Abstract
The gastrointestinal (GI) tract senses the ingestion of food and responds by signaling to the brain to promote satiation and satiety. Representing an important part of the gut–brain axis, enteroendocrine L-cells secrete the anorectic peptide hormones glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) in response to the ingestion of food. The release of GLP-1 has multiple effects, including the secretion of insulin from pancreatic β-cells, decreased gastric emptying, and increased satiation. PYY also slows GI motility and reduces food intake. At least part of the gut–brain response seems to be due to direct sensing of macronutrients by L-cells, by mechanisms including specific nutrient-sensing receptors. Such receptors may represent possible pathways to target to decrease appetite and increase energy expenditure. Designing drugs or functional foods to exploit the machinery of these nutrient-sensing mechanisms may offer a potential approach for agents to treat obesity and metabolic disease.
Keywords: enteroendocrine, appetite, glucagon-like peptide-1, peptide YY, macronutrient
Introduction
The gastrointestinal (GI) tract represents the largest endocrine organ in the human body. Enteroendocrine cells (EECs) are located throughout the GI tract, constituting <1% of the cell population in the intestinal epithelium, but playing critical physiological roles and representing an important component of the gut–brain axis (1). At least 15 types of EEC have been described, capable of secreting over 20 peptide hormones that influence processes including gut motility, gastric acid secretion, and energy intake. It was previously thought that EECs could be separated into discrete classes of cells with specific secretory profiles. Examples of previously characterized cell families include gastrin-secreting G-cells, cholecystokinin (CCK)-secreting I-cells, glucagon-like peptide (GLP-1), and peptide YY (PYY)-secreting L-cells, among others (2). However, recent work has suggested that EEC families may be less well defined, with EECs existing as a wide range of cell types that secrete various combinations of different peptides (3).
Nervous and endocrine signaling between the gut and the brain allows the modulation of GI functions to increase the efficiency of digestion, and the communication of energy and nutritional requirements to the brain to influence appetite. One key function of EECs is to sense luminal contents, which will then modulate their release of hormones that regulate food intake. To achieve this, open-type cells often have a distinct cone-shaped morphology with one extremity adjacent to the basal lamina and the other possessing microvilli on apical processes (Figure 1). Microvilli are thus in immediate contact with the luminal contents, sensing of which can lead to the release of hormones from secretory granules directly into the nearby blood vessels (4). G-protein coupled receptors (GPCRs) represent over one-third of therapeutic drug targets, and a number detect dietary components. When the products of food breakdown move through the GI tract, specific macronutrients stimulate the chemosensors of a variety of GPCRs. This leads to the modulation of gut hormone release, which will influence neuronal signaling in appetite centers in the brain to mediate the appropriate feeding behavior, by, for example, the termination of hunger and the induction of satiety (5). In contrast, closed-type EECs do not come into contact with luminal nutrients, and instead react to neural or circulating signals, though they also play a role in the regulation of food intake. In addition, the hormones released from EECs can have paracrine effects on nearby cells, including neurones. Recent evidence also suggests that EECs interact directly with neurones via synapse-like structures named neuropods (6, 7).
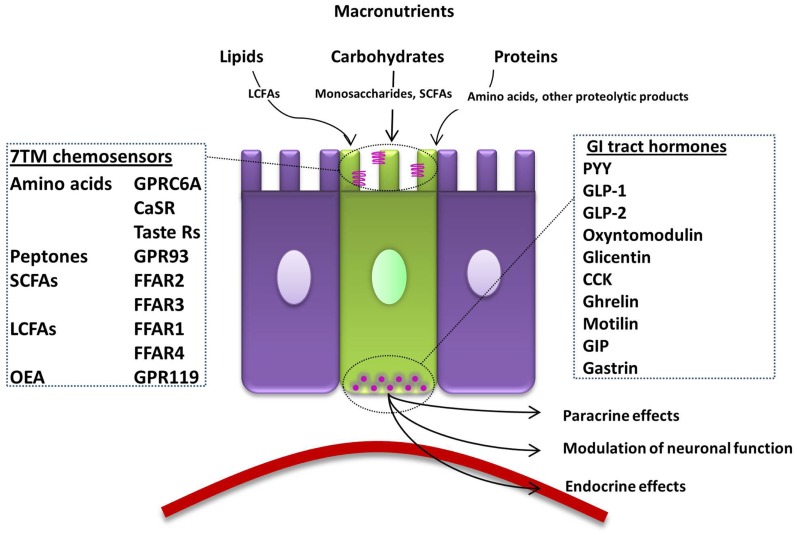
Figure 1.
Summary of the macronutrient sensing receptors expressed in enteroendocrine cells of the gut and the hormones they release. An open-type enteroendocrine cell possesses microvilli extending into the gut lumen, coming into direct contact with macronutrients. Food components are sensed by various GPCRs and transporters located on the apical border. GI tract hormones are released into the circulation, acting via paracrine, endocrine, and neural pathways to modulate food intake. LCFA; long-chain fatty acid, 7TM; 7-transmembrane, CaSR; calcium-sensing receptor, GPRC6A; G-protein coupled receptor family C group 6 subtype A, GPR93; G-protein coupled receptor 93, SCFA; short-chain fatty acid, FFAR; free-fatty-acid receptor, OEA; oleoylethanolamide, GPR119; G-protein coupled receptor 119, GI; gastrointestinal, PYY; peptide YY, GLP; glucagon-like peptide, CCK; cholecystokinin, GIP; gastric inhibitory peptide.
The human body stores excess energy intake as adipose tissue. The present obesity pandemic is a major global health issue, which has arisen due to the abundance of highly palatable, calorie-dense food combined with reduced levels of physical activity. The pharmacological agents for weight loss currently available are only modestly effective. Having previously been advocated for use in the morbidly obese, bariatric surgery is now recommended for obese patients with type II diabetes in UK (8). However, the number of patients that now qualify suggests that this is an impractical approach to dealing with obesity. Targeting gut hormone receptors to decrease appetite and increase energy expenditure is a major area of interest for the management of body weight (9–11), and the GLP-1 receptor agonist Liraglutide (Saxenda) has recently been approved by the U.S. Food and Drug Administration as a treatment for obesity (12). However, the formulation of foodstuffs, which contain targeted nutraceuticals to exploit the various nutrient-sensing systems, present on EECs represents another possible approach to the treatment of obesity, which might avoid the problems of administration, nausea, and tachyphylaxis that have been associated with gut hormone administration (Table 1) (13–15).
Table 1.
A summary of potential targets for the treatment of obesity and their mechanisms.
| Potential targets | Mechanism | Reference |
|---|---|---|
| Oxyntomodulin | GLP-1 and glucagon receptor agonism | (10, 16) |
| Peptide YY | Y2R agonism modulates central anorectic pathways and influences ileal brake | (17, 18) |
| Dietary supplementation with glutamine and l-arginine | Ingested glutamine and l-arginine potentiate the release of GLP-1 and PYY, via activation of AMPK and mTOR | (19–22) |
| Calcium-sensing receptor | Activation by specific l-amino acids stimulates the secretion of GLP-1 and PYY | (23) |
| G-protein coupled receptor 93 | Protein hydrolyzates stimulate the release of CCK | (24) |
| G-protein coupled receptor, class C, group 6, subtype A | Activation by specific l-amino acids stimulates the secretion of GLP-1 | (25, 26) |
| Sodium-glucose transporter 1 | Transport of ingested glucose into enterocytes stimulates the secretion of GLP-1 | (27) |
| Free fatty acid receptor 2 and 3 | Activation by short-chain fatty acids may stimulate the secretion of GLP-1 and PYY and inhibit gastrointestinal motility | (28–30) |
| Free fatty acid receptor 1 and 4 | Activation by medium and long-chain fatty acids stimulates the secretion of GLP-1 | (31–33) |
The Hormonal Products of L-Cells
Gastric distension and the release of upper-intestinal tract hormones, such as CCK from I-cells, trigger short-term satiation processes in the upper GI tract (4). However, longer-term satiety is likely driven by other mechanisms, which may include the direct sensing of nutrients leading to the release of anorectic gut hormones from enteroendocrine L-cells. Mature L-cells are commonly defined as EECs that express the preproglucagon gene. Posttranslational processing of preproglucagon is tissue-specific, and hence, yields different hormonal products in the pancreas and intestine. L-cells, which have traditionally been described as having a distinct cone-shaped morphology, secrete the products of cleavage by prohormone convertase 1; GLP-1, glucagon-like peptide 2 (GLP-2), glicentin, and oxyntomodulin (34–36). EECs have been suggested to express an overlapping spectrum of hormones dependent on spatial distribution and exposure to nutrients (3, 37). Immunostaining and fluorescence-activated cell sorted analysis (FACS) have revealed that L-cells co-secrete distinct peptides depending on their location. L-cells in the upper small intestine demonstrate co-localization with gastric inhibitory peptide (GIP), and thus, bear some resemblance to neighboring K-cells, while L-cells in the lower small intestine show high levels of co-localization with PYY and CCK. The GLP-1 and PYY co-expressing L-cells are typically considered to be involved in the regulation of energy homeostasis, in addition to other functions. GLP-1 mediates its effects via the GLP-1 receptor. PYY exists in two major circulating forms: the full-length peptide, PYY1–36, and a truncated form, PYY3–36. Full-length PYY acts on Y family receptors Y1, Y2, and Y5, whereas PYY3–36 is relatively selective for the Y2 receptor (38, 39).
L-cells that co-express GLP-1 and PYY are located along much of the length of the GI epithelium, starting at the proximal jejunum and increasing in density along the small intestine and then the large intestine. Thus, the contact of ingested nutrients with L-cells increases along the GI tract (34, 40). GLP-1 and PYY exhibit a two-phase release profile. The initial rapid rise in GLP-1 may partially represent release from L-cells in the upper small intestine. However, it is thought that most of this first phase response for GLP-1 and that of PYY is mediated via a neural reflex or a circulating factor (41, 42). The arrival of food in the distal gut is thought to drive the second phase of the release of GLP-1 and PYY into the circulation, by activation of specific nutrient receptors and other cellular machinery present on apical cell processes (43, 44).
Hormonal Products of L-Cells: Peripheral Effects
L-cell-secreted GLP-1 and PYY diffuse into the lamina propria and enter the systemic circulation via the hepatic portal vein. Systemic circulating levels of both hormones rise within 15 min of food ingestion in humans, with levels approximately proportional to the calories ingested. Following a mixed meal, plasma concentrations of GLP-1 and PYY peak at around 40 and 90 min, respectively, then reach a plateau (45, 46). GLP-1 and PYY circulate at basal levels in the fasting state, with concentrations rising rapidly postprandially; an effect that seems to be larger in humans than in rodents (41, 47–50). Following release, both GLP-1 and PYY undergo enzymatic cleavage in the intestinal endothelium and liver by dipeptidyl peptidase IV (DPPIV), which converts GLP-1 to an inactive form, and truncates PYY1–36 to its Y2 selective form, PYY3–36.
Glucagon-like peptide-1
The release of GLP-1 has several peripheral consequences, the most notable being its incretin effect. GLP-1 and GIP are reported to bind their respective receptors on β-cells in the pancreas in response to glucose (Figure 2). This leads to an increase in the concentration of intra-cellular calcium, and consequent exocytosis of insulin-containing vesicles (51). Glicentin has a similar but less potent effect, though a glicentin-specific receptor remains to be identified (52, 53). However, there is some controversy surrounding the incretin role of GLP-1 due to the relatively small increase in circulating GLP-1 observed postprandially, and the short lifespan of the peptide (54). Specific knockdown of Glp1r in the pancreatic β-cells of mice impairs glucose tolerance in response to hyperglycaemmia, and attenuates insulin secretion in response to exogenous GLP-1. However, a DPPIV inhibitor retained its glucose lowering effects in these mice, suggesting that the GLP-1R on the beta cell is not necessary for these effects, and that perhaps extra-islet receptors are responsible for the incretin effects of GLP-1. However, this may also reflect compensatory action by other DPPIV substrates (55).
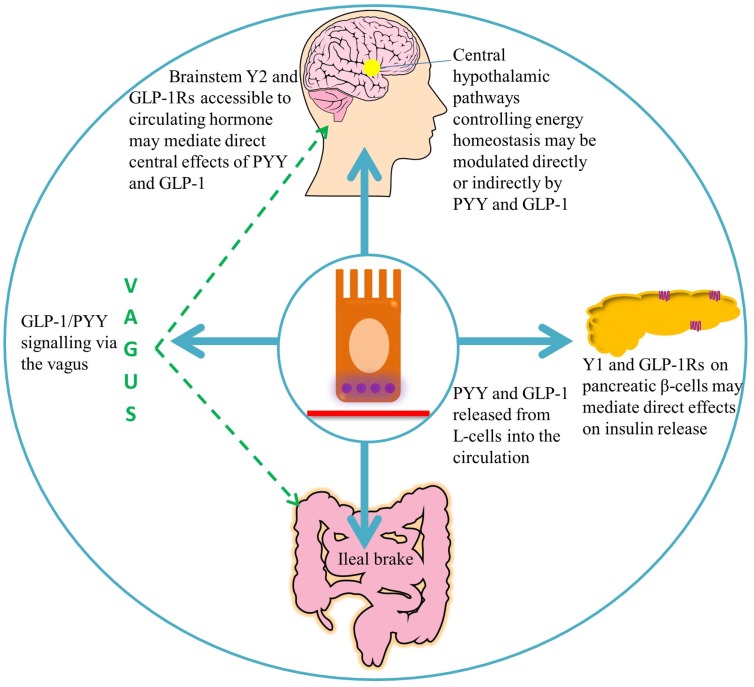
Figure 2.
A summary of the target areas of L-cell secreted PYY and GLP-1. Following nutrient ingestion, PYY and GLP-1 diffuse into the lamina propria and enter the circulation. GLP-1 binds its receptors on pancreatic β-cells, leading to insulin secretion (51). Full-length PYY binds pancreatic Y1 receptors and inhibits glucose-stimulated insulin secretion, while PYY3–36 may exert effects on glucose homeostasis via extra-islet Y2 receptors (56, 57). Circulating hormones are able to access areas of the hindbrain with a leaky blood–brain barrier, such as the area postrema, which communicates with the nucleus of the solitary tract (58). GLP-1 and PYY3–36 may signal via the vagus to central hypothalamic nuclei controlling energy homeostasis, where receptors for these hormones are widely expressed (46, 59–61).
In addition to acute effects, GLP-1 exhibits trophic effects on β-cells (62–65); a 2-day infusion of GLP-1 increased markers of proliferation, and decreased markers of apoptosis in the pancreas of Zucker diabetic rats (Figure 3) (66). This is thought to be mediated by the activation of transcription factors that result in enhanced proliferation and differentiation, and inhibition of apoptosis, thereby promoting islet growth (63, 66).
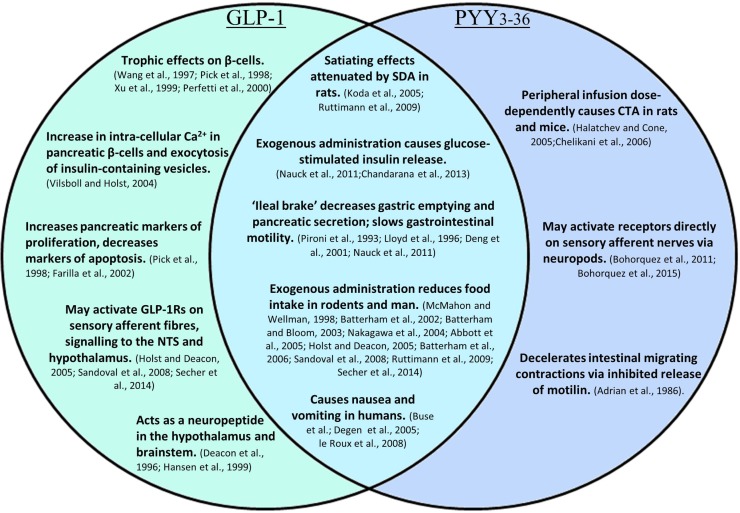
Figure 3.
A summary of the effects of GLP-1, PYY3–36, and the combined effects of both.
Vagal nervous activity can reduce gastric emptying, thus, slowing nutrient absorption. The slowing of gastric emptying by GLP-1 is subject to rapid tachyphylaxis following chronic exposure, likely at the level of the vagus nerve. In human, subjects given a continuous intravenous infusion of GLP-1, post-prandial concentrations of glucose, glucagon, and insulin progressively increased in subsequent meals. Therefore, it is possible that part of the glycemic control afforded by administration of GLP-1 occurs secondary to delayed gastric emptying (14). The related peptide, GLP-2, is co-secreted with GLP-1, and contributes to optimizing the local environment for nutrient absorption. GLP-2 induces crypt cell proliferation, while also preventing the apoptosis of intestinal cells and increasing GI blood flow (67, 68). Peripheral administration of GLP-2 also increases the expression of cellular transport machinery involved in glucose absorption in the rat jejunum, such as the sodium-glucose transporter 1 (SGLT-1) and glucose transporter 2 (GLUT2) (69, 70).
Originally demonstrated by an infusion of corn oil, administration of carbohydrate, lipid, and protein directly into the ileum of humans also stimulates the “ileal brake,” which results in the secretion of PYY and GLP-1, reduced food intake, and satiation (71–73). The ileal break is a feedback mechanism initiated by the presence of unabsorbed dietary components in the ileum, and acts to slow more proximal GI motility in order to allow efficient digestion and uptake of nutrients. GLP-1 and PYY appear to be important components of this system (Figure 2). Exogenous administration of PYY3–36 or GLP-1 decreases gastric emptying and pancreatic secretion (14, 18, 74) and decreases the speed of intestinal transit, an effect, which may partly be due to the inhibited release of motilin by PYY, and a consequent decrease in intestinal migrating contractions (17, 75). However, activation of Y1 receptors by PYY1–36 tonically accelerates colonic transit, an effect, which is attenuated by antagonism of this receptor (76), and which is perhaps designed to empty the colon to allow it to deal with the nutrients coming down the small intestine. Binding of PYY1–36 to the Y1 inhibitory receptor subtype in the gastric mucosa also regulates the function of gastric endocrine cells, inhibiting vagally stimulated gastric acid secretion (77).
Peptide YY
In addition to appetite regulation, PYY may regulate pancreatic islet function to regulate glucose homeostasis. Full-length PYY1–36 inhibits glucose-stimulated insulin secretion from murine islets, an effect thought to be mediated via neuropeptide Y (NPY) Y1 receptors. In accord with this, Pyy-null mice, or islets in which the Y1 receptor is absent, hypersecrete insulin (56, 57). The absence of the Y2 receptor in human and murine pancreatic islets corresponds to the lack of a direct effect of PYY3–36 on glucose-stimulated insulin secretion (78). However, the peripheral administration of exogenous PYY3–36 or Y2R agonist, administered either in the fed state or in combination with glucose, improved glucose tolerance, and stimulated insulin secretion in mice. This effect was abolished by the administration of a peripheral Y2R antagonist, implying that PYY3–36 exerts its effects on glucose homeostasis via extra-islet Y2 receptors in a nutrient-dependent manner (57). Endogenous PYY3–36 may act via the Y2R to increase hepato-portal GLP-1, as the effect was attenuated by administration of the GLP-1R antagonist, Exendin9-39 (57). However, a combined intravenous infusion of PYY3–36 and GLP-1 to overweight subjects increased first phase insulin secretion to the same level as GLP-1 alone; thus, the addition of PYY3–36 had no additive effects at the doses tested in an acute setting (79).
Oxyntomodulin
Oxyntomodulin, a longer isoform of glucagon, is a dual agonist of the GLP-1 and glucagon receptors, though with a 10- to 100-fold lower affinity than the native ligands (16, 80, 81). Unsurprisingly, the effects of oxyntomodulin combine those of GLP-1 and glucagon. Centrally injected oxyntomodulin reduces food intake to the same magnitude as GLP-1, despite a much lower receptor affinity (16). Exogenous administration reduces food intake in rodents and humans, an effect mediated by the GLP-1 receptor, and also modulates gastric acid and exocrine pancreatic secretion (82–84). Chronic pre-prandial administration of oxyntomodulin drove significant weight loss in overweight and obese human subjects (10). Studies in pair-fed rats suggested that, in addition to decreased energy intake, oxyntomodulin-stimulated weight loss may be the result of increased energy expenditure (84). Subsequent human data confirmed that oxyntomodulin increased activity-related energy expenditure, though not resting energy expenditure, following pre-prandial subcutaneous self-administration in overweight subjects (10). The beneficial actions of oxyntomodulin on food intake, energy expenditure, and body weight place it at the forefront of peptides of interest in the treatment of obesity. Modifying the N-terminus of oxyntomodulin, which confers resistance to enzymatic degradation by DPPIV, has shown significant therapeutic potential in terms of improved glycemic control and appetite suppression (85, 86).
Hormonal Products of L-Cells: The Gut–Brain Axis
A number of brain regions are involved in the central regulation of energy homeostasis, including the hypothalamus, the cortex, the limbic system, and the brain stem (87). The hypothalamus is divided into distinct nuclei that co-ordinate orexigenic and anorexigenic signals. The arcuate nucleus (ARC) is located proximate to the median eminence, an area with an incomplete blood–brain barrier, and is therefore in contact with circulating factors, including PYY and GLP-1. Within the ARC two distinct sets of neurons have opposing effects on appetite. Activation of pro-opio melanocortin (POMC) neurons inhibits appetite, while activation of NPY/agouti-related peptide (AgRP) neurons stimulates appetite (88–91). These neuronal populations project to intra- and extra-hypothalamic regions to regulate energy homeostasis. The nucleus of the solitary tract (NTS) in the brainstem receives input from the periphery via vagal afferent fibers, transmitting information, such as gastric distension, ingested dietary composition, and water content. Vagal efferent fibers are located in the dorsal motor nucleus, which is located ventral to the NTS in the caudal brainstem (92). These brain regions are critical to the control of energy homeostasis, and are thus the target regions for central pharmacological manipulation, with the hope that drugs acting specifically in these areas will display fewer unwanted side-affects.
GLP-1 in the gut–brain axis
The rapid breakdown of GLP-1 results in a half-life of approximately 2 min. Concentrations of GLP-1 are therefore highest in the intestinal submucosa, decreasing in the hepatic portal vein and systemic circulation. An estimated 15% of active GLP-1 secreted from the porcine intestine reaches the systemic circulation, before it is also degraded by DPPIV (93). Consequently, there is debate as to whether physiologically relevant levels in men are able to reach central GLP-1 receptors in the hypothalamus and brainstem, where centrally produced GLP-1 is also thought to act as a neuropeptide (94, 95). It has been suggested that peripheral GLP-1 may act via a neural rather than an endocrine route, activating receptors near its site of release before the peptide encounters endothelial DPPIV. Receptors for GLP-1 are located on enteric neurons that exhibit increased action potential firing in primary culture following the application of GLP-1, which may, for example, inhibit local muscle tone as part of the ileal brake (96). Receptors on sensory afferent fibers of the nodose ganglion may also be activated, relaying impulses to central regions important in energy homeostasis, such as the NTS and the hypothalamus (93). Expression of the GLP-1 receptor in neuronal cells of the ganglion has been confirmed by Nakagawa et al., who also demonstrated that intraportal injection of physiological levels of GLP-1 increased afferent signaling of the rat hepatic vagus, providing evidence for vagal chemoreception of peripheral GLP-1 (96, 97). Interestingly, rats that had undergone subdiaphragmatic vagal deafferation were less sensitive to the anorectic effects of intraperitoneally administered GLP-1, whereas the effects of GLP-1 administered into the vena cava and hepatic portal vein were not affected over a wide range of doses. This suggests that while intraperitoneal GLP-1 requires abdominal vagal afferent signaling to exert its anorectic effects, circulating GLP-1 mediates these effects via an alternative mechanism (Figure 3) (98). It has been suggested that exogenous intraperitoneal GLP-1 acts in a similar way to endogenous L-cell-secreted GLP-1, acting in a paracrine fashion, before DPPIV denatures the peptide within the capillary walls (96, 98).
As the satiating effects of intravenous GLP-1 are unaffected by vagotomy, it is possible that GLP-1 administered in this way is acting directly at central GLP-1 receptors (98, 99). Circulating GLP-1 is able to diffuse across the fenestrated capillaries of the circumventricular organs, binding receptors in the subfornical organ and area postrema (AP) (58). The AP is positioned in close proximity to the NTS, to which it communicates nutritional information. The AP also has efferent and afferent connections to the hypothalamus, allowing it to moderate feeding in response to the nutritional demands (58, 100). The GLP-1 receptor is widely expressed throughout the hypothalamus, with the highest expression in the PVN and ARC (60, 61). This coincides with dense connections from the NTS, which also expresses preproglucagon, highlighting the importance of the brainstem–hypothalamic pathway in GLP-1 signaling (60). The PVN is thought to be the primary mediator of brain-derived GLP-1-induced satiation, as direct injection into this nuclei elicits a robust anorectic response (101, 102). GLP-1 may also elicit its effects on food intake via the ARC, with some POMC neurons expressing the GLP-1 receptor (102). Indeed, evidence suggests that peripheral administration of liraglutide, a long-acting GLP-1 analog, acts on ARC POMC neurons to drive weight loss (61). The central expression of preproglucagon is highly conserved between rodents and non-human primates. Mapping of the GLP-1 receptor in the non-human primate brain is consistent with the functional role of GLP-1, with the most abundant expression in areas controlling energy homeostasis, specifically the hypothalamic and brainstem regions mentioned above. Interestingly, a higher level of expression of the GLP-1 receptor was present in the amygdala of the primate brain compared with the rodent brain, indicating a possible species difference in GLP-1 signaling in this region (103).
PYY in the gut–brain axis
Peptide YY has a half-life of approximately 10 min in humans, though plasma levels remain increased for up to 6 h postprandially due to sustained release (46, 104). The Y5 receptor is expressed throughout the brain, though most densely in the hypothalamus, where it is co-localized with the Y1 receptor. The Y5 receptor mediates the effects of NPY-induced food intake, with selective agonism of this receptor, resulting in stimulation of feeding (105). However, full-length PYY1–36 does not appear to influence appetite when administered peripherally, suggesting it is unable to access these orexigenic receptors (106).
The most notable role of PYY3–36 is as an anorectic peptide. Acute peripheral administration of PYY3–36 to rodents or humans reduces food intake (46, 107). Intermittent exogenous administration of PYY3–36 reduces food intake, body weight, and adiposity in rats, and prevents weight gain in diet-induced obese rats (108, 109). However, the primary mechanism by which PYY3–36 reduces food intake is unclear. It appears to be mediated by Y2 receptors; the anorectic effect of PYY is absent in mice with targeted gene deletion of the Y2R (46, 107). Initial studies found that PYY increased POMC mRNA and induced electrophysiological activation of these neurons. It was suggested that the anorectic effects were due to activation of pre-synaptic inhibitory Y2 receptors on arcuate NPY/AgRP neurons, resulting is a reduced inhibition of POMC neurons by the inhibitory neurotransmitter, γ-aminobutyric acid (GABA) (107). However, subsequent studies revealed that mice lacking functional POMC or MC4R were as susceptible as wild-type mice to the acute anorectic effects of peripheral PYY3–36 administration (110, 111). This indicates that although PYY3–36 may stimulate melanocortin production, melanocortin signaling is not necessary for its anorectic effects.
It has also been suggested that PYY3–36 acts to reduce food intake by signaling via peripheral neurons. The Y2R has been located on vagal afferent fibers, however, conflicting evidence has been found as to whether PYY3–36 requires an intact vagus to signal a reduction in food intake. Total subdiaphragmatic vagotomy did not attenuate the reduction in food intake compared to sham operated mice, and instead was found to prolong the anorectic effects of PYY3–36. Hence, vagal tone may modulate the duration of action of intestinally secreted PYY3–36, but may not be required for short-term signaling (59). However, in a separate study, bilateral subdiaphragmatic vagotomy abolished both the anorectic effects of PYY3–36 and its ability to induce Fos expression in the ARC of rats following peripheral administration, indicating blockade of hypothalamic activation (112). Interestingly, a novel mechanism by which PYY-releasing cells may directly influence neuronal function has recently been proposed. PYY-expressing EECs were found to exhibit long cytoplasmic processes with some characteristics of neuronal processes, named neuropods, which directly contact enteric neurons (Figure 3). Culturing PYY-expressing EECs isolated by FACS with sensory neurons resulted in the development of neuropods, which contacted the neuronal neurites, from where a putative axon develops. These EEC–neuron connections suggest that L-cells may have the ability to directly participate in the transmission of sensory signals from the gut lumen (6, 7).
It has been proposed that PYY3–36 induces hypophagia by causing non-specific malaise. Conditioned taste aversion (CTA) protocols are commonly used as a paradigm for nausea in rodents, which lack the necessary anatomy for vomiting. Infusions of PYY3–36 have been dose-dependently associated with CTA in rats and mice, thought in part to be due to inhibition of gastric emptying (59, 113). A similar dose-dependent effect on nausea and abdominal discomfort has been found following exogenous administration of PYY3–36 in humans (49). The aversive effects of this peptide potentially limit its therapeutic use as an anti-obesity agent. However, interestingly, there is little connection between the nausea experienced and the level of food intake inhibition, suggesting that perhaps the nausea occurs at a threshold level, but is unconnected to the physiological satiating effects of PYY3–36 (13).
Nutrient-Sensing by L-Cells
Currently, the most effective treatment for obesity is bariatric surgery; the Roux-en Y-Gastric bypass is the most commonly performed procedure, and results in sustained weight loss, though the popularity of the vertical sleeve gastrectomy is increasing (114). In healthy individuals, the post-prandial response involves a complex cocktail of hormones, their release reflecting the ingested and absorbed macronutrients (115). The post-prandial GLP-1 and PYY response is reported to be blunted in obese patients (116). However, following bariatric surgery, patients exhibit increased post-prandial levels of anorectic gut hormones GLP-1 and PYY, and attenuated levels of ghrelin, an orexigenic hormone released from the stomach (117). In addition, amelioration of type 2 diabetes frequently occurs within days of surgery. It has been widely postulated that altered post-prandial gut hormone levels may be responsible for at least some of the metabolic effects of bypass surgery. EECs are therefore a key area of interest in research into alternatives to bariatric surgery. Changes in intestinal morphology, in particular villus hyperplasia, have been implicated in the adaptive response following Roux-en Y-Gastric bypass and ileal interposition in rats (118, 119). A shift from absorptive to more secretory cell lineages, such as goblet cells, has also been observed (119, 120). In rats, villus proliferation occurs following the implantation of a duodenal–endoluminal sleeve, a device that acts as a physical barrier between nutrients and absorptive tissue (121). Increased villus length and surface area in these studies are associated with beneficial metabolic changes, including improved glucose homeostasis and increased post-prandial GLP-1 secretion (122). Exploiting the mechanisms by which various dietary macronutrients activate GPCRs on L-cells, and hence, the release of endogenous GLP-1 and PYY represents a possible therapeutic target. It is thus important to understand the specific mechanisms by which L-cells sense different types of nutrients (23).
Receptors on the apical surface of open-type L-cells directly sense dietary components in the intestinal lumen, and respond to produce the appropriate endocrine response (Figure 1). The contents of the intestinal lumen vary considerably with diet, requiring a number of specific receptors to detect the different macronutrients that modulate the secretion of hormones. Secretion of GLP-1 and PYY by L-cells is preceded by raised intracellular calcium and cyclic adenosine monophosphate (cAMP) levels. Calcium is released from intracellular stores following membrane depolarization due to increased sodium-dependent cell excitability and calcium influx, leading to the exocytosis of hormone-containing vesicles. This signal is potentiated downstream by intracellular cAMP, which is increased by the action of Gs protein coupled receptors, further augmenting hormone release (20, 123). Recently, super-resolution microscopy has demonstrated that secretory vesicles from L-cells of the mouse, rat, pig, and human contain primarily either GLP-1 or PYY. This raises the possibility that either hormone may be selectively released from a microdomain of a single EEC (124). A specific combination of cellular machinery may need to be activated in the L-cell to cause the differential release of GLP-1 or PYY. However, how these signals are integrated remains to be elucidated.
Protein-Sensing Receptors
Increasing dietary protein content by 10–15% promotes satiety, reduces overall calorie intake, and produces sustained weight loss in rodents and man, possibly due in part to the induced changes in circulating gut hormones (125–127). In both healthy and obese subjects, a high-protein meal increases plasma PYY levels significantly more than an isocaloric meal high in carbohydrate or fat, while PYY null mice are resistant to the satiating effects of protein (128). Chronic exposure to a high-protein diet also elevates post-prandial GLP-1 and increases satiety levels in healthy subjects (129).
The peptone receptor
It has been shown that peptones stimulate the release of CCK from I-cells, and stimulate GLP-1 release from ex vivo rat small intestine and colon, and from STC-1 cells. Activity of the proglucagon gene promoter is also enhanced, leading to increased transcription (130). The peptone GPCR, GPR93, is highly expressed by cells of the duodenal mucosa, including L-cells. Activation of GPR93 by protein hydrolyzates, results in the transcription and release of CCK (24, 131). Peptide-transporter 1 (PEPT1) is a brush-border transporter of di- and tri-peptides, located on L-cells of the small intestine and colon. Primary murine L-cell cultures have been shown to release GLP-1 in response to peptone administration, via PEPT1-dependent electrogenic uptake and activation of the CaSR (132). Hence, the larger fragments of protein hydrolysis may directly regulate proglucagon synthesis in the gut, and influence the secretion of its posttranslational products (130).
The mu-opioid receptor
The oligopeptides produced by protein breakdown are also active at the mu-opioid receptor (MOR), an inhibitory GPCR. The MOR is present in the small intestine and brain, particularly the nucleus accumbens. Agonism and antagonism of the central MOR has been shown to increase and decrease food intake, respectively (133, 134). Duraffourd et al. demonstrated that MORs located in the walls of the portal vein respond to the products of protein digestion in vivo, to induce intestinal gluconeogenesis (135). This occurs as a result of a protein-enriched diet, and leads to detection of increased portal glucose and signaling to the hypothalamic nuclei, which regulate food intake (136). These effects are abolished following denervation of the portal vein, providing a plausible link between the assimilation of dietary protein in the gut, and the central induction of satiation (136).
The CaSR
The CaSR seems to promote the secretion of GLP-1 and PYY, and has thus been identified as a potential therapeutic target in the treatment of diabetes and obesity (23). The CaSR is proposed to act as an l-amino acid sensor in the gut, and has been identified on rodent and human L-cells (Figure 1). The CaSR is able to bind a wide range of amino acids. However, the most potent are the aromatic amino acids, l-phenylalanine and l-tryptophan (26). Mace et al. reported that CaSR activation by the l-amino acids phenylalanine, tryptophan, asparagine, arginine, and glutamine, resulted in the secretion of the GLP-1 and PYY. These responses were abolished in the presence of a CaSR inhibitor or the absence of extracellular calcium, identifying amino acids as allosteric agonists that require the additional presence of calcium to initiate their effects (137). Voltage clamping of intact murine mucosa has shown that the response of the CaSR to l-glutamine is glucose sensitive. Peptone-triggered secretion of GLP-1 from murine primary colonic cultures is also abolished in the presence of CaSR antagonists and removal of extracellular calcium, though whether this effect represents the sensing of individual amino acids generated by initial protein breakdown, or the ability of small peptides to bind to the CaSR is unclear (132).
The GPRC6a
G-protein coupled receptor family C group 6 subtype A (GPRC6a) is closely related to the CaSR, though it preferentially binds the basic amino acids, l-arginine, l-lysine, and l-ornithine, whereas aromatic amino acids are inactive at this receptor (25). Similarly to the CaSR, GPRC6a possesses a calcium binding site on its extracellular domain, although with a weaker affinity than the aforementioned receptor, suggesting overlapping functions (138). GPRC6a is widely expressed throughout the body, including the gut, liver, spleen, lung, heart, kidney, skeletal muscle, brain, and bone (138–140). Exon II of GPRC6a encodes part of an orthosteric binding site for endogenous ligands. Deletion of exon II in GPRC6a knockout mice triggers the development of a complex metabolic-like syndrome. This affects multiple organs, and results in consequences including demineralization of bone, hyperglycemia, and obesity, suggesting that GPRC6a plays a role in the coordination of nutrient sensing and metabolism in these tissues (141). However, an additional GPRC6a knockout, involving disruption of the 7-transmembrane and C-terminal region by deletion of exon VI, displays a normal bone phenotype and glucose tolerance (142). The expression of GPRC6a in the gut, and its activation by l-amino acids indicated that this receptor may mediate the effects of fluctuating dietary protein on energy homeostasis. The GPRC6a, closely related to the CaSR, has been cloned from human and rodent cells, and subsequently localized on the GLUTag L-cell line (139). Application of l-ornithine to a homologous model of the human GPRC6a leads to coupling of the receptor to Gq. The consequent activation of phospholipase C and increased intracellular calcium then stimulates the secretion of GLP-1 (25, 26). Pharmacological inhibition of the downstream pathway decreases the resulting GLP-1 exocytosis from GLUTag cells. Furthermore, following administration of l-ornithine and l-lysine, depletion of endogenous GPRC6a by a small interfering RNA attenuated the intracellular calcium response (143). However, studies using mice with the entire GPRC6a gene deleted, found that this receptor is not necessary for the effects of a high- or low-protein diet on body weight (144).
The taste receptors
The capacity to sense the composition of food via taste allows for the selection of essential nutrients in the diet, in addition to the avoidance of harmful substances. Family C of the GPCRs also encompasses the type 1 taste receptors (T1Rs), formed of the subunits T1R1, T1R2, and T1R3. The combination of subunits T1R1 with T1R3 forms the umami taste receptor, a known l-amino acid receptor expressed in the lingual epithelium (26, 145). The umami receptor is broadly responsive to aliphatic amino acids, but is particularly sensitive to l-glutamine and l-aspartate, the taste of which gives the umami receptor its name and enhances food palatability (146). The rodent and human T1Rs are only approximately 70% homologous, resulting in varying agonist affinities (147). The human T1R1 is considerably more sensitive to glutamate than other amino acids, while the T1R2 is sensitive to artificial sweeteners, such as aspartame and cyclamate (148). Gustducin is a G-protein that plays a role in the downstream signaling transduction of taste from T1Rs. Coupling of the T1Rs to gustducin is thought to stimulate phosphodiesterase activity, leading to the hydrolysis of cAMP (149). Coexpression of T1R1/T1R3 with gustducin occurs in the fungiform and palatal taste buds and has also been reported in the GLP-1-producing STC1 cell line (150–152). However, it is currently unclear whether T1R1/T1R3 plays a physiological role in L-cell sensing of amino acids.
Specific receptors
In addition to promiscuous amino acid receptors, specific amino acid sensors are expressed in the gut. Glutamate is the primary excitatory neurotransmitter in the central nervous system; however, its receptors are also widely expressed in the periphery, including the GI tract. Metabotropic glutamate receptors are GPCRs that are classified into three groups. Group III includes the metabotropic glutamate receptor 4, mGluR4, which is highly expressed in the distal gut, with highest expression in the proximal colon of mice and humans (153). Activation of mGluR4 by glutamate decreases intracellular cAMP production, through coupling to G proteins, which inhibit adenylyl cyclase activity (154). Glutamate is found in many food sources, including legumes and dairy products, and is converted to glutamine in the gut, liver, and kidneys (155, 156).
As a major product of protein digestion, glutamine is an important fuel source for the gut that can enhance protein synthesis, particularly after injury (157). Glutamine acts as a GLP-1 secretagogue in primary cell cultures and the GLUTag cell line, causing both initiation and further amplification of GLP-1 secretion, even at physiologically relevant levels (20, 123). The mechanism is unclear, though the initial step involves electrogenic uptake of the amino acid, followed by a downstream enhancing step, which in primary murine L-cells involves an increase in cytosolic Ca2+ and cAMP (20, 123). In further human studies, oral glutamine was found to be well tolerated, and to result in a biphasic increase of GLP-1 in the circulation of lean, obese, and diabetic subjects (27). Glutamine is commonly delivered as part of enteral and parenteral nutrition, due to its ability to preserve the integrity of the gut, which may be attributable to the co-release of GLP-2 with GLP-1 (27, 158).
l-arginine is defined as a conditionally essential amino acid, as the bodies’ ability to synthesize it varies with age and injury status (159, 160). l-arginine is involved in the synthesis of nitric oxide, as well as that of several other amino acids, including l-glutamate, l-ornithine, proline, and creatine (161). A powerful secretagogue, it has long been known that l-arginine promotes the secretion of insulin and glucagon from the β- and α-cells of the pancreas, respectively, which may be partly due to its potent action at the GPRC6a receptor (162–164). Oral l-arginine stimulates the secretion of GLP-1 and insulin in lean- and diet-induced obese mice in vivo. This effect was abolished in Glp1r knockout mice, and hence, the improvement of l-arginine-stimulated glucose tolerance is dependent on this receptor (22). Furthermore, the addition of l-arginine to a low-protein diet was associated with a reduction in white adipose tissue and increased energy expenditure (165). Dietary supplementation of amino acids, such as glutamine and l-arginine, which potentiate the release of GLP-1 and PYY in vivo, may be useful as a nutritional therapy to enhance the response of the gut endocrine response in obese patients.
Mammalian target of rapamycin
Regulation of protein synthesis requires cells to sense nutrient availability. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that regulates cellular capacity for protein biosynthesis and cell growth by sensing intracellular amino acid levels. Autophagy involves the degradation of cellular protein into amino acids for use during conditions of starvation, a process inhibited by mTOR in an amino acid-rich environment. The levels of branched-chain amino acids, such as leucine, fall the most rapidly, with their depletion being the first to cause activation of mTOR (166). Dietary supplementation of branched-chain amino acids following exercise has anabolic effects on human muscle, involving phosphorylation of mTOR (167). The anticancer drug rapamycin mimics the conditions of low-amino acid availability or limited nitrogen, restricting cell growth and inducing autophagy (168). l-glutamine is the preferred nitrogen source in cells, the balance of which provides the rate-limiting step for anabolic conditions (168). Uptake, and subsequent efflux, of l-glutamine is required for the activation of mTOR by essential amino acids, leading to tissue growth (21).
Adenosine monophosphate-activated protein kinase
Further regulation of protein metabolism occurs via adenosine monophosphate-activated protein kinase (AMPK), a sensor of adenosine nucleotides. Hydrolysis of adenosine triphosphate (ATP) increases the intracellular ratio of AMP to ATP, leading to the activation of AMPK (19). This negatively regulates downstream targets of TOR complex 1 (TORC1), and thus, responds to energy deficits by suppressing cell growth (169, 170). These signaling pathways converge further, through the regulation of Forkhead box class O (FoxO) transcription factors. Activation of AMPK and TORC1 leads to the phosphorylation of FoxO and the elevated expression of its transcription factors, respectively, with both processes acting to inhibit cell proliferation (171, 172). Thus, AMPK acts as a nutritional sensor that influences protein metabolism and may interact with protein-sensing mechanisms.
A thorough understanding of the ability of the cell surface and intracellular sensors, which detect the products of protein digest to regulate energy balance by responding to nutritional and hormonal signals may lead to new ways of exploiting the benefits of high-protein diets without requiring patients to adopt such regimes. Simultaneous pharmacological targeting of receptors enriched in the gut and pancreas, which have dual functions in the release of anorectic peptides from EECs and incretin effects, might be beneficial in diabetic obese patients.
Carbohydrate-Sensing Receptors
Most mammalian cells rely on a steady glucose supply as an energy source, though circulating levels must be kept low in order to avoid the toxic state of hyperglycemia (173). Carbohydrate sensing is directly related to glucose homeostasis, beginning with taste receptors in the mouth and subsequently glucose sensors in the gut. Activation of sweet taste receptors stimulates incretin hormone release from EECs and promotes glucose absorption via increased intestinal expression of the transporters, GLUT2 and SGLT1 (119, 174). The tight, short-term control of glucose homeostasis allows sufficient flux of glucose to the brain, while avoiding states of hyperglycemia. However, there is no clear link between carbohydrate sensing and longer-term appetite regulation. An increase in blood glucose following either the consumption of carbohydrate in the form of breakfast cereal or intravenous infusion of glucose is not associated with decreased food intake, indicating that the glycemic response is not directly related to satiety (175, 176). Obese patients who have undergone Roux-en-Y gastric bypass are commonly reported to have resolution of the associated type 2 diabetes within days of the procedure. This precludes any weight loss, and may be partly due to the effects of duodenal isolation on acute intestinal glucose sensing and transport, resulting in the improved regulation of glycemia (119, 177).
Sodium-glucose transporter 1
Integrated glucose homeostasis relies on the ability of multiple tissue types to sense glucose levels. However, before glucose disposal can occur, dietary carbohydrate must first be metabolized and absorbed from the gut lumen. Hydrolysis of carbohydrates by small intestinal brush-border enzymes produces monosaccharides, such as glucose, that can then be absorbed. An essential component of this transepithelial transport system is the co-transporter, SGLT-1, which uses the sodium electrochemical gradient produced by the Na+/K+ ATPase pump to allow glucose entry across the apical membrane and into enterocytes (178, 179). Facilitated glucose transporters located on the basolateral membrane, such as GLUT2, subsequently allow passive diffusion of glucose into the interstitial space (180).
Enteroendocrine L-cells are directly glucose-responsive. Glucose-sensing leads to membrane depolarization and calcium entry through voltage-gated channels, leading to exocytosis of GLP-1-containing vesicles (27). Moriya et al. determined that in vivo injection of glucose into the upper intestine of mice increased circulating GLP-1 and GIP levels, and that this effect was prevented by coadministration of an SGLT-1 inhibitor. Injection of glucose into the colon did not affect incretin levels, consistent with localization of SGLT-1 mRNA in L-cells of the small intestine and very low detection in the colon (181). The non-metabolizable glucose analog and SGLT-1 agonist, α-methylglucopyranoside, is also a potent stimulator of GLP-1 release from primary intestinal L-cell cultures, highlighting this transporter as a major stimulator of hormone release from EECs (27).
ATP-sensitive potassium
The ATP-sensitive potassium (KATP) channel subunits, Kir6.2 and Sur1, have been identified in primary L-cells expressed at levels similar to those seen in pancreatic cells, where they are known to be involved in insulin release (182). Tolbutamide, which blocks these channels, triggered GLP-1 release from primary L-cells, confirming the presence of functional KATP channels in L-cells. However, the exact function of these channels is unknown (27).
The sweet taste receptor
Heterodimerization of subunits T1R2 and T1R3 of the abovementioned family C of the GPCRs, forms the sweet taste receptor. The sweet taste receptor is expressed on the lingual epithelium, but also acts as a carbohydrate sensor in the gut and STC1 cell line (151). Coupling of T1R2/T1R3 to the taste-associated G-protein, gustducin, mediates second messenger signaling cascades, and allows for the secretion of GLP-1 in the presence of glucose. This response is defective in gustducin-null mice, which have an impaired GLP-1 response to luminal glucose (152). Reimann et al. reported that although L-cells were unresponsive to artificial sweeteners at low concentrations, higher concentrations resulted in GLP-1 secretion in vitro, an effect that was additive with glucose, and may be due to activation of the sweet taste receptor (27). However, studies by Fujita et al. indicated that oral administration of a range of sweeteners active at sweet taste receptors on the lingual epithelium, did not result in incretin secretion in vivo in the rat (183). The role of carbohydrate sensing in the gut on the long-term regulation of energy homeostasis is thus currently unclear.
Fatty Acid-Sensing Receptors
Ethanolamide receptors
The varying chain length and saturation of fatty acids and their derivatives confer distinct receptor affinities. The Gs-associated GPCR, GPR119, detects the endogenous saturated fatty-acid ethanolamides, such as oleoylethanolamide (OEA) (Figure 1). OEA is produced in the small intestine, and has a chain length of 18 carbons, with one double bond. This high degree of saturation produces the greater efficacy of OEA at GPR119 than other ethanolamides (184). GPR119 is present on both pancreatic β-cells and intestinal L-cells. Activation in the pancreas mediates insulin secretion in the presence of glucose, via raised intracellular cAMP (185). Activation of GPR119 in the gut by the products of fat hydrolysis, leads to the release of GLP-1 and insulin secretion in a glucose-dependent manner (186). Conversely, GPR119 agonists are able to stimulate GLP-1 secretion in the absence of nutrients in GLUTag cells (187). Daily intraperitoneal administration of OEA to mice induces satiety and prevents weight gain, a feature that may be true of other GPR119 agonists (184, 188). Stimulation of GPR119 is coupled to increased proglucagon expression in GLUTag cells (189). This is further supported by the attenuated GLP-1 secretion in GPR119 knock-out mice in response to glucose. GPR119 may hence control GLP-1 synthesis, and pharmacological activation of this receptor may therefore enhance glycemic control and reduce food intake in diabetic patients (190).
SCFA receptors
Short-chain fatty acids (SCFAs) comprise a chain of fewer than six carbons in length. They are produced by the bacterial fermentation of undigested carbohydrates, the main products being acetate, propionate, and butyrate (191). Putative receptors for SCFAs, include the free-fatty acid receptors (FFARs) 2 and 3, also known as GPR43 and 41, respectively. Both receptors couple to Gi/o, and activation thus leads to raised intracellular calcium and decreased cAMP. FFAR2 also exhibits dual coupling to Gq, an activator of phospholipase C (192). Localized in the human colon and ileum, FFAR2 and 3 are expressed by enteroendocrine L-cells that secrete PYY. However, FFAR3 is much more enriched in L-cells of the small intestine (29), while FFAR2 is expressed at higher levels in the human colon. Coexpression of FFAR2 and 3 has not been observed in the same cells (191, 193). Recent evidence has suggested that increased intake of dietary fiber may reduce appetite by increasing the SCFAs produced by microbial fermentation in the colon, and thus, increasing the secretion of anorectic gut hormones (194). Propionate, in particular, may play an important role in satiety. Colonic infusion of SCFAs increases circulating PYY levels in vivo in the rat, inhibiting upper GI tract motility consistent with the ileal brake (28). Acetate and propionate enhance GLP-1 secretion in murine primary cell cultures. Propionate stimulates the secretion of GLP-1 and PYY from rodent colon in vivo and in vitro. This effect is significantly reduced in Ffar2-null mice, suggesting FFAR2 is important in SCFA-induced gut hormone secretion (30). The stimulatory effects of acetate on basal and glucose-stimulated GLP-1 release are also abolished in primary L-cell cultures from FFAR2 knock-out mice, though they were also found to be significantly reduced in FFAR3 knock-out tissue (29). Chronic delivery of propionate to the proximal colon of obese human subjects increases the production of PYY and GLP-1, reduces energy intake, and prevents weight gain. Targeted colonic propionate may therefore represent a novel and effective approach for weight management (195).
MCFA and LCFA receptors
Medium chain fatty acids (MCFAs) and long-chain fatty acids (LCFAs) are agonists of the FFAR1 and 4, previously known as GPR40 and 120. An MCFA is formed of a 6–12 carbon chain, and LCFAs of a chain containing more than 12 carbons (196). FFAR1 is expressed in the pancreatic β-cell, where evidence suggests it can influence glucose-stimulated insulin release (197). The effects of hyperlipidemia on glucose homeostasis may in part be mediated by this receptor (198, 199). FFAR1 is also expressed in GLP-1- and GIP-expressing cells of the GI tract, and the secretion of these hormones in response to oral fat is attenuated in Ffar1-null mice. The activation of FFAR1 in EECs therefore provides a route for the indirect regulation of insulin secretion (32). FFAR4 is expressed in the δ-cells of the pancreas, and widely in EECs. FFAR4 responds to unsaturated MCFAs and LCFAs by coupling to Gaq and increasing intracellular calcium, leading to GLP-1 secretion (31, 33, 37). FFAR4-deficient mice gain more weight than wild-type controls, and show higher fasting plasma levels of glucose and insulin when fed a high-fat diet (200). Human obesity has been associated with altered FFAR4 expression in adipose tissue, involving a deleterious Ffar4 mutation that is unable to transduce LCFA binding (200). While it feels counter intuitive to use lipids to treat obesity and metabolic disease, exploiting the pathways by which dietary lipid is sensed and drives satiety may be useful in the development of new pharmaceutical or nutraceutical agents.
Summary
Currently, the most effective treatment for obesity is bariatric surgery, specifically the Roux-en Y Gastric bypass. However, this method is not suitable for all, due the highly invasive nature and associated risks for patients, particularly those with cardiovascular problems. Due to the rapid degradation of endogenous PYY and GLP-1, and the difficulties in administration of peptide-based drugs, exploiting the mechanisms that lead to their continued release, such as the GLP-1 receptor agonist, Saxenda, might be a useful alternative approach to treating patients suffering from obesity and impaired glucose tolerance. Combination of pharmacological treatments that act by targeting the nutrient-sensing receptors and transporters discussed above may ameliorate the defective satiety and glucose homeostasis pathways present in these patient groups. Likewise, the potential formulation of nutraceuticals, containing specific combinations of amino acids and other nutrient receptor ligands, may represent another approach (Table 1). The continued receptor deorphanization and elucidation of the cellular machinery by which the L-cell detects nutrients may thus prove valuable in the development of new treatments for diabetes and obesity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
AgRP, agouti-related peptide; AMPK, adenosine monophosphate-activated protein kinase; AP, area postrema; ARC, arcuate nucleus; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CCK, cholecystokinin; CTA, conditioned taste aversion; DPPIV, dipeptidyl peptidase IV; EEC, enteroendocrine cell; FACS, fluorescence-activated cell sorted analysis; FFARs, free fatty-acid receptors; FoxO, forkhead box class O; GABA, γ-aminobutyric acid; GI, gastrointestinal; GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide 1; GLP-2, glucagon-like peptide 2; GLUT2, glucose transporter 2; GPCR, G-protein coupled receptors; KATP, ATP-sensitive potassium channel; LCFAs, long-chain fatty acids; MCFAs, medium chain fatty acids; MOR, mu-opioid receptor; mTOR, mammalian target of rapamycin; NPY, neuropeptide Y; NTS, nucleus of the solitary tract; OEA, oleoylethanolamide; PEPT1, peptide-transporter 1; POMC, pro-opio melanocortin; PYY, peptide YY; SCFAs, short-chain fatty acids; SGLT-1, sodium-glucose transporter 1; T1Rs, type 1 taste receptors; TORC1, TOR complex 1.
Acknowledgments
The Section is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7- HEALTH- 2009- 241592 EuroCHIP grant and is supported by the NIHR Imperial Biomedical Research Centre Funding Scheme. KM is funded by BBSRC project grant BB/I001816/1. ES is funded by a National Centre for the Replacement, Refinement, and Reduction of Animals in Research studentship.
References
- 1.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab (2012) 15:421–31. 10.1016/j.cmet.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 2.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci (2004) 1014:1–12. 10.1196/annals.1294.001 [DOI] [PubMed] [Google Scholar]
- 3.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, et al. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology (2012) 153:3054–65. 10.1210/en.2011-2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest (2007) 117:13–23. 10.1172/jci30227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab (2008) 8:447–9. 10.1016/j.cmet.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Bohorquez DV, Chandra R, Samsa LA, Vigna SR, Liddle RA. Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol (2011) 42:3–13. 10.1007/s10735-010-9302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest (2015) 125:782–6. 10.1172/jci78361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Obesity: Identification, Assessment and Management of Overweight and Obesity in Children, Young People and Adults [Online]. National Institute for Health and Care Excellence; (2014). Available from: http://www.nice.org.uk/guidance/cg189/chapter/1-recommendations#surgical-interventions (accessed April 2, 2015). [PubMed] [Google Scholar]
- 9.Dakin CL, Small CJ, Park AJ, Seth A, Ghatei MA, Bloom SR. Repeated ICV administration of oxyntomodulin causes a greater reduction in body weight gain than in pair-fed rats. Am J Physiol Endocrinol Metab (2002) 283:E1173–7. 10.1152/ajpendo.00233.2002 [DOI] [PubMed] [Google Scholar]
- 10.Wynne K, Park AJ, Small CJ, Meeran K, Ghatei MA, Frost GS, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) (2006) 30:1729–36. 10.1038/sj.ijo.0803344 [DOI] [PubMed] [Google Scholar]
- 11.Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes (2012) 61:2753–62. 10.2337/db11-1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA. FDA Approves Weight-Management Drug Saxenda [Online]. U.S. Department of Health and Human Services; (2014). Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427913.htm (accessed March 31, 2015). [Google Scholar]
- 13.le Roux CW, Borg CM, Murphy KG, Vincent RP, Ghatei MA, Bloom SR. Supraphysiological doses of intravenous PYY3-36 cause nausea, but no additional reduction in food intake. Ann Clin Biochem (2008) 45:93–5. 10.1258/acb.2007.007068 [DOI] [PubMed] [Google Scholar]
- 14.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes (2011) 60:1561–5. 10.2337/db10-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt JB, Gregersen NT, Pedersen SD, Arentoft JL, Ritz C, Schwartz TW, et al. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab (2014) 306:E1248–56. 10.1152/ajpendo.00569.2013 [DOI] [PubMed] [Google Scholar]
- 16.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, et al. Oxyntomodulin inhibits food intake in the rat. Endocrinology (2001) 142:4244–50. 10.1210/endo.142.10.8430 [DOI] [PubMed] [Google Scholar]
- 17.Adrian TE, Sagor GR, Savage AP, Bacarese-Hamilton AJ, Hall GM, Bloom SR. Peptide YY kinetics and effects on blood pressure and circulating pancreatic and gastrointestinal hormones and metabolites in man. J Clin Endocrinol Metab (1986) 63:803–7. 10.1210/jcem-63-4-803 [DOI] [PubMed] [Google Scholar]
- 18.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology (1993) 105:733–9. [DOI] [PubMed] [Google Scholar]
- 19.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5’-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem (1995) 270:27186–91. 10.1074/jbc.270.45.27186 [DOI] [PubMed] [Google Scholar]
- 20.Reimann F, Williams L, Da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia (2004) 47:1592–601. 10.1007/s00125-004-1498-0 [DOI] [PubMed] [Google Scholar]
- 21.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell (2009) 136:521–34. 10.1016/j.cell.2008.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemmensen C, Smajilovic S, Smith EP, Woods SC, Brauner-Osborne H, Seeley RJ, et al. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology (2013) 154:3978–83. 10.1210/en.2013-1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi S, Tough IR, Cox HM. Endogenous PYY and GLP-1 mediate l-glutamine responses in intestinal mucosa. Br J Pharmacol (2013) 170:1092–101. 10.1111/bph.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol (2007) 292:G1366–75. 10.1152/ajpgi.00516.2006 [DOI] [PubMed] [Google Scholar]
- 25.Wellendorph P, Hansen KB, Balsgaard A, Greenwood JR, Egebjerg J, Brauner-Osborne H. Deorphanization of GPRC6A: a promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol Pharmacol (2005) 67:589–97. 10.1124/mol.104.007559 [DOI] [PubMed] [Google Scholar]
- 26.Wellendorph P, Brauner-Osborne H. Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol (2009) 156:869–84. 10.1111/j.1476-5381.2008.00078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab (2008) 8:532–9. 10.1016/j.cmet.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol (1998) 275:G1415–22. [DOI] [PubMed] [Google Scholar]
- 29.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes (2012) 61:364–71. 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) (2014) 39(3):424–9. 10.1038/ijo.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med (2005) 11:90–4. 10.1038/nm1168 [DOI] [PubMed] [Google Scholar]
- 32.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes (2008) 57:2280–7. 10.2337/db08-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stone VM, Dhayal S, Brocklehurst KJ, Lenaghan C, Sorhede Winzell M, Hammar M, et al. GPR120 (FFAR4) is preferentially expressed in pancreatic delta cells and regulates somatostatin secretion from murine islets of Langerhans. Diabetologia (2014) 57:1182–91. 10.1007/s00125-014-3213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eissele R, Goke R, Willemer S, Harthus HP, Vermeer H, Arnold R, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest (1992) 22:283–91. 10.1111/j.1365-2362.1992.tb01464.x [DOI] [PubMed] [Google Scholar]
- 35.Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol (1996) 10:342–55. 10.1210/mend.10.4.8721980 [DOI] [PubMed] [Google Scholar]
- 36.Petersen N, Reimann F, Van Es JH, Van Den Berg BM, Kroone C, Pais R, et al. Targeting development of incretin-producing cells increases insulin secretion. J Clin Invest (2015) 125:379–85. 10.1172/jci75838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habib AM, Richards P, Rogers GJ, Reimann F, Gribble FM. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia (2013) 56:1413–6. 10.1007/s00125-013-2887-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandt D, Schimiczek M, Struk K, Shively J, Eysselein VE, Goebell H, et al. Characterization of two forms of peptide YY, PYY(1-36) and PYY(3-36), in the rabbit. Peptides (1994) 15:815–20. 10.1016/0196-9781(94)90035-3 [DOI] [PubMed] [Google Scholar]
- 39.Guarita DR, Deng X, Huh YB, Wood PG, Reeve JR, Jr, Whitcomb DC. PYY regulates pancreatic exocrine secretion through multiple receptors in the awake rat. Dig Dis Sci (2000) 45:1696–702. 10.1023/A:1005550732146 [DOI] [PubMed] [Google Scholar]
- 40.Larsson LI, Holst J, Hakanson R, Sundler F. Distribution and properties of glucagon immunoreactivity in the digestive tract of various mammals: an immunohistochemical and immunochemical study. Histochemistry (1975) 44:281–90. 10.1007/BF00490364 [DOI] [PubMed] [Google Scholar]
- 41.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol (1993) 138:159–66. 10.1677/joe.0.1380159 [DOI] [PubMed] [Google Scholar]
- 42.Nauck MA, Siemsgluss J, Orskov C, Holst JJ. Release of glucagon-like peptide 1 (GLP-1 [7-36 amide]), gastric inhibitory polypeptide (GIP) and insulin in response to oral glucose after upper and lower intestinal resections. Z Gastroenterol (1996) 34:159–66. [PubMed] [Google Scholar]
- 43.Pilichiewicz AN, Chaikomin R, Brennan IM, Wishart JM, Rayner CK, Jones KL, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab (2007) 293:E743–53. 10.1152/ajpendo.00159.2007 [DOI] [PubMed] [Google Scholar]
- 44.Pilichiewicz AN, Papadopoulos P, Brennan IM, Little TJ, Meyer JH, Wishart JM, et al. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol (2007) 293:R2170–8. 10.1152/ajpregu.00511.2007 [DOI] [PubMed] [Google Scholar]
- 45.Todd JF, Edwards CM, Ghatei MA, Mather HM, Bloom SR. Subcutaneous glucagon-like peptide-1 improves postprandial glycaemic control over a 3-week period in patients with early type 2 diabetes. Clin Sci (Lond) (1998) 95:325–9. 10.1042/CS19980051 [DOI] [PubMed] [Google Scholar]
- 46.Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann N Y Acad Sci (2003) 994:162–8. 10.1111/j.1749-6632.2003.tb03176.x [DOI] [PubMed] [Google Scholar]
- 47.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet (1987) 2:1300–4. 10.1016/S0140-6736(87)91194-9 [DOI] [PubMed] [Google Scholar]
- 48.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab (2003) 88:2706–13. 10.1210/jc.2002-021873 [DOI] [PubMed] [Google Scholar]
- 49.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, et al. Effect of peptide YY3-36 on food intake in humans. Gastroenterology (2005) 129:1430–6. 10.1053/j.gastro.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 50.Ronveaux CC, De Lartigue G, Raybould HE. Ability of GLP-1 to decrease food intake is dependent on nutritional status. Physiol Behav (2014) 135:222–9. 10.1016/j.physbeh.2014.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilsboll T, Holst JJ. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia (2004) 47:357–66. 10.1007/s00125-004-1342-6 [DOI] [PubMed] [Google Scholar]
- 52.Blache P, Kervran A, Bataille D. Oxyntomodulin and glicentin: brain-gut peptides in the rat. Endocrinology (1988) 123:2782–7. 10.1210/endo-123-6-2782 [DOI] [PubMed] [Google Scholar]
- 53.Ohneda A, Ohneda K, Nagasaki T, Sasaki K. Insulinotropic action of human glicentin in dogs. Metabolism (1995) 44:47–51. 10.1016/0026-0495(95)90288-0 [DOI] [PubMed] [Google Scholar]
- 54.D’Alessio DA. What if gut hormones aren’t really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology (2011) 152:2925–6. 10.1210/en.2011-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Li B, et al. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab (2014) 19:1050–7. 10.1016/j.cmet.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia (2006) 49:1360–70. 10.1007/s00125-006-0237-0 [DOI] [PubMed] [Google Scholar]
- 57.Chandarana K, Gelegen C, Irvine EE, Choudhury AI, Amouyal C, Andreelli F, et al. Peripheral activation of the Y2-receptor promotes secretion of GLP-1 and improves glucose tolerance. Mol Metab (2013) 2:142–52. 10.1016/j.molmet.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orskov C, Poulsen SS, Moller M, Holst JJ. Glucagon-like peptide I receptors in the subfornical organ and the area postrema are accessible to circulating glucagon-like peptide I. Diabetes (1996) 45:832–5. 10.2337/diab.45.6.832 [DOI] [PubMed] [Google Scholar]
- 59.Halatchev IG, Cone RD. Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab (2005) 1:159–68. 10.1016/j.cmet.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 60.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes (2014) 63:1224–33. 10.2337/db13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest (2014) 124:4473–88. 10.1172/jci75276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Perfetti R, Greig NH, Holloway HW, Deore KA, Montrose-Rafizadeh C, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest (1997) 99:2883–9. 10.1172/jci119482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes (1998) 47:358–64. 10.2337/diabetes.47.3.358 [DOI] [PubMed] [Google Scholar]
- 64.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes (1999) 48:2270–6. 10.2337/diabetes.48.12.2270 [DOI] [PubMed] [Google Scholar]
- 65.Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology (2000) 141:4600–5. 10.1210/endo.141.12.7806 [DOI] [PubMed] [Google Scholar]
- 66.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, et al. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology (2002) 143:4397–408. 10.1210/en.2002-220405 [DOI] [PubMed] [Google Scholar]
- 67.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A (1996) 93:7911–6. 10.1073/pnas.93.15.7911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology (2003) 125:136–47. 10.1016/S0016-5085(03)00667-X [DOI] [PubMed] [Google Scholar]
- 69.Cheeseman CI. Upregulation of SGLT-1 transport activity in rat jejunum induced by GLP-2 infusion in vivo. Am J Physiol (1997) 273:R1965–71. [DOI] [PubMed] [Google Scholar]
- 70.Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J (2002) 367:247–54. 10.1042/bj20020393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welch I, Saunders K, Read NW. Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology (1985) 89:1293–7. [DOI] [PubMed] [Google Scholar]
- 72.Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci (1995) 40:1074–82. 10.1007/BF02064202 [DOI] [PubMed] [Google Scholar]
- 73.van Avesaat M, Troost FJ, Ripken D, Hendriks HF, Masclee AA. Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obes (Lond) (2015) 39(2):235–43. 10.1038/ijo.2014.112 [DOI] [PubMed] [Google Scholar]
- 74.Deng X, Guarita DR, Wood PG, Kriess C, Whitcomb DC. PYY potently inhibits pancreatic exocrine secretion mediated through CCK-secretin-stimulated pathways but not 2-DG-stimulated pathways in awake rats. Dig Dis Sci (2001) 46:156–65. 10.1023/A:1012380004736 [DOI] [PubMed] [Google Scholar]
- 75.Persaud SJ, Bewick GA. Peptide YY: more than just an appetite regulator. Diabetologia (2014) 57:1762–9. 10.1007/s00125-014-3292-y [DOI] [PubMed] [Google Scholar]
- 76.Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y(1) and Y(2) receptors. Br J Pharmacol (2011) 164:471–84. 10.1111/j.1476-5381.2011.01401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lloyd KC, Grandt D, Aurang K, Eysselein VE, Schimiczek M, Reeve JR, Jr. Inhibitory effect of PYY on vagally stimulated acid secretion is mediated predominantly by Y1 receptors. Am J Physiol (1996) 270:G123–7. [DOI] [PubMed] [Google Scholar]
- 78.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther (2013) 139:359–91. 10.1016/j.pharmthera.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 79.Tan TM, Salem V, Troke RC, Alsafi A, Field BC, De Silva A, et al. Combination of peptide YY3-36 with GLP-1(7-36) amide causes an increase in first-phase insulin secretion after IV glucose. J Clin Endocrinol Metab (2014) 99:E2317–24. 10.1210/jc.2014-2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab (1983) 57:488–95. 10.1210/jcem-57-3-488 [DOI] [PubMed] [Google Scholar]
- 81.Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes (2009) 58:2258–66. 10.2337/db09-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dubrasquet M, Bataille D, Gespach C. Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci Rep (1982) 2:391–5. 10.1007/BF01119301 [DOI] [PubMed] [Google Scholar]
- 83.Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology (2004) 127:546–58. 10.1053/j.gastro.2004.04.063 [DOI] [PubMed] [Google Scholar]
- 84.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology (2004) 145:2687–95. 10.1210/en.2003-1338 [DOI] [PubMed] [Google Scholar]
- 85.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38). J Biol Chem (2003) 278:22418–23. 10.1074/jbc.M212355200 [DOI] [PubMed] [Google Scholar]
- 86.Lynch AM, Pathak N, Flatt YE, Gault VA, O’Harte FP, Irwin N, et al. Comparison of stability, cellular, glucose-lowering and appetite supressing effects of oxyntomodulin analogues modified at the N-terminus. Eur J Pharmacol (2014) 743:69–78. 10.1016/j.ejphar.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 87.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci (2014) 15:367–78. 10.1038/nrn3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron (1998) 21:1375–85. 10.1016/S0896-6273(00)80656-X [DOI] [PubMed] [Google Scholar]
- 89.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci (1998) 1:271–2. 10.1038/1082 [DOI] [PubMed] [Google Scholar]
- 90.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci (2011) 14:351–5. 10.1038/nn.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature (2012) 488:172–7. 10.1038/nature11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schwartz GJ. The role of gastrointestinal vagal afferents in the control of food intake: current prospects. Nutrition (2000) 16:866–73. 10.1016/S0899-9007(00)00464-0 [DOI] [PubMed] [Google Scholar]
- 93.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia (2005) 48:612–5. 10.1007/s00125-005-1705-7 [DOI] [PubMed] [Google Scholar]
- 94.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol (1996) 271:E458–64. [DOI] [PubMed] [Google Scholar]
- 95.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology (1999) 140:5356–63. 10.1210/endo.140.11.7143 [DOI] [PubMed] [Google Scholar]
- 96.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci (2004) 110:36–43. 10.1016/j.autneu.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 97.Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst (1996) 61:149–54. 10.1016/S0165-1838(96)00071-9 [DOI] [PubMed] [Google Scholar]
- 98.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology (2009) 150:1174–81. 10.1210/en.2008-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res (2005) 1044:127–31. 10.1016/j.brainres.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 100.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol (1985) 234:344–64. 10.1002/cne.902340306 [DOI] [PubMed] [Google Scholar]
- 101.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol (1998) 274:R23–9. [DOI] [PubMed] [Google Scholar]
- 102.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes (2008) 57:2046–54. 10.2337/db07-1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, et al. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology (2015) 156:255–67. 10.1210/en.2014-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lluis F, Fujimura M, Gomez G, Salva JA, Greeley GH, Jr, Thompson JC. [Cellular localization, half-life, and secretion of peptide YY]. Rev Esp Fisiol (1989) 45:377–84. [PubMed] [Google Scholar]
- 105.Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, et al. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature (1996) 382:168–71. 10.1038/382168a0 [DOI] [PubMed] [Google Scholar]
- 106.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab (2007) 292:E1062–8. 10.1152/ajpendo.00450.2006 [DOI] [PubMed] [Google Scholar]
- 107.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature (2002) 418:650–4. 10.1038/nature02666 [DOI] [PubMed] [Google Scholar]
- 108.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3-36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol (2006) 290:R298–305. 10.1152/ajpregu.00674.2005 [DOI] [PubMed] [Google Scholar]
- 109.Chelikani PK, Haver AC, Reidelberger RD. Intermittent intraperitoneal infusion of peptide YY(3-36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol (2007) 293:R39–46. 10.1152/ajpregu.00164.2007 [DOI] [PubMed] [Google Scholar]
- 110.Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci U S A (2004) 101:4695–700. 10.1073/pnas.0306931101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology (2004) 145:2585–90. 10.1210/en.2003-1754 [DOI] [PubMed] [Google Scholar]
- 112.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology (2005) 146:2369–75. 10.1210/en.2004-1266 [DOI] [PubMed] [Google Scholar]
- 113.Chelikani PK, Haver AC, Reidelberger RD. Dose-dependent effects of peptide YY(3-36) on conditioned taste aversion in rats. Peptides (2006) 27:3193–201. 10.1016/j.peptides.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 114.O’Brien PE. Controversies in bariatric surgery. Br J Surg (2015) 102(6):611–8. 10.1002/bjs.9760 [DOI] [PubMed] [Google Scholar]
- 115.Pedersen-Bjergaard U, Host U, Kelbaek H, Schifter S, Rehfeld JF, Faber J, et al. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest (1996) 56:497–503. 10.3109/00365519609088805 [DOI] [PubMed] [Google Scholar]
- 116.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg (2006) 243:108–14. 10.1097/01.sla.0000183349.16877.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK. Lower ghrelin levels and exaggerated postprandial peptide-YY, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg (2012) 22:1761–70. 10.1007/s11695-012-0738-5 [DOI] [PubMed] [Google Scholar]
- 118.Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol (2010) 299:G652–60. 10.1152/ajpgi.00221.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg (2010) 251:865–71. 10.1097/SLA.0b013e3181d96e1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol (2007) 292:G215–22. 10.1152/ajpgi.00188.2006 [DOI] [PubMed] [Google Scholar]
- 121.Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J, et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut (2014) 63:1238–46. 10.1136/gutjnl-2013-304583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology (2011) 141:950–8. 10.1053/j.gastro.2011.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology (2011) 152:405–13. 10.1210/en.2010-0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cho HJ, Robinson ES, Rivera LR, McMillan PJ, Testro A, Nikfarjam M, et al. Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res (2014) 357:63–9. 10.1007/s00441-014-1886-9 [DOI] [PubMed] [Google Scholar]
- 125.Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, et al. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr (2006) 83:211–20. [DOI] [PubMed] [Google Scholar]
- 126.Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr (2009) 139:514–21. 10.3945/jn.108.099440 [DOI] [PubMed] [Google Scholar]
- 127.Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. J Nutr (2013) 143:591–6. 10.3945/jn.112.167593 [DOI] [PubMed] [Google Scholar]
- 128.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab (2006) 4:223–33. 10.1016/j.cmet.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 129.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr (2006) 83:89–94. [DOI] [PubMed] [Google Scholar]
- 130.Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, et al. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes (1998) 47:1038–45. 10.2337/diabetes.47.7.1038 [DOI] [PubMed] [Google Scholar]
- 131.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol (2007) 292:G98–112. 10.1152/ajpgi.00295.2006 [DOI] [PubMed] [Google Scholar]
- 132.Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia (2013) 56:2688–96. 10.1007/s00125-013-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci (2003) 23:2882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sternini C, Patierno S, Selmer IS, Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil (2004) 16(Suppl 2):3–16. 10.1111/j.1743-3150.2004.00553.x [DOI] [PubMed] [Google Scholar]
- 135.Duraffourd C, De Vadder F, Goncalves D, Delaere F, Penhoat A, Brusset B, et al. Mu-opioid receptors and dietary protein stimulate a gut-brain neural circuitry limiting food intake. Cell (2012) 150:377–88. 10.1016/j.cell.2012.05.039 [DOI] [PubMed] [Google Scholar]
- 136.Mithieux G, Misery P, Magnan C, Pillot B, Gautier-Stein A, Bernard C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab (2005) 2:321–9. 10.1016/j.cmet.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 137.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol (2012) 590:2917–36. 10.1113/jphysiol.2011.223800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem (2005) 280:40201–9. 10.1074/jbc.M505186200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene (2004) 335:37–46. 10.1016/j.gene.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 140.Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem (2005) 93:383–91. 10.1111/j.1471-4159.2005.03025.x [DOI] [PubMed] [Google Scholar]
- 141.Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One (2008) 3:e3858. 10.1371/journal.pone.0003858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P, et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol (2009) 42:215–23. 10.1677/jme-08-0149 [DOI] [PubMed] [Google Scholar]
- 143.Oya M, Kitaguchi T, Pais R, Reimann F, Gribble F, Tsuboi T. The G protein-coupled receptor family C group 6 subtype A (GPRC6A) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem (2013) 288:4513–21. 10.1074/jbc.M112.402677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinsey-Jones J, Alamshah A, McGavigan A, Spreckley E, Banks K, Monteoliva N, et al. GPRC6A is not required for the effects of a high protein diet on body weight in mice. Obesity (Silver Spring) (2015) 23(6):1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell (1999) 96:541–51. 10.1016/S0092-8674(00)80658-3 [DOI] [PubMed] [Google Scholar]
- 146.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A (2002) 99:4692–6. 10.1073/pnas.072090199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell (2001) 106:381–90. 10.1016/S0092-8674(01)00451-2 [DOI] [PubMed] [Google Scholar]
- 148.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature (2002) 416:199–202. 10.1038/nature726 [DOI] [PubMed] [Google Scholar]
- 149.Kinnamon SC, Vandenbeuch A. Receptors and transduction of umami taste stimuli. Ann N Y Acad Sci (2009) 1170:55–9. 10.1111/j.1749-6632.2009.04106.x [DOI] [PubMed] [Google Scholar]
- 150.Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun (2003) 312:500–6. 10.1016/j.bbrc.2003.10.137 [DOI] [PubMed] [Google Scholar]
- 151.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans (2005) 33:302–5. 10.1042/bst0330302 [DOI] [PubMed] [Google Scholar]
- 152.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A (2007) 104:15069–74. 10.1073/pnas.0706890104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Symonds EL, Peiris M, Page AJ, Chia B, Dogra H, Masding A, et al. Mechanisms of activation of mouse and human enteroendocrine cells by nutrients. Gut (2014) 64(4):618–26. 10.1136/gutjnl-2014-306834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci (2003) 1003:12–21. 10.1196/annals.1300.002 [DOI] [PubMed] [Google Scholar]
- 155.Bertolo RF, Burrin DG. Comparative aspects of tissue glutamine and proline metabolism. J Nutr (2008) 138:2032s–9s. [DOI] [PubMed] [Google Scholar]
- 156.Delgado TC. Glutamate and GABA in appetite regulation. Front Endocrinol (2013) 4:103. 10.3389/fendo.2013.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr (2001) 131:2543S–9S. [DOI] [PubMed] [Google Scholar]
- 158.van der Hulst RR, Van Kreel BK, Von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, et al. Glutamine and the preservation of gut integrity. Lancet (1993) 341:1363–5. 10.1016/0140-6736(93)90939-E [DOI] [PubMed] [Google Scholar]
- 159.Barbul A. Arginine: biochemistry, physiology, and therapeutic implications. JPEN J Parenter Enteral Nutr (1986) 10:227–38. 10.1177/0148607186010002227 [DOI] [PubMed] [Google Scholar]
- 160.Visek WJ. Arginine needs, physiological state and usual diets. A reevaluation. J Nutr (1986) 116:36–46. [DOI] [PubMed] [Google Scholar]
- 161.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J (1998) 336(Pt 1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci (1969) 257:415–9. 10.1097/00000441-196906000-00008 [DOI] [PubMed] [Google Scholar]
- 163.Sener A, Lebrun P, Blachier F, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release. Insulinotropic action of agmatine. Biochem Pharmacol (1989) 38:327–30. 10.1016/0006-2952(89)90044-0 [DOI] [PubMed] [Google Scholar]
- 164.Clemmensen C, Smajilovic S, Wellendorph P, Brauner-Osborne H. The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol (2014) 171:1129–41. 10.1111/bph.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clemmensen C, Madsen AN, Smajilovic S, Holst B, Brauner-Osborne H. L-arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids (2012) 43:1265–75. 10.1007/s00726-011-1199-1 [DOI] [PubMed] [Google Scholar]
- 166.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A (2005) 102:14238–43. 10.1073/pnas.0506925102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr (2006) 136:269s–73s. [DOI] [PubMed] [Google Scholar]
- 168.Crespo JL, Hall MN. Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae. Microbiol Mol Biol Rev (2002) 66:579–91. 10.1128/MMBR.66.4.579-591.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell (2003) 115:577–90. 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- 170.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell (2008) 30:214–26. 10.1016/j.molcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem (2007) 282:30107–19. 10.1074/jbc.M705325200 [DOI] [PubMed] [Google Scholar]
- 172.Harvey KF, Mattila J, Sofer A, Bennett FC, Ramsey MR, Ellisen LW, et al. FOXO-regulated transcription restricts overgrowth of Tsc mutant organs. J Cell Biol (2008) 180:691–6. 10.1083/jcb.200710100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol (2009) 15:4137–42. 10.3748/wjg.15.4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol (2007) 582:379–92. 10.1113/jphysiol.2007.130906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rodin J, Wack J, Ferrannini E, Defronzo RA. Effect of insulin and glucose on feeding behavior. Metabolism (1985) 34:826–31. 10.1016/0026-0495(85)90106-4 [DOI] [PubMed] [Google Scholar]
- 176.Stewart SL, Black RM, Wolever TMS, Anderson GH. The relationship between the glycaemic response to breakfast cereals and subjective appetite and food intake. Nutr Res (1997) 17:1249–60. 10.1016/S0271-5317(97)00108-5 [DOI] [Google Scholar]
- 177.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA (2004) 292:1724–37. 10.1001/jama.292.14.1724 [DOI] [PubMed] [Google Scholar]
- 178.Hediger MA, Kanai Y, You G, Nussberger S. Mammalian ion-coupled solute transporters. J Physiol (1995) 482:7s–17s. 10.1113/jphysiol.1995.sp020559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dyer J, Hosie KB, Shirazi-Beechey SP. Nutrient regulation of human intestinal sugar transporter (SGLT1) expression. Gut (1997) 41:56–9. 10.1136/gut.41.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stumpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A (2001) 98:11330–5. 10.1073/pnas.211357698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moriya R, Shirakura T, Ito J, Mashiko S, Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab (2009) 297:E1358–65. 10.1152/ajpendo.00412.2009 [DOI] [PubMed] [Google Scholar]
- 182.Nielsen LB, Ploug KB, Swift P, Orskov C, Jansen-Olesen I, Chiarelli F, et al. Co-localisation of the Kir6.2/SUR1 channel complex with glucagon-like peptide-1 and glucose-dependent insulinotrophic polypeptide expression in human ileal cells and implications for glycaemic control in new onset type 1 diabetes. Eur J Endocrinol (2007) 156:663–71. 10.1530/eje-06-0756 [DOI] [PubMed] [Google Scholar]
- 183.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, et al. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab (2009) 296:E473–9. 10.1152/ajpendo.90636.2008 [DOI] [PubMed] [Google Scholar]
- 184.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab (2006) 3:167–75. 10.1016/j.cmet.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 185.Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology (2007) 148:2601–9. 10.1210/en.2006-1608 [DOI] [PubMed] [Google Scholar]
- 186.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes (2009) 58:1058–66. 10.2337/db08-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lan H, Lin HV, Wang CF, Wright MJ, Xu S, Kang L, et al. Agonists at GPR119 mediate secretion of GLP-1 from mouse enteroendocrine cells through glucose-independent pathways. Br J Pharmacol (2012) 165:2799–807. 10.1111/j.1476-5381.2011.01754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature (2003) 425:90–3. 10.1038/nature01921 [DOI] [PubMed] [Google Scholar]
- 189.Chepurny OG, Bertinetti D, Diskar M, Leech CA, Afshari P, Tsalkova T, et al. Stimulation of proglucagon gene expression by human GPR119 in enteroendocrine L-cell line GLUTag. Mol Endocrinol (2013) 27:1267–82. 10.1210/me.2013-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lan H, Vassileva G, Corona A, Liu L, Baker H, Golovko A, et al. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol (2009) 201:219–30. 10.1677/joe-08-0453 [DOI] [PubMed] [Google Scholar]
- 191.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res (2009) 30:149–56. 10.2220/biomedres.30.149 [DOI] [PubMed] [Google Scholar]
- 192.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem (2003) 278:25481–9. 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- 193.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol (2008) 39:135–42. 10.1007/s10735-007-9145-y [DOI] [PubMed] [Google Scholar]
- 194.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev (2001) 59:129–39. 10.1111/j.1753-4887.2001.tb07001.x [DOI] [PubMed] [Google Scholar]
- 195.Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SE, et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut (2014). 10.1136/gutjnl-2014-307913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ichimura A, Hasegawa S, Kasubuchi M, Kimura I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol (2014) 5:236. 10.3389/fphar.2014.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature (2003) 422:173–6. 10.1038/nature01478 [DOI] [PubMed] [Google Scholar]
- 198.Haber EP, Ximenes HM, Procopio J, Carvalho CR, Curi R, Carpinelli AR. Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol (2003) 194:1–12. 10.1002/jcp.10187 [DOI] [PubMed] [Google Scholar]
- 199.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab (2005) 1:245–58. 10.1016/j.cmet.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 200.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature (2012) 483:350–4. 10.1038/nature10798 [DOI] [PubMed] [Google Scholar]