Abstract
Background and Purpose
Eugenol, a vanilloid molecule found in some dietary plants, relaxes vasculature in part via an endothelium-dependent process; however, the mechanisms involved are unclear. Here, we investigated the endothelial cell-mediated mechanism by which eugenol modulates rat mesenteric artery contractility and systemic BP.
Experimental Approach
The isometric tension of rat mesenteric arteries (size 200–300 μm) was measured using wire myography; non-selective cation currents (ICat) were recorded in endothelial cells using patch clamp electrophysiology. Mean arterial pressure (MAP) and heart rate (HR) were determined in anaesthetized rats.
Key Results
Eugenol relaxed endothelium-intact arteries in a concentration-dependent manner and this effect was attenuated by endothelium denudation. L-NAME, a NOS inhibitor, a combination of TRAM-34 and apamin, selective blockers of intermediate and small conductance Ca2+-activated K+ channels, respectively, and HC-067047, a TRPV4 channel inhibitor, but not indomethacin, a COX inhibitor, reduced eugenol-induced relaxation in endothelium-intact arteries. Eugenol activated HC-067047-sensitive ICat in mesenteric artery endothelial cells. Short interfering RNA (siRNA)-mediated TRPV4 knockdown abolished eugenol-induced ICat activation. An i.v. injection of eugenol caused an immediate, transient reduction in both MAP and HR, which was followed by prolonged, sustained hypotension in anaesthetized rats. This sustained hypotension was blocked by HC-067047.
Conclusions and Implications
Eugenol activates TRPV4 channels in mesenteric artery endothelial cells, leading to vasorelaxation, and reduces systemic BP in vivo. Eugenol may be therapeutically useful as an antihypertensive agent and is a viable molecular candidate from which to develop second-generation TRPV4 channel activators that reduce BP.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Ion channelsb |
| TXA2 (TP) receptor | BK (KCa 1.1) channel |
| Enzymesc | IKCa (KCa 3.1) channel |
| COX | SKCa (KCa 2.1) channel |
| NOS | TMEM16A (CaCC) |
| TRPV4 |
| LIGANDS | ||
|---|---|---|
| ACh | GSK1016790A | Phenylephrine |
| Apamin | HC-067047 | PGI2 |
| Capsaicin | Indomethacin | TRAM-34 |
| Eugenol | L-NAME | U46619 |
| Nitric oxide (NO) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a, b, c).
Introduction
Endothelial cells line the luminal surface of all blood vessels and regulate vascular tone, coagulation and fibrinolysis, vascular inflammation and angiogenesis (Félétou and Vanhoutte, 2009). The endothelium regulates vascular tone by releasing factors, including NO, in response to both mechanical forces and soluble agonists. Endothelial intracellular calcium (Ca2+) concentration regulates vascular tone by controlling the generation of NO produced by NOS, prostacyclin (PGI2) generated by COX, and endothelium-derived hyperpolarizing factor (EDHF) (Favero et al., 2014). Endothelial cells also regulate vascular tone by controlling the membrane potential of electrically coupled smooth muscle cells (Yamamoto et al., 1999; Sandow and Hill, 2000; Haddock et al., 2006).
Several members of the transient receptor potential (TRP) family of non-selective cation channels are expressed in endothelial cells and are involved in endothelium-dependent vasodilatation (Garcia and Schilling, 1997; Earley and Brayden, 2010; Zhang and Gutterman, 2011). TRPV4, a vanilloid (TRPV) family member, is a Ca2+-permeable ion channel expressed in endothelial cells that stimulates vasodilator mechanisms (Hartmannsgruber et al., 2007; Saliez et al., 2008; Mendoza et al., 2010; Sonkusare et al., 2012). TRPV4 channels are activated by a broad range of chemical and physical stimuli (Filosa et al., 2013). Several studies have demonstrated that TRPV4 channels control vascular tone and influence BP (Watanabe et al., 2002; Kohler et al., 2006; Earley et al., 2009; Zhang et al., 2009; Ma et al., 2013).
Eugenol is a natural compound found in dietary plants including cloves, basil, cinnamon and nutmeg (Kamatou et al., 2012). These plants possess antihypertensive properties, although the chemicals mediating this effect have not been elucidated (Grover et al., 2002; Umar et al., 2010; Ranasinghe et al., 2013). Eugenol and capsaicin share a vanillyl group as an important structural motif for bioactivity. Therefore, eugenol, like capsaicin, can stimulate TRPV channels (Calixto et al., 2005). Eugenol activates TRPV1 channels in trigeminal ganglion neurons and TRPV3 channels in keratinocytes and endothelial cells (Yang et al., 2003; Xu et al., 2006; Earley and Brayden, 2010; Earley et al., 2010). In normotensive and deoxycorticosterone acetate-salt hypertensive rats, eugenol reduces BP and induces bradycardia. Both studies suggested that eugenol-induced hypotension may occur because of vasorelaxation (Lahlou et al., 2004; Interaminense et al., 2007). Eugenol also relaxed rat aorta and an intact mesenteric bed. The effects were shown to be partially dependent on the endothelium, but their mechanisms of action were unclear (Criddle et al., 2003; Damiani et al., 2003).
Here, we investigated endothelial-dependent eugenol-induced vasodilatation in rat mesenteric arteries, the mechanisms involved and functional significance. Our data indicate that eugenol activates TRPV4 currents in mesenteric artery endothelial cells, leading to vasodilatation and a reduction in systemic BP.
Methods
Tissue preparation
Animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center (Memphis, TN, USA) and Universidade Estadual do Ceará (Fortaleza, CE, Brazil). In this study, the total of 40 male Wistar rats (250 g) were used. Animals were killed by an i.p. injection of sodium pentobarbital solution (150 mg·kg−1). The mesenteric bed was removed and third-order branches (200–300 μm diameter) were harvested in ice-cold Krebs–Hanseleit solution (KHS) of composition (in mmol·l−1): NaCl 118; KCl 4.7; NaHCO3 25; CaCl2.2H2O 2.5; KH2PO4 1.2; MgSO4.7H2O 1.2; EDTA 0.01; glucose 11. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Vascular reactivity experiments
Mesenteric artery segments (2 mm in length) were mounted in a small vessel myograph chamber (model 610M; Danish Myo Tech, Aarhus, Denmark) for isometric tension recordings. Briefly, two steel wires (40 μm diameter) were introduced through the lumen and mounted according to methods previously described (Mulvany and Halpern, 1977). After a 15 min equilibration period in oxygenated KHS at 37°C and pH 7.4, segments were stretched to their optimal lumen diameter for active tension development. This was determined based on the internal circumference/wall tension ratio of the segments by setting the internal circumference, L1, to 90% of a value determined by a passive tension equivalent to a transmural pressure of 100 mmHg (Mulvany and Halpern, 1977). The diameter (I1) was determined according to the equation I1 = L1/π, using specific software for normalization of resistance arteries (DMT Normalization Module; AD Instruments, Sydney, Australia). Segments were left to equilibrate for 30 min. Vessel contractility was then tested by an initial exposure to a high-K+ (120 mmol·l−1) solution. Where required, endothelium was denuded by introducing an air bubble into the artery lumen for 1 min followed by a wash with KHS. Endothelium removal was confirmed by the absence of relaxation to ACh (10 μM) in phenylephrine (PE, 1–10 μM) precontracted arteries.
Arteries were precontracted using U46619 (1 μM) to produce a maximal, sustained contraction similar to that stimulated by KCl (120 mmol·l−1) (Rossoni et al., 2011). After the contraction stabilized, increasing cumulative concentrations of eugenol (1–1000 μM) were applied. Effects of eugenol (100 μM) were also studied on U46619 (1 μM) precontracted arteries in the presence of L-NAME (100 μM), a NOS inhibitor; indomethacin (10 μΜ), a COX inhibitor; a combination of TRAM-34 (1 μM) and apamin (1 μM), intermediate and small conductance Ca2+-activated K+ channel inhibitors, respectively, or HC-067047 (100 nM), a TRPV4 inhibitor.
Cell culture and TRPV4 knockdown
Rat mesenteric artery endothelial cells (Cell Biologics, Chicago, IL, USA) of passage 2 were maintained as recommended by the supplier under standard tissue culture condition (21% O2–5% CO2; 37°C). Short interfering RNAs (siRNA; Silencer® Select siRNA, Ambion®, Life Technologies, Carlsbad, CA, USA) specifically targeting TRPV4 were inserted into endothelial cells by transient transfection using Effectene® reagent (Qiagen, Valencia, CA, USA). Sense and anti-sense nucleotide sequences for TRPV4 siRNA were: AGAGAACCCUCACAAGAAAtt and UUUCUUGUGAGGGUUCUCUgt respectively. All experimental measurements were performed 36–48 h post-transfection. Non-targeting siRNA (Silencer® Select Negative Control n° 1, Ambion®, Life Technologies) was used as a negative control.
Western blotting
Cultured endothelial cell lysate protein concentration was determined spectrophotometrically with amido black solution. Proteins (<40 μg per lane) were separated on a 7.5% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. Blots were physically cut at 75 kDa to permit probing for TRPV4 at a higher MW and actin at the lower MW. Membranes were incubated with rabbit polyclonal anti-TRPV4 (1:1000, Abcam, Cambrigde, UK) or anti-actin (1:5000, EMD Millipore, Billerica, MA, USA) primary antibodies overnight at 4°C in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) and 5% non-fat dry milk. Proteins were visualized using HRP-conjugated secondary antibody (1:10,000 dilution; Pierce, Rockford, IL, USA) and a chemiluminescent detection kit (Pierce, Rockford, IL, USA).
Patch clamp electrophysiology
Non-selective cation currents (ICat) were recorded in cultured endothelial cells of rat mesenteric artery. Endothelial cells were trypsinized and allowed to adhere to glass coverslips for 2 h at 37°C. Coverslips were transferred to a recording chamber and whole-cell patch clamp recordings were acquired at room temperature using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) and pCLAMP 8.2 (Molecular Devices, Sunnyvale, CA, USA). Borosilicate glass electrodes of resistance 3–6 MΩ were filled with pipette solution containing (in mmol·L−1): 120 NaGlutamate, 20 NaCl, 1 MgCl2, 1 EGTA, 10 HEPES, 4 Na2ATP (pH 7.2). The extracellular bath solution contained (in mmol·L−1): 142 NaCl, 2 CaCl2, 6 KCl, 1 MgCl2, 10 HEPES and 10 glucose. Isolated cells with intact membranes that were not attached to neighbouring cells were used for patch clamp electrophysiology. Cell capacitance was measured by application of a 5 mV test pulse and correcting transients with series resistance compensation. ICat were recorded by applying voltage ramps (940 ms) every 20 s, ramping between −120 and +100 mV, from a 0 mV holding potential. Whole-cell currents were filtered at 1 kHz and digitized at 5 kHz.
In vivo BP measurement
Male Sprague-Dawley rats (250 g) were anaesthetized by an initial i.p. injection of sodium pentobarbital (50 mg·kg−1). Anaesthesia was maintained through i.v. injection of sodium pentobarbital based on constant monitoring of reflexes in response to hindlimb pinching or blinking evoked by a low-pressure corneal stimulation. Catheters (PE-10 fused to PE-50) filled with heparin (125 IU·mL−1)-treated saline solution were implanted in the abdominal aorta to record arterial BP and in the inferior vena cava for drug administration, through the left femoral artery and vein respectively. The aortic catheter was connected to a BP transducer (Living System Instrumentation, Burlington, VT, USA) coupled to an interface and software (Power Lab/8SP; AD Instruments) for systolic and diastolic pressure acquisition at a sampling frequency of 1 kHz. Mean arterial pressure (MAP) was calculated as diastolic pressure + [(systolic − diastolic)/3]. Heart rate was determined from pressure pulse intervals. Animals were allowed to stabilize for 15 min before starting experiments. Baseline values of MAP and heart rate were determined and changes measured during a 30 min post-injection period. Eugenol (5 mg·kg−1, i.v.) was administered as a bolus (100 μL) followed by a 50 μL flush with physiological saline. The effects of eugenol on BP took ∼45 min to reverse. Ten min after BP returned to baseline, HC-067047 (9 μg·kg−1, i.v.) was injected. Eugenol was then applied i.v. 5 min after HC-067047.
Chemicals
PE, ACh and L-NAME were first diluted in distilled water to 10−1 M. U46619 and HC-067047 were diluted in ethanol to 10−2 M. Indomethacin and apamin were first dissolved in acetic acid (0.05 M) and tris-HCl solution (pH 8.0), respectively, to 10−2 M. TRAM-34 was diluted in DMSO to 10−2 M. For in vitro experiments, eugenol (Sigma code #E51791) was first diluted in DMSO, brought to volume with KHS and sonicated immediately before use. For in vivo experiments, eugenol was dissolved in Tween 80 (2%), brought to final volume (100 μL) with sterile isotonic saline and sonicated before use. Final concentrations of ethanol and DMSO were ≥0.2%. Unless otherwise stated, all reagents were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). HC-067047 and TRAM-34 were purchased from Tocris Bioscience (Bristol, UK).
The drug/molecular target nomenclature conforms to BJP's Concise Guide to PHARMACOLOGY (Alexander et al., 2013a, b, c).
Statistical analysis
Data are expressed as means ± SEM. Individual eugenol concentration–response curves and mean concentration–response data were both fit with non-linear regression analysis using a four parameter logistic equation to calculate EC50 and Hill slope. Statistical significance was calculated by using Student's t-tests for paired or unpaired data and anova followed by Bonferroni's post hoc test for multiple datasets. P < 0.05 was considered significant.
Results
Eugenol induces endothelium-dependent relaxation of rat mesenteric arteries
Mesenteric artery rings with intact endothelium were mounted and a contraction was induced by U46619, a thromboxane A2 receptor agonist, with a mean tension development of 3.8 ± 0.2 mN·mm−1 (Figure 1A). Bath application of eugenol caused concentration-dependent relaxation of this contraction, which was significant at concentrations higher than 30 μM (Figure 1C). Non-linear regression analysis of individual concentration-response experiments produced a mean EC50 of 47.9 ± 6.3 μM and a slope of 4.0 ± 0.6. The vehicle (DMSO) for eugenol did not alter arterial contractility. The highest DMSO concentration used, which was that needed to dissolve 1 mM eugenol, alone did not alter contractility (94.8 ± 5.7% of control force, n = 8, P > 0.05). Endothelium denudation significantly attenuated eugenol-induced vasorelaxation (Figure 1B, C). In endothelium-denuded arteries, eugenol-induced relaxation was significant at concentrations higher than 100 μM, with an EC50 of 189.9 ± 19.3 μM and slope of 2.1 ± 0.4 (Figure 1B, C). These data indicate that eugenol relaxes mesenteric arteries, in part, via an endothelium-mediated mechanism.
Figure 1.

Eugenol relaxes endothelium-intact and -denuded mesenteric arteries. (A) Representative recording of concentration-dependent eugenol-induced relaxation in an endothelium-intact artery precontracted with U46619 (1 μM). The left trace shows the ACh (10 μM) -induced relaxation of a phenylephrine (PE)-induced contraction in the same artery. (B) Representative recording of concentration-dependent eugenol-induced relaxation in an endothelium-denuded artery precontracted by U46619 (1 μM). The left trace illustrates the lack of an ACh (10 μM)-induced relaxation of a PE-induced contraction in the same artery. (C) Mean data fit with non-linear regression analysis: endothelium-intact (+EC, n = 9) and -denuded (-EC, n = 5). *,§P < 0.05 versus control and +EC respectively.
Endothelium-dependent eugenol-induced relaxation involves NOS, KCa and TRPV4 channels
To investigate the endothelial cell-mediated mechanism that contributes to eugenol-induced relaxation, experiments were performed in the presence L-NAME, a NOS inhibitor, indomethacin, a COX inhibitor, TRAM-34 and apamin, inhibitors of intermediate (IKCa) and small conductance (SKCa) Ca2+-activated K+ channels, respectively, or HC-067047, a TRPV4 blocker. Relaxation to eugenol was measured in both the absence and presence of these inhibitors in the same endothelium-intact mesenteric arteries.
Each blocker caused a small relaxation, except L-NAME which increased tone (Supporting Information Fig. S1). L-NAME, a combination of TRAM-34 and apamin, or HC-067047 each attenuated eugenol-induced vasorelaxation to ∼55.5, 58.2 and 46.4%, respectively, without altering the relaxation rate (Figure 2A, B, Supporting Information Table S1). Eugenol-induced relaxation in HC-067047 + L-NAME or HC-067047 + TRAM/apamin was similar to that in the presence of each blocker alone, indicating a similar mechanism is involved (Figure 2B). In contrast, indomethacin did not alter the eugenol-induced relaxation (Figure 2A, B). Repetitive applications of eugenol caused similar-sized relaxation responses (first application, 55.7 ± 6.5% relaxation; second application, 62.2 ± 7.6% relaxation; n = 6, P > 0.05), indicating that the altered responses in the presence of the blockers was not due to desensitization of the eugenol effect (Figure 2D). To investigate whether eugenol-induced, endothelial-independent relaxation occurred through TRPV4, SKCa and IKCa channels, similar experiments were performed in endothelium-denuded arteries. HC-067047 reduced eugenol-induced relaxation in endothelium-denuded arteries to ∼73.6% of control (Figure 2C). In contrast, TRAM-34/apamin did not alter eugenol-induced relaxation in endothelium-denuded arteries (Figure 2C). These data indicate that endothelial NOS, SKCa and IKCa channels and TRPV4, but not COX, are involved in eugenol-induced endothelium-dependent vasorelaxation. Eugenol-induced TRPV4 channel activation also contributes to endothelium-independent relaxation.
Figure 2.
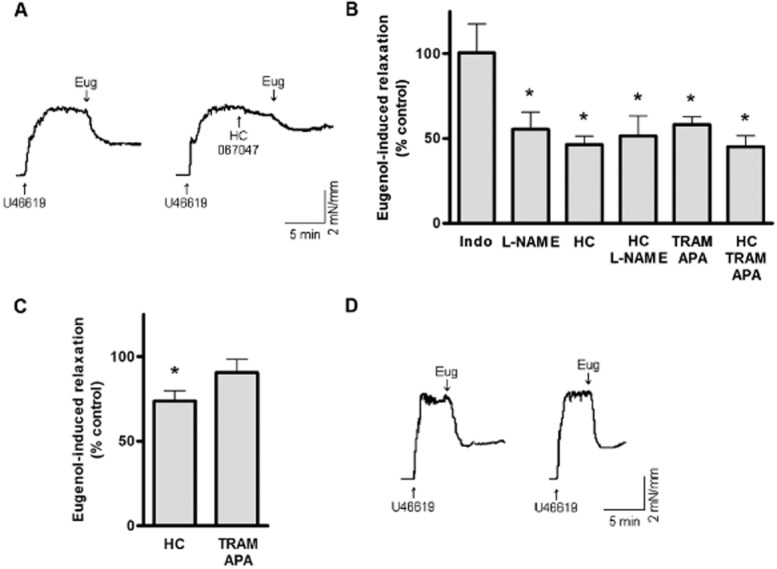
Inhibitors of endothelial signalling attenuate eugenol-induced vasorelaxation. (A) Representative recordings of eugenol (100 μM)-induced relaxation alone or in the presence of HC-067047 (100 nM) in the same endothelium-intact artery. (B) Mean data for the dilatation induced by eugenol (100 μM) in endothelium-intact arteries in the presence of: indomethacin (10 μM, n = 6), L-NAME (100 μM, n = 5), HC-067047 (100 nM, n = 6), HC-067047 plus L-NAME (n = 5), TRAM-34 plus apamin (1 μM each, n = 6), or HC-067047 plus TRAM-34/apamin (n = 6). *P < 0.05 versus eugenol alone. (C) Mean data for eugenol (100 μM)-induced dilatation in endothelium-denuded arteries precontracted by U46619 (1 μM) in the presence of HC-067047 (100 nM, n = 7) or TRAM-34 plus apamin (1 μM each, n = 6). *P < 0.05 versus eugenol alone. (D) Representative recording of two eugenol (100 μM)-induced relaxations in the same endothelium-intact artery.
Eugenol stimulates TRPV4-mediated currents in mesenteric artery endothelial cells
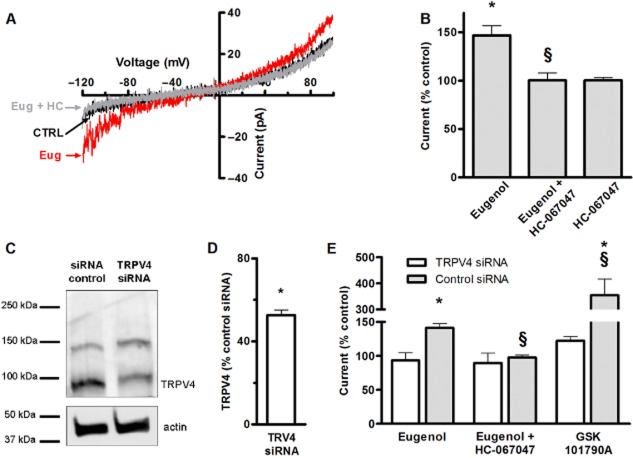
We focused on directly testing the hypothesis that eugenol activates TRPV4 channels by performing patch clamp electrophysiological experiments on mesenteric artery endothelial cells. ICat were stimulated by applying repetitive 940 ms ramp pulses from −120 to +100 mV from a holding potential of 0 mV. Eugenol increased mean ICat (at −100 mV) from −14.7 ± 2.17 pA to −21.8 ± 4.02 pA or 1.48-fold (Figure 3A, B). HC-067047, a TRPV4 inhibitor, alone did not alter ICat, but blocked eugenol activation of ICat (Figure 3A, B).
Figure 3.
Eugenol activates TRPV4 currents in mesenteric artery endothelial cells. (A) Representative recordings from the same cell illustrating ICat elicited by voltage ramps from −120 to +100 mV in control conditions, steady-state activation by eugenol (100 μM) before and after the addition of HC-067047 (100 nM). (B) Mean data illustrating the peak ICat at −100 mV in control conditions (n = 6), and in the presence of eugenol (100 μM, n = 8), eugenol + HC-067047 (100 nM, n = 8), and HC-067047 (100 nM, n = 7). *,§P < 0.05 versus control and eugenol respectively. (C) Representative blots illustrating both TRPV4 and actin total protein in mesenteric artery endothelial cells treated with control siRNA and TRPV4 siRNA. (D) Mean densitometry data of TRPV4 total protein in endothelial cells treated with TRPV4 siRNA (n = 5) versus control (n = 5). *P < 0.05 versus control. (E) Mean data illustrating ICat peak at −100 mV in cells treated with control siRNA (n = 5) and TRPV4 siRNA (n = 5), exposed to eugenol (100 μM), eugenol + HC-067047 (100 nM) or GSK101790A (10 nM). *,§P < 0.05 versus control and eugenol respectively.
Eugenol-induced ICat activation was also studied in endothelial cells in which TRPV4 expression was reduced using siRNA, with control siRNA used as a control. TRPV4 siRNA reduced TRPV4 protein to ∼52% of control, but did not alter basal current density (pA pF-1: control 1.5 ± 0.3, TRPV4 siRNA, 1.8 ± 0.1, n = 5 for each, Figure 3C, D). GSK1016790A (10 nM), a TRPV4 agonist, increased mean ICat ∼3.54-fold in TRPV4 siRNA-treated cells, but only 1.22-fold in control cells (Figure 3E). These data indicate that endothelial cells were functional after transfection and TRPV4 siRNA reduced TRPV4 expression and currents. Eugenol increased mean ICat ∼1.45-fold in cells transfected with control siRNA and this current was inhibited by HC-067047 in a similar manner to that observed in non-transfected cells (Figure 3B). In contrast, eugenol did not alter ICat and HC-067047 did not alter ICat when applied in the presence of eugenol in TRPV4 knockdown cells (Figure 3E). Collectively, these data indicate that eugenol activates TRPV4-mediated currents in mesenteric artery endothelial cells.
Eugenol reduces systemic BP via TRPV4 channel activation
The regulation of systemic BP by eugenol was studied in vivo in anaesthetized rats. An i.v. injection of eugenol caused a biphasic response composed of an immediate transient reduction in BP and a prolonged hypotension (Figure 4A). The first component peaked ∼10 s after eugenol injection and returned to baseline within 1 min. The second prolonged component was initiated ∼5 min after eugenol injection and was maintained for ∼25 min (Figure 4A).
Figure 4.
Eugenol reduces systemic BP by activating TRPV4 channels. (A) Representative recording illustrating the effects of eugenol (5 mg·kg−1, i.v.) on BP. (B) Representative trace showing that HC-067047 (9 μg·kg−1, i.v.) attenuates the prolonged reduction in BP induced by eugenol (5 mg·kg−1, i.v.). (C) Mean data for the effects of eugenol (5 mg·kg−1, i.v.) on MAP in the absence (n = 5) or presence (n = 5) of HC-067047 (9 μg·kg−1, i.v.). *§P < 0.05 versus control and Eug 5 mg·kg−1 respectively. (D) Mean data for effects of eugenol (5 mg·kg−1, i.v.) on heart rate in the absence (n = 5) or presence (n = 5) of HC-067047 (9 μg·kg−1, i.v.). *P < 0.05 versus control.
Specifically, mean control MAP was 91.4 ± 3.1 mmHg (Figure 4C). The eugenol injection almost immediately reduced MAP to 63.4 ± 5.8 mmHg, or to ∼68.9% of control (Figure 4A, C). This reduction in BP was associated with bradycardia with a reduction in HR from ∼283.9 to 67.3 beats min-1, or to ∼23.7 % of control ∼5 s after eugenol injection. HR and MAP returned to pre-eugenol levels after 10 s and ∼1 min respectively Figure 4C, D). The sustained second component of hypotension plateaued ∼20 min after eugenol injection to ∼84.1% of control (Figure 4C). HC-067047 injection alone (i.v.) did not alter MAP 10 s or 5 min after application (100.8 ± 1.0 and 101.7 ± 1.6% of control, respectively, n = 5, P > 0.05). Similarly, HR was unaltered 5 s and 5 min after HC-067047 injection (101.1 ± 1.8 and 101.2 ± 0.4% of control, respectively, n = 5, P > 0.05). HC-067047 did not alter the initial eugenol-induced transient reduction in MAP and HR, which were to ∼65.8 and 19.7% of control respectively (Figure 4C, D). In contrast, HC-067047 abolished the sustained reduction in BP induced by eugenol (Figure 4B, C). It was shown that the alterations in MAP and HR induced by eugenol were not caused by the vehicle. Ten seconds and 20 min after vehicle injection, MAPs were 101.0 ± 5.4 and 103.0 ± 0.7% of control respectively (n = 5 and P > 0.05 for each). Also the vehicle alone did not alter HR, which was 101.1 ± 0.5 % of control 5 s after injection. These data indicate that eugenol produces both acute bradycardia and an acute and chronic reduction in systemic BP. The data also indicate that TRPV4 activation contributes to the second chronic component of eugenol-induced hypotension.
Discussion
Here, we investigated the endothelial-dependent vasodilatation evoked by eugenol in rat mesenteric arteries, the mechanisms involved and its functional significance. The major findings were that: (i) endothelium denudation attenuates eugenol-induced vasorelaxation in mesenteric arteries; (ii) relaxation induced by eugenol is reduced by NOS, TRPV4, IKCa and SKCa inhibitors, but not by a COX inhibitor; (iii) eugenol activates TRPV4-mediated currents in mesenteric artery endothelial cells; and (iv) eugenol reduced systemic BP via TRPV4 channel activation. These results indicate that eugenol activates TRPV4 channels in endothelial cells, leading to vasodilatation, and a reduction in systemic BP.
Endothelial cells communicate with smooth muscle cells to control contractility, and are, thereby, involved in the regulation of blood flow and BP. A defect in the function of endothelial cells, which can occur in response to abnormal conditions such as ageing and metabolic disease, is a hallmark of vascular pathology and a predictor of major cardiovascular events (Vanhoutte et al., 2009; Davel et al., 2011). The function of endothelial cells is controlled by numerous physiological and exogenous molecules in the circulation, including medicines and dietary derivatives such as eugenol. Our data indicate that eugenol-induced relaxation in mesenteric arteries is mediated, in part, via the endothelium. These results agree with previous studies demonstrating that eugenol-induced vasorelaxation is partially dependent on the endothelium in rat aorta, a conduit vessel, and a whole mesentery preparation (Criddle et al., 2003; Damiani et al., 2003). Eugenol also relaxed endothelium-denuded arteries. One explanation for this effect is that eugenol blocks voltage-dependent Ca2+ channels in arterial smooth muscle cells to induce vasodilatation (Peixoto-Neves et al., 2014). In the present study, HC-067047, a selective TRPV4 channel blocker, attenuated eugenol-induced relaxation in endothelium-denuded arteries, suggesting that TRPV4 channels in cells other than endothelial cells are involved in this response. Activation of TRPV4 channels in arterial smooth muscle cells stimulates BK channels, leading to membrane hyperpolarization and vasodilatation, providing one explanation for this result (Earley et al., 2005). Eugenol inhibited TMEM16A, a Ca2+-activated chloride channel, in communication-deficient rat thyroid-derived cell line cells expressing recombinant human TMEM16A (Yao et al., 2012). Eugenol also inhibited the ileal contraction induced by Eact, a TMEM16A channel activator (Yao et al., 2012). TMEM16A channel activation leads to membrane depolarization and contraction of arterial smooth muscle cells (Bulley et al., 2012; Dam et al., 2014). Conceivably, an inhibitory effect on TMEM16A channels in smooth muscle cells may also contribute to the eugenol-induced relaxation observed in the present study.
We hypothesized that the activation of TRPV4 channels and downstream signalling pathways in endothelial cells underlies eugenol-induced vasorelaxation. TRPV4 is expressed in rat mesenteric artery endothelial cells (Bagher et al., 2012; Ma et al., 2013). Endothelium-derived NO, EDHF and PGI2 have been shown to relax mesenteric arteries (Mulvany and Aalkjaer, 1990). Also TRPV4 activation leads to endothelium-dependent vasodilatation via NO and EDHF release (Vriens et al., 2005; Kohler et al., 2006; Saliez et al., 2008; Earley et al., 2009; Zhang et al., 2009; Mendoza et al., 2010). TRPV4 channels generate local Ca2+ signals, which activate IKCa and SKCa in endothelial cells to induce hyperpolarization, leading to vasodilatation (Sonkusare et al., 2012). Studies have also suggested that the activation of IKCa and SKCa is associated with NOS activation and the production of NO (Sheng and Braun, 2007; Dalsgaard et al., 2010; Stankevicius et al., 2011). Furthermore, NOS, SKCa and TRPV4 channels are located in caveolae, which suggests their close spatial proximity and functional interaction (Sbaa et al., 2005; Absi et al., 2007; Saliez et al., 2008; Michel and Vanhoutte, 2010).
In the present study, eugenol-induced vasorelaxation was attenuated by HC-067047 in endothelium-intact arteries. HC067047 attenuated relaxation induced by GSK1016790A, a TRPV4 agonist, in endothelium-intact arteries, suggesting this blocker is selective for TRPV4 channels (Sukumaran et al., 2013; Zhang et al., 2013). We showed that L-NAME or a combination of TRAM-34 and apamin attenuated eugenol-induced relaxation. Furthermore, the combined inhibition of TRPV4 channels and eNOS or IKCa/SKCa did not further reduce the eugenol-induced relaxation response. In contrast, indomethacin did not alter eugenol-induced vasorelaxation. These data indicate that eugenol-induced vasorelaxation involves TRPV4 channel activation in mesenteric artery endothelial cells. We also consider it reasonable to propose that eugenol-induced dilatation occurs as a result of activation of TRPV4-mediated downstream NOS/IKCa/SKCa signalling pathways, as proposed by others (Kohler et al., 2006; Mendoza et al., 2010; Sonkusare et al., 2012).
In the present study, indomethacin, TRAM 34/apamin, and HC067047 each caused a small relaxation of U46619-contracted arteries. In contrast, L-NAME increased the tone of the precontracted arteries. Previous studies have demonstrated a similar effect for indomethacin, which attenuated phenylephrine-induced contractions and myogenic tone, demonstrating the involvement of prostanoids, such as thromboxane A2 and PGE2, in the regulation of the arterial contractility (Jarajapu et al., 2012; Aloysius et al., 2012). HC-067047 and TRAM-34/apamin were also demonstrated to reduce tension in pulmonary and cerebral arteries, which was associated with off-target effects, probably reducing Ca2+ entry to vascular smooth muscle cells (McNeish et al., 2010; Xia et al., 2013).
TRPV4 agonists, such as GSK1016790A, have been shown to damage endothelial cells (Bagher et al., 2012; Sonkusare et al., 2012). Here, repetitive eugenol applications produced relaxant responses of similar amplitude, indicating that endothelial cell damage did not occur. We showed that eugenol produced a smaller TRPV4 current than GSK1016790A at concentrations typically used to study the functional involvement of TRPV4 channels. Thus, eugenol is likely to produce a smaller elevation in intracellular Ca2+ concentration than GSK1016790A, which may explain the lack of damage to endothelial cells.
Eugenol increased HC-067047-sensitive ICat in mesenteric artery endothelial cells. In corroboration of these data, eugenol did not alter ICat in endothelial cells subjected to TRPV4 knockdown. As a positive control, GSK1016790A stimulated currents in control siRNA cells, but not in TRPV4 knockdown cells, demonstrating that ICat were functional after transfection and TRPV4 channels were knocked down by TRPV4 siRNA. Taken together, these data indicate that eugenol activates TRPV4 channels in mesenteric artery endothelial cells to induce vasorelaxation.
In the present study, i.v. administration of eugenol reduced both BP and heart rate. The reduction in BP consisted of two components; the first component is likely to be associated with the reduction in heart rate as these events were temporally aligned. Eugenol inhibits L-type Ca2+ channels in canine cardiomyocytes and induces a negative inotropic effect in rat left ventricle papillary muscle by inhibiting extracellular Ca2+ influx, providing one explanation for this observation (Damiani et al., 2004; Magyar et al., 2004). It has also been suggested that eugenol-induced bradycardia and hypotension are independent events (Lahlou et al., 2004). The bradycardia was attenuated in rats subjected to vagotomy and by hexamethonium and methylatropine, blockers of nicotinic and muscarinic receptors, respectively, suggesting bradycardia may be of vagal origin (Lahlou et al., 2004). Our data suggest that the second prolonged eugenol-induced reduction in BP is due to vasodilatation, as there was no concomitant alteration in heart rate during this phase, and this effect was attenuated by HC-067047. Providing further support for our observation, 4α-phorbol 12, 13-didecanoate, a TRPV4 channel agonist, reduces BP in part via KCa channel activation in resistance arteries and by the release of calcitonin gene-related peptide from sensory neurons (Gao and Wang, 2010). These results support the concept that eugenol-induced TRPV4 channel activation leads to vasorelaxation and a reduction in systemic BP in vivo.
It is appropriate to compare the potency of eugenol-induced smooth muscle cell relaxation in mesenteric arteries with that in other tissues, although different experimental conditions used among studies make such a comparison exploratory. Our results indicate an EC50 for eugenol of ∼48 μM in small mesenteric arteries. In rat endothelium-intact cerebral arteries and aorta, eugenol induced relaxation with EC50s of ∼235 and ∼140 μM respectively (Damiani et al., 2003; Peixoto-Neves et al., 2014). In tracheal smooth muscle, the eugenol EC50 was ∼570 μM (Lima et al., 2011). Although these studies were performed in a small number of smooth muscle containing tissues, the potency of eugenol appears to be higher in resistance size systemic arteries. Similarly, eugenol appears to be a better relaxant of vasculature than airway, which may be therapeutically beneficial for reducing BP in patients while limiting any side effects.
Our data suggest that eugenol is a more effective activator of TRPV4 than other TRPV channel family members. Eugenol activated an inward current with an EC50 of 0.73 mM that was partially reduced by capsazepine, a TRPV1 blocker, in dorsal root ganglia (Ohkubo and Kitamura, 1997). In HEK cells expressing recombinant TRPV1 channels and trigeminal ganglion neurons, 1 mM eugenol activated inward currents, which were blocked by capsazepine and ruthenium red (Yang et al., 2003). In keratinocytes and HEK cells expressing recombinant mTRPV3 and hTRPV3 channels, 2 mM eugenol activated currents (Xu et al., 2006). In the present study, endothelium denudation increased the EC50 of eugenol-induced relaxation from ∼48 to 190 μM. HC-067047 reduced eugenol-induced relaxation by ∼54%. At a concentration of 100 μM, eugenol also activated ICat in endothelial cells and this effect was inhibited by HC-067047. Collectively, these studies suggest that eugenol is more selective for TRPV4 channels than other TRPV family members.
In summary, our study demonstrates for the first time that eugenol-induced vasorelaxation occurs via TRPV4 channel activation in endothelial cells of resistance arteries. We also showed that eugenol reduces systemic BP via TRPV4 channel activation. Eugenol may be therapeutically useful as an antihypertensive agent and appears to be a viable molecular candidate from which to develop more potent TRPV4 channel activators that have the ability to reduce BP.
Glossary
- EDHF
endothelium-derived hyperpolarizing factor
- ICat
non-selective cation currents
- IKCa
intermediate conductance calcium-activated potassium channel
- MAP
mean arterial pressure
- PGI2
prostacyclin
- siRNA
short interfering RNA
- SKCa
small conductance calcium-activated potassium channel
- TRP
transient receptor potential
Acknowlegements
The authors thank Dr Pedro Jorge Caldas Magalhães (Universidade Federal do Ceará, Brazil) for kindly allowing the use of his laboratory for some experiments. This work was supported by the National Institutes of Health awards to J. H. J.
Author contributions
D. P.-N. and Q. W. performed the research. J. H. J. designed the research study. L. V. R. and J. H. L.-C. contributed essential reagents or tools. D. P.-N. and J. H. J. analysed the data. D. P.-N., J. H. J., J. H. L.-C. wrote the paper.
Conflict of interest
None.
Supporting Information
Figure S1 Regulation of U46619-induced contraction by endothelial signalling inhibitors. Indomethacin (10 μM, n = 6), L-NAME (100 μM, n = 5), HC-067047 (100 nM, n = 6), HC-067047 plus L-NAME (n = 5), TRAM-34 plus apamin (1 μM each, n = 6), or HC-067047 plus TRAM-34/apamin (n = 6). *P < 0.05 versus control.
Table S1 Relaxation rate (τ) of eugenol-induced relaxation alone and in presence of L-NAME (100 μM), HC-067047 (100 nM), HC-067047 plus L-NAME, TRAM-34 plus apamin (1 μM each), or HC-067047 plus TRAM-34/apamin in the same arteries.
References
- Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SKCa channels and caveolin-rich domains. Br J Pharmacol. 2007;151:332–340. doi: 10.1038/sj.bjp.0707222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The concise guide to pharmacology 2013/14: ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloysius UI, Achike FI, Mustafa MR. Mechanisms underlining gender differences in Phenylephrine contraction of normoglycaemic and short-term Streptozotocin-induced diabetic WKY rat aorta. Vascul Pharmacol. 2012;57:81–90. doi: 10.1016/j.vph.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res. 2012;111:1027–1036. doi: 10.1161/CIRCRESAHA.112.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto JB, Kassuya CA, Andre E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Criddle DN, Madeira SV, de Soares MR. Endothelium-dependent and -independent vasodilator effects of eugenol in the rat mesenteric vascular bed. J Pharm Pharmacol. 2003;55:359–365. doi: 10.1211/002235702694. [DOI] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Misfeldt M, Bek T, Simonsen U. Openers of small conductance calcium-activated potassium channels selectively enhance NO-mediated bradykinin vasodilatation in porcine retinal arterioles. Br J Pharmacol. 2010;160:1496–1508. doi: 10.1111/j.1476-5381.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam VS, Boedtkjer DM, Nyvad J, Aalkjaer C, Matchkov V. TMEM16A knockdown abrogates two different Ca2+-activated Cl− currents and contractility of smooth muscle in rat mesenteric small arteries. Pflugers Arch. 2014;466:1391–1409. doi: 10.1007/s00424-013-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani CE, Rossoni LV, Vassallo DV. Vasorelaxant effects of eugenol on rat thoracic aorta. Vascul Pharmacol. 2003;40:59–66. doi: 10.1016/s1537-1891(02)00311-7. [DOI] [PubMed] [Google Scholar]
- Damiani CE, Moreira CM, Zhang HT, Creazzo TL, Vassallo DV. Effects of eugenol, an essential oil, on the mechanical and electrical activities of cardiac muscle. J Cardiovasc Pharmacol. 2004;44:688–695. doi: 10.1097/00005344-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Davel AP, Wenceslau CF, Akamine EH, Xavier FE, Couto GK, Oliveira HT, et al. Endothelial dysfunction in cardiovascular and endocrine-metabolic diseases: an update. Braz J Med Biol Res. 2011;44:920–932. doi: 10.1590/s0100-879x2011007500104. [DOI] [PubMed] [Google Scholar]
- Earley S, Brayden JE. Transient receptor potential channels and vascular function. Clin Sci (Lond) 2010;119:19–36. doi: 10.1042/CS20090641. [DOI] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–H1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero G, Paganelli C, Buffoli B, Rodella LF, Rezzani R. Endothelium and its alterations in cardiovascular diseases: life style intervention. Biomed Res Int. 2014;2014:801896. doi: 10.1155/2014/801896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013;61:113–119. doi: 10.1097/FJC.0b013e318279ba42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wang DH. Hypotension induced by activation of the transient receptor potential vanilloid 4 channels: role of Ca2+-activated K+ channels and sensory nerves. J Hypertens. 2010;28:102–110. doi: 10.1097/HJH.0b013e328332b865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239:279–283. doi: 10.1006/bbrc.1997.7458. [DOI] [PubMed] [Google Scholar]
- Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D. Pharmacological studies on Myristica fragrans – antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Exp Clin Pharmacol. 2002;24:675–680. doi: 10.1358/mf.2002.24.10.802317. [DOI] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, et al. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–H2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, et al. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interaminense LF, Juca DM, Magalhaes PJ, Leal-Cardoso JH, Duarte GP, Lahlou S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam Clin Pharmacol. 2007;21:497–506. doi: 10.1111/j.1472-8206.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Grant MB, Knot HJ. Myogenic tone and reactivity of the rat ophthalmic artery. Invest Ophthalmol Vis Sci. 2004;45:253–259. doi: 10.1167/iovs.03-0546. [DOI] [PubMed] [Google Scholar]
- Kamatou GP, Vermaak I, Viljoen AM. Eugenol – from the remote Maluku Islands to the international market place: a review of a remarkable and versatile molecule. Molecules. 2012;17:6953–6981. doi: 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- Lahlou S, Interaminense LF, Magalhaes PJ, Leal-Cardoso JH, Duarte GP. Cardiovascular effects of eugenol, a phenolic compound present in many plant essential oils, in normotensive rats. J Cardiovasc Pharmacol. 2004;43:250–257. doi: 10.1097/00005344-200402000-00013. [DOI] [PubMed] [Google Scholar]
- Lima FC, Peixoto-Neves D, Gomes MD, Coelho-de-Souza AN, Lima CC, Araujo ZW, et al. Antispasmodic effects of eugenol on rat airway smooth muscle. Fundam Clin Pharmacol. 2011;25:690–699. doi: 10.1111/j.1472-8206.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, et al. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension. 2013;62:134–139. doi: 10.1161/HYPERTENSIONAHA.113.01500. [DOI] [PubMed] [Google Scholar]
- Magyar J, Szentandrassy N, Banyasz T, Fulop L, Varro A, Nanasi PP. Effects of terpenoid phenol derivatives on calcium current in canine and human ventricular cardiomyocytes. Eur J Pharmacol. 2004;487:29–36. doi: 10.1016/j.ejphar.2004.01.011. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish AJ, Altayo FJ, Garland CJ. Evidence both L-type and non-L-type voltage-dependent calcium channels contribute to cerebral artery vasospasm following loss of NO in the rat. Vascul Pharmacol. 2010;53:151–159. doi: 10.1016/j.vph.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, et al. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–H476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch. 2010;459:807–816. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany MJ, Aalkjaer C. Structure and function of small arteries. Physiol Rev. 1990;70:921–961. doi: 10.1152/physrev.1990.70.4.921. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Kitamura K. Eugenol activates Ca2+-permeable currents in rat dorsal root ganglion cells. J Dent Res. 1997;76:1737–1744. doi: 10.1177/00220345970760110401. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto-Neves D, Leal-Cardoso JH, Jaggar JH. Eugenol dilates rat cerebral arteries by inhibiting smooth muscle cell voltage-dependent calcium channels. J Cardiovasc Pharmacol. 2014;64:401–406. doi: 10.1097/FJC.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P, Pigera S, Premakumara GA, Galappaththy P, Constantine GR, Katulanda P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement Altern Med. 2013;13:275. doi: 10.1186/1472-6882-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossoni LV, Wareing M, Wenceslau CF, Al-Abri M, Cobb C, Austin C. Acute simvastatin increases endothelial nitric oxide synthase phosphorylation via AMP-activated protein kinase and reduces contractility of isolated rat mesenteric resistance arteries. Clin Sci. 2011;121:449–458. doi: 10.1042/CS20110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sbaa E, Frerart F, Feron O. The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc Med. 2005;15:157–162. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sheng JZ, Braun AP. Small- and intermediate-conductance Ca2+-activated K+ channels directly control agonist-evoked nitric oxide synthesis in human vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C458–C467. doi: 10.1152/ajpcell.00036.2007. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankevicius E, Dalsgaard T, Kroigaard C, Beck L, Boedtkjer E, Misfeldt MW, et al. Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat. J Pharmacol Exp Ther. 2011;339:842–850. doi: 10.1124/jpet.111.179242. [DOI] [PubMed] [Google Scholar]
- Sukumaran SV, Singh TU, Parida S, Narasimha Reddy CE, Thangamalai R, Kandasamy K, et al. TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery. Pharmacol Res. 2013;78:18–27. doi: 10.1016/j.phrs.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Umar A, Imam G, Yimin W, Kerim P, Tohti I, Berke B, et al. Antihypertensive effects of Ocimum basilicum L. (OBL) on blood pressure in renovascular hypertensive rats. Hypertens Res. 2010;33:727–730. doi: 10.1038/hr.2010.64. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, et al. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol. 2013;305:C704–C715. doi: 10.1152/ajpcell.00099.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Imaeda K, Suzuki H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J Physiol. 1999;514(Pt 2):505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, et al. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res. 2003;82:781–785. doi: 10.1177/154405910308201004. [DOI] [PubMed] [Google Scholar]
- Yao Z, Namkung W, Ko EA, Park J, Tradtrantip L, Verkman AS. Fractionation of a herbal antidiarrheal medicine reveals eugenol as an inhibitor of Ca2+-activated Cl− channel TMEM16A. PLoS ONE. 2012;7:e38030. doi: 10.1371/journal.pone.0038030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Gutterman DD. Transient receptor potential channel activation and endothelium-dependent dilation in the systemic circulation. J Cardiovasc Pharmacol. 2011;57:133–139. doi: 10.1097/FJC.0b013e3181fd35d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, et al. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension. 2009;53:532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer's disease. Br J Pharmacol. 2013;170:661–670. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Regulation of U46619-induced contraction by endothelial signalling inhibitors. Indomethacin (10 μM, n = 6), L-NAME (100 μM, n = 5), HC-067047 (100 nM, n = 6), HC-067047 plus L-NAME (n = 5), TRAM-34 plus apamin (1 μM each, n = 6), or HC-067047 plus TRAM-34/apamin (n = 6). *P < 0.05 versus control.
Table S1 Relaxation rate (τ) of eugenol-induced relaxation alone and in presence of L-NAME (100 μM), HC-067047 (100 nM), HC-067047 plus L-NAME, TRAM-34 plus apamin (1 μM each), or HC-067047 plus TRAM-34/apamin in the same arteries.