Significance
Despite the promise of personalized cancer medicine, most molecular therapies produce only modest and short-lived patient gains. In addition to drug resistance, it is also possible that tumors adaptively reprogram their signaling pathways to evade therapy-induced “stress” and, in the process, acquire more aggressive disease traits. We show here that small-molecule inhibitors of PI3K, a cancer node and important therapeutic target, induce transcriptional and signaling reprogramming in tumors. This involves the trafficking of energetically active mitochondria to subcellular sites of cell motility, where they provide a potent, “regional” energy source to support tumor cell invasion. Although this response may paradoxically increase the risk of metastasis during PI3K therapy, targeting mitochondrial reprogramming is feasible, and could provide a novel therapeutic strategy.
Keywords: mitochondria, molecular therapy, cytoskeleton, PI3K, cell invasion
Abstract
Molecular therapies are hallmarks of “personalized” medicine, but how tumors adapt to these agents is not well-understood. Here we show that small-molecule inhibitors of phosphatidylinositol 3-kinase (PI3K) currently in the clinic induce global transcriptional reprogramming in tumors, with activation of growth factor receptors, (re)phosphorylation of Akt and mammalian target of rapamycin (mTOR), and increased tumor cell motility and invasion. This response involves redistribution of energetically active mitochondria to the cortical cytoskeleton, where they support membrane dynamics, turnover of focal adhesion complexes, and random cell motility. Blocking oxidative phosphorylation prevents adaptive mitochondrial trafficking, impairs membrane dynamics, and suppresses tumor cell invasion. Therefore, “spatiotemporal” mitochondrial respiration adaptively induced by PI3K therapy fuels tumor cell invasion, and may provide an important antimetastatic target.
The phosphatidylinositol 3-kinase (PI3K) is a universal tumor driver (1) that integrates growth factor signaling with downstream circuitries of cell proliferation, metabolism, and survival (2). Exploited in nearly every human tumor, including through acquisition of activating mutations (3), PI3K signaling is an important therapeutic target, and several small-molecule antagonists of this pathway have entered clinical testing (4). However, the patient response to these agents has been inferior to expectations (5), dampened by drug resistance (6) and potentially other mechanisms of adaptation by the tumor (7).
In this context, there is evidence that therapeutic targeting of PI3K promotes tumor adaptation, paradoxically reactivating protein kinase B (PKB/Akt) in treated cells (8) and reprogramming mitochondrial functions in bioenergetics and apoptosis resistance (9). How these changes affect tumor traits, however, is unclear. Against the backdrop of a ubiquitous “Warburg effect” (10), where tumors switch from cellular respiration to aerobic glycolysis, a role of mitochondria in cancer has not been clearly defined (11) and at times has been proposed as that of a tumor suppressor (12).
In this study, we examined the impact of mitochondrial reprogramming induced by PI3K therapy on mechanisms of tumor progression.
Results
PI3K Therapy Reactivates Akt and Mammalian Target of Rapamycin Signaling.
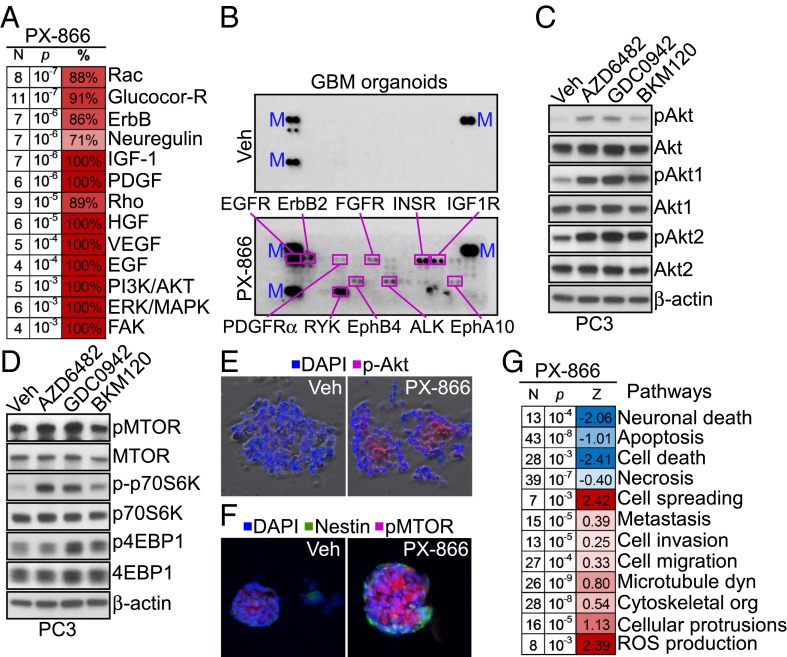
Treatment of patient-derived glioblastoma (GBM) organotypic cultures (13) with PX-866, an irreversible pan-PI3K antagonist currently in the clinic (4), caused transcriptional up-regulation of multiple growth factor receptor pathways (Fig. 1A). This was associated with widespread phosphorylation, namely activation of the GBM kinome in primary organotypic cultures (Fig. 1B and Table S1) as well as GBM LN229 cells (Fig. S1A). Consistent with previous observations (8), structurally diverse small-molecule PI3K antagonists induced robust (re)phosphorylation of Akt1 (S473) and Akt2 (S474) in tumor cells (Fig. 1C and Fig. S1B), as well as phosphorylation of downstream mammalian target of rapamycin (mTOR) and its effectors, 70S6K and 4EBP1 (Fig. 1D and Fig. S1C). Similar results were obtained in primary 3D GBM neurospheres, where PI3K therapy strongly induced Akt (Fig. 1E) and mTOR (Fig. 1F) phosphorylation. By transcriptome analysis, PI3K antagonists up-regulated two main gene networks of protection from apoptosis (9) and increased cell motility (Fig. 1G) in treated tumors.
Fig. 1.
PI3K therapy-induced tumor transcriptional reprogramming. (A) Heat map of changes in kinome functions in patient-derived GBM organotypic cultures treated with vehicle or PX-866 (10 μM for 48 h). N, number of genes; %, percentage of genes changed for any given function. (B) Extracts from GBM organotypic cultures treated with vehicle (Veh) or PX-866 (10 μM for 48 h) were incubated with a human phospho-RTK array followed by enhanced chemiluminescence detection. The position and identity of phosphorylated proteins are indicated. M, markers. (C and D) PC3 cells were treated with vehicle or the indicated PI3K inhibitors for 48 h and analyzed for changes in Akt (C) or mTOR (D) activation by Western blotting. p, phosphorylated. (E and F) Primary GBM spheroids treated with vehicle or 10 μM PX-866 for 48 h were imaged by phase-contrast and fluorescence microscopy for phosphorylated Akt (Ser473) (E) or mTOR (Ser2448) (F). DNA was counterstained with DAPI. Nestin is a GBM marker. (G) Heat map of changes in kinome pathways in GBM organotypic cultures treated with vehicle or PX-866 (10 μM) for 48 h. N, number of changed genes; Z, z score of the estimated function state: positive (red) indicates overall function is likely increased; negative (blue) indicates it is decreased.
Table S1.
Clinicopathological characteristics of the patients in this study
| Patient ID | Sex | Age, y | Grade | Ki-67, % | MGMT, % | LOH |
| GBM 1 | M | 66 | IV | 50 | 57 (M) | No |
| GBM 2 | F | 64 | IV | 30 | 54 (M) | 1p/19q |
| GBM 3 | F | 78 | IV | 35 | 62 (M) | No |
| GBM 4 | M | 64 | IV | 50 | 4 (UM) | No |
| GBM 5 | F | 63 | IV | 35 | 9 (UM) | 19q |
| GBM 6 | M | 53 | IV | 15 | 3 (UM) | No |
| GBM 7 | M | 36 | IV | 80 | 24 (M) | No |
The clinicopathological features of GBM patients used for organotypic glioma cultures (GBM 1 and 2) or neurosphere derivation (GBM 3–7) are indicated. F, female; M, male. Age (years) at diagnosis is indicated. Grade was estimated according to the fourth edition of the World Health Organization (WHO) classification of tumors of the central nervous system (48). The Ki-67 labeling index was considered in tumor hotspots as an indicator of glioma proliferation. The percentage of 06 methylguanine-DNA methyltransferase (MGMT) promoter methylation (by pyrosequencing analysis) and the resulting status (methylated if ≥20%, or unmethylated) is provided. M, methylated; UM, unmethylated. Loss of heterozygosity (LOH) at 1p/19q was analyzed.
Fig. S1.
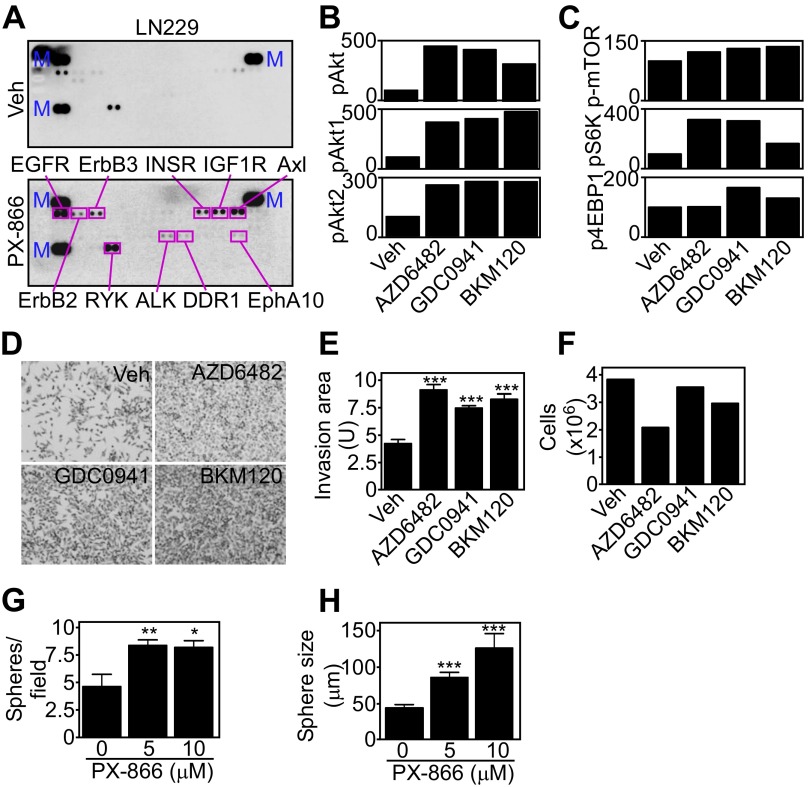
Molecular therapy-induced tumor reprogramming. (A) Extracts from vehicle (Veh)- or PX-866–treated (10 μM for 48 h) LN229 cells were incubated with a human phospho-RTK array and immunoreactive spots were visualized by enhanced chemiluminescence. The position and identity of phosphorylated proteins are indicated. M, markers. (B and C) PC3 cells were treated with the indicated PI3K inhibitors, and changes in the levels of the indicated phosphorylated proteins were quantified by densitometry and normalized to total levels. (D) PC3 cells were treated with vehicle or the indicated PI3K inhibitors for 48 h and analyzed for invasion across Matrigel. Representative images of invaded cells are shown. Magnification, 10×. (E and F) The experimental conditions are as in D, and the area occupied by invasive cells (E) or the number of cells before seeding (F) was quantified. Mean ± SEM (n = 3). ***P < 0.0001. (G and H) Patient-derived GBM spheroids were treated with vehicle or PX-866 (0–10 μM) for 48 h, stained with the vital dye PKH26, and the number of spheres per field (G) and sphere diameter (H) were quantified after 48 h. Mean ± SEM (n = 5 patients). *P = 0.01; **P = 0.0048; ***P = 0.0004.
Increased Tumor Cell Motility Mediated by PI3K Therapy.
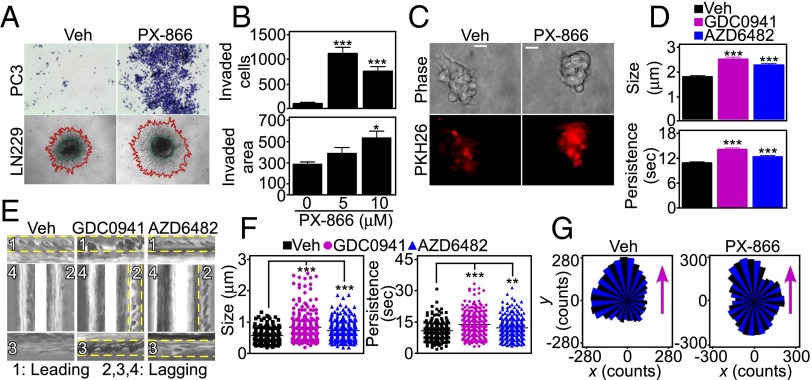
Consistent with these data, PI3K inhibitors vigorously stimulated tumor cell invasion across Matrigel-coated Transwell inserts (Fig. 2 A and B and Fig. S1 D and E) and in 3D tumor spheroids (Fig. 2 A and B). Tumor cell proliferation was not significantly affected (Fig. S1F) (9). In addition, PI3K therapy dose-dependently increased the number and size of 3D GBM neurospheres (Fig. 2C and Fig. S1 G and H).
Fig. 2.
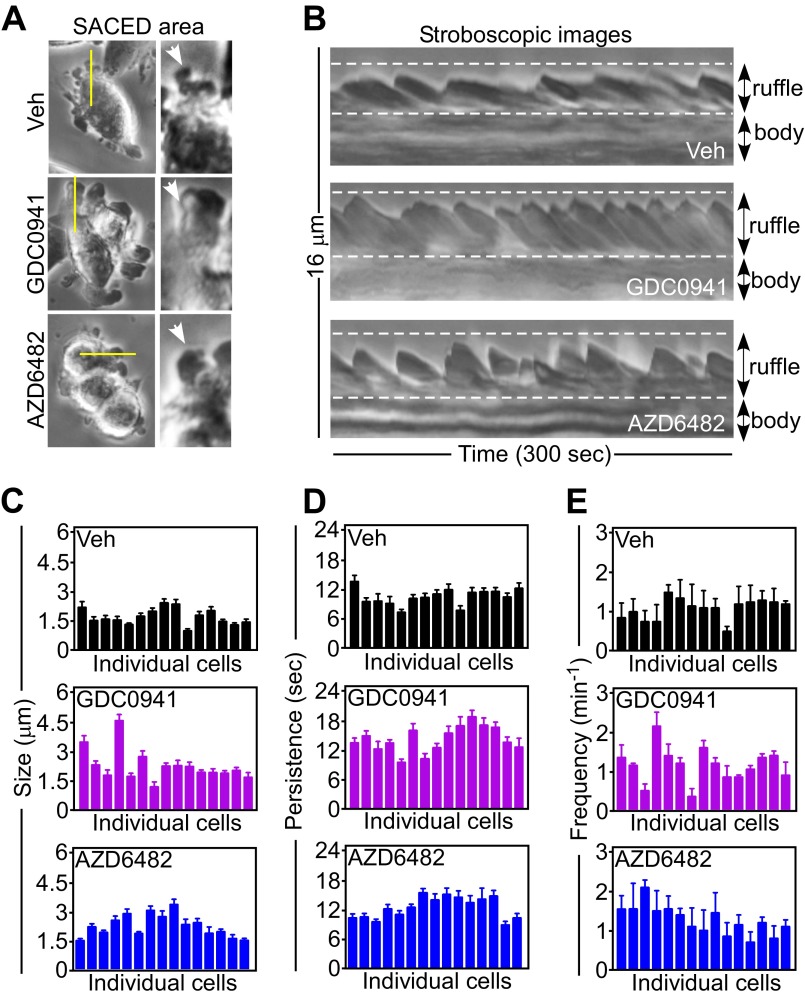
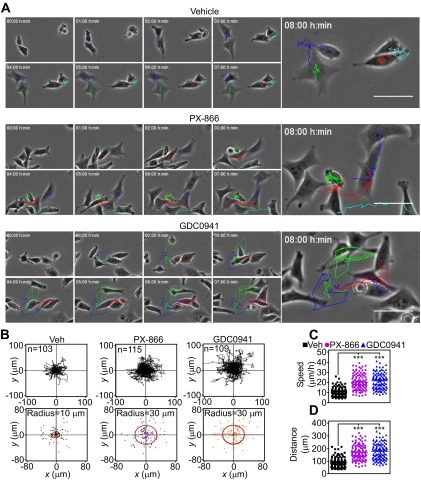
PI3K therapy induces adaptive tumor cell motility and invasion. (A) Tumor cells treated with vehicle or 10 μM PX-866 for 48 h were analyzed for invasion across Matrigel-coated Transwell inserts (Top) or in 3D spheroids (Bottom). Red, invasive edge; green, core. Representative images. Magnification, 10×. (B) PC3 (Top) or LN229 (Bottom) cells were treated with the indicated increasing concentrations of PX-866 and quantified for invasion across Matrigel (Top) or in 3D spheroids (Bottom). The distance between the core and edge of 3D spheroids was determined. Mean ± SEM of replicates from a representative experiment. *P = 0.02; ***P < 0.0001. (C) Patient-derived GBM spheroids were treated with vehicle or PX-866 (0–10 μM) for 48 h and analyzed by phase-contrast (Top) or fluorescence microscopy (Bottom). The vital dye PKH26 was used to counterstain live GBM neurospheres. (Scale bar, 20 μm.) (D) Membrane ruffling was quantified in PC3 cells treated with vehicle or PI3K inhibitors for 48 h by SACED microscopy. Average values from at least 330 ruffles per treatment are shown for ruffle size (Top) and time of ruffle persistence (Bottom). Mean ± SEM (n = 15). ***P < 0.0001. (E) Representative stroboscopic images from time-lapse video microscopy of PC3 cells treated with vehicle or PI3K inhibitors. Four SACED regions corresponding to the top (1), right (2), bottom (3), and left (4) of each cell are shown. The ruffling activity (broken yellow lines) is restricted to one main region (1) on the vehicle cell but is distributed equally between three regions (1–3) on cells treated with PI3K inhibitors. See also Movie S1. (F) PC3 cells were treated with vehicle or PI3K inhibitors, and membrane dynamics at lagging areas were quantified. Ruffle size (Left) or time of ruffle persistence (Right) from at least 205 individual lagging ruffles are shown. Mean ± SEM (n = 15). **P = 0.0047; ***P < 0.0001. (G) PC3 cells were treated with vehicle or PX-866 for 48 h and quantified for directional versus random cell migration by time-lapse video microscopy (8 h). Rose plots show the distribution of cells migrating along each position interval (range interval 10°, internal angle 60°). Arrows indicate the direction of chemotactic gradient.
To understand the basis of this cell invasion response, we next quantified the dynamics of membrane lamellipodia, which are required for cell motility, by single-cell stroboscopic microscopy (SACED, Fig. S2A) (14, 15). PI3K antagonists strongly stimulated lamellipodia dynamics (Fig. S2B), increasing the size (Fig. 2D, Top and Fig. S2C) and time of persistence (Fig. 2D, Bottom and Fig. S2D) of membrane ruffles compared with control cultures. Ruffle frequency was not affected (Fig. S2E). In addition, PI3K therapy changed the topography of membrane ruffles in tumor cells, with appearance of dynamic ruffles at lagging areas of the plasma membrane (Fig. 2E and Movie S1), potentially associated with random cell motility (16). These lateral ruffles were larger and persisted for a longer time in response to PI3K therapy compared with untreated cells (Fig. 2F), where membrane ruffles were instead polarized at the leading edge of migration (Fig. 2E). Consistent with these findings, PI3K antagonists strongly stimulated 2D tumor chemotaxis (Fig. S3A), extending the radius of cell migration (Fig. S3B) and promoting random, as opposed to directional, cell movements (Fig. 2G). Tumor cell movement in response to PI3K therapy proceeded at faster speed (Fig. S3C) and for longer distances (Fig. S3D) compared with untreated cultures.
Fig. S2.
PI3K therapy stimulates membrane ruffle dynamics. (A) PC3 cells treated with vehicle or the indicated PI3K inhibitors for 48 h were analyzed by time-lapse video microscopy. Four discrete regions per cell were followed over time to derive stroboscopic images by SACED. (Left) Still images at the beginning of the sequence. Yellow lines indicate the discrete areas analyzed for a cell. (Right) Zoomed-in micrographs of the ruffles being analyzed (arrows). (B) PC3 cells were treated with vehicle or the indicated PI3K inhibitors and analyzed by stroboscopic microscopy. Images representing the dynamics of the cell border over 300 s are shown. The positions of membrane ruffles and cell bodies are indicated. (C–E) PC3 cells (n = 15) as in B were analyzed for membrane ruffle dynamics by SACED with quantification of average ruffle size (C), time of ruffle persistence (D), and ruffle frequency (E). Each bar corresponds to an individual cell. Mean ± SEM (n = 4).
Fig. S3.
Adaptation-induced random tumor cell migration. (A) PC3 cells were treated with vehicle or the indicated PI3K inhibitors for 48 h, seeded onto 2D chemotaxis chambers, and analyzed by time-lapse video microscopy. The sequences were started immediately after setting up a gradient of NIH 3T3 conditioned media used as a chemoattractant. Videos were analyzed with WimTaxis software (Wimasis) and tracking data were exported into the Chemotaxis and Migration tool (Ibidi) for representation. Still images from the time lapse with trajectories overlaid at the indicated time intervals are shown. (Scale bars, 50 μm.) (B) PC3 cells treated with vehicle or PI3K inhibitors were analyzed for 2D chemotaxis. The trajectory (Top) and end points (Bottom) represented for all analyzed cells are shown; n ≥ 103. A circular region that splits the Euclidean distances 50:50 is indicated (radius). (C and D) PC3 cells were treated as in B, and speed of migration (C) or distance of migration (D) were quantitated by 2D chemotaxis analysis. ***P < 0.0001.
Mitochondrial Repositioning to the Cortical Cytoskeleton Supports Adaptive Tumor Cell Invasion.
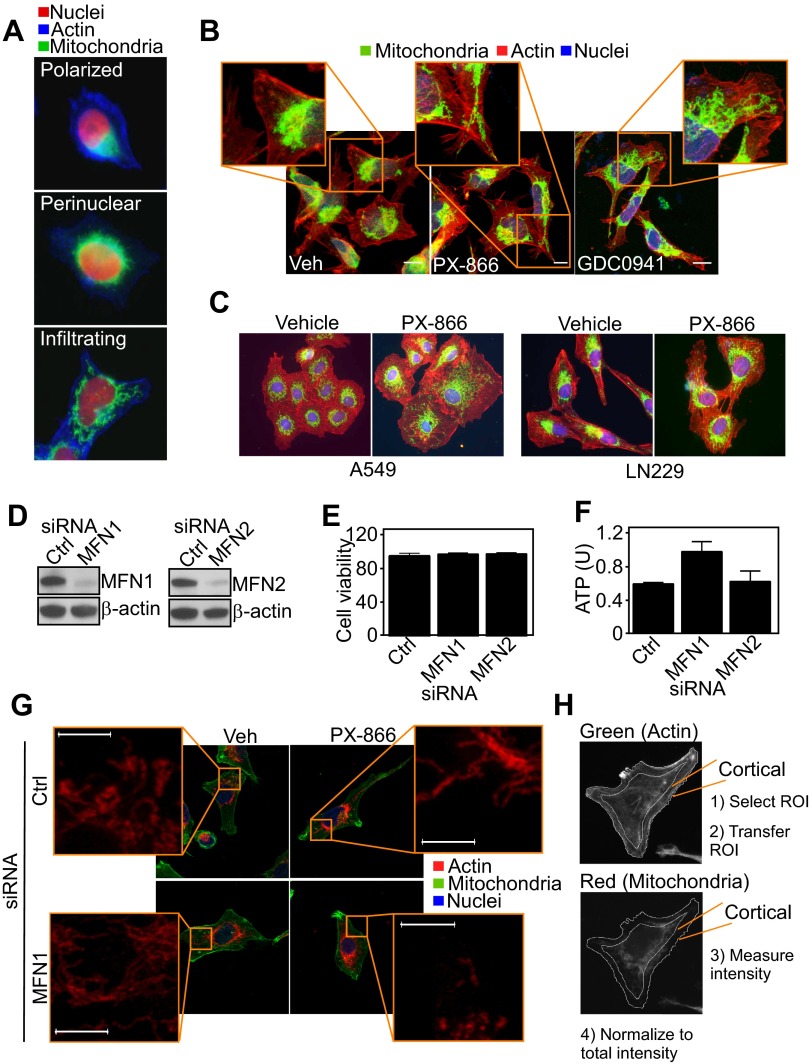
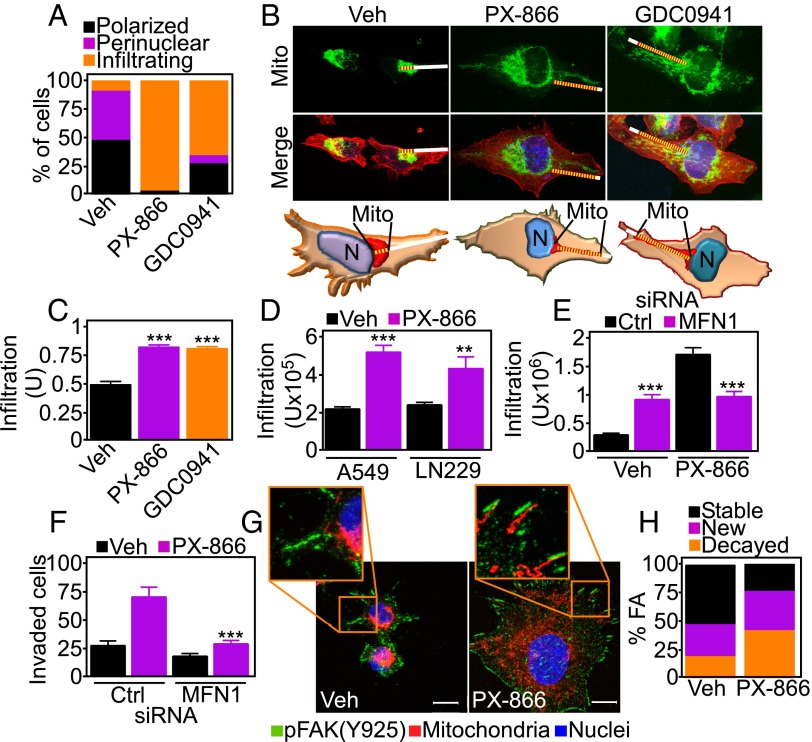
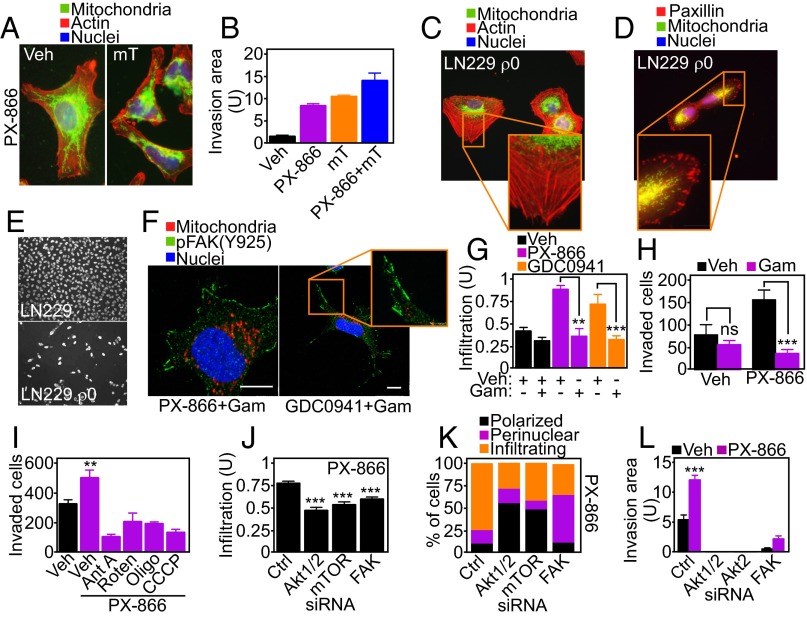
When analyzed by fluorescence microscopy, PI3K therapy induced profound changes in the morphology and distribution of mitochondria. Whereas untreated cells exhibited mitochondria that were polarized and mostly clustered around the nucleus (Fig. S4 A and B), PI3K inhibitors caused the appearance of elongated mitochondria (Fig. S4A) that “infiltrated” the cortical cytoskeleton of tumor cells, localizing in proximity of membrane protrusions implicated in cell motility (Fig. 3 A–C and Fig. S4B). This was a general response of heterogeneous tumor cell types, as lung adenocarcinoma A549 or glioblastoma LN229 cells comparably repositioned mitochondria to the cortical cytoskeleton in response to PI3K therapy (Fig. 3D and Fig. S4C). Mitochondria are highly dynamic organelles, regulated by cycles of fusion and fission (17). Small interfering RNA (siRNA) knockdown of effectors of mitochondrial fusion, mitofusin (MFN)1 or MFN2 (Fig. S4D), did not affect cell viability (Fig. S4E) or ATP production (Fig. S4F) in tumor cells. Under these conditions, MFN1 silencing suppressed mitochondrial trafficking to the cortical cytoskeleton (Fig. 3E and Fig. S4 G and H) as well as tumor cell invasion (Fig. 3F) induced by PI3K therapy. The combination of MFN2 knockdown plus PI3K inhibition induced extensive loss of cell viability (MFN1 siRNA+PX-866, 2.7 ± 0.05 × 105 cells; MFN2 siRNA+PX-866, 0.16 ± 0.13 × 105 cells; P = 0.0047), thus preventing additional studies of mitochondrial relocalization or tumor cell invasion.
Fig. S4.
Mitochondrial infiltration to the cortical cytoskeleton. (A) PC3 cells labeled with MitoTracker Red, phalloidin Alexa488, and DAPI were analyzed by fluorescence microscopy. Representative pseudocolored fluorescence microscopy images of different mitochondrial morphology (polarized, perinuclear, infiltrating) are shown. Magnification, 60×. (B) PC3 cells were treated with vehicle or PI3K inhibitors for 48 h, stained with MitoTracker Red, phalloidin Alexa488, and DAPI, and full cell stacks were used to generate 3D max projection images. Magnification, 63×. (Scale bars, 10 μm.) (C) The indicated tumor cell types were treated with vehicle or PX-866 for 48 h, stained as in B, and analyzed by fluorescence microscopy. (D) PC3 cells were transfected with control siRNA (Ctrl) or mitofusin (MFN)1- or MFN2-directed siRNA and analyzed by Western blotting. (E and F) PC3 cells transfected as in D were analyzed for cell viability by Trypan blue exclusion (E) or normalized ATP production (F). U, units. Mean ± SEM. (G) PC3 cells transfected with control siRNA or MFN1-directed siRNA were stained as in B and analyzed by confocal microscopy for changes in mitochondrial redistribution. Magnification, 63×. Insets were zoomed digitally at 20×. (Scale bar, 5 μm.) Full cell stacks were postprocessed for noise reduction using a median filter in LAS AF. (H) For cortical mitochondrial quantification, masks were manually created around the periphery of the cell based on the F-actin channel and subsequently applied to the mitochondrial channel to measure intensity at the cortical region. The intensity was normalized to total mitochondrial intensity per cell and background-subtracted. ROI, region of interest.
Fig. 3.
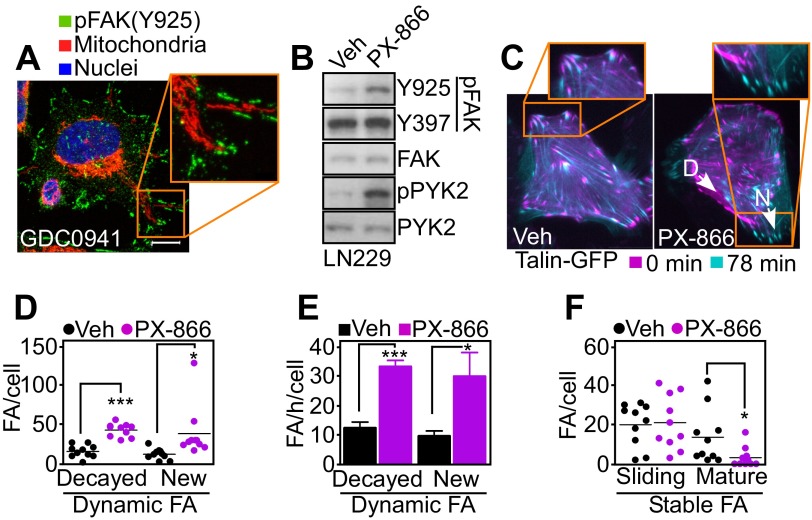
Mitochondria fuel focal adhesion dynamics. (A) PC3 cells treated with vehicle or PI3K inhibitors for 48 h were stained with MitoTracker Red, phalloidin Alexa488, and DAPI, and full cell stacks were used to generate 3D max projection images that were scored for mitochondrial morphology (polarized, perinuclear, infiltrating). (B) Representative confocal 3D max projection images of PC3 cells treated with vehicle or the indicated PI3K inhibitors and stained as in A. (Bottom) Models for quantification of mitochondrial trafficking. Mito, mitochondria. White lines indicate the distance from nuclei to the cell border. Yellow lines indicate the length of mitochondrial infiltration into membrane lamellipodia. Magnification, 63×. (C) PC3 cells treated with vehicle or the indicated PI3K inhibitors were labeled as in A and quantified for mitochondrial infiltration into lamellipodia. At least 18 cells were analyzed at two independent lamellipodia, and data were normalized to total lamellipodia length. Mean ± SEM (n = 36). ***P < 0.0001. (D) Lung adenocarcinoma A549 or glioblastoma LN229 cells were labeled as in A and scored for mitochondrial infiltration into membrane lamellipodia by fluorescence microscopy. Mean ± SEM. **P = 0.0056; ***P < 0.0001. (E) PC3 cells were transfected with control (Ctrl) or MFN1-directed siRNA, labeled as in A, and quantified for mitochondrial infiltration in the cortical cytoskeleton in the presence of vehicle or PX-866. Mean ± SEM. ***P < 0.0001. (F) PC3 cells transfected with control or MFN1-directed siRNA were treated with vehicle or PX-866 and analyzed for Matrigel invasion after 48 h. Mean ± SEM. ***P = 0.0002. (G) PC3 cells treated with vehicle or PI3K inhibitors for 48 h were replated onto fibronectin-coated slides for 5 h and labeled with an antibody to phosphorylated FAK (pY925) Alexa488, MitoTracker Red, and DAPI. Representative 1-µm extended-focus confocal images with localization of mitochondria near FA complexes are shown. Magnification, 63×. (Scale bar, 10 μm.) (H) PC3 cells expressing Talin-GFP to label FA were treated as indicated and quantified for decay, formation, and stability of FA complexes per cell over 78 min; n = 631. See also Movie S2.
Requirements of Mitochondrial Regulation of Tumor Cell Invasion.
A prerequisite of cell movements is the timely assembly/disassembly of focal adhesion (FA) complexes (14), and a role of mitochondrial trafficking in this process was next investigated. Mitochondria repositioned to the cortical cytoskeleton in response to PI3K antagonists colocalized with phosphorylated (Y925) focal adhesion kinase (FAK) (Fig. 3G and Fig. S5A). This was associated with increased FAK phosphorylation (Y925) compared with control cultures (Fig. S5B), suggesting deregulation of FA dynamics (18). By time-lapse video microscopy (Fig. S5C), PI3K therapy profoundly affected FA dynamics (Fig. 3H and Movie S2), increasing both the assembly and decay of FA complexes (Fig. S5D) and their turnover rate (Fig. S5E). In contrast, PI3K inhibition reduced the number of stable FA complexes (Fig. S5F).
Fig. S5.
PI3K therapy regulation of focal adhesion dynamics. (A) PC3 cells treated with the PI3K inhibitor GDC0941 for 48 h were replated onto fibronectin-coated slides for 5 h and labeled with an antibody to phosphorylated FAK (pY925) Alexa488, MitoTracker Red, or DAPI. Representative 1-µm extended-focus confocal images with localization of mitochondria near pFAK+ focal adhesion complexes are shown. Magnification, 63×. Inset was zoomed digitally at 10×. (Scale bar, 10 μm.) (B) LN229 cells were treated with vehicle or PX-866 for 48 h and protein extracts were analyzed by Western blotting. p, phosphorylated. (C) LN229 cells expressing Talin-GFP to label FA complexes were treated with vehicle or PX-866 for 48 h and analyzed by time-lapse microscopy. Representative images at initial (0 min) and final time points (78 min) were merged to visualize FA turnover. Arrows indicate representative decayed (D) or new (N) FAs. Magnification, 40×. (D–F) Individual FA complexes in LN229 cells expressing Talin-GFP as in C were manually tracked to determine FA dynamics. (D) Number of dynamic FA complexes per cell classified into disappearing (decayed) and newly formed (new). Mean ± SEM (n = 10). *P = 0.0353; ***P < 0.0001. (E) Turnover rates (number of FA complexes per h) of FA decay and formation. Mean ± SEM (n = 10). *P = 0.0235; ***P < 0.0001. (F) Stable FA complexes classified as sliding (moving) or mature (>50% overlay between initial and final localization). Mean ± SEM (n = 10). *P = 0.0393.
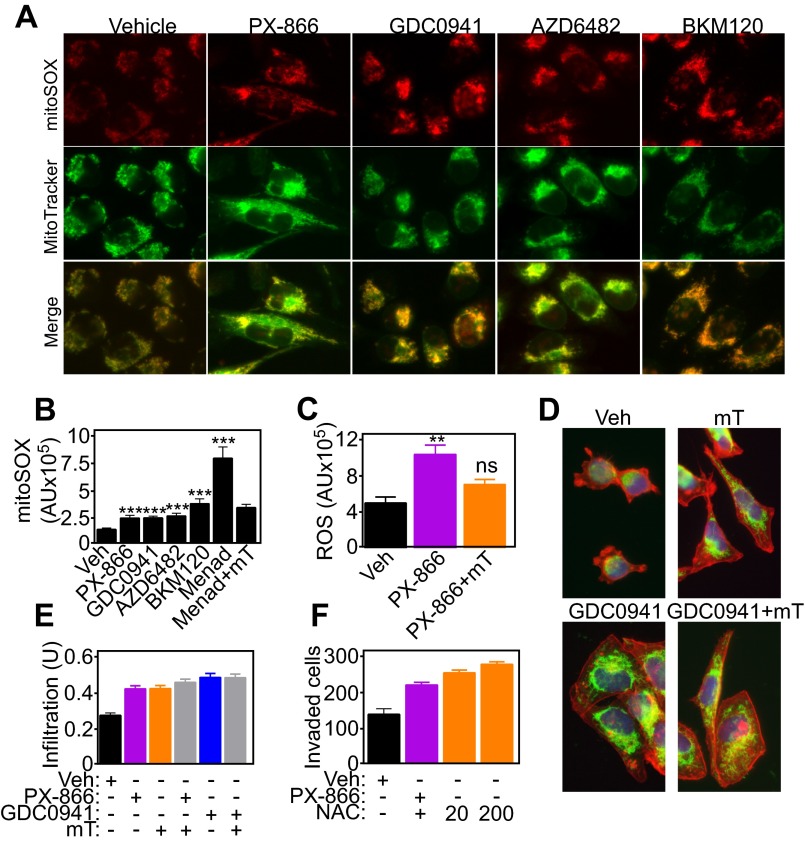
Mitochondria are a primary source of reactive oxygen species (ROS), and these moieties have been implicated in tumor cell motility. PI3K antagonists increased the production of mitochondrial superoxide in tumor cells compared with untreated cultures (Fig. S6 A and B), and this response was abolished by a mitochondrial-targeted ROS scavenger, mitoTEMPO (Fig. S6C). In contrast, ROS scavenging with mitoTEMPO did not affect mitochondrial repositioning to the cortical cytoskeleton (Fig. 4A and Fig. S6 D and E) or tumor cell invasion (Fig. 4B) mediated by PI3K inhibitors. Increasing concentrations of the pan-antioxidant N-acetyl cysteine (NAC) had no effect on PI3K therapy-mediated tumor cell invasion (Fig. S6F). The increase in basal cell motility in the presence of antioxidants may reflect release of ROS-regulated inhibitory mechanisms of mitochondrial trafficking.
Fig. S6.
Role of mitochondrial ROS in organelle trafficking and tumor cell invasion. (A and B) PC3 cells were treated with vehicle or the indicated PI3K inhibitors, stained with MitoTracker Green FM and MitoSOX Red mitochondrial superoxide probes, and analyzed by fluorescence microscopy (A) and quantified (B). Menadione (Menad, 100 μM, 1 h) was used as a control stimulus for ROS production. Mean ± SEM. ***P < 0.001; P (ANOVA) = 0.0002. AU, arbitrary units of fluorescence intensity. (C) PC3 cells were treated with vehicle or PX-866 for 48 h with or without the mitochondrial-targeted ROS scavenger mitoTEMPO (mT) and analyzed for ROS levels. Mean ± SEM. **P (ANOVA) = 0.0015. ns, not significant. AU, arbitrary units of fluorescence intensity. (D and E) PC3 cells were incubated with vehicle, mitoTEMPO alone, or in combination with the indicated PI3K inhibitors (E) and analyzed for mitochondrial repositioning to the cortical cytoskeleton by fluorescence microscopy (D) and quantified (E). Mean ± SEM. P (ANOVA) < 0.0001. U, units of fluorescence intensity in the cortical area, normalized to total cell intensity. (F) PC3 cells were incubated with vehicle or PX-866 for 48 h with or without the indicated concentrations (μM) of the pan-ROS scavenger NAC and analyzed for Matrigel invasion. Mean ± SEM. P (ANOVA) < 0.0001.
Fig. 4.
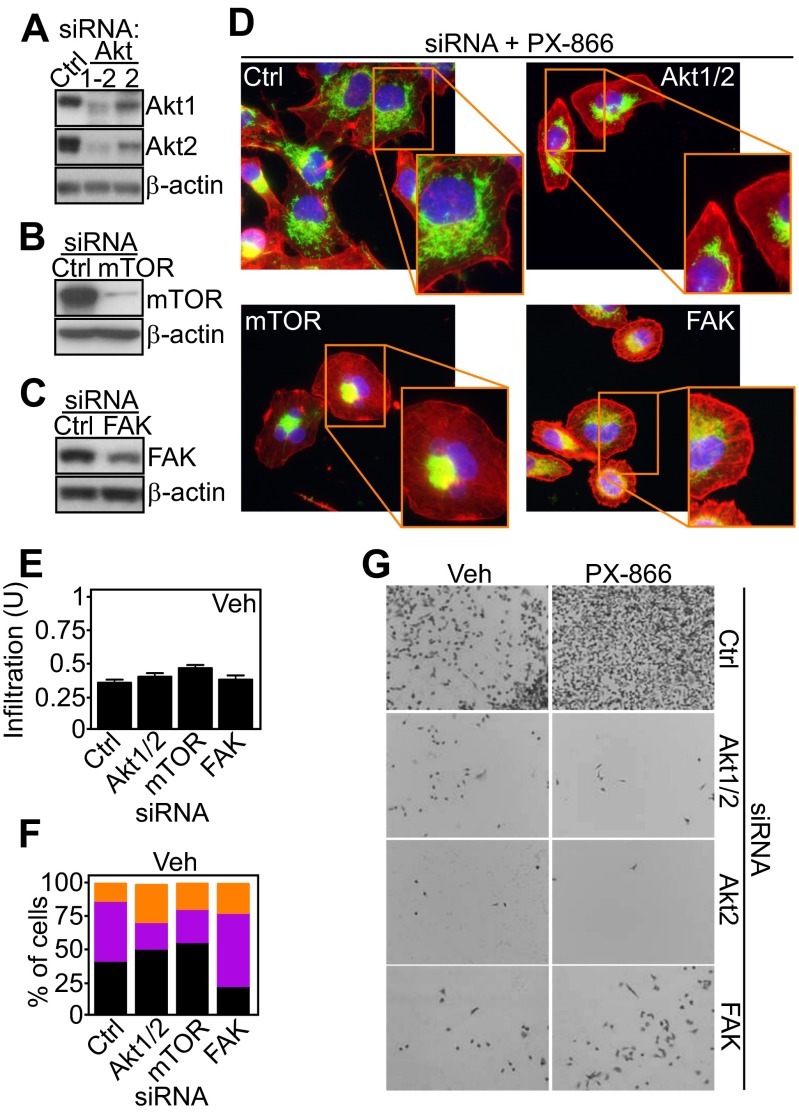
Control of tumor cell invasion by spatiotemporal mitochondrial bioenergetics. (A) PC3 cells were labeled with MitoTracker Red, phalloidin Alexa488, and DAPI, treated with PX-866, and analyzed for mitochondrial infiltration into the peripheral cytoskeleton in the presence of vehicle or the mitochondrial-targeted ROS scavenger mitoTEMPO (mT; 50 μM). (B) PC3 cells were incubated with the indicated agents alone or in combination (PX-866+mT) and analyzed for tumor cell invasion across Matrigel. Mean ± SEM. P (ANOVA) < 0.0001. (C and D) Mitochondrial (mt)DNA-depleted LN229 (ρ0) cells were stimulated with NIH 3T3 conditioned media for 2 h, labeled with MitoTracker Red, DAPI, and either phalloidin Alexa488 (C) or an antibody to FA-associated paxillin (D), and analyzed by fluorescence microscopy. Representative pseudocolored images are shown. Magnification, 60×. (E) WT or ρ0 LN229 cells were analyzed for invasion across Matrigel-coated Transwell inserts. Representative images of invasive cells stained with DAPI are shown. Magnification, 20×. (F) PC3 cells treated with vehicle or PI3K inhibitors in combination with the mitochondrial-targeted small-molecule Hsp90 inhibitor Gamitrinib (Gam) were labeled with anti–pY925-FAK Alexa488 followed by fluorescence microscopy. Representative 1-µm extended-focus confocal images are shown. Magnification, 63×. (Scale bar, 10 μm.) (G) PC3 cells treated with vehicle or PI3K inhibitors with or without Gamitrinib (1 μM) were labeled with MitoTracker Red, phalloidin Alexa488, and DAPI and quantified after 48 h for mitochondrial infiltration into lamellipodia by fluorescence microscopy; n = 48. Mean ± SEM. **P = 0.0044; ***P < 0.0009. (H) PC3 cells were treated with vehicle or PX-866 (5 μM) with or without Gamitrinib and quantified for invasion across Matrigel. Mean ± SEM of replicates (n = 2). ***P < 0.0001. ns, not significant. (I) PC3 cells were incubated with vehicle or PX-866 alone or in combination with the various mitochondrial respiratory chain inhibitors and analyzed for Matrigel invasion. Ant A, Antimycin A; Oligo, Oligomycin; Roten, Rotenone. Mean ± SEM. **P = 0.006. (J) PC3 cells transfected with control siRNA or siRNA to Akt1/2, mTOR, or FAK were labeled as in C, treated with PX-866, and quantified for mitochondrial infiltration into lamellipodia; n = 44. Mean ± SEM. ***P < 0.0001. (K) siRNA-transfected PC3 cells labeled as in C were treated with PX-866 (5 μM) and analyzed for mitochondrial morphology (polarized, perinuclear, infiltrating) by fluorescence microscopy; n = 21. (L) PC3 cells transfected with the indicated siRNAs were quantified for invasion across Matrigel in the presence of vehicle or PX-866. Mean ± SEM (n = 4). ***P < 0.0001.
Role of Bioenergetics in Mitochondrial Trafficking and Tumor Cell Invasion.
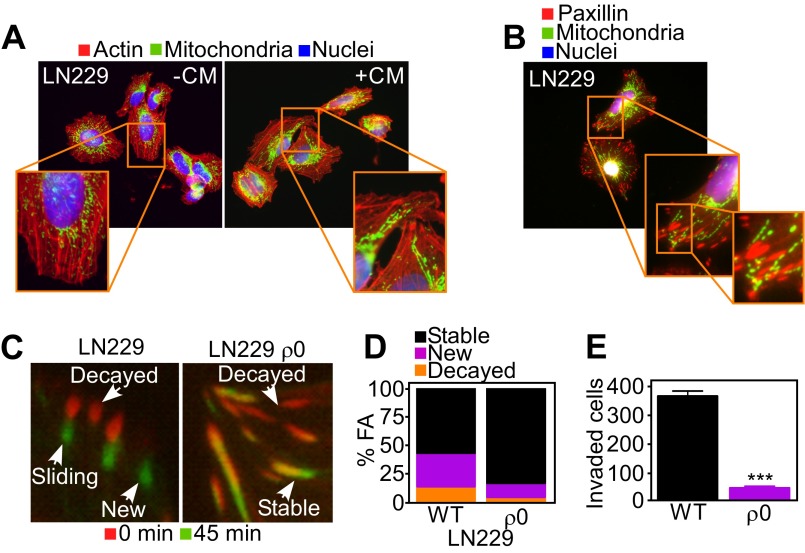
Next, we asked whether mitochondrial bioenergetics was important for this pathway, and generated LN229 cells devoid of oxidative phosphorylation (ρ0 cells). Chemoattractant stimulation of respiration-competent LN229 cells induced repositioning of mitochondria to the cortical cytoskeleton (Fig. S7A) that colocalized with paxillin+ FA complexes (Fig. S7B). In contrast, respiration-deficient LN229 ρ0 cells failed to reposition mitochondria to the cortical cytoskeleton (Fig. 4C). This absence of mitochondria proximal to FA complexes (Fig. 4D) was associated with loss of FA dynamics (Fig. S7 C and D and Movie S3) and suppression of tumor cell invasion across Matrigel-containing inserts (Fig. 4E and Fig. S7E).
Fig. S7.
Requirement of mitochondrial respiration for FA turnover. (A and B) Respiration-competent (WT) LN229 cells were incubated with Mitotracker Red, phalloidin Alexa488, and DAPI (A) or labeled for FA complexes with an antibody to paxillin (B) and analyzed by fluorescence microscopy. Magnification, 60×. CM, NIH3T3 conditioned media. (C) WT LN229 or mtDNA-depleted LN229 (ρ0) cells expressing Talin-GFP to label FA complexes were analyzed by time-lapse microscopy. Representative images at initial (0 min) and final (45 min) time points of the analysis were merged to visualize FA turnover. Representative images of the different stages of FA complexes (decayed, sliding, stable, or new) are indicated. (D) WT or mtDNA-depleted LN229 (ρ0) cells expressing Talin-GFP were analyzed by time-lapse microscopy and quantitated for decay, formation, and stability of FA complexes per cell over 45 min; n ≥ 431. See also Movie S3. (E) WT or ρ0 LN229 cells were analyzed for invasion across Matrigel-coated Transwell inserts. Mean ± SEM (n = 2). ***P < 0.0001.
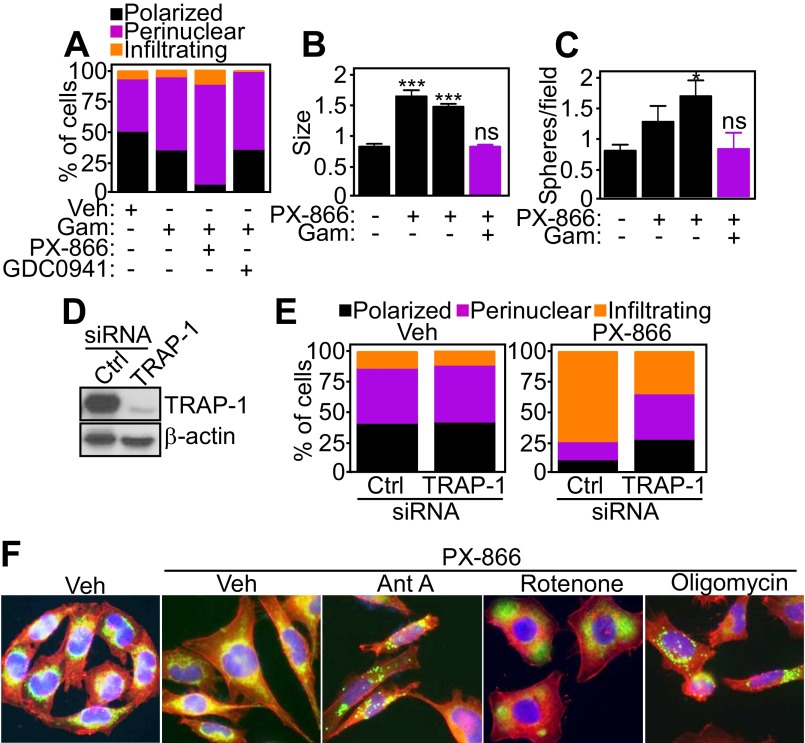
As an independent approach, we treated tumor cells with Gamitrinib, a mitochondrial-targeted small-molecule Hsp90 inhibitor that induces misfolding and degradation of the oxidative phosphorylation complex II subunit SDHB (19). Nontoxic concentrations of Gamitrinib abolished the trafficking of mitochondria to pFAK-containing FA complexes in response to PI3K antagonists (Fig. 4 F and G) and preserved a polarized and perinuclear mitochondrial distribution (Fig. S8A). Consistent with these findings, Gamitrinib abolished the increase in tumor cell invasion (Fig. 4H) and the expansion of primary GBM neurospheres (Fig. S8 B and C) mediated by PI3K antagonists. To validate these findings, we next silenced the expression of TRAP-1 (Fig. S8D), a mitochondrial Hsp90-like chaperone targeted by Gamitrinib and implicated in complex II stability (19). TRAP-1 silencing in vehicle-treated cells did not affect mitochondrial localization (Fig. S8E, Left). In contrast, knockdown of TRAP-1 abolished mitochondrial trafficking to the cortical cytoskeleton in the presence of PI3K antagonists, increasing the fraction of polarized and perinuclear organelles in these cells (Fig. S8E, Right). Finally, treatment with small-molecule inhibitors of mitochondrial complex I (Rotenone), complex III (Antimycin A), or complex V (Oligomycin) or a mitochondrial uncoupler (carbonyl cyanide m-chlorophenyl hydrazine; CCCP) inhibited mitochondrial repositioning to the cortical cytoskeleton (Fig. S8F) and tumor cell invasion (Fig. 4I) in the presence of PI3K therapy.
Fig. S8.
Requirement of oxidative phosphorylation for mitochondrial infiltration to the cortical cytoskeleton. (A) PC3 cells treated with vehicle or PI3K inhibitors in combination with the mitochondrial-targeted small-molecule Hsp90 inhibitor Gamitrinib (Gam) were labeled with MitoTracker Red, phalloidin Alexa488, and DAPI and scored for mitochondrial morphology (polarized, perinuclear, infiltrating) by fluorescence microscopy; n ≥ 42. (B and C) Patient-derived GBM spheroids were treated with increasing concentrations of PX-866 (0–10 μM) for 48 h and imaged by phase-contrast microscopy in the presence or absence of Gamitrinib (5 μM plus PX-866 at 10 μM). The size (B) or number (C) of neurospheres was quantified and normalized on initial size and number per each patient. Mean ± SEM (n = 4 patients). *P = 0.017; ***P < 0.0001. ns, not significant. (D) PC3 cells were transfected with control nontargeting siRNA or TRAP-1–directed siRNA and analyzed by Western blotting. (E) siRNA-transfected PC3 cells as in D were labeled with MitoTracker Red, phalloidin Alexa488, and DAPI with quantification of mitochondrial morphology (polarized, perinuclear, infiltrating) by fluorescence microscopy; n = 32. (F) PC3 cells were labeled as in A, treated with the indicated ETC inhibitors in the presence of PX-866, and analyzed by fluorescence microscopy. Ant. A, Antimycin A. Magnification, 60×.
To begin elucidating the signaling requirements of adaptive mitochondrial trafficking and tumor cell invasion, we next targeted the PI3K–Akt–mTOR axis, which becomes reactivated in response to PI3K therapy (8, 9). Knockdown of Akt1 or Akt2 (Fig. S9A), mTOR (Fig. S9B), or FAK (Fig. S9C) independently prevented the repositioning of mitochondria to the cortical cytoskeleton (Fig. 4 J and K and Fig. S9D) and suppressed tumor cell invasion (Fig. 4L and Fig. S9G) induced by PI3K antagonists. In contrast, knockdown of these molecules in the absence of PI3K inhibition had no effect on mitochondrial trafficking (Fig. S9E) or organelle morphology (Fig. S9F).
Fig. S9.
Requirement of Akt/mTOR signaling for mitochondrial redistribution to the cortical cytoskeleton. (A–C) PC3 cells were transfected with control nontargeting siRNA or siRNA targeting Akt1/2 (A), mTOR (B), or FAK (C) and analyzed by Western blotting. (D) PC3 cells transfected with control siRNA or siRNA directed to Akt1/2, mTOR, or FAK were treated with vehicle or PX-866, labeled with MitoTracker Red, phalloidin Alexa488, and DAPI and analyzed by fluorescence microscopy. Representative pseudocolored images are shown. Magnification, 60×. (E and F) siRNA-transfected PC3 cells were labeled with MitoTracker Red, phalloidin Alexa488, and DAPI, treated with vehicle, and scored for mitochondrial infiltration into lamellipodia (n = 44) (E) or changes in mitochondrial morphology (polarized, perinuclear, infiltrating) (n = 25) (F) by fluorescence microscopy. Mean ± SEM. (G) PC3 cells transfected with the indicated siRNAs were analyzed for invasion across Matrigel in the presence of vehicle (Veh) or PX-866. Representative images are shown. Magnification, 10×.
Discussion
In this study, we have shown that small-molecule PI3K inhibitors currently in the clinic induce global reprogramming of transcriptional and signaling pathways in tumor cells, paradoxically resulting in increased tumor cell motility and invasion. Mechanistically, this involves the trafficking of energetically active mitochondria to the cortical cytoskeleton of tumor cells, where they support membrane lamellipodia dynamics, turnover of FA complexes, and random cell migration and invasion. Conversely, interference with this spatiotemporal control of mitochondrial bioenergetics abolishes tumor cell invasion.
Although associated with important tumor traits, including “stemness” (20), malignant regrowth (21), and drug resistance (22), a general role of mitochondria in cancer has been difficult to determine (11). Whether these organelles play a role in tumor cell invasion and, therefore, metastatic competency has been equally controversial, with evidence that mitochondrial respiration is important (23), not important (24), or must be dysfunctional (25) to affect cell movements. Here disabling cellular respiration with depletion of mitochondrial DNA (26) or targeting an oxidative phosphorylation complex(es) (19) prevented mitochondrial trafficking to the cortical cytoskeleton, abolished membrane dynamics of cell motility, and suppressed cell invasion. Conversely, scavenging of mitochondrial ROS, which are increased in response to PI3K therapy, did not affect organelle dynamics and tumor cell invasion. Together, these data suggest that oxidative phosphorylation contributes to cancer metabolism and provides a “regional” and potent ATP source to fuel highly energy-demanding processes of cell movements and invasion (27).
This “spatiotemporal” model of mitochondrial bioenergetics is reminiscent of the accumulation of mitochondria at subcellular sites of energy-intensive processes in neurons (28), including synapses, active growth cones, and branches (29). Whether the cytoskeletal machinery that transports mitochondria along the microtubule network in neurons (30) is also exploited in cancer (this study) is currently unknown. However, there is evidence that comparable mechanisms of organelle dynamics (31) support mitochondrial redistribution in lymphocytes (32) and may contribute to directional migration of tumor cells (33). Consistent with this model (31), interference with the mitochondrial fusion machinery, namely mitofusins, suppressed mitochondrial repositioning to the cortical cytoskeleton and tumor cell invasion mediated by PI3K therapy.
In addition to oxidative phosphorylation, Akt/mTOR signaling was identified here as a key regulator of mitochondrial trafficking and tumor cell invasion. This is consistent with a pivotal role of PI3K in directional cell movements (34), supporting chemotaxis at the leading edge of migration (35) and Rac1 activation (36). A third signaling requirement of this pathway involved FAK activity (18), which has also been implicated in cytoskeletal dynamics (37).
Despite hopes for “personalized” medicine (4), small-molecule PI3K inhibitors have produced modest and short-lived patient responses in the clinic (5). Our data suggest that these agents potently activate global adaptive mechanisms in tumors (7), unexpectedly centered on mitochondrial reprogramming in cell survival/bioenergetics (9) and subcellular trafficking (this study). In this context, the increased tumor cell motility and invasion stimulated by PI3K inhibitors may create an “escape” mechanism for tumor cells to elude therapy-induced environmental stress, reminiscent of the heightened metastatic propensity associated with other unfavorable conditions of hypoxia (38), acidosis (39), and antiangiogenic therapy (40, 41). Although this adaptive response to PI3K therapy may paradoxically promote more aggressive tumor traits and further compromise clinical outcomes, disabling mitochondrial adaptation is feasible (19) and may provide a viable strategy to increase the anticancer efficacy of PI3K antagonists in the clinic.
Methods
Two-Dimensional Chemotaxis.
Cells were treated with PI3K inhibitors for 48 h and seeded in 2D chemotaxis chambers (Ibidi) in 10% (vol/vol) FBS medium. After a 6-h attachment, cells were washed and the reservoirs were filled with 0.1% BSA/RPMI, followed by gradient setup by addition of NIH 3T3 conditioned medium. Video microscopy was performed over 8 h, with a time-lapse interval of 10 min. At least 30 cells were tracked using the WimTaxis module (Wimasis), and the tracking data were exported into Chemotaxis and Migration Tool v2.0 (Ibidi) for graphing and statistical testing. Experiments were repeated twice (n = 3).
FA Dynamics.
Cells growing in high–optical-quality 96-well μ-plates (Ibidi) were transduced with Talin-GFP BacMam virus (50 particles per cell) for 18 h and imaged with a 40× objective on a Nikon TE300 inverted time-lapse microscope equipped with a video system containing an Evolution QEi camera and a time-lapse video cassette recorder. The atmosphere was equilibrated to 37 °C and 5% CO2 in an incubation chamber. Time-lapse fluorescence microscopy was carried out for the indicated times at 1 min per frame. Sequences were aligned in Image-Pro Plus 7 (Media Cybernetics) and imported into ImageJ (NIH) for further analysis. The initial and final frames were duplicated and assembled as composite images. FA complexes were manually counted and classified according to presence in some or all of the time frames: decaying, newly formed, stable sliding (FA moves to a different position over time), and stable mature (merged areas). The rate of decay and assembly of FA complexes was calculated for each cell as the number of FA complexes changing per h. At least 400 FA complexes from 10 cells were analyzed from 5 independent time lapses per condition.
Tumor Cell Invasion.
Experiments were carried out essentially as described (42). Briefly, 8-μm PET Transwell migration chambers (Corning) were coated with 150 μL 80 μg/mL Matrigel (Becton Dickinson). Tumor cells were seeded in duplicates onto the coated Transwell filters at a density of 1.25 × 105 cells per well in media containing 2% (vol/vol) FCS (FCIII; HyClone), and media containing 20% (vol/vol) FCS were placed in the lower chamber as chemoattractant. Cells were allowed to invade and adhere to the bottom of the plate, stained in 0.5% crystal violet/methanol for 10 min, rinsed in tap water, and analyzed by bright-field microscopy. Digital images were batch-imported into ImageJ, thresholded, and analyzed with the Analyze Particles function. For analysis of tumor cell invasion in 3D spheroids, tissue culture-treated 96-well plates were coated with 50 µL 1% Difco Agar Noble (Becton Dickinson). LN229 cells were seeded at 5,000 cells per well and allowed to form spheroids over 72 h. Spheroids were harvested, treated with PX-866 (0–10 μM), and placed in a collagen plug containing Eagle's minimum essential medium (EMEM), FBS, l-glutamine, sodium bicarbonate, and collagen type I (Gibco; 1.5 mg/mL). The collagen plug was allowed to set and 1 mL DMEM with 5% (vol/vol) FBS was added to the top of the plug. Cell invasion was analyzed every 24 h and quantified using Image-Pro Plus 7, as described (42).
Patient Samples.
For studies using human samples, informed consent was obtained from all patients enrolled, and the study was approved by an Institutional Review Board of the Fondazione IRCCS Ca' Granda. The clinicopathological features of GBM patients used in this study are summarized in Table S1.
Statistical Analysis.
Data were analyzed using either two-sided unpaired t test (for two-group comparisons) or one-way ANOVA test with Dunnett’s multiple comparison posttest (for more than two-group comparisons) using a GraphPad software package (Prism 6.0) for Windows. Data are expressed as mean ± SD or mean ± SEM of multiple independent experiments. A P value of <0.05 was considered statistically significant.
SI Methods
Antibodies and Reagents.
Antibodies to pan-Ser473/474–phosphorylated Akt1/2 (Cell Signaling), pan-Akt (Cell Signaling), Ser473-phosphorylated Akt1 (Cell Signaling), Akt1 (Cell Signaling), Ser474-phosphorylated Akt2 (Cell Signaling), Akt2 (Cell Signaling), Ser2448-phosphorylated mTOR (Cell Signaling), mTOR (Cell Signaling), Thr389-phosphorylated p70S6K (Cell Signaling), p70S6K (Cell Signaling), Thr37/46-phosphorylated 4EBP1 (Cell Signaling), MFN1 (Abcam), MFN2 (Abcam), Tyr397-phosphorylated FAK (Cell Signaling), Tyr925-phosphorylated FAK (Cell Signaling), FAK (Cell Signaling), Tyr402-phosphorylated PYK2 (Cell Signaling), PYK2 (Cell Signaling), TRAP-1 (BD Biosciences), and β-actin (Sigma-Aldrich) were used for Western blotting. Antibodies to Tyr925-phosphorylated FAK (Cell Signaling) and paxillin (Upstate) were used for immunofluorescence. The PI3K inhibitors (AZD6482, GCD0941, BKM120) were purchased from Selleckchem; PX-866 was from LC Laboratories. mitoTEMPO [(2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chloride], menadione (2-methyl-1,4-naphthoquinone sodium bisulfite), N-acetyl cysteine (NAC), and carbonyl cyanide m-chlorophenyl hydrazine (CCCP) were from Sigma-Aldrich. Antimycin A, Oligomycin, and Rotenone were obtained from Abcam Biochemicals. All chemicals were of the highest purity commercially available. The complete chemical synthesis, HPLC profile, and mass spectrometry of the mitochondrial-targeted Hsp90 antagonist Gamitrinib have been reported (43). The Gamitrinib variant containing triphenylphosphonium as a mitochondrial-targeting moiety was used throughout this study. Phalloidin Alexa488, MitoTracker Red CMH2XRos, MitoTracker Green FM, MitoSOX Red mitochondrial superoxide indicator, CellLight Talin-GFP BacMam 2.0, and secondary antibodies for immunofluorescence were from Molecular Probes.
Cell Culture and Drug Treatment.
Human glioblastoma (GBM) LN229, prostate adenocarcinoma PC3, lung carcinoma A549, and mouse fibroblast NIH 3T3 cells were obtained from the American Type Culture Collection and maintained in culture according to the supplier’s specifications. Conditioned media were prepared from exponentially growing cultures of NIH 3T3 cells, as previously described (42), in DMEM supplemented with 4.5 g/L d-glucose, 1 mM sodium pyruvate, 10 mM Hepes, and 10% (vol/vol) FBS for 48 h. Where indicated, starvation was carried out in DMEM without glucose for 24 h. PI3K inhibitors were dissolved in DMSO at 10 mM and added to the culture media for 48 h at the following concentrations: AZD6482 at 10 µM, GCD0941 at 2 µM, BKM120 at 0.4 µM, and PX-866 at 5 µM. mitoTEMPO was added to the culture media at 50–100 μM, NAC at 20 μM, Antimycin A at 30 μM, Oligomycin at 2.5 μM, Rotenone at 4 μM, and CCCP at 12.5 μM. For cell invasion assays, the antioxidants, ROS scavengers, and electron transport chain (ETC) inhibitors were also added to the top and bottom of the chambers at the moment of cell seeding.
Generation of LN229 ρ0 Cell Line.
Mitochondrial DNA-deficient LN229 cells were generated by a 3-wk culture in the presence of ethidium bromide (50 ng/mL) in medium supplemented with 1 mM sodium pyruvate and 50 μg/mL uridine. mtDNA depletion was confirmed by PCR to be >90%. Briefly, total DNA (both mitochondrial and genomic) was extracted from LN229 or LN229 ρ0 cultures using the QIAamp DNA Mini Kit (Qiagen). Real-time PCR was carried out in an Applied Biosystems ABI 7500 Fast Real Time PCR cycler using SYBR Green PCR Master Mix (Qiagen). Primers for two mtDNA markers (β-globin and 18S rRNA) were used. Amplifications were performed in 25-μL reactions composed of 15 ng total DNA, 1× SYBR Green PCR Master Mix (Qiagen) with 3.5 mM MgCl2 and 0.3 μM each primer. Triplicate reactions were performed for each marker in a 96-well plate.
Patients.
Fresh, patient-derived tissues obtained from surgical resections of grade IV GBM (seven cases) were used in this study. Informed consent was obtained from all patients, and the study was approved by an Institutional Review Board at the Fondazione IRCCS Ca’ Granda Hospital. The clinicopathological characteristics of the patient series used in this study are presented in Table S1.
Organotypic Cultures.
Short-term organotypic cultures from primary patient samples were established as described (13). Briefly, precise thick tissue slices (300-µm) were obtained by vibratome serial cutting (VT1200; Leica Microsystems) and cultured in six-well plates on organotypic inserts (Millicell PICM ORG; Merck Millipore) for up to 48 h in 1 mL Ham-F12 complete medium supplemented with vehicle (DMSO, 2.5 µL) or the pan-PI3K inhibitor PX-886 (10 µM). At the end of the experiment, one tissue slice per condition was formalin-fixed and paraffin-embedded and further processed for morphological and immunohistochemical analysis. An additional tissue slice was embedded in OCT and snap-frozen for molecular or immunofluorescence studies.
GBM Neurospheres.
Fresh GBM specimens collected from consenting patients (Division of Neurosurgery, Fondazione IRCCS Ca’ Granda) were immediately processed for the isolation of stem cell-containing neurospheres. Briefly, tissues were enzymatically processed with a Tumor Dissociation Kit (Miltenyi Biotec) at 37 °C in combination with gentleMACS Dissociators (Miltenyi Biotec) until achievement of a single-cell suspension. Then, dissociated GBM cells were grown as spheroid aggregates (neurospheres) as previously described (44) using NeuroCult NS-A Proliferation media (StemCell Technologies) supplemented with 20 ng/mL EGF and 10 ng/mL bFGF (StemCell Technologies) according to the manufacturer’s protocol. When pharmacological treatment was performed, neurospheres derived from five GBM patients were mechanically dissociated, stained with PKH26 (Sigma-Aldrich) as described (45), and plated at a density of 1,000 cells per well in 24-well plates. The intended use of the nontoxic dye PKH26 was for live-cell identification and staining. Twenty-four hours later, cells were treated with 5 or 10 µM PX-886 in the presence or absence of Gamitrinib (10 μM) and monitored for neurosphere formation for up to 3 d. During this interval, neurospheres were imaged every 24 h and their size (diameter) and number were assessed by direct counting using ImageJ software. PKH26 was monitored and recorded using a TRITC fluorescence filter. Experiments were performed in duplicates. For immunofluorescence experiments, control or PX-866 (5–10 μM)–treated neurospheres were fixed in 4% (vol/vol) paraformaldehyde for 15 min and placed on charged glasses using a Cytospin (Thermo Scientific). Samples were further incubated with primary antibodies to phosphorylated Akt (Ser473; Cell Signaling), phosphorylated mTOR (Ser2448; Cell Signaling), or Nestin (clone 196908; R&D Systems) and mounted in media containing DAPI (Abbott).
Small Interfering RNA.
Knockdown experiments were carried out as described (42). The following siRNAs were used: control, nontargeting siRNA pool (Dharmacon; D-001810) or specific siRNA pools targeting Akt1/2 (Santa Cruz; sc-43609), Akt2 (Santa Cruz; sc-29197), mTOR (Dharmacon; L-003008), FAK (Santa Cruz; sc-29310), TRAP-1 (Dharmacon; L-010104), MFN1 (Santa Cruz; sc-43927), and MFN2 (Santa Cruz; sc-43928). siRNAs were transfected at 10–30 nM concentrations in the presence of Lipofectamine RNAiMAX (Invitrogen) at a 1:1 ratio [vol siRNA (20 μM):vol Lipofectamine RNAiMAX]. Cells were incubated for 48 h, validated for target protein knockdown by Western blotting, and processed for subsequent experiments.
Kinome Profiling and Bioinformatics Analysis.
Transcriptional changes in the human kinome following PI3K inhibition were analyzed using OpenArray real-time PCR amplification (BioTrove). GBM organotypic cultures were treated with vehicle (DMSO) or PX-866 (10 μM) for 48 h. Total RNA was extracted from each sample and reverse-transcribed, and the derived cDNA was hybridized with TaqMan Array Human Protein Kinase Pathways (Applied Biosystems, cat. no 4414076). Ct values from the experiment were exported from BioTrove OpenArray Real-Time qPCR Analysis software v1.0.4. The ΔΔCt method (46) was used to calculate fold changes between sample pairs with the endogenous control Ct value calculated as the average among 15 endogenous controls on the array: ALAS1, B2M, CASC3, G6PD, GAPDH, GUSB, HMBS, HPRT1, IPO8, POLR2A, PPIA, RPLP0, TFRC, UBE2D2, and YWHAZ. The P values for differential expression were calculated using fold changes of 15 endogenous controls as a null distribution. False discovery rate (FDR) values were estimated using Benjamini–Hochberg correction for multiple testing (47), and results with FDR <20% were used for enrichment analysis with Ingenuity Pathway Analysis software (Ingenuity Systems) to identify a list of pathways and functions significantly overrepresented among the significantly changed genes.
Protein Analysis.
For Western blotting, protein lysates were prepared in Triton X-100 lysis buffer (20 mM Tris⋅HCl, pH 8.00, 137 mM NaCl, 1% Nonidet P-40, 10% (vol/vol) glycerol) containing EDTA-free Protease Inhibitor Mixture (Roche) and Phosphatase Inhibitor Mixtures 2 and 3 (Sigma-Aldrich), sonicated, and precleared by centrifugation at 20,000 × g for 10 min at 4 °C. Equal amounts of protein lysates were separated by SDS gel electrophoresis, transferred to PVDF membranes, blocked in 5% (wt/vol) low-fat milk/Tris buffered saline-0.1% Tween-20 (TBST), and incubated with primary antibodies of various specificities diluted 1:1,000 in 5% (wt/vol) BSA/TBST overnight at 4 °C. After washing in TBST, membranes were incubated with secondary antibodies conjugated to HRP [1:1,000–1:5,000 dilution in 5% (wt/vol) BSA/TBST] for 1 h at room temperature and washed with TBST, and protein bands were visualized by enhanced chemiluminescence.
Membrane Ruffle Dynamics in Live Cells.
Quantification of membrane ruffle dynamics in live cells was carried out as described previously (42). Briefly, 3–5 × 104 cells were grown on high–optical-quality 96-well μ-plates (Ibidi) and imaged with a 40× objective on a Nikon TE300 inverted time-lapse microscope equipped with a video system containing an Evolution QEi camera and a time-lapse video cassette recorder. The atmosphere was equilibrated to 37 °C and 5% CO2 in an incubation chamber. Phase-contrast images were captured at 0.5-s intervals for 250 s (500 frames) and merged into sequence files using Image-Pro Plus 7. To monitor dynamics of a particular region by stroboscopic analysis of cell dynamics (SACED) (15), the sequence files were imported into ImageJ and a particular region of 16.2 μm × 0.162 μm (“SACED line”) was selected, duplicated, and montaged in sequence to display the region over time in a stroboscopic image. This process was repeated to get four SACED lines and therefore four stroboscopic images per cell (n = 15), and structures such as protruding lamellipodia and ruffles were manually labeled and the length and angle obtained were used to calculate the distance and time of each event by trigonometry. For each cell, the frequency of ruffles per min, ruffling retraction speed (μm/min), and ruffle migration distance (μm) and persistence (s) were calculated. Mean values were calculated from at least 15 cells from three or four independent time lapses, as described (42).
Detection of Mitochondrial Superoxide in Live Cells.
The production of superoxide by mitochondria was visualized by fluorescence microscopy. Briefly, 1.5 × 104 cells were grown on high–optical-quality eight-well μ-slides (Ibidi) and stained with MitoTracker Green FM (50 nM, 1 h) and MitoSOX Red mitochondrial superoxide indicator (5 μM, 10 min) in complete medium, followed by extensive washing in warm complete medium. Stained cells were imaged with a 40× objective on a Nikon TE300 inverted time-lapse microscope equipped with a video system containing an Evolution QEi camera and a time-lapse video cassette recorder. The atmosphere was equilibrated to 37 °C and 5% CO2 in an incubation chamber. Phase, red fluorescence (TRITC filter cube, excitation wavelength 532–554 nm, emission wavelength 570–613 nm) and green fluorescence (FITC filter cube, excitation wavelength 467–498 nm, emission wavelength 513–556 nm) images were captured. To quantitate superoxide levels, files were imported into ImageJ and masks were manually created around the periphery of the cell based on the phase image and subsequently applied to the TRITC channel to measure intensity. A minimum of 100 cells was analyzed in each independent experiment to obtain mean values.
Mitochondrial Localization.
After the indicated transfections or treatments, cells were stained with MitoTracker Red CMH2XRos (100 nM, 2 h) at 37 °C. After fixation with 4% (vol/vol) formalin in PBS for 15 min at room temperature, cells were permeabilized with 0.1% Triton X-100/PBS for 5 min, washed, and incubated for 30 min with 1% BSA/0.3 M glycine/PBS. F-actin was stained with phalloidin Alexa488 (1:1,000 dilution) for 30 min at room temperature. Slides were washed and mounted in DAPI-containing ProLong Gold mounting medium (Invitrogen). At least seven random fields were analyzed by fluorescence microscopy on a Nikon E600 microscope. Composite images were analyzed in ImageJ; for each cell, two lamellipodia were measured to calculate the infiltration index, defined as the radial distance from the nucleus to the farthest mitochondrial string, and normalized to the radial distance from the nucleus to the cell border. In parallel, all cells on the seven fields were scored into three categories according to gross localization of mitochondria: polarized, perinuclear, and infiltrating (Fig. S4A). For cortical mitochondrial quantification, a mask was manually created around the periphery of the cell based on the F-actin channel and subsequently applied to the mitochondrial channel to measure intensity at the cortical region (Fig. S4H). The intensity was normalized to total mitochondrial intensity per cell and background-subtracted. A minimum of 20 cells was analyzed in each independent experiment to obtain mean values. For high-resolution imaging of mitochondria in MFN1-depleted cells, slides were sequentially scanned for red, green, and blue fluorescence by a Leica SP5 confocal microscope with a 63× objective and a 20× digital zoom. Full cell z stacks (about 5–8 μm) were postprocessed in Leica Application Suite Advanced Fluorescence (LAS AF) to reduce noise by applying a median filter (two iterations, radius 3) and used to generate max projections.
Focal Adhesion Complexes and Mitochondrial Dual Labeling.
Coverslides were coated with fibronectin (10 μg/mL in PBS) overnight at 4 °C, rinsed in PBS, and equilibrated at 37 °C with 500 μL 10% (vol/vol) FBS/RPMI. Cells were treated as indicated, trypsinized, washed with 10% (vol/vol) FBS/RPMI, and plated on fibronectin-coated slides in the presence of MitoTracker Red CMH2XRos (100 nM). After 5 h, cells were fixed with 4% (vol/vol) formalin in PBS for 15 min at room temperature, permeabilized with 0.1% Triton X-100/PBS for 5 min, and washed, and FA complexes were labeled with primary antibodies diluted in 1% BSA/0.3 M glycine/PBS overnight at 4 °C. Either one of the following antibodies was used: rabbit anti–pY925-FAK (1:50; Cell Signaling) or mouse anti-paxillin (1:100; Upstate). Next, slides were washed, incubated with secondary antibodies (1:500; Alexa Fluor 488-conjugated) for 1 h at room temperature, washed, and mounted in DAPI-containing ProLong Gold mounting medium (Invitrogen). Slides were sequentially scanned for red, green, and blue fluorescence by a Leica SP5 confocal microscope with a 100× objective. Libraries were imported into LASlite, and a three-step z stack centered at the plane of focal adhesions (±0.5 μm) was used to generate 3D max projections.
Supplementary Material
Acknowledgments
We thank James Hayden and Frederick Keeney of the Wistar Imaging Facility for outstanding help with time-lapse imaging. This work was supported by National Institutes of Health Grants P01 CA140043 (to D.C.A. and L.R.L.), R01 CA78810 and CA190027 (to D.C.A.), F32 CA177018 (to M.C.C.), and R01 CA089720 (to L.R.L.), the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award W81XWH-13-1-0193 (to D.C.A.), and a Joint Grant in Molecular Medicine 2013 from Fondazione IRCCS Ca’ Granda and Istituto Nazionale Genetica Molecolare (to V.V.). Support for the core facilities used in this study was provided by Cancer Center Support Grant CA010815 to The Wistar Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500722112/-/DCSupplemental.
References
- 1.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 2.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10(3):143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 5.Fruman DA, Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13(2):140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jänne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8(9):709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AA, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322(5907):1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarty A, Sánchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA. 2012;109(8):2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh JC, et al. Adaptive mitochondrial reprogramming and resistance to PI3K therapy. J Natl Cancer Inst. 2015;107(3):dju502. doi: 10.1093/jnci/dju502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward PS, Thompson CB. Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12(10):685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frezza C, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477(7363):225–228. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 13.Vaira V, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci USA. 2010;107(18):8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11(8):573–587. doi: 10.1038/nrc3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinz B, Alt W, Johnen C, Herzog V, Kaiser H-W. Quantifying lamella dynamics of cultured cells by SACED, a new computer-assisted motion analysis. Exp Cell Res. 1999;251(1):234–243. doi: 10.1006/excr.1999.4541. [DOI] [PubMed] [Google Scholar]
- 16.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10(8):538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: Mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14(9):598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YC, et al. Landscape of the mitochondrial Hsp90 metabolome in tumours. Nat Commun. 2013;4:2139. doi: 10.1038/ncomms3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janiszewska M, et al. Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 2012;26(17):1926–1944. doi: 10.1101/gad.188292.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haq R, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23(3):302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBleu VS, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiraishi T, et al. 2015. Glycolysis is the primary bioenergetic pathway for cell motility and cytoskeletal remodeling in human prostate and breast cancer cells. Oncotarget 6(1):130–143.
- 25.Porporato PE, et al. A mitochondrial switch promotes tumor metastasis. Cell Reports. 2014;8(3):754–766. doi: 10.1016/j.celrep.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Olgun A, Akman S. Mitochondrial DNA-deficient models and aging. Ann N Y Acad Sci. 2007;1100:241–245. doi: 10.1196/annals.1395.025. [DOI] [PubMed] [Google Scholar]
- 27.De Bock K, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154(3):651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Lee CW, Peng HB. Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol. 2006;66(6):522–536. doi: 10.1002/neu.20245. [DOI] [PubMed] [Google Scholar]
- 29.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125(Pt 9):2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birsa N, Norkett R, Higgs N, Lopez-Domenech G, Kittler JT. Mitochondrial trafficking in neurons and the role of the Miro family of GTPase proteins. Biochem Soc Trans. 2013;41(6):1525–1531. doi: 10.1042/BST20130234. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J, et al. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32(40):4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morlino G, et al. Miro-1 links mitochondria and microtubule dynein motors to control lymphocyte migration and polarity. Mol Cell Biol. 2014;34(8):1412–1426. doi: 10.1128/MCB.01177-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai SP, Bhatia SN, Toner M, Irimia D. Mitochondrial localization and the persistent migration of epithelial cancer cells. Biophys J. 2013;104(9):2077–2088. doi: 10.1016/j.bpj.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kölsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121(Pt 5):551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiger MC, et al. Spontaneous phosphoinositide 3-kinase signaling dynamics drive spreading and random migration of fibroblasts. J Cell Sci. 2009;122(Pt 3):313–323. doi: 10.1242/jcs.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraynov VS, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290(5490):333–337. doi: 10.1126/science.290.5490.333. [DOI] [PubMed] [Google Scholar]
- 37.Fabry B, Klemm AH, Kienle S, Schäffer TE, Goldmann WH. Focal adhesion kinase stabilizes the cytoskeleton. Biophys J. 2011;101(9):2131–2138. doi: 10.1016/j.bpj.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennacchietti S, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 39.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 40.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pàez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caino MC, et al. Metabolic stress regulates cytoskeletal dynamics and metastasis of cancer cells. J Clin Invest. 2013;123(7):2907–2920. doi: 10.1172/JCI67841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang BH, et al. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119(3):454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortensi B, et al. Rai is a new regulator of neural progenitor migration and glioblastoma invasion. Stem Cells. 2012;30(5):817–832. doi: 10.1002/stem.1056. [DOI] [PubMed] [Google Scholar]
- 45.Vaira V, et al. miR-296 regulation of a cell polarity-cell plasticity module controls tumor progression. Oncogene. 2012;31(1):27–38. doi: 10.1038/onc.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 48.Louis DN, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neurophatol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.