Abstract
Background and Purpose
Concentrations of 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3], the active ligand of the vitamin D receptor, are tightly regulated by CYP27B1 for synthesis and CYP24A1 for degradation. However, the dose-dependent pharmacokinetic (PK)-pharmacodynamic (PD) relationship between these enzymes and 1,25(OH)2D3 concentrations has not been characterized.
Experimental Approach
The pharmacokinetics of 1,25(OH)2D3 were evaluated after administration of single (2, 60 and 120 pmol) and repeated (2 and 120 pmol q2d ×3) i.v. doses to male C57BL/6 mice. mRNA expression of CYP27B1 and CYP24A1 was examined by quantitative PCR and 1,25(OH)2D3 concentrations were determined by enzyme immunoassay.
Key Results
CYP27B1 and CYP24A1 changes were absent for the 2 pmol dose and biexponential decay profiles showed progressively shorter terminal half-lives with increasing doses. Fitting with a two-compartment model revealed decreasing net synthesis rates and increasing total clearances with dose, consistent with a dose-dependent down-regulation of renal CYP27B1 and the induction of renal/intestinal CYP24A1 mRNA expression. Upon incorporation of PD parameters for inhibition of CYP27B1 and induction of CYP24A1 to the simple two-compartment model, fitting was significantly improved. Moreover, fitted estimates for the 2 pmol dose, together with the PD parameters as modifiers, were able to predict profiles reasonably well for the higher (60 and 120 pmol) doses. Lastly, an indirect response model, which considered the synthesis and degradation of enzymes, adequately described the PK and PD profiles.
Conclusions and Implications
The unique PK of exogenously administered 1,25(OH)2D3 led to changes in PD of CYP27B1 and CYP24A1, which hastened the clearance of 1,25(OH)2D3.
Tables of Links
| TARGETS | |
|---|---|
| Ion channelsa | Enzymesc |
| TRPV5 | CYP24A1 |
| TRPV6 | CYP27A1 |
| Nuclear hormone receptorsb | CYP27B1 |
| Vitamin D receptor | CYP2R1 |
| LIGANDS |
|---|
| 1α,25-dihydroxyvitamin D3 |
| 25-hydroxyvitamin D3 |
| PTH |
| Vitamin D |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guideto PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
Vitamin D, formed from 7-dehydrocholesterol in skin upon exposure to sunlight, is metabolized by CYP2R1 and CYP27A1 in liver to its major circulating form, 25-hydroxyvitamin D3 [25(OH)D3]. This relatively inactive metabolite is transported by the vitamin D binding protein (DBP) for activation by 1α-hydroxylase (CYP27B1) in the kidney to form the active ligand of the vitamin D receptor, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Jones et al., 1998). A major physiological role of 1,25(OH)2D3 is to regulate plasma calcium concentrations through the calcium ion channels, TRPV5 and TRPV6, in the kidney and intestine (den Dekker et al., 2003) and the calcium-sensing receptor (Carrillo-Lopez et al., 2008). Continuous bone turnover, including resorption of existing bone and deposition of new bone, is another process that is stimulated by 1,25(OH)2D3 and the parathyroid hormone (PTH) (Jones et al., 1998; Hoenderop et al., 2005).
Calcium and 1,25(OH)2D3 homeostasis is tightly controlled by 1,25(OH)2D3, calcium and PTH (Shinki et al., 1992; Masuda et al., 2005; Turunen et al., 2007). Plasma 1,25(OH)2D3 concentrations are regulated by two major enzymes: CYP27B1 for synthesis and CYP24A1 for degradation. CYP27B1, expressed predominantly in the kidney, responds positively to PTH at low plasma calcium concentrations (Shinki et al., 1992), but is down-regulated by high concentrations of 1,25(OH)2D3 (Brenza and DeLuca, 2000; Turunen et al., 2007). CYP24A1, distributed abundantly in the kidney and intestine, is responsible for the metabolism of 25(OH)D3 to 24,25-dihydroxyvitamin D3 and 1,25(OH)2D3 to 1α,24,25-trihydroxyvitamin D3 (Holick et al., 1972; Kumar et al., 1978; Halloran and Castro, 1989). Because elevated concentrations of 1,25(OH)2D3 are known to cause hypercalcaemia (Jones et al., 1987; Makin et al., 1989), CYP24A1 expression in the kidney is up-regulated as a feedback mechanism to increase 1,25(OH)2D3 catabolism and reduce 1,25(OH)2D3 and 25(OH)D3 stores (Clements et al., 1992). In contrast, intestinal CYP24A1 is regulated by 1,25(OH)2D3 and not PTH (Henry, 2001), suggesting that the induction of intestinal CYP24A1 is an acute response to the vitamin D receptor (Akeno et al., 1994).
Pharmacokinetic (PK) studies of 1,25(OH)2D3 are challenging due to assay sensitivity in measuring low 1,25(OH)2D3 concentrations and studies in rodents are further hampered by the limited plasma volume for sampling. Masuda et al. (2005) examined the decay of radiolabelled 1,25(OH)2D3 over 96 h in CYP24A1(+/−) and CYP24A1(−/−) mice, confirming that CYP24A1 is the major enzyme involved in the metabolism of 1,25(OH)2D3. CYP24A1(−/−) mice exhibited a longer t1/2 compared with CYP24A1(+/−) mice. In a phase I clinical trial, where 2 to 10 μg 1,25(OH)2D3 was administered s.c., the derived t1/2 proved to be ill-defined due to inadequate sampling over 12 h (Smith et al., 1999). In a human study in which prolonged sampling was conducted following p.o. and i.v. doses of 4 μg 1,25(OH)2D3, a t1/2 of 26 h was observed, along with a plasma clearance (dose/AUC∞) of 0.17 mL·min−1·kg−1 and bioavailability of 0.71 after 72 h of sampling (Brandi et al., 2002). C3H/HeJ mice treated with 0.125 or 0.5 μg 1,25(OH)2D3 i.p., with sampling up to 24 h, produced an apparent clearance (dose/AUC0→24), but a debatable terminal t1/2 due to limited sampling (Muindi et al., 2004). Chow et al. (2013) reported an apparent terminal t1/2 of 6.8 h after sampling for 48 h in mice treated with 0.05 μg 1,25(OH)2D3 i.p. and showed that both plasma and tissue 1,25(OH)2D3 concentrations fell below basal levels at 24 h due to the induction of CYP24A1. None of these studies provided an in-depth interpretation of the PK when describing the net rate of synthesis (Rsyn) of endogenous 1,25(OH)2D3 nor accounted for pharmacodynamic (PD) changes on the inhibition of CYP27B1 or induction of CYP24A1.
In this study, we examined the PD changes driven by 1,25(OH)2D3 PK in relation to basal concentrations of 1,25(OH)2D3. Single and repeated i.v. doses were administered to mice to appraise the dose- and time-dependent PK of 1,25(OH)2D3. Fitting with a simple two-compartment model yielded a decreasing Rsyn and increasing total plasma clearance (CLtotal), observations consistent with the inhibition of CYP27B1 and induction of CYP24A1 with dose. Inclusion of parameters associated with PD changes in mRNA expression for the down-regulation of CYP27B1 and the induction of CYP24A1, obtained upon regression of mRNA expression fold change (FC) of enzymes in tissue versus 1,25(OH)2D3 plasma concentration, significantly improved model fitting criteria. Lastly, an indirect response model was able to provide similar parameters as the PD-linked model and predicted the temporal PK and PD data for the different doses administered. The composite data show that changes in the PD of CYP27B1 and CYP24A1 with increasing 1,25(OH)2D3 doses resulted in altered PK of 1,25(OH)2D3.
Methods
Pharmacokinetic study
The concentration of 1,25(OH)2D3 in anhydrous ethanol was assayed spectrophotometrically at 265 nm (UV-1700, Shimadzu Scientific Instruments, Mandel Scientific, Guelph, Ontario, Canada) and diluted with sterile 0.9% saline containing 1% ethanol. Male C57BL/6 mice (8 weeks old), weighing 25.5 ± 1.6 g (mean ± SD), were purchased from Charles River Canada (Saint-Constant, Quebec, Canada). Mice were given water and food ad libitum and maintained under a 12:12 h light and dark cycle in accordance with approved protocols by the Animal Care and Use Committee at the University of Toronto. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 130 mice were used in the experiments described. Mice were randomly assigned to treatment with single (0, 2, 60 or 120 pmol) or repeated (0, 2 or 120 pmol q2d ×3) i.v. doses of 1,25(OH)2D3 on days 0, 2 and 4 at 0900 h. Serial blood sampling from the saphenous vein was performed at 1, 5, 15, 30 or 60 min. Thereafter, mice were anaesthetized with ketamine and xylazine i.p. (150 and 10 mg·kg−1 respectively) before blood collection by cardiac puncture with a 1 mL syringe-23G 3/4″ needle set that was pre-rinsed with heparin (1000 IU·mL−1). The depth of anaesthesia was assessed by monitoring the heart rate and pedal reflex. Tissues were harvested at each sampling point (3, 6, 9, 12, 24 and 48 h) from the treated mice (n = 3–4 per time point). For the vehicle-treated group (n = 9), sampling was conducted at 0 h on days 0 and 4 and averaged, as described previously (Chow et al., 2013), to provide basal concentrations. Plasma was obtained by centrifugation of blood at 3000× g for 10 min. After flushing the lower vena cava with ice-cold saline, the kidneys and ileum (6 cm proximal to the ileocecal junction) were removed over ice as outlined previously (Chow et al., 2011). Samples were snap-frozen in liquid nitrogen and stored at −80°C.
Plasma 1,25(OH)2D3 analysis
Plasma 1,25(OH)2D3 concentrations were measured by enzyme immunoassay (EIA) (Chow et al., 2013).
Quantitative real-time PCR (qPCR)
Total RNA, obtained from kidney tissues and scraped ileal enterocytes, was extracted using the TRIzol extraction method (Sigma-Aldrich, Mississauga, Ontario, Canada) in accordance with the manufacturer's protocol, with modifications (Chow et al., 2011). A total of 1.5 μg of cDNA was synthesized from RNA using the high capacity cDNA reverse transcription kit (Applied Biosystems® by Life Technologies, Burlington, Ontario, Canada) and qPCR was performed with SYBR Green detection system. Kidney and intestinal mRNA data were normalized to cyclophilin and villin, respectively, for calculation of the relative change in gene expression (Chow et al., 2009).
PK and PD analysis
Non-compartmental analysis
The AUC from 0 to 48 h (AUC0→48h) was estimated by the trapezoidal rule. The extrapolated area from the last datum point to time infinity (AUC48h→∞) was calculated upon dividing the measured plasma concentration, C48h, by the terminal slope (β). Other parameters included: t1/2β or terminal half-life, estimated from data between 6 and 48 h and calculated as 0.693/β, and CLtotal, estimated as dose/AUC∞.
Estimation of PD parameters for inhibition and induction
The mRNA expression of renal CYP27B1 and renal and ileal CYP24A1 was normalized to basal levels (vehicle-treated mice) and the FC was plotted against the plasma 1,25(OH)2D3 concentration. For CYP27B1, the inhibition function or FC of CYP27B1 (CYP27B1FC) was
![]() (1)
(1)
For CYP24A1, the induction function or FC of CYP24A1 (CYP24A1FC) was
![]() (2) with Cp as the plasma 1,25(OH)2D3 concentration, the maximal FC as Imax and Emax for inhibition and induction factors, and IC50 and EC50 as the plasma concentrations that result in 50% of Imax and Emax (Mager et al., 2009). The Emax, EC50, Imax and IC50 estimates were obtained upon non-linear regression of single and repeated dose and combined data from all doses with Equations 1994 and 2013a using Scientist® (version 2.0; Micromath, St. Louis, Missouri, USA).
(2) with Cp as the plasma 1,25(OH)2D3 concentration, the maximal FC as Imax and Emax for inhibition and induction factors, and IC50 and EC50 as the plasma concentrations that result in 50% of Imax and Emax (Mager et al., 2009). The Emax, EC50, Imax and IC50 estimates were obtained upon non-linear regression of single and repeated dose and combined data from all doses with Equations 1994 and 2013a using Scientist® (version 2.0; Micromath, St. Louis, Missouri, USA).
Fitting without consideration of PD: simple two-compartment model
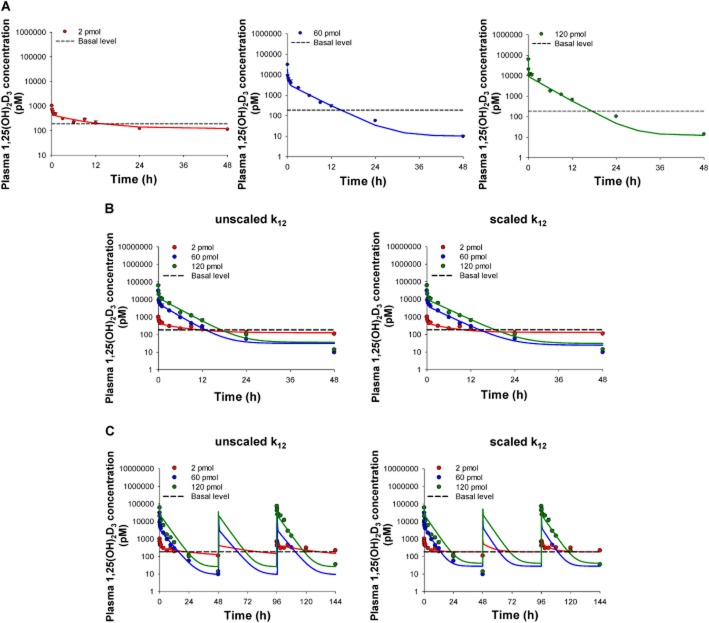
Fitting was conducted with Scientist with appropriate weighting schemes (unity, 1/observation and 1/observation2) or with ADAPT5 (version 5; Biomedical Simulations Resource, University of Southern California, Los Angeles, California, USA). Assuming stationary kinetics and dynamics, we estimated the volume of the central compartment (V1), net synthesis rate (Rsyn) controlled by CYP27B1 and the micro-rate constants (k12, k21, k10) during the first 48 h of sampling. The fit for each of the single 2, 60 or 120 pmol dose levels was obtained with a simple two-compartment model (Figure A and equations in Appendix A). The steady-state volume of distribution (Vss) was estimated as V1·(1 + k12/k21). Values of k12 for the 60 and 120 pmol doses are expressed as multiples of k12, relative to the 2 pmol dose, for the combined fit of all data (1.5× and 5.7× the k12 value of the 2 pmol dose). Parameter estimates obtained from the single doses were used as initial estimates for the combined fit to all data upon repeated dosing.
Figure 1.
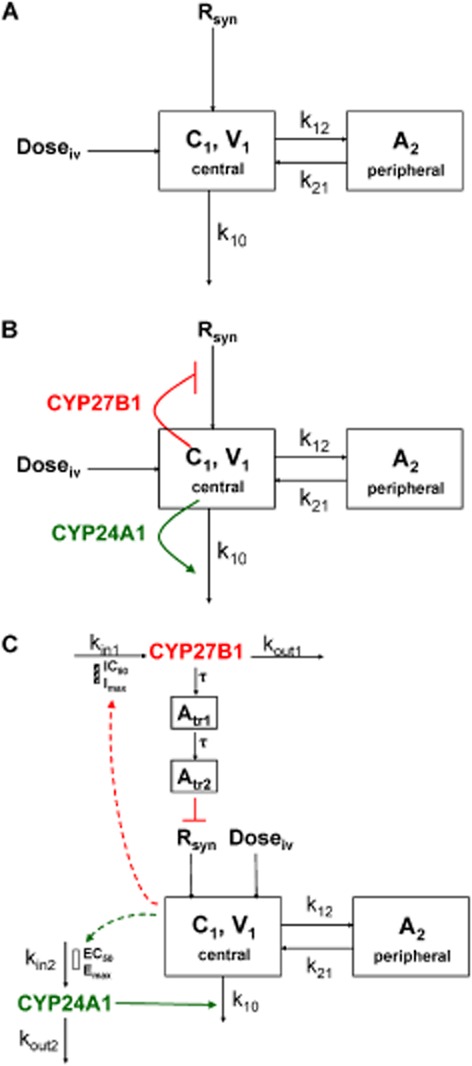
Fitting of 1,25(OH)2D3 data using (A) a simple two-compartment model, (B) a PD-linked model and (C) an indirect response model. For the compartmental models, it is assumed that synthesis and elimination of 1,25(OH)2D3 are occurring from the central compartment.
Integrated PKPD model
Parameters governing changes in CYP27B1 and CYP24A1 expression were incorporated into a two-compartment model (Figure B and Appendix B) to modify Rsyn and k10. Based on the assumption that there was no down-regulation of CYP27B1 nor induction of CYP24A1 for the 2 pmol dose, fitted parameters for this dose (V1, k12, k21, Rsyn and k10) from the two-compartment model were used as initial estimates, together with averaged estimates of Imax, IC50, Emax and EC50 (Table 3), for combined fitting of data for the first doses (2, 60 and 120 pmol) and data from all doses. Fitting was repeated upon addition of scaling factors, then Hill coefficients (Appendix B). Again, k12 values for the 60 and 120 pmol doses were scaled to account for possible changes in the distribution of 1,25(OH)2D3 in the simultaneous fits. The PD-linked models were compared with the simple two-compartment model using the F-test (Boxenbaum et al., 1974).
Table 3.
Pharmacodynamic parameters estimated from CYP27B1 or CYP24A1 fold change versus plasma 1,25(OH)2D3 concentration
| Renal CYP27B1 | Renal CYP24A1 | Ileal CYP24A1 | ||||
|---|---|---|---|---|---|---|
| Imaxa | IC50 (pM) | Emaxa | EC50 (pM) | Emaxa | EC50 (pM) | |
| For first dose | 8 ± 2b | 100 ± 9 | 82 ± 19 | 300 ± 23 | 1000 ± 129 | 500 ± 62 |
| For repeated third dose | 24 ± 2 | 150 ± 15 | 107 ± 16 | 1480 ± 90 | 939 ± 126 | 3630 ± 220 |
| For first and third doses | 24 ± 4 | 150 ± 24 | 107 ± 19 | 1480 ± 189 | 970 ± 218 | 3630 ± 272 |
Imax and Emax values are expressed as fold change relative to baseline.
SD of parameter estimate.
Fitting with the indirect response model
The indirect response model (Figure C), which describes an indirect mechanism of action and incorporates transit compartments (Atransit1 and Atransit2) containing a time-delay function (τ) (Appendix C), relates temporal differences between drug concentrations and responses (Mager et al., 2003). The indirect response model was used to explain the time delay for the CYP27B1 effects, assuming CYP27B1 is indirectly stimulated by PTH-induced activation of the vitamin D receptor. Similar to the PD-linked model, scaling factors and Hill coefficients were incorporated for fitting purposes.
Statistical analysis
The mRNA data are expressed as mean ± SEM. One-way anova and a post hoc Tukey honest significant difference test were used to evaluate differences between mean mRNA expression of groups at each time point using GraphPad Prism Software (version 6; GraphPad Software Inc., La Jolla, California, USA). The goodness of fit was appraised by the weighted sum of square residuals (WSSR), Akaike information criterion (AIC), and SD of the parameter estimate, while the F-test was used for comparing the models. Significance was defined as P < 0.05.
Materials
1,25(OH)2D3 in powder form was obtained from Sigma-Aldrich. The EIA kit (Cat# AC-62F1) for 1,25(OH)2D3 measurements was manufactured by Immunodiagnostics Systems Inc. (Scottsdale, Arizona, USA) and purchased from Inter Medico (Markham, Ontario, Canada). All other reagents were obtained from Sigma-Aldrich and Fisher Scientific (Mississauga, Ontario, Canada).
Results
Dose-dependent PK of 1,25(OH)2D3
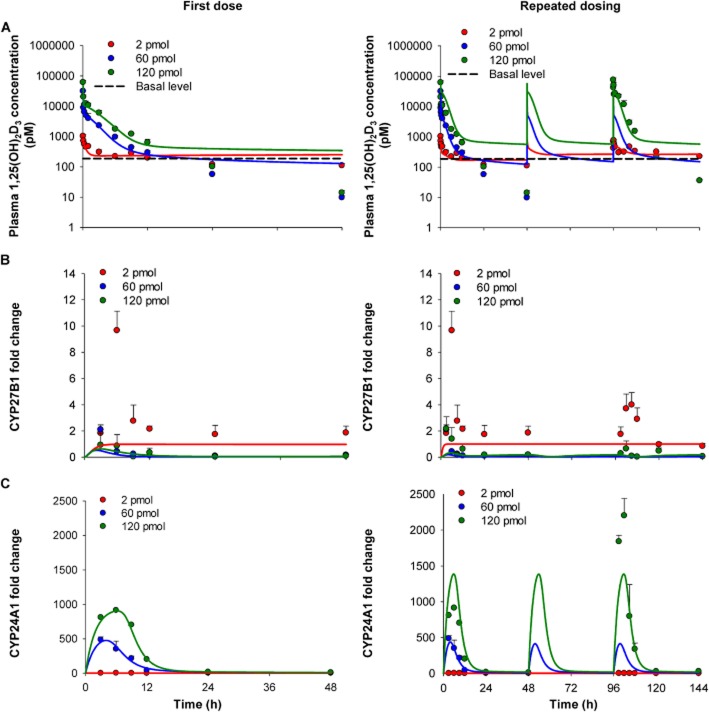
1,25(OH)2D3 concentrations for the 60 and 120 pmol doses fell below basal values (187 ± 48.5 pM) by 24 h whereas those for the 2 pmol dose remained relatively unchanged. Plasma 1,25(OH)2D3 concentrations decayed biexponentially at each dose level (Figure 2). There was a prolonged t1/2β (36.7 h) for the lowest dose and a dramatically shorter t1/2β (∼6 h) for higher doses (Table 1). Non-compartmental values of AUC∞/dose decreased with increasing dose, yielding greater clearance values at higher doses. CLtotal increased from 0.1 to 2.0 mL·min−1·kg−1 (Table 1), an observation compatible with induction of CYP24A1 for the metabolism of 1,25(OH)2D3.
Figure 2.
Plasma 1,25(OH)2D3 concentrations after i.v. administration of 2, 60 and 120 pmol doses versus basal levels. The two-compartment model was used to fit the 1,25(OH)2D3 data for each single 2, 60 or 120 pmol dose (A) individually, (B) combined fit of data for the first doses, with unscaled or scaled k12, and (C) combined fit of data for all doses, with unscaled or scaled k12. Observed plasma 1,25(OH)2D3 concentrations are shown as mean ± SEM (n = 3–4 different mice) with fitted values shown as a solid line. Data for vehicle-treated mice (basal level) were averaged and joined by the dashed line (n = 9).
Non-compartmental pharmacokinetic parameters following administration of first doses of 1,25(OH)2D3 to mice
| Dose (pmol) | |||
|---|---|---|---|
| 2 | 60 | 120 | |
| t1/2β (h)a | 36.7 | 6.6 | 6.0 |
| AUC∞ (pM·h)b | 14 900 | 24 500 | 57 200 |
| AUC∞/dose (h·L−1) | 7 460 | 409 | 477 |
| CLtotal (mL·min−1·kg−1)c | 0.112 | 2.04 | 1.75 |
t1/2β was calculated as 0.693/β, where β is the terminal slope of ln(concentration) versus time data between 6 and 48 h.
AUC∞ was determined as (AUC0→48 + Clast/β) and AUC0→48 was estimated by the trapezoidal rule.
CLtotal was estimated as dose/AUC∞.
For the simple two-compartment model that contained Rsyn for 1,25(OH)2D3 formation from its vitamin D precursors, fits to the single dose data individually revealed a decreasing Rsyn and an increasing k10 with increasing dose (Figure A; Table 2). Furthermore, fitted values of k12 and Vss increased with dose, suggesting a larger distribution volume with increasing dose levels. These distributional changes could not be explained by a saturation of protein binding sites as the 5 μM concentration of DBP in plasma (Chun, 2012) greatly exceeds the plasma 1,25(OH)2D3 concentrations from i.v. dosing. We then performed combined fitting of all data from the first doses and compared the parameter estimates obtained to those from individual fits. Accommodation of the changing k12 was accomplished by scaling k12 with dose (Table 2; Appendix A). The model predicted the data for the first dose well, whether or not k12 was scaled (Figure 2B). For the two-compartment model, the fitted k12 value for the 2 pmol dose (2.77 ± 1.42 h−1), obtained from the individual fit of the 2 pmol data (Figure 2A), was about half that from forced fitting of data from the first doses (4.15 ± 1.23 h−1) and with scaling of k12 (Table 2). For fitting of the first and repeated doses, the fit to the third 120 pmol dose was better than the fit to the first dose, with or without k12 scaled (Figure 2C). For the forced fit to all data from single and repeated dosing, the averaged value of the fitted k12 was halved when k12 was scaled, k21 and k10 were lower and V1 and Vss were higher (Table 2). The WSSR was smaller with scaled k12 (although the AICs were similar), suggesting that scaling of k12 was an improvement (Table 2). The lack of a significant improvement in the forced fit for scaled k12 versus unscaled k12 was likely due to the inability of this model to account for the dose-dependent nature of Rsyn and k10.
Table 2.
Fitted pharmacokinetic parameters for first doses of 1,25(OH)2D3 according to the simple two-compartment model
| Individual fitting to first dose | Forced fitting to first doses | ||||
|---|---|---|---|---|---|
| 2 pmol | 60 pmol | 120 pmol | 2, 60 and 120 pmol k12 unscaled | 2, 60 and 120 pmol k12 scaled | |
| k12 (h−1) | 2.77 ± 1.42c | 4.17 ± 3.57 | 15.8 ± 4.36 | 10.8 ± 4.67 | 4.15 ± 1.23 |
| k21 (h−1) | 2.15 ± 1.14 | 1.41 ± 0.74 | 2.09 ± 0.40 | 2.93 ± 0.10 | 1.89 ± 0.49 |
| k10 (h−1) | 0.27 ± 1.10 | 0.93 ± 0.44 | 2.21 ± 0.71 | 1.11 ± 0.71 | 0.75 ± 0.37 |
| V1 (mL·kg−1) | 112 ± 17.0 | 167 ± 81.5 | 61.5 ± 21.0 | 72.0 ± 1.6 | 117 ± 10.5 |
| Vss (mL·kg−1)a | 255 | 659 | 526 | 337 | 409 |
| Rsyn (fmol·h−1)b | 71.9 ± 39.8 | 31.1 ± 10.8 | 32.6 ± 5.91 | 85.5 ± 4.78 | 81.6 ± 16.4 |
| WSSR | 9.05 | 5.73 | |||
| AIC | 494 | 523 | |||
Vss was calculated as V1 + V2, where V2, peripheral compartment = V1 (k12/k21).
Rsyn initial estimate (49.6 fmol·h−1) was obtained from Hsu et al. (1987).
SD of parameter estimate.
CYP27B1 and CYP24A1 mRNA expression
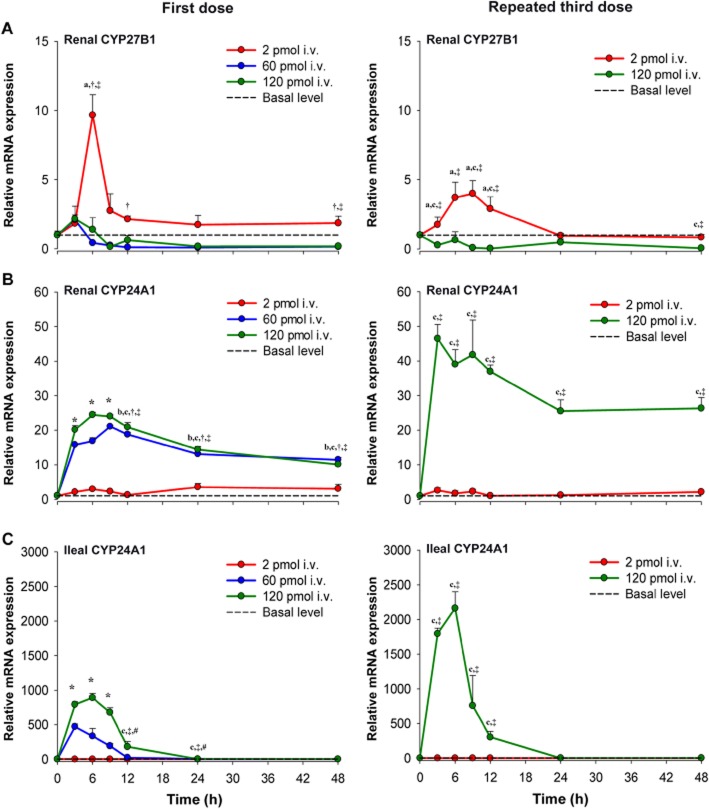
The mRNA expression of the synthetic enzyme in kidney and degradation enzyme in intestine and kidney also displayed dose-dependent changes (Figure 3). For the 2 pmol single dose, there was an absence of any notable trend for the mRNA expression of renal CYP27B1 or renal and ileal CYP24A1, as levels remained relatively unchanged in relation to basal values (Figure 3, left panel). Furthermore, hypercalcaemia was not observed for the low dose (data not shown). Thus, the Rsyn value obtained for the 2 pmol dose should represent the net synthesis rate of endogenous 1,25(OH)2D3 formation from its vitamin D precursors. In contrast, markedly lower CYP27B1 mRNA expression at 9 h after 1,25(OH)2D3 administration was noted with higher doses, wherein levels fell and remained below basal levels. Maximal induction of renal CYP24A1 expression occurred at 6–9 h and was sustained until 48 h. In ileum, maximal induction of CYP24A1 mRNA expression occurred at 3 h following the 60 pmol dose and at 6 h following the 120 pmol dose (Figure 3, left panel). Repeated administration of 120 pmol 1,25(OH)2D3 led to a greater down-regulation (CYP27B1) and induction (CYP24A1) of renal mRNA levels when compared with the single dose and a similar pattern was observed for ileal CYP24A1 mRNA expression (Figure 3, right panel).
Figure 3.
Relative mRNA expression for synthesis and degradation enzymes following single or repeated i.v. administration of 1,25(OH)2D3. (A) Renal CYP27B1 mRNA expression is reduced by both single and repeated administration of 60 and 120 pmol 1,25(OH)2D3. (B) Renal and (C) ileal CYP24A1 mRNA expression are induced by 1,25(OH)2D3 in a dose-dependent manner for single and repeated dosing. Data for vehicle-treated mice (basal level) were averaged and joined by the dashed line (n = 9), whereas data for treated mice are mean ± SEM and joined by a solid line (n = 3–4 different mice). Significant differences between groups were denoted by: abasal level versus 2 pmol; bbasal level versus 60 pmol; cbasal level versus 120 pmol; †2 pmol versus 60 pmol; ‡2 pmol versus 120 pmol; #60 pmol versus 120 pmol; *all groups except basal level versus 2 pmol.
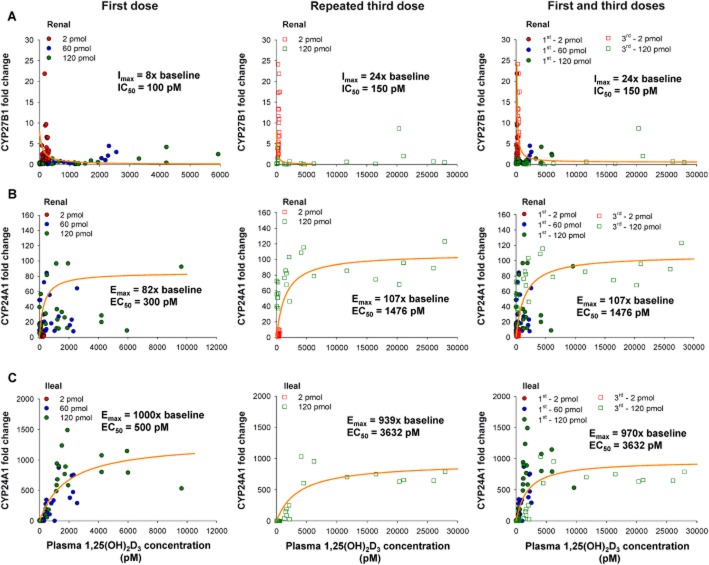
PD response versus concentration curves
Figure 4 shows the concentration-response relationship for FC of renal CYP27B1 and CYP24A1 and intestinal CYP24A1 mRNA expression in mice receiving single (2, 60 and 120 pmol) and repeated (2 and 120 pmol) doses of 1,25(OH)2D3. A plateau was reached for CYP27B1 and CYP24A1 within the dose range and FC for CYP27B1 down-regulation or CYP24A1 induction remained constant at 1,25(OH)2D3 concentrations >5000 pM. Upon fitting of Equation 1994, a threefold increase in Imax was observed after repeated dosing (Table 3; Figure 4A), although the fitted IC50 values were similar for the single and repeated doses. Composite Imax and IC50 values were also obtained upon regression of pooled data from the first and repeated doses with Equation 1994 and these values were similar to those obtained for the repeated third dose (Table 3). Estimated Emax values (expressed over basal level) obtained for CYP24A1 (Equation 2013a) for the first and the third doses were virtually identical (Figure 4B,C), whereas EC50 values for both renal and intestinal CYP24A1 increased significantly upon repeated dosing compared with that for the first dose (Table 3). Values for Emax and EC50 for the pooled data were similar to those for the third dose. For fitting of Equation 1974 of Appendix B, the Emax values obtained from the pooled data for renal and ileal CYP24A1 were summed to provide the total Emax; the EC50 was estimated as the average of the EC50 values for renal and intestinal CYP24A1.
Figure 4.
Inhibition of CYP27B1 and induction of CYP24A1 by 1,25(OH)2D3. Plots of renal CYP27B1 and renal and intestinal CYP24A1 mRNA FC versus plasma concentration of 1,25(OH)2D3 following first or third dose of 2, 60 and 120 pmol, with equations for inhibition 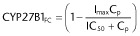 and induction
and induction 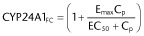 .
.
Fitting of the PD-linked model to 1,25(OH)2D3 concentrations
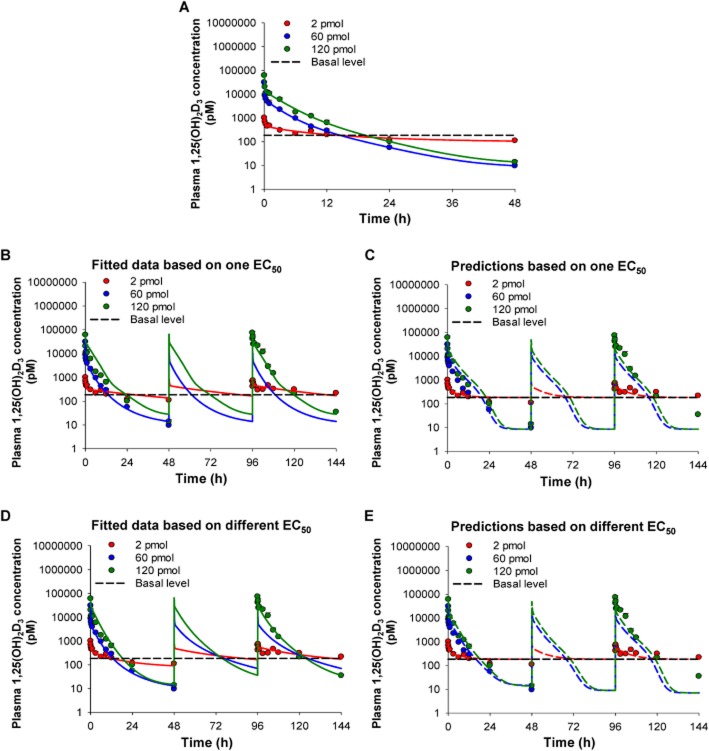
Owing to the dose discrepancy and progressive changes that occur for Rsyn and k10 with dose (Table 2), there was a need to incorporate the PD changes of CYP27B1 and CYP24A1 into the two-compartment model shown in Figure A (Appendix B). Hence, a PD-linked model (Figure 1B) was developed using scaled k12 values for greater doses. From fitting with data from the first doses, the EC50 (average of renal and ileal CYP24A1 EC50) and the summed Emax for the kidney and intestine were used as initial estimates. Additionally, scaling factors and Hill coefficients for the inhibition and induction functions were added stepwise to monitor improvement of fit in order to determine the best predictive model. For data from the single doses, forced fitting with the PD-linked model showed significant improvement compared with the simple two-compartment model (Figure 5A and Table 4). The best outcome was obtained when all of these modifications were incorporated into the model (Table 4). Values of the Hill coefficients were close to unity, Rsyn was 61.5 ± 5.24 fmol·h−1 (a value lower than that estimated from the two-compartment model), V1 was similar to plasma volume and k10 [representing the basal elimination rate constant of 1,25(OH)2D3] was 0.128 ± 0.021 h−1, a value lower than but reasonable to that estimated from the simple two-compartment model.
Figure 5.
Fitting of single and repeated dose plasma 1,25(OH)2D3 data using a PD-linked model. (A) Simultaneous fitting of single dose plasma 1,25(OH)2D3 data. Simultaneous fitting of combined single and repeated dose plasma 1,25(OH)2D3 using (B) one EC50 and (C) simulations to predict data for higher doses using parameters from the 2 pmol dose. Simultaneous fitting with (D) different EC50s and (E) simulations to predict data for higher doses. Observed plasma 1,25(OH)2D3 concentrations are shown as mean ± SEM (n = 3–4 different mice) with fitted values shown as a solid line and simulated values shown as a dashed line. Data for vehicle-treated mice (basal level) were averaged and joined by the dashed line (n = 9).
Table 4.
Simultaneous fitting of first dose or pooled first and third dose data to different models with k12 scaled
| Combined fit to data of first dose | Combined fit to data of all doses (first and repeated third dose) | ||||||
|---|---|---|---|---|---|---|---|
| Fitted parameters | Two-compartment model | PD-linked model | Indirect response model | Two-compartment model | PD-linked model (one EC50) | PD-linked model (different EC50) | Indirect response model |
| k12 (h−1)a | 4.15 ± 1.23d | 4.71 ± 2.22 | 3.88 ± 3.52 | 3.23 ± 0.77 | 2.75 ± 1.42 | 2.74 ± 1.34 | 6.17 ± 4.81 |
| k21 (h−1) | 1.89 ± 0.49 | 3.01 ± 0.24 | 1.70 ± 1.21 | 2.22 ± 0.55 | 2.83 ± 1.40 | 3.25 ± 1.42 | 3.75 ± 2.68 |
| k10 (h−1) | 0.75 ± 0.37 | 0.128 ± 0.021 | 0.349 ± 0.096 | 0.55 ± 0.27 | 0.091 ± 0.034 | 0.092 ± 0.007 | 0.133 ± 0.118 |
| V1 (mL) | 2.33 ± 0.21 | 1.38 ± 0.30 | 1.79 ± 0.55 | 2.43 ± 0.31 | 1.86 ± 0.49 | 1.86 ± 0.49 | 1.43 ± 0.83 |
| Rsyn (fmol·h−1) | 81.6 ± 16.4 | 61.5 ± 5.24 | 49.1 ± 9.2 | 78.9 ± 42.8 | 47.2 ± 16.1 | 44.5 ± 6.47 | 29.4 ± 19.0 |
| Emax | 398 ± 54.6 | 802 ± 175 | 404 ± 80.7 | 407 ± 47.4 | 1050 ± 515 | ||
| EC50(1) (pM) | 713 ± 137 | 1530 ± 1280 | 790 ± 248 | 616 ± 296 | 3110 ± 2990 | ||
| EC50(2) (pM) | 3810 ± 326 | 4640 ± 6110 | |||||
| Imax | 11.3 ± 3.30 | 23.4 ± 5.41 | 11.6 ± 2.56 | 10.0 ± 3.07 | 45.2 ± 14.6 | ||
| IC50 (pM) | 276 ± 91.0 | 143 ± 218 | 306 ± 49.7 | 310 ± 74.8 | 388 ± 701 | ||
| SM1 | 4.24 ± 0.42 | 4.47 ± 0.87 | 5.41 ± 0.81 | 7.15 ± 1.38 | 0.81 ± 0.52 | ||
| SM2 | 25.8 ± 2.87 | 75.4 ± 16.6 | 25.9 ± 4.66 | 26.8 ± 6.20 | 9.64 ± 10.4 | ||
| γ1 | 1.25 ± 0.44 | 2.80 ± 0.96 | 1.85 ± 1.82 | 0.783 ± 0.391 | 2.12 ± 0.49 | ||
| γ2 | 0.983 ± 0.249 | 2.14 ± 0.21 | 1.33 ± 0.16 | 1.15 ± 0.154 | 2.50 ± 0.53 | ||
| kin1 (h−1)bc | 0.390 ± 0.163 | 0.343 ± 0.083 | |||||
| kin2 (h−1)bc | 0.195 ± 0.110 | 0.362 ± 0.175 | |||||
| τ (h) | 0.809 ± 0.114 | 1.36 ± 1.07 | |||||
| WSSR | 5.73 | 0.47 | 709 | 30.2 | 23.6 | 11.8 | 676 |
| AIC | 523 | 649 | 982 | 913 | 1067 | 1086 | 1547 |
| F-test value | 28.3* | −1.53 | 1.48 | 7.09* | −1.32 | ||
| Fcritical (df1, df2) | 2.45 (8, 20) | 2.41 (11, 17) | 2.17 (8, 42) | 2.12 (9, 41) | 2.43 (12, 16) | ||
Values of k12 for the 60 and 120 pmol doses were assigned as 1.5× and 5.7× the value of k12 for the 2 pmol dose.
kin1 and kin2 are the zero-order synthesis rate constants for CYP27B1 and CYP24A1, respectively.
kout1 and kout2 are the first-order degradation rate constants for CYP27B1 and CYP24A1, respectively, and are equal to kin1 × baseline or kin2 × baseline, where baseline is 1.
SD of parameter estimate.
Significant difference between PD-linked versus two-compartment model at P < 0.05 compared with Fcritical.
For fitting of data from all doses (Table 4), we further adopted a second strategy as there were changes in the EC50 upon repeated dosing. We used one EC50 [obtained from regression of FC vs. 1,25(OH)2D3 plasma concentrations from the first dose] or two different EC50 values [EC50(1) from regression of data from first dose and EC50(2) from pooled data]. When using one EC50, it was assumed that the EC50 for the first dose was identical to that for the second and third doses and the regressed value from data from the first dose was used as the initial estimate. When using different EC50 values, EC50(1) obtained from the first dose and EC50(2) from pooled data were used as initial estimates. These EC50 values, together with scaled k12 functions, scaling factors and/or Hill coefficients in the PD-linked model, were incorporated in a stepwise manner for fitting (data not shown). From fits based on one or two EC50 values, we found that the parameters (k12, k21, k10, V1 and Rsyn) and the scaling factors remained similar. The Hill coefficients using two EC50 values remained similar to unity, whereas those for the one EC50 model were greater (Table 4). Overall, it was concluded that the best fit was attained when all of these modifications, including different EC50 values, were added to the PD-linked model (Table 4). We also used parameters obtained from the 2 pmol first dose to simulate plasma 1,25(OH)2D3 concentration time data for other single and repeated dosing regimens, with use of one EC50 (Figure 5C) or two different EC50s (Figure 5E). The simulations predicted the first dose reasonably well, but were unable to fully capture the later concentrations upon repeated dosing, presumably due to tolerance.
Indirect response model with inhibition and induction functions to explain 1,25(OH)2D3 PK
The PD components of the indirect response model incorporate both an indirect inhibitory (CYP27B1) and stimulatory (CYP24A1) response model. We added transit compartments with lag time (τ) to explain the time delay for CYP27B1 effects, as CYP27B1 was indirectly affected by vitamin D receptor activation by PTH (Figure C). A zero-order rate constant for formation (kin), a first-order decay rate constant (kout) and the appropriate inhibition and induction functions were added to account for decreased CYP27B1 and increased CYP24A1 production (Appendix C; Dayneka et al., 1993; Sharma and Jusko, 1996). Preliminary fits showed that two additional transit compartments were needed to improve model fitting (data not shown). Improved fits were obtained when scaling factors and Hill coefficients were incorporated into the model. For the single dose data, the fitted parameter values for k12, k21, k10, V1 and Rsyn were usually within twofold (Table 4). For the pooled data, when different EC50 values were used, greater values for k12 and EC50(1) values were obtained, whereas Rsyn and the scaling factors were smaller (Table 4). Model fitting criteria were not statistically improved compared with the two-compartment model, although the indirect response model was able to reveal correlations between PD responses to 1,25(OH)2D3 concentrations in a temporal fashion (Figure 6).
Figure 6.
The indirect response model for simultaneous fitting of all data on plasma 1,25(OH)2D3 and FC of vitamin D receptor target genes, CYP27B1 and CYP24A1. (A) Simultaneous fitting of single dose (left) and single and repeated dose (right) plasma 1,25(OH)2D3 concentration. Simultaneous fitting for (B) inhibition of CYP27B1 FC and (C) stimulation of CYP24A1 FC. Observed plasma 1,25(OH)2D3 concentrations and CYP27B1 and CYP24A1 FC are shown as mean ± SEM (n = 3–4 different mice) with fitted values shown as a solid line. Data for vehicle-treated mice (basal level) were averaged and joined by the dashed line (n = 9).
Comparison of the two-compartment, PD-linked and indirect response models
Fitting of the two-compartment model to individual data sets revealed dose-dependent kinetics and reasonably good fits (Figure 2). However, fitting with the PD-linked model for the single dose data proved to be superior (Figure 5; Table 2013c). For repeated dosing, we found that the PD-linked model with different EC50 values was associated with smaller WSSR values with a significant F-value compared with the two-compartment model (P < 0.05), despite the larger AIC (Figure 5D; Table 4). Furthermore, incorporation of scaled k12 constants, scaling factors and Hill coefficients into the PD-linked model improved model performance (data not shown). Although the indirect response model did not improve the fit statistically compared with the two-compartment model, the model showed reasonable utility and was adequate in predicting changes in the temporal PD profiles against 1,25(OH)2D3 concentrations at the different administered dose levels (Figure 6).
Discussion and conclusions
1,25(OH)2D3 is used extensively for the treatment of secondary hyperparathyroidism in uraemic patients (Slatopolsky et al., 1984; Kimura et al., 1991; Brandi et al., 2002) and has demonstrated therapeutic potential for anticancer therapy (Hershberger et al., 2002; Rassnick et al., 2008; Ramnath et al., 2013). The therapeutic use of 1,25(OH)2D3 is often limited by the propensity of 1,25(OH)2D3 to cause hypercalcaemia and adverse effects. Recent studies have suggested that 1,25(OH)2D3 may also play a beneficial role in lowering cholesterol (Chow et al., 2014) as well as enhancement of amyloid-β efflux and reduction of cerebral plaque in transgenic mice expressing the human amyloid-β precursor protein (Durk et al., 2014). Thus, an understanding of the PK and PD of 1,25(OH)2D3 is critical for the prediction of a proper dose and dosing regimen for potential therapeutic uses.
Information on the PK of 1,25(OH)2D3 is equivocal. Some of the discrepancies in the reported PK parameters of 1,25(OH)2D3 may exist due to species differences, inadequate sampling or dose-dependent PK and altered PD with dose and route. An examination of available literature regarding the PK properties of 1,25(OH)2D3 shows substantially different t1/2 values with respect to dose and route of administration among species. In humans, a 4 μg dose of 1,25(OH)2D3 administered i.v. or p.o. resulted in a t1/2 of 25.9 and 28.2 h, respectively (Brandi et al., 2002), whereas a similar i.v. dose of 0.06 μg·kg−1 led to a shorter t1/2 of 16.5 h (Salusky et al., 1990). An equal dose of 20 μg·kg−1 administered i.p. and p.o. to the rat resulted in different t1/2 values of 5.0 and 10.4 h (Vieth et al., 1990). Administration of 10 and 50 μg·kg−1 of 1,25(OH)2D3 i.v. resulted in t1/2 of 3.8 and 2.3 h respectively (Kissmeyer and Binderup, 1991). These shorter t1/2 values are due to greater clearances and are the consequence of a greater induction of CYP24A1. In contrast, mice treated with single doses of 0.125 or 0.5 μg of 1,25(OH)2D3/mouse i.p. did not exhibit a clear dose-dependency, with t1/2 of 7.6 and 7.8 h (Muindi et al., 2004). Chow et al. (2013) also reported a t1/2 of 6.8 h in mice treated i.p. with repeated doses of 0.05 μg per mouse 1,25(OH)2D3.
Our present study with 2, 60 and 120 pmol (0.00083, 0.025 and 0.05 μg per mouse) 1,25(OH)2D3 doses revealed dose-dependent t1/2β values ranging from 36.7 to 6.6 h (Table 1). Accordingly, CLtotal values of 0.1, 2.0 and 1.8 mL·min−1·kg−1 for 2, 60 and 120 pmol doses of 1,25(OH)2D3 were found (Table 1). Changes in enzymes were virtually absent for the 2 pmol dose, rendering the fitted parameters according to the two-compartment model pertinent to basal conditions, whereas maximal inhibitory (CYP27B1) and stimulatory (CYP24A1) responses were observed for the higher doses. Important information was revealed from fitted results from the lowest dose. For example, the volume of distribution of 1,25(OH)2D3 was low and Rsyn was 71.9 ± 39.8 fmol·h−1, a basal value that is higher than that (49.6 fmol·h−1) previously reported by Hsu et al. (1987). Both the 60 and 120 pmol doses produced greater CLtotal values (2.0 and 1.8 mL·min−1·kg−1) that were similar to i.p. mouse studies, with apparent CLtotal values (CLtotal/F where F is bioavailability of i.p. dose) of 1.2 and 2.8 mL·min−1·kg−1, estimated graphically from the data of Muindi et al. (2004), for doses of 0.125 and 0.5 μg per mouse.
Gene profiling of CYP27B1 and CYP24A1 in the kidney and intestine, two major vitamin D receptor-containing tissues, confirmed the inhibition and induction associated with 1,25(OH)2D3 administration (Figure 3). The dose-dependent down-regulation of renal CYP27B1 mRNA expression readily explains the decreased Rsyn of 1,25(OH)2D3 for higher doses. Moreover, a dose-dependent induction of renal and ileal CYP24A1 mRNA expression further explains the increased CLtotal and decreased t1/2 with increasing dose. Interestingly, the concentration-response curve for CYP24A1 suggests tolerance as EC50 values were increased in mice given repeated doses of 1,25(OH)2D3 without a change in Emax (Figure 4). The observed tolerance may be attributed to a decreased binding affinity of 1,25(OH)2D3 to the vitamin D receptor. We also observed a sustained induction of renal CYP24A1 mRNA expression at 48 h, whereas ileal CYP24A1 expression returned to basal levels by 24 h post-injection for both single and repeated dosing. Long-term treatment with 1,25(OH)2D3 has been shown to lower CYP24A1 mRNA expression, suggesting a lack of activation of vitamin D receptor upon repeated dosing (unpublished data). Accordingly, the change in intestinal CYP24A1 mRNA level in response to 1,25(OH)2D3 has been described to be more short-lived than in kidney and becomes refractory to the continued administration of 1,25(OH)2D3, suggesting the presence of intestinal adaptation mechanisms which down-regulate the responsiveness of the enzyme (Lemay et al., 1995). The sustained induction of renal, and not intestinal CYP24A1, and the fact that little to no intestinal removal occurs for systemically administered compounds (due to shunting of intestinal flow away from the enterocyte region) (Doherty and Pang, 1997; Cong et al., 2000) suggest that renal CYP24A1 is the major enzyme for the catabolism of 1,25(OH)2D3 given i.v. Collectively, tolerance of CYP24A1 to repeated 1,25(OH)2D3 administration explains the inconsistency of the parameters derived from the PD-linked model for the 2 pmol dose in predicting 1,25(OH)2D3 profiles for higher doses and upon repeated dosing.
The present comprehensive analysis of 1,25(OH)2D3 quantification, together with gene profiling and data fitting using PD-linked and indirect response models, brings a new perspective as to how 1,25(OH)2D3 levels affect vitamin D receptor target genes and how these PD changes affect the PK of exogenously administered 1,25(OH)2D3. We recommend that PK studies involving 1,25(OH)2D3 incorporate PD effects and Rsyn in order to fully capture the disposition of 1,25(OH)2D3. Changes in CYP27B1 and CYP24A1 expression alter the synthesis and degradation of 1,25(OH)2D3, and these events can be explained with appropriate PKPD models. By incorporating the PD changes, we were able to integrate the gene changes that affect 1,25(OH)2D3 concentrations, showing the mutual interaction of kinetics and dynamics. With our findings, we caution against merely reporting t1/2, especially when the data do not cover the time course describing the decay. Our findings could explain the array of reported t1/2 with different doses in the literature, as we show that clearance is dose-dependent and that CYP27B1 and CYP24A1 work closely to affect 1,25(OH)2D3 levels and vice versa. Clearly, the simple two-compartment model, although adequately showing dose-dependency for k12, Vss, Rsyn and CLtotal, could not fully explain the intricacies of the dose-dependent PK of 1,25(OH)2D3. Using the derived parameters of Emax, EC50, Imax and IC50, with or without incorporating changes in EC50 for single and repeated doses, we could adequately predict the dose-dependent PKPD profiles of 1,25(OH)2D3. Further modifications to the model, including scaled k12, scaling factors and Hill coefficients significantly improved model performance. Although there is no apparent improvement with the indirect response model statistically, we could demonstrate the direct correlation between 1,25(OH)2D3 and vitamin D receptor target genes and essential time delays using transit compartments in the model (Mager and Jusko, 2001; Mager et al., 2003).
The unique PK of exogenously administered 1,25(OH)2D3 rests on the inhibition and induction of its own synthesis and metabolism, although there has been other evidence for altered 1,25(OH)2D3 efficacy due to single nucleotide polymorphisms in the CYP24A1 gene (Chen et al., 2011; Ramnath et al., 2013). We have demonstrated that future studies involving the administration of 1,25(OH)2D3 as a therapeutic agent should incorporate Rsyn and PD effects of CYP27B1 and CYP24A1 in order to fully explain the disposition of 1,25(OH)2D3 in the body. This present investigation on the PKPD relationships with respect to renal and intestinal handling of synthesis and metabolism via down-regulation of CYP27B1 and induction of CYP24A1 has expanded ways to view changes of other vitamin D receptor target genes and their PD effects. A physiologically based PKPD model could extend this model to different routes of administration to describe the regulatory effects of the vitamin D receptor on vitamin D receptor target genes in other tissues in the body.
Acknowledgments
We thank Dr. Matthew R. Durk for assistance in the study. This work was supported by the Canadian Institutes of Health Research (K. S. P.), the National Sciences and Engineering Research Council of Canada (H. P. Q. and E. C. C.) and the Ontario Graduate Scholarship Program (H. P. Q.).
Glossary
- AIC
Akaike information criterion
- AUC
area under the plasma concentration time curve
- CL
clearance
- DBP
vitamin D binding protein
- k
rate constant
- PD
pharmacodynamic
- PK
pharmacokinetic
- PTH
parathyroid hormone
- qPCR
quantitative real-time PCR
- Rsyn
net rate of synthesis
- V1,
volume of central compartment
- Vss
volume of distribution at steady state
- WSSR
weighted sum of square residuals
- 1,25(OH)2D3
1α,25-dihydroxyvitamin D3
- 25(OH)D3
25-hydroxyvitamin D3
Appendix A
For the simple two-compartment model (Figure 1A):
| A1 |
| A2 |
Note: k12 for the 60 pmol dose is 1.5× k12 of the 2 pmol dose; k12 for the 120 pmol dose is 5.7× k12 of the 2 pmol dose.
Appendix B
For PD-linked model with scaling factors (SM1 and SM2) and Hill coefficients (γ1 and γ2) (Figure 1B)
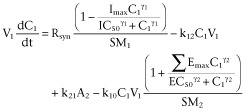 |
B1 |
Appendix C
For the indirect response model, with scaling factors (SM1 and SM2) to adjust for fold changes (FC) (Figure 1C)
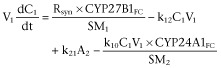 |
C1 |
The rates of change in PD responses (CYP27B1 or CYP24A1) can be described as
| C2 |
where kin is the zero-order rate constant for production of the response and kout is the first-order rate constant for loss of the response. The response variable, R, in this study is the same as the FC of vitamin D receptor target gene mRNA expression. The inhibition (Equation 1994) and induction (Equation 2013a) functions were included, respectively, to account for the inhibition of CYP27B1 and induction of CYP24A1. Inhibition of the CYP27B1 response variable (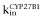 ) and induction of the CYP24A1 response variable (
) and induction of the CYP24A1 response variable (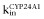 ) were described as
) were described as
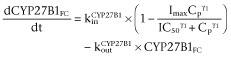 |
C3 |
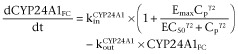 |
C4 |
where Cp is the plasma 1,25(OH)2D3 concentration; kin = kout × baseline, with baseline = 1; Imax and Emax are the maximal FC for inhibition and induction factors; and IC50 and EC50 are the plasma concentrations that result in 50% of Imax and Emax. The Emax is the sum of the Emax from the intestine and kidney.
A time-delay function was required to provide a better fit for CYP27B1 expression, where τ is the time delay in h and Atransit1 and Atransit2 are the amounts in transit compartments 1 and 2 respectively:
| C5 |
| C6 |
Author contributions
H. P. Q., E. C. C. and S. Y. H. performed the experiments. H. P. Q., E. C. C. and K. S. P. designed the research study. H. P. Q., Q. J. Y., D. E. M. and K. S. P. analysed the data and wrote the manuscript. H. P. Q., E. C. C. and K. S. P. revised the manuscript.
Conflicts of interest
None.
Supporting Information
Figure S1 Weighted sum of squares residuals versus time plots for the (A) two-compartment and (B) PD-linked models. The dashed line represents y = 0.
References
- Akeno N, Saikatsu S, Kimura S, Horiuchi N. Induction of vitamin D 24-hydroxylase messenger RNA and activity by 22-oxacalcitriol in mouse kidney and duodenum. Possible role in decrease of plasma 1α,25-dihydroxyvitamin D3. Biochem Pharmacol. 1994;48:2081–2090. doi: 10.1016/0006-2952(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol. 2013a;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: nuclear hormone receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2:123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Brandi L, Egfjord M, Olgaard K. Pharmacokinetics of 1,25(OH)2D3 and 1α(OH)D3 in normal and uraemic men. Nephrol Dial Transplant. 2002;17:829–842. doi: 10.1093/ndt/17.5.829. [DOI] [PubMed] [Google Scholar]
- Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D3 1α-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;381:143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- Carrillo-Lopez N, Alvarez-Hernandez D, Gonzalez-Suarez I, Roman-Garcia P, Valdivielso JM, Fernandez-Martin JL, et al. Simultaneous changes in the calcium-sensing receptor and the vitamin D receptor under the influence of calcium and calcitriol. Nephrol Dial Transplant. 2008;23:3479–3484. doi: 10.1093/ndt/gfn338. [DOI] [PubMed] [Google Scholar]
- Chen G, Kim SH, King AN, Zhao L, Simpson RU, Christensen PJ, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin Cancer Res. 2011;17:817–826. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow EC, Maeng HJ, Liu S, Khan AA, Groothuis GM, Pang KS. 1α,25-Dihydroxyvitamin D3 triggered vitamin D receptor and farnesoid X receptor-like effects in rat intestine and liver in vivo. Biopharm Drug Dispos. 2009;30:457–475. doi: 10.1002/bdd.682. [DOI] [PubMed] [Google Scholar]
- Chow EC, Durk MR, Cummins CL, Pang KS. 1α,25-dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(−/− and fxr(+/+ mice and increased renal and brain efflux of digoxin in mice in vivo. J Pharmacol Exp Ther. 2011;337:846–859. doi: 10.1124/jpet.111.179101. [DOI] [PubMed] [Google Scholar]
- Chow EC, Quach HP, Vieth R, Pang KS. Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. Am J Physiol Endocrinol Metab. 2013;304:E977–E989. doi: 10.1152/ajpendo.00489.2012. [DOI] [PubMed] [Google Scholar]
- Chow EC, Magomedova L, Quach HP, Patel R, Durk MR, Fan J, et al. Vitamin D receptor activation down-regulates the small heterodimer partner and increases CYP7A1 to lower cholesterol. Gastroenterology. 2014;146:1048–1059. doi: 10.1053/j.gastro.2013.12.027. [DOI] [PubMed] [Google Scholar]
- Chun RF. New perspectives on the vitamin D binding protein. Cell Biochem Funct. 2012;30:445–456. doi: 10.1002/cbf.2835. [DOI] [PubMed] [Google Scholar]
- Clements MR, Davies M, Hayes ME, Hickey CD, Lumb GA, Mawer EB, et al. The role of 1,25-dihydroxyvitamin D in the mechanism of acquired vitamin D deficiency. Clin Endocrinol. 1992;37:17–27. doi: 10.1111/j.1365-2265.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Cong D, Doherty M, Pang KS. A new physiologically based, segregated-flow model to explain route-dependent intestinal metabolism. Drug Metab Dispos. 2000;28:224–235. [PubMed] [Google Scholar]
- Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–478. doi: 10.1007/BF01061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dekker E, Hoenderop JG, Nilius B, Bindels RJ. The epithelial calcium channels, TRPV5 & TRPV6: from identification towards regulation. Cell Calcium. 2003;33:497–507. doi: 10.1016/s0143-4160(03)00065-4. [DOI] [PubMed] [Google Scholar]
- Doherty MM, Pang KS. First-pass effect: significance of the intestine for absorption and metabolism. Drug Chem Toxicol. 1997;20:329–344. doi: 10.3109/01480549709003891. [DOI] [PubMed] [Google Scholar]
- Durk MR, Han K, Chow EC, Ahrens R, Henderson JT, Fraser PE, et al. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer's disease. J Neurosci. 2014;34:7091–7101. doi: 10.1523/JNEUROSCI.2711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, Castro ME. Vitamin D kinetics in vivo: effect of 1,25-dihydroxyvitamin D administration. Am J Physiol. 1989;256:E686–E691. doi: 10.1152/ajpendo.1989.256.5.E686. [DOI] [PubMed] [Google Scholar]
- Henry HL. The 25(OH)D3/1α,25(OH)2D3-24R-hydroxylase: a catabolic or biosynthetic enzyme? Steroids. 2001;66:391–398. doi: 10.1016/s0039-128x(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Hershberger PA, McGuire TF, Yu WD, Zuhowski EG, Schellens JH, Egorin MJ, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1:821–829. [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972;11:4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- Hsu CH, Patel S, Young EW, Simpson RU. Production and degradation of calcitriol in renal failure rats. Am J Physiol. 1987;253:F1015–F1019. doi: 10.1152/ajprenal.1987.253.5.F1015. [DOI] [PubMed] [Google Scholar]
- Jones G, Vriezen D, Lohnes D, Palda V, Edwards NS. Side-chain hydroxylation of vitamin D3 and its physiological implications. Steroids. 1987;49:29–53. doi: 10.1016/0039-128x(87)90078-x. [DOI] [PubMed] [Google Scholar]
- Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Nakayama M, Kuriyama S, Watanabe S, Kawaguchi Y, Sakai O. Pharmacokinetics of active vitamins D3, 1α-hydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 in patients on chronic hemodialysis. Clin Nephrol. 1991;35:72–77. [PubMed] [Google Scholar]
- Kissmeyer AM, Binderup L. Calcipotriol (MC 903): pharmacokinetics in rats and biological activities of metabolites. A comparative study with 1,25(OH)2D3. Biochem Pharmacol. 1991;41:1601–1606. doi: 10.1016/0006-2952(91)90160-7. [DOI] [PubMed] [Google Scholar]
- Kumar R, Schnoes HK, DeLuca HF. Rat intestinal 25-hydroxyvitamin D3- and 1α,25-dihydroxyvitamin D3-24-hydroxylase. J Biol Chem. 1978;253:3804–3809. [PubMed] [Google Scholar]
- Lemay J, Demers C, Hendy GN, Delvin EE, Gascon-Barre M. Expression of the 1,25-dihydroxyvitamin D3-24-hydroxylase gene in rat intestine: response to calcium, vitamin D3 and calcitriol administration in vivo. J Bone Miner Res. 1995;10:1148–1157. doi: 10.1002/jbmr.5650100803. [DOI] [PubMed] [Google Scholar]
- Mager DE, Jusko WJ. Pharmacodynamic modeling of time-dependent transduction systems. Clin Pharmacol Ther. 2001;70:210–216. doi: 10.1067/mcp.2001.118244. [DOI] [PubMed] [Google Scholar]
- Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos. 2003;31:510–518. doi: 10.1124/dmd.31.5.510. [DOI] [PubMed] [Google Scholar]
- Mager DE, Woo S, Jusko WJ. Scaling pharmacodynamics from in vitro and preclinical animal studies to humans. Drug Metab Pharmacokinet. 2009;24:16–24. doi: 10.2133/dmpk.24.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262:173–180. doi: 10.1042/bj2620173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda S, Byford V, Arabian A, Sakai Y, Demay MB, St-Arnaud R, et al. Altered pharmacokinetics of 1α,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 2005;146:825–834. doi: 10.1210/en.2004-1116. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi JR, Modzelewski RA, Peng Y, Trump DL, Johnson CS. Pharmacokinetics of 1α,25-dihydroxyvitamin D3 in normal mice after systemic exposure to effective and safe antitumor doses. Oncology. 2004;66:62–66. doi: 10.1159/000076336. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnath N, Daignault-Newton S, Dy GK, Muindi JR, Adjei A, Elingrod VL, et al. A phase I/II pharmacokinetic and pharmacogenomic study of calcitriol in combination with cisplatin and docetaxel in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2013;71:1173–1182. doi: 10.1007/s00280-013-2109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick KM, Muindi JR, Johnson CS, Balkman CE, Ramnath N, Yu WD, et al. In vitro and in vivo evaluation of combined calcitriol and cisplatin in dogs with spontaneously occurring tumors. Cancer Chemother Pharmacol. 2008;62:881–891. doi: 10.1007/s00280-008-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salusky IB, Goodman WG, Horst R, Segre GV, Kim L, Norris KC, et al. Pharmacokinetics of calcitriol in continuous ambulatory and cycling peritoneal dialysis patients. Am J Kidney Dis. 1990;16:126–132. doi: 10.1016/s0272-6386(12)80566-x. [DOI] [PubMed] [Google Scholar]
- Sharma A, Jusko WJ. Characterization of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1996;24:611–635. doi: 10.1007/BF02353483. [DOI] [PubMed] [Google Scholar]
- Shinki T, Jin CH, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, et al. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1α,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992;267:13757–13762. [PubMed] [Google Scholar]
- Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, Trump DL. A phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin Cancer Res. 1999;5:1339–1345. [PubMed] [Google Scholar]
- Turunen MM, Dunlop TW, Carlberg C, Vaisanen S. Selective use of multiple vitamin D response elements underlies the 1α,25-dihydroxyvitamin D3-mediated negative regulation of the human CYP27B1 gene. Nucleic Acids Res. 2007;35:2734–2747. doi: 10.1093/nar/gkm179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth R, Kooh SW, Balfe JW, Rawlins M, Tinmouth WW. Tracer kinetics and actions of oral and intraperitoneal 1,25-dihydroxyvitamin D3 administration in rats. Kidney Int. 1990;38:857–861. doi: 10.1038/ki.1990.282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Weighted sum of squares residuals versus time plots for the (A) two-compartment and (B) PD-linked models. The dashed line represents y = 0.