Abstract
Background and Purpose
The ability of a chronic treatment with indacaterol, a new ultra-long-acting β2-adrenoceptor agonist, to reverse cardiac remodelling and its effects in combination with metoprolol, a selective β1-adrenoceptor antagonist, were investigated on myocardial infarction in a rat model of heart failure (HF).
Experimental Approach
We investigated the effects of indacaterol and metoprolol, administered alone or in combination, on myocardial histology, β-adrenoceptor-mediated pathways, markers of remodelling and haemodynamic parameters in a rat model of HF. Five groups of rats were assessed: sham-operated rats; HF rats; HF + indacaterol 0.3 mg·kg−1·day−1; HF + metoprolol 100 mg·kg−1·day−1; HF + metoprolol + indacaterol. All pharmacological treatments continued for 15 weeks.
Key Results
Treatment with either indacaterol or metoprolol significantly reduced the infarct size in HF rats. However, the combination of indacaterol and metoprolol reduced the infarct size even further, reduced both BP and heart rate, reversed the decrease in ejection fraction, normalized left ventricular systolic and diastolic internal diameters, normalized the decreased β1 adrenoceptor mRNA expression as well as cardiac cAMP levels and reduced cardiac GPCR kinase 2 expression, compared with the untreated HF group.
Conclusion and Implications
The results of our study demonstrated an additive interaction between indacaterol and metoprolol in normalizing and reversing cardiac remodelling in our experimental model of HF. The translation of these findings to clinical practice might be of interest, as this combination of drugs could be safer and more effective in patients suffering from HF and COPD.
Tables of Links
| TARGETS | |
|---|---|
| GPCRsa | Enzymesb |
| β1-adrenoceptor | AC (adenylyl cyclase) |
| β2-adrenoceptor | GRK2 |
| LIGANDS | |
|---|---|
| Adrenaline | Collagen type 1 |
| ANP | Indacaterol |
| BNP | Metoprolol |
| cAMP | Noradrenaline (NA) |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a, b).
Introduction
In advanced heart failure (HF), increased arterial elastance and arterio-ventricular uncoupling, excessive sympathetic activation and extensive abnormalities of β-adrenoceptor-mediated signalling contribute to cardiac remodelling; these include desensitization of cardiac β-adrenoceptors and the development of left ventricular (LV) dysfunction (Cohn et al., 2000; Floras, 2002; Kubanek et al., 2013).
The term ‘ventricular remodelling’ includes a complex of anatomical, functional, cellular and molecular changes in the myocardium in response to injury. Haemodynamic and echocardiographic changes are markers of cardiac remodelling, which correlate with cardiac impairment (Cohn et al., 2000; Kubanek et al., 2013). In animal models of HF, the process of LV remodelling begins rapidly, and then continues to progress. LV remodelling is characterized by dilatation of the cardiac chamber, increased systolic and diastolic volume index, and a progressive decline in the ejection fraction (EF) (Eaton and Bulkley, 1981; Korup et al., 1997; Cohn et al., 2000; Kubanek et al., 2013).
It has been documented that the changes induced by the increased adrenergic overdrive in HF patients might contribute to myocyte injury. Increased plasma levels of noradrenaline have been related to prognosis (Floras, 2002; Adameova et al., 2009). Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), secreted by the heart in response to cardiac transmural pressures, are sensitive indicators of cardiac dysfunction and remodelling, and severity in HF (Cohn et al., 2000; Sato et al., 2012). In addition, the stimulation of fibroblasts increases collagen synthesis and causes fibrosis of both the infarcted and non-infarcted regions of the ventricle in both human and animal models, thus contributing to remodelling. The role of collagen as a marker of the turnover of interstitium has been further confirmed in previous studies (Cohn et al., 2000; Sun and Weber, 2000; Sato et al., 2004).
The down-regulation and uncoupling of cardiac β1- and β2-adrenoceptors is related to the enhanced and sustained sympathetic drive responsible for the up-regulation of cardiac β-adrenoceptor-associated GPCR kinase 2 (GRK2). GRK2 levels are elevated in the early stages of the disease and inversely correlate with AC activity (Bristow et al., 1986; Floras, 2002), which suggests that it could be a potential new biomarker of cardiac dysfunction in human HF (Raake et al., 2008; Lymperopoulos, 2011; Aguero et al., 2012). In recent years, it has become evident that chronic treatment with β-blockers improves the functioning of failing LV, prevents or reverses progressive LV dilation, chamber sphericity and hypertrophy, and consequently positively affects cardiac remodelling in patients suffering from HF (Sabbah, 2004). Although, acute stimulation of β2-adrenoceptors contributes to sustaining the failing heart, the chronic activation of these receptors may also alter cardiac electrical stability, increasing the propensity for the formation of harmful arrhythmias (Bristow et al., 1986; Matera et al., 2010; 2013; 2014). Therefore, β2-adrenoceptor agonists are contraindicated in patients suffering from HF. Nevertheless, the cardioprotective and survival benefits of long-term combination therapy with β2-adrenoceptor agonists and β1-adrenoceptor blockers have been demonstrated, at least, in experimental models of HF (Ahmet et al., 2004; 2008).
Indacaterol is a novel, chirally pure, ultra-long-acting β2-adrenoceptor agonist (ultra-LABA), which has a high affinity for β2-adrenoceptors. Within a series of 8-hydroxyquinoline 2-aminoindan derived β2-adrenoceptor agonists, lipophilicity was used as the basis for the design and rationalization of the onset and duration of their action profiles. Indacaterol appears to have a high intrinsic activity at human β2-adrenoceptors in vitro, and interestingly, no tachyphylaxis has been demonstrated for it (Battram et al., 2006; Cazzola et al., 2013). In addition, preclinical and clinical data suggest that, for a given degree of bronchodilator activity, indacaterol has a greater cardiovascular safety margin than formoterol or salmeterol (Battram et al., 2006; Matera et al., 2010; Cazzola et al., 2011; 2013).
In the present study, we investigated the effect and the mechanism of action of a chronic treatment with indacaterol on cardiac remodelling and its effects when given in combination with metoprolol, a selective β1-adrenoceptor antagonist, in an experimental model of HF. To be more specific, we investigated the effects of indacaterol and metoprolol on histological changes, β-adrenoceptor-mediated pathways, markers of remodelling and haemodynamic parameters in this experimental model.
Methods
Animals
All experimental procedures were approved by the Animal Ethics Committee of the Second University of Naples. Animal care was in compliance with Italian (D.L. 116/92) and European Community (E.C. L358/1 18/12/86) guidelines on the use and protection of laboratory animals. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). As the Animal Ethics Committee of the Second University of Naples stipulated that animal suffering should be kept to a minimum and the number of animals used reduced, only one dose of each studied drug was approved. Therefore, we used indacaterol at a dose that induced a submaximal effect and metoprolol was administered at a dose used in previous studies by Ahmet and colleagues (Ahmet et al., 2009; EMEA, 2009).
Male Wistar rats (225–250 g; n = 50; Harlan Laboratories, Bresso, Italy) were randomly assigned to five experimental groups (n = 10 for each group): sham-operated rats (SHAM); rats with HF; HF + indacaterol 0.3 mg·kg−1·day−1 (I); HF + metoprolol 100 mg·kg−1·day−1 (M); HF + metoprolol + indacaterol (M + I). Treatment was started the day after the surgical procedure and continued for 15 weeks. Metoprolol and indacaterol were dissolved in the drinking water. Four and 15 weeks postoperatively, echocardiography was performed to monitor HF. At the same time points, the BP was evaluated by use of the tail-cuff method (Kubota et al., 2006; Rinaldi et al., 2014).
Experimental procedure
HF was performed by permanent ligation of the left anterior descending coronary artery, according to previously described procedures (Rinaldi et al., 2014). Briefly, after an i.p. injection of ketamine hydrochloride (100 mg·kg−1) and xylazine (2.5 mg·kg−1) supplemented as needed, the rats were intubated and ventilated with room air by a small animal ventilator (Harvard Apparatus, Holliston, MA, USA; Model 623). The depth of anaesthesia was assessed by monitoring EEG. After performing the thoracotomy in the third and fourth intercostal space, the pericardium was incised and a 6-0 silk suture (Johnson & Johnson, Somerville, NJ, USA) was placed around the proximal portion of the left coronary artery (Rinaldi et al., 2014).
Tail cuff method with heating and haemodynamic measurements
Mean arterial BP (MAP, mmHg) and heart rate (HR, beats min-1) were measured in conscious rats using the non-invasive tail-cuff system (model BP 2000 Blood Pressure Analysis System; Visitech Systems, Apex, NC, USA) (Kubota et al., 2006; Rinaldi et al., 2013). MAP and HR were measured before, during (week 4) and at the end of the study in all rats after placing the animals on a restraint platform maintained at 33–34°C.
Transthoracic echocardiography measurements
Four and 15 weeks after surgery, the left cardiac morphology and functions were evaluated using non-invasive transthoracic M-Mode echocardiography in order to ascertain heart function (VisualSONICSVeVo 770 imaging system with a RMV710B; Toronto, ON, Canada) in anaesthetized rats. Transthoracic echocardiographic determinations (EF%; LV systolic internal diameter: LVIDs, mm; LV diastolic internal diameter: LVIDd, mm) were performed in the lateral decubitus position (Rinaldi et al., 2014).
Histology
Myocardial sections (5 μm thick) were obtained at the midpillary muscle level; serial sections from formalin-fixed multiple slices of constant thickness cut perpendicularly to the cardiac axis were stained with haematoxylin and eosin and Masson trichrome (Spitalieri et al., 2012) and then photographed. The extent of LV myocardial infarction was calculated as the percentage of fibrotic (blue) areas (Suurmeijer et al., 2003). Immunostaining for anti-actin cardiac antibody (Sigma-Aldrich, St Louis, MO, USA) was used as a control (Ferlosio et al., 2012).
Real-time reverse transcriptase PCR
Total RNA was isolated from the heart of all rats. Frozen tissues were powdered and homogenized with l mL of TRIzol (Sigma-Aldrich Company, Milan, Italy) twice using a rotator-stator and a 23 G needle (Invitrogen, Milan, Italy) according to the protocol recommended by the manufacturer and described elsewhere. The expression levels of the mRNAs for cAMP, ANP, BNP, β-adrenoceptors, collagen type 1, GRK2 and GAPDH were quantified by real-time RT-PCR using SYBR Green (Bio-Rad Laboratories, Milan, Italy). mRNA concentrations are expressed as ratio over GAPDH, which was amplified as a housekeeping gene, as described elsewhere (Rinaldi et al., 2014).
Western blot
Heart samples were used to quantify the protein expression of GRK2. Tissues were homogenized on ice in 1 mL ice-cold RIPA lysis buffer (Tris HCl pH 7.8, 0.1% sodium deoxycholate, 1% SDS, NP-40 1X) containing protease and phosphatase inhibitors using a Politron PT 13 000 D tissue homogenizer (Kinematica, Bohemia NY, USA) and the tubes were vigorously shaken at 4°C for 30 min on a shaking platform. Lysates were centrifuged at 12 000× g for 10 min to remove the insoluble debris. After centrifugation the supernatants were frozen in aliquots at −80°C until use. Protein contents were determined by the Bradford method (Bradford MM, 1976). Protein samples (40 μg per lane) were separated on denaturing 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. Non-specific binding to the membrane was blocked for 1 h at room temperature with 5% milk in T-TBS (Tris buffer saline with 0.1% Tween 20). Membranes were then incubated at 4°C overnight with primary antibody for GRK2 (Santa Cruz Biotechnology, Milan, Italy), washed three times with 0.1% T-TBS solution, and then incubated for 1 h at room temperature with a secondary antibody (anti-rabbit IgG peroxidase conjugated from Santa Cruz Biotechnology). Tubulin monoclonal antibody was used as an internal standard. The immunoreactive bands were visualized using an enhanced chemilumunescence system (SuperSignal West Femto Maximum Sensitivity Substrate, Pierce, Rockford, IL, USA). The protein bands were scanned and quantified with Gel Doc-2000 (Bio-Rad; Rinaldi et al., 2011; 2013).
cAMP detection
The quantification of cAMP was measured using an elisa Kit (Abcam plc, Cambridge, UK), according to the manufacturers' instructions. Briefly, a goat anti-rabbit IgG antibody was precoated onto 96-well plates. Standards or test samples were added to the wells, along with an alkaline phosphatase conjugated-cAMP antigen and a polyclonal rabbit antibody specific to cAMP. After a 2 h incubation, the excess reagents were washed away. para-Nitrophenylphosphate substrate was added and after a 1 h incubation the enzyme reaction was stopped and the yellow colour generated was read at 405 nm. The intensity of the yellow colour was inversely proportional to the amount of cAMP captured in the plate.
Myocardial and plasma catecholamines detection
Noradrenaline and adrenaline concentrations were measured in samples by electrochemical detection as described previously (Rinaldi et al., 2014).
Data analysis
All values are presented as arithmetic mean and SEM of 10 animals for each treatment group. Statistical significance was assessed by Student's t-test or one-way anova, with Dunnett's or Bonferroni's post tests. All data analyses were performed using computer software (GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, USA). The level of statistical significance was defined as P < 0.05.
Analysis of drug interaction
The analysis of drug interaction between metoprolol and indacaterol was measured by applying the Bliss Independence (BI) theory, as previously described (Calzetta et al., 2013; Rogliani et al., 2013). Briefly, the BI theory for understanding the action of two agents is expressed by the following equation: E(x, y) = Ex + Ey − (Ex × Ey), where E is the fractional effect, and x and y are the doses of two compounds in a combination experiment. If the combination effect is higher than the expected value from the equation shown earlier, the interaction is considered synergistic, while if this effect is lower, the interaction is antagonistic. Otherwise, the effect is additive and there is no interaction. In this study, when the BI equation was applied, x = metoprolol and y = indacaterol.
Results
Haemodynamic and ecocardiographic measurements
The haemodynamic and ecocardiographic values obtained at the beginning of the experiments from all the animal groups were not significantly different (data not shown). After 15 weeks, MAP and HR were significantly increased in HF compared with SHAM (≈+23%, P < 0.05); a treatment with indacaterol and metoprolol significantly reduced both MAP and HR (≈−31%, P < 0.05) and a better result was obtained with the combination (≈−36%, P < 0.05) (Table 1). Ecocardiographic data indicated that EF was significantly reduced in HF (≈−24% vs. SHAM, P < 0.05); 15 weeks of treatment with indacaterol alone or in combination reversed these decreased EF values (≈+15% vs. HF, P < 0.05); both LVIDs and LVIDd where increased in HF (≈+71% vs. SHAM; P < 0.005); indacaterol induced a non-significant more pronounced increase of both systolic and diastolic LVID compared with metropolol (≈+26%, P > 0.05), and the combination normalized both values (≈−30% vs. HF, P < 0.05) (Figure 1A, Table 1).
Table 1.
Haemodynamic and ecocardiographic data
| SHAM | HF | M | I | M + I | |
|---|---|---|---|---|---|
| EF (%) | 73.5 ± 0.1 | 49.8 ± 0.2 | 65.3 ± 0.5 | 60.8 ± 0.9 | 69 ± 0.4 |
| HR (beats min−1) | 345.5 ± 1.4 | 405.4 ± 1.6 | 303.3 ± 2.5 | 286.2 ± 2.8 | 259.6 ± 1.5 |
| MAP (mmHg) | 105.6 ± 1.4 | 135.5 ± 1.6 | 89.9 ± 1.0 | 88.2 ± 1.9 | 86.4 ± 1.0 |
| LVIDd (mm) | 7.2 ± 0.1 | 11.6 ± 0.3 | 9.2 ± 0.1 | 10.9 ± 0.4 | 8.2 ± 0.1 |
| LVIDs (mm) | 4.9 ± 0.1 | 8.9 ± 0.3 | 6.2 ± 0.2 | 8.3 ± 0.3 | 6.2 ± 0.3 |
Figure 1.
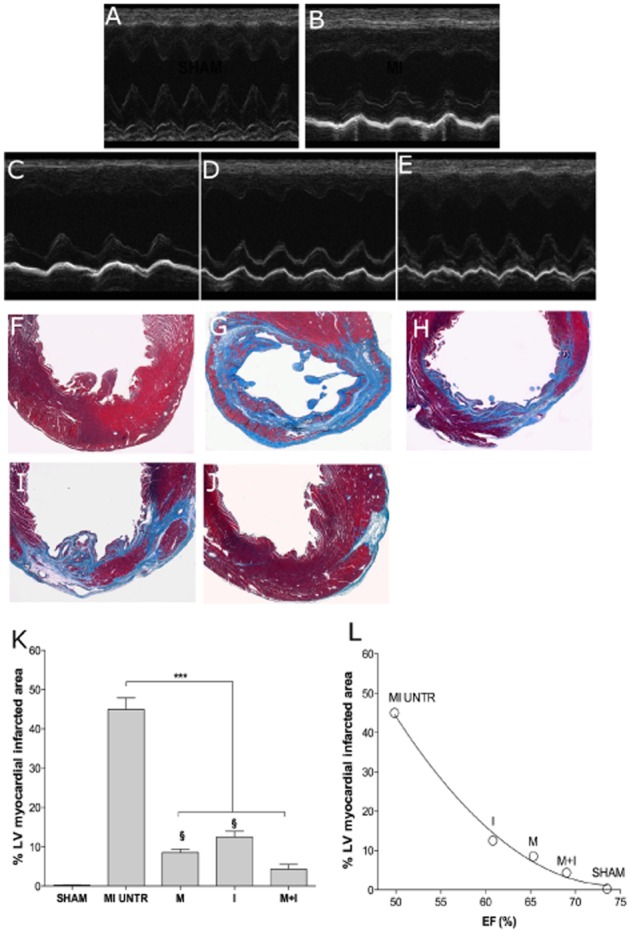
Raw data for dynamic changes of LV investigated by echocardiography in basal condition (A), after 15 weeks of myocardial infarction (B), after indacaterol (C), after metoprolol (D) and after the combination of metoprolol and indacaterol (E) compared with haemodynamic data after myocardial infarction. Myocardial-infarcted area in SHAM (F), HF untreated (G), metoprolol (H), indacaterol (I) and indacaterol plus metoprolol (J) treated groups (A). Bar graph of the % of myocardial-infarcted area (K). Correlation between myocardial infarction detected in the groups studied and EF (L). MI: myocardial infarction, M: metoprolol, I: indacaterol. §P < 0.05 versus MI untreated, ***P < 0.001 between selected groups.
Histological infarct size
The infarct size (% of LV myocardial-infarcted area) at 15 weeks was significantly larger in the untreated HF group compared with the SHAM group (45.0 ± 02.89 and 0.25 ± 0.03%, respectively; P < 0.001). Treatment with either metoprolol or indacaterol alone significantly reduced the infarct size (8.50 ± 0.87% and 12.50 ± 1.44%, respectively; P < 0.001) with respect to untreated animals. However, the combined administration of metoprolol plus indacaterol significantly reduced the infarct size even more (4.33 ± 1.20%; P < 0.05), compared with the groups treated with metoprolol or indacaterol alone (Figure 1B). The infarct size of SHAM, untreatedand treated groups significantly correlated with HF (EF%: Pearson's r −0.96, −0.997 to −0.503; R2 0.92; P < 0.01) (Figure 1B). The relationship between infarct size and EF is presented in Figure 1C.
Myocardial β-adrenoceptor-mediated pathways and remodelling biomarker
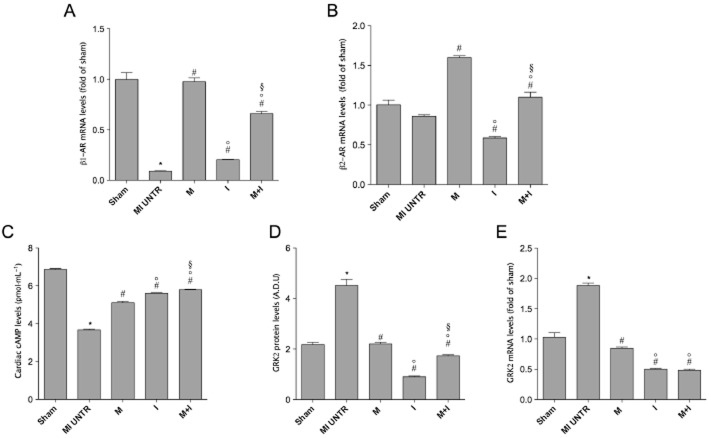
Cardiac β1-, but not β2-adrenoceptor mRNA levels (fold of sham), measured by real-time PCR as well as cardiac levels of cAMP (pmol·mL−1), measured by elisa, were significantly decreased 15 weeks after HF (Figure 2A, B, C), compared with SHAM. Treatment with indacaterol or metoprolol significantly increased β1– adrenoceptor mRNA expression and cardiac cAMP levels (Figure 2A, B, C) with respect to the untreated HF group. Indacaterol decreased and metoprolol increased β2-adrenoceptor mRNA expression, compared with HF. The combination of indacaterol and metoprolol normalized the increased β1- and β2-adrenoceptor mRNA expression, as well as cardiac cAMP levels (Figure 2A, B, C), when compared with results obtained from the untreated HF group. Finally, myocardial mRNA and protein GRK2 levels were significantly increased in the untreated HF group compared with the SHAM group. Metoprolol or indacaterol alone or in combination significantly reduced cardiac GRK2 expression after 15 weeks in the failing rat hearts (Figure 2D, E).
Figure 2.
(A) β1- and (B) β2-adrenoceptor (AR) mRNA levels in all experimental groups. All values were normalized to GAPDH. Data are presented as mean ± SEM. *P < 0.05 versus SHAM; #P < 0.05 versus MI untreated (UNTR); °P < 0.05 versus M; §P < 0.05 versus I. (C) Cardiac cAMP. Data are presented as mean ± SEM. *P < 0.05 versus SHAM; #P < 0.05 versus MI untreated; °P < 0.05 versus M; §P < 0.05 versus I. (D) mRNA and (E) protein levels of GRK2; data obtained from real-time PCR were confirmed by Western blot analysis. Data are presented as mean ± SEM. MI, myocardial infarction. *P < 0.05 versus SHAM; #P < 0.05 versus MI UNTR; °P < 0.05 versus M; §P < 0.05 versus I.
Plasma catecholamine levels
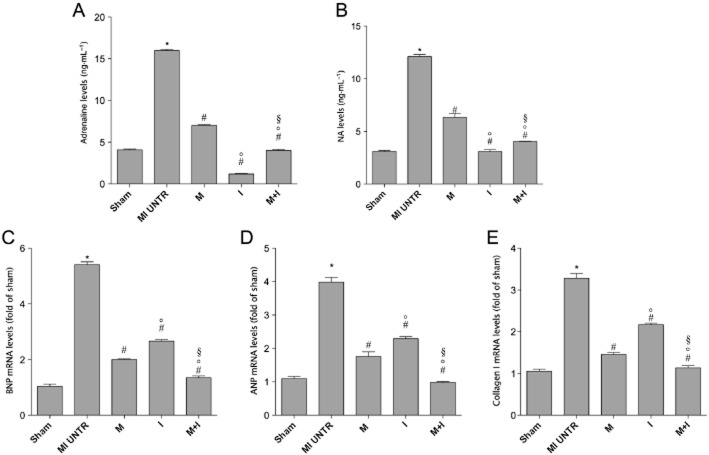
Plasma adrenaline and NA were measured in all experimental groups by HPLC. Fifteen weeks after HF, activation of sympathetic nervous system and outflow was observed and was characterized by an increase in the circulating levels of catecholamines compared with the SHAM group. The treatment with indacaterol or metoprolol alone or in combination significantly reduced catecholamine values to basal levels (Figure 3A, B).
Figure 3.
Plasma catecholamine levels in the different HF groups; (A) adrenaline, (B) noradrenaline. Data are presented as mean ± SEM. *P < 0.05 versus SHAM; #P < 0.05 versus MI untreated (UNTR); °P < 0.05 versus M; §P < 0.05 versus I. (C) BNP, (D) BNP and (E) collagen I mRNA levels. All values were normalized to GAPDH. Data are presented as mean ± SEM. MI, myocardial infarction. *P < 0.05 versus SHAM; #P < 0.05 versus MI untreated; °P < 0.05 versus M; §P < 0.05 versus I.
Natriuretic peptides and collagen 1 expression
To evaluate LV remodelling and hypertrophy, cardiac mRNA levels of ANP, BNP and collagen 1 were measured 15 weeks after HF by real-time PCR. In accord with the echocardiographic data, ANP, BNP and collagen 1 mRNA levels were markedly increased in the untreated HF group compared with the SHAM group and they were significantly reduced in all treated groups (Figure 3C, D, E).
Drug interaction
The results of the BI analysis for the pharmacodynamic interaction of metoprolol and indacaterol on LV myocardial infarction, EF, cAMP, BNP and collagen indicated an additive effect when the drugs were administered in combination (expected effect: 0.86 ± 0.08, observed effect 0.83 ± 0.12; delta effect: 0.03 ± 0.10), confirming the BI zero-interaction hypothesis (P > 0.05).
Discussion
The changes in heart size, shape and mass, EF, end-diastolic and end-systolic volumes, and peak force of contraction, in our experimental model, could be considered the most logical surrogate markers for assessing the extent of remodelling (Hochman and Bulkley, 1982; Weisman et al., 1985; Cohn et al., 2000; Ahmet et al., 2004; 2008; Kubanek et al., 2013). Fifteen weeks after HF, we observed a decrease in MAP and EF and an increase in HR and LVID compared with the SHAM group, as indicated by echocardiographic and histological data. The HW/BW ratio was significantly increased in control HF and the infarct size at 15 weeks was significantly more extensive in the untreated HF group than in the SHAM group and correlated with the decrease in EF.
Although, functional and anatomical markers are frequently assessed in large randomized studies, they may not be sufficient to evaluate the degree of LV remodelling, as technical factors and differences of interpretation lead to variations in the results. (Pfeffer and Braunwald, 1990; Cohn et al., 2000; Kubanek et al., 2013). Moreover, other factors that lead to remodelling could be the major determinants of HF prognosis and not ventricular dilation itself.
Neurohormonal activation in HF mediates several compensatory changes in response to the reduction in cardiac output, but it is also a major component of disease progression and of the remodelling process (Floras, 2002; Adameova et al., 2009). In the early stage of HF, activation of the adrenergic nervous system provides support for the failing myocardium; chronically, the continued activation of neurohormonal systems becomes deleterious to the heart and leads to excessive vasoconstriction, a deterioration of cardiac function and LV remodelling (Adameova et al., 2009). In line with the studies mentioned earlier, we found higher plasma levels of catecholamines in the HF groups than in the SHAM group, and these results were consistent with the histological, haemodynamic and echocardiographic data.
In order to analyse the molecular aspects of LV remodelling, we also assessed mRNA expression of specific factors, which are known to play a role in the pathogenesis of HF, such as ANP, BNP and collagen I. Natriuretic peptides are secreted by the heart in response to cardiac transmural pressures; the levels of the various natriuretic peptides could predict subsequent haemodynamic deterioration and adverse events in cardiovascular disease, and they can be used to monitor those at high risk and act as a guide to the effectiveness of a treatment. The most important stimulus for the secretion of ANP is an increase in arterial wall tension, HR and catecholamines, while the major determinant of BNP secretion is the degree of ventricular stretch and cardiac work (Cohn et al., 2000; Sun and Weber, 2000; Sato et al., 2012). The growth and activity of extracellular matrix producing cells are critical factors involved in tissue fibrosis; it has been demonstrated, in human and animal models, that during HF cardiac fibroblasts continue to generate fibrillar type I collagen, which causes fibrosis of both the infarcted and non-infarcted regions of the ventricle, thus contributing to remodelling (Sun and Weber, 2000). Consistent with the echocardiographic data that suggested LV remodelling, we also observed a significant increase in other cardiac remodelling markers, such as ANP, BNP and collagen I in the HF groups compared with the sham-operated rats at 15 weeks after HF.
At least two different β-adrenoceptor subtypes are present in the myocardium, β1- and β2-adrenoceptors; both receptors are coupled to the Gs pathway, which leads to the generation of second messengers, such as cAMP. This G-protein mediated signalling can be terminated by a process known as ‘desensitization’ through the activation of a family of kinases, known as GRKs, which inhibit further G-protein signalling by blocking the GPCR–G-protein interaction and the recruitment of enzymes that degrade second messenger molecules (Clark and Rich, 2003; Lymperopoulos, 2011; Aguero et al., 2012; Gurevich et al., 2012; Kassner et al., 2012).
Theoretically, the up-regulation of GRK2 observed in HF could be interpreted as a homeostatic protective mechanism that aims to defend the heart from the toxic effects of excessive catecholaminergic stimulation by reducing signalling through β-adrenoceptors (Lymperopoulos, 2011). Moreover, GRK2 levels are increased in failing myocardium, and studies have shown that not only does enhanced GRK2 activity lead to the aforementioned β-adrenoceptor-mediated changes in HF, but it also appears to contribute to the progression of HF (Rengo et al., 2009; Lymperopoulos, 2011; Aguero et al., 2012).
It has been demonstrated that cardiac-specific GRK2 knockout mice exhibited enhanced inotropic sensitivity to the β-adrenoceptor agonist isoprenaline, with impairment of normal inotropic and lusitropic tachyphylaxis, and accelerated development of catecholamine toxicity with chronic isoprenaline treatment (Matkovich et al., 2006).
In the present study, we observed a significant decrease in both β1- and β2-adrenoceptor mRNA levels in hearts derived from animals with HF, compared with those obtained from sham-operated animals; the decreased number of β-adrenoceptors correlated with a similar decrease in the amount of cardiac cAMP.
The extent of this receptor down-regulation observed in individual animals was correlated significantly to the increase in GRK2 mRNA levels and proteins. This result may have been related to the increased levels of plasma NA and adrenaline, which stimulated the desensitization of the β-adrenoceptors caused by GRK-mediated phosphorylation that leads to β-adrenoceptor internalization. This receptor down-regulation has also been documented by a large body of evidence, which demonstrates that NA and adrenaline are the most potent agonists for inducing β-adrenoceptor desensitization and internalization (Lymperopoulos, 2011; Aguero et al., 2012).
Taken together our results demonstrate the presence of ventricular remodelling in our experimental model of HF.
Although clinical trials have documented detrimental effects in HF patients after long-term treatment with β2-adrenoceptor agonists, more recently in some models of chronic ischaemic HF, some β2-adrenoceptor agonists, such as clenbuterol and fenoterol, have been found to attenuate ventricular remodelling. This effect was more pronounced if a β2-adrenoceptor agonist was combined with a β1-adrenoceptor blocker (Xydas et al., 2006; Ahmet et al., 2008; Soppa et al., 2008). Additionally, clenbuterol, used in conjunction with LV assistant devices, promotes reverse remodelling (Soppa et al., 2005; 2008). Moreover, adjuvant treatment of HF patients with clenbuterol along with bisoprolol, a selective β1-adrenoceptor antagonist, improved myocardial recovery, thus supporting the idea that selective stimulation of β2-adrenoceptors might be protective for the failing myocardium (George et al., 2006).
As the common coexistence of chronic obstructive pulmonary disease (COPD) and HF presents several therapeutic constraints that have not been comprehensively investigated, and pharmacological modulation of β-adrenoceptor function is one of the critical issues in the treatment of these patients (Cazzola et al., 2011; de Miguel Diez et al., 2013), our study aimed to evaluate the effect of a new β2-adrenoceptor agonist, indacaterol, alone or in combination with metoprolol, a selective β1-adrenoceptor antagonist on ventricular remodelling.
In this study, we observed that the development of HF was attenuated by single therapies, and that the combined therapy arrested its progression. Indacaterol treatment induced a decrease in both MAP (16%) and HR (29.4%) as did metoprolol MAP (14.4%) and HR (25.2%). However, the combination induced a greater reduction of MAP (17.7%) and HR (36%). EF and LVID, which indicate contractility of left ventricle, increased by 31% after metoprolol compared with EF recorded 15 weeks after HF. After indacaterol, EF improved by 22%; however, the best result, 38%, was obtained using both drugs. LVIDs, which is also an expression of mechanical performance, improved after treatment compared with HF 15 weeks without treatment. LVID was reduced by 20.5% after metoprolol, 9.7% after indacaterol; but by 28.9% when both drugs were administered.
For molecular LV remodelling at 15 weeks after treatment, we observed a greater reduction with indacaterol on plasma NA and adrenaline and also of ANP, BNP and collagen 1, than in the untreated HF group, but also a significant change in the expression of both β1-, and β2-adrenoceptors in the LV myocardium, as well as in cardiac cAMP. However, the combination with metoprolol normalized all these values. Interestingly, both treatments, either alone or in combination, significantly reduced the level of GRK2.
It is not surprising that metoprolol induced beneficial effects on cardiac remodelling as chronic treatment with β-adrenoceptor blockers improves the functioning of a failing LV, morbidity, mortality and consequently, reverse cardiac remodelling (Sabbah, 2004).
Furthermore, our results with indacaterol are in contrast with previous findings on chronic treatment with other β2-adrenoceptor agonists. Although in our experiments we measured the β2-adrenoceptor mRNA expression and not the protein expression, long-term experiments have documented that single therapy with fenoterol has some beneficial effects, but decreases β2-adrenoceptor density, probably because of tachyphylaxis (Talan et al., 2011). In line with our results, Ahmet and co-workers (2008) found that all the beneficial effects of a single treatment with fenoterol only lasted for the first 3 months of therapy, suggesting that the loss of efficacy after a prolonged single therapy with fenoterol was associated with a reduction in myocardial β2-adrenoceptor density (these receptors are not reduced in untreated animals). However, this reduction in β2-adrenoceptor density was not observed when therapy with fenoterol was combined with a β1-adrenoceptor blocker, metoprolol (Ahmet et al., 2008; Talan et al., 2011). These observations are consistent with several clinical studies in patients suffering from asthma or COPD, which have documented a reduction in the bronchoprotective effect of β2-adrenoceptors with regular treatment (Larj and Bleecker, 2002; Matera et al., 2010; Cazzola et al., 2011).
The differences in these results represent a peculiar novelty of this study, in which we did not observe any tachyphylaxis with indacaterol administered alone after 3 months of treatment. The discrepancies between indacaterol and fenoterol could be due to the peculiar properties of indacaterol compared with other β2-adrenoceptor agonsits. It has been suggested that desensitization, associated with down-regulation of β-adrenoceptors induced by a β-adrenoceptor agonist, is related to the translocation of the β2-adrenoceptor out of caveolae and that this translocation might cause a sequestration of the receptor from the effector, contributing to a reduction in the efficiency of β2-adrenoceptor coupling to AC (Ostrom et al., 2001). Caveolae play a pivotal role in compartmentalizing GRKs, potentially providing a mechanism for rapid activation/inactivation of GRKs that may be necessary in certain signalling situations, such as receptor desentitization (Robey et al., 2001). In addition, indacaterol shows a greater affinity for caveolae and could maintain the efficiency of β-adrenoceptor-linked effectors, reducing desensitization by interacting with caveolae (Lombardi et al., 2009). Moreover, as there is a considerable lack of data in the literature concerning the in vivo interaction between metoprolol and indacaterol, this study appears to be innovative as, to the best of our knowledge, it is the first work that investigated the interaction between these two drugs in an animal model of cardiac remodelling. Therefore, we believe that our results obtained by investigating the ultra-long-acting a β2-adrenoceptor agonist indacaterol instead of a shorter-acting β2-adrenoceptor agonist represent a significant novelty of this study. Also, the last Global Initiative for Chronic Obstructive Lung Disease (GOLD, 2014) recommendations suggest that all groups of COPD patients should be treated with a long-acting β2-adrenoceptor agonist, alone or in combination with other classes of long-acting bronchodilator drugs.
Finally, we observed an additive effect of the combination of indacaterol plus metoprolol; this combination normalized and reversed the cardiac remodelling in our experimental setting. The lack of a synergistic interaction between these compounds might be attributed to the level of doses that induced submaximal effectiveness when they were administered alone, a condition that may hide the potential pharmacological synergism when compounds are administered in combination (Calzetta et al., 2013; Rogliani et al., 2013). Hence, we cannot exclude the possibility of a synergistic interaction between the two drugs investigated when they are administered at lower dose combinations, as previously suggested by studies demonstrating that pharmacological synergism is mainly evidenced at low concentrations inducing less that 50% of the maximal effectiveness, in both ex vivo and in vivo settings (Cazzola et al., 2014a, b).
In conclusion, the translation to clinical practice of these results might suggest that the combination investigated, indacaterol and metoprolol, could be safer in patients suffering from both HF and COPD, than these drugs administered alone.
Acknowledgments
This work was supported by Novartis Farma S.p.A., Origgio (VA). The authors thank Dr Martin Brimble, English Lector, Department Humanities University of Calabria (Cosenza, Italy) for having edited this paper.
Glossary
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- EF
ejection fraction (%)
- GRK2
GPCR kinase 2
- HF
heart failure
- LV
left ventricular
- LVIDd
LV diastolic internal diameter
- LVIDs
LV systolic internal diameter
Authorship contribution
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: B. R., M. D., L. S., G. G., E. M., A. O., C. R., F. R., L. C., A. C., M. G. M.. Drafting the work or revising it critically for important intellectual content: B. R., M. D., L. S., G. G., E. M., A. O., C. R., F. R., L. C., A. C., M. G. M.. Final approval of the version to be published: B. R., M. D., L. S., G. G., E. M., A. O., C. R., F. R., L. C., A. C., M. G. M.. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: B. R., M. D., L. S., G. G., E. M., A. O., C. R., F. R., L. C., A. C., M. G. M.. B. R., L. S., M. D., G. G., C. R. performed the research. M. G. M. and L. C. designed the research study. M. G. M., E. M., A. O. contributed essentially reagents or tools. L. C., B. R., A. C., F. R. analysed the data. M. G. M., L. C., B. R. wrote the paper.
Conflict of interest
No conflict of interest exists for any of authors.
References
- Adameova A, Abdellatif Y, Dhalla NS. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol. 2009;87:493–514. doi: 10.1139/y09-042. [DOI] [PubMed] [Google Scholar]
- Aguero J, Almenar L, Monto F, Oliver E, Sanchez-Lazaro I, Vicente D, et al. Myocardial G protein receptor-coupled kinase expression correlates with functional parameters and clinical severity in advanced heart failure. J Card Fail. 2012;18:53–61. doi: 10.1016/j.cardfail.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, et al. Cardioprotective and survival benefits of long-term combined therapy with beta2 adrenoreceptor (AR) agonist and beta1 AR blocker in dilated cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther. 2008;325:491–499. doi: 10.1124/jpet.107.135335. [DOI] [PubMed] [Google Scholar]
- Ahmet I, Morrell C, Lakatta EG, Talan MI. Therapeutic efficacy of a combination of a beta1-adrenoreceptor (AR) blocker and beta2-AR agonist in a rat model of postmyocardial infarction dilated heart failure exceeds that of a beta1-AR blocker plus angiotensin-converting enzyme inhibitor. J Pharmacol Exp Ther. 2009;331:178–185. doi: 10.1124/jpet.109.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-o ne (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–770. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- Calzetta L, Page CP, Spina D, Cazzola M, Rogliani P, Facciolo F, et al. Effect of the mixed phosphodiesterase 3/4 inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346:414–423. doi: 10.1124/jpet.113.204644. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Calzetta L, Matera MG. Beta(2)-adrenoceptor agonists: current and future direction. Br J Pharmacol. 2011;163:4–17. doi: 10.1111/j.1476-5381.2011.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M, Rogliani P, Ruggeri P, Segreti A, Proietto A, Picciolo S, et al. Chronic treatment with indacaterol and airway response to salbutamol in stable COPD. Respir Med. 2013;107:848–853. doi: 10.1016/j.rmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Calzetta L, Page CP, Rogliani P, Facciolo F, Gavaldà A, et al. Pharmacological characterization of the interaction between aclidinium bromide and formoterol fumarate on Human isolated bronchi. Eur J Pharmacol. 2014a;745:135–143. doi: 10.1016/j.ejphar.2014.10.025. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Calzetta L, Segreti A, Facciolo F, Rogliani P, Matera MG. Translational study searching for synergy between glycopyrronium and indacaterol. 2014b. pp. 1–7. COPD. [DOI] [PubMed]
- Clark RB, Rich TC. Probing the roles of protein kinases in g-protein-coupled receptor desensitization. Mol Pharmacol. 2003;64:1015–1017. doi: 10.1124/mol.64.5.1015. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling – concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- Eaton LW, Bulkley BH. Expansion of acute myocardial infarction: its relationship to infarct morphology in a canine model. Circ Res. 1981;49:80–88. doi: 10.1161/01.res.49.1.80. [DOI] [PubMed] [Google Scholar]
- EMEA. Assessment report for hirobriz breezhaler. 2009. European Medicines Agency, Evaluation of Medicines for Human Use Doc.Ref.: EMA/814999/2009; Procedure No. EMEA/H/C/001211.
- Ferlosio A, Arcuri G, Doldo E, Scioli MG, De Falco S, Spagnoli LG, et al. Age-related increase of stem marker expression influences vascular smooth muscle cell properties. Atherosclerosis. 2012;224:51–57. doi: 10.1016/j.atherosclerosis.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Floras JS. The ‘unsympathetic’ nervous system of heart failure. Circulation. 2002;105:1753–1755. doi: 10.1161/01.cir.0000013788.71817.16. [DOI] [PubMed] [Google Scholar]
- George I, Xydas S, Mancini DM, Lamanca J, DiTullio M, Marboe CC, et al. Effect of clenbuterol on cardiac and skeletal muscle function during left ventricular assist device support. J Heart Lung Transplant. 2006;25:1084–1090. doi: 10.1016/j.healun.2006.06.017. [DOI] [PubMed] [Google Scholar]
- GOLD. 2014. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) (updated 2014). Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jan23.pdf (accessed 10/3/2015)
- Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman JS, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation. 1982;65:1446–1450. doi: 10.1161/01.cir.65.7.1446. [DOI] [PubMed] [Google Scholar]
- Kassner A, Toischer K, Bohms B, Kolkhof P, Abraham G, Hasenfubeta G, et al. Regulation of cyclic adenosine monophosphate release by selective beta2-adrenergic receptor stimulation in human terminal failing myocardium before and after ventricular assist device support. J Heart Lung Transplant. 2012;31:1127–1135. doi: 10.1016/j.healun.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korup E, Dalsgaard D, Nyvad O, Jensen TM, Toft E, Berning J. Comparison of degrees of left ventricular dilation within three hours and up to six days after onset of first acute myocardial infarction. Am J Cardiol. 1997;80:449–453. doi: 10.1016/s0002-9149(97)00393-7. [DOI] [PubMed] [Google Scholar]
- Kubanek M, Sramko M, Maluskova J, Kautznerova D, Weichet J, Lupinek P, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. J Am Coll Cardiol. 2013;61:54–63. doi: 10.1016/j.jacc.2012.07.072. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Umegaki K, Kagota S, Tanaka N, Nakamura K, Kunitomo M, et al. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol Pharma Bull. 2006;29:1756–1758. doi: 10.1248/bpb.29.1756. [DOI] [PubMed] [Google Scholar]
- Larj MJ, Bleecker ER. Effects of beta2-agonists on airway tone and bronchial responsiveness. J Allergy Clin Immunol. 2002;110(6 Suppl):S304–S312. doi: 10.1067/mai.2002.130045. [DOI] [PubMed] [Google Scholar]
- Lombardi D, Cuenoud B, Kramer SD. Lipid membrane interactions of indacaterol and salmeterol: do they influence their pharmacological properties? Eur J Pharm Sci. 2009;38:533–547. doi: 10.1016/j.ejps.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A. GRK2 and beta-arrestins in cardiovascular disease: something old, something new. Am J Cardiovasc Dis. 2011;1:126–137. [PMC free article] [PubMed] [Google Scholar]
- Matera MG, Martuscelli E, Cazzola M. Pharmacological modulation of beta-adrenoceptor function in patients with coexisting chronic obstructive pulmonary disease and chronic heart failure. Pulm Pharmacol Ther. 2010;23:1–8. doi: 10.1016/j.pupt.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Matera MG, Calzetta L, Cazzola M. beta-Adrenoceptor modulation in chronic obstructive pulmonary disease: present and future perspectives. Drugs. 2013;73:1653–1663. doi: 10.1007/s40265-013-0120-5. [DOI] [PubMed] [Google Scholar]
- Matera MG, Rogliani P, Cazzola M. Muscarinic receptor antagonists for the treatment of chronic obstructive pulmonary disease. Expert Opin Pharmacother. 2014;15:961–977. doi: 10.1517/14656566.2014.899581. [DOI] [PubMed] [Google Scholar]
- Matkovich SJ, Diwan A, Klanke JL, Hammer DJ, Marreez Y, Odley AM, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel Diez J, Chancafe Morgan J, Jimenez Garcia R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305–312. doi: 10.2147/COPD.S31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, et al. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi B, Donniacuo M, Esposito E, Capuano A, Sodano L, Mazzon E, et al. PPARalpha mediates the anti-inflammatory effect of simvastatin in an experimental model of zymosan-induced multiple organ failure. Br J Pharmacol. 2011;163:609–623. doi: 10.1111/j.1476-5381.2011.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi B, Donniacuo M, Sodano L, Gritti G, Signoriello S, Parretta E, et al. Effects of sildenafil on the gastrocnemius and cardiac muscles of rats in a model of prolonged moderate exercise training. PLoS ONE. 2013;8:e69954. doi: 10.1371/journal.pone.0069954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi B, Capuano A, Gritti G, Donniacuo M, Scotto Di Vettimo A, Sodano L, et al. Effects of chronic administration of beta-blockers on airway responsiveness in a murine model of heart failure. Pulm Pharmacol Ther. 2014;28:109–113. doi: 10.1016/j.pupt.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Robey RB, Ruiz OS, Baniqued J, Mahmud D, Espiritu DJ, Bernardo AA, et al. SFKs, Ras, and the classic MAPK pathway couple muscarinic receptor activation to increased Na-HCO(3) cotransport activity in renal epithelial cells. Am J Physiol Renal Physiol. 2001;280:F844–F850. doi: 10.1152/ajprenal.2001.280.5.F844. [DOI] [PubMed] [Google Scholar]
- Rogliani P, Calzetta L, Rendina EA, Massullo D, Dauri M, Rinaldi B, et al. The influence of propofol, remifentanil and lidocaine on the tone of human bronchial smooth muscle. Pulm Pharmacol Ther. 2013;26:325–331. doi: 10.1016/j.pupt.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Sabbah HN. Biologic rationale for the use of beta-blockers in the treatment of heart failure. Heart Fail Rev. 2004;9:91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kita T, Takatsu Y, Kimura T. Biochemical markers of myocyte injury in heart failure. Heart. 2004;90:1110–1113. doi: 10.1136/hrt.2003.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Fujiwara H, Takatsu Y. Biochemical markers in heart failure. J Cardiol. 2012;59:1–7. doi: 10.1016/j.jjcc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Soppa GK, Smolenski RT, Latif N, Yuen AH, Malik A, Karbowska J, et al. Effects of chronic administration of clenbuterol on function and metabolism of adult rat cardiac muscle. Am J Physiol Heart Circ Physiol. 2005;288:H1468–H1476. doi: 10.1152/ajpheart.00624.2004. [DOI] [PubMed] [Google Scholar]
- Soppa GK, Lee J, Stagg MA, Felkin LE, Barton PJ, Siedlecka U, et al. Role and possible mechanisms of clenbuterol in enhancing reverse remodelling during mechanical unloading in murine heart failure. Cardiovasc Res. 2008;77:695–706. doi: 10.1093/cvr/cvm106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitalieri P, Quitadamo MC, Orlandi A, Guerra L, Giardina E, Casavola V, et al. Rescue of murine silica-induced lung injury and fibrosis by human embryonic stem cells. Eur Respir J. 2012;39:446–457. doi: 10.1183/09031936.00005511. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- Suurmeijer AJ, Clement S, Francesconi A, Bocchi L, Angelini A, Van Veldhuisen DJ, et al. Alpha-actin isoform distribution in normal and failing human heart: a morphological, morphometric, and biochemical study. J Pathol. 2003;199:387–397. doi: 10.1002/path.1311. [DOI] [PubMed] [Google Scholar]
- Talan MI, Ahmet I, Xiao RP, Lakatta EG. beta(2) AR agonists in treatment of chronic heart failure: long path to translation. J Mol Cell Cardiol. 2011;51:529–533. doi: 10.1016/j.yjmcc.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman HF, Bush DE, Mannisi JA, Bulkley BH. Global cardiac remodeling after acute myocardial infarction: a study in the rat model. J Am Coll Cardiol. 1985;5:1355–1362. doi: 10.1016/s0735-1097(85)80348-x. [DOI] [PubMed] [Google Scholar]
- Xydas S, Kherani AR, Chang JS, Klotz S, Hay I, Mutrie CJ, et al. beta(2)-Adrenergic stimulation attenuates left ventricular remodeling, decreases apoptosis, and improves calcium homeostasis in a rodent model of ischemic cardiomyopathy. J Pharmacol Exp Ther. 2006;317:553–561. doi: 10.1124/jpet.105.099432. [DOI] [PubMed] [Google Scholar]