Abstract
Counter-regulation afforded by specialized regulatory cell populations and immunosuppressive cytokines is critical for balancing immune outcome. The inhibitory potential of the established suppressive cytokines, IL-10 and TGFβ, has been well elucidated in diverse inflammatory scenarios in conjunction with their key roles in Treg development and function. Despite the early predictions for an immunomodulatory role for the Ebi3/p35 heterodimer in placental trophoblasts, IL-35 biology remained elusive until 2007 when it was established as a Treg-restricted inhibitory cytokine. Since then, Treg-derived IL-35 has been shown to exhibit its suppressive activities in a range of autoimmune diseases and cancer models. Recent studies are beginning to explore other cellular sources of IL-35, such as Bregs and CD8+ Tregs. Despite these new cellular sources and targets, the mode of IL-35 suppression remains restricted to inhibition of proliferation and induction of an IL-35-producing induced regulatory T cell population referred to as iTr35. In this review, we explore the early beginnings, status quo, and future prospects of IL-35 biology. The unparalleled opportunity of targeting multiple immunosuppressive populations (Tregs, Bregs, CD8+ Tregs) through IL-35 is highly exciting and offers tremendous promise from a translational standpoint, particularly for cancer immunotherapies.
Cytokines and Immune Regulation
Immune protection is orchestrated as a balanced interplay of events without triggering aberrant responses to self or foreign antigens that underlie autoimmunity, allergies, chronic infections, and cancer. These varied responses are shaped largely by intercellular communication mediated by messenger molecules called cytokines. Cytokines are small soluble proteins secreted by immune cells in response to diverse external stimuli. Lymphocyte activation through receptor engagement (signal 1) and appropriate costimulation (signal 2) initiates the immune response and drives clonal expansion of antigen-specific cells. Cytokine signaling (signal 3) is critical for functional maturation of this response into appropriate effector lineages with helper, cytotoxic, memory, or antibody-secreting potential.
Cytokines are members of several distinct families based on their structure and receptor composition (hematopoietins, interleukins, interferons, TNF family, immunoglobulin supergene family, chemokines, and adipokines). They function in an autocrine or paracrine manner to coordinate a plethora of biological events ranging from embryonic development, cellular differentiation, migration, disease pathogenesis, and even cognitive functions and aging. Cytokine biology is extremely complex owing to the pleiotropic nature, functional redundancy, and also the growing addition of new members to an existing family of more than 100 cytokines and their receptors (Dinarello 2007; Yoshimoto and Yoshimoto 2013).
These cytokine families encompass both proinflammatory and suppressive members, and often the net effect of the cytokine milieu determines the immune outcome. Any trigger to the immune system elicits the release of proinflammatory cytokines and chemokines by the innate immune cells. This initial innate response holds the enemy at bay until adaptive immunity kicks in with its specialized armor of effector cells exhibiting distinct cytokine profiles and functions. These cytokine-driven cellular influxes and expansions promote inflammation that ultimately leads to the clearance of infection. Cytokine storms typically subside once the infection is eliminated or when the autoimmune response is curtailed by negative feedback circuits provided by suppressive cytokines (Banchereau and others 2012) and specialized regulatory cells (Tregs) (Sakaguchi and others 2010; Josefowicz and others 2012). Suppressive cytokines help restore the immune equilibrium and homeostasis with minimal collateral damage to the host (Banchereau and others 2012). A better understanding of the immune networks established by these positive and negative regulators will allow for effective cytokine modulation for therapeutic intervention.
Immune Modulation by Suppressive Cytokines
The established suppressive cytokines (IL-10 and TGFβ) and the newcomers (IL-27 and IL-35) are critical constituents of the regulatory, negative feedback loops and tolerance-promoting pathways that are integral to the immune system. These cytokines differ in their expression patterns, cellular sources, signaling circuits, and targets of suppression (Yoshimoto and Yoshimoto 2013). They typically act in concert for maximal suppressive potential, although different members may be more or less active under homeostatic or diverse inflammatory scenarios.
TGFβ is highly expressed in most tissues under basal conditions (Li and others 2012). TGFβ signaling is indispensible for limiting T-cell reactivity to self and maintenance of steady-state immune homeostasis and tolerance. Thus, mice with germ line TGFβ deletion or T-cell-specific deficiency in the TGFβ receptor develop spontaneous multifocal inflammatory disease associated with exuberant T-cell activation and Th1/Th2 cytokine release (Shull and others 1992; Li and Flavell 2008; Tran 2012). The same is true for patients with Sezary syndrome whose CD4+ T cells have reduced expression of TGFβ receptor and consequently unrestrained T-cell proliferation (Capocasale and others 1995).
In contrast to TGFβ, IL-10 is minimally expressed by unstimulated cells and often requires commensal- or pathogen-driven upregulation (Banchereau and others 2012). Although originally identified as a Th2 cytokine, it is now clear that many immune cell types can produce IL-10 (Moore and others 2001; Sabat and others 2010; Saraiva and O'Garra 2010). Whether activated through innate or adaptive pathways, IL-10 plays a major regulatory role in dampening most inflammatory responses. Indeed, mice that lack Il10 are highly susceptible to acute inflammation, exhibit profound immune polarization, and succumb to colitis, similar to the early onset of IBD observed in humans that harbor mutations in the genes encoding IL-10 or its 2 receptor components (Glocker and others 2009, 2011).
Together, this established suppressive cytokine duo plays a dominant role in maintaining steady-state immune equilibrium and taming exuberant responses at environmental interfaces. While TGFβ plays crucial roles in the induction of Foxp3 and the thymic development of Tregs (Xu and others 2010; Mahmud and others 2013), TGFβ and IL-10 also harbor another unique trait that further aids their immunomodulatory properties. Both these cytokines can transmit their suppressive potential to effector T cells causing them to gain a regulatory phenotype as well as target dendritic cells to promote conversion of effector cells to potent suppressors. This propagation of the tolerance-promoting state between cells is referred to as infectious tolerance (Gershon and Kondo 1971; Qin and others 1993; Gravano and Vignali 2012). IL-10 and TGFβ can drive naïve T-cell conversion to Tr1 and iTreg cells, respectively. Tr1 cells are identified by their ability to secrete large amounts of IL-10 and mild to moderate levels of TGFβ (Roncarolo and others 2001, 2006), while Foxp3+ iTregs are induced in the periphery through many different mechanisms and contribute to immunity at mucosal interfaces (Chen and others 2003; Sun and others 2007; Yadav and others 2013).
The recently discovered cytokines endowed with suppressive activity, IL-27 and IL-35, belong to the IL-12 family of cytokines. Unlike TGFβ, but similar to IL-10, IL-27 and IL-35 are minimally expressed in human tissues and are mainly induced in inflammatory conditions (Li and others 2012). This is well supported by lack of lethal defects in mice with genetic deletions in IL-35 and IL-27 components (p35, Ebi3, and p28), unlike mice with TGFβ1 deficiency. Thus, TGFβ functions as a housekeeping anti-inflammatory cytokine, while IL-10, along with IL-27 and IL-35, can be categorized as inducible suppressive members (Li and others 2012).
IL-35: Latest Addition to the IL-12 Cytokine Family
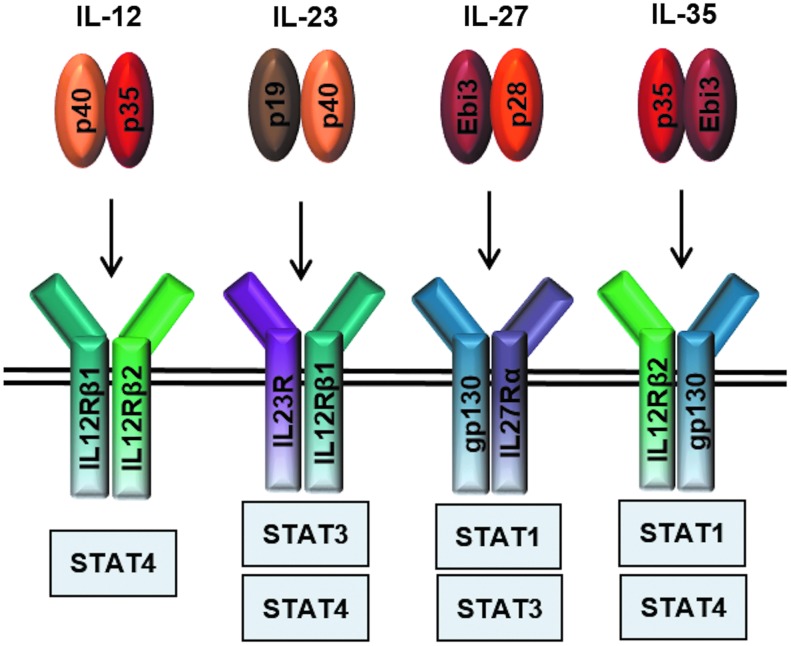
The IL-12 cytokine family belongs to the IL-6 superfamily of type I cytokines. Unlike the IL-6 family members that are secreted as single-subunit monomers, the IL-12 family is characterized by heterodimeric members formed from chain sharing interactions of 3 alpha chains (p19, p28, p35), classic 4-helix bundle cytokines, and 2 beta chains (p40 and Ebi3), which resemble soluble versions of cytokine receptors (Jones and Vignali 2011; Vignali and Kuchroo 2012). This gives rise to the 4 current members: IL-12 (p35/p40), IL-23 (p19/p40), IL-27 (p28/Ebi3), and IL-35 (p35/Ebi3) (Fig. 1). IL-12 and IL-23 are predominantly proinflammatory, while IL-27 and IL-35 are primarily suppressive cytokines (Vignali and Kuchroo 2012). Additionally, the existence of monomers and homodimers has been reported, which function as natural antagonists of bioactive cytokine signaling (Jones and Vignali 2011; Yoshimoto and Yoshimoto 2013).
FIG. 1.
IL-12 family cytokines and their receptors. Four current members of the IL-12 cytokine family and their downstream signaling pathways: IL-12 (p35/p40) signals through IL-12Rβ1 and IL-12Rβ2, IL-23 (p19/p40) signals through IL-23R and IL-12Rβ1, IL-27 (p28/Ebi3) signals through gp130 and WSX-1, and IL-35 (p35/Ebi3) utilizes IL-12Rβ2 and gp130 heterodimers and homodimers, respectively.
These cytokines signal through unique pairings of 5 receptor chains: IL-12Rβ1, IL-12Rβ2, IL-23R, IL-27R or WSX-1, and gp130 (Fig. 1). IL-12 signals through IL-12Rβ1 and IL-12Rβ2 and IL-23 through IL-23R and IL-12Rβ1, while IL-27 utilizes gp130 and WSX-1. In T cells, IL-35 signals through IL-12Rβ2 and gp130, although it can also signal through IL-12Rβ2-IL-12Rβ2 and gp130-gp130 homodimers (Collison and others 2012). Signal transduction through these receptor chains is mediated through the JAK-STAT pathway. The key Janus kinases mediating STAT phosphorylation for the IL-12 family members are JAK2 and either JAK1 or TYK2. STAT utilization varies between receptor chains such that IL-12 signals primarily through pSTAT4, IL-23 through pSTAT3 and pSTAT4, and IL-27 through pSTAT1 and pSTAT3. IL-35 signaling through gp130 and IL-12Rβ2 homodimers is mediated by pSTAT1 and pSTAT4, respectively (Collison and others 2012). These homodimeric receptors enable suppression of T-cell proliferation, but cannot induce iTr35 conversion (Fig. 2). In contrast, T-cell suppression and iTr35 induction can be mediated through an IL-12Rβ2:gp130 heterodimeric receptor that drives formation of pSTAT1:pSTAT4 heterodimers, which in turn bind to unique sites in the Ebi3 and Il12a promoters (Collison and others 2012). As discussed in detail below, recent studies have also suggested that IL-35 signaling in B cells is mediated through an IL-12Rβ2:WSX-1 heterodimer and induces pSTAT1 and pSTAT3 (Shen and others 2014).
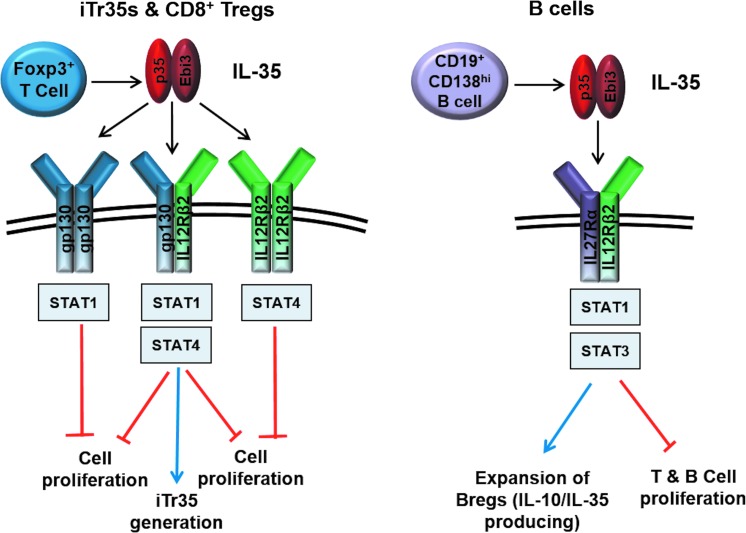
FIG. 2.
IL-35 signaling in T and B cells. Production of IL-35 by Foxp3+ T cells and CD19+CD138hi B cells inhibits T- and B-cell proliferation. Signaling through gp130 and IL12Rβ2 homodimers inhibits T-cell proliferation, while formation of the gp130/IL12Rβ2 heterodimer is required for both inhibition of T-cell proliferation and generation of iTr35 cells (left panel). B cells respond to IL-35 through the IL27Rα/IL12Rβ2 heterodimer leading to expansion of IL-10 and IL-35-producing Bregs that function to inhibit cellular proliferation (right panel).
Despite the obvious structural similarities in the cytokine subunits, receptor components, and downstream signaling, the IL-12 family members display surprisingly diverse but balanced functional outcomes. IL-12, IL-23, and IL-27 are secreted by activated antigen-presenting cells (B cells, monocyte/macrophages, dendritic cells), in response to bacteria, bacterial products, or intracellular parasites (Ma and Trinchieri 2001; O'Shea and Paul 2002; Hunter 2005). IL-12 stimulates IFN-γ production from both NK and T cells, thereby bridging the innate and adaptive immune arms and concurrently downregulating IL-4, thus inhibiting Th2 propagation. IL-12 also enhances the cytolytic activity of macrophages, T cells, and NK cells. Similar to IL-12, IL-23 can also drive Th1 proliferation (Oppmann and others 2000). However, IL-23 has been mainly linked to inflammatory processes associated with Th17 differentiation (Aggarwal and others 2003). IL-23 potently enhances and stabilizes differentiation to the Th17 lineage, promotes memory T-cell proliferation, and can also aid in DC cytokine production and antigen presentation (Zeng and others 2013). Thus, IL-12 and IL-23 represent the strictly proinflammatory members of the IL-12 family with key roles in Th1 and Th17 development.
IL-27, first discovered in 2002, was suggested to be a proinflammatory IL-12 family member due to its role in supporting Th1 development and IFN-γ production, while inhibiting Th2 and Th17 differentiation programs (Pflanz and others 2002; Villarino and others 2006). However, recent studies have revealed a broader immunomodulatory role for this cytokine. IL-27 was demonstrated to inhibit Th17 differentiation by targeting the Th17 transcription factors, RORγt, RORα, and RORc, as well as inhibiting IL-23-induced IL-17 production (Diveu and others 2009). IL-27 also promotes the generation of IL-10-producing Tr1 cells through induction of the transcription factor, cMaf, cytokine IL-21, and costimulatory receptor, ICOS, thus extending its ability to dampen inflammatory processes (Awasthi and others 2007; Fitzgerald and others 2007; Stumhofer and others 2007; Batten and others 2008; Pot and others 2009). These accumulating studies support a role for IL-27 in modulating immune responses.
IL-35, the newest IL-12 family member and focus of this review, is unique in its expression pattern and function. Unlike the other members, IL-35 is predominantly secreted by regulatory T cells, has been shown to suppress T-cell proliferation and function in a number of in vitro and in vivo disease models, and appears to be required for maximal suppressor function of mouse and human Tregs (Collison and others 2007, 2009; Pillai and others 2011). As recently reported, it can also be produced by regulatory B cells (Shen and others 2014; Wang and others 2014). Like IL-10 and TGFβ, IL-35 can also induce the conversion of naïve T cells into an IL-35-dependent regulatory population referred to as iTr35 cells (Collison and others 2010). iTr35 cells function independently of Foxp3, IL-10, and TGFβ and have been demonstrated to exhibit tolerance-promoting abilities in mouse models of parasite infection and tumors. Since its discovery in 2007, IL-35 has been shown to contribute to the regulatory milieu in a wide variety of disease states by several groups, thereby warranting detailed characterization of its cellular source, targets of suppression, and mechanism of action. Interestingly, thus far, it only seems to have a suppressive function, suggesting that it has a relatively restricted pattern of activity.
Early Beginnings of IL-35 Biology and Its Addition to the Treg Arsenal
IL-12, the archetypal member of the IL-12 family, was identified and purified from cell culture media of Epstein-Barr virus (EBV)-transformed B cell lines (Devergne and others 1997). Macrophages and EBV-transformed B cells could produce this 70 kDa p35/p40 heterodimer and typically an excess of p40. IL-12α/p35 expression was ubiquitous, while p40 seemed restricted and inducible. This dissociation between p35 and p40 gene regulation had hinted at the possibility that either or both of these subunits might have additional partners. Thus, the discovery of the other p40-related beta chain (Ebi3) in EBV-infected B cells in 1996 initiated experiments to determine if it was an alternate binding partner for p35 (Devergne and others 1996; Niedobitek and others 2002). This led to the first demonstration in 1997 that Ebi3 can heterodimerize with p35 to form a novel potentially naturally occurring hematopoietin (Devergne and others 1997). As Ebi3 is expressed in EBV-transformed B lymphocytes, tonsil, spleen, and placental trophoblasts and the Ebi3/p35 heterodimer was also coimmunoprecipitated from trophoblasts of a human full-term placenta (Devergne and others 2001), it was proposed that this Ebi3/p35 heterodimer might be an important immune modulator. However, its function and physiological relevance were unknown.
Despite these early observations, it was not until 2007 that a name and function were given to this p35/Ebi3 heterodimer when it was shown by the Vignali laboratory that IL-35 was produced by regulatory T cells and was required for their maximal suppressive function (Collison and others 2007). This rather came as a surprise to the field since both the IL-35 subunits are constituents of the IL-12 family of cytokines that were thought to be exclusively produced by APCs (Collison and Vignali 2008). Ebi3 was also noted as a candidate gene upregulated in Treg cell-specific gene arrays and was proposed as a potential Foxp3 target (Knoechel and others 2006; Collison and others 2007; Hill and others 2007; Zheng and others 2007). IL-35 appeared to be constitutively secreted by mouse Foxp3+ Tregs, but not effector T cells, and its expression was further boosted following coculture of Tregs with effector T cells (Collison and others 2007, 2009). Importantly, Treg cells deficient in either IL-35 subunit (Ebi3−/− or Il12a−/−), stimulated alone or following coculture with effector T cells, displayed reduced suppressive function in vitro and failed to control homeostatic proliferation and colitis in vivo (Collison and others 2007). Furthermore, recombinant IL-35 was able to suppress T-cell proliferation and its ectopic expression conferred suppressive potential to naïve T cells (Collison and others 2007). These studies were the first to delineate a defined function for this long-known Ebi3/p35 heterodimer as a Treg-restricted suppressive cytokine needed for maximal function, joining ranks with IL-10 and TGFβ as soluble, cell contact-independent mediators of suppression.
Around the same time, another group generated an Ebi3 and p35 Fc fusion protein and also demonstrated that this heterodimer suppressed CD4+CD25− effector T-cell proliferation and IFN-γ production (Niedbala and others 2007). It also inhibited Th17 cell differentiation and was noted to alleviate development of collagen-induced arthritis, a mouse model of Th1/Th17 inflammation. Interestingly, this group reported that this Ebi3:p35:Fc fusion protein could expand CD4+CD25+ Treg cells without induction of additional Foxp3 expression while retaining their suppressive function (Niedbala and others 2007). Taken together, these initial studies validated the immunomodulatory potential of IL-35 and paved the beginning for an exciting series of future studies to assess the physiological and pathophysiological roles of IL-35 in health and disease.
Regulatory Potential of Treg-Derived IL-35
The addition of IL-35 to the Treg cell arsenal in 2007 sparked tremendous enthusiasm in the Treg field as there has been continued interest in identifying novel mechanisms of Treg suppression that can be optimally channeled to either boost or inhibit Treg activity. While Treg suppression through inhibitory receptor expression (PD1, Lag3), cytolysis (Granzyme, Perforin), and metabolic disruption (IL-2 deprivation, adenosine) have all been documented, release of suppressive cytokines has prevailed as a major cell contact-independent mechanism of suppression. The inclusion of IL-35 to this important category of Treg suppressive mechanisms was particularly fascinating as IL-35 was the only known inhibitory mediator that appeared to be exclusively secreted by Treg cells alone, unlike IL-10 and TGFβ that are also secreted by other immune cells in addition to Tregs. Although this view has begun to change with the recent identification of new cellular sources of IL-35, this critical difference brought IL-35 at the forefront in this category and triggered a series of investigations into its mechanism of suppression.
Thus far, IL-35 has only been shown to exhibit suppressive activities and mechanisms. (Collison and others 2007; Gravano and Vignali 2012; Vignali and Kuchroo 2012). Regarding its suppressive activity, this is supported by experiments demonstrating the failure of Ebi3- and IL-12α-deficient Tregs to suppress T-cell proliferation in vitro and in a wide variety of inflammatory scenarios in vivo (Collison and others 2007; Wirtz and others 2011; Bettini and others 2012). This has also been validated by gain-of-function approaches using recombinant IL-35 in cell culture systems or ectopically expressed single-chain Ebi3:p35:Fc fusion proteins. In all these settings, IL-35 decreased T-cell proliferation. IL-35 appears to induce cell cycle arrest, but how this is mediated remains unknown. This has been confirmed in NOD transgenic mice with islet-specific expression of IL-35 (Bettini and others 2012). These mice exhibit substantially reduced diabetes incidence and inflammatory pathology owing to reductions in islet-resident CD4+ and CD8+ T cells. Even though the proportion of islet-infiltrating T cells in the G1 phase was comparable, beta cell-secreted IL-35 blocked their progression at the G1:S transition.
In addition to suppressing proliferation, IL-35 has also been shown to suppress differentiation to effector T cell lineages. An Ebi3:p35:Fc fusion protein was shown to suppress Th17 differentiation and attenuate collagen-induced arthritis (Niedbala and others 2007). Mice lacking Ebi3 also exhibit increased Th17 responses (Yang and others 2008; Liu and others 2012). In addition, there have been several studies demonstrating that recombinant IL-35 administration reduces Th17 differentiation and function. IL-35 also limited collagen-induced arthritis in another study by suppressing both Th1 and Th17 cells (Niedbala and others 2007; Wirtz and others 2011). Furthermore, intratracheal delivery of single-chain recombinant IL-35 significantly attenuated allergic airway inflammation by reducing the Th2 effector and humoral responses (Huang and others 2011). Thus, IL-35 can modulate effector T-cell differentiation pathways depending upon the nature of the inflammatory insult.
Another proposed mode of IL-35 action is through Treg expansion. Exposure to an Ebi3:p35:Fc fusion protein was shown to induce the proliferation of a population of CD4+CD25+ Foxp3+ T cells (Niedbala and others 2007). These IL-35-expanded Tregs expressed IL-10 and were able to suppress effector T-cell proliferation, potentially contributing to its inhibitory effects in a mouse model of arthritis. Similarly, in another report, IL-35 treatment expanded Foxp3+CD39+ CD4+ T cells that were dependent on IL-10 for mediating autoimmune protection following adoptive transfer in a collagen-induced arthritis model (Kochetkova and others 2010). However, it remains to be determined if IL-35 mediates expansion of Tregs in a physiological setting.
Last, similar to IL-10 and TGFβ, IL-35 seems to mediate infectious tolerance (Belkaid and Chen 2010; Gravano and Vignali 2012). Thus, naïve T cells stimulated in the presence of IL-35 are not only suppressed but also converted into an IL-35-producing regulatory population termed iTr35 cells. The promotion of infectious tolerance by IL-35 also derived from studies by the Vignali group who demonstrated that Treg cell-mediated suppression induces, through the concerted actions of IL-10 and IL-35, a distinct regulatory population characterized by IL-35 secretion that they termed iTr35 cells (Collison and others 2010). Notably, these cells are phenotypically and functionally distinct from the other known induced regulatory populations (TGFβ-induced Tregs [iTregs] and IL-10-induced Tregs [Tr1]), in that they do not express Foxp3, and suppress exclusively through IL-35 and independently of IL-10, TGFβ, CTLA-4, or any other known Treg suppressive mediators. Transcription profiling suggested that these iTr35 cells exhibit a highly restricted gene signature (CD4+Foxp3−Ebi3+p35+IL-10−TGFβ−), indicative of limited rather than global changes following IL-35 treatment of T cells. Mouse iTr35 cells exhibited potent suppressive potential in 5 distinct disease models. Importantly, inflammatory milieu generated following parasite infection (T. muris) and tumor inoculation (B16 and MC38) induced iTr35 cells, which seemed to provide a substantial physiological contribution to the regulatory milieu established by nTregs in the B16 melanoma model. Even naïve human CD4 T cells could be induced to develop into iTr35 cells in the presence of IL-35 (Collison and others 2010) or viral-exposed DCs (Seyerl and others 2010), and these human iTr35 cells also suppressed primary human T-cell proliferation through IL-35. One of the more exciting features of this induced regulatory population was its demonstrated stability and efficiency relative to TGFβ-iTr and IL-10-iTr cells. iTr35 cells were almost as efficient as nTregs in restoring homeostasis and preventing autoimmunity in Foxp3−/− mice, limiting T-cell proliferation in lymphopenic settings, curtailing inflammation in experimental autoimmune encephalitis (EAE) and colitis models, and promoting tumor growth in the B16 melanoma model. iTr35 cells also displayed increased stability and retained suppressor potential relative to TGFβ-induced counterparts following recovery 3 weeks post-transfer into immune-competent hosts. These intriguing aspects of the IL-35-induced regulatory population make these cells a safe and attractive therapeutic candidate considering the complexities associated with generation, short life span, and stability of ex vivo-generated TGFβ-iTr and IL-10-iTr cells (Collison and others 2010). However, further studies are warranted to fully elucidate the contribution of iTr35 cells in diverse disease scenarios, and the availability of purified rIL-35 remains a major limitation.
Thus, inhibition of effector T-cell proliferation through cell cycle arrest, modulating T-cell differentiation programs, expansion of regulatory T cells, and induction of iTr35 cells emerge as the key mechanisms for IL-35-mediated regulatory functions. As new cellular targets and sources of IL-35 will be deciphered, more information will likely emerge, expanding its scope of influence in immune regulation.
Identification of a Regulatory B Cell Subset Secreting IL-35
Analogous to the regulatory T cell (Treg) counterpart, the existence of regulatory B cells (Bregs) and their role in control of autoimmune diseases (EAE, CIA) has been described by several groups (Fillatreau and others 2002; Mauri and Ehrenstein 2008; Yanaba and others 2008; Barr and others 2012; Mauri and Bosma 2012). A key focus of the Breg cell field is to identify phenotypic markers to better characterize the subsets that have been identified thus far [CD1dhiCD5hi B10 cells (Yanaba and others 2008), CD21hiCD23hiCD24hi transitional type 2 marginal zone precursors (Evans and others 2007), Tim1-expressing B cells (Ding and others 2011), and CD138-expressing plasma cells (Neves and others 2010)]. Despite the lack of a unifying signature, IL-10 has accounted for most of the regulatory function ascribed to these regulatory B cell subsets (Fillatreau and others 2002; Banchereau and others 2012; Kalampokis and others 2013). In fact, IL-10-producing Breg cells have also been identified in humans, and numerical and/or functional perturbations in these IL-10-secreting Breg cell subsets have been reported in human autoimmune diseases (multiple sclerosis and type 1 diabetes) (Blair and others 2010; Mauri and Nistala 2014).
However, B cells have also been shown to mediate regulatory effects independent of IL-10 secretion (Wilson and others 2010; Su and others 2011). B cells can also induce Tregs in an IL-10-independent manner (Flores-Borja and others 2013). It is therefore not too hard to conceive that, similar to Tregs, Bregs may also harbor a range of suppressive mediators that can account for their broader involvement in immune regulation. Two recent studies have focused on elucidating other regulatory mediators with the potential to induce Bregs, drive B cell IL-10 secretion, and act independently or synergistically with IL-10 to elicit maximal Breg function in vivo (Shen and others 2014; Wang and others 2014). Interestingly, Ebi3, the beta chain of the Treg inhibitory cytokine, IL-35, emerged as one of the differentially regulated genes in a transcriptome analysis of B cells with TLR4 and CD40 costimulation relative to TLR4 priming alone (Shen and others 2014). Analysis of B cell-specific expression of other IL-12 family constituents showed that B cells also constitutively express the IL-35 alpha chain, p35, but do not express p40. Thus, BCR engagement along with TLR4 and CD40 stimulation results in increased Ebi3 and p35 transcription and IL-35 secretion, identifying B cells as a novel source of IL-35.
To test the in vivo functional relevance of B-cell-derived IL-35, bone marrow chimeric mice were generated with B cells lacking either the p35 or Ebi3 chain of IL-35. These B-cell-restricted IL-35-deficient mice developed exacerbated EAE and were protected from S. typhimurium (Salmonella)-induced sepsis relative to controls (Shen and others 2014). These responses were largely accounted for the enhanced antigen-presenting potential of IL-35-deficient B cells (associated with higher expression of MHCII, CD80, CD86), thereby stimulating higher proliferation and inflammatory cytokine production by their cognate CD4+ T cells. Thus, the suppressive effects of B-cell-derived IL-35 mainly stemmed from its ability to regulate the APC function of B cells, restraining inflammatory cell infiltration (T cells, macrophages) and thus limiting autoimmunity or mediating enhanced pathogen clearance.
The regulatory potential of B-cell-derived IL-35 was further confirmed and complemented by another independent study that showed that recombinant IL-35 treatment induced IL-10-secreting Bregs and these Bregs efficiently suppressed proliferation of CD19+ B cells (Wang and others 2014). Furthermore, IL-35 also mediated conversion of B cells into a Breg cell population that secreted IL-35 (IL-35+ Bregs). Administration of recombinant IL-35 or IL-35+ Breg cells efficiently controlled initial and established stages of experimental autoimmune uveitis (EAU). This IL-35+ Breg-mediated protection was dependent on increased induction and expansion of endogenous Bregs and Foxp3+ Tregs and inhibition of pathogenic Th1 and Th17 effector cells (Wang and others 2014). Thus, these 2 studies provide support for the existence of comparable regulatory modules within the T and B cell lineages, with IL-35 and IL-10 serving as a common link.
To assess the relationship between IL-35- and IL-10-producing B cells, Ebi3 and Il10 transcripts were quantified between CD19+CD138− B cells and CD138hi plasma cells. This analysis revealed that CD138hi plasma cells were the major source of IL-10 and IL-35 in vivo, although very few plasma cells cotranscribed Il10, Ebi3, and Il12a together, suggesting distinct regulatory plasma cell subsets secreting IL-10 and IL-35 (Shen and others 2014). Both studies demonstrated IL-35 enrichment within CD1d- and Tim1-expressing B cells (Shen and others 2014; Wang and others 2014). One of the major differences between the 2 studies was the relative importance of IL-10 or IL-35 to the regulatory function of B cells. At the peak of Breg expansion, almost 3% and 12% of the CD138hi plasma cells secreted IL-35 and IL-10, respectively. Despite this difference, deletion of either p35 or IL-10 resulted in similar exacerbations in EAE severity, and animals capable of producing either cytokine, but not both, had similar disease scores as wild-type animals, indicating that the cytokines functioned independent of each other (Shen and others 2014). In complete contrast, when B cells deficient in IL-12α, IL-12Rβ2, and IL-10 were cultured in the presence of rIL-35 and then tested for their capacity to control autoimmune uveitis (EAU), loss of IL-10 completely abrogated the suppressive effects of IL-35, suggesting that IL-10 is the final mediator of suppression of IL-35+ Bregs (Wang and others 2014). This was compatible with their in vitro studies demonstrating that addition of the neutralizing IL-10 antibody abrogated the suppressive effects of rIL-35.
The finding that IL-35 treatment of human B cells also induces IL-10 expression while inhibiting B-cell proliferation, suggests that IL-35 may hold promise for autologous Breg induction in the near future in conjunction with IL-21, which is currently used for large-scale B10 cell expansion ex vivo (Tedder and Leonard 2014).
IL-35 Signaling in T and B Cells
Considering that IL-35 shares its subunits with IL-12 and IL-27 and the fact that chain sharing of the IL-12 family also extends to the receptor chains and downstream signaling, it was likely that the IL-35 receptor and STAT utilization would involve components of the IL-12 and/or IL-27 signaling cascades. However, since the biological activity of IL-35 contrasts with its other siblings, utilization of a completely novel signal transduction mechanism was also possible. Using cells deficient in the IL-12 family receptor chains, it was demonstrated that IL-35 signals through gp130 and IL12Rβ2 in T cells (Collison and others 2012). Interestingly, while cells deficient for both receptor chains were completely resistant to the suppressive effects of IL-35, activated Il6st−/−(gp130-deficient) or Il12rb2−/− T cells were only partially resistant to IL-35 or iTr35 cell-mediated suppression. Thus, unlike IL-12 and IL-27, IL-35 was capable of signaling through a single receptor chain (Garbers and others 2012; Vignali and Kuchroo 2012). However, its capacity to upregulate Ebi3 and p35 expression and iTr35 conversion required the IL-35 heterodimeric receptor (IL-12Rβ2:gp130). IL-35 was demonstrated to signal through 3 receptor combinations in T cells: gp130 homodimers and IL-12Rβ2 homodimers, which are sufficient to mediate suppression, and gp130:IL-12Rβ2 heterodimers, which are essential for iTr35 conversion and required for maximal suppression. A unique STAT1:STAT4 heterodimer was identified as the major downstream mediator of IL-35 signaling and bound to many sites in the Il12a and Ebi3 promoters to initiate transcription. It is intriguing that the STAT1:STAT4 heterodimer predominates only after IL-35 stimulation and not after simultaneous stimulation with IFN-γ and IL-12 (activators of STAT1 and STAT4, respectively), which in contrast induces a proinflammatory Th1 response.
The recent Breg studies have proposed a new composition for the functional IL-35 receptor on B cells (Wang and others 2014). Unlike its T cell counterparts, silencing of gp130 through siRNA-mediated knockdown or using neutralizing antibodies did not affect IL-35-mediated inhibition of B-cell proliferation or IL-10 induction. In contrast, silencing of IL-12Rβ2 and IL-27Rα completely abrogated the inhibitory effects of IL-35 in B cells. Further analysis revealed that IL-35 signals through a receptor comprising IL-12Rβ2 and IL-27Rα and activates STAT1 and STAT3 in B cells (Wang and others 2014). These findings indicate that IL-35 may mediate its distinct biological activities in diverse cell types through differential receptor and STAT utilization (Fig. 2).
The composition of IL-35 receptor sheds some light on its potential cellular targets. Although gp130 is ubiquitous in its expression, IL-12Rβ2 is fairly restricted (namely activated T cells, NK cells, B cells, and dendritic cells) (Presky and others 1996; Grohmann and others 1998). IL-12Rβ2 is undetectable on most resting T cells, but gets rapidly induced in the presence of cytokines, such as IL-2, IFN-γ, IL-12, IL-27, and TNFα (Collison and others 2012). Indeed, pretreatment of activated T cells with IL-2 or IL-27 increases their sensitivity to IL-35-mediated suppression (Collison and others 2012). It is thus possible that IL-27-mediated upregulation of IL-12Rβ2 may prime T cells to the suppressive effects of IL-35, which may underlie the perceived inhibitory role of IL-27 (Vignali and Kuchroo 2012). The expression of IL-27Rα, a component of the IL-35 receptor on B cells, is also primarily restricted to immune cells (T, B, NK, mast cells, monocytes, neutrophils) (Pflanz and others 2004). Since gp130 is ubiquitous and has a broad expression pattern, gp130 homodimers may facilitate IL-35-mediated suppression of a broad array of cell types. Thus, the ability of IL-35 to signal through multiple receptor combinations expands its sphere of influence and also varies the suppressive impact and consequences it has on different cell types.
Regulatory Role of IL-35 in Inflammation and Tumors
The regulatory potential of IL-35 has been highlighted in multiple disease models. Earlier studies were mainly performed using mouse strains deficient in p35, p40, and Ebi3 (Collison and Vignali 2008). However, the mice that lacked p40 were deficient in both IL-12 and IL-23 and thus any observation made could be attributed to effects from either cytokine. This was evident in the different susceptibilities of Il12a−/−and Il12b−/− mouse strains in EAE and CIA models. With the discovery of p19, some of the inexplicable differences were attributed to the proinflammatory role of IL-23 following reevaluation of the phenotypic differences in Il12a−/−, Il12b−/−, and Il23a−/−mouse strains. The discovery of IL-35 suggested even more complexity as Il12a−/− mice lack IL-12 and IL-35, while Ebi3−/− mice lack IL-27 and IL-35. These observations warranted reassessment of the phenotypes attributed to either IL-12 or IL-27, which, in fact, could be features attributed to IL-35 (Collison and Vignali 2008).
Despite these complications, studies utilizing Il12a−/− and Ebi3−/− mouse strains provided substantial evidence confirming a role for IL-35 in ameliorating inflammation in various autoimmune and chronic inflammatory settings. Thus, IL-35 mediates suppressive effects in models of inflammatory bowel disease, EAE, collagen-induced arthritis, myocarditis, and liver fibrosis to name a few (Collison and others 2007; Niedbala and others 2007; Kochetkova and others 2010; Tirotta and others 2013). Several recent studies have complemented these genetic deletion approaches with gene therapy-based tools utilizing either plasmid DNA encoding single-chain IL-35 fusion protein, intraperitoneal injections of recombinant IL-35 or IL-35 transgenic mice. Ectopic IL-35 expression through these approaches conferred protection in mouse models of allergic airway inflammation, colitis, and diabetes (Huang and others 2011; Wirtz and others 2011; Bettini and others 2012). More recent studies have further expanded the protective effects of IL-35 overexpression to murine acute graft-versus-host disease, viral myocarditis, and atherosclerosis (Huang and others 2013; Hu and others 2014; Liu and others 2014).
Involvement of IL-35 in tumor progression and tumor immune surveillance can be traced back to earlier observations demonstrating increased Ebi3 expression in EBV-associated Hodgkin lymphoma, diffuse large B-cell lymphoma, nasopharyngeal carcinoma, and acute myeloid leukemia cells (Niedobitek and others 2002). The IL-12 p35 subunit, but not IL-27p28, was associated with Ebi3+ tumor cells, providing early implications for a role for IL-35 in the tumor microenvironment (Niedobitek and others 2002; Larousserie and others 2005). Tregs, inhibitory cytokines, and induced regulatory populations are highly prevalent in peripheral blood and the tumor microenvironment in both mouse tumor models and cancer patients (Liyanage and others 2002; Wolf and others 2005). Furthermore, recent studies have suggested a role for Treg-derived IL-35 in limiting antitumor immunity (Collison and others 2010). The IL-35-induced regulatory population, iTr35 cells, was shown to contribute significantly to the regulatory milieu in the tumor microenvironment, thereby confirming a critical role for the regulatory triad of IL-35, Tregs, and iTr35 cells in tumor progression (Collison and others 2010). In addition to Tregs and induced regulatory populations, tumor-infiltrating dendritic cells have also been shown to express Ebi3, although IL-35 production by tolerogenic dendritic cells remains to be explored (Niedobitek and others 2002; Larousserie and others 2005). Stable Ebi3 expression in lung cancer cells promoted tumor progression, while siRNA-mediated Ebi3 gene silencing inhibited cancer cell proliferation (Nishino and others 2011). In fact, high Ebi3 expression in lung cancer cells was shown to be associated with poor prognosis (Nishino and others 2011). Similarly, Ebi3-deficient mice are protected from lung metastasis upon intravenous injection of the B16-F10 tumor cell line and this is mainly associated with induction of CD11c+B220+NK1.1+Gr1− (IFN-γ-releasing) killer dendritic cells (IK-DCs) in the lungs that augment the local CD8+ T-cell antitumor response (Sauer and others 2008). Another recent report demonstrated a role for tumor-derived IL-35 in promoting tumor growth through the accumulation of suppressive myeloid cell populations (MDSCs) and increasing angiogenesis (Wang and others 2013). IL-35-producing cancer cell lines considerably increased tumorigenesis in both immune-competent and immune-deficient mice in 2 tumor models (J558 plasmacytoma and B16.F10 melanoma). Treatment with an IL-35-neutralizing antibody abrogated tumor enhancement, confirming the specificity of these effects to IL-35. IL-35+ tumors showed significant infiltration with CD11b+Gr1+ MDSCs that have been linked to promoting tumor angiogenesis through production of vascular endothelial growth factor and other proangiogenesis factors. MDSCs also mediate immune suppression by inhibiting cytotoxic T-cell responses. In line with this, IL-35+ tumors had reduced cytotoxic T lymphocyte (CTL) responses, although IL-35 did not directly affect CTL activation and effector function. Indeed, depletion of MDSCs with anti-Gr1 mAb abrogated tumor progression, confirming a critical role for MDSC accumulation in the tumorigenesis mediated by IL-35 (Wang and others 2013). IL-35 produced by tumor cells was also shown to play a role in making the tumor cells resistant to CTL-mediated destruction through upregulation of gp130 expression on tumor cells. Importantly, gp130 signaling has been associated with cancer cell resistance to chemotherapy. IL-35 overexpression in tumor cells can also induce cell cycle arrest at the G1 phase and sensitize tumor cells and other infiltrating cells to serum starvation-induced apoptosis (Long and others 2013). Thus, through varied mechanisms, IL-35 may actually help the tumor cells evade immune surveillance and establish long-term tolerance, thereby promoting tumorigenesis. Since IL-35 can be produced by tumor cells as well as the tumor-infiltrating Tregs, induced regulatory cells, and possibly other populations in the tumor microenvironment, a better understanding of the cross talk between these populations would allow for effective targeting of the IL-35-mediated regulatory milieu for therapeutic purposes.
Serum IL-35 as a Diagnostic Biomarker for Human Cancers and Chronic Inflammation
While there is considerable literature highlighting the suppressive potential of IL-35 in various mouse models, comparable validation for the human counterpart is limited. Earlier studies failed to detect IL-35 production by human Tregs, both direct ex vivo and following T cell receptor (TCR) stimulation (Bardel and others 2008). Ectopic Foxp3 expression could not induce Ebi3 and p35 expression in human T cells (Allan and others 2008). These differences could be partially explained by the observations from a data mining study suggesting that IL-35 is not constitutively expressed in human tissues unlike TGFβ, but may get induced in certain tissues and cell types in response to various inflammatory stimuli (Li and others 2012). This may indeed be true since it has been shown that human Tregs in the face of strong inflammatory stimuli (eg, following rhinovirus and hepatitis C virus infection) secrete IL-35, suggesting that IL-35 induction maybe a novel activation program in human T cells responding to viral infections (Langhans and others 2010; Seyerl and others 2010). Human CD4+ T cells exposed to IL-35 also express and mediate suppressive effects through IL-35 analogous to mouse iTr35 counterparts (Collison and others 2010). In addition, a human CD8+ regulatory T cell population expressing CTLA-4 and secreting IL-35 has been shown to regulate the antitumor immune response in prostate cancer (Olson and others 2012).
Interestingly, there seems to be an increasing number of reports documenting increased or decreased IL-35 levels in human samples from a variety of disease states (Table 1). Although warranting rigorous assessment in terms of the reagents and controls used for IL-35 detection, the overall trend suggests that increased serum IL-35 levels correlate with the severity of malignancy and the clinical stage of the tumor (Wu and others 2012; Zeng and others 2013; Jin and others 2014). In contrast to observations made with human malignancies, reduced IL-35 levels seem to be associated with human autoimmunity and chronic infections (Lin and others 2012; Mao and others 2013; Chen and others 2014; Jafarzadeh and others 2015; Li and others 2014; Ma and others 2014; Ozkan and others 2014, 2015; Wan and others 2014; Yang and others 2014).
Table 1.
Serum IL-35 as a Diagnostic Biomarker for Cancer and Autoimmune Diseases
| Disease/model | IL-35 expression | References |
|---|---|---|
| Elevated circulating IL-35 levels in human cancer | ||
| Colorectal cancer | Serum IL-35 levels are positively correlated with Treg numbers in the peripheral blood of colorectal cancer patients and reduction in serum IL-35 following tumor resection | Zeng and others (2013) |
| Pancreatic ductal adenocarcinoma | Increased plasma IL-35 levels associated with lymph node metastasis and late-stage tumor | Jin and others (2014) |
| Acute myeloid leukemia | Increased plasma IL-35 concentration has also been correlative in newly diagnosed acute myeloid leukemia patients, one of the most common hematological malignancy in adults | Wu and others (2012) |
| Reduced circulating IL-35 levels in human autoimmune diseases | ||
| Multiple sclerosis (MS) | Serum IL-35 levels were lower in MS patients and treatment with interferon β, methylprednisolone, or combination of both offered beneficial effects through the upregulation of IL-35 production | Jafarzadeh and others (2015) |
| Asthma and chronic obstructive pulmonary disease (COPD) | Expression of IL-35 mRNA and protein were both downregulated in asthmatic children, and serum IL-35 level was inversely related to serum IL-4 and positively correlated with serum IFN-γ levels | Chen and others (2014) and Ma and others (2014) |
| Allergic rhinitis | Serum IL-35 and Ebi3 expression was also significantly reduced in allergic rhinitis patients relative to normal controls | Wan and others (2014) |
| Ulcerative colitis (UC) and Crohn's disease (CD) | Serum IL-35 levels were also significantly reduced in ulcerative colitis (UC) and Crohn's disease (CD) patients suggestive of insufficient anti-inflammatory activity in vivo. | Li and others (2014) |
| Immune thrombocytopenia (ITC) | Reduced plasma IL-35 levels in active ITC patients than those in remission | Yang and others (2014) |
| Fetomaternal tolerance, recurrent pregnancy loss (RPL), and preeclampsia (PE) | Decreased plasma IL-35 levels were noted in females with RPL and PE | Ozkan and others (2014, 2015) |
| Atherosclerosis and coronary artery diseases (CAD) | Plasma IL-35 levels were significantly decreased in patients with stable and unstable angina pectoris (SAP and UAP) and acute myocardial infarction (AMI) | Lin and others (2012) |
These different correlative studies offer a consistent theme—increased IL-35 expression in tumors and malignancies and decreased IL-35 expression in autoimmune and chronic inflammatory conditions (Fig. 3). These observations can be reconciled to highlight critical information about this inhibitory cytokine. First, IL-35 levels may correlate with the degree of Treg infiltration in these different diseases. Thus, Tregs and other induced regulatory populations heavily infiltrate or are induced in the tumor microenvironment, impeding tumor immune surveillance, while autoimmunity and chronic inflammation are associated with reduced Treg numbers. Second, IL-35 expression may be associated with maximal Treg function or may mark highly suppressive Tregs. This can explain the high functionality of tumor-associated Tregs, while Tregs from autoimmune and inflammatory environments have impaired suppressor potential. Third, IL-35 levels may be highly indicative of the degree of Treg stability across a spectrum of immunological diseases. High IL-35 expression or increased IL-35+ Tregs in tumors may be an indication of highly stable Tregs impairing the antitumor immune response. On the other hand, reduced IL-35 levels or decreased proportion of IL-35+ Tregs may underlie the basis for development of autoimmunity and chronic inflammation. While these observations are encouraging, more rigorous assessments of the functional scope of human IL-35 will be required to fully evaluate its therapeutic potential through blockade in cancer or administration in autoimmune and inflammatory diseases.
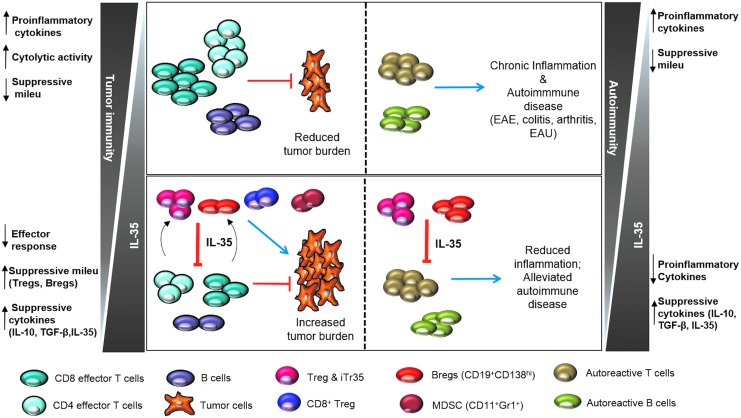
FIG. 3.
Proposed model for the role of IL-35 in health and disease. In the tumor microenvironment, replete with IL-35, the increased induction of IL-35+ populations (Tregs, iTr35, Bregs, CD8+ Tregs) impairs the antitumor immune response (CD8 effector, CD4 effector, and B cells), resulting in tumorigenesis (left panel). On the other end of the spectrum lie autoimmunity and chronic inflammation, wherein IL-35 deficiency results in activation of autoreactive cells, leading to the exacerbated inflammation associated with experimental autoimmune encephalitis (EAE), colitis, arthritis, and experimental autoimmune uveitis (EAU) (right panel).
Concluding Model for IL-35-Driven Regulatory Milieu and Infectious Tolerance
The recent studies on B-cell-derived IL-35 have expanded the regulatory scope of IL-35 to other cellular lineages apart from Tregs. Given that the Breg field focused entirely on IL-10 as the sole mechanism of suppression over past several years, this discovery that Breg cells can also suppress through IL-35 secretion marks significant progress. In addition, given recent observations that IL-35 expression is inducible in many human tissues under conditions promoting inflammation, it is reasonable to predict that new cellular sources and targets of IL-35 may be identified, expanding the range of cells and tissues that are susceptible to IL-35-dependent immune regulation.
Importantly, IL-35+ Breg cells can induce Tregs while Treg-derived IL-35 can also sustain Breg cells. Thus, in inflammatory scenarios that elicit the IL-35-dependent regulatory milieu, these different IL-35-producing populations may promote reciprocal activity through a positive feed-forward mechanism, resulting in induction of iTr35 cells and IL-35+ Bregs through infectious tolerance (Fig. 3). This may be particularly relevant in tumor settings that are heavily infiltrated with IL-35-producing Tregs, Bregs, and other suppressive populations. If this is true, approaches that boost or target IL-35 may result in recruitment or blockade, respectively, of both the B and T cell arms of the immune response, offering broader therapeutic benefits.
Perspectives, Challenges, and Future Directions
There are 7 key challenges that need to be resolved and questions that need to be addressed before a greater understanding of the physiological importance and therapeutic promise of IL-35 can be established.
(1) Progress in the IL-35 field has been relatively slow due to significant challenges related to its unique biology. Given that IL-35 and the various IL-35 receptors share components with other IL-12 family cytokines and their receptors, dissecting and determining the true contributions of IL-35 has been challenging.
(2) A major hindrance to progress in the field has been the lack of critical reagents. The lack of mutant mouse models to conditionally and temporally delete Ebi3, p35, and its receptor chains has been a major limitation. It would also be highly desirable to have reporter and Cre lines to further assess the expression and fate of IL-35, IL35R, and cells that express them. Another major limitation has been the lack of purified recombinant mouse and human IL-35 and the questionable activity and purity of the recombinant versions that are available. Last, the lack of availability of good antibodies for IL-35, and in particular the IL-35 receptor, has also been a significant limitation. The availability and quality of these key reagents will dictate the pace of future progress.
(3) It will be important to determine the physiological relevance and contribution of IL-35 in vivo from the known cellular sources, especially Tregs, iTr35 cells, and Bregs. It will also be important to determine if there are any other cellular sources of physiological significance.
(4) Given the apparent complexity of IL-35 receptor utilization, further studies are needed to clearly establish whether all these combinations are physiologically relevant and required. We also need to gain a greater understanding of the distribution of the IL-35 receptor and the cells that are impacted by IL-35.
(5) The intracellular mechanism of action of IL-35 remains largely unknown. How does IL-35 inhibit cell cycle progression? How can a receptor that induces STAT1, STAT4, and/or STAT3 in different cell types mediate suppression and IL-35 (or IL-10) induction, while the same STATs can mediate proinflammatory events when activated by different cytokine receptors? In addition, are these receptors and signaling mechanisms really different in T versus B cells? Simply put, we need to know how IL-35 works.
(6) The importance of IL-35 in humans also needs to be fully and rigorously established. We also need to determine if its mechanism of action is the same in human cells as it is in mouse cells.
(7) Several clinically relevant questions remain. Is IL-35 a viable therapeutic target in cancer? Could recombinant IL-35 be used to treat autoimmunity and inflammatory diseases? Last, there needs to be a more rigorous assessment of IL-35 expression by different tissues and cell types in disease settings to determine the utility of IL-35 as a diagnostic biomarker.
Overall, while the expanded job profile of IL-35 generates enthusiasm, it also raises significant questions and challenges that need to be resolved. With expansion in its sources and targets, new roles have been ascribed to IL-35 beyond inhibition of proliferation and infectious tolerance, namely modulating T-cell differentiation programs, expansion of Tregs, and regulating APC function of B cells, thus far. Nevertheless, IL-35 remains an inhibitory cytokine and does not seem to have the opposing and pleiotropic activities of many other cytokines. More in-depth studies are warranted using recombinant IL-35 and specialized mouse models proposed above in appropriate cellular lineages to fully appreciate the physiological impact of IL-35. Understanding the cross talk between the various IL-35+ cell populations (Tregs, Bregs, CD8+ Tregs) in diverse disease settings and the overall regulatory network established by this unique suppressive cytokine will greatly aid in effective manipulation of IL-35 for translational benefit.
Acknowledgments
The authors thank members of the Vignali laboratory for critical reading of the manuscript. This work was supported by the National Institutes of Health (AI091977 and AI039480 to D.A.A.V) and the NCI Comprehensive Cancer Center Support CORE grant (CA047904 to D.A.A.V.). D.A.A.V. declares competing financial interests with patents covering IL-35, with others pending, and is entitled to a share in net income generated from licensing of these patent rights for commercial development.
Author Disclosure Statement
No competing financial interests exist.
References
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278(3):1910–1914 [DOI] [PubMed] [Google Scholar]
- Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. 2008. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol 38(12):3282–3289 [DOI] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. 2007. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8(12):1380–1389 [DOI] [PubMed] [Google Scholar]
- Banchereau J, Pascual V, O'Garra A. 2012. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 13(10):925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L'Hermine A, Devergne O. 2008. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol 181(10):6898–6905 [DOI] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O'Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209(5):1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. 2008. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol 180(5):2752–2756 [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Chen W. 2010. Regulatory ripples. Nat Immunol 11(12):1077–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettini M, Castellaw AH, Lennon GP, Burton AR, Vignali DA. 2012. Prevention of autoimmune diabetes by ectopic pancreatic beta-cell expression of interleukin-35. Diabetes 61(6):1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. 2010. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32(1):129–140 [DOI] [PubMed] [Google Scholar]
- Capocasale RJ, Lamb RJ, Vonderheid EC, Fox FE, Rook AH, Nowell PC, Moore JS. 1995. Reduced surface expression of transforming growth factor beta receptor type II in mitogen-activated T cells from Sezary patients. Proc Natl Acad Sci U S A 92(12):5501–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Deng Y, Chen H, Wu X, Cheng S, Xu Y, Xiong W, Xie J. 2014. Decreased concentration of IL-35 in plasma of patients with asthma and COPD. Asian Pac J Allergy Immunol 32(3):211–217 [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198(12):1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. 2010. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11(12):1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG, Murray PJ, Vignali DA. 2012. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol 13(3):290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Pillai MR, Chaturvedi V, Vignali DA. 2009. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol 182(10):6121–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Vignali DA. 2008. Interleukin-35: odd one out or part of the family? Immunol Rev 226:248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450(7169):566–569 [DOI] [PubMed] [Google Scholar]
- Devergne O, Birkenbach M, Kieff E. 1997. Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc Natl Acad Sci U S A 94(22):12041–12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Coulomb-L'Hermine A, Capel F, Moussa M, Capron F. 2001. Expression of Epstein-Barr virus-induced gene 3, an interleukin-12 p40-related molecule, throughout human pregnancy: involvement of syncytiotrophoblasts and extravillous trophoblasts. Am J Pathol 159(5):1763–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devergne O, Hummel M, Koeppen H, Le Beau MM, Nathanson EC, Kieff E, Birkenbach M. 1996. A novel interleukin-12 p40-related protein induced by latent Epstein-Barr virus infection in B lymphocytes. J Virol 70(2):1143–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 2007. Historical insights into cytokines. Eur J Immunol 37 (Suppl 1):S34–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. 2011. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 121(9):3645–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. 2009. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 182(9):5748–5756 [DOI] [PubMed] [Google Scholar]
- Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. 2007. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol 178(12):7868–7878 [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. 2002. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3(10):944–950 [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. 2007. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 8(12):1372–1379 [DOI] [PubMed] [Google Scholar]
- Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. 2013. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med 5(173):173ra23. [DOI] [PubMed] [Google Scholar]
- Garbers C, Hermanns HM, Schaper F, Muller-Newen G, Grotzinger J, Rose-John S, Scheller J. 2012. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev 23(3):85–97 [DOI] [PubMed] [Google Scholar]
- Gershon RK, Kondo K. 1971. Infectious immunological tolerance. Immunology 21(6):903–914 [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361(21):2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. 2011. IL-10 and IL-10 receptor defects in humans. Ann N Y Acad Sci 1246:102–107 [DOI] [PubMed] [Google Scholar]
- Gravano DM, Vignali DA. 2012. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci 69(12):1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. 1998. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity 9(3):315–323 [DOI] [PubMed] [Google Scholar]
- Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. 2007. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 27(5):786–800 [DOI] [PubMed] [Google Scholar]
- Hu Y, Dong C, Yue Y, Xiong S. 2014. In vivo delivery of interleukin-35 relieves coxsackievirus-B3-induced viral myocarditis by inhibiting Th17 cells. Arch Virol 159(9):2411–2419 [DOI] [PubMed] [Google Scholar]
- Huang CH, Loo EX, Kuo IC, Soh GH, Goh DL, Lee BW, Chua KY. 2011. Airway inflammation and IgE production induced by dust mite allergen-specific memory/effector Th2 cell line can be effectively attenuated by IL-35. J Immunol 187(1):462–471 [DOI] [PubMed] [Google Scholar]
- Huang Y, Lin YZ, Shi Y, Ji QW. 2013. IL-35: a potential target for the treatment of atherosclerosis. Pharmazie 68(10):793–795 [PubMed] [Google Scholar]
- Hunter CA. 2005. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 5(7):521–531 [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A, Jamali M, Mahdavi R, Ebrahimi HA, Hajghani H, Khosravimashizi A, Nemati M, Najafipour H, Sheikhi A, Mohammadi MM, Daneshvar H. 2015. Circulating levels of interleukin-35 in patients with multiple sclerosis: evaluation of the influences of FOXP3 gene polymorphism and treatment program. J Mol Neurosci 55(4):891–897 [DOI] [PubMed] [Google Scholar]
- Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J. 2014. Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol 75(1):29–33 [DOI] [PubMed] [Google Scholar]
- Jones LL, Vignali DA. 2011. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res 51(1):5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. 2012. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalampokis I, Yoshizaki A, Tedder TF. 2013. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 15 (Suppl. 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoechel B, Lohr J, Zhu S, Wong L, Hu D, Ausubel L, Abbas AK. 2006. Functional and molecular comparison of anergic and regulatory T lymphocytes. J Immunol 176(11):6473–6483 [DOI] [PubMed] [Google Scholar]
- Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. 2010. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol 184(12):7144–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, Vidovic N, Hoerauf A, Oldenburg J, Sauerbruch T, Spengler U. 2010. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond) 119(2):97–109 [DOI] [PubMed] [Google Scholar]
- Larousserie F, Bardel E, Pflanz S, Arnulf B, Lome-Maldonado C, Hermine O, Bregeaud L, Perennec M, Brousse N, Kastelein R, Devergne O. 2005. Analysis of interleukin-27 (EBI3/p28) expression in Epstein-Barr virus- and human T-cell leukemia virus type 1-associated lymphomas: heterogeneous expression of EBI3 subunit by tumoral cells. Am J Pathol 166(4):1217–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Flavell RA. 2008. TGF-beta: a master of all T cell trades. Cell 134(3):392–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. 2012. IL-35 is a novel responsive anti-inflammatory cytokine—a new system of categorizing anti-inflammatory cytokines. PLoS One 7(3):e33628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Y, Liu Y, Wang Y, Zuo X, Li Y, Lu X. 2014. The possible role of the novel cytokines il-35 and il-37 in inflammatory bowel disease. Mediators Inflamm 2014:136329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Huang Y, Lu Z, Luo C, shi Y, Zeng Q, Cao Y, Liu L, Wang X, Ji Q. 2012. Decreased plasma IL-35 levels are related to the left ventricular ejection fraction in coronary artery diseases. PLoS One 7(12):e52490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW, Jr, Yu C, Shi FD, Whitacre CC, Trgovcich J, Bai XF. 2012. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J Immunol 188(7):3099–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu Y, Wang Y, Cai Y, Hu B, Bao G, Fang H, Zhao L, Ma S, Cheng Q, Song Y, Liu Y, Zhu Z, Chang H, Yu X, Sun A, Zhang Y, Vignali DA, Wu D, Liu H. 2014. IL-35 mitigates murine acute graft-versus-host disease with retention of graft-versus-leukemia effects. Leukemia [Epub ahead of print]; DOI: 10.1038/leu.2014.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. 2002. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169(5):2756–2761 [DOI] [PubMed] [Google Scholar]
- Long J, Zhang X, Wen M, Kong Q, Lv Z, An Y, Wei XQ. 2013. IL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cells. Biochem Biophys Res Commun 430(1):364–369 [DOI] [PubMed] [Google Scholar]
- Ma X, Trinchieri G. 2001. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol 79:55–92 [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu X, Wei Z, Wang X, Xu D, Dai S, Li Y, Gao M, Ji C, Guo C, Zhang L, Wang X. 2014. The expression of a novel anti-inflammatory cytokine IL-35 and its possible significance in childhood asthma. Immunol Lett 162(1 Pt A):11–17 [DOI] [PubMed] [Google Scholar]
- Mahmud SA, Manlove LS, Farrar MA. 2013. Interleukin-2 and STAT5 in regulatory T cell development and function. JAKSTAT 2(1):e23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Gao W, Ma C, Sun J, Liu J, Shao Q, Song B, Qu X. 2013. Human placental trophoblasts express the immunosuppressive cytokine IL-35. Hum Immunol 74(7):872–877 [DOI] [PubMed] [Google Scholar]
- Mauri C, Bosma A. 2012. Immune regulatory function of B cells. Annu Rev Immunol 30:221–241 [DOI] [PubMed] [Google Scholar]
- Mauri C, Ehrenstein MR. 2008. The 'short' history of regulatory B cells. Trends Immunol 29(1):34–40 [DOI] [PubMed] [Google Scholar]
- Mauri C, Nistala K. 2014. Interleukin-35 takes the 'B' line. Nat Med 20(6):580–581 [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765 [DOI] [PubMed] [Google Scholar]
- Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. 2010. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 33(5):777–790 [DOI] [PubMed] [Google Scholar]
- Niedbala W, Wei XQ, Cai B, Hueber AJ, Leung BP, McInnes IB, Liew FY. 2007. IL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cells. Eur J Immunol 37(11):3021–3029 [DOI] [PubMed] [Google Scholar]
- Niedobitek G, Pazolt D, Teichmann M, Devergne O. 2002. Frequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cells. J Pathol 198(3):310–316 [DOI] [PubMed] [Google Scholar]
- Nishino R, Takano A, Oshita H, Ishikawa N, Akiyama H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y. 2011. Identification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancer. Clin Cancer Res 17(19):6272–6286 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Paul WE. 2002. Regulation of T(H)1 differentiation—controlling the controllers. Nat Immunol 3(6):506–508 [DOI] [PubMed] [Google Scholar]
- Olson BM, Jankowska-Gan E, Becker JT, Vignali DA, Burlingham WJ, McNeel DG. 2012. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol 189(12):5590–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13(5):715–725 [DOI] [PubMed] [Google Scholar]
- Ozkan ZS, Deveci D, Simsek M, Ilhan F, Risvanli A, Sapmaz E. 2015. What is the impact of SOCS3, IL-35 and IL17 in immune pathogenesis of recurrent pregnancy loss? J Matern Fetal Neonatal Med 28(3):324–328 [DOI] [PubMed] [Google Scholar]
- Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. 2014. Plasma IL-17, IL-35, interferon-gamma, SOCS3 and TGF-beta levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med 27(15):1513–1517 [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. 2004. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 172(4):2225–2231 [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. 2002. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16(6):779–790 [DOI] [PubMed] [Google Scholar]
- Pillai MR, Collison LW, Wang X, Finkelstein D, Rehg JE, Boyd K, Szymczak-Workman AL, Doggett T, Griffith TS, Ferguson TA, Vignali DA. 2011. The plasticity of regulatory T cell function. J Immunol 187(10):4987–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. 2009. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol 183(2):797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. 1996. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci U S A 93(24):14002–14007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. 1993. “Infectious” transplantation tolerance. Science 259(5097):974–977 [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. 2001. Type 1 T regulatory cells. Immunol Rev 182:68–79 [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. 2006. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev 212:28–50 [DOI] [PubMed] [Google Scholar]
- Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J. 2010. Biology of interleukin-10. Cytokine Growth Factor Rev 21(5):331–344 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. 2010. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10(7):490–500 [DOI] [PubMed] [Google Scholar]
- Saraiva M, O'Garra A. 2010. The regulation of IL-10 production by immune cells. Nat Rev Immunol 10(3):170–181 [DOI] [PubMed] [Google Scholar]
- Sauer KA, Maxeiner JH, Karwot R, Scholtes P, Lehr HA, Birkenbach M, Blumberg RS, Finotto S. 2008. Immunosurveillance of lung melanoma metastasis in EBI-3-deficient mice mediated by CD8+ T cells. J Immunol 181(9):6148–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyerl M, Kirchberger S, Majdic O, Seipelt J, Jindra C, Schrauf C, Stockl J. 2010. Human rhinoviruses induce IL-35-producing Treg via induction of B7-H1 (CD274) and sialoadhesin (CD169) on DC. Eur J Immunol 40(2):321–329 [DOI] [PubMed] [Google Scholar]
- Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S. 2014. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507(7492):366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. . 1992. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359(6397):693–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. 2007. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 8(12):1363–1371 [DOI] [PubMed] [Google Scholar]
- Su Y, Zhang AH, Noben-Trauth N, Scott DW. 2011. B-cell gene therapy for tolerance induction: host but not donor B-cell derived IL-10 is necessary for tolerance. Front Microbiol 2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204(8):1775–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder TF, Leonard WJ. 2014. Autoimmunity: regulatory B cells—IL-35 and IL-21 regulate the regulators. Nat Rev Rheumatol 10(8):452–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirotta E, Duncker P, Oak J, Klaus S, Tsukamoto MR, Gov L, Lane TE. 2013. Epstein-Barr virus-induced gene 3 negatively regulates neuroinflammation and T cell activation following coronavirus-induced encephalomyelitis. J Neuroimmunol 254(1–2):110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ. 2012. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol 4(1):29–37 [DOI] [PubMed] [Google Scholar]
- Vignali DA, Kuchroo VK. 2012. IL-12 family cytokines: immunological playmakers. Nat Immunol 13(8):722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. 2006. IL-27 limits IL-2 production during Th1 differentiation. J Immunol 176(1):237–247 [DOI] [PubMed] [Google Scholar]
- Wan J, Luo Y, Yang C, Liu J, Du Y, Wang K. 2014. [Expression of IL-35, Epstein-Barr virus-induced gene 3 mRNA and IL-12A mRNA in peripheral blood of patients with allergic rhinitis]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 28(13):952–954 [PubMed] [Google Scholar]
- Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, Wingfield PT, Kim SH, Egwuagu CE. 2014. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 20(6):633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. 2013. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 190(5):2415–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. 2010. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol 40(6):1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Billmeier U, McHedlidze T, Blumberg RS, Neurath MF. 2011. Interleukin-35 mediates mucosal immune responses that protect against T-cell-dependent colitis. Gastroenterology 141(5):1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. 2005. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 11(23):8326–8331 [DOI] [PubMed] [Google Scholar]
- Wu H, Li P, Shao N, Ma J, Ji M, Sun X, Ma D, Ji C. 2012. Aberrant expression of Treg-associated cytokine IL-35 along with IL-10 and TGF-beta in acute myeloid leukemia. Oncol Lett 3(5):1119–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitani A, Strober W. 2010. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol 3(3):230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Stephan S, Bluestone JA. 2013. Peripherally induced tregs - role in immune homeostasis and autoimmunity. Front Immunol 4:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28(5):639–650 [DOI] [PubMed] [Google Scholar]
- Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J, Xiong H. 2008. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol 38(5):1204–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xuan M, Zhang X, Zhang D, Fu R, Zhou F, Ma L, Li H, Xue F, Zhang L, Yang R. 2014. Decreased IL-35 levels in patients with immune thrombocytopenia. Hum Immunol 75(8):909–913 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yoshimoto T. 2013. Cytokine Frontiers: Regulation of Immune Responses in Health and Disease. Tokyo, Japan: Springer Japan [Google Scholar]
- Zeng JC, Zhang Z, Li TY, Liang YF, Wang HM, Bao JJ, Zhang JA, Wang WD, Xiang WY, Kong B, Wang ZY, Wu BH, Chen XD, He L, Zhang S, Wang CY, Xu JF. 2013. Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol 6(9):1806–1816 [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. 2007. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445(7130):936–940 [DOI] [PubMed] [Google Scholar]