Abstract
Background: The increasing incidence of thyroid cancer has resulted in the rate tripling over the past 30 years. Reasons for this increase have not been established. Geostatistics and geographic information system (GIS) tools have emerged as powerful geospatial technologies to identify disease clusters, map patterns and trends, and assess the impact of ecological and socioeconomic factors (SES) on the spatial distribution of diseases. In this study, these tools were used to analyze thyroid cancer incidence in a rural population.
Methods: Thyroid cancer incidence and socio-demographic factors in Vermont (VT), United States, between 1994 and 2007 were analyzed by logistic regression and geospatial and temporal analyses.
Results: The thyroid cancer age-adjusted incidence in Vermont (8.0 per 100,000) was comparable to the national level (8.4 per 100,000), as were the ratio of the incidence of females to males (3.1:1) and the mortality rate (0.5 per 100,000). However, the estimated annual percentage change was higher (8.3 VT; 5.7 U.S.). Incidence among females peaked at 30–59 years of age, reflecting a significant rise from 1994 to 2007, while incidence trends for males did not vary significantly by age. For both females and males, the distribution of tumors by size did not vary over time; ≤1.0 cm, 1.1–2.0 cm, and >2.0 cm represented 38%, 22%, and 40%, respectively. In females, papillary thyroid cancer (PTC) accounted for 89% of cases, follicular (FTC) 8%, medullary (MTC) 2%, and anaplastic (ATC) 0.6%, while in males PTC accounted for 77% of cases, FTC 15%, MTC 1%, and ATC 3%. Geospatial analysis revealed locations and spatial patterns that, when combined with multivariate incidence analyses, indicated that factors other than increased surveillance and access to healthcare (physician density or insurance) contributed to the increased thyroid cancer incidence. Nine thyroid cancer incidence hot spots, areas with very high normalized incidence, were identified based on zip code data. Those locations did not correlate with urban areas or healthcare centers.
Conclusions: These data provide evidence of increased thyroid cancer incidence in a rural population likely due to environmental drivers and SES. Geospatial modeling can provide an important framework for evaluation of additional associative risk factors.
Introduction
Thyroid cancer incidence is increasing at an annual rate of 3–5%, resulting in the rate tripling over the past 30 years in the United States as well as in other countries (1–5). In the United States, the number of cases has risen from 4.3 cases per 100,000 in 1980 to 12.9 cases per 100,000 individuals in 2008. Mortality rates have slightly increased (+0.8% annual percent change [APC]) (6–8). A recent study noted a disproportional increase in women (9). The basis for the increase in thyroid cancer incidence is not known. Some studies suggest enhanced diagnostic scrutiny and better detection of subclinical cancers result in widespread overdiagnosis and thus not a true increase in incidence (4,8,10–15). Other studies note that an increase in both large tumors and microcarcinomas as well as a change in relative frequencies of histological types implicate other contributing factors (1,16–19). Of note, recent reports of aggressive, metastatic microcarcinomas of the thyroid that correlate with the risk of second cancers (20) suggest that microcarcinomas once considered subclinical might emerge as important new healthcare concerns and reflect an important dimension of the increase in thyroid cancer incidence.
Environmental and demographic factors may be critical determinants in the increase in thyroid cancer incidence (3,21–23). A recognized risk factor for thyroid cancer is ionizing radiation exposure through medical procedures, including x-rays, as well as radioactive fallout (24–26). A study of the overall geographic distribution of thyroid cancer in the United States revealed a higher incidence in areas proximate to nuclear power reactors (27). High levels of nitrate in public drinking water supplies have been linked to increased thyroid cancer incidence (28), and environmental endocrine disruptors including polyhalogenated aromatic hydrocarbons (PHAHs), notably polybrominated diphenyl ethers (PBDEs) and organochlorine insecticides, are postulated factors (21,29–31). Leux and Guenel (21) noted that many environmental chemicals interfere with thyroid function and increase the risk of goiters, nodules, and possibly neoplasia. Additional known risk factors include family history, sex, and age (3). Socioeconomic factors (SES) may also indicate that access to healthcare affects incidence (4,32). Thus, novel analyses are needed to elucidate both incidence and contributing factors.
With the capability to visualize, analyze, interpret, and map geo-located data, the field of geostatistics, notably the geographic information system (GIS) tool, has emerged as a powerful geospatial technology that is gaining prominence in healthcare applications (33). GIS-based cancer mortality maps produced by the National Cancer Institute and Centers for Disease Control and Prevention (CDC) are widely used by public health officials to guide disease surveillance and control activities throughout the United States (34). Beyond traditional GIS mapping capabilities, more sophisticated spatial statistical analyses have been utilized to identify spatial disease clusters (i.e., nonrandom spatial distributions of disease cases, incidence, or prevalence), map and monitor disease patterns and trends over time and space, and assess the impact of ecological and SES on the spatial distribution of diseases. Although there are still many technical (e.g., knowledgeable users, data quality control) and organizational (e.g., access and sharing) barriers to the wide-scale adoption of geospatial technologies in the healthcare sector (35), recent advances in the understanding of disease dynamics, healthcare management has demonstrated the power of geospatial technologies to identify new drivers of public health concerns and advance the field of public health research. The present objective was to examine the characteristics of thyroid cancer incidence and determine the geospatial distribution in the state of Vermont, United States.
This study postulated that geospatial analyses would reveal important risk factors of thyroid cancer incidence in a rural population that would provide the framework for investigation of potential drivers of disease patterns. It was determined that the characteristics of thyroid cancer incidence, including significant nonrandom clusters, are most likely due to environmental and lifestyle factors. Spatial statistical analyses revealed that the overall distribution of thyroid cancer incidence and higher APC in these rural regions provide the framework for evaluating demographic and environmental drivers that may contribute to thyroid cancer incidence.
Methods
Data sources
Data on thyroid cancer (1994–2007) were obtained from the Vermont Department of Health, and U.S. data on thyroid cancer were obtained from the National Cancer Institute at the United States National Institutes of Health Surveillance, Epidemiology, and End Results (SEER) Program. State mandated data collection began in 1994 and included year of initial diagnosis, age at diagnosis, sex, primary site of disease at diagnosis, histology code, histological grade, behavior code, size of tumor, postal code at diagnosis, year last contacted, vital status, and death place code. Data exchange agreements between neighboring states minimize underreporting in border counties. Data pertaining to residents of neighboring states were not included in this study. Thyroid cancers were grouped based on histology codes, including papillary (8050, 8052, 8130, 8260, 8340–8344, 8450, 8452), follicular (8290, 8330–8332, 8335), medullary (8345, 8346), anaplastic (8021), and other/indeterminate/not specified (8012, 8032, 8046, 8070, 8140, 8190, 8335, 8337, 8347, 8350) (36).
Population data, used to calculate incidence, were obtained from the Vermont Department of Health's intercensal population estimates (37). The Vermont population in 1994, 2000, and 2007 was 585,544, 608,827, and 623,481, respectively. Incidence and mortality rates were age adjusted to the U.S. 2000 Standard Population (as per SEER practice (38)) and normalized per 100,000 person-years (39). For the geospatial analyses, zip code boundaries were downloaded from the U.S. Census Bureau, and all map layers projected to the Vermont State Plane Coordinate System North American Datum 1983. Information regarding SES was obtained from the 2000 U.S. Census variables, which included percent of the population by age, length of household occupancy, median household income, and post–high school education. The percent of the population with health insurance was obtained from Vermont Household Health Insurance Survey, Department of Financial Regulation, State of Vermont (40).
The study was approved by the Institutional Review Board of the University of Vermont Committee on Human Research and the Vermont Cancer Center.
Statistical analyses
Age-adjusted incidence (also known as age-standardized rate) was calculated as described by Boyle and Parkin (41). This method adjusts each age group's contribution to the overall population incidence so that incidence is based on the same age structure. Proportional age-adjusted incidence was also calculated that quantified the contribution of various age strata (e.g., 30–39 year olds) to the age-adjusted incidence. The proportional age-adjusted incidence for each age group of interest was calculated by summing the product of the crude incidence and the respective frequency of the standard population for each single year of age within the age group of interest (e.g., for age group 30–39, sum product for ages 30, 31,…,39). The standard errors of the overall age-adjusted incidence and proportional age-adjusted incidence were calculated using the Poisson approximation method (41).
The estimated APC is a summary statistic used to measure trends over time by taking the average rate of change in incidence over several years (39). The values were calculated by fitting a regression line to the natural logarithm of the incidence using the calendar year as the independent variable (42). The estimated APC is equal to 100×(eslope − 1). The statistical significance (p<0.05) of the linear slope was compared to zero, and confidence intervals (CI) were calculated from the standard error of the slope. The time period was split into 1994–2000 and 2001–2007 in order to compare trends from the first half of the study period to the second half of the study period, and the estimated APC was calculated for incidence for time periods 1994–2007, 1994–2000, and 2001–2007 for males, females, and both sexes combined, respectively.
The age-adjusted incidence for each county was compared to the overall age-adjusted incidence of Vermont by creating a standardized rate ratio (SRR) (41). To determine whether national incidence was significantly different from the incidence in Vermont, the confidence interval of each SRR was approximated as described by Smith (43). There was a significant difference between incidences if the confidence interval did not include SRR 1.0, indicating equal incidence. All statistical analysis, including estimation of the APC and age-adjusted incidence, were performed using Excel 2013 (Microsoft Corp., Redmond, WA), JMP® Pro v10.0.0 (SAS Institute, Cary, NC), ArcGIS® v10.2 (esri®, Redlands, CA), and MATLAB® 2014a (MathWorks, Natick, MA). All incidence data were age adjusted to the U.S. 2000 Standard Population baseline.
Trend analyses
Significant (p<0.05) annual trends in the age-adjusted incidence for Vermont females, males, and the total population of Vermont were performed using the Ljung-Box Q analysis in JMP® Pro. The same analysis was used to test for significant annual trends for sex-specific proportional age-adjusted incidence for three age groups (<30 years old, 30–59 years old, and >59 years old). In addition, the study tested for significant proportional annual trends in thyroid cancer tumors ≤1.0 cm, 1.1–2.0 cm, and >2.0 cm in size.
Socioeconomic analyses
Socioeconomic data from the 2000 U.S. census was analyzed at both the zip code and county scale. As a result, the study used both logistic and linear regression analysis to test for significance between the annual age-adjusted incidence of thyroid cancer and with socioeconomic variables related to income, education, length of residency, and access to healthcare at both the zip code and county scales.
Geospatial analyses
ArcGIS® v10.2 software was used to perform geospatial analyses and map visualization. The number of thyroid cancer cases in each zip code was mapped to show their spatial locations in Vermont. The cases were normalized per 100,000 to the population for each zip code based on the Vermont Department of Health's intercensal population estimates. Due to the nature of zip code data and inconsistencies between the 2010 census zip code boundaries and zip code census data, some zip codes were combined. For two zip codes with recorded thyroid cancer cases and no zip code associated with those zip codes, the cases were added to the zip code that shared the greatest area of the zip code. Calculated normalized incidence was mapped to illustrate the effect of population on incidence distribution. The cases and incidence distributions for each image were classified based on Jenks Natural Breaks. This method of classification partitions data into the specified number of classes based on natural groups or clusters of data values.
Spatial statistics use inferential statistics to test a null hypothesis that the features are randomly distributed in space. In this case, the feature tested is the average annual age-adjusted incidence of thyroid cancer for each zip code. A p-value and z-score are computed to determine the statistical significance of observed spatial patterns. A p-value calculates the probability that the observed patterns were due to random chance; statistically significant clustering is evident at a p-value of<0.05. The z-score is the standard deviation of the result, which is calculated using the logistic regression model. Very high (>1.96) and very low (<–1.96) z-scores correspond to low p-values (0.05) and indicate the spatial distribution of age-adjusted incidence is not random.
The Getis-Ord Gi* statistic was calculated for each age-adjusted incidence in a weighted set of zip codes using the Hot Spot Analyses tool. Although a particular zip code may have high incidence, Hot Spot Analysis identifies those zip codes with statistically higher incidence of cancer cases, that is, those zip codes that have significantly higher values than can be expected by chance. The Gi* local statistic identifies individual members (zip codes) of local clusters by looking at each target zip code compared to neighboring zip codes within a specified “Zone of Indifference.” This distance metric calculation enables each age-adjusted incidence within the critical distance to be equally weighted and the age-adjusted incidence of each zip code outside the specified distance with diminishing weights as distance increases. A significant Hot Spot (p<0.05) is identified if the sum of a zip code's value and the values of all its neighboring zip codes is proportionally higher than expected when compared to the sum of all zip codes in the state. Likewise, a zip code is a significant Cold Spot (p<0.05) if the sum of its value and the values of its neighboring zip codes is proportionally lower than expected.
The Hot Spot Analysis tool requires the input of a specified distance, which determines the scale of the analysis. This value was calculated using the “Calculate Distance Band from Neighbor Count” geoprocessing tool to determine the distance between every zip code and, in this work, its eight nearest neighbors, and returns the minimum, maximum, and average distance. The minimum value is the distance (in meters) one would travel away from a zip code to ensure that at least one zip code has eight neighbors, the maximum value is the distance one would travel away from a zip code to ensure that each zip code has at least eight neighbors, and the average value is the average distance between each zip code and its eight nearest neighbors. Maximum and average distances were chosen to test for clustering at multiple scales across the state (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/thy).
Results
Incidence trends
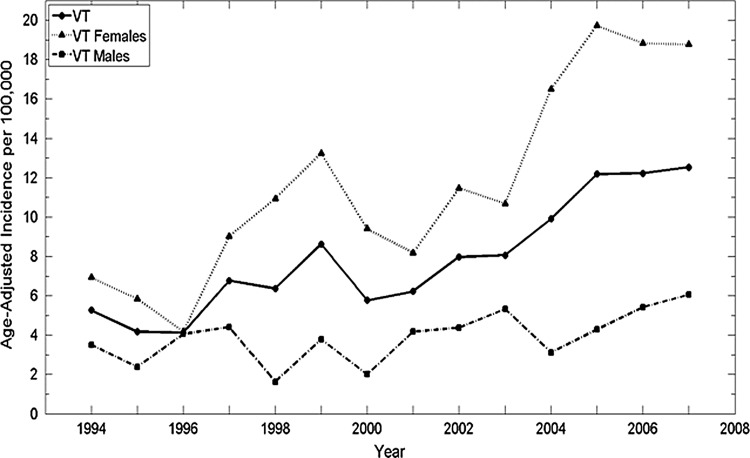
The age-adjusted thyroid cancer incidence in Vermont rose significantly 2.4-fold from 5.3 in 1994 to 12.6 in 2007 with a significant estimated APC of 8.3% [CI 5.7–11.0] compared to the national estimated APC of 5.7% [CI 5.2–6.3] (Table 1 and Supplementary Fig. S2). Although the overall average annual aged-adjusted incidence for females in Vermont was similar to that in the United States (11.8 and 12.3, respectively), the estimated APC was higher at 9.9 for Vermont and 5.9 for the United States. For males, both the average annual age-adjusted incidence and the estimated APC were similar to national trends, with both significantly increased over time (Table 1 and Supplementary Fig. S1). The thyroid cancer age-adjusted incidence in Vermont (8.0 per 100,000) was comparable to the national incidence (8.4 per 100,000). Also, the overall mortality rate was 0.5 per 100,000 for males and females, which is similar to the national rate (6).
Table 1.
Age-Adjusted Incidence of Thyroid Cancer per 100,000 People for the United States (U.S.) and Vermont (VT), 1994–2007
| Age-adjusted incidence 1994–2007 | Annual percent change | Confidence interval | t-Test | |
|---|---|---|---|---|
| VT | 8.0 | 8.3 | [5.7–11.0] | p<0.001 |
| U.S. | 8.4 | 5.7 | [5.2–6.1] | p<0.001 |
| VT females | 11.8 | 9.9 | [5.9–14.0] | p<0.001 |
| U.S. females | 12.3 | 5.9 | [5.4–6.3] | p<0.001 |
| VT males | 4.1 | 4.9 | [0.2–9.9] | p<0.05 |
| U.S. males | 4.4 | 5.1 | [4.4–5.7] | p<0.001 |
Annual percent changes were significant at p<0.001 (df=12) or p<0.05 (df=12) as indicated.
Trends by sex and age
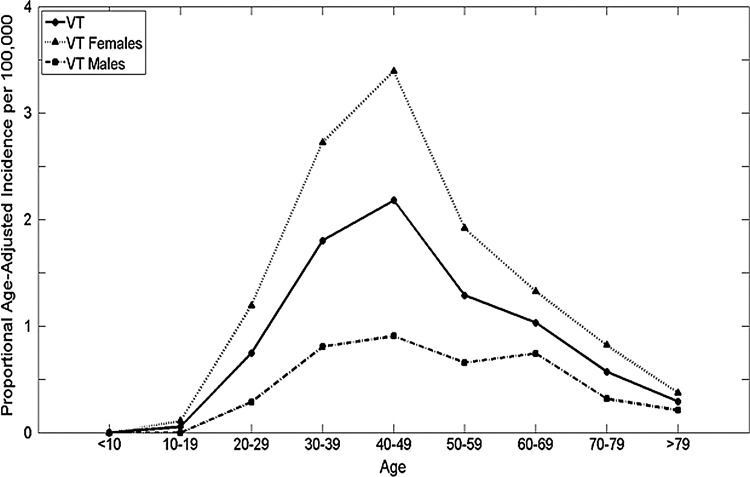
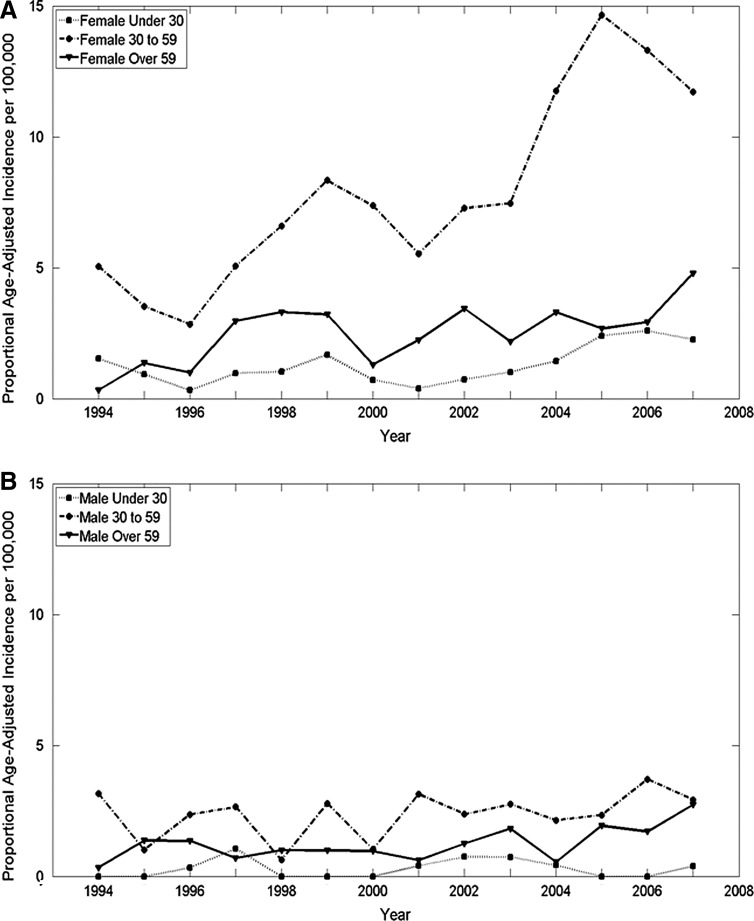
Using the Ljung-Box Q analysis, increasing trends for annual age-adjusted thyroid cancer incidence in Vermont were significant between 1994 and 2000 and 2002 and 2007 for the total population, and between 1994 and 1999 and 2002 and 2007 for females, reflecting changes within the overall increase (Fig. 1). While the overall ratio of age-adjusted incidence for females to males is 3.1 to 1, the rate of change differed during the time frame. The estimated APC among females was a little more than double that of males: 9.9 versus 4.9, respectively. The estimated APC for both females and males was higher for more recent years (2001–2007) at 13.2% for females [CI 7.3–19.1] and 11% for males [CI 0.7–21.2]. The proportional age-adjusted incidence was higher among females than males for all ages except those younger than 10 years of age (Fig. 2). From 1994–2000, the peak age of diagnosis was between 30 and 49 years for females and between 40 and 49 years for males. However, from 2001 to 2007, the peak age of diagnosis was between 40 and 49 years for females and between 30 and 69 years for males. Overall, 29.8% of the cases were diagnosed below the age of 40 years, and 57.7% of the cases below the age of 50 years. The overall increase in incidence for females was in the 30–59 year age group for females, while no overall change in incidence by age was noted for males (Fig. 3). There is no significant difference in the statewide distribution of the population by age or sex.
FIG. 1.
Annual age-adjusted thyroid cancer incidence significantly increased in Vermont, 1994–2007. Significant annual trends are noted for Vermont (1994–2000, 2002–2007) and Vermont females (1994–1999, 2002–2007). Significance is p<0.05, n=14, using Ljung-Box Q analysis in JMP® Pro v10.0.0.
FIG. 2.
Average annual proportional age-adjusted incidence (1994–2007) for Vermont overall, Vermont females, and Vermont males. For Vermont females, the age groups with the three highest annual average age-adjusted incidence are ages 30–39 years, 40–49 years, and 50–59 years.
FIG. 3.
Proportional age-adjusted incidence of thyroid cancer differed by age and sex in Vermont, 1994–2007. Significant trends were identified for females (A) younger than 30 years of age (1994–1996), females aged 30–59 years old (1994–2007), females older than 59 years old (2006–2007), and males (B) younger than 30 years of age (1997–2007) by Ljung-Box Q analysis in JMP® Pro v10.0.0 (p<0.05, n=14).
Incidence by tumor size and type
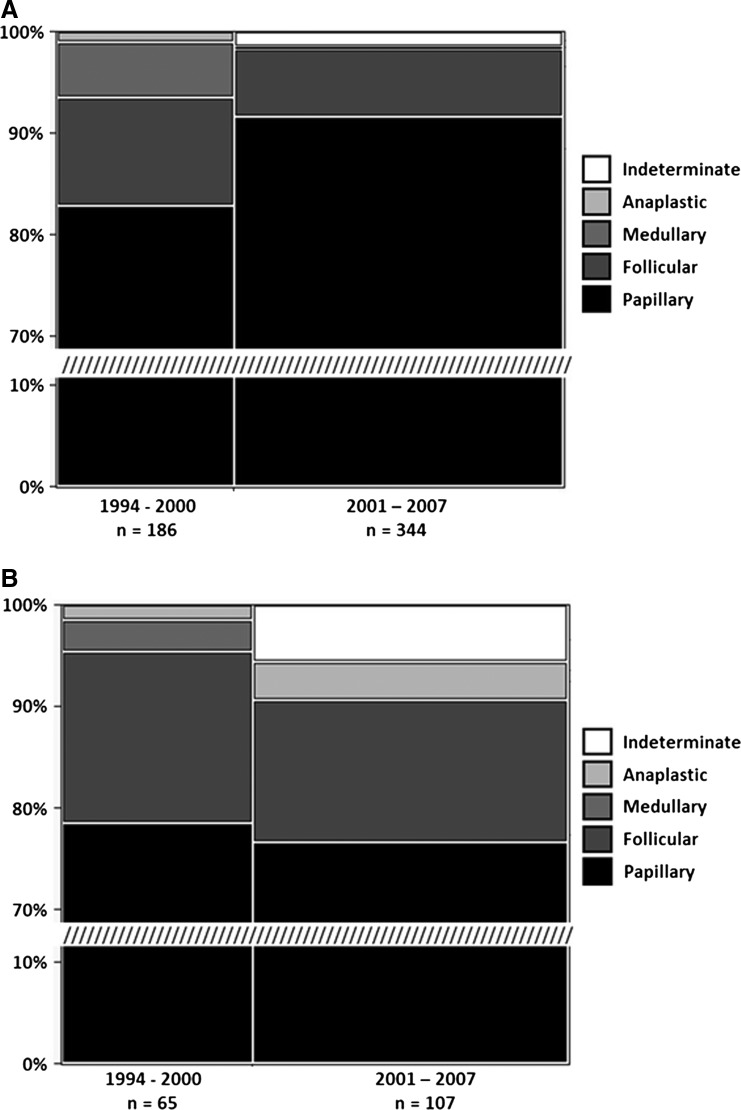
In Vermont, during 1994–2007, 86% of thyroid cancer cases were papillary, 9% follicular, 2% medullary, and <2% anaplastic comparable to national data. Of particular note, the findings reveal that sex is a factor in the distribution of cases by histological type (Fig. 4). In females, papillary thyroid cancer (PTC) incidence was 89%, follicular (FTC) 8%, medullary (MTC) 2%, and anaplastic (ATC) 0.6%, while in males, PTC was 77%, FTC 15%, MTC 1%, and ATC 3%, respectively. The increase in females encompasses primarily PTCs with a small increase in follicular cancer types, but in males the increase is primarily in differentiated follicular cancers (Table 2). National data (18) indicate that PTC and FTC increased for both males and females, whereas data from the present study indicate an increase in PTC for females and FTC and ATC for males.
FIG. 4.
The percent of thyroid cancer types between females and males in VT differ significantly. Females (A) have proportionally more cases of papillary cancer and fewer cases of follicular and anaplastic cancer than males (B). (Pearson chi square test; p<0.001, n=702, df=4).
Table 2.
Thyroid Cancer Histological Type Varies by Age and Sex
| Age group | ||||||
|---|---|---|---|---|---|---|
| <30 years, n | <30 years, % | 30–59 years, n | 30–59 years, % | >59 years, n | >59 years, % | |
| Both sexes: | ||||||
| Papillary | 60 | 92.3 | 415 | 88.7 | 127 | 75.1 |
| Follicular | 2 | 3.1 | 41 | 8.8 | 26 | 15.4 |
| Medullary | 3 | 4.6 | 6 | 1.3 | 3 | 1.8 |
| Anaplastic | 0 | 0 | 1 | 0.2 | 7 | 4.1 |
| Indeterminate | 0 | 0 | 5 | 1.1 | 6 | 3.6 |
| Total | 65 | 100 | 468 | 100 | 169 | 100 |
| Males: | ||||||
| Papillary | 10 | 90.9 | 87 | 83.7 | 36 | 63.2 |
| Follicular | 1 | 9.1 | 14 | 13.5 | 11 | 19.3 |
| Medullary | 0 | 0 | 1 | 1 | 1 | 1.8 |
| Anaplastic | 0 | 0 | 1 | 1 | 4 | 7 |
| Indeterminate | 0 | 0 | 1 | 1 | 5 | 8.8 |
| Total | 11 | 100 | 104 | 100 | 57 | 100 |
| Females: | ||||||
| Papillary | 50 | 92.6 | 328 | 90.1 | 91 | 81.3 |
| Follicular | 1 | 1.9 | 27 | 7.4 | 15 | 13.4 |
| Medullary | 3 | 5.6 | 5 | 1.4 | 2 | 1.8 |
| Anaplastic | 0 | 0 | 0 | 0 | 3 | 2.7 |
| Indeterminate | 0 | 0 | 4 | 1.1 | 1 | 0.9 |
| Total | 54 | 100 | 364 | 100 | 112 | 100 |
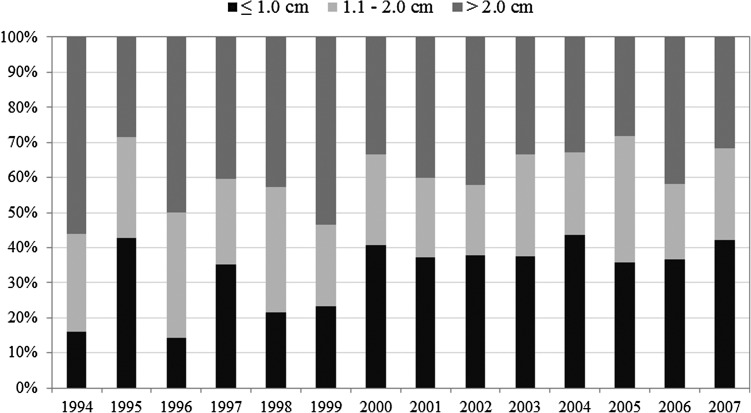
Although some studies have indicated that the increase in thyroid cancer could be attributed to an increase in detection of small tumors and microcarcinomas, using the Ljung-Box Q analysis, the present data for Vermont indicate no significant difference in tumor size over time (Fig. 5). For both females and males, the distribution of tumors by size did not vary over time; ≤1.0 cm, 1.1–2.0 cm, and >2.0 cm represented 38%, 22%, and 40%, respectively. While the distribution of tumors ≤1.0 cm, 1.1–2.0 cm, and >2.0 cm varies from year to year, the increase in thyroid cancer incidence is not due to a significant increase in small tumors but to an overall increase in cases diagnosed with tumors.
FIG. 5.
Thyroid cancer incidence classified by tumor size in Vermont, 1994–2007. The minimum number of tumors measured in any given year was 14 (1995); the maximum was 79 (2006). Using Ljung-Box Q analysis, the only significant trend occurred for tumors 1.1–2.0 cm in size in 2001–2004. When the 1.1–2.0 cm category was combined with either of the other two categories, there were no significant trends.
Geospatial distribution of thyroid cancer incidence
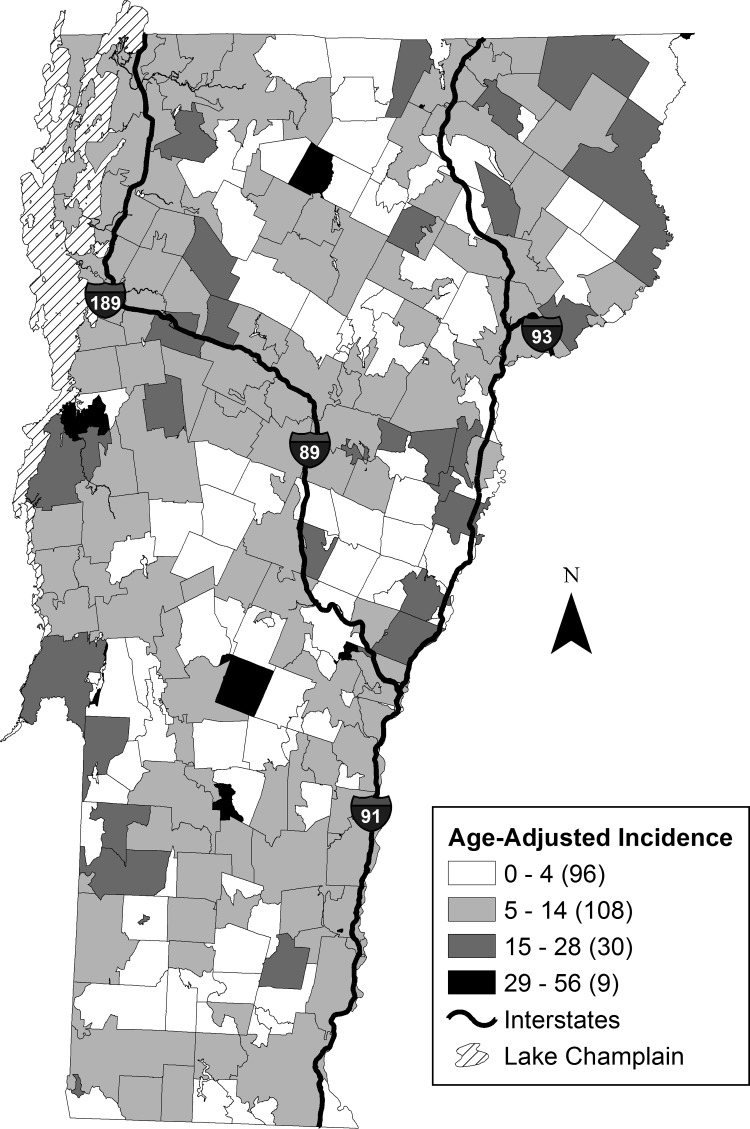
Between 1994 and 2007, thyroid cancer age-adjusted incidence varied widely throughout Vermont, ranging from no incidence to >30 per 100,000. The wide variability in incidence is striking as noted across adjacent zip codes (Fig. 6). This was further supported by no spatial autocorrelation being detected between the annual age-adjusted thyroid cancer incidence at the zip code scale, indicating the high spatial heterogeneity of incidence across the state. Even with the high spatial variability of incidence, nine zip code Hot Spots were identified, highlighting specific focus areas that could provide insight into future research regarding SES and environmental drivers of thyroid cancer. No other significant relationships between thyroid cancer incidence and other U.S. census variables were found.
FIG. 6.
Geospatial distribution of thyroid cancer incidence. Average annual age-adjusted incidence for Vermont (1994–2007) mapped to the U.S. 2010 Census zip code tabulation areas (zip codes). Jenks Natural Breaks was used to create the four classification categories of cancer incidence.
At the county scale, Vermont health data showed a significant (df=13, F=12.82, p=0.004, R2adj=0.48) negative linear relationship between thyroid cancer incidence and the number of medical practices per 100,000 people. In addition, no significant linear relationship was found between thyroid cancer incidence and the percent insured or the number of primary care physicians per 100,000 people at the county scale. Several nonrandom clusters of high thyroid cancer incidence were revealed by Getis-Ord Gi* analyses. These clusters are located in 8 of 14 counties, and include northern and central regions of the state. The geographic distribution of the clusters occurred predominantly in the regions of highest elevation along the north–south axis of the state, which encompasses the Green Mountain Range.
When SES and demographic factors and measures of health care access were analyzed, thyroid cancer incidence was not correlated with mean family income, education at more than high school level, mean travel time to work, and long-term residents (in residence prior to 1979). At the county scale, the high thyroid cancer incidence was negatively correlated with access to healthcare, as measured by location and concentration of primary care physicians compared to the population (HISA-VT 2008). No Hot Spots were identified in the highest income counties whether by per capita or median household income. According to Vermont Health Insurance Survey, >92% of the population has health insurance coverage (reference BISHCA) since 1990 when the surveys were initiated.
Discussion
Between 1994 and 2007, the incidence of thyroid cancer more than doubled in Vermont. The present findings suggest that during 1994–2007, the thyroid cancer incidence in Vermont (8.0%) was comparable to the national trend (8.4%). However, the estimated APC for women was higher in Vermont (9.9%) compared with the national APC (5.9%) as also reflected in the total estimated APC in Vermont and the United States (8.3% and 5.7%, respectively). Strikingly, the estimated APC for females in Vermont was double that for males (9.9% and 4.9%, respectively). When categorized by age groups, the thyroid cancer incidence more than doubled for females aged between 30 and 59 years over the study period, while all other categories increased but less dramatically. The total incidence increased for males, but there was no significant difference among age groups. Various studies have indicated a relation between reproductive factors and hormone use that may partially explain the increasing thyroid cancer incidence in younger women (44). Although the overall health insurance rate in Vermont (>92%) is near complete, it is unclear in this study whether female access to healthcare is greater than for males, which might contribute to the sex difference in estimated APC.
Overall, PTC accounts for more than 85% and FTC 10% of the tumors detected, as anticipated. However, the distribution varies by age (Table 2); PTC represents >92% of the tumors in those younger than 30 years of age, but only 75% in patients older than 59 years of age. The incidence of FTC and ATC increases for those older than 59 years of ages for both men and women. For men, PTC is most common in those younger than 59 years of age (>90%), but in those older than 59 years of age, PTC drops to <63%, and FTC and ATC increase to 19% and 7%, respectively. For females, the change in distribution of thyroid cancer type is less pronounced such that in those aged 59 years and older, PTC accounts for >81% of cases, while FTC and ATC increase to 13.4% and 2.7%, respectively. Aschebrook–Kilfoy et al. recently reported an increase in age-adjusted FTC in women and men, with an increase in aggressive tumors as well as small tumors particularly in women (18). Unfortunately, the grade of tumor and metastatic lesions were not reported in the Vermont registry in >80% of cases, so a comparison of aggressive tumors is not possible.
While previous studies have reported a significant increase in small (≤1.0 cm) tumors (4,10,22,45), the present findings did not reveal a significant selective increase in these tumors. An increase in small tumors and a decrease in larger tumors (>2.0 cm) would be predicted if increased diagnostic scrutiny accounted for the increase in thyroid cancer incidence. An incidence of ≤1.0 cm tumor size that does not significantly increase over time would argue against an increased detection due to improved diagnosis.
The present findings for the entire state do not show concordance with higher SES and increase in thyroid cancer incidence as has been previously shown (4,23,32). Unexpectedly, the higher thyroid cancer incidence by county was not located in the counties with the highest per capita income, family income, and education as would have been predicted from previous studies. No correlation was observed between zip codes with high incidence of thyroid cancers and SES or access to enhanced medical diagnostics. Data on tumor characteristics by zip code would be necessary to determine a potential correlation between SES and tumor size and stage at diagnosis. Nevertheless, the distribution of higher incidence of thyroid cancer incidence is not consonant with higher diagnostic scrutiny that would be expected with higher SES and access to healthcare. Aside from healthcare access, variation in healthcare provider culture and practices could contribute to the geospatial and temporal patterns that were observed in thyroid cancer incidence, but this could not be addressed in this study. Future studies could examine variation across healthcare provider networks.
This study was unable to determine causal relations between healthcare access, diagnostic approaches, environmental factors, and thyroid cancer incidence based on the geospatial analyses, but regions were identified where an assessment of possible environmental and demographic drivers may be focused. Although the geostatistical analysis did not identify a spatial autocorrelation at the zip code scale, the possibility of autocorrelation cannot be ruled out. As with any geo-referenced data set, there is always the possibility that the scale or range of autocorrelation will be missed if the spacing between observations is too large (46). As a result, it is suggested that a database geo-referenced at the household scale is needed to identify spatial correlations better between environmental factors and risk of thyroid cancer. Future studies are necessary to evaluate the role of diagnostic evaluation, environmental factors directly in thyroid cancer incidence trends.
This study may also be limited by the usual concerns of population-based studies, including nonreview of histopathological diagnoses, incomplete data collection, and variations in tumor classifications related to analyses of registry data. The 1994–2007 data collection time frame is subsequent to the World Health Organization recommended change in thyroid tumor classification that occurred in 1988 (47). Further, the population of Vermont is generally racially homogenous (>95% white Caucasian), and thus caution must be taken in generalizing the results to other populations with greater representation of racial groups. The finding that variation in access to healthcare does not fully explain temporal and spatial trends in thyroid cancer incidence in Vermont warrants further investigation in other study populations, particularly those with increased racial diversity. Healthcare insurance coverage is high (>92%) in Vermont and should be taken into consideration when generalizing to other states or population groups.
In summary, in rural Vermont with nearly complete healthcare coverage and a relatively stable population, the incidence of thyroid cancer is increasing among both women and men. The increase is most profound for women between the ages of 30 and 59 years. The increase in thyroid cancer is reflected in both small and large tumors; there is no significant difference in tumor size detected over the time period studied. Furthermore, geospatial analysis revealed a distribution of thyroid cancer incidence across the state that did not correlate with proximity to tertiary healthcare centers or SES. Similarly, the data did not support the often-reported hypothesis of increased incidence over time due to improved diagnostic scrutiny. These findings strongly suggest that other SES and environmental factors may likely contribute to the increase in thyroid cancer incidence. Investigation into naturally occurring and man-made environmental factors as well as lifestyle impact on thyroid cancer development is clearly warranted.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Alison Johnson, Cancer Registry Chief, Vermont Department of Health for insightful discussions. This publication was made possible by the Vermont Department of Health's provision of information from the Vermont Cancer Registry under a data use agreement. This work was supported, in part, by National Institutes of Health grant 3P01CA082834-15S1 (FEC) and Vermont EPSCoR with funds from the National Science Foundation Grant EPS-110131 (DMR) and DBC-EID-1216193 (JPH). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Vermont Department of Health, the National Science Foundation, or the National Institutes of Health.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N, Zhang Y. 2009. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control 20:525–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curado MP. Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. 2007. Cancer Incidence in Five Continents. Volume 9 IARC Scientific Publications, Lyon, France [Google Scholar]
- 3.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. 2013. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris LGT SA, Tosteson TD, Davies L. 2013. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 23:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute 2012. Surveillance, Epidemiology and End Results (SEER) Stat Fact Sheets: Thyroid Cancer. Available at: http://seer.cancer.gov/statfacts/html/thyro.html (accessed September2013)
- 7.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. 2010. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 148:1147–1152; discussion 1152–1153. [DOI] [PubMed] [Google Scholar]
- 8.Enewold L, Zhu K, Ron E, Marrogi AJ, Stojadinovic A, Peoples GE, Devesa SS. 2009. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev 18:784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards BK. Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. 2010. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies L, Welch HG. 2006. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 11.Ross DS. 2006. Predicting thyroid malignancy. J Clin Endocrinol Metab 91:4253–4255 [DOI] [PubMed] [Google Scholar]
- 12.Grodski S, Brown T, Sidhu S, Gill A, Robinson B, Learoyd D, Sywak M, Reeve T, Delbridge L. 2008. Increasing incidence of thyroid cancer is due to increased pathologic detection. Surgery 144:1038–1043; discussion 1043. [DOI] [PubMed] [Google Scholar]
- 13.Yu GP, Li JC, Branovan D, McCormick S, Schantz SP. 2010. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid 20:465–473 [DOI] [PubMed] [Google Scholar]
- 14.Reitzel LR, Nguyen N, Li N, Xu L, Regan SD, Sturgis EM. 2014. Trends in thyroid cancer incidence in Texas from 1995 to 2008 by socioeconomic status and race/ethnicity. Thyroid 24:556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall SF, Walker H, Siemens R, Schneeberg A. 2009. Increasing detection and increasing incidence in thyroid cancer. World J Surg 33:2567–2571 [DOI] [PubMed] [Google Scholar]
- 16.Pazaitou-Panayiotou K, Iliadou PK, Chrisoulidou A, Mitsakis P, Doumala E, Fotareli A, Boudina M, Mathiopoulou L, Patakiouta F, Tziomalos K. 2013. The increase in thyroid cancer incidence is not only due to papillary microcarcinomas: a 40-year study in 1778 patients. Exp Clin Endocrinol Diabetes 121:397–401 [DOI] [PubMed] [Google Scholar]
- 17.Ward EM, Jemal A, Chen A. 2010. Increasing incidence of thyroid cancer: is diagnostic scrutiny the sole explanation? Future Oncol 6:185–188 [DOI] [PubMed] [Google Scholar]
- 18.Aschebrook-Kilfoy B, Grogan RH, Ward MH, Kaplan E, Devesa SS. 2013. Follicular thyroid cancer incidence patterns in the United States, 1980–2009. Thyroid 23:1015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AY, Jemal A, Ward EM. 2009. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115:3801–3807 [DOI] [PubMed] [Google Scholar]
- 20.Kim C, Bi X, Pan D, Chen Y, Carling T, Ma S, Udelsman R, Zhang Y. 2013. The risk of second cancers after diagnosis of primary thyroid cancer is elevated in thyroid microcarcinomas. Thyroid 23:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leux C, Guénel P. 2010. Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev Epidemiol Sante Publique 58:359–367 [DOI] [PubMed] [Google Scholar]
- 22.Morris LG, Myssiorek D. 2010. Improved detection does not fully explain the rising incidence of well-differentiated thyroid cancer: a population-based analysis. Am J Surg 200:454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. 2013. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid 23:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Thyroid cancer. Available at: www.cancer.gov/cancertopics/types/thyroid (accessed September2013)
- 25.Wartofsky L. 2010. Increasing world incidence of thyroid cancer: increased detection or higher radiation exposure? Hormones 9:103–108 [DOI] [PubMed] [Google Scholar]
- 26.Richardson DB. 2009. Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology 20:181–187 [DOI] [PubMed] [Google Scholar]
- 27.Mangano JJ. 2009. Geographic variation in U.S. thyroid cancer incidence and a cluster near nuclear reactors in New Jersey, New York, and Pennsylvania. Int J Health Serv 39:643–661 [DOI] [PubMed] [Google Scholar]
- 28.Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. 2010. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology 21:389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grimalt JO, Sunyer J, Moreno V, Amaral OC, Sala M, Rosell A, Anto JM, Albaiges J. 1994. Risk excess of soft-tissue sarcoma and thyroid cancer in a community exposed to airborne organochlorinated compound mixtures with a high hexachlorobenzene content. Int J Cancer 56:200–203 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Guo GL, Han X, Zhu C, Kilfoy BA, Zhu Y, Boyle P, Zheng T. 2008. Do polybrominated diphenyl ethers (PBDE) increase the risk of thyroid cancer? Bioscience Hypotheses 1:195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu C, Zheng T, Kilfoy BA, Han X, Ma S, Ba Y, Bai Y, Wang R, Zhu Y, Zhang Y. 2009. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid 19:1061–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprague BL, Warren Andersen S, Trentham-Dietz A. 2008. Thyroid cancer incidence and socioeconomic indicators of health care access. Cancer Causes Control 19:585–593 [DOI] [PubMed] [Google Scholar]
- 33.Musa GJ, Chiang P-H, Sylk T, Bavley R, Keating W, Lakew B, Tsou H-C, Hoven CW. 2013. Use of GIS mapping as a public health tool—from cholera to cancer. Health Serv Insights 6:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw NT. 2012. Geographical information systems and health: current state and future directions. Healthc Inform Res 18:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulos DN. Ghali RR, Ibrahim EM, Boulos MN, AbdelMalik P. 2011. An eight-year snapshot of geospatial cancer research (2002–2009): clinico-epidemiological and methodological findings and trends. Med Oncol 28:1145–1162 [DOI] [PubMed] [Google Scholar]
- 36.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. 2000. International Classification of Diseases for Oncology. World Health Organization, Geneva [Google Scholar]
- 37.Vermont Population Estimates. Available at: http://healthvermont.gov/research/pop_estimate.aspx (accessed September2013)
- 38.Standard Populations (Millions) for Age-Adjustment. Available at: http://seer.cancer.gov/stdpopulations/ (accessed September2013)
- 39.Breslow NE, Day NE. 1987. Statistical methods in cancer research: the design and analysis of cohort studies. IARC Sci Publ 82:1–415 [PubMed] [Google Scholar]
- 40.Vermont Department of Banking ISaHCA 2010. Vermont Household Health Insurance Survey (2009). Presentation to the State Legislature. Market Decisions, pp 1–69 [Google Scholar]
- 41.Boyle P, Parkin DM. 1991. Cancer registration: statistical methods for registries. IARC Sci Publ 95:126–158 [PubMed] [Google Scholar]
- 42.Ries LAG, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, Vernon SW, Cronin K, Edwards BK. 2000. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer 88:2398–2424 [DOI] [PubMed] [Google Scholar]
- 43.Smith P. 1987. Comparison Between Registries: Age-Standardized Rates. Vol VI IARC Scientific Publications, Lyon, France: [PubMed] [Google Scholar]
- 44.Negri E, Dal Maso L, Ron E, La Vecchia C, Mark SD, Preston-Martin S, McTiernan A, Kolonel L, Yoshimoto Y, Jin F, Wingren G, Rosaria Galanti M, Hardell L, Glattre E, Lund E, Levi F, Linos D, Braga C, Franceschi S. 1999. A pooled analysis of case-control studies of thyroid cancer. II. Menstrual and reproductive factors. Cancer Causes Control 10:143–155 [DOI] [PubMed] [Google Scholar]
- 45.Davies L, Ouellette M, Hunter M, Welch HG. 2010. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope 120:2446–2451 [DOI] [PubMed] [Google Scholar]
- 46.Goovaerts P. 1998. Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biol Fertil Soils 27:315–334 [Google Scholar]
- 47.Hedinger C, Williams ED, Sobin LH. 1988. Histological Typing of Thyroid Tumours. World Health Organization. Second edition. Springer Verlag, Berlin [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.