Abstract
AIM: To investigate the prevalence and the clinical characteristics of Asian patients with eosinophilic esophagitis.
METHODS: We conducted a systematic search of the PubMed and Web of Science databases for original studies, case series, and individual case reports of eosinophilic esophagitis in Asian countries published from January 1980 to January 2015. We found 66 and 80 articles in the PubMed and Web of Science databases, respectively; 24 duplicate articles were removed. After excluding animal studies, articles not written in English, and meeting abstracts, 25 articles containing 217 patients were selected for analysis.
RESULTS: Sample size-weighted mean values were determined for all pooled prevalence data and clinical characteristics. The mean age of the adult patients with eosinophilic esophagitis was approximately 50 years, and 73% of these patients were male. They frequently presented with allergic diseases including bronchial asthma, allergic rhinitis, food allergy, and atopic dermatitis. Bronchial asthma was the most frequent comorbid allergic disease, occurring in 24% of patients with eosinophilic esophagitis. Dysphagia was the primary symptom reported; 44% of the patients complained of dysphagia. Although laboratory blood tests are not adequately sensitive for an accurate diagnosis of eosinophilic esophagitis, endoscopic examinations revealed abnormal findings typical of this disease, including longitudinal furrows and concentric rings, in 82% of the cases. One-third of the cases responded to proton pump inhibitor administration.
CONCLUSION: The characteristics of eosinophilic esophagitis in Asian patients were similar to those reported in Western patients, indicating that this disease displays a similar pathogenesis between Western and Asian patients.
Keywords: Eosinophilic esophagitis, Allergy, Prevalence, Symptom, Endoscopy, Asia, Treatment
Core tip: We conducted a systematic literature search of eosinophilic esophagitis in Asian countries. More than 200 patients with eosinophilic esophagitis were found, and their clinical characteristics were summarized. All clinical characteristics of the Asian patients, except for the prevalence of food impaction, were similar to those of Western patients. Eosinophilic esophagitis may share the same pathogenetic mechanisms between Asian and Western patients.
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic inflammatory esophageal disease that induces dense intra-epithelial infiltration of eosinophils in the esophageal mucosa[1,2]. Affected patients develop esophageal fibrostenotic complications after chronic inflammation and often suffer from dysphagia, swallowing discomfort, and heartburn. More than half of patients with EoE have concomitant atopic diseases, which may play a role in its development. The pathogenetic mechanism of EoE is considered to be a Th2-type chronic allergic reaction, primarily to food allergens, and IL-5, -13, and -15, eotaxin-3, periostin, and TGF-β are considered to be the principal participants in the development of EoE[3,4]. As with many allergic diseases, including bronchial asthma, the incidence and the prevalence of EoE have been shown to be rapidly increasing, with a reported incidence in Western countries ranging from 10 to 50 per 100000 individuals in the general population[5].
Published reports of EoE from Asian countries are limited, including only observational studies using a small sample size and case reports. Because of this limited information, the prevalence of EoE in Asian countries, as well as similarities and differences in regards to the clinical characteristics of affected patients, in Western and Asian countries have not been clarified. In the present study, we surveyed original publications and case reports of EoE from Asian countries and performed a systematic review.
MATERIALS AND METHODS
Systematic literature search
Systematic searches of PubMed and Web of Science for reports published from January 1980 to January 2015 were conducted using the following search strings: [eosinophilic esophagitis] and ([Asia] or [Asian] or [Japan] or [Japanese] or [Korea] or [Korean] or [China] or [Chinese] or [Taiwan] or [India] or [Indian] or [Indonesia] or [Cambodia] or [Singapore] or [Sri Lanka] or [Thailand] or [Nepal] or [Pakistan] or [Bangladesh] or [Timor] or [Bhutan] or [Philippines] or [Brunei] or [Viet Nam] or [Malaysia] or [Myanmar] or [Maldives] or [Mongolia] or [Laos]). All articles written in English, including original articles, case series, and individual case reports, were analyzed.
Data extraction and analysis
Because we were interested in the prevalence and the clinical characteristics of EoE in Asian countries, we focused on the number of cases investigated via upper gastrointestinal endoscopy. In addition, we recorded possible accompanying atopic diseases, symptoms, laboratory test results, endoscopic findings, and therapeutic responses.
Statistical analysis
Sample size-weighted mean values were determined for all pooled prevalence data and clinical characteristics. The statistical methods of this study were reviewed by Akira Yasuda, Department of Medical Informatics, Shimane University, Izumo, Japan.
RESULTS
Literature search
The literature search identified 25 articles, including 8 case reports, that fulfilled the inclusion criteria for this review (Figure 1)[6-30]. Most studies were performed in Japan (n = 15), followed by Korea, Turkey, Saudi Arabia, China, and Taiwan. We did not find any reports from other Asian countries, including central and western Asian countries.
Figure 1.
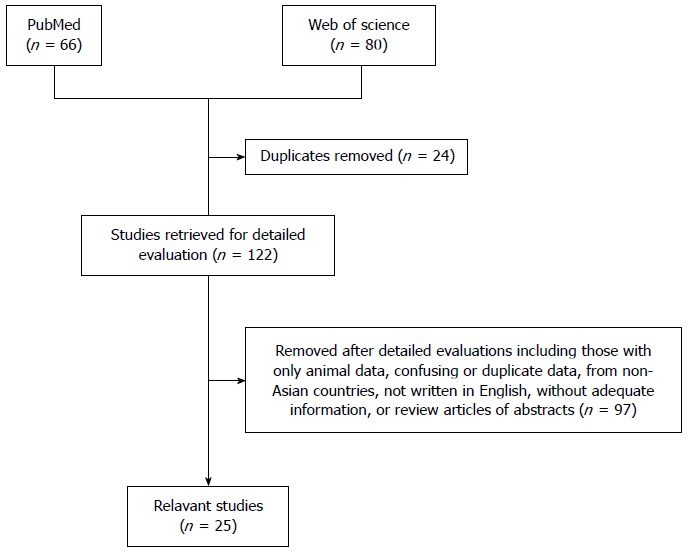
Flow chart of our systematic literature search.
Demographic characteristics
The identified reports included a total of 217 Asian patients with EoE, of whom 73% were male. All studies except for 2 contained more males than females, although one report of 4 patients did not indicate their age or gender. The age of the patients in these studies ranged from 1 to 83 years, with a mean age of 40.4 years, and the mean age of the adult patients was approximately 50 years (Figure 2).
Figure 2.
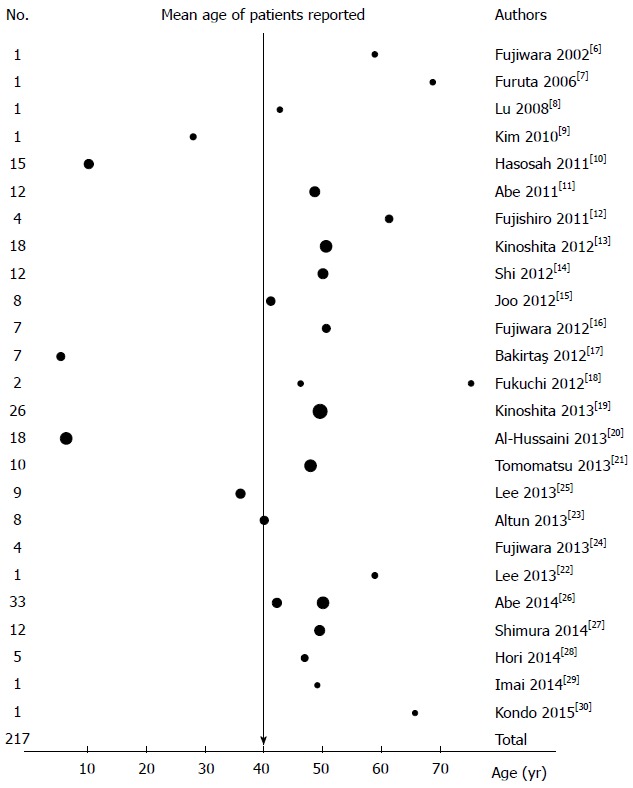
Number of patients with eosinophilic esophagitis in all 25 studies included in this review. The dots indicate the mean age, and the dot size indicates the number of patients. One report did not describe the ages of the included patients.
Prevalence of EoE
Nine studies investigated the prevalence of EoE in upper gastrointestinal cases investigated via endoscopy. Of 117946 cases in which endoscopy was used, EoE was found in 77 cases. The reported prevalence of EoE in these studies displayed wide variability, from 17 to 6557 per 105 endoscopy-investigated cases. However, the possibility of inclusion bias in the studies using a small sample size should be considered, as the studies using a small sample size showed a higher prevalence of EoE (Figure 3). Two studies that included more than 20000 endoscopy-investigated cases showed an EoE prevalence of approximately 20 in 105 cases, suggesting a lower prevalence in Asian countries than in Western countries. No studies investigated the population-based prevalence or incidence of EoE in Asian countries.
Figure 3.
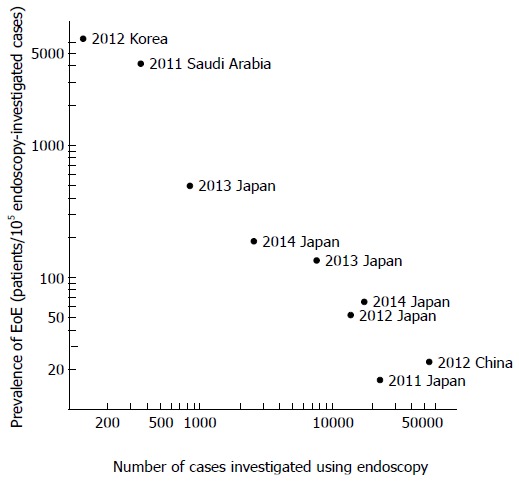
Reported prevalence of eosinophilic esophagitis in patients examined via upper gastrointestinal endoscopy. In the studies using a smaller sample size, the prevalence of eosinophilic esophagitis (EoE) was higher. Different indication criteria for endoscopic examination between institutions may be partially responsible for the large variation in the reported incidence of EoE.
History of allergic diseases
A history of allergic diseases was reported for 175 cases (Figure 4). Bronchial asthma was reported in 42 patients, allergic rhinitis in 39 patients, food allergies in 23 patients, and atopic dermatitis in 20 patients. More than 50% of the patients with EoE had some type of allergic disease or a medical history of an allergic disease.
Figure 4.
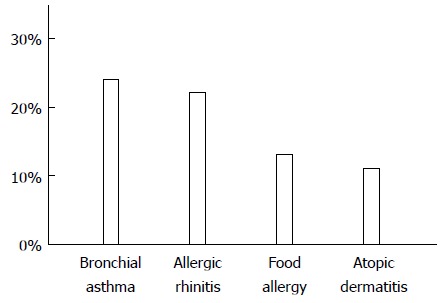
Allergic diseases reported by patients with eosinophilic esophagitis. Twenty-one articles contained descriptions of the histories of allergic diseases among the reported patients. The most frequently reported allergic disease was bronchial asthma, followed by allergic rhinitis.
Symptoms reported by patients
Symptoms were reported in 213 cases (Figure 5), and multiple symptoms were often noted. Dysphagia and/or swallowing discomfort, the most frequently noted symptoms, were reported by 93 patients. Other reported symptoms included heartburn (n = 47), epigastralgia (n = 24), failure to thrive (n = 20, only pediatric cases), vomiting (n = 13), chest pain (n = 11), food impaction (n = 9), regurgitation (n = 8), abdominal pain (n = 6), and back pain (n = 4). Of the 40 pediatric cases, 18 reported dysphagia or swallowing discomfort.
Figure 5.
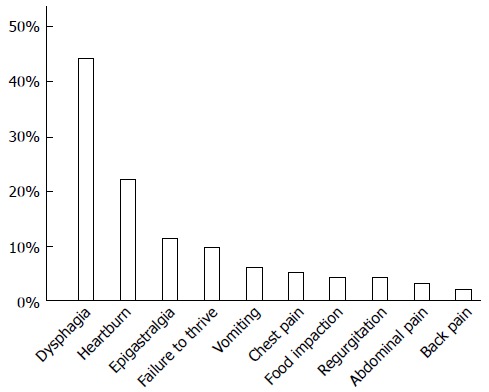
Symptoms reported by patients with eosinophilic esophagitis. Twenty-four articles contained descriptions of symptoms reported by patients. The most frequently reported symptom was dysphagia, followed by heartburn.
Laboratory test results
The possible presence of peripheral blood eosinophilia was reported in 102 patients, 34 (33%) of whom had eosinophilia (> 500 eosinophils/μL), although high-grade eosinophilia (> 1000 eosinophils/μL) was rarely encountered. The total serum IgE concentration was reported for 76 cases, of which 45 (59%) of the cases displayed elevated IgE levels.
Serum anti-Helicobacter pylori (H. pylori) IgG antibody levels were reported for 24 patients, although only 4 (17%) were found to be infected by H. pylori, suggesting a lower rate of infection in EoE cases than in healthy individuals.
Endoscopy
Endoscopic findings were reported for 188 patients (Figure 6), of whom 154 (82%) had some endoscopic abnormalities suggesting the presence of EoE, including furrows, concentric rings, and white plaques. Longitudinal furrows were the most frequently reported abnormality, observed in 52% of the patients, followed by white plaques and concentric rings. Shimura and co-workers investigated the sensitivity and the specificity of various endoscopic abnormalities in Asian patients and found that longitudinal furrows corresponded to the highest positive and negative predictive values[27]. Non-specific findings such as edema, erythema, and decreased visibility of the vasculature were reported in a small number of patients.
Figure 6.
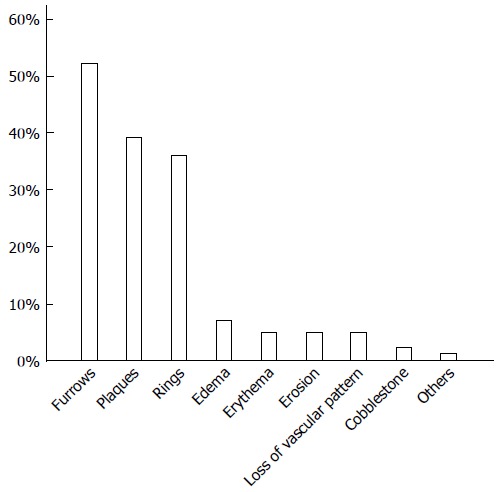
Endoscopic abnormalities found in patients with eosinophilic esophagitis. Longitudinal furrows, white plaques, and fixed/transient concentric rings were most frequently detected.
Regarding the histological diagnosis of EoE, 7 studies employed 20 or 24 eosinophils/high power field as the minimal threshold to indicate eosinophil infiltration for EoE diagnosis. In the remaining studies, the patients were diagnosed with histological EoE when 15 or more eosinophils/high power field were found in esophageal mucosa biopsy specimens. Eosinophil micro-abscess, fibrosis in the esophageal sub-epithelial layer, and infiltration of other immunocytes into the epithelial layer have been reported to be possible characteristic histological findings of EoE, although these histological abnormalities were not used for the diagnosis of EoE in the collected publications. Additionally, the sites of biopsy sampling were not clearly described in the collected publications, despite that the sampling site may affect the sensitivity and the specificity of histological diagnosis.
Therapeutic response
Topical glucocorticoid therapy, elemental or elimination diet, and proton pump inhibitor (PPI) therapy were generally employed for the treatment of EoE. Some studies included PPI-responsive cases, whereas others excluded such cases. In 7 studies that included PPI-responsive cases, 23 of the 61 patients (38%) responded favorably to PPI administration and were diagnosed with PPI-responsive EoE (PPI-REE) according to the 2011 Eosinophilic Esophagitis Updated Consensus Recommendations[1].
All symptomatic patients with EoE were reported to be successfully treated via glucocorticoid or PPI administration or dietary therapy. A small number of patients had negligible symptoms that did not require therapeutic intervention or experienced spontaneous disease remission, at least temporarily, without any treatment. None of the reported patients required balloon dilatation treatment for esophageal stenosis.
DISCUSSION
Our review of the literature identified over 200 patients with EoE in Asian countries during the search period. An increasing trend of publication was observed since 2011, as 21 of the 25 studies surveyed were published after 2011. The age and gender ratios of the reported cases in Asian countries were very similar to those reported in Western countries[1,2,31], although the reason for the male preponderance of EoE has yet to be clarified.
The prevalence of EoE in Asian countries has not been appropriately investigated via population studies, in contrast to the studies performed in Western countries[5,32]. The prevalence of cases that utilized endoscopy varied remarkably among the studies reviewed here (Figure 3), and this variability may be partially due to the different indications for which upper gastrointestinal endoscopy is used and partially because of different levels of familiarity with EoE among endoscopists in different countries. In Western countries, EoE has been reported to be found in approximately 1 out of 200 endoscopy examinations[33,34]. In contrast, studies using large a sample size performed in Asian countries have reported EoE in approximately 1 out of 5000 endoscopy examinations, indicating a much lower prevalence of EoE[12,14]. Because the accessibility of and the indication for endoscopic examination are known to differ between Western and Asian countries, a direct comparison of the EoE prevalence based on endoscopic findings between these 2 regions of the world is difficult. We believe that population-based studies in Asian countries are needed.
Similar to Western EoE patients, Asian EoE patients frequently presented with comorbid atopic and allergic diseases[1,2,31]. In both regions, bronchial asthma has been shown to be the allergic disease most frequently associated with EoE. Thus, the atopic condition is considered to be at least partially involved in the development of EoE not only in Western countries but also in Asian countries. The occurrence of allergic diseases may be prevented by bacterial or parasitic infections[35,36]. In Western countries, the infection rate of H. pylori is reported to be lower in patients with EoE[33,37], and in the Asian EoE patients, eosinophilic gastrointestinal diseases were reported to be infrequently accompanied by H. pylori infection[38,39]. The infection rate observed in the present review also suggests a lower rate of H. pylori infection in Asian EoE patients than in the general Asian population.
For accurate diagnosis of EoE, a biopsy performed via upper gastrointestinal endoscopy is absolutely necessary. The presence of characteristic symptoms and endoscopic abnormalities is important for determining whether biopsy specimens should be collected. Dysphagia, the most frequently reported symptom of EoE in both Asian and Western countries, was found in nearly half of the patients reported in the studies covered by this review. Alternatively, food impaction was reported in less than 5% of Asian EoE cases, and this value was lower than that in Western countries[40,41]. Endoscopy revealed longitudinal furrows in half of the Asian patients; thus, longitudinal furrows were the most frequently observed endoscopic abnormality in both Asian and Western EoE patients[42-44]. As in Western studies, more than 80% of the Asian EoE patients showed endoscopic abnormalities characteristic of EoE, including longitudinal furrows, plaques, and concentric rings. These findings clearly indicate that Western and Asian EoE patients share similar symptoms and endoscopic abnormalities. This information is important for the accurate diagnosis of EoE. However, in Asian patients, fixed concentric rings/stenosis were not frequently found, although transient concentric rings were reported[12,19,27]. The rarity of fixed concentric rings in Asian EoE patients is reasonable given the observation that food impaction is a rare symptom reported by Asian patients with EoE. The low grade of inflammation and the early stage of EoE disease at the time of diagnosis in Asian patients may be responsible for these differences in the clinical characteristics of EoE between Asian and Western patients.
EoE can be divided into two types, EoE and PPI-REE, based on the response of the disease to PPI administration. When administration of a PPI results in the resolution of EoE together with endoscopic abnormalities and symptoms, the appropriate diagnosis is PPI-REE[1,2]. Alternatively, when a response to a PPI is not observed, the patient should be diagnosed with EoE according to the consensus of the published recommendations[1,2]. However, some investigators have questioned the appropriateness of this classification. PPI-REE and EoE do not differ in regards to their clinical, endoscopic, or histological characteristics[45-47], and the messenger RNA expression profile in the esophageal mucosa of PPI-REE and EoE cases has been reported to be nearly identical[48]. In addition, PPIs have recently been shown to suppress Th2-type inflammatory responses, which are important for the development of EoE[49,50]. Together, these findings suggest the limited importance and difficultly of PPI responsiveness in the classification of EoE. As a result, some investigators included PPI-responsive EoE cases in their study population, whereas others did not. Seven studies covered by our review classified their study populations into PPI-REE and PPI-resistant EoE cases, and 23 (38%) of the 61 patients analyzed in those 7 reports exhibited PPI responsiveness. Although the percentage of PPI-REE cases reported by various studies differs remarkably in Western countries, 38% of the Asian cases were responsive to PPI administration, and this finding was generally similar to the results reported in Western countries[46,51,52]. Additionally, PPI-resistant EoE patients in Asian countries were reported to be successfully treated via administration of glucocorticoid or dietary therapies, as has been reported in Western countries.
In summary, patients with EoE in Asian countries share similar clinical, endoscopic, and histopathological characteristics to those reported in Western countries, although the prevalence of EoE is lower in Asia. One-third of the Asian EoE patients responded favorably to PPI administration, whereas the others showed a good response to glucocorticoid or dietary therapy; these results were identical to those reported in Western countries. We conclude that EoE patients in Asian countries exhibit similar characteristics to those in Western countries.
COMMENTS
Background
The prevalence of eosinophilic esophagitis (EoE), a disease that is primarily caused by food allergies, is rapidly increasing in Western countries. EoE decreases health-related quality of life by causing dysphagia. The prevalence of EoE was reported to be approximately 50 per 100,000 individuals in the USA. However, the prevalence, the incidence, and the clinical characteristics of Asian patients with EoE have not been thoroughly investigated.
Research frontiers
Based on twin studies, the role of genetic factors in the development of EoE is approximately 20%. Therefore, with the westernization of social environments in Asian countries, EoE is expected to increase in a manner similar to that in Western countries. Accordingly, the prevalence and the clinical characteristics of Asian EoE patients need to be summarized and compared with those of Western EoE patients.
Innovations and breakthroughs
We have conducted a systematic literature search for studies on Asian patients with EoE. More than 200 such patients were found in the literature. The prevalence of EoE in Asian countries is lower than that reported in Western countries. The clinical characteristics of EoE are similar between Asian and Western countries, and common pathogenetic mechanisms are suggested. Possibly because of the lower grade of inflammation or the early stage of EoE at the time of diagnosis in Asian patients, endoscopic abnormalities and symptoms associated with EoE were less frequently observed in Asian patients than in Western patients.
Applications
The results of this study suggest that information concerning Western patients with EoE can be applied to Asian patients with EoE and that similar treatment strategies used in Western patients can be administered to Asian patients.
Terminology
The eosinophil is a type of leukocyte that increases and accumulates in allergic diseases. These cells, together with other inflammatory cells, cause inflammation in the esophageal mucosa, resulting in unpleasant symptoms. Dysphagia, or difficulty in swallowing, is the symptom most frequently reported by patients with EoE. Dysphagia significantly decreases patient quality of life.
Peer-review
Endoscopy examination is more popular in Asian countries, however, the diagnosis of EoE is rare than that in Western countries. The authors reviewed the clinical characteristics of Asian EoE patients, which is helpful for the understanding of this disease in the East.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare concerning this manuscript.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 21, 2015
First decision: April 23, 2015
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20.e6; quiz 21-22. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692; quiz 693. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148:1143–1157. doi: 10.1053/j.gastro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:281–296. doi: 10.1016/j.gtc.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, Locke GR, Talley NJ. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055–1061. doi: 10.1016/j.cgh.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiwara H, Morita A, Kobayashi H, Hamano K, Fujiwara Y, Hirai K, Yano M, Naka T, Saeki Y. Infiltrating eosinophils and eotaxin: their association with idiopathic eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2002;89:429–432. doi: 10.1016/S1081-1206(10)62047-9. [DOI] [PubMed] [Google Scholar]
- 7.Furuta K, Adachi K, Kowari K, Mishima Y, Imaoka H, Kadota C, Koshino K, Miyake T, Kadowaki Y, Furuta K, et al. A Japanese case of eosinophilic esophagitis. J Gastroenterol. 2006;41:706–710. doi: 10.1007/s00535-006-1827-9. [DOI] [PubMed] [Google Scholar]
- 8.Lu HC, Lu CL, Chang FY. Eosinophilic esophagitis in an asymptomatic Chinese. J Chin Med Assoc. 2008;71:362–364. doi: 10.1016/S1726-4901(08)70140-0. [DOI] [PubMed] [Google Scholar]
- 9.Kim NI, Jo Y, Ahn SB, Son BK, Kim SH, Park YS, Kim SH, Ju JE. A case of eosinophilic esophagitis with food hypersensitivity. J Neurogastroenterol Motil. 2010;16:315–318. doi: 10.5056/jnm.2010.16.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasosah MY, Sukkar GA, Alsahafi AF, Thabit AO, Fakeeh ME, Al-Zahrani DM, Satti MB. Eosinophilic esophagitis in Saudi children: symptoms, histology and endoscopy results. Saudi J Gastroenterol. 2011;17:119–123. doi: 10.4103/1319-3767.77242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe Y, Iijima K, Ohara S, Koike T, Ara N, Uno K, Asano N, Imatani A, Kato K, Shibuya D, et al. A Japanese case series of 12 patients with esophageal eosinophilia. J Gastroenterol. 2011;46:25–30. doi: 10.1007/s00535-010-0295-4. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro H, Amano Y, Kushiyama Y, Ishihara S, Kinoshita Y. Eosinophilic esophagitis investigated by upper gastrointestinal endoscopy in Japanese patients. J Gastroenterol. 2011;46:1142–1144. doi: 10.1007/s00535-011-0435-5. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Furuta K, Ishimura N, Ishihara S. Elevated plasma cytokines in Japanese patients with eosinophilic esophagitis and gastroenteritis. Digestion. 2012;86:238–243. doi: 10.1159/000341421. [DOI] [PubMed] [Google Scholar]
- 14.Shi YN, Sun SJ, Xiong LS, Cao QH, Cui Y, Chen MH. Prevalence, clinical manifestations and endoscopic features of eosinophilic esophagitis: a pathological review in China. J Dig Dis. 2012;13:304–309. doi: 10.1111/j.1751-2980.2012.00593.x. [DOI] [PubMed] [Google Scholar]
- 15.Joo MK, Park JJ, Kim SH, Kim KH, Jung W, Yun JW, Lee BJ, Kim JH, Yeon JE, Kim JS, et al. Prevalence and endoscopic features of eosinophilic esophagitis in patients with esophageal or upper gastrointestinal symptoms. J Dig Dis. 2012;13:296–303. doi: 10.1111/j.1751-2980.2012.00589.x. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara Y, Sugawa T, Tanaka F, Tatsuwaki H, Okuyama M, Hayakawa T, Yamamori K, Wada R, Ohtani K, Uno H, et al. A multicenter study on the prevalence of eosinophilic esophagitis and PPI-responsive esophageal eosinophilic infiltration. Intern Med. 2012;51:3235–3239. doi: 10.2169/internalmedicine.51.8670. [DOI] [PubMed] [Google Scholar]
- 17.Bakirtaş A, Arga M, Eğrıtaş O, Topal E, Sari S, Poyraz A, Dalgiç B, Demırsoy MS, Türktaş I. The first experience of eosinophilic esophagitis in Turkish children. Turk J Gastroenterol. 2012;23:1–7. [PubMed] [Google Scholar]
- 18.Fukuchi M, Sakurai S, Fukasawa T, Suzuki M, Naitoh H, Tabe Y, Kiriyama S, Horiuchi K, Yuasa K, Kuwano H. Two Janese patients with esophageal eosinophilia detected by routine medical examination. Esophagus. 2012;9:25–28. [Google Scholar]
- 19.Kinoshita Y, Furuta K, Ishimaura N, Ishihara S, Sato S, Maruyama R, Ohara S, Matsumoto T, Sakamoto C, Matsui T, et al. Clinical characteristics of Japanese patients with eosinophilic esophagitis and eosinophilic gastroenteritis. J Gastroenterol. 2013;48:333–339. doi: 10.1007/s00535-012-0640-x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hussaini A, Al-Idressi E, Al-Zahrani M. The role of allergy evaluation in children with eosinophilic esophagitis. J Gastroenterol. 2013;48:1205–1212. doi: 10.1007/s00535-012-0741-6. [DOI] [PubMed] [Google Scholar]
- 21.Tomomatsu Y, Yoshino J, Inui K, Wakabayashi T, Kobayashi T, Miyoshi H, Kosaka T, Yamamoto S, Torii Y. Clinical features of eosinophilic esophagitis: ten Japanese cases. Dig Endosc. 2013;25:117–124. doi: 10.1111/j.1443-1661.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee BE, Kim GH. Magnifying endoscopy with narrow band imaging and endoscopic ultrasonography for assessing eosinophilic esophagitis. J Neurogastroenterol Motil. 2013;19:104–106. doi: 10.5056/jnm.2013.19.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altun R, Akbas E, Yıldırım AE, Öcal S, Korkmaz M, Selcuk H. Frequency of eosinophilic esophagitis in patients with esophageal symptoms: a single-center Turkish experience. Dis Esophagus. 2013;26:776–781. doi: 10.1111/j.1442-2050.2012.01395.x. [DOI] [PubMed] [Google Scholar]
- 24.Fujiwara Y, Tanigawa T, Yamagami H, Watanabe K, Tominaga K, Watanabe T, Arakawa T. Eosinophilic esophagitis-like endoscopic findings in patients with erosive esophagitis. Esophagus. 2013;10:199–204. [Google Scholar]
- 25.Lee JH, Kim MJ, Kim JH, Youn YH, Kim N, Bak YT, Jo Y, Park H. Clinical analysis of primary eosinophilic esophagitis. J Neurogastroenterol Motil. 2013;19:204–209. doi: 10.5056/jnm.2013.19.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe Y, Iijima K, Ohara S, Koike T, Kikuchi R, Kato K, Shibuya D, Inomata Y, Oikawa K, Ueno Y. Localized esophageal eosinophilia: Is it an early manifestation of eosinophilic esophagitis or a subtype of gastroesophageal reflux disease? Dig Endosc. 2014;26:337–343. doi: 10.1111/den.12150. [DOI] [PubMed] [Google Scholar]
- 27.Shimura S, Ishimura N, Tanimura T, Yuki T, Miyake T, Kushiyama Y, Sato S, Fujishiro H, Ishihara S, Komatsu T, et al. Reliability of symptoms and endoscopic findings for diagnosis of esophageal eosinophilia in a Japanese population. Digestion. 2014;90:49–57. doi: 10.1159/000365209. [DOI] [PubMed] [Google Scholar]
- 28.Hori K, Watari J, Fukui H, Tanaka J, Tomita T, Sakurai J, Kondo T, Oshima T, Toyoshima F, Yamasaki T, et al. Do endoscopic features suggesting eosinophilic esophagitis represent histological eosinophilia? Dig Endosc. 2014;26:156–163. doi: 10.1111/den.12091. [DOI] [PubMed] [Google Scholar]
- 29.Imai J, Suzuki T, Mine T, Imai Y. Radiographic findings of eosinophilic esophagitis. Intern Med. 2014;53:515–516. doi: 10.2169/internalmedicine.53.1577. [DOI] [PubMed] [Google Scholar]
- 30.Kondo T, Uehara T, Takada T, Terada K, Ikusaka M. Recurrent back pain of eosinophilic esophagitis. Am J Med. 2015;128:e1–e2. doi: 10.1016/j.amjmed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME; First International Gastrointestinal Eosinophil Research Symposium (FIGERS) Subcommittees. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–419. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Ronkainen J, Talley NJ, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Walker MM, Agréus L. Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population-based Kalixanda study. Gut. 2007;56:615–620. doi: 10.1136/gut.2006.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. doi: 10.1053/j.gastro.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Ami Shor D, Harel M, Eliakim R, Shoenfeld Y. The hygiene theory harnessing helminths and their ova to treat autoimmunity. Clin Rev Allergy Immunol. 2013;45:211–216. doi: 10.1007/s12016-012-8352-9. [DOI] [PubMed] [Google Scholar]
- 36.Rautava S, Ruuskanen O, Ouwehand A, Salminen S, Isolauri E. The hygiene hypothesis of atopic disease--an extended version. J Pediatr Gastroenterol Nutr. 2004;38:378–388. doi: 10.1097/00005176-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, Genta RM. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141:1586–1592. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Duan L, Ding S, Lu J, Jin Z, Cui R, McNutt M, Wang A. Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol. 2011;46:1074–1080. doi: 10.3109/00365521.2011.579998. [DOI] [PubMed] [Google Scholar]
- 39.Furuta K, Adachi K, Aimi M, Ishimura N, Sato S, Ishihara S, Kinoshita Y. Case-control study of association of eosinophilic gastrointestinal disorders with Helicobacter pylori infection in Japan. J Clin Biochem Nutr. 2013;53:60–62. doi: 10.3164/jcbn.13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudson S, Sampson C, Muntz HR, Jackson WD, Smith ME. Foreign body impaction as presentation of eosinophilic esophagitis. Otolaryngol Head Neck Surg. 2013;149:679–681. doi: 10.1177/0194599813500462. [DOI] [PubMed] [Google Scholar]
- 41.Sperry SL, Crockett SD, Miller CB, Shaheen NJ, Dellon ES. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc. 2011;74:985–991. doi: 10.1016/j.gie.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller S, Pühl S, Vieth M, Stolte M. Analysis of symptoms and endoscopic findings in 117 patients with histological diagnoses of eosinophilic esophagitis. Endoscopy. 2007;39:339–344. doi: 10.1055/s-2007-966216. [DOI] [PubMed] [Google Scholar]
- 43.Kim HP, Vance RB, Shaheen NJ, Dellon ES. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:988–96.e5. doi: 10.1016/j.cgh.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62:489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 45.Dranove JE, Horn DS, Davis MA, Kernek KM, Gupta SK. Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J Pediatr. 2009;154:96–100. doi: 10.1016/j.jpeds.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 46.Dellon ES, Speck O, Woodward K, Gebhart JH, Madanick RD, Levinson S, Fritchie KJ, Woosley JT, Shaheen NJ. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol. 2013;108:1854–1860. doi: 10.1038/ajg.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moawad FJ, Schoepfer AM, Safroneeva E, Ally MR, Chen YJ, Maydonovitch CL, Wong RK. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther. 2014;39:603–608. doi: 10.1111/apt.12636. [DOI] [PubMed] [Google Scholar]
- 48.Wen T, Dellon ES, Moawad FJ, Furuta GT, Aceves SS, Rothenberg ME. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol. 2015;135:187–197. doi: 10.1016/j.jaci.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS One. 2012;7:e50037. doi: 10.1371/journal.pone.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013;62:824–832. doi: 10.1136/gutjnl-2012-302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, Dueñas C, Fernandez-Gonzalez N, Quintana EM, Gonzalez-Nuñez MA. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110–117. doi: 10.1016/j.cgh.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Vazquez-Elizondo G, Ngamruengphong S, Khrisna M, Devault KR, Talley NJ, Achem SR. The outcome of patients with oesophageal eosinophilic infiltration after an eight-week trial of a proton pump inhibitor. Aliment Pharmacol Ther. 2013;38:1312–1319. doi: 10.1111/apt.12513. [DOI] [PubMed] [Google Scholar]


