Abstract
Background and Purpose
Sativex® is an oromucosal spray, containing equivalent amounts of Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD)-botanical drug substance (BDS), which has been approved for the treatment of spasticity and pain associated to multiple sclerosis (MS). In this study, we investigated whether Sativex may also serve as a disease-modifying agent in the Theiler's murine encephalomyelitis virus-induced demyelinating disease model of MS.
Experimental Approach
A Sativex-like combination of phytocannabinoids and each phytocannabinoid alone were administered to mice once they had established MS-like symptoms. Motor activity and the putative targets of these cannabinoids were assessed to evaluate therapeutic efficacy. The accumulation of chondroitin sulfate proteoglycans (CSPGs) and astrogliosis were assessed in the spinal cord and the effect of Sativex on CSPGs production was evaluated in astrocyte cultures.
Key Results
Sativex improved motor activity – reduced CNS infiltrates, microglial activity, axonal damage – and restored myelin morphology. Similarly, we found weaker vascular cell adhesion molecule-1 staining and IL-1β gene expression but an up-regulation of arginase-1. The astrogliosis and accumulation of CSPGs in the spinal cord in vehicle-infected animals were decreased by Sativex, as was the synthesis and release of CSPGs by astrocytes in culture. We found that CBD-BDS alone alleviated motor deterioration to a similar extent as Sativex, acting through PPARγ receptors whereas Δ9-THC-BDS produced weaker effects, acting through CB2 and primarily CB1 receptors.
Conclusions and Implications
The data support the therapeutic potential of Sativex to slow MS progression and its relevance in CNS repair.
Tables of Links
| TARGETS |
|---|
| GPCRsa |
| CB1 receptors |
| CB2 receptors |
| Nuclear hormone receptorsb |
| PPAR-γ |
| Enzymesc |
| Arg-1, arginase 1 |
| LIGANDS |
|---|
| AM251 |
| AM630 |
| CBD, cannabidiol |
| IFN-γ |
| IL-10 |
| T0070907 |
| TNF-α |
| Δ9-THC, Δ9-tetrahydrocannabinol- |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a, b, c).
Introduction
Multiple sclerosis (MS), the most common cause of neurological disability in young adults, is a complex autoimmune disease characterized by inflammation, demyelination and axonal damage (Compston and Coles, 2008). Most patients with MS initially develop a relapsing-remitting disease course that it is followed by a secondary progressive clinical form. By contrast, 10–15% of MS patients develop a primary progressive form from the onset of the disease. Nevertheless, effective treatments for patients with either primary or secondary MS remain elusive. Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) is a well-established model of MS (Lipton and Dal Canto, 1976) that is unique in reproducing the putative viral aetiology of MS and for studying virus-induced autoimmunity (Miller et al., 1997). TMEV is a single-stranded RNA virus, which when inoculated intracranially in susceptible mouse strains, provokes the development of a chronic-progressive demyelinating disease. Moreover, as TMEV-IDD progresses, epitope spreading produces T-cell responses against myelin peptides.
The cannabinoids are currently being studied for their potential benefits in the treatment of MS and other neuroinflammatory/neurodegenerative diseases (Pryce and Baker, 2012; Velayudhan et al., 2014). Preclinical studies have demonstrated that cannabinoids can alleviate MS-associated symptoms, such as spasticity (Baker et al., 2000), and they exhibit anti-inflammatory (Arévalo-Martín et al., 2003; Croxford and Miller, 2003), antioxidant, anti-excitotoxic and neuroprotective properties (Pryce et al., 2003; Fernández-Ruiz et al., 2010; Loría et al., 2010). Cannabinoids are also protective to oligodendrocytes in vitro and in vivo (Molina-Holgado et al., 2002; Gómez et al., 2010; Mecha et al., 2012). Sativex® is an oromucosal spray that contains an equimolecular combination of Δ9-tetrahydrocannabinol-botanical drug substance (Δ9-THC-BDS) and cannabidiol-botanical drug substance (CBD-BDS) and it has been approved for use in patients with MS-related spasticity and neuropathic pain. Indeed, safety studies with Sativex have indicated a low risk for serious adverse drug reactions (Serpell et al., 2013). Evidence for a neuroprotective effect of Sativex was recently reported in inflammatory models of Huntington disease and in a transgenic murine model of amyotrophic lateral sclerosis (Valdeolivas et al., 2012; Moreno-Martet et al., 2014). Moreover, both components of Sativex have been described to independently modify immune responses in MS animal models (Maresz et al., 2007; Kozela et al., 2011; Mecha et al., 2013b). However, there are still no clinical studies that have investigated the potential of Sativex as a disease-modifying treatment for progressive MS.
The potential of cannabinoids to influence the progression of MS also includes the possibility that they may be used for repair processes. In MS, repair and remyelination occur in shadow plaques (Chang et al., 2012) but, as the disease progresses, remyelination becomes less efficient (Chang et al., 2002; Franklin, 2002; Goldschmidt et al., 2009). It is unlikely that the inability to remyelinate results from the absence of oligodendrocyte precursor cells (OPCs) in the adult CNS, as OPCs are present in elderly patients even decades after having been diagnosed with the disease (Chang et al., 2012). Alternatively, the failure in remyelination might reflect changes in the local extracellular environment, specifically those affecting the extracellular matrix (ECM) proteins and chondroitin sulfate proteoglycans (CSPGs). Post-injury deposition of CSPGs may be a protective CNS response to limit damage (Silver and Miller, 2004), yet CSPGs up-regulation in chronic MS provokes scar formation (Sobel and Ahmed, 2001; Mohan et al., 2010), which inhibits both axon regeneration and remyelination (Siebert and Osterhout, 2011; Lau et al., 2012). This is why CSPGs and hyaluronan are considered to be inhibitory molecules, the accumulation of which is thought to be the main reason for the failure of remyelination (Harlow and Macklin, 2014).
TMEV represents a suitable model to elucidate the role of glial scar formation in chronic inflammatory and demyelinating CNS lesions. Chronic demyelination in the TMEV model is accompanied by alterations in spinal cord ECM and astrogliosis (Haist et al., 2012) which in turn contribute to the failure in regeneration. Accordingly, limited remyelination in TMEV has been associated with insufficient oligodendroglial differentiation (Ulrich et al., 2008). While cannabinoids have been shown to improve remyelination (Arévalo-Martín et al., 2003), there is a little information about their effects on the ECM and CSPGs.
Thus, we set out to investigate the therapeutic potential of a Sativex-like combination of phytocannabinoids (Sativex) as a disease-modifying therapy in a model of primary progressive MS. TMEV-infected mice with established symptomatology were treated with Sativex or individual Δ9-THC-BDS or CBD-BDS, which improved the motor deficits associated with the disease. Indeed, Sativex reduced cellular infiltrates, decreased microglial activity and diminished axonal damage in the TMEV-infected mice. Moreover, myelin morphology was restored and the expression of proinflammatory cytokines and adhesion molecules were down-regulated by Sativex. Astrogliosis, the accumulation of CSPGs and the expression of the synthetic enzyme xylosyltransferase-I (XT-I), was also reduced by Sativex treatment. In addition, our results support the involvement of CB1 and CB2 receptors in the beneficial effects of Δ9-THC-BDS and of PPARγ receptors in the effects of CBD-BDS in TMEV-IDD.
Methods
Animals and Theiler's virus infection
All animal care and experimental procedures were performed in accordance with EU (2010/63/EU) and governmental guidelines (Royal Decree 53/2013 BOE n°34 and Comunidad de Madrid: ES 280790000184) and were approved by the Ethics Committee on Animal Experimentation of the Cajal Institute (CSIC) (protocol number: 2013/03 CEEA-IC). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 90 animals were used in the experiments described here.
TMEV-IDD-susceptible female SJL/J mice from Harlan (Barcelona, Spain) were maintained at our in-house colony (Cajal Institute, Madrid, Spain) on a 12 h light/dark cycle with ad libitum access to food and water. Four-week-old mice were intracerebrally inoculated in the right hemisphere with 2 × 106 plaque forming units of the Daniel strain of TMEV diluted in 30 μL of DMEM supplemented with 10% FCS (Lledó et al., 1999). Sham-operated mice received 30 μL of DMEM + 10% FCS alone.
Pharmacological treatments
Sham or TMEV-IDD mice were treated daily with vehicle alone or a 1:1 combination of botanical extracts enriched with either Δ9-THC botanical extract (Δ9-THC-BDS) containing 67.1% Δ9-THC, 0.3% CBD, 0.9% cannabigerol, 0.9% cannabichromene and 1.9% other phytocannabinoids or CBD: CBD botanical extract (CBD-BDS) containing 64.8% CBD, 2.3% Δ9-THC, 1.1% cannabigerol, 3.0% cannabichromene and 1.5% other phytocannabinoids (kindly provided by GW Pharmaceuticals Ltd., Cambridgeshire, UK). The dose of Sativex administered was 10 mg·kg−1 which corresponds to 5 mg·kg−1 of Δ9-THC-BDS plus 5 mg·kg−1 of CBD-BDS. Cannabinoids were prepared in a Tween-80 saline solution (1:16) gassing it with N2 to avoid oxidation and they were administered i.p. once daily from days 70 to 80 post-infection when the signs of disease were evident. The duration of the treatment was based on previous studies using the TMEV-IDD model (Docagne et al., 2007). Additional groups of mice received Δ9-THC-BDS (5 mg·kg−1) or CBD-BDS (5 mg·kg−1) administered separately. In an additional experiment, mice treated with Δ9-THC-BDS also received the CB1 receptor antagonist AM251 (2 mg·kg−1) or the CB2 receptor antagonist AM630 (2 mg·kg−1) (Tocris Bioscience, Bristol, UK) 30 min previously, whereas mice administered with CBD-BDS were treated 30 min beforehand with the PPARγ inhibitor T0070907 (5 mg·kg−1) (Cayman Chem, Ann Arbor, MI, USA). In all experiments and after 10 days of treatment, the motor activity was evaluated and the animals were killed with an overdose of anaesthetic for tissue collection.
Spontaneous motor activity
Locomotor activity was evaluated in mice using an activity cage (Activity Monitor System, Omnitech Electronics Inc., Columbus, OH, USA) coupled to a digiscan analyser. The number of times that the animals broke the horizontal or vertical sensor beams was measured in two 5 min sessions.
Tissue processing
Mice were anaesthetized with pentobarbital (Dolethal, 50 mg·kg−1 body weight, i.p.) and perfused transcardially. The spinal cord was fixed overnight in 4% paraformaldehyde (PFA) prepared in 0.1 M phosphate buffer (PB) and cryoprotected in sucrose solution in 0.1 M PB (15% followed by a 30%). Coronal and longitudinal spinal cord cryostat sections (15 and 30 μm thick; cryostat Leica Microsystems CM1900, Barcelona, Spain) were then processed for immunohistochemistry.
Immunohistochemistry
Free-floating spinal cord cryostat sections were washed with 0.1 M phosphate buffer (PB), PB + 0.2% Triton X-100 (PBT) and then blocked for 1 h at room temperature in blocking buffer (PBT and 5% normal goat serum: Vector Laboratories, Inc., Burlingame, CA, USA) after inhibiting the endogenous peroxidase for 3,3′ diaminobenzidine tetrahydrochloride (DAB) staining. The sections were then incubated overnight at 4°C with the primary antibody (Table 1). For colourimetric immunohistochemistry, microglial cells in spinal cord sections (30 μm thick) were stained with Iba-1 in blocking buffer. T lymphocytes were stained for CD4 and CD8 expression. We also assessed vascular cell adhesion molecule-1 (VCAM-1) staining. For immunofluorescence, SMI32 antibody was used to detect axonal damage (sections of 15 μm thick). In longitudinal and transversal 30 μm sections, axons were stained with Neurofilament-H and RIP antibodies. Astrocytes were stained with GFAP antibody, proliferative astrocyte CSPGs were stained with vimentin antibody and CSPGs with an anti-chondroitin sulfate antibody (CS56). After incubation with the primary antibody, the sections were rinsed with PBT and were incubated for 1 h with biotinylated antibodies (Vector Laboratories, Inc.). For immunofluorescence, an Alexa 488 Fluor-conjugated goat anti-rabbit antibody (for Neurofilament-H), goat anti-mouse antibody (for SMI-32 and GFAP; Molecular Probes Inc., Eugene, OR, USA) and Texas Red-conjugated donkey anti-mouse IgM (for CS56; Jackson Immunoresearch Europe, Suffolk, UK) were used. For DAB immunostaining, the sections were incubated for 1 h with a biotin–peroxidase complex (Vector Laboratories, Inc.) and with the chromogen DAB (Sigma-Aldrich, St. Louis, MO, USA). In all cases, the specificity of staining was confirmed by omitting the primary antibody.
Table 1.
Primary antibodies used for immunochemistry and Western blot analysis
| Antibody | Host | Dilution | Source |
|---|---|---|---|
| lba-1 | Rabbit | 1:1000 | Wako, Osaka, Japan |
| CD4 | Rat IgG | 1:1000 | Pharmingen, San Diego, CA, USA |
| CD8 | Rat IgG | 1:1000 | Pharmingen, San Diego, CA, USA |
| VCAM-1 | Mouse IgG | 1:500 | DSHB, University of Iowa, Ames, IA, USA |
| SMI32 | Mouse IgG | 1:1000 | Covance, Emeryville, CA, USA |
| Neurofilament-H | Rabbit IgG | 1:1000 | Millipore, Billerica, MA, USA |
| RIP | Mouse IgG | 1:1000 | DSHB, University of Iowa, Ames, IA, USA |
| GFAP | Mouse IgG | 1:1000 | Sigma-Aldrich; St Louis, MO, USA |
| Vimentin | Mouse IgG | 1:500 | Abcam, Cambridge, UK |
| CS56 | Mouse IgM | 1:250 | DSHB, University of Iowa, Ames, IA, USA |
| Neurocan-1F6 | Mouse IgG | 1:500/1:1000 (Western blot) | DSHB, University of Iowa, Ames, IA, USA |
| Mouse IgG | 1:10000 | Sigma-Aldrich, St Louis, MO, USA |
For immunocytochemistry, astrocytes maintained in culture for 48 h were fixed with PFA 4% for 20 min, incubated with blocking buffer and stained overnight at 4°C for neurocan, using an antibody that recognizes the N-terminal and full-length protein. The following day, the cells were rinsed and incubated for 1 h with Alexa 594 Fluor-conjugated goat anti-mouse antibody (Molecular Probes Inc.).
Microscopy and image analysis
Immunofluorescence images were acquired on a Leica TCS SP5 confocal microscope and for immunohistochemistry, with a Zeiss Axiocam high resolution digital colour camera. Individual optical sections were examined analysing five to six sections from at least five to six animals per group. Staining was quantified using Image J software (NIH, Bethesda, MD, USA), maintaining the threshold intensity constant to compare the experimental and control images obtained within the experiments. The data are presented as the percentage of the total area stained with respect to the sham-operated animals.
Inflammatory infiltrates analysis and Luxol fast blue (LFB) staining
The spinal cord sections were stained with haematoxylin and eosin (H&E) to analyse the infiltrates in the parenchyma and with LFB to define myelin integrity. Inflammatory infiltrates were evaluated by scoring the infiltrate in the spinal cord on a scale of 0 to 4: a score of 0 reflects the absence of infiltrates; 4 reflects the maximal infiltrate; while the intermediate scores of 1, 2 and 3 define the increasing infiltrate density in the tissue.
For LFB staining, free-floating spinal cord sections (15 μm thick) were washed with 0.1 M PB. Tissue samples were dehydrated successively in an ethanol series from 70 to 95% and the sections were then incubated in LFB solution at 56oC in an oven overnight. The following day, the excess stain was rinsed off from the sections with 95% ethanol and the slides were contrasted in lithium carbonate solution for 30 s. Finally, the sections were dehydrated, cleared with xylene and cover slipped.
Astrocyte cell cultures
Astrocyte cultures were prepared from postnatal Wistar pups (0–2 days of age) as described previously (Mecha et al., 2011). After isolation, the astrocytes were plated in poly-D-lysine-coated 6-well plates at a density of 6 × 105 cells·mL−1 for Western blotting and PCR analysis or in 24-well plates with coverslips at a density of 5 × 104 cells·mL−1 for immunocytochemistry and grown in DMEM medium supplemented with 5% horse serum, 5% FBS, 100 U·mL−1 penicillin and 100 mg·mL−1 streptomycin. The medium was replaced 3 h later, adding cytosine-d-arabinofuranoside (Sigma-Aldrich). After 4 days in vitro, the cells were maintained for 1 h in DMEM before they were then exposed for 24, 48 or 72 h to TGFβ1 and bFGF (10 ng·mL−1 each; Peprotech, Rocky Hill, NJ, USA) and with Sativex (100 nM: 50 CBD-BDS + 50 Δ9-THC-BDS; 0.50 μM: 0.25 CBD-BDS + 0.25 Δ9-THC-BDS; 1 μM: 0.5 CBD-BDS + 0.5 Δ9-THC-BDS) or the vehicle alone. The cells harvested at 24 h were processed for PCR analysis to analyse the mRNA expression of brevican and XT-I. Immunocytochemical studies were performed on astrocytes harvested after 48 h, whereas the supernatants were taken from cells incubated with the stimuli for 72 h. These supernatants were analysed by Western blots to evaluate the amount of ECM secreted into the medium.
Reverse transcription (RT) and real-time PCR
Total RNA was extracted from spinal cords or astrocyte cultures using RNeasy mini columns (Qiagen, Manchester, UK), avoiding genomic DNA contamination by DNase I degradation (DNase I, Sigma-Aldrich). The RNA yield was determined using a Nanodrop spectrophotometer (Thermo Scientific; Wilmington, DE, USA) and the total RNA (1 μg in 20 μL) was reverse transcribed into cDNA using poly-dT primers and a reverse transcription kit (Promega Biotech Ibérica, S.L., Madrid, Spain). Real-time PCR was carried out with SYBR® and the oligonucleotide primer sequences (Applied Biosystems, Warrington, UK) used are given in Table 2. After an initial incubation at 50°C for 2 min and 95°C for 10 min, PCR amplification was performed over 40 cycles of 95°C for 15 s and 60°C for 1 min. The samples were assayed in triplicate on an Applied Biosystems PRISM 7500 Sequence detection system. To rule out genomic DNA contamination, a control sample using RNA that had not been reversed transcribed was used as the template for each set of extractions. Gene expression was calculated using the 2−ΔΔCt method and the relative expression was quantified by calculating the ratio between the values obtained for each gene of interest and those of the 18S gene. The results are expressed as a percentage with respect to the sham operated mice for each time point.
Table 2.
The primer sequences used in quantitative PCRs
| Genes | Forward | Reverse |
|---|---|---|
| TNF-α | 5′-GACTCCCCCCTCCGTCTAAG-3′ | 5′-CGCAGTAAAGCCCACGTTGT-3′ |
| IL-1β | 5′-TGGTGTGTGACGTTCCCATT-3′ | 5′-TCCATTGAGGTGGAGAGCTTTC-3′ |
| IFN-γ | 5′-GGCCATCAGCAACAACATAAGCGT-3′ | 5′-TGGGTTGTTGACCTCAAACTTGGC-3′ |
| ICAM-1 | 5′-CACCCCAAGGACCCCAAGGAGAT-3′ | 5′-CGACGCCGCTCAGAAGAACCA-3′ |
| Brevican | 5′-ACCC ACC ACTCCGTAATTCC-3′ | 5′-CCATCCAGAACCCACGAGA-3′ |
| XT-1 | 5′-GGGAATGCAGAAATGGGGGA-3′ | 5′-GAAGGTCAGAGGTGCGACAA-3′ |
| 18S | 5′-ATGCTCTTAGCTGAGTGTCCCG-3′ | 5′-ATTCCTAGCTGCGGTATCCAGG-3′ |
| CB1 receptor | 5′-CCAAGAAAAGATGACGGCAG-3′ | 5′-AGGATGACACATAGCACCAG-3′ |
| CB2 receptor | 5′-TCGCTTAC ATCCTTCAGACAG-3′ | 5′-TCTTCCCTCCCAACTCCTTC-3′ |
| PPARγ | 5′-TCACAAGAGCTGACCCAATG-3′ | 5′-TGAGGCCTGTTGTAGAGCTG-3′ |
| Arginase-1 | 5′-GAACACGGCAGTGGCTTTAAC-3′ | 5′-TGCTTAGCTCTGTCTGCTTTG-3′ |
| IL-10 | 5′-TGAATTCCCTGGGTGAGAAGCTGA-3′ | 5′-TGGCCTTGTAGACACCTTGGTCTT-3′ |
Supernatant protein concentration and Western blotting analysis
Identical amounts (1 mL) of astrocyte supernatant were precipitated overnight at −20°C in 4 vol acetone. The proteins were pelleted by centrifugation at 11,180× g, dried and resuspended in 50 μL chondroitinase ABC buffer (50 mM Tris pH 8.0, 60 mM sodium acetate, 0.02% BSA) and treated for 3 h at 37°C with 0.1 U·mL−1 chondroitinase ABC from Proteus vulgaris (Sigma-Aldrich). This reaction was stopped by adding Laemmli sample buffer and boiling for 10 min. The proteins (15 μL of the protein supernatant) were then resolved on a 6% SDS-polyacrylamide gel and transferred at 4°C to a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ, USA). The membranes were incubated for 20 min in citrate buffer at 95°C for antigen retrieval and then washed with Tris-buffered saline (TBS) followed by further washes with TBS with 0.1% Tween® 20 (TBST). The membranes were blocked for 1 h in 5% BSA (Gibco-Invitrogen S.A., Barcelona, Spain) in TBST and they were probed overnight for neurocan (Table 1). The membranes were then washed in blocking solution and incubated with a secondary HRP conjugated goat anti-mouse IgG antibody for 1 h (1:8000; Bio-Rad, Hercules, CA, USA). The membranes were washed with TBST and TBS. Protein bands were visualized by enhanced chemiluminescence detection and the amount of protein was estimated by densitometry (GS800 calibrated densitometer; Bio-Rad).
Data analysis
Data are expressed as the mean ± SEM. One-way anova followed by the Bonferroni and Tukey's post hoc test or non-parametric Kruskal–Wallis test or unpaired two-tailed Student's t-test were used to determine the statistical significance. The level of significance was set at P ≤ 0.05.
Results
The treatment with Sativex, Δ9-THC-BDS or CBD-BDS alone significantly improved motor function in TMEV-IDD: the involvement of the CB1, CB2 receptors and PPARγ
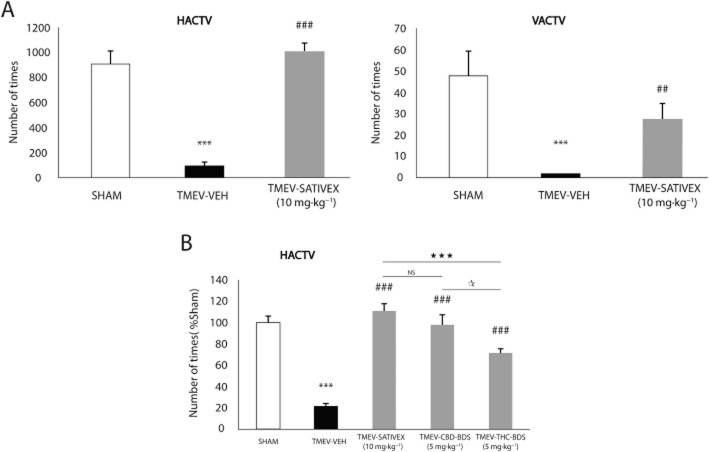
We studied the effect of Sativex and of the two components, Δ9-THC-BDS and CBD-BDS, individually in the TMEV-IDD model of murine primary progressive MS. To this end, 70 days after TMEV infection, mice were treated for 10 consecutive days with Sativex (10 mg·kg−1: 5 mg·kg−1 Δ9-THC-BDS and 5 mg·kg−1 CBD-BDS), with CBD-BDS (5 mg·kg−1) or with Δ9-THC-BDS (5 mg·kg−1) or an equivalent amount of the vehicle alone and their motor activity was then assessed in an activity cage. As expected, TMEV infection dramatically reduced both horizontal and vertical activity in the chronic phase of the model, whereas Sativex treatment abolished these motor deficits (Figure 1A). The same degree of motor improvement was seen after the treatment with CBD-BDS alone, whereas Δ9-THC-BDS provoked a weaker improvement (Figure 1B). These data indicate that Δ9-THC-BDS and CBD-BDS, used independently or in combination (in a Sativex-like mixture), restored motor function in TMEV-infected mice, although a greater influence in the effect of Sativex can be attributed to CBD-BDS. These treatments had no effect on sham animals (data not shown).
Figure 1.
A Sativex®-like combination of phytocannabinoids, Δ9-THC-BDS or CBD-BDS treatments alone, significantly improved motor deficits in the chronic phase of TMEV infection. SJL/J mice were intracranially inoculated with TMEV or the vehicle alone and after 70 days, once the symptomatology was established, the mice were treated i.p. for 10 consecutive days with a Sativex-like combination of phytocannabinoids (10 mg·kg−1), CBD-BDS (5 mg·kg−1), Δ9-THC-BDS (5 mg·kg−1) or the vehicle alone. On the last day of treatment, motor function was evaluated by measuring horizontal (HACTV) and vertical activity (VACTV) in the activity cage test (A) which revealed a significant attenuation of motor deficits in the TMEV-IDD model following Sativex administration. In a comparative analysis, Sativex and CBD-BDS alone were the most effective treatments as they abolished the decreased motor activity and restored the normal levels of HACTV (B). Activity parameters were recorded for 10 min and the results are represented as the mean ± SEM of nine mice per group: ***P ≤ 0.001 versus Sham; ##P ≤ 0.01; ###P ≤ 0.001 versus TMEV-VEH animals; ⋆⋆⋆P ≤ 0.001 versus TMEV-Sativex; *P ≤ 0.05 TMEV-CBD-BDS versus TMEV-THC-BDS (non parametric Kruskal–Wallis test). NS, not significant.
The different contribution of each phytocannabinoid to the activity of Sativex may be related to differences in their potential targets. Thus, to determine how the CB1 and CB2 receptors influenced the effects of Δ9-THC-BDS, this phytocannabinoid was co-administered with a selective antagonist for the CB1 (AM251) or CB2 (AM630) receptor. In addition, as CBD appears to act through PPARγ (Esposito et al., 2011), and only negligibly through the CB1 and CB2 receptors (Izzo et al., 2009; Mecha et al., 2013b), CBD-BDS was also administered along with the PPARγ antagonist, T0070907. Our data indicated that the positive effect of Δ9-THC-BDS on motor deterioration was significantly blocked by the CB1 receptor antagonist AM251 and only partially by the CB2 receptor antagonist AM630 (Figure 2A). Similarly, the beneficial effect of CBD-BDS was significantly attenuated by the PPARγ antagonist (Figure 2B). These data suggest the involvement of CB1 receptors and, to a lesser extent, of CB2 receptors in ameliorating the effect of motor disturbances in TMEV-IDD mice provoked by Δ9-THC-BDS and an equivalent role of PPARγ in the effects of CBD-BDS. Moreover, we saw that Sativex treatment tended to up-regulate the gene expression of CB1 and CB2 receptors and of PPARγ in TMEV animals (data not shown). Accordingly, these three targets might be involved in the action of Sativex on TMEV-IDD consistent with the idea of a broad-spectrum effect of this combination of phytocannabinoids.
Figure 2.
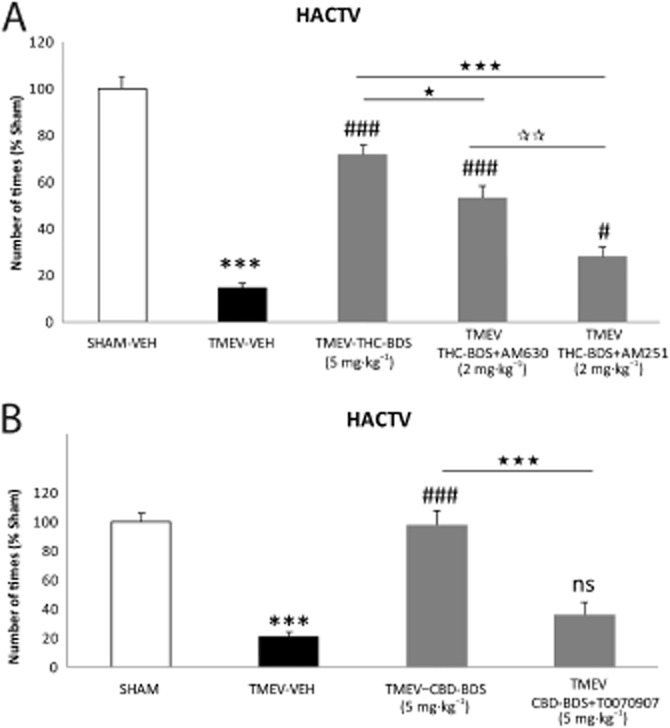
The involvement of the CB1, CB2 receptors and PPARγ in the effect of the Sativex®-like phytocannabinoid combination. To determine the receptors involved in the beneficial effects of Sativex, we administered Δ9-THC-BDS (5 mg·kg−1) together with a selective antagonist for the CB2 receptor (AM630, 2 mg·kg−1) and an antagonist of the CB1 receptor (AM251, 2 mg·kg−1). CBD-BDS (5 mg·kg−1) was administered in combination with a PPARγ receptor antagonist (T0070907, 5 mg·kg−1) for 10 days once the symptomatology had been established and motor function was evaluated by measuring horizontal activity (HACTV) on day 80 post-infection. The positive effect of Δ9-THC-BDS was significantly blocked by the administration of the CB1 receptor antagonist and less so by the CB2 receptor antagonist (A). Likewise, the beneficial effect of CBD-BDS was significantly attenuated by the antagonist of PPARγ (B). Activity parameters were recorded for 10 min and the results represent the mean ± SEM of six mice per group: ***P ≤ 0.001 versus Sham; #P ≤ 0.05 ###P ≤ 0.001 versus TMEV-VEH animals; ⋆P ≤ 0.05 versus TMEV-THC-BDS; ⋆⋆⋆P ≤ 0.001 versus TMEV-Δ9-THC-BDS; versus TMEV-CBD-BDS; **P ≤ 0.01 TMEV-THC-BDS-AM630 versus TMEV-THC-BDS-AM251 (one-way anova followed by Tukey's and Bonferroni's test: CBD-BDS and antagonist analysis; non-parametric Kruskal–Wallis test: Δ9-THC-BDS and antagonist analysis). ns, not significant.
Sativex reduced leukocyte infiltration and expression of adhesion molecules in the spinal cord of TMEV-infected animals
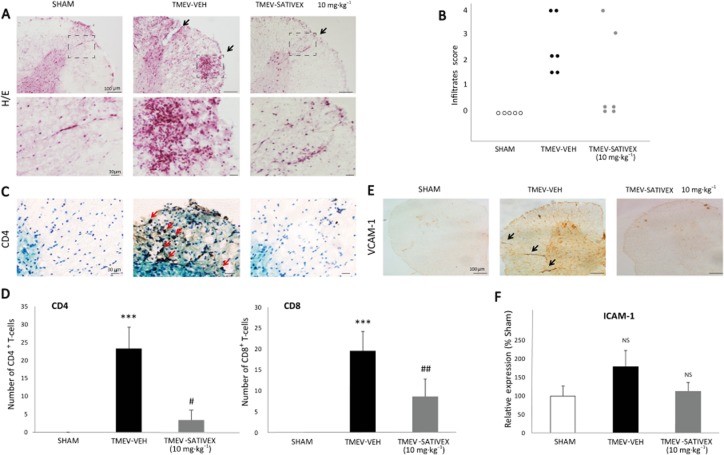
H&E staining revealed that infection with TMEV provoked the infiltration of immune cells into the spinal cord (Figure 3A). Treatment with Sativex partly diminished this infiltration (Figure 3B) and specifically decreased the accumulation of CD4 and CD8 lymphocytes into the parenchyma (Figure 3C, D). Sativex also reduced the expression of VCAM-1 (Figure 3E) and ICAM-1 (Figure 3F), both involved in peripheral immune cell transmigration. These results suggest that Sativex may restrict the permissiveness of immune cell infiltration into the CNS parenchyma. Indeed, no infiltrates were detected in sham-operated mice treated with Sativex (data not shown).
Figure 3.
A Sativex®-like phytocannabinoid combination treatment decreased leukocyte infiltration and down-regulated the adhesion molecules in the spinal cord of TMEV-infected animals. Transverse cervical spinal cord sections (30 μm thick) obtained at day 80 post-infection were stained with H&E (A). TMEV infection increased leukocyte infiltration, an effect that was attenuated by Sativex (10 mg·kg−1), as indicated by the infiltrate score (B) and by the analysis of CD4 and CD8 lymphocyte staining (representative microphotographs of CD4 staining, C; quantification of CD4 and CD8 number of positive cells, D). Sativex also showed a tendency to decrease mRNA expression of the ICAM-1 adhesion molecule as determined by RT-PCR on spinal cord of TMEV-infected animals (n = 6 per group) normalizing mRNA expression to that of the 18S gene, (F). Sativex significantly reduced the expression of VCAM-1 adhesion molecule (representative microphotographs of VCAM-1 staining, E). The data represent the mean ± SEM: ***P ≤ 0.001 versus Sham; #P ≤ 0.05, ##P ≤ 0.01 versus TMEV-VEH animals (non-parametric Kruskal–Wallis test: CD4 and CD8 analysis). For histology analysis, five to six spinal cord slices per animal were analysed (n = 5–6 animals per group): Scale bar = 100 μm; 30 μm. NS, not significant.
Sativex decreased microglial activity, down-regulated the expression of proinflammatory cytokines and up-regulated arginase-1 (Arg-1) and IL-10 in the spinal cord of TMEV-infected mice
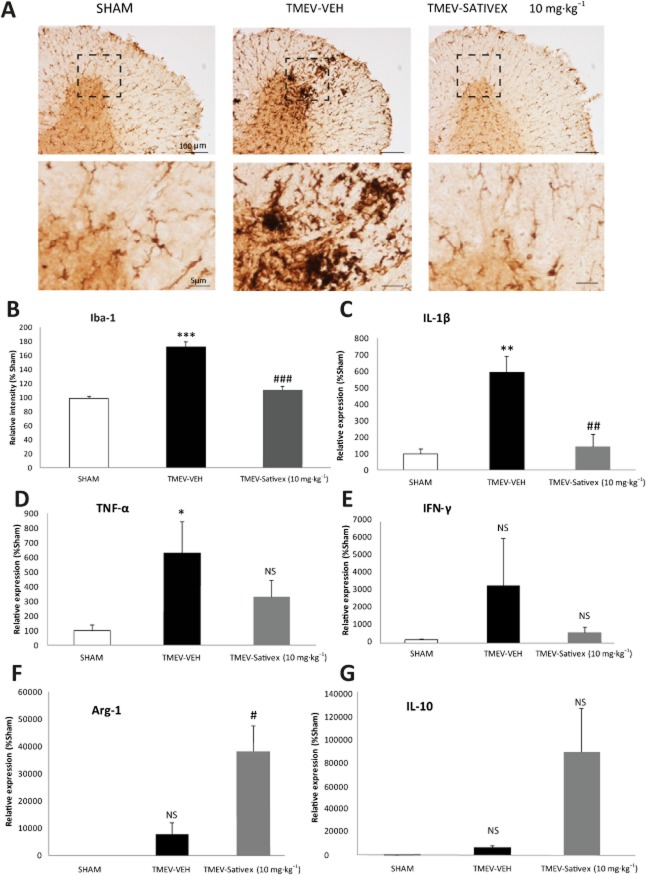
Microglial/macrophage activation plays a critical role in TMEV-IDD and thus, we analysed the effect of Sativex on Iba-1 immunoreactivity in the spinal cord of TMEV-infected mice. As expected, Iba-1 staining revealed increased microglial reactivity in the spinal cord at chronic phases of the disease following TMEV infection. Sativex administration significantly reduced the intensity of this microglial labelling (Figure 4A, B). Moreover, no microglia reactivity was detected in Sham mice administered Sativex (data not shown).
Figure 4.
A Sativex®-like phytocannabinoid combination attenuated the microglial response, down-regulated proinflammatory cytokines and up-regulated Arg-1 and IL-10 in the chronic phases of TMEV-IDD. Transverse cervical spinal cord sections (30 μm thick) obtained at day 80 post-infection were stained for Iba-1. (A) Representative microphotographs of Iba-1 immunostaining showing morphological changes in the microglial cells of infected animals that were reversed by Sativex treatment (10 mg·kg−1). (B) Quantification of the percentage area occupied by microglia in the spinal cord white matter per field (n = 5–6 animals per group). Sativex treatment also decreased IL-1β (C) TNF-α (D) and IFN-γ (E) mRNA induction and increased Arg-1 (F) and IL-10 (G) as determined by RT-PCR in the spinal cord of TMEV-infected animals, normalizing mRNA expression to that of the 18S gene (n = 6 per group). The data represent the mean ± SEM: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus Sham; #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 versus TMEV-VEH animals (one-way anova followed by Tukey's and Bonferroni's test: IL-1β analysis; non-parametric Kruskal–Wallis test: Iba-1 analysis; and unpaired two-tailed Student's t-test: TNF-α and Arg-1 analysis). For histology analysis, five to six spinal cord slices were examined per animal (n = 6 animals per group): Scale bar = 100 μm; 5 μm. NS, not significant.
The reduction of microglia reactivity was accompanied by a significant reduction of IL-1β mRNA levels (Figure 4C) and a tendency to reduce TNF-α and IFN-γ (Figure 4D, E). Sativex treatment also up-regulated the expression of Arg-1 and IL-10 mRNA showed a tendency to increase (Figure 4F, G). These data suggest that Sativex could act as an immunomodulator, not only limiting the inflammation in TMEV-IDD mice but also promoting an anti-inflammatory environment. No effect on these genes was observed in Sham animals treated with the Sativex (data not shown).
Sativex restored myelin morphology and prevented the axonal damage detected in TMEV-infected mice
Demyelinated areas that are evident upon LFB staining can be observed in TMEV-IDD infected mice at chronic phases of the disease. However, demyelination was diminished in TMEV-infected mice treated with Sativex as it preserved the morphology of the myelin sheaths in these infected mice (Figure 5A). To further assess the neuroprotective potential of Sativex, we evaluated axonal damage in TMEV-infected mice, as reflected by Neurofilament-H and SMI32 staining. Axonal damage in the white matter tracts was clearly evident in the TMEV-infected animals that received the vehicle alone, yet it was absent in the Sham mice. Treatment with Sativex restored Neurofilament-H staining (Figure 5B), reflecting the preservation of the axonal package (Figure 5C). Similarly, the axonal swelling evident when longitudinal spinal cord sections of TMEV-infected animals that received the vehicle alone were stained with Neurofilament-H (Figure 6A) was less frequent and milder following Sativex administration, as confirmed in longitudinal spinal cord sections labelled with SMI32 (Figure 6B).
Figure 5.
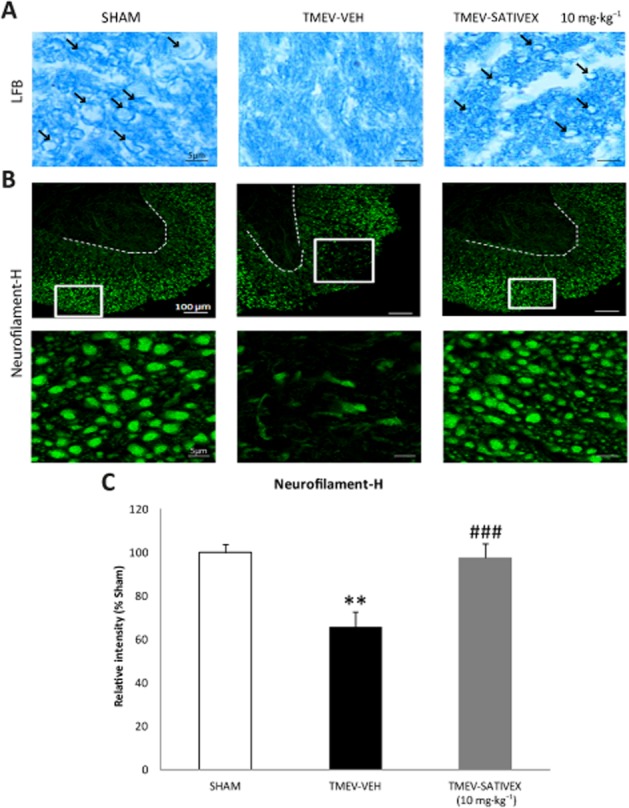
Sativex®-like phytocannabinoid combination treatment restored myelin morphology and prevented axonal damage in TMEV-infected mice. Transverse cervical spinal cord sections were obtained at day 80 post-infection and stained with LFB (15 μm thick) and Neurofilament-H (30 μm thick). The myelin was clearly disrupted in cervical spinal cord sections from infected animals treated with the vehicle while Sativex (10 mg·kg−1) treatment contributed to maintain the myelin structure (A, arrows indicate myelin sheaths. (B) Representative images of Neurofilament-H staining showing that there is prominent axonal damage in the spinal cord white matter of vehicle-treated infected animals that diminished significantly following Sativex treatment. The area occupied by Neurofilament-H was quantified in the spinal cord white matter of each field (5–6 slices, n = 5–6 animals per group) (C). The data represent the mean ± SEM: **P ≤ 0.01 versus Sham; ###P ≤ 0.001 versus TMEV-VEH animals (non-parametric Kruskal–Wallis test): Scale bar = 5 μm; 100 μm.
Figure 6.
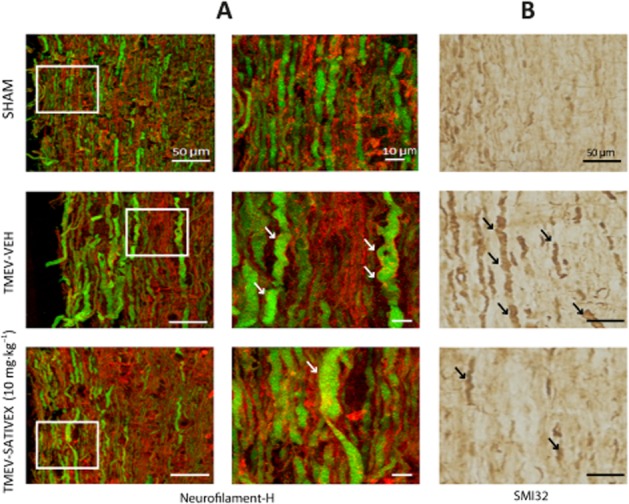
A Sativex®-like phytocannabinoid combination prevented the swelling and ovoid formations in axons from TMEV-infected animals. Longitudinal cervical spinal cord sections (30 μm thick) stained for Neurofilament-H (green) and RIP (red) (A) revealed the axons to be swollen, deformed and with ovoid formation in TMEV vehicle-treated mice. Lesions in the axons of the Sativex-treated group (10 mg·kg−1) were less frequent and axons displayed markedly reduced damage as confirmed in longitudinal spinal cord sections labelled with SMI32 (B): Scale bar = 10 μm; 50 μm.
Sativex reduced astrocyte reactivity and the accumulation of CSPGs in the spinal cord of TMEV-infected mice
To address whether the treatment with Sativex affected astrogliosis and the expression of CSPGs in TMEV-IDD at the chronic phases of the disease, we analysed its effects on astroglial reactivity using GFAP and vimentin staining as an indicator of reactive astrocytes (Nash et al., 2011). Likewise, the accumulation of CSPGs was studied by staining with CS56 and analysing the brevican gene expression in the spinal cord. There was prominent astrogliosis [Figure 7: GFAP (A,B) and vimentin (C) staining] associated with the accumulation of CSPGs (CS56) in the spinal cord of vehicle-treated TMEV-infected mice (Figure 7D). However, treatment with Sativex reduced both astrogliosis (Figure 7B) CS56 staining (Figure 7D) and brevican gene expression (Figure 7E) in TMEV-infected mice, suggesting that it may modulate glial scar formation.
Figure 7.
A Sativex®-like phytocannabinoid combination reduced astrocyte reactivity and the accumulation of CSPGs. Transverse cervical spinal cord sections (30 μm) were obtained on day 80 post-infection and stained for GFAP, vimentin and CS56. There was a prominent astrogliosis in the spinal cord of vehicle-treated infected animals evident in the representative microphotographs of GFAP staining (A). This is also reflected in the area occupied by GFAP+ astrocytes in the spinal cord white matter in each field (5–6 slices, n = 5–6 animals per group) (B), as well as in the vimentin staining (C). This astrogliosis is associated with an accumulation of CSPGs (CS56 staining, D; brevican gene expression, E) which is impeded by Sativex treatment (10 mg·kg−1). The data represent the mean ± SEM: **P ≤ 0.01, ***P ≤ 0.001 versus Sham; #P ≤ 0.05, ###P ≤ 0.001 versus TMEV-VEH animals (one-way anova followed by Tukey's and Bonferroni test: GFAP analysis; Non parametric Kruskal–Wallis test: brevican). WM, white matter; GM, gray matter. Scale bar = 100 μm; 20 μm.
Sativex reduced CSPGs synthesis and release by astrocytes in vitro
As astrocytes are the main source of CSPGs (Susarla et al., 2011), we assessed the capability of Sativex to alter the production of CSPGs by astrocytes in culture. Exposure to the Sativex not only reduced the mRNA expression of brevican and XT-I in activated astrocytes (TGFβ1 and bFGF) after 24 h (Figure 8A), but it also reduced the neurocan protein staining at an intracellular and extracellular level in astrocytes by immunocytochemistry (Figure 8B). Most importantly, Sativex diminished the release of neurocan by activated astrocytes when assessed at 72 h (Figure 8C, D). An overall inhibitory effect of Sativex on CSPG production by astrocytes occurred at a dose of 0.50 μM. However, a dose of 100 nM (50 nM for each phytocannabinoid) also achieved a significant reduction in the expression of brevican mRNA and the dose of 1.0 μM (0.5 μM for each phytocannabinoid) diminished the expression of XT-I mRNA within 24 h. Together, these data suggest that astrocytes could represent a new target for Sativex activity, modulating the production of CSPG.
Figure 8.
A Sativex®-like phytocannabinoid combination treatment reduced the synthesis and release of CSPGs by astrocytes in vitro. CSPG, brevican and XT-I enzyme syntheses are up-regulated in primary rat astrocytes stimulated with TGFβ1 (10 ng·mL−1) plus bFGF (10 ng·mL−1) and significantly down-regulated by Sativex at a dose of 100 nM and 0.5 μM or 1 μM in the case of XT-I after 24 h of treatment. Brevican and XT-I mRNA induction was determined by RT-PCR, normalizing mRNA expression (n = 10 per group) to that of the 18S gene (A). Representative microphotographs of primary astrocytes after 48 h in the presence of Sativex (0.5 μM) show that neurocan immunocytochemistry is less intense than in control astrocytes, both at the intracellular level and in the culture substrate (B). In the presence of Sativex (0.5 μM) for 72 h, activated astrocytes release less neurocan to the supernatant, as evident in Western blots. Representative Western blots (C) and quantification of the OD (D). Every assay was performed in duplicate, in three independent experiments. The data represent the mean ± SEM: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 versus CTL (control); #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 versus TGFβ1+ bFGF (non-parametric Kruskal–Wallis test). ns, not significant.
Discussion
Despite intense research, there are still no treatments available to achieve clinical modification of progressive MS. The approval of Sativex for treatment of spasticity and neuropathic pain in MS opens the door to study the therapeutic potential of Sativex as a disease-modifying agent. Accordingly, we have assessed the efficacy of Sativex in controlling disease progression in a model of primary progressive MS, the TMEV-IDD model (Mecha et al., 2013a).
There is a wealth of data from clinical trials that support the efficacy and safety of Sativex in patients with MS-related spasticity (Collin et al., 2007; Wade et al., 2010; Sastre-Garriga et al., 2011; García-Merino, 2013). However, to date, there are no clinical studies evaluating Sativex as a disease-modifying agent of progressive MS. The closest clinical study is the CUPID trial, the results of which were recently published (Zajicek et al., 2013). This study showed that dronabinol (Δ9-THC) administration to patients with progressive MS did not slow the course of the disease. However, the failure to detect such an effect may reflect the high attrition rate and the lower rate of disease progression than initially expected.
The study performed here is the first to address the effect of Sativex on primary progressive MS in a recognized experimental model, providing highly promising results in terms of reduced axonal damage. Indeed, this result is consistent with the proposed neuroprotective profile of Sativex and its components (Valdeolivas et al., 2012; Moreno-Martet et al., 2014). When administered alone, Δ9-THC or CBD has positive effects on disease progression in EAE, CREAE and TMEV-IDD models. A clinical and histological improvement provoked by Δ9-THC, predominantly reducing inflammation, was first described in EAE rats (Lyman et al., 1989). Here, we also found that Δ9-THC-BDS ameliorated the progression of disease in TMEV-IDD mice, with a major participation of CB1 receptors, even though blocking CB2 receptors partly prevented the benefits elicited by this phytocannabinoid. The activation of CB1 receptors probably mediates the neuroprotective effects of Δ9-THC as this receptor fulfils an important role in the defence against excitotoxicity (Marsicano et al., 2003) and excitotoxic mechanisms are prominent in TMEV-IDD (Docagne et al., 2007). Nevertheless, the participation of CB2 receptors is not unexpected given their key involvement in the control of glial activation and given that inflammatory events are also important in TMEV-IDD mice (Arévalo-Martín et al., 2003; 2008). Indeed, it was previously demonstrated that Δ9-THC suppressed CNS autoimmune inflammation with the participation of both CB1 and CB2 receptors in CREAE mice (Maresz et al., 2007).
With regard to CBD, the other component of Sativex, this phytocannabinoid provokes a reduction in disease severity in both autoimmune (EAE mice) and viral (TMEV-IDD mice) models of MS, as well as in a murine collagen-induced arthritis model (Malfait et al., 2000; Kozela et al., 2011; Mecha et al., 2013b). The attenuation of disease progression in MS models involved the reduction of immune cell infiltrates and the decrease in microglial activation following systemic CBD treatment (5 mg·kg−1), the same dose of CBD-BDS used in this study. These findings contrast with the earlier failure to find an effect of CBD in the CREAE model of MS (Maresz et al., 2007), which might reflect differences in the strain of mice and/or in the antigen used. The influence of genetic background on EAE in mice deficient in some key endocannabinoid genes has recently been shown (Sisay et al., 2013). It should be noted that CBD is not particularly active at classic CB1 and CB2 receptors and, while it appears to be able to inhibit the inactivation of endocannabinoids (Bisogno et al., 2001), its mechanisms of action are quite diverse and extend to targets beyond the cannabinoid system (see Fernández-Ruiz et al., 2013). One of these targets includes the nuclear receptors of the PPAR family, in particular PPARγ (O'Sullivan and Kendall, 2010; Esposito et al., 2011). Here, we found that the inhibition of PPARγ completely blocked the motor benefits of CDB-BDS, clearly indicating the participation of these nuclear receptors in the effects of CBD-BDS on TMEV-IDD and presumably when it is combined with Δ9-THC-BDS in Sativex. This is consistent with studies showing that activation of PPARγ by selective agonists ameliorated MS symptomatology (Feinstein et al., 2002; Klotz et al., 2009; Mestre et al., 2009).
In accordance with the diminished axonal damage, we found reduced astrogliosis and CSPGs accumulation during the late chronic phase of TMEV-IDD. It is well known that CPGSs are up-regulated in demyelinated MS lesions (Haylock-Jacobs et al., 2011; Haist et al., 2012). In particular, astrocytes at the edge of active MS lesions up-regulate CSPGs such as versican, aggrecan and neurocan (Haylock-Jacobs et al., 2011; Haist et al., 2012; Lau et al., 2012). Significantly, there are several in vitro studies indicating that neurocan and aggrecan impair the outgrowth of oligodendrocyte process, OPC differentiation and myelination (Siebert and Osterhout, 2011; Pendleton et al., 2013). Here, the enhanced expression of CSPGs in astrocytes following exposure to TGFβ (Gris et al., 2007; Susarla et al., 2011) was down-regulated by Sativex administration. We focused on neurocan and brevican, core proteins that carry inhibitory glycosaminoglycan (GAG) chains. CSPG side chain synthesis is initiated by the addition of xylose to a serine moiety of the core protein, a step carried out by XT-I, among other enzymes (Gris et al., 2007). Significantly, the up-regulation of XT-I expression in stimulated astrocytes was dampened by Sativex, which could impair CSPG synthesis and thereby provide a permissive environment for regeneration in accordance with previous studies in which XT-I targeting promotes neurite outgrowth (Hurtado et al., 2008).
The inhibitory effect of Sativex in the production of brevican and neurocan as well as in the synthesis enzyme XT-I by activated astrocytes confirm our in vivo results and support the interest of Sativex as a putative therapy in promoting repair myelin processes by reducing astroglial scar. Further work is necessary to delineate the effects of Sativex-like combination of phytocannabinoids on remyelination in experimental models of MS.
In conclusion, Sativex improved the neurological deficits evident at the chronic phases of the TMEV-IDD model of MS. These effects were fully reproduced by CBD-BDS alone, acting through PPARγ and, to a lesser extent, by Δ9-THC-BDS, acting through CB2 and primarily CB1 receptors. One potential explanation for the beneficial effects of Sativex could be the reduced transmigration of cell infiltrates into the parenchyma. Sativex also acted as an immunomodulator, decreasing microglial reactivity, the expression of proinflammatory cytokine genes and increasing levels of Arg-1 and IL-10. Moreover, Sativex diminished axonal damage and restored myelin morphology, again supporting its cytoprotective potential. Finally, we postulate that Sativex could modulate glial scar formation, reducing astrogliosis and the accumulation of CSPGs in vivo, as well as in astrocyte cultures. As CSPGs associated with inflammation have been implicated in the failure of remyelination in human MS and in murine models of demyelination, our results highlight the potential benefits of Sativex in CNS repair.
Acknowledgments
This work was supported by grants from the Ministry of the Economy and Competition SAF2010-17501 (C. G.) and SAF2009-11847 (J. F.-R.), the Comunidad de Madrid, S2011/BMD-2308, the Red Española de Esclerosis Múltiple RD12/0032/0008 (C. G.) sponsored by the Fondo de Investigación Sanitaria. GW Pharmaceuticals Ltd provided the combination of phytocannabinoids. None of the funding bodies played any role in the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Glossary
- Δ9-THC-BDS
Δ9-tetrahydrocannabinol-botanical drug substance
- Arg-1
arginase-1
- CBD-BDS
cannabidiol-botanical drug substance
- CSPGs
chondroitin sulfate proteoglycans
- ECM
extracellular matrix
- GAG chains
glycosaminoglycan chains
- OPCs
oligodendrocyte precursor cells
- TMEV-IDD
Theiler's murine encephalomyelitis virus-induced demyelinating disease
- VCAM-1
vascular cell adhesion molecule-1
- Sativex
Sativex-like combination of phytocannabinoids
- XT-I
xylosyltransferase-I
Author contributions
A. F. carried out the TMEV-IDD model, prepared the astrocytes cultures, determined the ECM proteins and analysed the data. M. M. performed the qRT-PCR assays. M. M.-M. was involved in motor tests. F. J. C.-S. was involved in perfusion and sections staining. M. M.-M., E. L., J. F.-R. and C. G. participated in the experimental design. A. F. and C. G. wrote the manuscript.
Conflict of interest
The authors have formal links with GW Pharmaceuticals that fund some of their research.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo-Martín A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo-Martín A, García-Ovejero D, Gómez O, Rubio-Araiz A, Navarro-Galve B, Guaza C, et al. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: from neuroimmune interactions to cell replacement strategies. Br J Pharmacol. 2008;153:216–225. doi: 10.1038/sj.bjp.0707466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control tremor and spasticity in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. 6773. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effects on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chang A, Staugaitis SM, Dutta R, Batt CE, Easley KE, Chomyk AM, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72:918–926. doi: 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Davies P, Mutiboko IK, Ratcliffe S Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290–296. doi: 10.1111/j.1468-1331.2006.01639.x. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Miller SD. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R + WIN55,212. J Clin Invest. 2003;111:1231–1240. doi: 10.1172/JCI17652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docagne F, Muñetón V, Clemente D, Ali C, Loría F, Correa F, et al. Excitotoxicity in a chronic model of multiple sclerosis: neuroprotective role of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Esposito G, Scuderi G, Valenza M, Togna GI, Latina V, De Filippis D, et al. Cannabidiol reduces Ab-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE. 2011;6:e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, et al. Peroxisome proliferator-activated receptor gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, García C, Sagredo O, Gómez-Ruiz M, de Lago E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin Ther Targets. 2010;14:387–404. doi: 10.1517/14728221003709792. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Sagredo O, Pazos MR, García RG, Pertwee R, Mechoulam R, et al. Cannabidiol for neurodegenerative disorders: important new clinical applications for this phytocannabinoid. Br J Clin Pharmacol. 2013;75:323–333. doi: 10.1111/j.1365-2125.2012.04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- García-Merino A. Endocannabinoid system modulator use in everyday clinical practice in the UK and Spain. Expert Rev Neurother. 2013;13:9–13. doi: 10.1586/ern.13.4. [DOI] [PubMed] [Google Scholar]
- Gómez O, Arevalo-Martin A, Garcia-Ovejero D, Ortega-Gutierrez S, Cisneros JA, Almazan G, et al. The constitutive production of 2-arachidonoylglycerol participates in oligodendrocyte differentiation. Glia. 2010;58:1913–1927. doi: 10.1002/glia.21061. [DOI] [PubMed] [Google Scholar]
- Goldschmidt T, Antel J, König FB, Brück W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- Gris P, Tighe A, Levin D, Sharma R, Brown A. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55:1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- Haist V, Ulrich R, Kalkuhl A, Deschl U, Baumgartner W. Distinct spatio-temporal extracellular matrix accumulation within demyelinated spinal cord lesions in Theiler's murine encephalomyelitis. Brain Pathol. 2012;22:188–204. doi: 10.1111/j.1750-3639.2011.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Macklin WB. Inhibitors of myelination: ECM changes, CSPGs and PTPs. Exp Neurol. 2014;251:39–46. doi: 10.1016/j.expneurol.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylock-Jacobs S, Keough MB, Lau L, Yong VW. Chondroitin sulphate proteoglycans: extracellular matrix proteins that regulate immunity of the central nervous system. Autoimmun Rev. 2011;10:766–772. doi: 10.1016/j.autrev.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Podinin H, Oudega M, Grimpe B. Deoxyribozyme-mediated knockdown of xylosyltransferase-1 mRNA promotes axon growth in the adult rat spinal cord. Brain. 2008;131:2596–2605. doi: 10.1093/brain/awn206. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-Psychotropic plant cannabinoids: new therapeutic opportunities for an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Lev N, Kaushansky N, Eilam R, Rimmerman N, Levy R, et al. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol. 2011;163:1507–1519. doi: 10.1111/j.1476-5381.2011.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Döring A, Sloka S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair demyelination. Ann Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- Lipton HL, Dal Canto MC. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J Neurol Sci. 1976;30:201–207. doi: 10.1016/0022-510x(76)90267-7. [DOI] [PubMed] [Google Scholar]
- Lledó A, Borrell J, Guaza C. Dexamethasone regulation of interleukin-1 receptors in the hippocampus of Theiler's virus infected mice: effects on virus-mediated demyelination. Eur J Pharmacol. 1999;372:75–83. doi: 10.1016/s0014-2999(99)00187-9. [DOI] [PubMed] [Google Scholar]
- Loría F, Petrosino S, Hernangómez M, Mestre L, Spagnolo A, Correa F, et al. An endocannabinoid tone limits excitotoxicity in vitro and in a model of multiple sclerosis. Neurobiol Dis. 2010;37:166–176. doi: 10.1016/j.nbd.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Delta 9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23:73–81. doi: 10.1016/0165-5728(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The non-psychotropic cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Iñigo PM, Mestre L, Hernangómez M, Borrel J, Guaza C. An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: a beginners approach. Protoc Exch. 2011 doi: 10.1038/protex.2011.218. [Google Scholar]
- Mecha M, Torrao AS, Mestre L, Carrillo-Salinas FJ, Mechoulam R, Guaza C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012;3:e331. doi: 10.1038/cddis.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Carrillo-Salinas FJ, Feliu A, Guaza C. Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler's virus. Prog Neurobiol. 2013a;102:46–64. doi: 10.1016/j.pneurobio.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Feliú A, Iñigo PM, Mestre L, Carrillo-Salinas FJ, Guaza C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: a role for A2A receptors. Neurobiol Dis. 2013b;59:141–150. doi: 10.1016/j.nbd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Mestre L, Docagne F, Correa F, Loría F, Hernangómez M, Borrell J, et al. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulation the adhesion molecules. Mol Cell Neurosci. 2009;40:258–266. doi: 10.1016/j.mcn.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, et al. Persistent infection with TMEV leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Mohan H, Krumbholz M, Sharma R, Eisele S, Junker A, Sixt M, et al. Extracellular matrix in multiple sclerosis lesions: fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. doi: 10.1111/j.1750-3639.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J, et al. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Martet M, Espejo-Porras F, Fernández-Ruiz J, de Lago E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1G93A transgenic mice and evaluation of a Sativex®-like combination of phytocannabinoids: interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci Ther. 2014;20:809–815. doi: 10.1111/cns.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash B, Ioannidou K, Barnett SC. Astrocyte phenotypes and their relationship to myelination. J Anat. 2011;219:44–52. doi: 10.1111/j.1469-7580.2010.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol. 2013;247:113–121. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Pryce G, Baker D. Potential control of multiple sclerosis by cannabis and the endocannabinoid system. CNS Neurol Disord Drug Targets. 2012;11:624–641. doi: 10.2174/187152712801661310. [DOI] [PubMed] [Google Scholar]
- Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM, et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126:2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J, Vila C, Clissold S, Montalban X. THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Expert Rev Neurother. 2011;11:627–637. doi: 10.1586/ern.11.47. [DOI] [PubMed] [Google Scholar]
- Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial study in patients with spasticity due to multiple sclerosis. J Neurol. 2013;260:285–295. doi: 10.1007/s00415-012-6634-z. [DOI] [PubMed] [Google Scholar]
- Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119:176–188. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sisay S, Pryce G, Jackson SJ, Tanner C, Ross RA, Michael GJ, et al. Genetic background can result in a marked or minimal effect of gene knockout (GPR55 and CB2 receptor) in experimental autoimmune encephalomyelitis models of multiple sclerosis. PLoS ONE. 2013;8:e76907. doi: 10.1371/journal.pone.0076907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- Susarla BT, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-β-mediated induction of chondroitin sulfate proteoglycans. J Neurochem. 2011;119:868–878. doi: 10.1111/j.1471-4159.2011.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Seeliger F, Kreutzer M, Germann PG, Baumgärtner W. Limited remyelination in Theiler's murine encephalomyelitis due to insufficient oligodendroglial differentiation of nerve/glial antigen 2 (NG2)-positive putative oligodendroglial progenitor cells. Neuropathol Appl Neurobiol. 2008;34:603–620. doi: 10.1111/j.1365-2990.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- Valdeolivas S, Satta V, Pertwee RG, Fernández-Ruiz J, Sagredo O. Sativex like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Hungtinton's disease: role of CB1 and CB2 receptors. ACS Chem Neurosci. 2012;3:400–406. doi: 10.1021/cn200114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudhan L, Van Diepen E, Marudkar M, Hands O, Suribhatla S, Prettyman R, et al. Therapeutic potential of cannabinoids in neurodegenerative diseases: a selective review. Curr Pharm Des. 2014;20:2218–2230. doi: 10.2174/13816128113199990434. [DOI] [PubMed] [Google Scholar]
- Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler. 2010;16:707–714. doi: 10.1177/1352458510367462. [DOI] [PubMed] [Google Scholar]
- Zajicek J, Ball S, Wright D, Vickery J, Nunn A, Miller D, et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12:857–865. doi: 10.1016/S1474-4422(13)70159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]