel, prednisone Cabazitaxel (25 mg/m2 iv) is administered on a 3-wk cycle with daily prednisone (10 mg PO) until disease progression, unacceptable toxicity, or completion of 10 cycles | ||
| SYNERGY Comparison of docetaxel/prednisone to docetaxel/prednisone in combination with OGX-011 in men with prostate cancer (NCT01188187) | Drugs: custirsen sodium, docetaxel, prednisone Docetaxel/prednisone on a 3-wk cycle with weekly OGX-011 640 mg infusions until disease progression, unacceptable toxicity, or completion of 10 cycles. Drugs: docetaxel, prednisone Docetaxel/prednisone on a 3-wk cycle until disease progression, unacceptable toxicity, or completion of 10 cycles | 1023 | April, 2014 |
One completed phase III trial
The phase III SYNERGY trial was completed in April 2014, and was designed to confirm that adding custirsen to standard first-line docetaxel/prednisone treatment could slow tumor progression and enhance survival outcomes compared to standard first-line docetaxel/prednisone treatment alone. In this trial, 1000 male patients with prostate cancer were randomly assigned with equal probability to two arms. In the first half of 2014, the results from this trial were announced by the company OncoGenex Pharmaceuticals. The results suggested that custirsen plus standard first-line docetaxel/prednisone therapy did not display a statistically significant improvement in overall survival in male patients with metastatic CRPC, compared to docetaxel/prednisone alone (median survival: 23.4 mo and 22.2 mo, respectively). A thorough analysis of this trial must be performed to explore the potential key factors that might have contributed its results. Although the results of this phase III trial were unexpected, there are still two phase III trials and one phase II trial ongoing. We need to await the results from the ongoing trials to perform a comprehensive analysis of data from completed trials (Table 3).
Preclinical studies of sCLU in HCC
The CLU gene was first detected in ram rete testis fluid and was subsequently found in many different tissues[30-32]. Thus, at the beginning of study into the gene, it had many different names, such as SGP-2[33], SP40-40[31], TRPM-2[34], and ApoJ[35]. After complete cDNA cloning, sequencing and comparison, it was proved that SGP-2 and TRPM-2 were identical to CLU[36,37]. Today, this protein is officially named CLU. It was detected for the first time in HCC by Tobe et al[38], who found that SP40-40 could be expressed in hepatoma cells and also provided further evidence for the assignment of the human SP40-40 gene to chromosome 8. sCLU is a unique glycoprotein thought to be involved in numerous physiological processes, including lipid transport, the regulation of complement function, programmed cell death and membrane recycling by regulating some potential pathways (Figure 1)[39]. The Burkey group found that human HCC cells (HepG2) could secrete Apo lipoprotein J (Apo-J), another name for sCLU, in association with a significant amount of lipid, providing unequivocal evidence that Apo-J could transport lipids[35]. This research also demonstrated that Apo-J was a secreted lipid transport protein associated with all of the major lipid classes that could play an important role in lipid transportation. In addition, other researchers found that TRPM-2 mRNA could be induced by heat shock treatment of the human culture cell line HepG2[40]; and this heat shock induction was observed in culture cells derived from different animal species. Because there might be a relationship between heat shock and apoptosis[41], TRPM-2 might play a role in the process of apoptosis[40]. As research continued, sCLU was found to be expressed in many human tumors, including breast, lung, bladder, kidney and prostate cancers. Its expression has been documented to lead to broad-based resistance to other unrelated chemotherapeutic agents such as doxorubicin and cisplatin[14-16,18]. To explore the expression of clusterin in HCC, Kang et al[42] examined 100 surgically resected HCCs using the tissue microarray method, and they found that a total of 89 HCCs exhibited clusterin overexpression, which was associated with poor Edmondson’s histological grades and high TNM stages. Another group demonstrated that CLU overexpression was an important factor for metastasis and this might be related to YKL-40 in playing the metastatic role in HCC. However, the study by Aigelsreiter et al[43] found the opposite results after analysis, and the authors reported that the reasons for this discrepancy might be methodological differences or differences in antigen preservation. In addition, Hsieh et al[44] suggested that dysregulation of the clusterin gene in human hepatoma was most likely due to cellular responses to external stresses, particularly during sample collection procedures, rather than due to any correlations with hepatoma development or progression. External stresses or micro-environmental changes can greatly affect gene or protein expression. Although there is varying sCLU protein expression in a number of different liver cancer tissues, sCLU might also contribute to resistance of HCC to chemotherapy and could play a role in promoting HCC metastasis[45]. Our previous results demonstrated that overexpression of sCLU abrogated OXA-induced inhibition of cell growth and cell apoptosis, but depletion of sCLU synergized with OXA to inhibit cell growth and enhance cell apoptosis, by regulating the proteins involved in mitochondrial apoptosis pathways. sCLU could be a novel molecular target for overcoming OXA resistance in HCC[21,22]. In recent years, an increasing number of reports have provided evidence that a proteomic approach is a promising method for discovering and identifying novel biomarkers for HCC. In Asako Kimura’s research, three-step proteome analyses were performed in serum samples, and 83 up-regulated proteins, the most significantly overexpressed of which was sCLU, were found in the serum of HCC patients. The overexpression of serum sCLU was confirmed by ELISA using another group of HCC samples, and the author confirmed that sCLU was a potential novel serum marker for HCC[46]. The same conclusion was also verified by another research center[47]. However, opposite conclusions were obtained from three different research groups[48-50], all of which demonstrated that serum sCLU levels in HCC patients were significantly lower than those in healthy patients but statistically higher than in cirrhosis patients. The reasons for this discrepancy might include that the total number of cases was insufficient and that the selection criteria for patients were different. Therefore, a large number of clinical trials in different research centers are necessary to explore the potential of serum clusterin as a biomarker in HCC.
Figure 1.
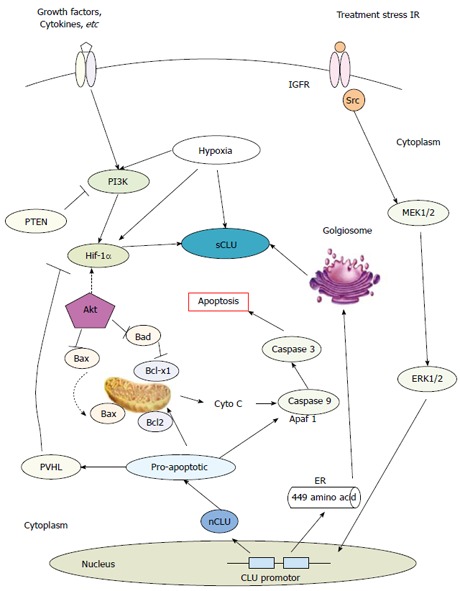
Potential mechanisms of secretory clusterin in tumors[21,22,39]. ER: Endoplasmic reticulum; Cyto C: Cytochrome c; IGFR: Insulin-like factor receptor.
CONCLUSION
Custirsen (OGX-011) is a second-generation antisense molecule designed to block the expression of the protein sCLU, which is up-regulated in tumor cells in response to treatment interventions, such as chemotherapy, hormone ablation and radiation therapy. In recent years, studies of the Clusterin gene have led to the understanding of entirely new mechanisms of cancer drug resistance (Figure 1). Studies from multiple canters worldwide have indicated the potential for the development of therapeutic strategies that aim to overcome cancer cell drug resistance and the targeting of clusterin as an early diagnostic serum biomarker in HCC. However, in the coming years, questions remain regarding the role of Clusterin gene in HCC that must be explored. First, additional experiments exploring the expression of sCLU in HCC tissues are required to confirm the relationships between sCLU expression and clinicopathologic parameters in HCC patients, and more samples are needed from HCC patients with HBV or HCV infection from multiple research centers. Second, in recent years, an increasing number of reports have provided evidence that clusterin could be a novel biomarker for HCC. However, the research about the pertinence of sCLU expression in tumor tissues and blood serum is limited. If we could find the relationship between the sCLU expression in tumor tissues and blood serum, in other words, if the contents of sCLU in blood serum could predict the expression of sCLU in tumor tissues, there will be of great significance for the individualized treatment in HCC. Third, understanding the mechanisms and the signaling pathways regulated by sCLU could be critical to unravelling the solution for multi-drug resistance in HCC. Then, further investigations should be targeted at the mechanisms and signaling pathways regulated by sCLU in HCC. Furthermore, although additional preclinical trials and clinical trials are necessary to explore the role of sCLU in HCC, targeting the sCLU with custirsen could also validate the approach as a systemic therapy to increase chemotherapy sensitivity. A deeper understanding of the mechanisms and the role of sCLU in anticancer drug resistance might reveal that custirsen is a valuable therapeutic agent for overcoming anticancer drug resistance in advanced HCC.
Footnotes
Supported by National Nature Science Foundation of China, No. 81172331 and No. 30972890; and Shandong Provincial Medicine and Health Science Technology Development Planning, China, No. 2013WS0145.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 23, 2014
First decision: March 10, 2015
Article in press: May 21, 2015
P- Reviewer: Lei M, Yang Y S- Editor: Yu J L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Scaggiante B, Kazemi M, Pozzato G, Dapas B, Farra R, Grassi M, Zanconati F, Grassi G. Novel hepatocellular carcinoma molecules with prognostic and therapeutic potentials. World J Gastroenterol. 2014;20:1268–1288. doi: 10.3748/wjg.v20.i5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 5.Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: present and future. J Gastroenterol Hepatol. 2012;27:862–872. doi: 10.1111/j.1440-1746.2012.07096.x. [DOI] [PubMed] [Google Scholar]
- 6.Chi KN, Zoubeidi A, Gleave ME. Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin for the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1955–1962. doi: 10.1517/13543780802528609. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski R, Chi KN. Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin in development for the treatment of prostate cancer. Future Oncol. 2012;8:1239–1251. doi: 10.2217/fon.12.129. [DOI] [PubMed] [Google Scholar]
- 8.Wong P, Borst DE, Farber D, Danciger JS, Tenniswood M, Chader GJ, van Veen T. Increased TRPM-2/clusterin mRNA levels during the time of retinal degeneration in mouse models of retinitis pigmentosa. Biochem Cell Biol. 1994;72:439–446. doi: 10.1139/o94-058. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Zhou W, Kundu S, Jang TL, Yang X, Pins M, Smith N, Jovanovic B, Xin D, Liang L, et al. The leader sequence triggers and enhances several functions of clusterin and is instrumental in the progression of human prostate cancer in vivo and in vitro. BJU Int. 2006;98:452–460. doi: 10.1111/j.1464-410X.2006.06263.x. [DOI] [PubMed] [Google Scholar]
- 10.Leskov KS, Araki S, Lavik JP, Gomez JA, Gama V, Gonos ES, Trougakos IP, Matsuyama S, Boothman DA. CRM1 protein-mediated regulation of nuclear clusterin (nCLU), an ionizing radiation-stimulated, Bax-dependent pro-death factor. J Biol Chem. 2011;286:40083–40090. doi: 10.1074/jbc.M111.252957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki S, Israel S, Leskov KS, Criswell TL, Beman M, Klokov DY, Sampalth L, Reinicke KE, Cataldo E, Mayo LD, et al. Clusterin proteins: stress-inducible polypeptides with proposed functions in multiple organ dysfunction. BJR supplement/BIR. 2005;27:106–113. [Google Scholar]
- 12.Leskov KS, Klokov DY, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin, a cell death protein. J Biol Chem. 2003;278:11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- 13.Kususda Y, Miyake H, Gleave ME, Fujisawa M. Clusterin inhibition using OGX-011 synergistically enhances antitumour activity of sorafenib in a human renal cell carcinoma model. Br J Cancer. 2012;106:1945–1952. doi: 10.1038/bjc.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sintich SM, Steinberg J, Kozlowski JM, Lee C, Pruden S, Sayeed S, Sensibar JA. Cytotoxic sensitivity to tumor necrosis factor-alpha in PC3 and LNCaP prostatic cancer cells is regulated by extracellular levels of SGP-2 (clusterin) Prostate. 1999;39:87–93. doi: 10.1002/(sici)1097-0045(19990501)39:2<87::aid-pros2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein j gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64:1834–1842. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Jin RJ, Kwak C, Jeong H, Park MS, Lee NK, Lee SE. Suppression of clusterin expression enhanced cisplatin-induced cytotoxicity on renal cell carcinoma cells. Urology. 2002;60:516–520. doi: 10.1016/s0090-4295(02)01806-x. [DOI] [PubMed] [Google Scholar]
- 17.Tang Y, Liu F, Zheng C, Sun S, Jiang Y. Knockdown of clusterin sensitizes pancreatic cancer cells to gemcitabine chemotherapy by ERK1/2 inactivation. J Exp Clin Cancer Res. 2012;31:73. doi: 10.1186/1756-9966-31-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleave M, Miyake H. Use of antisense oligonucleotides targeting the cytoprotective gene, clusterin, to enhance androgen- and chemo-sensitivity in prostate cancer. World J Urol. 2005;23:38–46. doi: 10.1007/s00345-004-0474-0. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan L, Whyte L, Chatterjee N, Tenniswood M. Effects of clusterin over-expression on metastatic progression and therapy in breast cancer. BMC Cancer. 2010;10:107. doi: 10.1186/1471-2407-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao C, Shinohara ET, Li H, Niermann KJ, Kim KW, Sekhar KR, Gleave M, Freeman M, Lu B. Clusterin as a therapeutic target for radiation sensitization in a lung cancer model. Int J Radiat Oncol Biol Phys. 2005;63:1228–1236. doi: 10.1016/j.ijrobp.2005.07.956. [DOI] [PubMed] [Google Scholar]
- 21.Xiu P, Dong X, Dong X, Xu Z, Zhu H, Liu F, Wei Z, Zhai B, Kanwar JR, Jiang H, et al. Secretory clusterin contributes to oxaliplatin resistance by activating Akt pathway in hepatocellular carcinoma. Cancer Sci. 2013;104:375–382. doi: 10.1111/cas.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiu P, Xu Z, Liu F, Li Z, Li T, Zou F, Sun X, Li J. Downregulating sCLU enhances the sensitivity of hepatocellular carcinoma cells to gemcitabine by activating the intrinsic apoptosis pathway. Dig Dis Sci. 2014;59:1798–1809. doi: 10.1007/s10620-014-3111-9. [DOI] [PubMed] [Google Scholar]
- 23.Visser ME, Witztum JL, Stroes ES, Kastelein JJ. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J. 2012;33:1451–1458. doi: 10.1093/eurheartj/ehs084. [DOI] [PubMed] [Google Scholar]
- 24.Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J, Tu D, Gleave ME. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2’-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst. 2005;97:1287–1296. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- 25.Chi KN, Siu LL, Hirte H, Hotte SJ, Knox J, Kollmansberger C, Gleave M, Guns E, Powers J, Walsh W, et al. A phase I study of OGX-011, a 2’-methoxyethyl phosphorothioate antisense to clusterin, in combination with docetaxel in patients with advanced cancer. Clin Cancer Res. 2008;14:833–839. doi: 10.1158/1078-0432.CCR-07-1310. [DOI] [PubMed] [Google Scholar]
- 26.Chi KN, Hotte SJ, Yu EY, Tu D, Eigl BJ, Tannock I, Saad F, North S, Powers J, Gleave ME, et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:4247–4254. doi: 10.1200/JCO.2009.26.8771. [DOI] [PubMed] [Google Scholar]
- 27.Saad F, Hotte S, North S, Eigl B, Chi K, Czaykowski P, Wood L, Pollak M, Berry S, Lattouf JB, et al. Randomized phase II trial of Custirsen (OGX-011) in combination with docetaxel or mitoxantrone as second-line therapy in patients with metastatic castrate-resistant prostate cancer progressing after first-line docetaxel: CUOG trial P-06c. Clin Cancer Res. 2011;17:5765–5773. doi: 10.1158/1078-0432.CCR-11-0859. [DOI] [PubMed] [Google Scholar]
- 28.Laskin JJ, Nicholas G, Lee C, Gitlitz B, Vincent M, Cormier Y, Stephenson J, Ung Y, Sanborn R, Pressnail B, et al. Phase I/II trial of custirsen (OGX-011), an inhibitor of clusterin, in combination with a gemcitabine and platinum regimen in patients with previously untreated advanced non-small cell lung cancer. J Thorac Oncol. 2012;7:579–586. doi: 10.1097/JTO.0b013e31823f459c. [DOI] [PubMed] [Google Scholar]
- 29.Chia S, Dent S, Ellard S, Ellis PM, Vandenberg T, Gelmon K, Powers J, Walsh W, Seymour L, Eisenhauer EA. Phase II trial of OGX-011 in combination with docetaxel in metastatic breast cancer. Clin Cancer Res. 2009;15:708–713. doi: 10.1158/1078-0432.CCR-08-1159. [DOI] [PubMed] [Google Scholar]
- 30.Fritz IB, Burdzy K, Sétchell B, Blaschuk O. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol Reprod. 1983;28:1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- 31.Choi NH, Mazda T, Tomita M. A serum protein SP40,40 modulates the formation of membrane attack complex of complement on erythrocytes. Mol Immunol. 1989;26:835–840. doi: 10.1016/0161-5890(89)90139-9. [DOI] [PubMed] [Google Scholar]
- 32.James RW, Hochstrasser AC, Borghini I, Martin B, Pometta D, Hochstrasser D. Characterization of a human high density lipoprotein-associated protein, NA1/NA2. Identity with SP-40,40, an inhibitor of complement-mediated cytolysis. Arterioscler Thromb. 1991;11:645–652. doi: 10.1161/01.atv.11.3.645. [DOI] [PubMed] [Google Scholar]
- 33.Collard MW, Griswold MD. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987;26:3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- 34.Montpetit ML, Lawless KR, Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8:25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- 35.Burkey BF, Stuart WD, Harmony JA. Hepatic apolipoprotein J is secreted as a lipoprotein. J Lipid Res. 1992;33:1517–1526. [PubMed] [Google Scholar]
- 36.Bettuzzi S, Hiipakka RA, Gilna P, Liao ST. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989;257:293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng CY, Chen CL, Feng ZM, Marshall A, Bardin CW. Rat clusterin isolated from primary Sertoli cell-enriched culture medium is sulfated glycoprotein-2 (SGP-2) Biochem Biophys Res Commun. 1988;155:398–404. [PubMed] [Google Scholar]
- 38.Tobe T, Minoshima S, Yamase S, Choi NH, Tomita M, Shimizu N. Assignment of a human serum glycoprotein SP-40,40 gene (CLI) to chromosome 8. Cytogenet Cell Genet. 1991;57:193–195. doi: 10.1159/000133144. [DOI] [PubMed] [Google Scholar]
- 39.Zoubeidi A, Ettinger S, Beraldi E, Hadaschik B, Zardan A, Klomp LW, Nelson CC, Rennie PS, Gleave ME. Clusterin facilitates COMMD1 and I-kappaB degradation to enhance NF-kappaB activity in prostate cancer cells. Mol Cancer Res. 2010;8:119–130. doi: 10.1158/1541-7786.MCR-09-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura K, Asami K, Yamamoto M. Effect of heat shock treatment on the production of variant testosterone-repressed prostate message-2 (TRPM-2) mRNA in culture cells. Cell Biochem Funct. 1997;15:251–257. doi: 10.1002/(SICI)1099-0844(199712)15:4<251::AID-CBF748>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Mailhos C, Howard MK, Latchman DS. Heat shock protects neuronal cells from programmed cell death by apoptosis. Neuroscience. 1993;55:621–627. doi: 10.1016/0306-4522(93)90428-i. [DOI] [PubMed] [Google Scholar]
- 42.Kang YK, Hong SW, Lee H, Kim WH. Overexpression of clusterin in human hepatocellular carcinoma. Hum Pathol. 2004;35:1340–1346. doi: 10.1016/j.humpath.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Aigelsreiter A, Janig E, Sostaric J, Pichler M, Unterthor D, Halasz J, Lackner C, Zatloukal K, Denk H. Clusterin expression in cholestasis, hepatocellular carcinoma and liver fibrosis. Histopathology. 2009;54:561–570. doi: 10.1111/j.1365-2559.2009.03258.x. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh SY, Chen WY, Shih TC, Yeh JY, Jeng JT. Dys-regulation of clusterin in human hepatoma is not associated with tumorigenesis but is secondary to cell response to external tresses. Mol Carcinog. 2005;43:100–107. doi: 10.1002/mc.20095. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Jiang K, Kang X, Gao D, Sun C, Li Y, Sun L, Zhang S, Liu X, Wu W, et al. Tumor-derived secretory clusterin induces epithelial-mesenchymal transition and facilitates hepatocellular carcinoma metastasis. Int J Biochem Cell Biol. 2012;44:2308–2320. doi: 10.1016/j.biocel.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Kimura A, Sogawa K, Satoh M, Kodera Y, Yokosuka O, Tomonaga T, Nomura F. The application of a three-step serum proteome analysis for the discovery and identification of novel biomarkers of hepatocellular carcinoma. Int J Proteomics. 2012;2012:623190. doi: 10.1155/2012/623190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nafee AM, Pasha HF, Abd El Aal SM, Mostafa NA. Clinical significance of serum clusterin as a biomarker for evaluating diagnosis and metastasis potential of viral-related hepatocellular carcinoma. Clin Biochem. 2012;45:1070–1074. doi: 10.1016/j.clinbiochem.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 48.Comunale MA, Wang M, Rodemich-Betesh L, Hafner J, Lamontagne A, Klein A, Marrero J, Di Bisceglie AM, Gish R, Block T, et al. Novel changes in glycosylation of serum Apo-J in patients with hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1222–1229. doi: 10.1158/1055-9965.EPI-10-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Jia W, Sun W, Jin W, Guo L, Wei J, Ying W, Zhang Y, Xie Y, Jiang Y, et al. Combination of improved (18)O incorporation and multiple reaction monitoring: a universal strategy for absolute quantitative verification of serum candidate biomarkers of liver cancer. J Proteome Res. 2010;9:3319–3327. doi: 10.1021/pr9011969. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Liu YH, Mai SJ, He LJ, Liao YJ, Deng HX, Guan XY, Zeng YX, Kung HF, Xie D. Evaluation of serum clusterin as a surveillance tool for human hepatocellular carcinoma with hepatitis B virus related cirrhosis. J Gastroenterol Hepatol. 2010;25:1123–1128. doi: 10.1111/j.1440-1746.2009.06205.x. [DOI] [PubMed] [Google Scholar]


