Abstract
Hirsutella rhossiliensis and H. minnesotensis are endoparasitic fungi of the second-stage juvenile (J2) of the soybean cyst nematode (Heterodera glycines) in nature. They also parasitize both H. glycines J2 and Caenorhabditis elegans on agar plates. Agrobacterium tumefaciens-mediated transformation conditions were established for these Hirsutella spp. The resulting transformants were similar to the corresponding wild-type strains. The infection processes of H. glycines J2 and C. elegans second larval stage (L2) by H. minnesotensis expressing ZsGreen were microscopically analyzed. Conidia of H. minnesotensis adhered to passing nematodes within 8 h post-inoculation (hpi), formed an infection peg between 8 and 12 hpi, and penetrated the nematode cuticle between 12 and 24 hpi for C. elegans L2 and between 12 and 32 hpi for H. glycines J2. Hyphal proliferation inside of the nematode coelom was observed at approximately 32 hpi for C. elegans L2 and at approximately 40 hpi for H. glycines J2. The fungus consumed the whole body and grew out to produce conidia at approximately 156 and 204 hpi for C. elegans L2 and H. glycines J2, respectively. The efficient transformation protocol and a better understanding of infection process provide a solid foundation for studying the molecular and cellular mechanisms underlying fungal parasitism of nematodes.
The soybean cyst nematode (SCN, Heterodera glycines Ichinohe) is one of the most destructive plant-parasitic nematodes of soybean in many regions of the world1,2. This nematode reduces yield both directly by siphoning off nutrients from infected plants and indirectly by creating wound sites that facilitate secondary fungal infection3. The endoparasitic fungi Hirsutella rhossiliensis and H. minnesotensis produce conidia that adhere to the cuticles of passing nematodes. Attachment leads to hyphal penetration and eventually to nematode death4,5. Only conidia that are attached to conidiogenous cells are infectious6. Once successful penetration occurs, they proliferate in the nematode body, filling it with mycelia, and produce new conidia within a few days to initiate a new infection cycle. Three endoparasitic Hirsutella spp. have been identified to date, including H. rhossiliensis Minter & Brady4, H. minnesotensis Chen, Liu & Chen7, and H. vermicola Xiang & Liu8. H. rhossiliensis has been detected worldwide1,9,10 and infects a wide range of hosts, including nematodes in the genera Heterodera, Meloidogyne, Xiphinema, and Rotylenchus and soil mites11,12. H. minnesotensis has been found in the U.S., Germany, Poland and China12,13, and it also infects diverse nematodes in the genera Aphelenchoides, Heterodera, Mesocriconema, Belonolaimus, Hoplolaimus, Steinernema, and Heterorhabditis12,14 and Collembola15. H. vermicola was recently discovered in bacteria-feeding nematodes8.
Because second-stage juveniles (J2s) of H. glycines are infectious, their presence in soil is directly associated with soybean yield reduction16. Both H. rhossiliensis and H. minnesotensis parasitize high percentages of SCN juveniles in diverse agricultural soils, supporting their potential as biocontrol agents. The H. rhossiliensis isolate OWVT-1 can reduce the density of H. glycines eggs by 95% and the J2 density by 98% in pots containing field soil17. Both species have also displayed high efficiencies in suppressing H. glycines density in greenhouse experiments18. As the dominant parasite of H. glycines J2 in China, H. minnesotensis is considered a main contributor to the suppression of SCN in field soils19. Field trials have demonstrated its efficiency for SCN biological control20.
Enhanced understanding of the infection process of H. glycines by H. minnesotensis is crucial to exploit this fungus as an effective biocontrol agent and also to study the molecular mechanisms underlying its interaction with nematodes. Caenorhabditis elegans has been widely used as a model organism to study diverse fundamental biological processes, mainly because of its experimental tractability (e.g., easy manipulation on agar, short generation time, and well-established experimental tools) and rich resources, such as genome sequences and diverse mutants21. Although the parasitism of bacteria-feeding nematodes, such as C. elegans by H. minnesotensis has not been detected in nature, the successful infection of C. elegans, by this fungus at different larval stages on agar plates has been documented22, suggesting that this model nematode can potentially help enhance the current understanding of the molecular and cellular mechanisms underlying H. minnesotensis–nematode interactions. To realize this potential, it is critical to develop genetic manipulation tools for H. minnesotensis.
Fluorescent proteins have been expressed in a wide variety of organisms, including bacteria, fungi, plants, and animals23, and have facilitated the imaging of diverse organismal interactions and cellular processes24,25,26. In this study, we established an Agrobacterium tumefaciens-mediated transformation (ATMT) protocol for H. rhossiliensis and H. minnesotensis and generated transformants expressing fluorescent proteins with non-overlapping spectral properties. One H. minnesotensis transformant was used to investigate the manner by which it infects H. glycines J2s and C. elegans L2s using fluorescence microscopy.
Results
Transformation of Hirsutella spp. with three fluorescent protein genes
The sensitivities of Hirsutella spp. to hygromycin B and geneticin were determined. The minimum concentrations of hygromycin B and geneticin resulting in complete growth inhibition were 150 μg/ml and 400 μg/ml, respectively, for H. minnesotensis AS3.9869 and 100 μg/ml and 400 μg/ml, respectively, for H. rhossiliensis AS6.0004. Growth responses of these isolates to hygromycin B on potato dextrose agar (PDA) and cornmeal agar (CMA) were indistinguishable. Based on their higher sensitivity to hygromycin B than geneticin, we chose the hph gene, which confers resistance to hygromycin B, to select transformants. Binary vectors containing hph and the AsRed, AsCyan or ZsGreen gene were used for transformation. PCR analysis of 12 randomly selected transformants revealed that the hph gene in the inserted T-DNA was detected in all selected transformants but not in the wild-type (wt) AS3.9869 or AS6.0004 strains (Fig. 1). Microscopic observations of slide cultures showed that all three fluorescent proteins were expressed strongly in hyphae and conidia of AS3.9869 and AS6.0004 transformants (Fig. 2).
Figure 1. Detection of the hph gene in twelve randomly selected transformants using PCR.
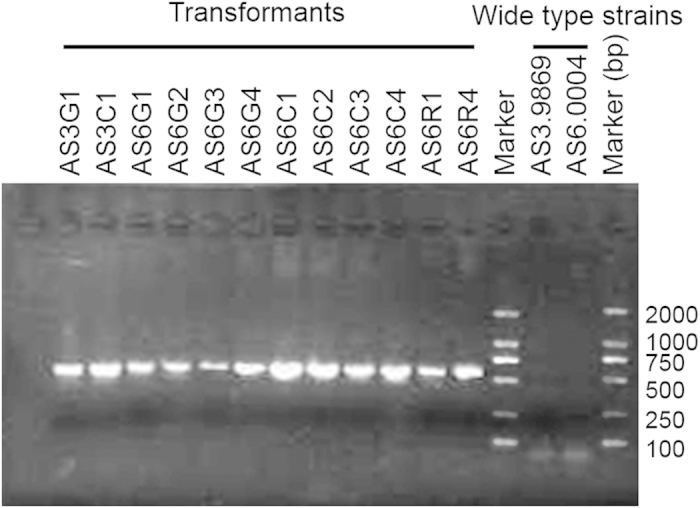
The presence of the hph gene in transformants of H. minnesotensis AS3.9869 and H. rhossiliensis AS6.0004 was detected using PCR. This PCR product was absent in AS3.9869 and AS6.0004.
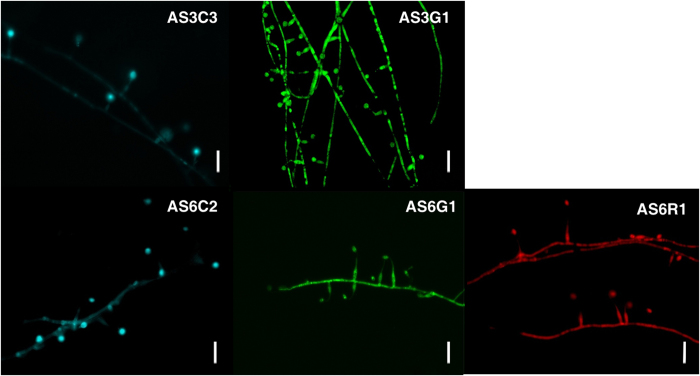
Figure 2. Transformants of H.minnesotensis AS3.9869 and H.rhossiliensis AS6.0004 expressing a fluorescent protein.
Expression of cyan (AsCyan) and green (ZsGreen) fluorescent proteins was detected in hyphae and conidia of transformants AS3C3 and AS3G1 of H. minnesotensis AS3.9869, respectively. Expression of AsCyan, ZsGreen and AsRed, a red fluorescent protein, in hyphae and conidia of transformants AS6C2, AS6G1 and AS6R1 of H. rhossiliensis AS6.0004, respectively, by slide culture are shown. Scale bar = 20 μm.
Morphology, growth characteristics and the capability to parasitize nematodes were evaluated for 50 transformants (10 ZsGreen and 10 AsCyan transformants of AS3.9869; and 10 ZsGreen, 10 AsCyan and 10 AsRed transformants of AS6.0004). Compared with the corresponding wt strains, several transformants parasitized fewer nematodes, which may be due to genetic changes that occurred during transformation. However, most transformants showed no significant differences from the wt strains. The transformant AS3G1, a ZsGreen transformant of H. minnesotensis AS3.9869, was selected to examine the manner by which it infects C. elegans and H. glycines (Figs. 2 and 3). Both AS3.9869 and AS3G1 parasitized substantial percentages of H. glycines J2 and C. elegans at four larval stages (L1-L4). The percentages of colonization for H. glycines J2 and C. elegans L1-L4s were 96.5%, 87.6%, 68.3%, 66.4% and 79.1%, respectively, for AS3.9869 and 96.4%, 84.3%, 69.9%, 67.2% and 78.0%, respectively, for AS3G1. There were no significant differences detected between AS3.9869 and AS3G1 (t0.05/2 = −0.715; p = 0.485). However, as shown in Fig. 3, the percentage of parasitized nematodes differed significantly between H. glycines J2 and C. elegans L1-L4 (F = 62.56; df = 4, 25; p < 0.001).
Figure 3. Efficiencies of parasitizing nematodes by H.minnesotensis AS3.9869 and its transformant AS3G1.
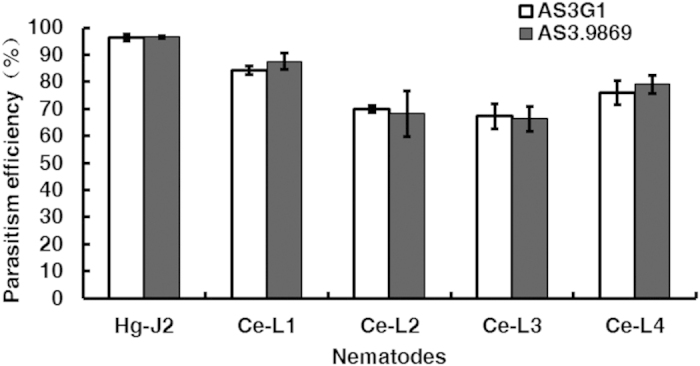
The percentages of nematodes parasitized by AS3.9869 and AS3G1 were analyzed 24 hours after inoculation, which showed no significant differences in their ability to parasitize H. glycines J2 (Hg-J2) and four different larval stages of C. elegans (Ce-L1, Ce-L2, Ce-L3, and Ce-L4). Error bar indicates the standard deviations within three replications.
Infection cycle of nematodes by H. minnesotensis
The infection cycle consists of four stages: recognition and adhesion; penetration; consumption of the nematode body; and sporulation27. Notable features observed at each of these stages of nematode colonization by AS3G1 are outlined below.
Recognition and adhesion
Interactions of AS3G1 with H. glycines J2s and C. elegans L2s at 4, 8, 16, and 24 h post inoculation (hpi) showed that even at 4 hpi, more than 90% of H. glycines J2s had attached conidia (Fig. 4). The percentage of C. elegans L2s with attached conidia at this stage was approximately 65%, which was significantly lower than that observed for the H. glycines J2s. The percentage of parasitized H. glycines J2s did not vary greatly during this period (Fig. 4), suggesting that unattached H. minnesotensis conidia quickly recognized and adhered to passing H. glycines J2s. There was a noticeable increase in the colonization of C. elegans L2s between 4 and 8 hpi, after which it remained stable at approximately 75%. More conidia adhered to individual nematodes as time progressed, with up to 20 adhered spores for each nematode until mobility was lost. These results suggest that the stage of recognition and adhesion was mostly completed within 8 hpi for both nematodes (Fig. 5, 6, 7).
Figure 4. Percentages of nematodes infected by H.minnesotensis AS3.9869 and its transformant AS3G1 within 24 h post inoculation.
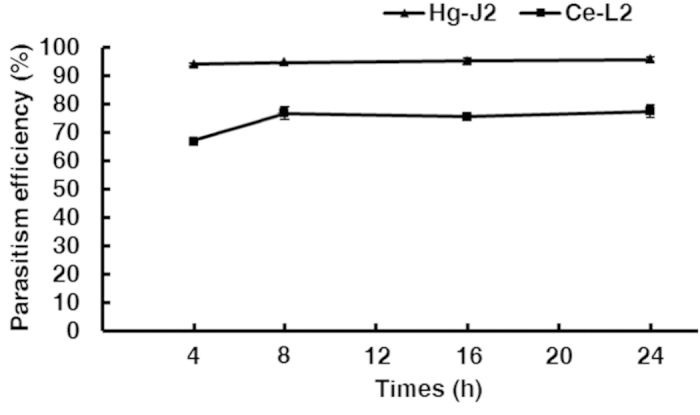
Parasitism efficiencies of Hg-J2 and Ce-L2 by these strains at 4, 8, 16, and 24 h post inoculation are shown. Error bar indicates the standard deviation within three replications.
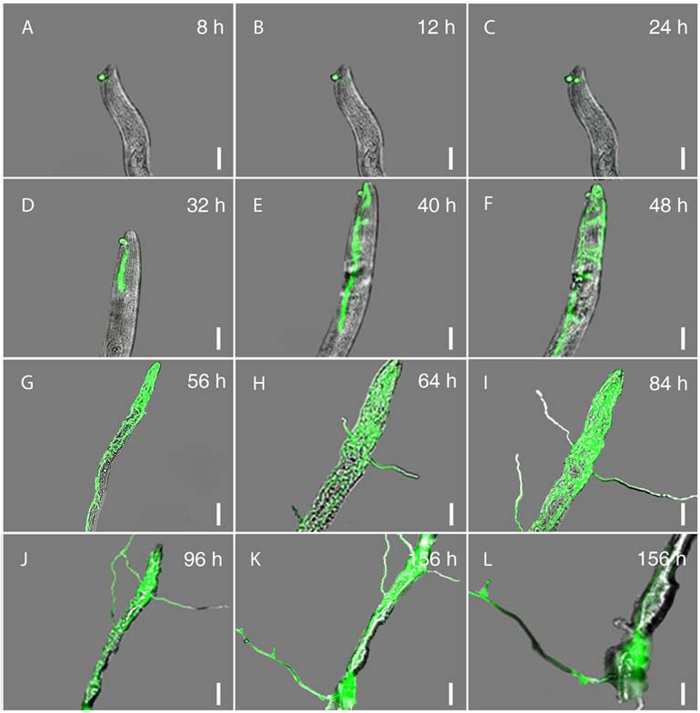
Figure 5. Progression of H. glycinesJ2 infection by H.minnesotensis.
A, a spore of AS3G1 (a H. minnesotensis transformant expressing ZsGreen) attached to H. glycines second stage juvenile (J2); B, formation of an infection peg; C, the apex of the infection peg becoming globose; D, initial colonization of coelom by hyphae; E-G, hyphal growth through coelom; H-J, hyphal growth out of coelom; K-L, formation of new conidia. The scale bar in A-F, H-I, and L corresponds to 20 μm, whereas the scale bar in G, J and K equals 40 μm.
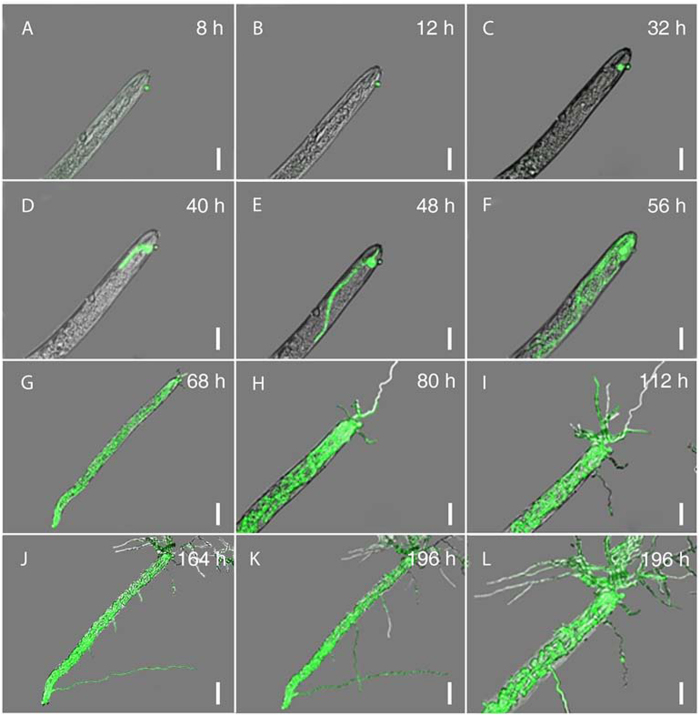
Figure 6. Progression of C.elegans L2 infection by H. minnesotensis.
A, a spore of AS3G1 attached to C. elegans second stage larvae (L2); B, formation of an infection peg; C, the apex of the infection peg becoming globose; D, initial colonization of coelom by hyphae; E-G, hyphal growth through coelom; H-J, hyphal growth out of coelom; K-L, formation of new conidia. The scale bar in A-F, H-I, and L corresponds to 20 μm, whereas the scale bar in G, J and K equals 40 μm.
Figure 7. Comparison of the infection cycle of H. glycines J2s andC.elegans L2s by H.minnesotensis AS3G1.
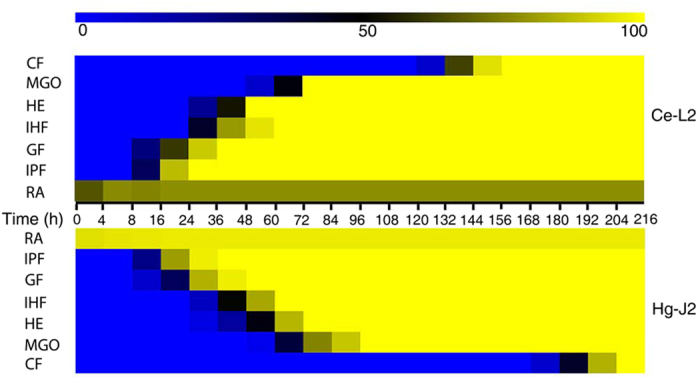
A heatmap was used to facilitate the comparison of the infection cycle of H. glycines J2s (Hg-J2) and C. elegans L2s (Ce-L2) by H. minnesotensis on agar plates. Patterns observed in 30 individuals for each nematode species were used to draw the map. The background color, changing from blue to black to yellow, indicates the percentage of nematodes parasitized at individual infection stages at the given observation point. The infection stages include recognition and adhesion (RA), infection peg formation (IPF), globus hyphal formation (GHF), initial hyphal formation (IHF), hyphal extension (HE), mycelia growing out (MGO) and conidial formation (CF). The infection cycle of Ce-L2s and Hg-J2s analyzed here were 156 h and 204 h, respectively.
Penetration
Following the germination of the conidia adhered to the nematodes, an infection peg was formed, and penetration of the nematode cuticle occurred between 8 and 12 hpi (Figs. 5, 6, 7). After penetration, the apex of the infection hypha became globus between 12 and 24 hpi for C. elegans L2 (Fig. 5C, Fig. 7) and between 12 and 32 hpi for H. glycines J2 (Fig. 6C, Fig. 7). Although C. elegans L2s were alive and could move slowly at 24 hpi, more than 80% of them were observed to be penetrated by one or more conidia. H. glycines J2s appeared dead at 32 hpi because their stylets were no longer moving, and more than 90% of them were penetrated by one or more conidia during this period (Fig. 7).
Consumption of the nematode body
Once the fungus penetrated the nematode, the initial hypha started to grow in the nematode body. The fungus began to develop an initial hypha inside of C. elegans L2 (Fig. 5D) at 32 hpi and inside of H. glycines J2 at 40 hpi (Fig. 6D). Eighty percent of the examined nematodes with developed initial hyphae were found at 48 hpi for C. elegans L2 and at 60 hpi for H. glycines J2. The initial hyphae quickly extended inside of C. elegans L2 (Fig. 5E-G) and H. glycines J2 (Fig. 6E-G), and the coeloms of both nematodes were completely filled with mycelia at 84 hpi (Fig. 7).
Sporulation
Hyphae began growing out of the colonized nematode coelom at approximately 56 hpi for C. elegans L2 (Fig. 5G) and at 68 hpi for H. glycines J2 (Fig. 6G), and hyphae were clearly visible at 64 hpi for C. elegans L2 (Fig. 5H-J, Fig. 7) and at 80 hpi for H. glycines J2 (Fig. 6H-J, Fig. 7). Subsequently, the fungus produced new conidia (Fig. 5K,L, Fig. 6K,L) from up to 94% of C. elegans L2s at 156 hpi and 84% of H. glycines J2s at 204 hpi (Fig. 7). In summary, the infection cycles of C. elegans L2 and H. glycines J2 by H. minnesotensis on agarose slides were completed in approximately 156 and 204 h, respectively.
Discussion
Green fluorescent protein (GFP) and its color variants as molecular labels have been applied to studying diverse filamentous fungi28. To assess the interactions between nematophagous fungi and nematodes, attempts have been made to transform Pochonia chlamydosporia23, Clonostachys rosea26 and Dactylellina cionopaga29 with a gene encoding GFP. However, no gfp transformant of P. chlamydosporia was obtained23 because of its slower growth and limited sporulation, and the material coating its spores resulted in a very low transformation efficiency26. The goal of our study was to establish a robust transformation protocol for the dominant nematode endoparasitic fungi Hirsutella spp. in nature using fluorescent protein genes as markers to assess the manner by which they infect nematodes. ATMT was successful in transforming both H. rhossiliensis and H. minnesotensis, and most transformants expressed three fluorescent proteins with high efficiency and displayed growth, morphology, and pathogenicity that were indistinguishable from the corresponding wt strains. These transformants will facilitate ecological studies, such as the monitoring of the spatial and temporal distribution of Hirsutella spp. in soil and plant roots, competition between H. rhossiliensis and H. minnesotensis, and the elucidation of the molecular and cellular mechanisms of fungal interactions with nematodes.
Due to the movement of free-living nematodes, their microscopic observation is challenging. Conventional techniques for immobilizing nematodes include the uses of cyanoacrylate glue30 and anesthetic compounds, such as sodium azide (a metabolic inhibitor)31 and levamisole (a cholinergic agonist)32. Recently, a computer-controlled micro-fluidic device was developed to limit nematode mobility33. Although these methods have been successfully applied, some limitations remain. The use of the glue is labor-intensive and low-throughput. Anesthetic compounds have negative effects on H. minnesotensis growth. Further, microfluidic device cannot provide enough air for this fungus. Here, we successfully used the agarose fixation method to immobilize H. glycines J2 and C. elegans larvae without negatively affecting fungal infection.
H. minnesotensis is a cold-adapted fungus that preferentially parasitizes cyst nematodes, even in the presence of bacterial-feeding nematodes in nature19,34. Both H. minnesotensis and H. rhossiliensis can parasitize various nematodes, including C. elegans, on agar plates15,22. However, they cannot parasitize bacterial-feeding nematodes in natural soil, or C. elegans in autoclaved soil, as demonstrated in our recent study. The differences in parasitism between agar plates and soil suggest that some soil factors may interfere with fungal recognition and/or colonization of nematodes. Follow-up studies to address this supposition will be conducted using the transformants generated in this study.
Several characteristics of C. elegans, including its small size, short generation time, invariant developmental lineage, and sequenced genome, along with the powerful genetic manipulation tools available for its analysis and its ease of handling, have made it a model organism for studying the mechanisms underlying bacterial and fungal pathogenesis in animals and innate immunity35. Studies with bacterial pathogens, such as Pseudomonas, Salmonella, or Serratia, have been highly fruitful in revealing the existence of an innate immune system in C. elegans, similar to that in vertebrates36. This model organism has also been used to study innate immune responses to fungal infections37,38. Interactions between nematophagous fungi and nematodes have not been extensively studied.
The capability of H. minnesotensis, a dominant H. glycines J2 parasite, to infect C. elegans on agar plates presents a new model system for investigating the molecular and cellular mechanisms underlying the pathogenesis of nematophagous fungi and host defenses at different infection stages. The infection process of H. minnesotensis against H. glycines J2 and C. elegans L2 observed in this study has guided transcriptomics analyses of C. elegans L2 parasitized by H. minnesotensis39, as well as an ongoing study of the experimental evolution of parasitism of Hirsutella spp.
Materials and Methods
Strains and nematodes
H. minnesotensis AS3.9869 (strain HLJ1-10) was isolated from H. glycines J2 collected in Jiamusi, Heilongjiang, China in 2006. H. rhossiliensis AS6.0004 (strain OWVT-1) was isolated from H. glycines J2 collected in Waseca, Minnesota, USA in 19971. Single-spore isolates of these fungi that parasitized a high percentage of H. glycines J2s18 were used for transformation. These strains were cultured on PDA at 25 °C and maintained in 15% glycerol at −80 °C until needed.
Three A. tumefaciens strains, including SK2245 (A. tumefaciens strain EHA105 that carries a pBHt2 binary vector containing the AsRed gene), SK2247 (EHA105 that carries a pBHt2 binary vector containing the AsCyan gene) and SK2251 (EHA105 that carries a pBHt2 binary vector containing the ZsGreen gene), were used for fungal transformation. The expression of all three fluorescent protein genes was driven by the promoter of the Fusarium verticillioides elongation factor 1α gene. These A. tumefaciens strains were cultured at 28 °C for 48 h in minimal medium40 supplemented with 50 μg/ml kanamycin before transformation.
Soybean cyst nematode race 4 was isolated from a soybean field near Beijing. For H. glycines egg preparation, newly formed cysts in soybean roots cultivated in the greenhouse were extracted using the sucrose floatation and centrifugation method18. Individuals at the J2 stage were prepared as described by Liu and Chen1 and used immediately.
Bristol strain N2 of C. elegans in mixed-stages was cultured on nematode growth medium (NGM) plates and seeded with Escherichia coli stain OP5041 at 24 °C. Fresh C. elegans specimens at the four larval stages were harvested following the method described by Xie et al.42 and used immediately.
Preparation of fungal spores
Spores of H. minnesotensis AS3.9869 and H. rhossiliensis AS6.0004 were harvested by the scraping of 15-day-old cultures on PDA after the flooding of plates (5 plates per strain) with 15 ml of sterile water. After passing individual spore suspensions through two layers of Miracloth (Calbiochem, Cat. #475855, La Jolla, CA) to remove large debris, they were centrifuged at 10,000 rpm for 10 min and washed twice with sterile distilled water. The harvested spores were re-suspended in sterile water to produce a solution of 1 × 106 spores/ml.
Fungal transformation
To determine the sensitivities of the fungal strains to antibiotics prior to transformation, PDA and CMA plates with different amounts of hygromycin (50, 100, 150, 200, 250 μg/ml) or geneticin (50, 100, 200, 400, 800 μg/ml) and control plates without antibiotics were prepared. Small amounts of mycelia from the tested strains were inoculated on each plate and incubated for 15 days. The amounts of antibiotics that completely inhibited fungal growth were chosen for transformant selection. Agrobacterium tumefaciens-mediated transformation was performed according to Khang et al.40 with minor modifications. Each of the three A. tumefaciens strains described above was incubated in 1 ml of minimal medium (MM) supplemented with kanamycin (50 μg/ml) for 1-2 days at 28 °C. The resulting A. tumefaciens cultures were inoculated into induction medium (IM) at OD600 = 0.15. After the culturing of A. tumefaciens cells for 6 h at 28 °C at 200 rpm, a 100-μl suspension of A. tumefaciens cells and 100 μl of fungal spores (1 × 106 spores/ml) were mixed and spread onto a nitrocellulose membrane (Whatman Cat. #7141 104; 47-mm diameter; 0.45-μm pore size) placed on co-cultivation medium in the presence of 200 μM AS. After 2 days of incubation at room temperature in the dark, the membrane was transferred to CMA selection medium containing hygromycin B (150 μg/ml for AS3.9869 and 100 μg/ml for AS6.0004) and cefotaxime (0.2 mM). The membrane was incubated for 2-3 weeks at room temperature until colonies formed.
Putative hygromycin B-resistant colonies were transferred to 24-well plates containing CMA selection medium with hygromycin B using sterile toothpicks. After the plates were incubated at 25 °C for 10 days, individual wells were flooded with sterile water. The water was pipetted up and down several times to dislodge the conidia. The conidia were spread onto water agar plates with hygromycin B and incubated for 24 h, and 10 single germinating spores for each transformation were then picked up with a sterile needle under a microscope and transferred to PDA plates for subsequent analysis and preservation.
Characterization of putative transformants
A simple slide culture technique was used for detecting the fluorescence of the growing transformants. A PDA agar block (approximately 4 × 4 mm2 and 4 mm thick) inoculated with fungus was placed on a sterile slide with a coverslip43. The slide culture was placed into a 90-mm Petri dish sealed with parafilm to avoid contamination and to maintain humidity. After 7-10 days of incubation at room temperature, the mycelia were observed with a Zeiss Axio Imager A1 fluorescent microscope (Carl Zeiss, Germany) with AxioVision 4.6 software to confirm the expression of each of the three fluorescent proteins. Three filter sets were used as follows: (i) excitation at 460-480 nm and emission at 505-530 nm for ZsGreen; (ii) excitation at 540-552 nm and emission at 575-640 nm for AsRed; and (iii) excitation at 365 nm and emission at 420-470 nm for AsCyan.
Twelve randomly selected fluorescent transformants were cultured on cellophane membranes placed on PDA plates containing hygromycin B at 25 °C for 10 days. Mycelia were harvested from the cellophane, frozen in liquid nitrogen and ground to a fine powder. Fungal DNA was extracted using the CTAB method and purified using a Wizard® Genomic DNA Purification Kit (Promega, USA) according to the manufacturer’s instruction. The primers hph-F (5’-GAGCCTGACCTATTGCATCTC) and hph-R (5’-CCGTCAACCAAGCTCTGATAG) were used for PCR analysis to confirm the presence of the integrated hph gene following the protocol recommended by White and Chen44, with an initial denaturation step at 95 °C for 5 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 72 °C for 30 s and a final elongation step at 72 °C for 10 min. PCR products were separated in a 1% agarose gel in TAE buffer. The D2000 DNA ladder (TIANGEN Biotech, China) was used as a size marker.
Evaluation of transformant stability and capacity to parasitize nematodes
Single-spore purified transformants were cultured on PDA plates without hygromycin B for at least five generations, and they were then evaluated for the stable production of individual fluorescent proteins.
To assess the pathogenicity of the selected transformants, wt strains and their transformants were inoculated on PDA plates at 25 °C. Individual conidial suspensions were prepared by washing 2-week-old colonies with sterilized 0.1% Tween-20 solution. The number of conidia was determined using a hemacytometer, and the conidial concentration was adjusted to 2×105 spores/ml. An aliquot of the conidial suspension (200 μl) was spread onto the surface of each CMA plate. The plates were left uncovered for 30 min in a laminar flow hood to allow excess water to evaporate. After 2 weeks of incubation at room temperature, approximately 300 nematodes in 100 μl of 4.5 mM KCl solution were added to the center of each plate45. After 3 days of incubation, the nematodes were washed off using 5 ml of 0.1% Tween-20 solution. The percentage of nematodes with attached conidia or of those colonized by each strain was determined by observing 100 randomly chosen nematodes from each plate using an Olympus CK 40 microscope (Olympus, Japan) at 40–200× magnifications18. Four plates were used for each strain.
Microscopic observation of infection process
To determine the effect of inoculation time on the percentage of parasitism by H. minnesotensis, 200 H. glycines J2s or C. elegans L2s were inoculated in each well of a 12-well plate containing 2-week-old fungal colonies growing on CMA at 25 °C. The nematodes were washed out with 1 ml of sterile 0.1% Tween-20 at different time points (4, 8, 16, and 24 h). The nematodes that were parasitized or possessed adhered conidia were counted.
After 4 h of incubation, the nematodes from each well of the 12-well tissue culture plates were collected using sterile water and transferred into each well of a 6-well tissue culture plate to observe parasitism and to select nematodes with a single conidium attached. One drop of 4% boiled agarose was placed on the center of a sterilized slide, and a sterilized coverslip was placed onto the agarose drop immediately and pressed down to spread the agarose46. Once the agarose was solidified, the coverslip was removed. One nematode with a single spore attached was picked up and transferred to the center of the agarose pad and covered with a coverslip. Five slides were prepared for each nematode species. The location of the nematode was marked, and three edges of the coverslip were sealed with Vaseline. Another 30 nematodes with single or multiple spores attached were picked up in the same manner and transferred to 5 different agarose pads as described above. The slides were kept in a container at a relative humidity of 98% (saturated solution of K2SO4)47 at 20 °C. The infection process was observed and photographed with a Zeiss fluorescence microscope (Axio Imager A1) with Apochromat 20× and 40× objective lenses, and AxioVision 4.6 software was used for image acquisition.
Statistical analysis
The effects of the nematode species, stages and fungal species and isolates on the percentages of parasitized nematodes were determined using analysis of variance (ANOVA). The significances of the differences between the percentages of nematodes parasitized by AS3.9869 and AS3G1 were analyzed using the t-test. The SPSS (Statistical Package for the Social Sciences) software package (SPSS 16.0) was used for all statistical analyses. The data pertaining to the infection cycles of H. minnesotensis in C. elegans L2 and H. glycines J2 were analyzed using the MeV software package (MeV 4.8.1).
Additional Information
How to cite this article: Sun, J. et al. Development of a transformation system for Hirsutella spp. and visualization of the mode of nematode infection by GFP-labeled H. minnesotensis. Sci. Rep. 5, 10477; doi: 10.1038/srep10477 (2015).
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (31430071 and 30800732) and the 973 Program (2013CB127506).
Footnotes
Author Contributions X.L., S.K. and J.Q. initiated and coordinated the project. M.X. and S.P. performed the ATMT. J.S. and M.X. characterized resulting transformants, studied the infection process and initially wrote the manuscript. X.L. and S.K. edited the manuscript.
References
- Liu X. Z. & Chen S. Y. Parasitism of Heterodera glycines by Hirsutella spp. in Minnesota soybean fields. Biol. Control 19:161–166 (2000). [Google Scholar]
- Niblack T. L., Lambert K. N. & Tylka G. L. A model plant pathogen from the kingdom animalia: Heterodera glycines, the soybean cyst nematode. Annu. Rev. Phytopathol. 44:283–303 (2006). [DOI] [PubMed] [Google Scholar]
- Wrather J. A. et al. Soybean disease loss estimates for the top 10 soybean producing countries in 1994. Plant Dis. 81, 107–117 (1997). [DOI] [PubMed] [Google Scholar]
- Sturhan D. & Schneider R. Hirsutella heteroderae, a new nematode-parasitic fungus. J. Phytopathol. 99, 105–115 (1980). [Google Scholar]
- Jaffee B. A. & Zehr E. I. Parasitism of the nematode Criconemella xenoplax by the fungus Hirsutella rhossiliensis. Phytopathology 72, 1378–1381 (1982). [Google Scholar]
- McInnis T. M. & Jaffee B. A. An assay for Hirsutella rhossiliensis spores and the importance of phialides for nematode inoculation. J Nematol. 21, 229–234 (1989). [PMC free article] [PubMed] [Google Scholar]
- Chen S.Y., Liu X. Z. & Chen F. J. Hirsutella minnesotensis sp. nov., a new pathogen of the soybean cyst nematode. Mycologia 92, 819–824 (2000). [Google Scholar]
- Xiang M. C., Yang E. C., Xiao Q. M., Liu X. Z. & Chen S. Y. Hirsutella vermicola sp nov., a new species parasitizing bacteria-feeding nematodes. Fungal Divers 22, 255–265 (2006). [Google Scholar]
- Jaffee B. A., Muldoon A. E., Anderson C. E. & Westerdahl B. B. Detection of the nematophagous fungus Hirsutella rhossiliensis in California sugarbeet fields. Biol. Control 1, 63–67 (1991). [Google Scholar]
- Tedford E. C., Jaffee B. A. & Muldoon A. E. Variability among isolates of the nematophagous fungus Hirsutella rhossiliensis. Mycol. Res. 98, 1127–1136 (1994). [Google Scholar]
- Velvis H. & Kamp P. Infection of second stage juveniles of potato cyst nematodes by the nematophagous fungus Hirsutella rhossiliensis in Dutch potato field. Nematologica 41, 617–627 (1995). [Google Scholar]
- Ma R., Liu. X. Z., Jian H. & Li S. D. Detection of Hirsutella spp. and Pasteuria sp parasitizing second-stage juveniles of Heterodera glycines in soybean fields in China. Biol. Control 33, 223–229 (2005). [Google Scholar]
- Balazy S., Wrzosek M., Sosnowska D., Tkaczuk C. & Muszewska A. Laboratory trials to infect insects and nematodes by some acaropathogenic Hirsutella strains (Mycota : Clavicipitaceous anamorphs). J. Invertebr. Pathol. 97, 103–113 (2008). [DOI] [PubMed] [Google Scholar]
- Liu S. F. & Chen S. Y. Nematode hosts of the fungus Hirsutella minnesotensis. Phytopathology 91, S138 (2001). [Google Scholar]
- Xiang M. C. et al. Variability of morphology, parasitism, and nucleotide sequences among isolates and species of nematophagous Hirsutella. Biol. Control 41, 110–119 (2007). [Google Scholar]
- Liu X. Z., Li J. Q. & Zhang D. S. History and status of soybean cyst nematode in China. Int. J. Nemat . 7, 18–25 (1997). [Google Scholar]
- Liu X. Z. & Chen S. Y. Screening isolates of Hirsutella species for biocontrol of Heterodera glycines. Biocontrol Sci. Techn. 11, 151–160 (2001). [Google Scholar]
- Chen S. Y. & Liu X. Z. Control of the soybean cyst nematode by the fungi Hirsutella rhossiliensis and Hirsutella minnesotensis in greenhouse studies. Biol. Control 32, 208–219 (2005). [Google Scholar]
- Xiang M. C., Xiang P. A., Jiang X. Z., Duan W. J. & Liu X. Z. Detection and quantification of the nematophagous fungus Hirsutella minnesotensis in soil with real-time PCR. Appl. Soil Ecol. 44, 170–175 (2010). [Google Scholar]
- Liu S. F. & Chen S. Y. Efficacy of the fungi Hirsutella minnesotensis and H. rhossiliensis from liquid culture for control of the soybean cyst nematode Heterodera glycines. Nematology 7, 149–157 (2005). [Google Scholar]
- Kamath R. S. et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421, 231–237 (2003). [DOI] [PubMed] [Google Scholar]
- Sun J. Z., Xie H. Y., Qiu J. Z., Liu X. Z. & Xiang M. C. Parasitism of secondary-stage juvenile of Heterodera glycines and four larva stages of Caenorhabditis elegans by Hirsutella spp. Exp. Parasitol. 135, 96–101 (2013). [DOI] [PubMed] [Google Scholar]
- Atkins S. D., Mauchline T. H., Kerry B. R. & Hirsch P. R. Development of a transformation system for the nematophagous fungus Pochonia chlamydosporia. Mycol. Res. 108, 654–661 (2004). [DOI] [PubMed] [Google Scholar]
- Cantone F. A. & Vandenberg J. D. Use of the green fluorescent protein for investigations of Paecilomyces fumosoroseus in insect hosts. J. Invertebr. Pathol. 74, 193–197 (1999). [DOI] [PubMed] [Google Scholar]
- Czymmek K. et al. In vivo time-lapse documentation using confocal and multi-photon microscopy reveals the mechanisms of invasion into the Arabidopsis root vascular system by Fusarium oxysporum. Fungal Genet. Biol. 44, 1011–1023 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl. Microbiol. Biotechnol. 78, 983–990 (2008). [DOI] [PubMed] [Google Scholar]
- Jansson H. B., Jeyaprakash A. & Zuckerman B. M. Differential adhesion and infection of nematodes by the endoparasitic fungus Meria coniospora (Deuteromycetes). Appl. Environ. Microb. 49, 552–555 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliego C. et al. GFP sheds light on the infection process of avocado roots by Rosellinia necatrix. Fungal Genet Biol. 46, 137–145 (2009). [DOI] [PubMed] [Google Scholar]
- Yu H. Y., Wei X. & Gao X. X. Development of a transformation method for the nematophagous fungus Dactylellina cionopaga. Afr. J. Biotechnol. 11, 3370–3378 (2012). [Google Scholar]
- Kerr R. et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26, 583–594 (2000). [DOI] [PubMed] [Google Scholar]
- Denbelder E. & Jansen E. Saprophytic and predacious abilities in Arthrobotrys oligospora in relation to dead and living root-knot nematodes. Fund. Appl. Nematol. 17, 423–431 (1994). [Google Scholar]
- Watson H., Lee D. L. & Hudson P. J. The effect of Trichostrongylus tenuis on the caecal mucosa of young, old and anthelmintic-treated wild red grouse, Lagopus lagopus scoticus. Parasitology 94, 405–411 (1987). [DOI] [PubMed] [Google Scholar]
- Chung K. H., Crane M. M. & Lu H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 5, 637–643 (2008). [DOI] [PubMed] [Google Scholar]
- Xiang M.C., Xiang P. A., Liu X. Z. & Zhang L. M. Effect of environment on the abundance and activity of the nematophagous fungus Hirsutella minnesotensis in soil. FEMS Microbiol. Ecol. 71, 413–417 (2010). [DOI] [PubMed] [Google Scholar]
- Powell J. & Ausubel F. Models of Caenorhabditis elegans infection by bacterial and fungal pathogens in Innate Immunity (eds. Ewbank J. & Vivier E.) 403–427 (Humana, 2008). [DOI] [PubMed] [Google Scholar]
- Kim D. H. et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297, 623–626 (2002). [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Ayyadevara S., Mcewen J. E. & Reis R. J. S. Histoplasma capsulatum and Caenorhabditis elegans: a simple nematode model for an innate immune response to fungal infection. Med. Mycol. 47, 808–813 (2009). [DOI] [PubMed] [Google Scholar]
- Pukkila-Worley R., Ausubel F. M. & Mylonakis E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 7, e1002074 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y. L. et al. Comparative genomics and transcriptomics analyses reveal divergent lifestyle features of nematode endoparasitic fungus Hirsutella minnesotensis. Genome Biol. Evol . 6, 3077–3093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khang C. H., Park S. Y., Rho H. S., Lee Y. H. & Kang S. Filamentous fungi (Magnaporthe grisea and Fusarium oxysporum) in Agrobacterium Protocols (ed .Wang K.) 403–420 (Humana, 2007). [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Ceanorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. Y. et al. Trap induction and trapping in eight nematode-trapping fungi (Orbiliaceae) as affected by juvenile stage of Caenorhabditis elegans. Mycopathol. 169, 467–473 (2010). [DOI] [PubMed] [Google Scholar]
- Coetzee J. C. & Eicker A. A simple slide culture technique facilitating the viewing of growing fungi. Phytophylact. 22, 361–362 (1990). [Google Scholar]
- White D. & Chen W. Genetic transformation of Ascochyta rabiei using Agrobacterium-mediated transformation. Curr. Genet. 49, 272–280 (2006). [DOI] [PubMed] [Google Scholar]
- Jaffee B. A. & Zehr E. I. Sporulation of the fungus Hirsutella rhossiliensis from the nematode Criconemella xenoplax. Plant Dis. 67, 1265–1267 (1983). [Google Scholar]
- Schwartz H. T. A protocol describing pharynx counts and a review of other assays of apoptotic cell death in the nematode worm Caenorhabditis elegans. Nat Protoc. 2, 705–714 (2007). [DOI] [PubMed] [Google Scholar]
- Winston P. W. & Bates D. H. Saturated solutions for the control of humidity in biological-research. Ecology 41, 232–237 (1960). [Google Scholar]