Abstract
Superficial zone protein (SZP), also known as lubricin and proteoglycan 4 (PRG4), plays an important role in the boundary lubrication of articular cartilage and is regulated by transforming growth factor (TGF)-β. Here, we evaluate the role of cell surface glycosaminoglycans (GAGs) during TGF-β1 stimulation of SZP/lubricin/PRG4 in superficial zone articular chondrocytes. We utilized primary monolayer superficial zone articular chondrocyte cultures and treated them with various concentrations of TGF-β1, in the presence or absence of heparan sulfate (HS), heparin, and chondroitin sulfate (CS). The cell surface GAGs were removed by pretreatment with either heparinase I or chondroitinase-ABC before TGF-β1 stimulation. Accumulation of SZP/lubricin/PRG4 in the culture medium in response to stimulation with TGF-β1 and various exogenous GAGs was demonstrated by immunoblotting and quantitated by enzyme-linked immunosorbent assay. We show that TGF-β1 and exogenous HS enhanced SZP accumulation of superficial zone chondrocytes in the presence of surface GAGs. At the dose of 1 ng/mL of TGF-β1, the presence of exogenous heparin inhibited SZP accumulation whereas the presence of exogenous CS stimulated SZP accumulation in the culture medium. Enzymatic depletion of GAGs on the surface of superficial zone chondrocytes enhanced the ability of TGF-β1 to stimulate SZP accumulation in the presence of both exogenous heparin and CS. Collectively, these results suggest that GAGs at the surface of superficial zone articular chondrocytes influence the response to TGF-β1 and exogenous GAGs to stimulate SZP accumulation. Cell surface GAGs modulate superficial zone chondrocytes' response to TGF-β1 and exogenous HS.
Introduction
Superficial zone protein (SZP), also known as lubricin and proteoglycan 4 (PRG4), is a mucinous glycoprotein involved in the boundary lubrication of articular cartilage.1 The majority of SZP is secreted into the synovial fluid in vivo with minimal retention in the extracellular matrix (ECM), as demonstrated by the fact that most of SZP synthesized by superficial zone articular chondrocytes in culture is secreted into the media.2 SZP plays an important role in articular joint physiology, and the loss of accumulation of SZP may have a role in the pathology of osteoarthritis (OA). Mice lacking the Prg4 gene display alterations of the articular surface and subsequent degradation of articular cartilage reminiscent of osteoarthritic degeneration.3,4 The regulation of SZP by transforming growth factor (TGF)-β in superficial zone articular chondrocytes has previously been studied.1,2,5,6 Exposure of articular chondrocytes to TGF-β enhances SZP expression and accumulation.5,6 Interestingly, conditional mutant mice lacking Ext-1, which encodes a subunit of the Ext1/Ext2 protein complex responsible for heparan sulfate (HS) synthesis, develop an uneven articulating superficial zone that expresses very low levels of lubricin.7 Achieving optimal lubrication remains one of the primary goals of tissue engineering of articular cartilage.8
TGF-β, a potent pleiotropic regulator, plays an important role in the development and maintenance of articular cartilage.9,10 TGF-β1 stimulates the cell growth rate and mediates cell survival and matrix synthesis in articular chondrocytes.11–14 Lack of TGF-β1 or abnormalities in its signaling pathways result in a cartilage phenotype closely resembling pathological osteoarthritic tissue.15 Proteoglycans decorin and biglycan, both with chondroitin sulfate (CS) chains, bind to TGF-β. This serves to keep TGF-β sequestered in the pericellular matrix surrounding cells and regulates the activity of TGF-β in the synthesis of matrix components.16,17 TGF-β1 binds to heparin, a highly sulfated analog of HS, under physiological conditions.18 HS proteoglycans potentiate the activity of TGF-β1 by providing protection from enzymatic proteolysis at the cell surface, leading to the accumulation of TGF-β1 in the pericellular environment.19 Transfection with syndecan-2, a cell surface proteoglycan containing both HS and CS,20 increases the expression of TGF-β receptors on the cell surface.21 In addition, heparin and HS have been shown to enhance the binding of TGF-β1 to TGF-β type II receptor.22 TGF-β1 may be used to enhance synthesis of SZP/lubricin in articular chondrocytes; however, the synergic effects of exogenous glycosaminoglycans (GAGs), specifically in the presence and absence of cell surface proteoglycans, require further investigation.
GAGs are linear polysaccharides—linked covalently to a protein core to form proteoglycans—with diverse functions in cell growth, migration, and differentiation present in the cell surface and ECM.16,23 GAGs bind and provide localization of growth factors at the cell surface or ECM and promote their biological activities.24,25 The ECM of articular cartilage—composed of water, collagen, and GAGs—contributes to its biomechanical and low friction properties. OA involves a combination of abnormal mechanical stresses and biochemical imbalances that lead to a loss of proteoglycans and disruption of the collagen network in articular cartilage.4 In articular cartilage, the ECM contains primarily CS while the pericellular matrix is abundant in HS.26 Addition of exogenous CS to superficial zone chondrocytes is shown to augment mRNA expression of PRG4.27 Mice with impaired secretion of perlecan—the most abundant HS proteoglycan present in articular cartilage—exhibit postnatal joint abnormalities, including degeneration of articular cartilage.28 In addition, mice deficient in Ext-1 exhibit ectopic formation of hypertrophic-like chondrocytes within the articular cartilage of synovial joints similar to those often seen in the joints of OA.29
The aim of this study was to investigate the accumulation of SZP in superficial zone articular chondrocytes in response to stimulation with TGF-β1 using cell culture. We investigated the effects of TGF-β1 on SZP accumulation in the presence of exogenous HS, heparin, or CS. We further demonstrated the role of cell surface GAGs during stimulation of SZP accumulation by TGF-β1 and exogenous GAGs.
Materials and Methods
Isolation of superficial zone articular chondrocytes
Bovine stifle joints from 3-month-old calves were obtained from an abattoir and dissected under aseptic conditions to expose the femoral condyles.5 The superficial zone of articular cartilage constitutes 5–7% (∼100–500 μm) of the top surface thickness.30 Superficial zone articular cartilage (∼100 μm thick) was harvested using a dermatome. The cartilage was enzymatically digested for 2.5 h with 0.2% collagenase P (Roche) in culture medium (Dulbecco's modified Eagle's medium/F-12; Gibco) containing 50 μg/mL ascorbate-2-phosphate (Sigma-Aldrich), 0.1% bovine serum albumin (BSA; Sigma-Aldrich), and 1% penicillin/streptomycin supplemented with 3% heat-inactivated fetal bovine serum (FBS; Gibco) at 37°C. Isolated superficial zone articular chondrocytes were plated at a density of 100,000 cells/well (25,000 cells/cm2) in 12-well dishes (Corning), and incubated in 5% CO2 at 37°C overnight in culture medium containing 10% FBS.
Monolayer culture of primary articular chondrocytes
Superficial zone chondrocytes were cultured in serum-free, defined conditions consisting of culture medium supplemented with 1% ITS+Premix (BD Biosciences) and 0.5% fungizone in a humidified, 5% CO2 incubator at 37°C. After the media exchange, various concentrations of TGF-β1 (0.3, 1 ng/mL) and GAGs (30 μg/mL) mentioned earlier were added to the cell culture. Bovine kidney HS, bovine trachea CS, and porcine intestinal mucosa heparin were purchased from Sigma-Aldrich. TGF-β1 was purchased from R&D Systems. Heparinase I (HEP-I) and chondroitinase-ABC (C-ABC) enzymes, which selectively degrade HS/heparin and CS, respectively, were used to deplete surface GAGs. For HEP-I and C-ABC treatments, chondrocytes were pretreated with either HEP-I (0.05 μg/mL) or C-ABC (0.1 μg/mL) (both from Sigma-Aldrich) for 2 h in a humidified, 5% CO2 incubator at 37°C and washed with phosphate-buffered saline (PBS) before the addition of TGF-β1 (0.3, 1 ng/mL) and/or GAGs (30 μg/mL). Preliminary studies on chondrocyte cultures were conducted to optimize dosage of TGF-β1, exogenous HS, heparin and CS, HEP-I, and C-ABC. Chondrocytes were harvested after 4 days of culture. Viable cell counts were taken on the day of initial seeding and at day 4 of treatment using 0.4% Trypan blue (Sigma-Aldrich) exclusion assay with a hemocytometer. Viable cell count values are presented as the percent difference between treated and untreated controls.
Immunoblot analysis of SZP
The culture media was collected from chondrocyte cultures at day 4 for qualitative analysis. Equal volumes of media sample were denatured and electrophoretically separated under reducing conditions in 4–12% Bis–Tris polyacrylamide gels using MOPS buffer (Life Technologies). Proteins were then transferred to polyvinylidene fluoride membranes using a semidry transfer cell (Bio-Rad). Membranes were blocked with 5% nonfat dry milk in TBST (25 mM Tris–HCl, 125 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature and incubated overnight at 4°C with mouse monoclonal anti-SZP S6.79 antibody (1:5000). After a washing step, the membrane was incubated with a goat anti-mouse IgG conjugated with horseradish peroxidase (1:3000; Bio-Rad) for 4 h at 4°C. The SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) was added after a subsequent wash, and the immunoblot was imaged using film.
Quantitation of SZP by enzyme-linked immunosorbent assay
SZP accumulation in the culture medium was quantified by sandwich enzyme-linked immunosorbent assay (ELISA). The capture reagent peanut lectin (EY Laboratories) was prepared as a 1 μg/mL solution in a 50 mM sodium carbonate buffer (pH 9.5), and used to coat black 96-well MaxiSorp plates (50 μL/well; Nalge Nunc International) overnight at 4°C. The wells were blocked with 1% BSA in the same buffer at room temperature for 2 h. SZP standards and samples were serially diluted in PBS and incubated for 1 h at room temperature.31 Plates were incubated overnight at 4°C with monoclonal antibody S6.79 (1:5000) followed by incubation with goat anti-mouse IgG conjugated with horseradish peroxidase (1:3000; Bio-Rad) for 1 h at room temperature. The SuperSignal ELISA Femto Maximum Sensitivity Substrate (Thermo Scientific) was used and the chemiluminescent signal was recorded using a chemiluminescent imaging system (Alpha Innotech). Wells were washed with PBS containing 0.05% Tween 20 (Sigma-Aldrich) between all steps. SZP levels from samples were quantified using a bovine SZP standard. Purity of standard SZP was verified by immunoblot analysis, and the SZP standard concentration was determined using a Micro BCA Protein Assay Kit (Thermo Scientific) based on a BSA protein standard.32
Statistical analysis
Each treatment group consisted of a sample size of n=6. Results were evaluated using a one-way analysis of variance followed by a Tukey's honestly significant difference. Statistical significance was established at p-values less than 0.05. All the quantitative data are presented as an SZP fold increase compared with untreated control groups. Results are presented as the mean±standard error.
Results
Characterization of superficial zone articular chondrocytes
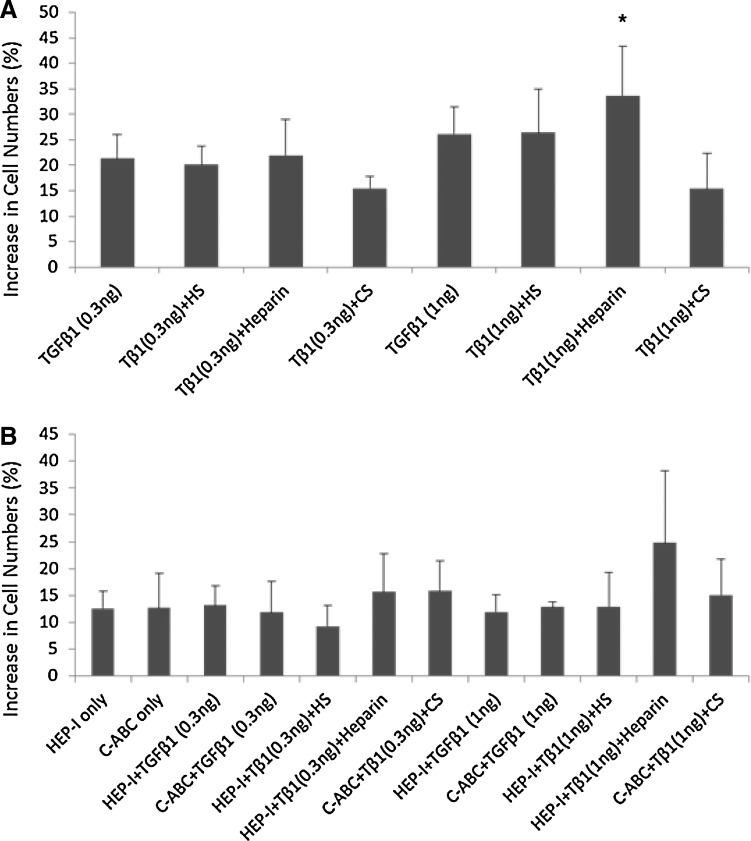
In articular cartilage, SZP is localized in the cells of the superficial zone.33 The secretion of SZP is characteristic of superficial zone chondrocytes, as SZP accumulation is not detected in cultures of middle and deep zone chondrocytes, even when stimulated by TGF-β1.5,33 Superficial zone chondrocytes have a flattened, fibroblast-like appearance. By day 4 of culture, untreated and treated chondrocytes maintained their phenotypical appearance under a light microscope (Fig. 1). To address the concern that our treatments could have an inhibitory impact on chondrocytes by increasing cell death, the viability of the chondrocytes was examined. Trypan blue exclusion assay was used to distinguish the live cells from the dead ones in the cell cultures of superficial zone articular chondrocytes. The differences in cell numbers between treatment groups and untreated controls were determined. Treatments did not result in cell death nor did they inhibit the chondrocyte proliferation (Fig. 2). Treatment with TGF-β1 (1 ng/mL) and exogenous heparin significantly increased chondrocyte proliferation as a 34% increase in cell numbers was observed compared with untreated controls (Fig. 2A). No significant differences in cell numbers between the other experimental groups and the untreated controls were observed.
FIG. 1.
The effect of treatments on the cellular appearance of superficial zone articular chondrocytes. Primary bovine superficial zone chondrocytes were treated and cultured for 4 days. Chondrocyte morphology was examined under a light microscope at day 4 of treatment. The control displayed a flattened, fibroblast-like appearance. Treated chondrocytes had a similar appearance to the controls. Chondrocytes were treated according to the corresponding treatment labels above the image. Scale bar=50 μm.
FIG. 2.
The effect of treatment on chondrocyte proliferation. Viable cell counts were taken using Trypan blue exclusion assay with a hemocytometer. The values, given as percentage, represent the difference in cell numbers at the end of the experiment (day 4) between the treatment groups and untreated controls. (A) Concurrent treatment with transforming growth factor (TGF)-β1 (1 ng/mL) and exogenous heparin (30 μg/mL) significantly increased chondrocyte proliferation as a 34% increase in cell numbers was observed compared with untreated controls. (B) No significant increase in cell numbers between experimental and untreated control groups was observed after enzymatic removal of surface glycosaminoglycans (GAGs) with heparinase I (HEP-I) or chondroitinase-ABC (C-ABC). Values presented as percent mean±standard error. *p<0.05 compared with untreated control.
TGF-β1 actions on SZP accumulation in the presence of exogenous GAGs
The dose response of TGF-β1 with regard to the accumulation of SZP was demonstrated by Niikura and Reddi.5 The study showed that TGF-β1 behaved in a biphasic manner with maximal effects observed with a dose of 3 ng/mL. Our preliminary data on TGF-β1 dose response confirmed the published study. Therefore, based on the published and preliminary results, the submaximal doses of TGF-β1 at 0.3 and 1 ng/mL were selected for this study. Monolayer superficial zone articular chondrocyte cultures were established and treated for 4 days with either 0.3 or 1 ng/mL of TGF-β1 in the absence or presence of 30 μg/mL of GAGs. Since the majority of SZP synthesized by superficial zone chondrocytes is secreted,2 the amount of SZP in the culture media was quantified to determine the actions of TGF-β1 on SZP accumulation in the presence of exogenous HS, heparin, or CS. In the absence of exogenous GAGs, TGF-β1 was able to stimulate the accumulation of SZP in superficial zone articular chondrocytes. Compared with untreated controls, the maximum increase in SZP accumulation was threefold with TGF-β1 at 0.3 ng/mL and 3.4-fold with TGF-β1 at 1 ng/mL (Fig. 3). No significant SZP fold difference was observed between the TGF-β1 doses. TGF-β1 in the presence of exogenous HS increased the accumulation of SZP compared with that observed on treatment of TGF-β1 in the absence of exogenous HS (Fig. 3). In the presence of exogenous HS, TGF-β1 at the dose 0.3 ng/mL was significantly more effective at increasing SZP accumulation than at the dose of 1 ng/mL, when compared with untreated controls. Compared with its absence, the presence of exogenous HS increased SZP accumulation by about onefold with 0.3 ng/mL of TGF-β1. The maximum increase in SZP accumulation compared with untreated control was 4.1-fold with 0.3 ng/mL of TGF-β1 and 3.6-fold with 1 ng/mL of TGF-β1. In the presence of exogenous heparin, TGF-β1 stimulated the accumulation of SZP in a dose-dependent manner (Fig. 3). The presence of exogenous heparin significantly increased SZP accumulation by 1.2-fold with 0.3 ng/mL of TGF-β1 (Fig. 3A) but decreased SZP accumulation by onefold with 1 ng/mL of TGF-β1 (Fig. 3B) compared with that observed on treatment of TGF-β1 in the absence of exogenous heparin. When compared with untreated controls, the maximum response in SZP accumulation in the presence of exogenous heparin was 4.2-fold with TGF-β1 at 0.3 ng/mL and 2.4-fold with TGF-β1 at 1 ng/mL. In the presence of exogenous CS, the action of TGF-β1 on the accumulation of SZP in superficial zone articular chondrocytes was independent of TGF-β1 dose (Fig. 3). TGF-β1 at 1 ng/mL in the presence of exogenous CS increased the accumulation of SZP by half fold compared with that observed on treatment of TGF-β1 at 1 ng/mL alone (Fig. 3B). The maximum increase in SZP accumulation compared with the untreated controls was 3.4-fold with 0.3 ng/mL of TGF-β1 and 3.9-fold with 1 ng/mL of TGF-β1.
FIG. 3.
Accumulation of superficial zone protein (SZP) stimulated by TGF-β1 in the presence of exogenous GAGs. Monolayer superficial zone articular chondrocyte cultures were treated for 4 days with either 0.3 or 1 ng/mL of TGF-β1 in the absence or presence of 30 μg/mL of GAGs. (A) Treatment of TGF-β1 (0.3 ng/mL) in the presence of exogenous heparan sulfate (HS) or heparin significantly increased SZP accumulation compared with untreated control groups. (B) Treatment of TGF-β1 (1 ng/mL) in the presence of exogenous chondroitin sulfate (CS) significantly increased SZP accumulation compared with untreated control groups. Results presented as SZP protein fold increase compared with untreated controls. Values presented as mean±standard error. *p<0.05 compared with untreated control.
TGF-β1 actions on SZP accumulation after enzymatic removal of endogenous GAGs
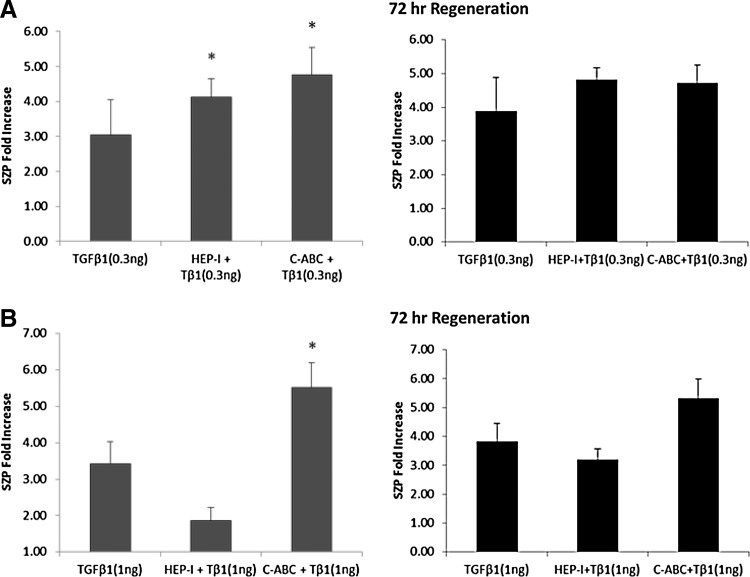
GAGs are commonly present on the cell surface of superficial zone articular chondrocytes. Endogenous GAGs are known to promote the biological activity of growth factors by binding and concentrating them at the cell surface or ECM.25,34 To investigate the interaction of endogenous GAGs with TGF-β1 during its stimulation of SZP, endogenous GAGs were enzymatically removed and the accumulation of SZP in the culture medium was quantified. Monolayer cultures of superficial zone articular chondrocyte were treated with either HEP-I to enzymatically remove surface HS or C-ABC to enzymatically remove surface CS. After enzymatic treatment, chondrocytes were treated with either 0.3 or 1 ng/mL of TGF-β1. Accumulation of SZP in the absence of endogenous GAGs was stimulated by TGF-β1 in a dose-dependent manner. In the absence of endogenous GAGs, TGF-β1 at 0.3 ng/mL significantly increased the accumulation of SZP compared with untreated controls and with that observed on treatment of TGF-β1 at 0.3 ng/mL in the presence of endogenous GAGs (Fig. 4A). The absence of endogenous HS and endogenous CS increased SZP accumulation by 1.1- and 1.8-fold, respectively, with 0.3 ng/mL of TGF-β1 when compared with TGF-β1 at 0.3 ng/mL in the presence of endogenous GAGs. The maximum increase in SZP accumulation with 0.3 ng/mL of TGF-β1 compared with untreated controls was 4.1-fold in the absence of endogenous HS and 4.8-fold in the absence of endogenous CS. TGF-β1 at 1 ng/mL significantly increased the accumulation of SZP by 2.1-fold in the absence of endogenous CS compared with untreated controls and with that observed on treatment of TGF-β1 at 1 ng/mL in the presence of endogenous GAGs (Fig. 4B). The maximum increase in SZP accumulation compared with untreated control was 5.5-fold with 1 ng/mL TGF-β1. On the other hand, a 1.5-fold decrease in SZP accumulation by TGF-β1 at 1 ng/mL was observed in the absence of endogenous HS (Fig. 4B). Compared with untreated control, the maximum increase in SZP accumulation in the absence of endogenous HS was 1.9-fold with 1 ng/mL of TGF-β1. Hence, endogenous HS may be involved in the ability of TGF-β1 at 1 ng/mL to enhance SZP. These results suggest that TGF-β1 may not require the presence of endogenous CS to exercise its actions on SZP accumulation in superficial zone articular chondrocytes. Together, the data suggest that the ability of TGF-β1 to stimulate SZP is modulated by endogenous GAGs.
FIG. 4.
Accumulation of SZP stimulated by TGF-β1 after enzymatic removal and regeneration of endogenous GAG. Monolayer superficial zone articular chondrocyte cultures were treated with HEP-I or C-ABC for the enzymatic removal of endogenous HS or CS, respectively. After enzymatic treatment, cultures were treated with either 0.3 or 1 ng/mL of TGF-β1 immediately or incubated for 72 h to allow regeneration of endogenous GAGs before treatment with either 0.3 or 1 ng/mL of TGF-β1. Cultures were treated for 4 days after initial TGF-β1 treatment. (A) SZP accumulation significantly increased with TGF-β1 (0.3 ng/mL) in the absence of endogenous GAGs. (B) SZP accumulation significantly increased with TGF-β1 (1 ng/mL) in the absence of endogenous CS. The replenishment of endogenous HS enhanced SZP accumulation by 1.3-fold. Results presented as SZP protein fold increase compared with untreated controls. Values presented as mean±standard error. *p<0.05 compared with untreated control.
TGF-β1 actions on SZP accumulation in the presence of exogenous GAGs after enzymatic removal of endogenous GAGs
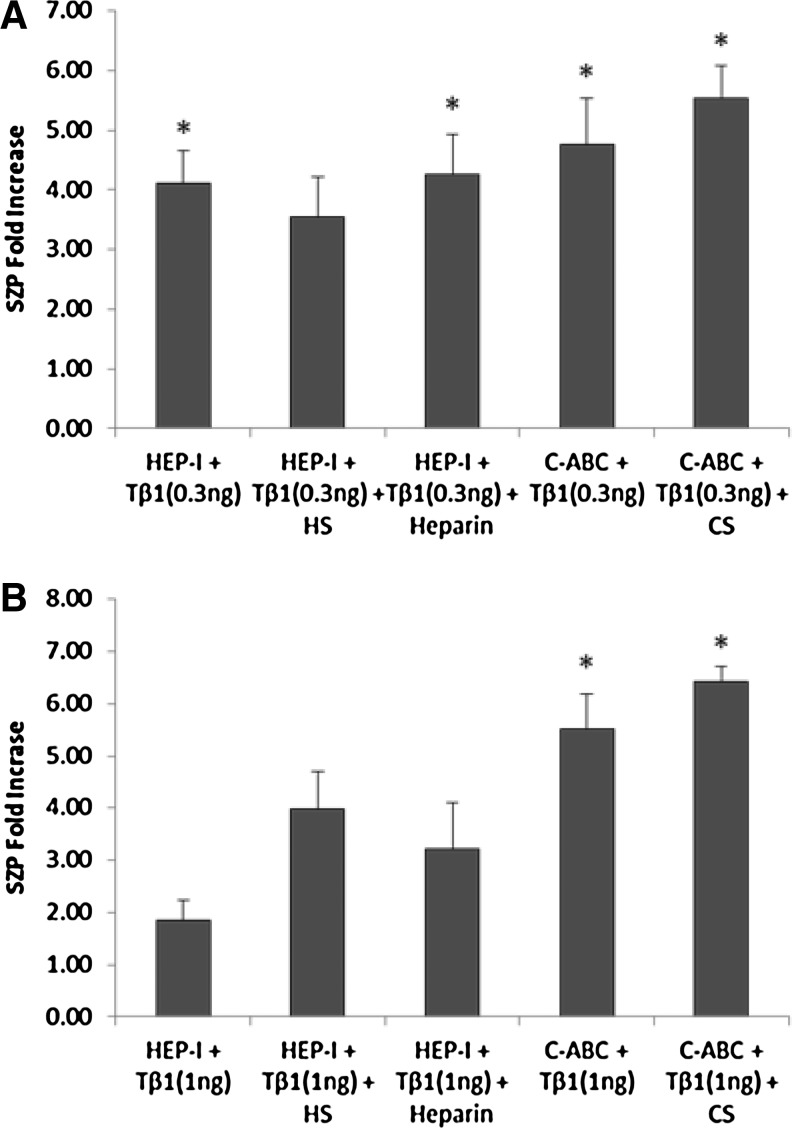
After enzymatic removal of endogenous GAGs, exogenous HS, heparin, and CS were added to replenish the GAGs to further evaluate the actions of TGF-β1 on SZP accumulation in superficial zone articular chondrocytes (Fig. 5). Monolayer cultures of superficial zone articular chondrocyte were treated with either HEP-I or C-ABC. After enzymatic treatment, cultures were treated for 4 days with either 0.3 or 1 ng/mL of TGF-β1 in the presence of 30 μg/mL of GAGs. Accumulation of SZP after exogenous GAGs were replenished was stimulated by TGF-β1 in a dose-dependent manner (Fig. 5). The replenishment of exogenous HS reduced the ability of TGF-β1 at 0.3 ng/mL to stimulate SZP accumulation by 0.6-fold compared with that observed with TGF-β1 at 0.3 ng/mL after enzymatic removal of endogenous HS. The maximum fold increase in SZP accumulation compared with untreated controls was 3.6-fold (Fig. 5A). On the other hand, SZP accumulation increased by 2.1-fold with TGF-β1 at 1 ng/mL after exogenous HS was replenished compared with that observed with TGF-β1 at 1 ng/mL after enzymatic removal of endogenous HS (Fig. 5B). The maximum fold increase in SZP accumulation was similar to that observed with 1 ng/mL of TGF-β1 in the presence of exogenous HS before enzymatic removal of endogenous GAG (Figs. 5B and 3B). Taken together, these results suggest that exogenous HS enhances the ability of TGF-β1 to stimulate SZP. In addition, both endogenous GAGs and exogenous HS are utilized by TGF-β1 at 1 ng/mL to enhance SZP accumulation.
FIG. 5.
Accumulation of SZP stimulated by TGF-β1 in the presence of exogenous GAGs after enzymatic removal of endogenous GAGs. Monolayer superficial zone articular chondrocyte cultures were treated with HEP-I or C-ABC for the enzymatic removal of endogenous HS or CS, respectively. After enzymatic treatment, cultures were treated for 4 days with either 0.3 or 1 ng/mL of TGF-β1 in the presence of 30 μg/mL of GAGs. (A) TGF-β1 (0.3 ng/mL) in the presence of replenished exogenous heparin and CS significantly increased SZP accumulation in the culture media. (B) TGF-β1 (1 ng/mL) in the presence of replenished exogenous CS significantly increased SZP accumulation in the culture media. Results presented as SZP protein fold increase compared with untreated controls. Values presented as mean±standard error. *p<0.05 compared with untreated control.
Replenishment of exogenous heparin enhanced the ability of TGF-β1 to stimulate SZP accumulation. Notably, the accumulation of SZP increased by 1.3-fold with TGF-β1 at 1 ng/mL after exogenous heparin was replenished compared with that observed on treatment of TGF-β1 at 1 ng/mL after enzymatic removal of endogenous GAGs (Fig. 5B). The maximum fold increase in SZP accumulation compared with untreated control was similar to that observed with TGF-β1 at 1 ng/mL in the absence of exogenous heparin (Figs. 5B and 3B). These observations imply that with TGF-β1 at 1 ng/mL, the presence of both exogenous heparin and endogenous GAGs produces an inhibitory response on SZP accumulation. Replenishment of exogenous CS significantly increased the ability of TGF-β1 to stimulate SZP accumulation in superficial zone articular chondrocytes compared with untreated controls (Fig. 5). Accumulation of SZP increased 0.7-fold with TGF-β1 at 0.3 ng/mL and about onefold with TGF-β1 at 1 ng/mL after exogenous CS was replenished compared with that observed with TGF-β1 after enzymatic removal of endogenous CS. Thus, the presence of endogenous CS appears to have an inhibitory effect on SZP accumulation. These results suggest that in the presence of TGF-β1, endogenous CS restricts the ability of exogenous CS to enhance SZP accumulation in superficial zone articular chondrocytes.
TGF-β1 actions on SZP accumulation after regeneration of endogenous GAGs
The production and turnover of proteoglycans and endogenous GAGs are a general property of animal cells.16 After enzymatic removal of endogenous GAGs, endogenous GAGs were allowed to regenerate and replenish on the cell surface. The actions of TGF-β1 on the accumulation of SZP in superficial zone articular chondrocytes in the presence of regenerated endogenous GAGs were determined. Monolayer cultures of superficial zone articular chondrocyte were treated with either HEP-I or C-ABC to enzymatically remove cell surface HS or CS, respectively. After enzymatic treatment, chondrocytes were incubated for 72 h in serum-free conditions to allow for endogenous GAGs to regenerate and replenish the cell surface. After the 72 h incubation period, cultures were treated for 4 days with either 0.3 or 1 ng/mL of TGF-β1. The replenishment of endogenous HS enhanced the ability of TGF-β1 to stimulate SZP accumulation. In particular, the accumulation of SZP increased by 1.3-fold with TGF-β1 at 1 ng/mL compared with that observed after enzymatic removal of endogenous HS (Fig. 4B). The maximal increase in SZP accumulation compared with untreated control was 4.8-fold with 0.3 ng/mL of TGF-β1 and 3.2-fold with 1 ng/mL of TGF-β1. These results further support the notion that endogenous HS may be involved in the ability of TGF-β1 at 1 ng/mL to enhance SZP. Compared with its absence, the regeneration of endogenous CS caused a slight decrease in SZP accumulation by 0.1-fold with TGF-β1 at 0.3 ng/mL and by 0.2-fold with TGF-β1 at 1 ng/mL, providing further evidence to an inhibitory role for endogenous CS. Compared with untreated controls, the maximum increase in the accumulation of SZP was 4.7-fold with 0.3 ng/mL of TGF-β1 and 5.3-fold with 1 ng/mL of TGF-β1 (Fig. 4). Overall, these results further suggest that endogenous GAGs modulate the ability of TGF-β1 to stimulate SZP in superficial zone articular chondrocytes.
Discussion
SZP, also known as lubricin, and PRG4 are encoded by the PRG4 gene and function as a boundary lubricant in the synovial joints.2,35 In this study, we established that TGF-β1 can stimulate SZP accumulation in superficial zone articular chondrocytes in the presence of exogenous GAGs. Further, we demonstrate that endogenous GAGs present on the surface of articular chondrocytes influence the ability of TGF-β1 to stimulate the accumulation of SZP. Next, addition of exogenous GAGs influences the ability of TGF-β1 to regulate the accumulation of SZP in superficial zone chondrocytes depleted of endogenous GAGs. These findings will have useful applications in the tissue engineering of functional articular cartilage constructs.
TGF-β is a critical regulator of SZP in superficial zone articular chondrocytes. Exposure to TGF-β induces the accumulation of SZP in both superficial zone articular chondrocytes and synoviocytes in vitro.5,6 In this study, we utilized superficial zone articular chondrocytes and investigated the actions and modulations of TGF-β1 on SZP accumulation in the presence of exogenous GAGs. SZP accumulation regulated by TGF-β1 increased in the presence of exogenous HS. TGF-β1 at 0.3 ng/mL was more effective at stimulating SZP accumulation than at 1 ng/mL in the presence of exogenous HS. Previous work on the actions of TGF-β on SZP accumulation demonstrated the duration of phosphorylation of Smad2 in superficial zone articular chondrocytes to be inversely proportional to the dose of TGF-β.36 A longer duration of persistence of Smad phosphorylation in TGF-β1 at 0.3 ng/mL explains the higher increase in SZP accumulation observed with the lower TGF-β1 dose. In the presence of exogenous heparin or CS, the ability of TGF-β1 to regulate SZP accumulation was in a dose-dependent manner. TGF-β1 at 0.3 ng/mL in the presence of exogenous heparin increased the accumulation of SZP found in the culture medium. However, TGF-β1 at a dose of 1 ng/mL produced an inhibitory response toward the accumulation of SZP in the presence of exogenous heparin. This decrease was not attributed to a loss or death of cells in culture, as an increase in cell numbers was noted. This inhibitory response might be due to increased cell density. Addition of exogenous heparin along with endogenous GAGs present on chondrocytes may result in an oversaturation of GAGs, reducing the ability of TGF-β1 to interact with its receptors on the cell surface. The presence of exogenous CS may not affect TGF-β1 at 0.3 ng/mL, as the response in SZP accumulation was similar to the response observed in the absence of exogenous CS. However, the accumulation of SZP increased with TGF-β1 at 1 ng/mL in the presence of exogenous CS. The presence of exogenous GAGs may aid TGF-β1 in prolonging its binding interactions with its receptor and increasing the duration of phosphorylation of signal transducing Smad proteins.
Endogenous GAGs are commonly expressed on the cell surface of articular chondrocytes and deposited in the ECM. The ECM of articular cartilage contains primarily CS, while the pericellular matrix is abundant in HS.26 Enzymatic removal of endogenous CS increased the ability of TGF-β1 to stimulate SZP and was further enhanced by the replenishment of exogenous CS. Enzymatic treatment of articular cartilage with C-ABC alters matrix characteristics and promotes matrix maturation.37,38 Thus, alterations in the matrix induced by C-ABC and the depletion of endogenous CS may change the way in which TGF-β1 is perceived by chondrocytes, enhance the actions of TGF-β1 by allowing access for exogenous CS to interact with TGF-β1, and help contribute toward stimulating SZP accumulation. Cartilage has demonstrated the ability to restore endogenous GAG content after enzymatic digestion.39 Regeneration of endogenous CS decreased the ability of TGF-β1 to stimulate SZP, further supporting an inhibitory role for endogenous CS. In conclusion, endogenous CS is not involved in the actions of TGF-β1 and may have an inhibitory effect on SZP accumulation in articular chondrocytes.
Enzymatic removal of endogenous HS increased the ability of TGF-β1 to stimulate SZP and was further enhanced when exogenous HS and heparin were replenished. However, replenished exogenous HS reduced the ability of TGF-β1 at 0.3 ng/mL to stimulate SZP, indicating that endogenous HS may be involved in the activity of TGF-β1 at low doses. This was supported by the fact that replenishment of endogenous HS enhanced the ability of TGF-β1 to stimulate SZP accumulation. Endogenous HS proteoglycans bind to and localize growth factors at their site of action and potentiate their biological activities.25 Depletion of endogenous HS may limit the ability of TGF-β1 to properly interact with its receptors at the cell surface and reduce the activity of TGF-β1. Exogenous HS enhances the ability of TGF-β1 to stimulate SZP, as it may aid in sequestering TGF-β1 at the cell surface to facilitate binding to its receptor, supporting the role of HS as a growth factor modulator and co-receptor.24,25,40
It is well accepted that SZP plays an important role in the integrity of synovial joints as the key modulator in boundary lubrication.3,5,31,32,41,42 In this study, we investigated the accumulation of SZP in superficial zone articular chondrocytes in response to stimulation with TGF-β1 in the presence of exogenous GAGs. Our findings demonstrate that the presence of exogenous HS enhances the capacity of TGF-β1 to regulate SZP accumulation in superficial zone articular chondrocytes. Furthermore, enzymatic removal of endogenous GAG enhanced the ability of TGF-β1 to stimulate SZP. Enhancing the capacity of superficial zone articular chondrocytes to produce SZP may aid in reducing the friction coefficient and contribute to the tissue engineering of functional articular cartilage constructs.
Acknowledgments
This work was supported by funds from the Lawrence J. Ellison Endowed Chair held by A. Hari Reddi. The authors thank Dr. Thomas M. Schmid of Rush University for generously providing the monoclonal antibody S6.79. This investigation was supported in part by the NIH grant RO1 AR 061496.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Jones A.R., and Flannery C.R. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cells Mater 13, 40, discussion 45, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Flannery C.R., Hughes C.E., Schumacher B.L., Tudor D., Aydelotte M.B., Kuettner K.E., et al. . Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Comm 254, 535, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Jay G.D., Torres J.R., Rhee D.K., Helminen H.J., Hytinnen M.M., Cha C.J., et al. . Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum 56, 3662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coles J.M., Zhang L., Blum J.J., Warman M.L., Jay G.D., Guilak F., et al. . Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum 62, 1666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niikura T., and Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum 56, 2312, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Darling E.M., and Athanasiou K.A. Growth factor impact on articular cartilage subpopulations. Cell Tissue Res 322, 463, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Mundy C., Yasuda T., Kinumatsu T., Yamaguchi Y., Iwamoto M., Enomoto-Iwamoto M., et al. . Synovial joint formation requires local Ext1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol 351, 70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNary S.M., Athanasiou K.A., and Reddi A.H. Engineering lubrication in articular cartilage. Tissue Eng Part B Rev 18, 88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Kraan P.M. Osteoarthritis and a high-fat diet: the full ‘OA syndrome’ in a small animal model. Arthritis Res Ther 12, 130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol 6, 597, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Yonekura A., Osaki M., Hirota Y., Tsukazaki T., Miyazaki Y., Matsumoto T., et al. . Transforming growth factor-beta stimulates articular chondrocyte cell growth through p44/42 MAP kinase (ERK) activation. Endocr J 46, 545, 1999 [DOI] [PubMed] [Google Scholar]
- 12.van Beuningen H.M., van der Kraan P.M., Arntz O.J., and van den Berg W.B. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest 71, 279, 1994 [PubMed] [Google Scholar]
- 13.Jahng J.S., Lee J.W., Han C.D., Kim S.J., and Yoo N.C. Transforming growth factor-beta 1 responsiveness of human articular chondrocytes in vitro: normal versus osteoarthritis. Yonsei Med J 38, 40, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Brenner R.E., Nerlich A., Heinze E., Vetter U., and Teller W.M. Different regulation of clonal growth by transforming growth factor-beta 1 in human fetal articular and costal chondrocytes. Pediatr Res 33, 390, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Blaney Davidson E.N., van der Kraan P.M., and van den Berg W.B. TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15, 597, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Esko J.D., Kimata K., and Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A., Cummings R.D., Esko J.D., Freeze H.H., Stanley P., Bertozzi C.R., et al., eds. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 2009. [PubMed] [Google Scholar]
- 17.Hildebrand A., Romaris M., Rasmussen L.M., Heinegard D., Twardzik D.R., Border W.A., et al. . Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J 302, 527, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaffrey T.A., Falcone D.J., and Du B. Transforming growth factor-beta 1 is a heparin-binding protein: identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-beta 1. J Cell Physiol 152, 430, 1992 [DOI] [PubMed] [Google Scholar]
- 19.McCaffrey T.A., Falcone D.J., Brayton C.F., Agarwal L.A., Welt F.G., and Weksler B.B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol 109, 441, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan D., and Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol 1, a002493, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Klass C., and Woods A. Syndecan-2 regulates transforming growth factor-beta signaling. J Biol Chem 279, 15715, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Kou I., Nakajima M., and Ikegawa S. Binding characteristics of the osteoarthritis-associated protein asporin. J Bone Miner Metab 28, 395, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Ruoslahti E., and Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell 64, 867, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Capila I., and Linhardt R.J. Heparin-protein interactions. Angew Chem 41, 391, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Jackson R.L., Busch S.J., and Cardin A.D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev 71, 481, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Farach-Carson M.C., Hecht J.T., and Carson D.D. Heparan sulfate proteoglycans: key players in cartilage biology. Crit Rev Eukaryot Gene Expr 15, 29, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Coates E.E., Riggin C.N., and Fisher J.P. Matrix molecule influence on chondrocyte phenotype and proteoglycan 4 expression by alginate-embedded zonal chondrocytes and mesenchymal stem cells. J Orthop Res 30, 1886, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Rodgers K.D., Sasaki T., Aszodi A., and Jacenko O. Reduced perlecan in mice results in chondrodysplasia resembling Schwartz-Jampel syndrome. Hum Mol Genet 16, 515, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sgariglia F., Candela M.E., Huegel J., Jacenko O., Koyama E., Yamaguchi Y., et al. . Epiphyseal abnormalities, trabecular bone loss and articular chondrocyte hypertrophy develop in the long bones of postnatal Ext1-deficient mice. Bone 57, 220, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattori S., Oxford C., and Reddi A.H. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun 358, 99, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neu C.P., Khalafi A., Komvopoulos K., Schmid T.M., and Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum 56, 3706, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Neu C.P., Reddi A.H., Komvopoulos K., Schmid T.M., and Di Cesare P.E. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum 62, 2680, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.Y., Niikura T., and Reddi A.H. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A 14, 1799, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Bernfield M., Gotte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., et al. . Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68, 729, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt T.A., Schumacher B.L., Klein T.J., Voegtline M.S., and Sah R.L. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum 50, 2849, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Khalafi A., Schmid T.M., Neu C., and Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res 25, 293, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Natoli R.M., Revell C.M., and Athanasiou K.A. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 15, 3119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Responte D.J., Arzi B., Natoli R.M., Hu J.C., and Athanasiou K.A. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials 33, 3187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asanbaeva A., Masuda K., Thonar E.J., Klisch S.M., and Sah R.L. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum 56, 188, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kuo W.J., Digman M.A., and Lander A.D. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell 21, 4028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jay G.D., Harris D.A., and Cha C.J. Boundary lubrication by lubricin is mediated by O-linked beta(1–3)Gal-GalNAc oligosaccharides. Glycoconj J 18, 807, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Schumacher B.L., Block J.A., Schmid T.M., Aydelotte M.B., and Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 311, 144, 1994 [DOI] [PubMed] [Google Scholar]