Abstract
Bone morphogenetic protein-7 (BMP-7) promotes not only osteogenesis but also matrix production in chondrocytes and tenocytes. However, because of its short half-life, maintaining local concentrations of BMP-7 is difficult. We examined the use of a gelatin hydrogel sheet (GHS) for the sustained release of BMP-7 in stimulating rotator cuff repair at the tendon-to-bone insertion. Twelve-week-old male Sprague-Dawley rats were used. Radiolabeled BMP-7 (125I-BMP-7) was injected into the subacromial bursa in the 125I-BMP-7 group, whereas a GHS impregnated with 125I-BMP-7 was implanted on the tendon attached to the tendon-to-bone insertion in the 125I-BMP-7+GHS group. Levels of 125I-BMP-7 in the tendon-to-bone insertion were assessed at 1, 3, 7, 14, and 21 postoperative days. The BMP-7 concentrations were significantly higher in the 125I-BMP-7+GHS group than in the 125I-BMP-7 group. Next, the bilateral supraspinatus tendons were resected and sutured to the greater tuberosity of the humerus using the Mason-Allen technique. Treatment groups were created as follows: either phosphate-buffered saline (PBS) or BMP-7 was injected into the subacromial bursa in the PBS and BMP-7 groups, whereas a GHS impregnated with either PBS or BMP-7 was implanted on the repaired tendon attached to the tendon-to-bone insertion in the PBS+GHS and BMP-7+GHS groups. The resected specimens were stained at 2, 4, and 8 postoperative weeks with hematoxylin and eosin as well as Safranin O, and tissue repair was evaluated histologically by using the tendon-to-bone maturing score. Tissue repair was assessed biomechanically at 4 and 8 postoperative weeks. The BMP-7+GHS group at 8 postoperative weeks demonstrated a favorable cartilage matrix production and tendon orientation; moreover, the tendon-to-bone maturing score and the ultimate force-to-failure were the highest in this group. The ability of GHS to provide controlled release of various growth factors has been previously reported. We confirmed that the GHS releases BMP-7 in a sustained manner in the rat shoulder joint. At 8 postoperative weeks, the repaired tissue was mostly restored, both histologically and biomechanically, in the BMP-7+GHS group. We therefore conclude that the sustained release of BMP-7 from a GHS can stimulate rotator cuff repair.
Introduction
Rotator cuff tears often affect the shoulder joint, causing pain, limited range of motion, muscle weakness, and difficulty in performing daily activities. When conservative therapy fails to improve symptoms, surgery is performed to suture the tear to the enthesis; however, the retear rate is high, occurring in 18.6–47.6% of cases.1–4 After surgery, repair of the tendon-to-bone insertion takes several months.5 If range of motion and muscle training is resumed too soon after surgery, retearing at the tendon-to-bone insertion can occur.6,7 Therefore, good repair of the tendon-to-bone insertion is necessary.
A healthy tendon-to-bone insertion is composed of four layers: tendon, a noncalcified fibrocartilage layer, a calcified fibrocartilage layer, and bone. Fibrocartilage absorbs the mechanical stress placed on the tendon-to-bone insertion, and thus, reconstruction of the fibrocartilage layers is considered an important factor in rotator cuff repair.8
Rotator cuff repair involves many types of cells, such as mesenchymal cells, synovial cells, and tenocytes.9–11 Expression levels of various growth factors such as basic fibroblast growth factor, bone morphogenetic protein (BMP)-12, BMP-13, BMP-14, connective tissue growth factor, platelet-derived growth factor-B, and transforming growth factor β (TGF-β)-1 are increased at the repair site.12
BMP-7, which belongs to the TGF-β superfamily,13 promotes not only osteogenesis but also matrix production in chondrocytes14 and tenocytes.15–18
However, growth factors such as BMP are unstable and have short life spans in vivo, which make the maintenance of local concentrations difficult. Tabata et al. have developed a biodegradable gelatin hydrogel sheet (GHS) that has attracted attention as a potentially safe drug delivery system (DDS).19–23 The impregnation of a GHS with growth factors and the subsequent adsorption using electrostatic force allow for the preservation of biological activity. Therefore, growth factors with preserved biological activity can be released in a sustained manner until degradation of the GHS occurs.
We hypothesized that the use of a GHS, which maintains local concentrations of BMP-7, can promote rotator cuff repair. The objective of the present study was to examine the rotator cuff repair-stimulating effect of the sustained release of BMP-7 from a GHS.
Materials and Methods
Animals
Twelve-week-old Sprague-Dawley (SD) male rats (Shimizu Laboratory Supplies Co., Ltd., Kyoto, Japan) were raised in the animal house at our institution. A total of 95 SD rats (190 shoulders) were used in this study, which was reviewed and approved by the Animal Experiment Institutional Review Board of our institution.
GHS production
After preparing a 5 wt% aqueous solution of gelatin hydrogel (Nitta Gelatin Co., Osaka, Japan), we added 25% glutaraldehyde (GA; Wako Pure Chemical Ltd., Kyoto, Japan) and let it settle in a dish for 12 h at 4°C to induce crosslinking. The gelatin hydrogel was removed from the dish, and the unreacted GA was inactivated and washed with 0.1 M glycine solution (at room temperature for 1 h). Next, the gelatin hydrogel was washed twice with double-distilled water and frozen at −80°C. Freeze-drying and ethylene oxide gas sterilization were then performed.19,24 Before the operation, the gelatin hydrogel was cut into 1.0×1.0 cm segments and arranged in a sterile dish. Two hundred microliters of preprepared phosphate-buffered saline (PBS) or recombinant human BMP-7 (Pepro Tech, Inc., Rocky Hill, NJ) was instilled, and the gelatin hydrogel was allowed to sit for 2 h for sufficient impregnation with the solution.
Assessment of GHS-sustained release capability (radioactive isotope gamma scintigraphy)
BMP-7 labeled with a radioactive isotope (iodine 125) by the chloramine-T method25,26 was used. We created two groups in 15 SD rats (30 shoulders); in each rat, a GHS impregnated with 200 μL BMP-7 (2.5 ng/μL) was implanted on the tendon attached to the tendon-to-bone insertion in the right shoulder (the 125I-BMP-7+GHS group), and 200 μL BMP-7 (2.5 ng/μL) was injected into the subacromial bursa in the left shoulder (the 125I-BMP-7 group). BMP-7 dosing was determined according to the study by Hayashi et al.27
The rats were euthanized with a pentobarbital overdose at 1, 3, 7, 14, and 21 postoperative days (POD). The tissues surrounding both shoulder joints, including the deltoid, rotator cuff tissue, proximal humerus, and scapula, were harvested. Using a Packard Cobra E5002 Auto-Gamma Counter (PerkinElmer Japan Co., Ltd., Kanagawa, Japan), levels of 125I-BMP-7 in the rat shoulder joints were measured, and local residual rates of BMP-7 were calculated.
Rotator cuff repair
General anesthesia was induced through an intraperitoneal injection of pentobarbital, and both shoulders were operated on under sterile conditions. We made lateral-to-posterior skin incisions on the shoulders and elevated the acromion to expand the operative field. The supraspinatus tendon was resected from the greater tuberosity of the humerus with a sharp-pointed scalpel. Soft tissue and cartilage were removed as much as possible at the insertion site. Next, a drilling procedure was performed at the insertion site using a 0.5-mm-diameter drill bit attached to an electric drill until we observed bleeding from the marrow.28 A bone tunnel was made sagittally to the distal greater tuberosity. The end of the torn rotator cuff was grasped by the Mason-Allen technique29,30 with a 5-0 nylon suture as previously described.28,31 The suture was then placed through the bone tunnel and was tied to fix the rotator cuff to the greater tuberosity. The skin incision was closed with a 5-0 nylon suture. After surgery, all rats were allowed to move freely inside a cage in a temperature-controlled environment with a 12-h light–12-h dark cycle.
Creation of treatment groups
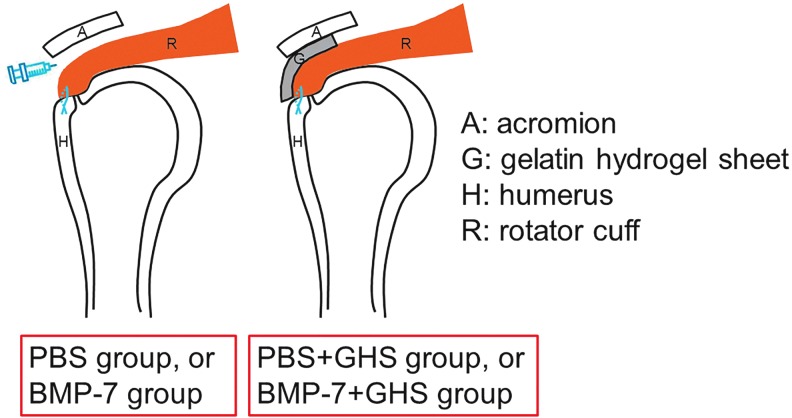
After rotator cuff repair, 80 SD rats (160 shoulders) were divided into groups as follows: 200 μL PBS was injected into the subacromial bursa before incision closure in the PBS group; similarly, 200 μL BMP-7 (2.5 ng/μL) was injected in the BMP-7 group, and a GHS impregnated with 200 μL PBS was implanted on the repaired tendon attached to the tendon-to-bone insertion before incision closure in the PBS+GHS group; and similarly, a GHS impregnated with 200 μL BMP-7 (2.5 ng/μL) was implanted on the repaired tendon in the BMP-7+GHS group (Fig. 1).
FIG. 1.
Operative methods: Schema of coronal sections. After rotator cuff repair, either PBS or BMP-7 was injected into the subacromial bursa in the PBS and BMP-7 groups, whereas a GHS impregnated with either PBS or BMP-7 was implanted on the repaired tendon attached to the tendon-to-bone insertion in the PBS+GHS and BMP-7+GHS groups. BMP-7, bone morphogenetic protein-7; GHS, gelatin hydrogel sheet; PBS, phosphate-buffered saline.
Microcomputed tomography assessment
The rats were euthanized with a pentobarbital overdose at 2, 4, and 8 postoperative weeks. The tissues surrounding both shoulder joints, including the deltoid, rotator cuff tissue, proximal humerus, and scapula, were taken. The harvested tissues of 48 SD rats (96 shoulders) were immediately fixed in 4% formaldehyde for 24 h at 4°C.
We randomly selected 18 SD rats (36 shoulders) from among the treatment groups described above and images of their shoulder joints were obtained with microcomputed tomography (μCT, micro focus 2D/3D, ScanXmate-E090S40; Comscantecno Co., Ltd., Kanagawa, Japan). The μCT images were scanned at an electric potential and current of 60 kV and 40 μA. Three-dimensional (3D) reconstructions were performed using an image reconstruction software program (FanCT ver. 1.3; Comscantecno Co., Ltd.), and these reconstructions were converted into images with an image analysis software program (TRI/3D-BON; Ratoc System Engineering Co., Ltd., Tokyo, Japan). We then checked for the presence of heterotopic ossification in the shoulder joints and the surrounding tissues.
Creation and observation of tissue samples
The harvested tissues of 48 SD rats (96 shoulders) were immediately decalcified at room temperature in ethylenediaminetetraacetic acid solution (pH 7.5) for 6 weeks.
An automated vacuum infiltration processor (Tissue-Tek VIP5Jr.; Sakura Finetek Japan Co., Ltd., Tokyo, Japan) was used to initially embed the tissue samples in paraffin, and an embedding center (Tissue-Tek Dispensing console IV and Tissue-Tek Cryo console; Sakura Finetek Japan Co., Ltd.) was used to complete the process.
Next, a sliding microtome (LS-113, Yamato Kohki Industrial Co., Ltd., Saitama, Japan; or IVS-410, Sakura Seiki Co., Ltd., Tokyo, Japan) was used to create thin serial sections (4 μm thickness) from coronal slices parallel to the long axis of the supraspinatus tendon from the paraffin block. These sections were subsequently fixed onto glass slides.
After staining the specimen sections with hematoxylin and eosin, we studied the cell morphology and tissue construction in the tendon-to-bone insertion with a light microscope. For adjacent sections, we performed Safranin O staining and assessed cartilage matrices with a light microscope.
Histological assessments of tendon-to-bone insertion repair
Repair in the tendon-to-bone insertion was assessed using the method devised by Ide et al. (the tendon-to-bone maturing score).32 This score comprises eight items (cellularity, vascularity, proportion of parallel oriented fibers, proportion of fibers with a large diameter, continuity, bone ingrowth, fibrocartilage cells, and tidemark) that are compared to the normal tendon-to-bone insertion. The first two items are graded as marked, moderate, mild, or minimal, while the remaining six items are graded as <25%, 25–50%, 50–75%, or >75%. Each item is given a score of 1, 2, 3, or 4 points, with a maximum overall score of 32 points. The scoring was performed by two coauthors who were blinded to information such as group and time point.
Biomechanical testing
At 4 and 8 postoperative weeks, specimens of 32 SD rats (64 shoulders) were used to perform biomechanical testing with a conventional tensile tester (LSC-200/30-2; Tokyo Testing Machine, Tokyo, Japan).28 The supraspinatus was separated from the scapular end, and the surrounding soft tissues were removed, except for the humerus and the repaired supraspinatus. The repaired rotator cuff was trimmed toward the tendon tissues for the measurement of force-to-failure by leaving 2 mm of the center to standardize the area of cross-section samples of the repaired. Calipers were used to measure the width of the trimmed tendon. After removing the surrounding scar tissue, the thickness of the tendon cross section was 1 mm, and biomechanical testing was performed immediately. Specimens were kept hydrated with PBS until biomechanical testing. For soft tissue biomechanical testing of each specimen, the supraspinatus and the humeral shaft were carefully gripped by using a custom-made clamping device with liquid nitrogen, such that the tendon could be stabilized to the clamp and was aligned parallel to the direction of load application. Each tendon was loaded to failure at a rate of 10 mm/min, and the maximum load supported by the repair was recorded.
Statistical analysis
All results are displayed as mean with standard deviation. To assess the controlled-release capability of the GHS, a two-way analysis of variance (ANOVA) was performed using groups as a within-subjects factor and POD as a between-subjects factor. For conducting histological assessments, a three-way ANOVA was performed using groups and postoperative weeks as between-subjects factors. A one-way ANOVA was then performed for each postoperative week. For conducting biomechanical assessments, a three-way ANOVA was performed with groups and postoperative weeks as between-subjects factors. A one-way ANOVA was then performed for each postoperative week. A p value of <0.05 was set as the level of statistical significance. Statistical analysis was performed using SPSS software (Windows ver.20; SPSS, Inc., Chicago, IL).
Results
Assessment of GHS sustained release capability
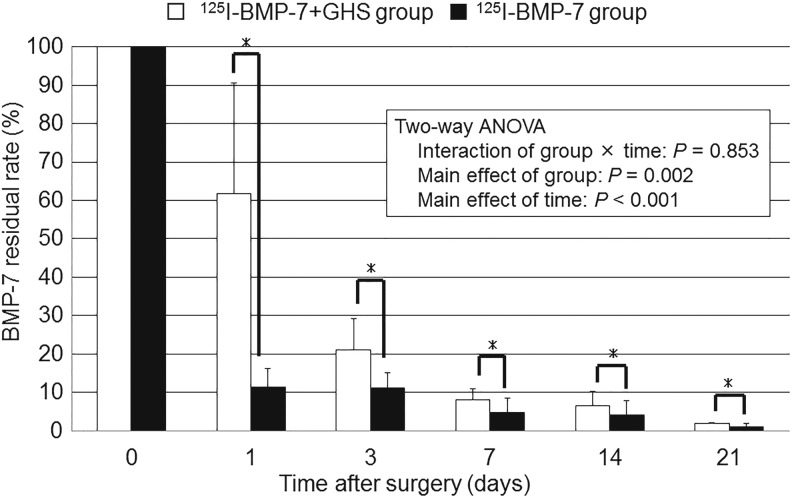
The local residual rates of BMP-7 in the shoulder joint were compared between the 125I-BMP-7 group and the 125I-BMP-7+GHS group. A significant main effect of groups (F(1,20)=12.28, p=0.002) and the number of POD (F(4,20)=21.64, p<0.001) was observed. A significant interaction between groups and the number of POD (F(4,20)=0.332, p=0.853) was not observed.
At every POD examined, the local residual rate of BMP-7 in the shoulder joint was significantly higher in the 125I-BMP-7+GHS group than in the 125I-BMP-7 group (Fig. 2).
FIG. 2.
Assessment of the sustained release of BMP-7 from a GHS using the chloramine-T method: All results are displayed as mean±standard deviation. The local residual rate of BMP-7 was significantly higher in the 125I-BMP-7+GHS group than in the 125I-BMP-7 group for 21 postoperative days. *p<0.05, the 125I-BMP-7+GHS group versus the 125I-BMP-7 group. ANOVA, analysis of variance.
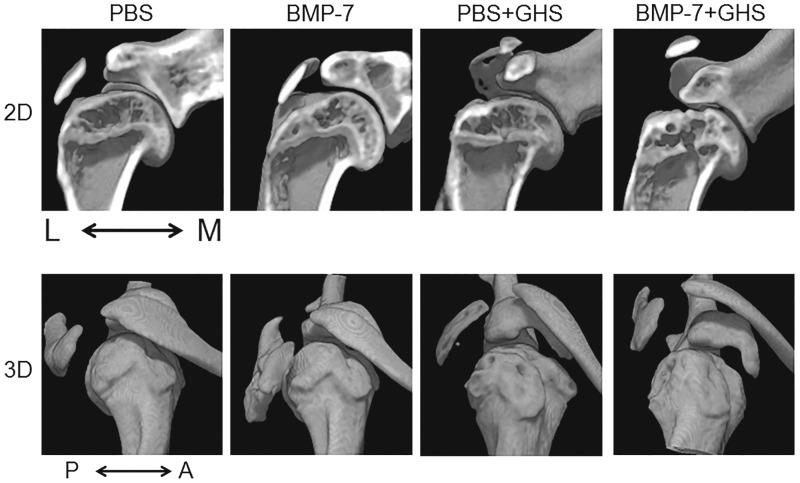
μCT assessment
In Figure 3, the upper row shows 2D coronal images of rat shoulder joints, while the lower row shows 3D profiles. No evidence of heterotopic ossification in the shoulder joints was observed in any group.
FIG. 3.
μCT assessment of ectopic calcification: All images are right shoulders. 2D coronal images (upper row) and 3D profiles (lower row) are shown. Heterotopic ossification was not observed in any group. A, anterior; L, lateral; M, medial; P, posterior; μCT, microcomputed tomography.
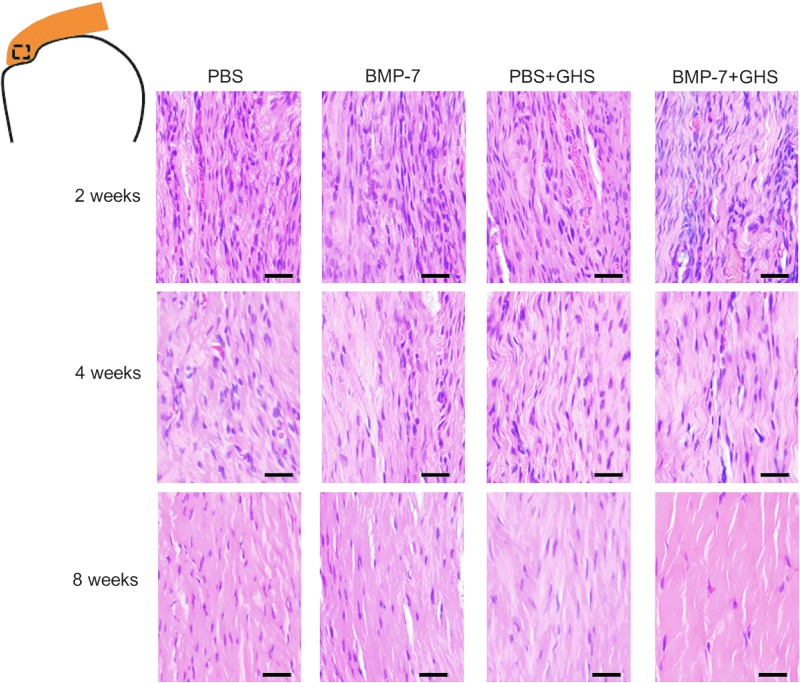
Histological assessments
At 2 postoperative weeks, an increase in fibroblast-like cells and vessels was observed in the tendon parenchyma near the tendon-to-bone insertion. A scattered orientation in the tendon was observed, and the difference between groups was not significant (Fig. 4). At 4 postoperative weeks, there were fewer fibroblast-like cells and blood vessels than at 2 postoperative weeks, along with an improvement in the orientation of rotator cuff collagen fibers. At 8 postoperative weeks, there were fewer fibroblast-like cells and blood vessels than at 4 postoperative weeks, and the BMP-7+GHS group had the most favorable orientation of rotator cuff collagen fibers among all the groups.
FIG. 4.
Histological findings in the tendon parenchyma near the tendon-to-bone insertion in each group at 2, 4, and 8 postoperative weeks (HE staining): Over time, cell density and the number of blood vessels decreased, while tendon orientation improved. Magnification is 400×, and the scale bar indicates 25 μm. HE, hematoxylin and eosin.
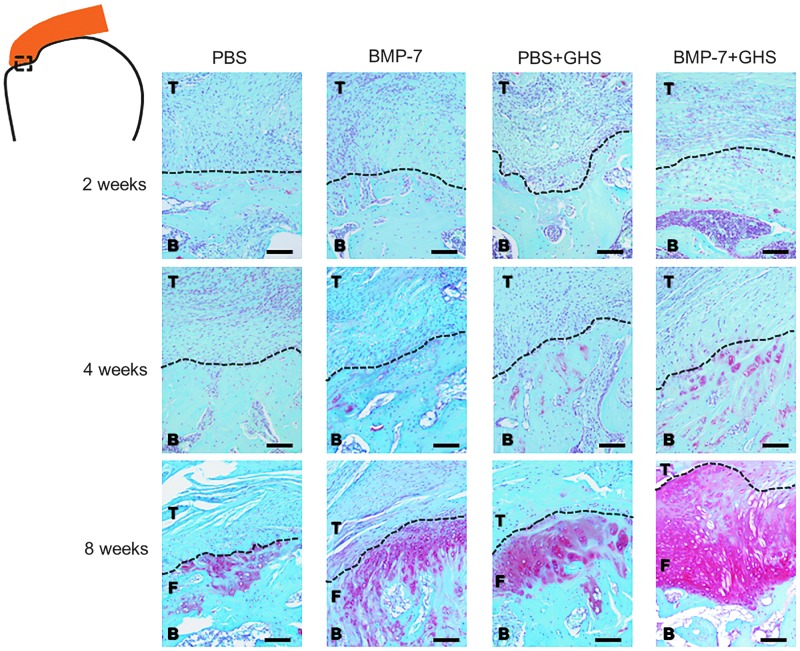
Only scattered cells were stained red by Safranin O in the tendon-to-bone insertion at 2 and 4 postoperative weeks, and there was no significant difference between the groups (Fig. 5). At 8 postoperative weeks, regions of red stained cells were observed in the tendon-to-bone insertion in all groups. Compared with the PBS, BMP-7, and PBS+GHS groups, the BMP-7+GHS group had a significant increase in the size of red-stained regions.
FIG. 5.
Histological findings in the tendon-to-bone insertion in each group at 2, 4, and 8 postoperative weeks (Safranin O staining): At 8 postoperative weeks, tendon (T), fibrocartilage (F) and bone (B) were observed in each group. The BMP-7+GHS group showed an increase in chondrogenesis compared to the other groups. Magnification is 100×, and the scale bar indicates 100 μm. Dotted line indicates the boundary line between tendon and bone or tendon and fibrocartilage.
Tendon-to-bone maturing scores
The tendon-to-bone maturing scores in the PBS, BMP-7, PBS+GHS, and BMP-7+GHS groups at 2 postoperative weeks were 12.4±1.8, 11.0±1.2, 11.9±1.4, and 12.0±1.8 points, respectively. At 4 postoperative weeks, the scores were 14.4±1.4, 12.3±1.8, 12.9±1.7, and 14.4±2.1 points, respectively. At 8 postoperative weeks, the scores were 17.6±1.8, 18.3±3.4, 19.3±1.0, and 22.3±2.4 points, respectively (Table 1).
Table 1.
The Tendon-to-Bone Maturing Score in Each Group (Points)
| Groups | ||||
|---|---|---|---|---|
| Time after surgery (weeks) | PBS | BMP-7 | PBS+GHS | BMP-7+GHS |
| 2 | 12.4±1.8 | 11.0±1.2 | 11.9±1.4 | 12.0±1.8 |
| 4 | 14.4±1.4 | 12.3±1.8 | 12.9±1.7 | 14.4±2.1 |
| 8 | 17.6±1.8 | 18.3±3.4 | 19.3±1.0 | 22.3±2.4a |
The maximum score was 32 points. All results are displayed as mean±standard deviation. In all four groups, tissue repair was achieved over time following the operation. The scores in the presence of GHS were significantly higher than those in the absence of GHS. At 8 postoperative weeks, the score of the BMP-7+GHS group was higher than those of the other groups.
p<0.05, the BMP-7+GHS group versus the PBS group and the BMP-7 group at 8 postoperative weeks.
BMP-7, bone morphogenetic protein-7; GHS, gelatin hydrogel sheet; PBS, phosphate-buffered saline.
In comparisons of the PBS, BMP-7, PBS+GHS, and BMP-7+GHS groups, a significant interaction was not observed between the presence or absence of BMP-7, the presence or absence of GHS, and the number of postoperative weeks (F(2,84)=0.308, p=0.736). Significant interactions were observed between the following: the presence or absence of BMP-7 and the presence or absence of GHS (F(1,84)=8.311, p=0.005), the presence or absence of GHS and the number of postoperative weeks (F(2,84)=5.062, p=0.008), and the presence or absence of BMP-7 and the number of postoperative weeks (F(2,84)=3.705, p=0.029). Significant main effects were observed for the number of postoperative weeks (F(2,84)=136.5, p<0.001) and the presence or absence of GHS (F(1,84)=6.567, p=0.012). No significant main effect was observed for the presence or absence of BMP-7 (F(1,84)=1.14, p=0.289).
In all four groups, scores improved over time after the operation. In between-group comparisons, according to the number of postoperative weeks, no significant differences were observed at 2 or 4 postoperative weeks. At 8 postoperative weeks, the score of the BMP-7+GHS group was significantly higher than those of the PBS and BMP-7 groups (p=0.003 and 0.013, respectively) and was also higher than the score of the PBS+GHS group, although this did not reach statistical significance (p=0.073).
Biomechanical testing
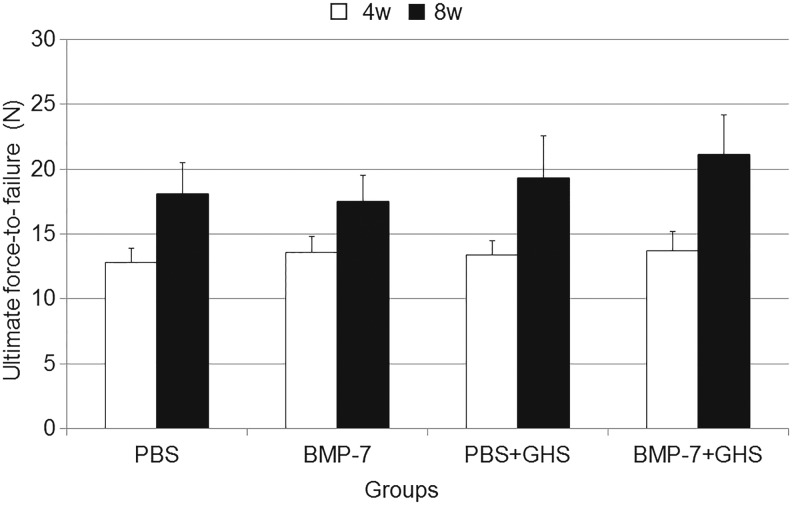
The ultimate force-to-failure was evaluated to test the biomechanical strength of the repaired rotator cuff in each group over time (Fig. 6). The ultimate force-to-failure values in the PBS, BMP-7, PBS+GHS, and BMP-7+GHS groups were 12.8±1.1, 13.6±1.2, 13.4±1.1, and 13.7±1.5 N, respectively, at 4 postoperative weeks and 18.0±2.4, 17.5±2.0, 19.3±3.3, and 21.1±3.1 N, respectively, at 8 postoperative weeks.
FIG. 6.
Ultimate force-to-failure in each group at 4 and 8 postoperative weeks: All results are displayed as mean±standard deviation. Significant main effects were observed for the number of postoperative weeks and the presence or absence of GHS. At 8 postoperative weeks, the ultimate force-to-failure in the BMP-7+GHS group was the highest among all groups, however, significant differences were not observed among any groups.
There was no significant interaction between the presence or absence of BMP-7, the presence or absence of GHS, and the number of postoperative weeks (F(1,56)=1.564, p=0.216). Significant interactions were not observed between the following: the presence or absence of BMP-7 and the presence or absence of GHS (F(1,56)=0.666, p=0.418), the presence or absence of GHS and the number of postoperative weeks (F(1,56)=3.832, p=0.056), and the presence or absence of BMP-7 and the number of postoperative weeks (F(1,56)=0.012, p=0.912). Significant main effects were observed for the number of postoperative weeks (F(1,56)=110.588, p<0.001) and the presence or absence of GHS (F(1,56)=6.889, p=0.011). No significant main effect was observed for the presence or absence of BMP-7 (F(1,56)=1.232, p=0.272).
In all four groups, the ultimate force-to-failure improved over time after the operation. At 8 postoperative weeks, the ultimate force-to-failure of the BMP-7+GHS group was the highest among all groups. However, although it was higher than the BMP-7 group (p=0.070), it was not significantly higher than those of the PBS group and the PBS+GHS group (p=0.135 and 0.587, respectively).
Discussion
BMPs are members of the TGF-β superfamily, which comprises groups of proteins with structures resembling TGF-β. The existence of BMPs from 1 to 16 has been documented to date. All BMPs except for BMP-1, BMP-3, and BMP-12 induce alkaline phosphatase production in osteoblasts.33,34 Although BMP-7 is already being used clinically in some countries (primarily for the promotion of osteogenesis), it has also been reported to promote matrix production in chondrocytes and tenocytes. Therefore, the present study used BMP-7, since it may exert multifaceted effects in tendons, cartilage, and bone (the constituents of rotator cuff tendon-to-bone insertion).
Because rotator cuff repair is a lengthy process, BMP-7 administered for this purpose must exert a continuous effect on the tendon-to-bone insertion. The effects of a single intra-articular administration of BMP-7 have been previously described by Hayashi et al., who administered 500 ng of BMP dissolved in 200 μL PBS into the knee joints of Japanese white rabbits to examine the efficacy of BMP-7 for knee osteoarthritis. They reported that BMP-7 administration stimulated cartilage matrix production and effectively inhibited knee osteoarthritis progression; however, weekly administration was required.27 Thus, growth factors like BMPs have short half-lives and require a noninvasive DDS that can maintain their effects continuously. Growth factors to be released are incorporated into the gelatin hydrogel by various intermolecular interactions, such as electrostatic and hydrophobic interactions. As the gelatin hydrogel is enzymatically degraded in vivo to generate water-soluble gelatin fragments, factors are released in a sustained manner around the hydrogel over the course of few weeks.35,36 The gelatin hydrogel is degraded and absorbed with time in vivo and does not remain in the body after the factor release finishes. Gelatin has been used clinically and its safety is approved for clinical and pharmaceutical applications. The gelatin hydrogel can be produced easily and economically. Thus, clinical applications are already taking place.37 Gelatin hydrogel can also be processed into various forms such as sheets and granules. In the present study, we created sheets of gelatin hydrogel, which we were able to implant stably into the rotator cuff repair site. Asamura et al. impregnated a GHS with BMP-2 for use in a canine orbital floor fracture model and reported the sustained release capability of GHS; in the BMP-2 single administration group, the residual rate of BMP-2 on day 1 was <4%, whereas in the BMP-2+GHS group, the residual rate of BMP-2 on day 14 was 17.1%.38 In the present study, the group implanted with a GHS impregnated with 125I-labeled BMP-7 showed a significantly higher residual rate up to day 21 after administration compared with the BMP-7 single administration group. This result demonstrated that GHS was capable of releasing BMP-7 in a sustained manner in rat shoulder joints.
Pauly et al. reported that the administration of BMP-7 to tenocyte-like cells taken from a human rotator cuff stimulated the production of type I collagen.18 In the present study, the BMP-7+GHS group had the favorable collagen fiber orientation at 8 postoperative weeks. Thus, the postoperative sustained release of BMP-7 may have stimulated tenocyte matrix production. Repair of the tendon-to-bone insertion involves the infiltration of undifferentiated mesenchymal cells from the subacromial bursa and bone marrow.5 These cells are therefore considered important factors in the reconstruction of the fibrocartilage layers in the tendon-to-bone insertion. BMP-2, BMP-7, and BMP-13 have been reported to induce chondrocyte differentiation in undifferentiated mouse mesenchymal cells.39 In the present study, compared with the other groups, the BMP-7+GHS group had more chondrocytes in the tendon-to-bone insertion at 8 postoperative weeks. This indicates that the sustained release of BMP-7 may have induced the differentiation of previously migratory undifferentiated mesenchymal cells into chondrocytes. Flechtenmacher et al. have reported that BMP-7 administration to chondrocytes stimulated the synthesis of proteoglycans and type II collagen.14 In our study, histological findings demonstrated a significant difference in cartilage matrix production at 8 postoperative weeks between the BMP-7 group and the BMP-7+GHS group. This result suggests that the sustained release of BMP-7 has stimulated cartilage matrix production at 8 postoperative weeks.
Some scoring systems of tendon-to-bone insertion are available for histological assessments.40 The tendon-to-bone maturing score32 in this study developed a modified Watkins score to assess the regeneration of the equine superficial digital flexor.41 This score assesses the repair for tendon-to-bone insertion of the rat rotator cuff and includes items related to the four layers consisting of tendon-to-bone insertion, the tendon (cellularity, vascularity, proportion of fibers oriented parallel, proportion of fibers of large diameter, and continuity), fibrocartilage layer (fibrocartilage cells and tidemark), and bone (bone ingrowth). Thus, we used this tendon-to-bone maturing score.
The reconstruction of the four zone layers in the tendon-to-bone insertion is important biomechanically.5,42 In this study, results of biomechanical testing were similar to those of histological assessment. No significant main effect was observed for the presence or absence of BMP-7, and significant main effects were observed for the presence or absence of GHS both histologically and biomechanically. These results suggested that the local application of BMP-7 alone is insufficient, and that GHS might have served as a reservoir of growth factors that are useful for the tissue repair at the tendon-to-bone insertion. There is a possibility that the GHS acted not only in the sustained release of growth factors but also in tissue repair. Both histological and biomechanical assessments demonstrated that, at 8 postoperative weeks, the BMP-7+GHS group had the best results among all the groups tested, although significant differences were not observed. These results suggest that the sustained release of BMP-7 from a GHS stimulates rotator cuff repair. In the future, further examination of the biodegradation rate of the GHS and the kind of growth factors should be undertaken to identify which conditions might result in a significant histological and biomechanical effect.
It has been previously reported that, as local concentrations of BMP-7 increase, the risk of heterotopic ossification also increases. 27 Heterotopic ossification has also been reported in clinical cases.33,34 In the present study, however, μCT did not reveal evident heterotopic ossification in the BMP-7+GHS group. The GHS used in this study released BMP-7 in a sustained manner over a period of ∼3 weeks, which may explain why there were no issues concerning safety.
There are limitations to the animal model used in this study. First, the rat shoulder differs from the human shoulder43 in that the portion of the rat supraspinatus muscle, which passes under the acromial arch, is muscular not tendinous, as in humans44; moreover, retear after rotator cuff repair in rats has not been observed postoperatively.45 Second, this model does not reflect the degenerative age-related rotator cuff tear that is commonly seen in humans.46
In this study, the repaired tissue especially in the tendon and cartilage was histologically most favorable in the BMP-7+GHS group at 8 postoperative weeks. In addition, the ultimate force-to-failure of the BMP-7+GHS group was the highest among all groups at the same time point, although a significant difference was not observed. These results suggest that the sustained release of BMP-7 from a GHS stimulates rotator cuff repair.
In conclusion, the GHS effectively maintained local levels of BMP-7 during tissue repair and contributed to the reconstruction of the tendon-to-bone insertion.
Acknowledgment
The authors would like to express their sincere gratitude to Dr. Yosuke Yamada of the Section of Energy Metabolism Department of Nutritional Science National Institute of Health and Nutrition.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chung S.W., Huong C.B., Kim S.H., and Oh J.H. Shoulder stiffness after rotator cuff repair: risk factors and influence on outcome. Arthroscopy 29, 290, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Kim K.C., Shin H.D., Cha S.M., and Kim J.H. Repair integrity and functional outcomes for arthroscopic margin convergence of rotator cuff tears. J Bone Joint Surg Am 95, 536, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Park J.Y., Lhee S.H., Oh K.S., Moon S.G., and Hwang J.T. Clinical and ultrasonographic outcomes of arthroscopic suture bridge repair for massive rotator cuff tear. Arthroscopy 29, 280, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Rhee Y.G., Cho N.S., and Parke C.S. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med 40, 2440, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Koike Y., Trudel G., and Uhthoff H.K. Formation of new enthesis after attachment of the supraspinatus tendon: a quantitative histologic study in rabbits. J Orthop Res 23, 1433, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Mahar A., Allred D.W., Wedemeyer M., Abbi G., and Pedowitz R. A biomechanical and radiographic analysis of standard and intracortical suture anchors for arthroscopic rotator cuff repair. Arthroscopy 22, 130, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Barber F.A., Coons D.A., and Ruiz-Suarez M. Cyclic load testing of biodegradable suture anchors containing 2 high-strength sutures. Arthroscopy 23, 355, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hayashi K., Kumai T., Higashiyama Y., Matsuda T., and Takakura Y. Repair process after fibrocartilagious enthesis drilling: histological study in a rabbit model. J Orthop Sci 14, 76, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Schon L.C., Gill N., Thorpe M., Davis J., Kim J., Molligan J., and Zhang Z. Efficacy of a mesenchymal stem cell loaded surgical mesh for tendon repair in rats. J Transl Med 12, 110, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju Y.J., Muneta T., Yoshimura H., Koga H., and Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res 332, 469, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Cheng B., Ge H., Zhou J., and Zhang Q. TSG-6 mediates the effect of tendon derived stem cells for rotator cuff healing. Eur Rev Med Pharmacol Sci 18, 247, 2014 [PubMed] [Google Scholar]
- 12.Wurgler-Hauri C.C., Dourte L.M., Baradet T.C., Williams G.R., and Soslowsky L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 16, S198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelrad T.W., and Einhorn T.A. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev 20, 481, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Flechtenmacher J., Huch K., Thonar E.J., Mollenhauer J.A., Davies S.R., Schmid T.M., Puhl W., Samapath T.K., Aydelotte M.B., and Kuettner K.E. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum 39, 1896, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Tsai A.D., Yeh L.C., and Lee J.C. Effects of osteogenic protein-1 (OP-1, BMP-7) on gene expression in cultured medial collateral ligament cells. J Cell Biochem 90, 777, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Yamada M., Akeda K., Asanuma K., Thonar E.J., An H.S., Uchida A., and Masuda K. Effect of osteogenic protein-1 on the matrix metabolism of bovine tendon cells. J Orthop Res 26, 42, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Yeh L.C., Tsai A.D., and Lee J.C. Bone morphogenetic protein-7 regulates differentially the mRNA expression of bone morphogenetic proteins and their receptors in rat achilles and patellar tendon cell cultures. J Cell Biochem 104, 2107, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Pauly S., Klatte F., Strobel C., Schmidmaier G., Greiner S., Scheibel M., and Wildemann B. BMP-2 and BMP-7 affect human rotator cuff tendon cells in vitro. J Shoulder Elbow Surg 21, 464, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Tabata Y., and Ikeda Y. Protein release from gelatin matrices. Adv Drug Deliv Rev 31, 287, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Tabata Y. Biomaterial technology for tissue engineering applications. J R Soc Interface 6, S311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi G., Miyamoto M., Tara S., Takagi I., Takano H., Yasutake M., Tabata Y., and Mizuno K. Controlled-release basic fibroblast growth factor for peripheral artery disease: comparison with autologous bone marrow-derived stem cell transfer. Tissue Eng Part A 17, 2787, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Hato N., Nota J., Komobuchi H., Teraoka M., Yamada H., Gyo K., Yanagihara N., and Tabata Y. Facial nerve decompression surgery using bFGF-impregnated biodegradable gelatin hydrogel in patients with Bell palsy. Otolaryngol Head Neck Surg 146, 641, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Nagae M., Ikeda T., Mikami Y., Hase H., Ozawa H., Matsuda K., Sakamoto H., Tabata Y., Kawata M., and Kubo T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng 13, 147, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M., Li H., Roybal J., Santore M., Radu A., Jo J., Kaneko M., Tabata Y., and Flake A. A tissue engineering approach for prenatal closure of myelomeningocele: comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Eng Part A 17, 1099, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Hunter W.M., and Greenwood F.C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature 194, 495, 1962 [DOI] [PubMed] [Google Scholar]
- 26.Kanematsu A., Yamamoto S., Ozeki M., Noguchi T., Kanatani I., Ogawa O., and Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials 25, 4513, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hayashi M., Muneta T., Ju Y.J., Mochizuki T., and Sekiya I. Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther 10, R118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kida Y., Morihara T., Matsuda K., Kajikawa Y., Tachiiri H., Iwata Y., Sawamura K., Yoshida A., Oshima Y., Ikeda T., Fujiwara H., Kawata M., and Kubo T. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg 22, 197, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Mason M.L., and Allen H.S. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg 113, 424, 1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J.L., Carreira D., Ponnappan R., Volz B., and Cole B.J. Use of bipolar radiofrequency energy in delayed repair of acute supraspinatus tears in rats. J Shoulder Elbow Surg 16, 640, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Chechik O., Dolkart O., Mozes G., Rak O., Alhajajra F., and Maman E. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg 134, 515, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Ide J., Kikukawa K., Hirose J., Iyama K., Sakamoto H., Fujimoto T., and Mizuta H. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. J Shoulder Elbow Surg 18, 391, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Axelrad T.W., Steen B., Lowenberg D.W., Creevy W.R., and Einhorn T.A. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br 90, 1617, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Wysocki A.M., and Cohen M.S. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to distal humerus for recalcitrant nonunion: a case report. J Hand Surg 32, 647, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Tabata Y., Nagano A., and Ikeda Y. Biodegradation of hydrogel carrier incorporating fibroblast growth factor. Tissue Eng 5, 127, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M., Takahashi Y., and Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 24, 4375, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Marui A., Tabata Y., Kojima S., Yamamoto M., Tambara K., Nishina T., Saji Y., Inui K., Hashida T., Yokoyama S., Onodera R., Ikeda T., Fukushima M., and Komeda M. A novel approach to therapeutic angiogenesis for patients with critical limb ischemia by sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogel: an initial report of the phase I-IIa study. Circ J 71, 1181, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Asamura S., Mochizuki Y., Yamamoto M., Tabata Y., and Isogai N. Bone regeneration using a bone morphogenetic protein-2 saturated slow-release gelatin hydrogel sheet: evaluation in a canine orbital floor fracture model. Ann Plastic Surg 64, 496, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Dorman L.J., Tucci M., and Benqhuzzi H. In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentiation of mouse mesenchymal stem cells. Biomed Sci Instrum 48, 81, 2012 [PubMed] [Google Scholar]
- 40.Loppini M., Longo U.G., Niccoli G., Khan W.S., Maffulli N., and Denaro V. Histopathological scores for tissue-engineered, repaired and degenerated tendon: a systematic review of the literature. Curr Stem Cell Res Ther 10, 43, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Watkins J.P., Auer J.A., Morgan S.J., and Gay S. Healing of surgically created defects in the equine superficial digital flexor tendon: collagen-type transformation and tissue morphologic reorganization. Am J Vet Res 46, 2091, 1985 [PubMed] [Google Scholar]
- 42.Thomopoulos S., Hattersley G., Rosen V., Mertens M., Galatz L., Williams G.R., and Soslowsky L.J. The localized expression of extracellular matrix components in healing tendon insertion site: an in situ hybridization study. J Orthop Res 20, 454, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Longo U.G., Forriol F., Campi S., Maffulli N., and Denaro V. Animal models for translational research on shoulder pathologies: from bench to bedside. Sports Med Arthrosc 19, 184, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Schneeberger A.G., Nyffeler R.W., and Gerber C. Structural changes of the rotator cuff caused by experimental subacromial impingement in the rat. J Shoulder Elbow Surg 7, 375, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Galatz L.M., Charlton N., Das R., Kim H.M., Havlioglu N., and Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 18, 669, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Lui P.P., Maffulli N., Rolf C., and Smith R.K. What are the validated animal models for tendinopathy. Scand J Med Sci Sports 21, 3, 2011 [DOI] [PubMed] [Google Scholar]