Significance
Leukocytes partition certain proteins into cholesterol- and sphingolipid-rich membrane regions (lipid rafts) that function as signaling platforms. Inflammatory stimuli cause leukocytes to elongate to form lamellipodia and uropods at opposite ends that facilitate migration. Many raft-associated proteins move to uropods. Proteins are typically thought to use their transmembrane and cytoplasmic domains to associate with rafts. Here, we found that some leukocyte adhesion proteins used carbohydrate modification (glycosylation) of their extracellular domains to associate with lipid rafts. These proteins required preassociation with rafts to transduce signals but, unexpectedly, not to move to uropods. These data define a mechanism for localizing proteins to critical membrane regions of leukocytes.
Keywords: cell adhesion, cell signaling, inflammation, uropod, neutrophil
Abstract
Palmitoylated cysteines typically target transmembrane proteins to domains enriched in cholesterol and sphingolipids (lipid rafts). P-selectin glycoprotein ligand-1 (PSGL-1), CD43, and CD44 are O-glycosylated proteins on leukocytes that associate with lipid rafts. During inflammation, they transduce signals by engaging selectins as leukocytes roll in venules, and they move to the raft-enriched uropods of polarized cells upon chemokine stimulation. It is not known how these glycoproteins associate with lipid rafts or whether this association is required for signaling or for translocation to uropods. Here, we found that loss of core 1-derived O-glycans in murine C1galt1−/− neutrophils blocked raft targeting of PSGL-1, CD43, and CD44, but not of other glycosylated proteins, as measured by resistance to solubilization in nonionic detergent and by copatching with a raft-resident sphingolipid on intact cells. Neuraminidase removal of sialic acids from wild-type neutrophils also blocked raft targeting. C1galt1−/− neutrophils or neuraminidase-treated neutrophils failed to activate tyrosine kinases when plated on immobilized anti–PSGL-1 or anti-CD44 F(ab′)2. Furthermore, C1galt1−/− neutrophils incubated with anti–PSGL-1 F(ab′)2 did not generate microparticles. In marked contrast, PSGL-1, CD43, and CD44 moved normally to the uropods of chemokine-stimulated C1galt1−/− neutrophils. These data define a role for core 1-derived O-glycans and terminal sialic acids in targeting glycoprotein ligands for selectins to lipid rafts of leukocytes. Preassociation of these glycoproteins with rafts is required for signaling but not for movement to uropods.
Lipid rafts are ordered membrane domains that assemble cholesterol, sphingolipids, and selected proteins (1). They were first defined by resistance to solubilization in cold nonionic detergents, which maintains raft proteins in the lighter fractions of density gradients (2). On intact cells, lateral crosslinking with antibodies or other probes copatches lipid and protein constituents of rafts (3). High-resolution imaging confirms that rafts are small, dispersed structures that can be oligomerized (1). Importantly, rafts serve as signaling platforms, notably on immune cells (4).
How proteins partition to rafts is incompletely understood (5). Hydrophobic residues in some transmembrane domains may interact with sphingolipids and/or cholesterol. Cysteines modified with saturated fatty acids, usually palmitic acid, direct cytosolic proteins such as Src family kinases (SFKs) to raft inner leaflets. Palmitoylated cysteines in transmembrane and cytoplasmic domains of some membrane proteins also interact with rafts. In polarized epithelial cells, apical transport vesicles are enriched in cholesterol and sphingolipids (1). N- and O-glycans on some apical proteins act as sorting determinants, probably through multiple mechanisms. Glycans may enhance association of some apically destined proteins with rafts (6). Whether glycans direct proteins to rafts of hematopoietic cells is unknown.
At sites of infection or injury, circulating leukocytes adhere to activated endothelial cells and platelets and to adherent leukocytes. The adhesion cascade includes tethering, rolling, deceleration (slow rolling), arrest, intraluminal crawling, and transendothelial migration (7). Selectins mediate rolling, whereas β2 integrins mediate slow rolling, arrest, and crawling. Selectins are lectins that form rapidly reversible, force-regulated bonds with glycosylated ligands under flow (8). Leukocytes express L-selectin, activated platelets express P-selectin, and activated endothelial cells express P- and E-selectin. The dominant leukocyte ligand for P- and L-selectin is P-selectin glycoprotein ligand-1 (PSGL-1). Major leukocyte ligands for E-selectin include PSGL-1, CD44, and CD43, although other ligands contribute to adhesion (9). PSGL-1 and CD43 are mucins with multiple O-glycans attached to serines and threonines. Although not a mucin, CD44 is modified with both N- and O-glycans (10, 11). The selectins bind, in part, to the sialyl Lewis x (sLex) determinant (NeuAcα2–3Galβ1–4[Fucα1–3]GlcNAcβ1-R), which caps some N-glycans and mucin-type O-glycans (8, 12). CD44 uses N-glycans to interact with E-selectin (13, 14), whereas PSGL-1 uses mucin-type, core 1-derived O-glycans to interact with all three selectins (14–16). The enzyme core 1 β1–3-galactosyltransferase forms the core 1 backbone (Galβ1–3GalNAcα1-Ser/Thr) to which more distal determinants such as sLex are added (17). Neutrophils from mice lacking core 1 β1–3-galactosyltransferase in endothelial and hematopoietic cells (EHC C1galt1−/−) have markedly impaired rolling on P- or E-selectin (14).
PSGL-1 (18, 19), CD44 (20), and CD43 (21) associate with lipid rafts on leukocytes, but how they do so is unclear. In knockin mice, PSGL-1 lacking the cytoplasmic domain still associates with leukocyte rafts (19). In transfected nonhematopoietic cells, detergent resistance of CD44 is reversed by mutating cysteines in the transmembrane and cytoplasmic domains and an ezrin/radixin/moesin (ERM)-binding site in the cytoplasmic domain (22). Whether similar mechanisms operate in primary leukocytes is unknown. Determining how these proteins target to rafts is relevant because of their important signaling functions in leukocytes. Selectin binding to PSGL-1 or CD44 on neutrophils triggers phosphorylation of SFKs and downstream mediators that convert β2 integrins to an extended, intermediate-affinity state, slowing rolling and contributing to arrest (23–25). Disrupting lipid rafts by depleting or sequestering cholesterol blocks signaling (23). Binding of P-selectin to PSGL-1 on myeloid cells causes shedding of microparticles with proinflammatory and procoagulant properties (26). The microparticles are enriched in raft-associated proteins such as PSGL-1 and tissue factor, but not in nonraft proteins such as CD45 (18). Disrupting rafts by chelating or sequestering cholesterol blocks microparticle generation (18). However, it is not known whether PSGL-1 or other selectin ligands must preassociate with rafts to trigger integrin activation or microparticle shedding.
Leukocytes stimulated with chemokines or bacterial peptides polarize to form leading-edge lamellipodia and trailing-edge uropods. PSGL-1, CD43, and CD44 redistribute to the uropods (27–29). Studies in transfected cells suggest that PSGL-1 moves to uropods through interactions of its cytoplasmic domain with ERM proteins (30). In knockin mice, however, PSGL-1 lacking the cytoplasmic domain relocates normally to uropods of polarized neutrophils (19). It has also been proposed that PSGL-1 moves to uropods by interacting with flotillin, a raft-resident protein (31). Notably, disrupting lipid rafts by chelating cholesterol blocks uropod formation (21). It is not known whether PSGL-1 or other proteins must associate with rafts before moving to uropods.
Here, we found that loss of core 1-derived O-glycans in leukocytes from EHC C1galt1−/− mice blocked raft targeting of PSGL-1, CD43, and CD44, but not of other glycosylated proteins. Treating leukocytes with neuraminidase to remove terminal sialic acids had similar effects. Failure to partition into rafts prevented PSGL-1 or CD44 from activating SFKs and generating microparticles. However, O-glycans were not required to redistribute PSGL-1, CD43, or CD44 to the uropods of polarized leukocytes.
Results
PSGL-1 Does Not Require Its Transmembrane Domain to Associate with Lipid Rafts.
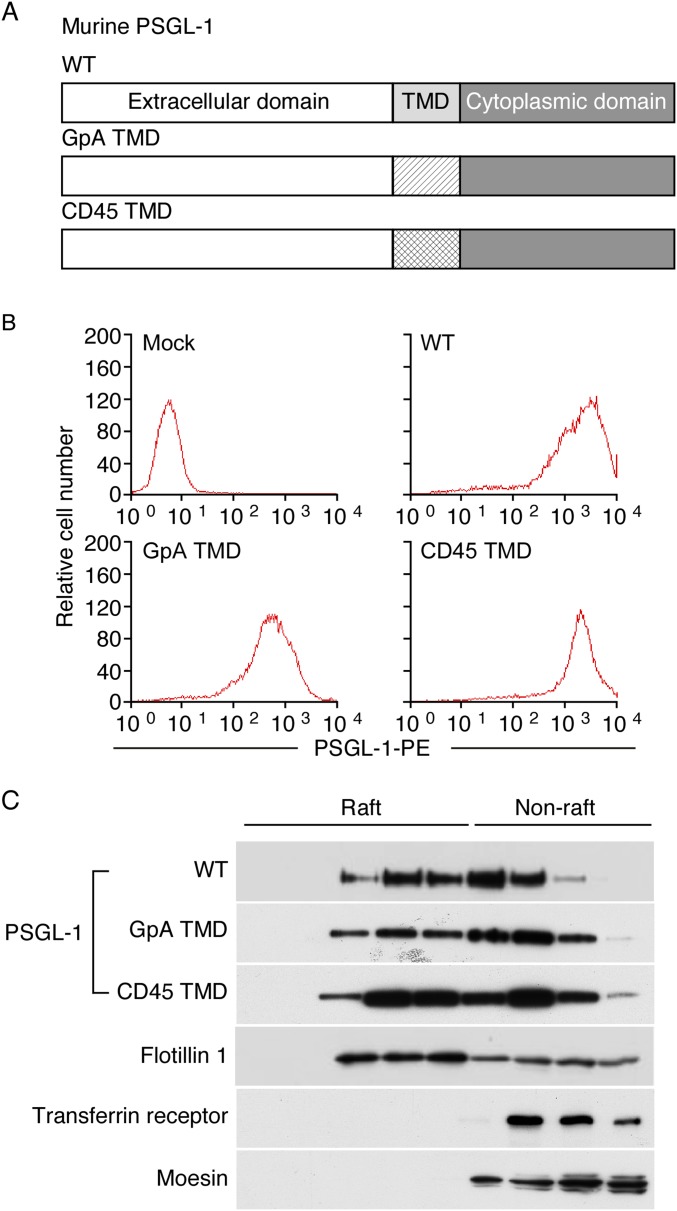
Deleting the cytoplasmic domain of PSGL-1 does not prevent its partitioning into detergent-resistant membranes (DRMs, lipid rafts) (19). We asked whether PSGL-1 requires its transmembrane domain to associate with rafts. We generated PSGL-1 chimeras that substituted the transmembrane domain of PSGL-1 with the transmembrane domain of glycophorin A or of CD45, which do not partition into rafts (18, 32, 33) (Fig. S1A). Wild-type (WT) PSGL-1 and PSGL-1 chimeras were expressed in transfected Chinese hamster ovary cells at similar densities (Fig. S1B). The cells were also transfected with vectors that express glycosyltransferases required to construct selectin ligands (34). We lysed the cells in cold 1% Triton X-100 and fractionated the extracts by ultracentrifugation in an OptiPrep gradient. Western blotting revealed that significant portions of WT PSGL-1 and both PSGL-1 chimeras were in lighter-density DRMs that colocalized with the raft-resident protein flotillin 1. In contrast, the nonraft proteins transferrin receptor and moesin were found only in higher-density fractions (Fig. S1C). Thus, PSGL-1 does not require its cytoplasmic or transmembrane domain to associate with rafts.
Fig. S1.
PSGL-1 does not require its transmembrane domain to associate with lipid rafts. (A) Schematic of wild-type (WT) murine PSGL-1 or of PSGL-1 constructs containing the transmembrane domain (TMD) of glycophorin A (GpA) or CD45. (B) Flow cytometry of transfected cells expressing the indicated PSGL-1 construct labeled with PE-conjugated anti–PSGL-1 mAb. (C) Transfected cells were lysed in cold 1% Triton X-100 and centrifuged in an OptiPrep gradient. Fractions collected from Top to Bottom (Left to Right, corresponding to lower to higher density) were analyzed by Western blotting with antibodies to the indicated proteins. Results are representative of at least three experiments.
PSGL-1, CD43, and CD44 Require Core 1-Derived O-Glycans to Associate with Lipid Rafts.
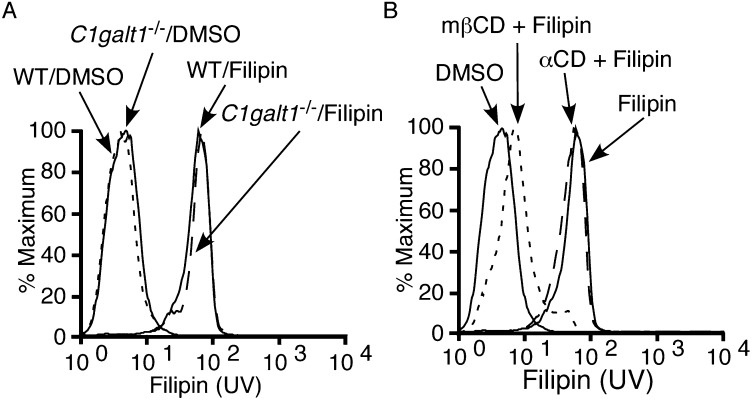
We next considered whether PSGL-1 uses its extracellular domain to associate with rafts. Some epithelial cell proteins use N- or O-glycans for transport into raft-enriched apical domains (6). Therefore, we asked whether the multiple O-glycans on the extracellular domain of PSGL-1 contribute to raft targeting. Leukocytes from EHC C1galt1−/− mice attach GalNAc to serines and threonines but lack core 1-derived O-glycans, including core 1, extended core 1, and core 2 structures (14). They express normal surface levels of PSGL-1, CD43, CD44, and other glycoproteins (14). The cholesterol probe filipin (35) bound similarly to plasma membranes of WT and C1galt1−/− neutrophils (Fig. S2A). Filipin binding was specific for cholesterol, because it was eliminated by treating neutrophils with methyl-β-cyclodextrin, a cholesterol chelator, but not with α-cyclodextrin, an inactive analog (Fig. S2B).
Fig. S2.
The cholesterol probe filipin binds similarly to WT and C1galt1−/− neutrophils. (A) WT or C1galt1−/− neutrophils or (B) WT neutrophils pretreated with the cholesterol chelator methyl-β-cyclodextrin (MβCD) or its inactive analog α-cyclodextrin (αCD) were incubated with DMSO vehicle or filipin. Flow cytometry was used to measure filipin binding by UV light emission. Results are representative of three experiments.
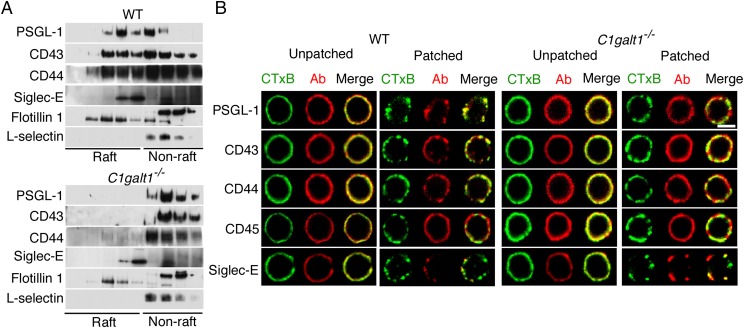
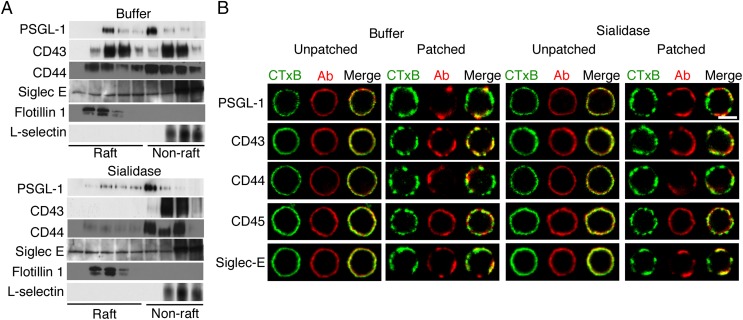
As in transfected Chinese hamster ovary cells, a significant portion of PSGL-1 in detergent extracts of WT neutrophils was in lighter-density DRMs that colocalized with flotillin 1 (Fig. 1A). The O-glycosylated proteins CD43 and CD44 from WT neutrophils were also enriched in raft fractions. However, virtually all PSGL-1, CD43, and CD44 in extracts from C1galt1−/− neutrophils were in higher-density, nonraft fractions (Fig. 1A). In contrast, the N-glycosylated protein siglec-E was enriched in lower-density fractions of both genotypes, and the N-glycosylated protein L-selectin was enriched in higher-density fractions of both genotypes (Fig. 1A).
Fig. 1.
PSGL-1, CD43, and CD44 require core 1-derived O-glycans to associate with lipid rafts. (A) WT or C1galt1−/− neutrophils were lysed in cold 1% Triton X-100 and centrifuged in an OptiPrep gradient. Fractions collected from Top to Bottom (Left to Right, corresponding to lower to higher density) were analyzed by Western blotting with antibodies to the indicated proteins. (B) WT or C1galt1−/− neutrophils were incubated with Alexa 488-conjugated CTxB (green) to label GM1-containing lipid rafts. The cells were then incubated with anti-CTxB antibodies at 4 °C as control (unpatched) or at 37 °C to aggregate the rafts (patched). The cells were then fixed and labeled with antibodies to the indicated protein, followed by Alexa 647-conjugated secondary antibody (red). Representative cells were visualized with confocal microscopy to identify CTxB, antibody (Ab), or both CTxB and Ab (merge). Results are representative of at least three experiments. (Scale bar, 5 μm.)
To identify proteins in lipid rafts of intact cells, we used confocal microscopy to visualize copatching of proteins with rafts by crosslinking cholera toxin B (CTxB) bound to the raft-enriched ganglioside GM1. Before crosslinking (without incubation at 37 °C to cause patching), antibodies to CTxB, PSGL-1, CD43, CD44, CD45, and siglec-E homogeneously stained the plasma membranes of both WT and C1galt1−/− neutrophils (Fig. 1B). After crosslinking CTxB at 37 °C, lipid rafts clustered in discrete aggregates on neutrophils of both genotypes (Fig. 1B). Siglec-E, but not the nonraft protein CD45, copatched with CTxB on both WT and C1galt1−/− neutrophils. PSGL-1, CD43, and CD44 also copatched with CTxB on WT neutrophils. In sharp contrast, they remained homogeneously distributed on C1galt1−/− neutrophils (Fig. 1B). Thus, both detergent resistance and copatching assays demonstrate that PSGL-1, CD43, and CD44 require core 1-derived O-glycans to associate with lipid rafts.
PSGL-1, CD43, and CD44 Require Sialic Acids to Associate with Lipid Rafts.
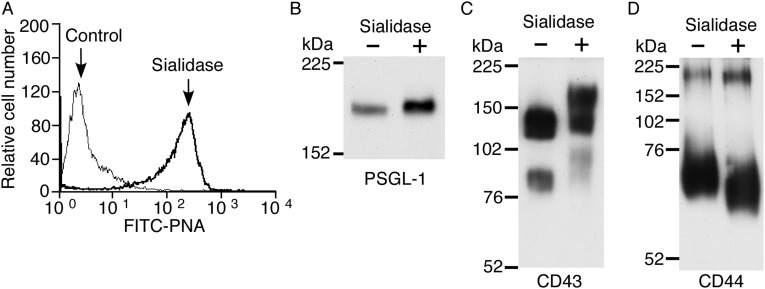
Sialic acids cap most N- and O-glycans on mammalian cells, including neutrophils (36). We asked whether sialic acids contribute to raft targeting of PSGL-1, CD43, and CD44. For this purpose, we treated WT neutrophils with neuraminidase (sialidase). This treatment effectively removed sialic acids, as measured by increased binding of the lectin, peanut agglutinin, to neutrophil surfaces (Fig. S3A), and by altered mobility of PSGL-1, CD43, and CD44 during SDS/PAGE (Fig. S3 B–D). Neuraminidase treatment markedly reduced the amount of each protein in lighter-density DRMs (Fig. 2A). Neuraminidase did not alter basal homogeneous staining of PSGL-1, CD43, CD44, CD45, and siglec-E (Fig. 2B), but it substantially decreased copatching of PSGL-1, CD43, and CD44 with the raft marker CTxB (Fig. 2B). However, it did not alter the distribution of siglec-E (Fig. 2 A and B) or CD45 (Fig. 2B). These data demonstrate that PSGL-1, CD43, and CD44 require sialic acids, most likely on O-glycans, to associate with lipid rafts.
Fig. S3.
Neuraminidase effectively removes sialic acids from glycans on neutrophils. (A) WT neutrophils were treated with control buffer or neuraminidase (sialidase) and then stained with FITC-conjugated peanut agglutinin (PNA). (B–D) WT neutrophils treated with or without sialidase were lysed and analyzed by Western blotting with mAbs against PSGL-1, CD43, or CD44 under nonreducing conditions. Results are representative of three experiments.
Fig. 2.
PSGL-1, CD43, and CD44 require sialic acids to associate with lipid rafts. WT neutrophils were incubated with buffer or neuraminidase (sialidase). (A) The cells were lysed, fractionated on OptiPrep gradients, and analyzed by Western blotting with antibodies to the indicated protein as in Fig. 1. (B) CTxB-bound rafts and antibodies to the indicated protein were visualized by confocal microscopy as in Fig. 1. Results are representative of at least three experiments. (Scale bar, 5 μm.)
PSGL-1 and CD44 Require Core 1-Derived O-Glycans and Sialic Acids to Initiate Signaling.
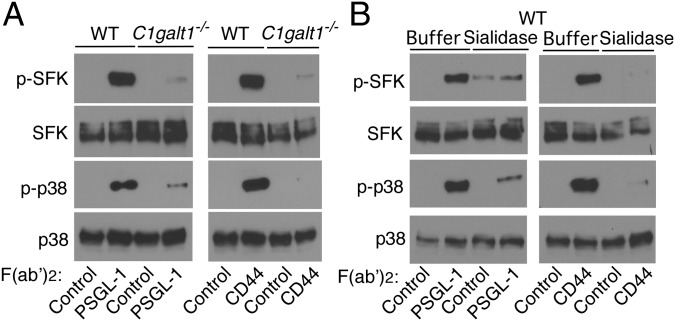
Selectin binding to PSGL-1 and CD44 on neutrophils induces tyrosine phosphorylation of SFKs and downstream kinases, including p38 MAPK, which convert β2 integrins to an extended, intermediate-affinity conformation that mediates slow rolling (9, 23, 24, 37). Disrupting lipid rafts by depleting or sequestering cholesterol blocks signaling (23). We asked whether PSGL-1 and CD44 must preassociate with lipid rafts to initiate signaling. We used mAbs to PSGL-1 or CD44 as selectin surrogates. WT neutrophils plated on F(ab′)2 fragments of anti–PSGL-1 or anti-CD44 mAb, but not isotype-control F(ab′)2, phosphorylated tyrosines on SFKs, and p38 MAPK (Fig. 3A). In marked contrast, C1galt1−/− neutrophils plated on anti–PSGL-1 or anti-CD44 F(ab′)2 did not activate SFKs or p38 MAPK. Furthermore, neuraminidase-treated WT neutrophils plated on anti–PSGL-1 or anti-CD44 F(ab′)2 did not activate SFKs or p38 MAPK (Fig. 3B). These results indicate that selectin-triggered signaling in neutrophils requires O-glycan– and sialic acid-dependent association of PSGL-1 and CD44 with lipid rafts.
Fig. 3.
PSGL-1 and CD44 require core 1-derived O-glycans and sialic acids to initiate signaling. (A) WT or C1galt1−/− neutrophils were incubated on immobilized F(ab′)2 fragments of isotype control, anti–PSGL-1, or anti-CD44 mAb. Lysates were probed by Western blotting with antibodies to phospho-SFK (p-SFK), total SFK, phospho-p38 (p-p38), or total p38. (B) WT neutrophils were incubated with buffer or neuraminidase (sialidase) and then incubated on immobilized F(ab′)2 fragments of isotype control, anti–PSGL-1, or anti-CD44 mAb. Lysates were probed by Western blotting with antibodies to p-SFK, total SFK, p-p38, or total p38. Results are representative of three experiments.
PSGL-1 Requires Core 1-Derived O-Glycans to Trigger SFK-Dependent Generation of Microparticles.
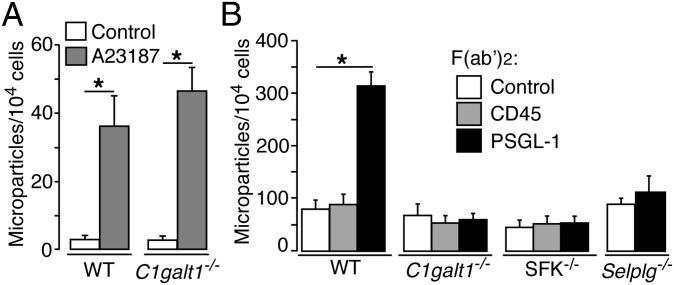
Neutrophils stimulated with LPS or the Ca2+ ionophore A23187 or by P-selectin binding to PSGL-1 generate microparticles enriched in lipid raft-associated proteins (18, 26). We labeled the membranes of WT or C1galt1−/− neutrophils with a fluorescent dye and measured agonist-induced release of fluorescent microparticles. The Ca2+ ionophore A23187, but not vehicle control, generated equivalent numbers of microparticles from WT and C1galt1−/− neutrophils (Fig. 4A). By contrast, F(ab′)2 fragments of anti–PSGL-1 mAb, but not of anti-CD45 or isotype-control mAb, generated microparticles from WT but not C1galt1−/− neutrophils (Fig. 4B). Anti–PSGL-1 F(ab′)2 did not generate microparticles from PSGL-1–deficient neutrophils, confirming its specificity (Fig. 4B). Furthermore, anti–PSGL-1 F(ab′)2 did not generate microparticles from SFK-deficient neutrophils (Fig. 4B). These data indicate that PSGL-1 requires O-glycan–dependent association with lipid rafts to generate microparticles through an SFK-dependent signaling pathway.
Fig. 4.
PSGL-1 requires core 1-derived O-glycans to trigger SFK-dependent generation of microparticles. (A) Fluorescent WT or C1galt1−/− neutrophils were incubated with vehicle control or with the Ca2+ ionophore A23187. The number of microparticles generated was measured by flow cytometry. (B) Fluorescent neutrophils of the indicated genotype were incubated with F(ab′)2 fragments of isotype control, anti-CD45, or anti–PSGL-1 mAb. The number of microparticles generated was measured by flow cytometry. The data represent the mean ± SEM of five experiments. *P < 0.01.
PSGL-1, CD43, and CD44 Do Not Require Core 1-Derived O-Glycans to Redistribute to the Uropods of Polarized Neutrophils.
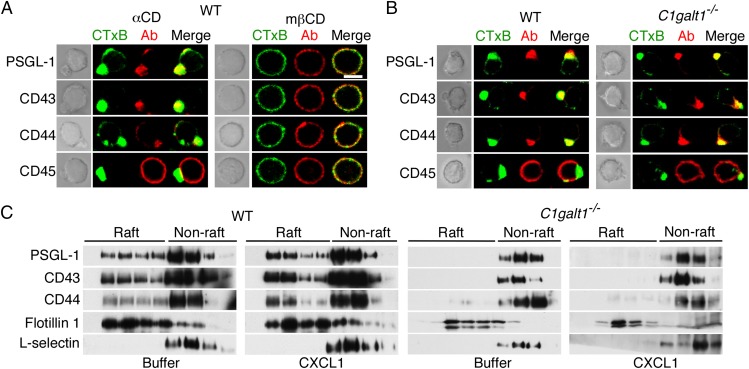
Chemokine-stimulated leukocytes polarize to form leading-edge lamellipodia and trailing-edge uropods (38). We visualized the distribution of membrane proteins on neutrophils after stimulation with CXCL1. The raft-associated proteins PSGL-1, CD43, and CD44, but not the nonraft protein CD45, redistributed to the uropods of WT neutrophils (Fig. 5 A and B). Disrupting lipid rafts with the cholesterol chelator, methyl-β-cyclodextrin, but not with the inactive analog, α-cyclodextrin, blocked polarization, confirming previous studies (21) (Fig. 5A). Unexpectedly, PSGL-1, CD44, and CD43 also redistributed to the uropods of C1galt1−/− neutrophils (Fig. 5B). However, CXCL1 did not alter the density distribution of raft and nonraft proteins in detergent extracts from WT or C1galt1−/− neutrophils (Fig. 5C). Thus, PSGL-1, CD43, and CD44 do not require preassociation with lipid rafts to move to the uropods of polarized neutrophils.
Fig. 5.
PSGL-1, CD43, and CD44 do not require core 1-derived O-glycans to redistribute to the uropods of polarized neutrophils. (A) WT neutrophils preincubated with methyl-β-cyclodextrin (mβCD) or control α-cyclodextrin (αCD) were stimulated with the chemokine CXCL1. After fixation, the cells were labeled with CTxB (green) or antibodies to the indicated protein (red). Representative cells were visualized by phase-contrast microscopy (Left) or confocal microscopy (Right). (B) WT or C1galt1−/− neutrophils were stimulated with CXCL1, fixed, labeled, and visualized as in A. (C) WT or C1galt1−/− neutrophils were incubated with buffer or CXCL1. The cells were lysed, fractionated on OptiPrep gradients, and analyzed by Western blotting with antibodies to the indicated protein as in Fig. 1. Results are representative of at least three experiments. (Scale bar, 5 μm.)
Discussion
We defined a critical role for core 1-derived O-glycans and terminal sialic acids in targeting glycoprotein ligands for selectins to lipid rafts on leukocytes. We used complementary assays to identify glycoproteins in rafts: resistance to solubilization in nonionic detergent and copatching with a raft-resident sphingolipid on intact cells. Both assays yielded congruent results that strengthen our conclusions. We further demonstrated that these glycoproteins must preassociate with rafts to transduce biologically important signals.
PSGL-1 lacking its cytoplasmic domain still associates with lipid rafts (19). Here we ruled out a requirement for the transmembrane domain of PSGL-1 for raft targeting. This argues against palmitoylation of cysteines in either domain as an essential mechanism for moving PSGL-1 to rafts. Instead, extension of sialylated core 1-derived O-glycans on the extracellular domain of PSGL-1, and of CD44 and CD43, enabled targeting. Global loss of O-glycans or terminal sialic acids did not indirectly impair raft association of all proteins, because flotillin-1 and N-glycosylated siglec-E remained in rafts.
PSGL-1 and CD43 are extended mucins with O-glycans attached to many serines and threonines (9, 15, 39). Clustered, sialylated O-glycan “patches” on these proteins are possible raft-targeting signals. However, the less clustered O-glycans on CD44 also mediated raft targeting, whereas the O-glycans on CD45 (40) did not. Thus, the structural features of the signal require further definition. Raft association could involve interactions of glycan determinants on PSGL-1, CD43, and CD44 with a raft-resident lectin. Candidates are siglecs and the structurally related paired Ig-like type 2 receptors (PILRs), which bind terminal sialic acids in particular contexts (41, 42). Siglec-E, the siglec CD33, and PILRα are expressed on murine myeloid cells. However, all three lectins have cytoplasmic immunoreceptor tyrosine-based inhibitory motifs that negatively regulate inflammation (43, 44), whereas raft association of PSGL-1, CD43, and CD44 promotes proinflammatory signaling. CD33 and PIRLα prefer sialic acid linked α2–6 to N-acetylgalactosamine (45, 46), not the sialic acid linked α2–3 to galactose that caps core 1-derived O-glycans. Alternatively, desialylation or truncation of O-glycans could indirectly affect the conformation of targeting signals on the protein backbone. However, a single N-acetylgalactosamine attached to serines and threonines, as occurs on C1galt1−/− leukocytes, is sufficient to extend the polypeptide backbone of mucins such as PSGL-1 and CD43 (47, 48).
In epithelial cells, similarly complex signals target glycoproteins to apical membrane domains that are enriched in cholesterol and sphingolipids (1). Both N- and O-glycans have been implicated in apical targeting (6). Glycosylation of some proteins enhances raft association as well as apical targeting (49), whereas glycosylation of other proteins mediates apical targeting independently of rafts (50).
During neutrophil rolling, selectin engagement of PSGL-1 or CD44 triggers a signaling cascade similar to that used by the T-cell receptor (9). The cascade activates SFKs and downstream kinases and recruits multiple adaptors. Disrupting lipid rafts by depleting or sequestering cholesterol blocks signaling (23). Lipid rafts function as signaling platforms by assembling signaling components such as SFKs. Ligand clustering may merge T-cell receptors in nonraft domains with coreceptors in raft domains to initiate signaling (4). By contrast, we found that PSGL-1 and CD44 must associate with rafts before engaging a selectin surrogate to trigger signaling. These rafts are probably too small to contain a full complement of SFKs or other signaling proteins. During cell adhesion, selectin binding to PSGL-1 or CD44 likely clusters small rafts into larger domains with sufficient kinases, substrates, and adaptors to trigger signaling. PSGL-1 also requires its cytoplasmic domain to signal (19), suggesting that it directly recruits one or more signaling components. Perhaps PSGL-1 and CD44 require preassociation with rafts because, unlike the T-cell receptor, they lack coreceptors that facilitate movement from nonraft to raft domains. Although not yet tested, E-selectin engagement of CD43 on rolling effector T cells (51, 52) may induce signaling by a similar mechanism.
The best characterized effector response to PSGL-1– or CD44-mediated signaling is conversion of β2 integrins to an extended, intermediate-affinity form that mediates slow rolling on ICAM-1 (9). However, P-selectin binding to PSGL-1 also triggers release of prothrombotic and proinflammatory microparticles (18, 26, 53). We found that PSGL-1 required preassociation with lipid rafts to generate microparticles through an SFK-dependent signaling pathway. Thus, raft-dependent signaling was required to generate raft-enriched microparticles. A downstream event in PSGL-1–induced signaling is activation of phospholipase C (9), which generates intracellular Ca2+ that was probably the proximal inducer of microparticle release. By directly elevating cytosolic Ca2+, the ionophore A23187 bypassed the upstream components of this receptor-mediated signaling cascade.
During polarization of activated leukocytes, membrane domains enriched in cholesterol and sphingolipids, including GM1, coalesce in uropods with a subset of transmembrane glycoproteins that include PSGL-1, CD43, and CD44 (38). Surprisingly, these glycoproteins also moved to uropods of chemokine-stimulated C1galt1−/− neutrophils, even though, before stimulation, they did not copatch with GM1 in lipid rafts, and after stimulation, they remained in higher-density, detergent-soluble “nonraft” fractions. Uropods form through membrane interactions with flotillins 1 and 2 and with the actin cytoskeleton (54, 55), in part through binding of ERM adaptors to the cytoplasmic domains of membrane glycoproteins (56). PSGL-1 associates with flotillins as measured by coimmunoprecipitation in detergent extracts and by a proximity-ligation assay in intact cells (31, 57). However, direct binding of PSGL-1 to flotillins has not been demonstrated. Direct interactions, if they occur, may have low affinity, because flotillins dissociated from PSGL-1, CD43, and CD44 in gradients of C1galt1−/− neutrophil extracts. On intact C1galt1−/− neutrophils, however, low-affinity interactions with flotillins might sweep PSGL-1, CD43, and CD44 into uropods as rafts coalesce into larger domains that increase binding avidity. These interactions might synergize with binding of the cytoplasmic domains of PSGL-1, CD43, and CD44 to ERM proteins that link to the cytoskeleton. Because of clustered, high-avidity interactions, uropods might form even if only some cytoplasmic domains bind directly to ERM proteins. This could explain why PSGL-1 lacking its cytoplasmic domain still moves to uropods of stimulated neutrophils (19).
In addition to selectin ligands, other glycoproteins may use sialylated O-glycans to associate with lipid rafts on hematopoietic cells. Thus, O-glycosylation may influence how membrane domains regulate diverse functions during hematopoiesis, immune responses, and hemostasis.
Materials and Methods
All mouse experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation. Details including reagents, mice, cells, isolation of murine neutrophils from bone marrow, detergent-resistant membrane preparation, Western blot, flow cytometry, patching of lipid rafts, neutrophil polarization, activation of SFKs or p38 MAPK by crosslinking PSGL-1 or CD44, neutrophil microparticle preparation, and statistical analysis are given in SI Materials and Methods.
SI Materials and Methods
Reagents.
Rat monoclonal antibodies (mAbs) to murine PSGL-1 (clone 4RA10), CD44 (clone KM114), CD45 (clone 30-F11), and L-selectin (clone Mel-14), murine mAb to flotillin-1 (clone 18/flotillin-1, crossreacts with human and murine flotillin), CD71 (clone 2/transferrin), and L-selectin (clone DREG-56), murine mAb to moesin (clone 38/moesin, crossreacts with human and murine moesin), R-phycoerythrin (PE)-labeled rat mAb to murine PSGL-1 (clone 2PH1), and fluorescein isothiocyanate (FITC)-labeled goat polyclonal antibody to rat IgG were from BD Biosciences. Goat polyclonal antibody to murine CD43 was from Santa Cruz Biotechnology. Sheep polyclonal antibody to mouse siglec E was prepared as described previously (58). Rabbit mAb to murine Src (clone 36D10), rabbit mAb to murine phospho-Src (Tyr-416, clone D49G4), and rabbit polyclonal antibody to murine phospho-p-38 MAPK (T180/Y182) were from Cell Signaling. Rabbit polyclonal antibody to murine p-38 MAPK was from BioLegend. Alexa Fluor 647-conjugated goat polyclonal antibody to rat IgG, donkey polyclonal antibody to goat IgG, donkey polyclonal antibody to sheep IgG, and Alexa Fluor 488-conjugated cholera toxin subunit B (CTxB) were from Life Technologies. Goat polyclonal antibody to cholera toxin subunit B was from Calbiochem. Horseradish peroxidase-conjugated anti-mouse IgG, anti-rat IgG, anti-rabbit IgG, anti-goat IgG, and anti-sheep IgG were from Thermo Scientific. Percoll was from GE Healthcare Life Sciences. Filipin III was from Cayman Chemical. α2–3,6,8-Sialidase from Arthrobacter ureafaciens was from New England BioLabs or Sigma, and α2–3,6,8-sialidase from Vibrio cholerae was from Roche Lifescience. FITC-conjugated peanut agglutinin was from Vector Laboratories. Complete protease inhibitor mixture was from Roche. OptiPrep density gradient, methyl-β-cyclodextrin (mβCD), α-cyclodextrin (αCD), A23187, fibronectin, PKH67 green fluorescent cell linker kit, and poly-l-lysine were from Sigma Aldrich. Recombinant murine CXCL1 was from R&D Systems.
Mice.
EHC C1galt1−/− mice (59), Selplg−/− mice (60), and Hck/Fgr/Lyn-deficient (SFK−/−) mice (61) were of mixed genetic background (129S1/SvIm and C57BL/6J). Littermate controls were used. All experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Cells.
Transfected Chinese hamster ovary (CHO) cells expressing core 2 GlcNAcT-I, FucT-VII, and WT or mutant murine PSGL-1 were generated as previously described (62).
Isolation of Murine Neutrophils from Bone Marrow.
Murine bone marrow leukocytes, isolated as described (60), were suspended in 45% (vol/vol) Percoll. Percoll concentrations of 81%, 62%, 55%, and 50% were layered from the bottom sequentially. The cells were laid on the top of the four-layer Percoll gradient. After centrifugation (1,200 × g, 30 min, room temperature, no braking), neutrophils at the interface between 62% and 81% Percoll solutions were collected. The cells were washed twice with Hank’s balanced salt solution (HBSS) without Ca2+ and Mg2+ and then suspended in the indicated buffer for experiments.
Detergent-Resistant Membrane Preparation.
Membranes were isolated as described previously (63). Briefly, chilled murine neutrophils or transfected CHO cells were lysed with 1% Triton X-100. After homogenization and centrifugation, the cell lysate was mixed with OptiPrep solution to a final concentration of 40% (vol/vol). This mixture was put at the bottom of a 2.4-mL centrifuge tube. On the top of the mixture, OptiPrep solutions at concentrations of 30%, 20%, and 5% were layered sequentially. After centrifugation at 100,000 × g for 4 h at 4 °C, nine equal fractions were collected from top to bottom of the tube. In some experiments, neutrophils were preincubated with 10 units/mL α2–3,6,8-sialidase from A. ureafaciens at 37 °C for 1 h before lysis.
Western Blot.
Western blots were performed as described previously (60).
Flow Cytometry.
Flow cytometry was performed as described previously (60). Cholesterol in the plasma membrane of neutrophils was detected by binding of filipin as described (35). Briefly, after fixation with 2% paraformaldehyde, neutrophils were washed, stained with 50 μg/mL filipin III at room temperature for 2 h, and then immediately analyzed by flow cytometry. In some experiments, cells were treated with mβCD or αCD before fixation.
Patching of Lipid Rafts.
Patching was performed as described previously (3). Briefly, neutrophils were incubated with Alexa 488-conjugated CTxB in PBS with 0.1% BSA for 30 min on ice. Cells were washed at 4 °C and incubated with polyclonal antibody to CTxB for 30 min on ice. To patch lipid rafts, the cells were incubated at 37 °C for 15 min and then fixed with 2% paraformaldehyde in PBS at room temperature. After fixation, neutrophils were stained with primary antibodies to the indicated membrane proteins and with Alexa 647-conjugated secondary antibodies. Immunofluorescence was detected with a confocal laser-scanning microscope equipped with an argon/krypton laser light (LSM 510, Zeiss). In some experiments, neutrophils were preincubated with 10 units/mL α2–3,6,8-sialidase from A. ureafaciens at 37 °C for 1 h before patching.
Neutrophil Polarization.
Neutrophils were plated on fibronectin-coated coverslips in the presence or absence of 20 ng/mL CXCL1 at 37 °C for 30 min (19). The polarized cells were fixed with 2% (wt/vol) paraformaldehyde at room temperature for 10 min. The fixed cells were stained with Alexa 488-conjugated CTxB and primary antibodies to indicated proteins, followed by Alexa 647-conjugated secondary antibodies. Immunofluorescence was detected with a confocal laser-scanning microscope equipped with an argon/krypton laser light (LSM 510, Zeiss).
Activation of SFKs or p38 MAPK by Crosslinking PSGL-1 or CD44.
F(ab′)2 fragments of anti–PSGL-1 mAb 4RA10, anti-CD44 mAb IM7, or isotype control rat IgG were generated with an F(ab′)2 preparation kit (Thermo Scientific). Fifty μg/mL F(ab′)2 were coated in 48-well plates overnight at 4 °C. The plates were blocked with 1% human serum albumin for 1 h at room temperature. Bone marrow neutrophils from WT or EHC C1galt−/− mice (5 × 106) in 200 μL HBSS with 0.5% human serum albumin were incubated in F(ab′)2-coated wells for 10 min at room temperature. In some experiments, neutrophils were preincubated with 0.6 units/mL α2–3,6,8-sialidase from A. ureafaciens and 5 μg/mL α2–3,6,8-sialidase from Vibrio cholerae in HBSS with 0.5% human serum albumin) at 37 °C for 1 h before incubation in F(ab′)2-coated wells. The cells were lysed in 1% Triton X-100, 125 mM NaCl, 50 mM Tris, pH 8.0, 10 mM EDTA, 2 mM PMSF, and 1/100 protease and phosphatase inhibitor mixture. The cell lysates were probed by Western blotting under reducing conditions with antibodies against SFK, phospho-SFK (Y416), p38, or phospho-p38.
Neutrophil Microparticle Preparation.
Neutrophil microparticles were prepared as described (18, 26), with minor modifications. Briefly, neutrophils isolated from murine bone marrow were labeled with PKH67 fluorescent membrane dye. The cells were incubated with 20 µg/mL F(ab′)2 fragments of anti–PSGL-1, anti-CD45, or isotype control mAb in RPMI 1640, 10% (vol/vol) FBS at 37 °C for 12 h. The cell suspension was centrifuged sequentially at 500 × g, 1,500 × g, and 5,000 × g to remove cells and debris. The supernatant was then spun at 20,000 × g for 20 min to pellet microparticles for quantification by flow cytometry. In some assays, neutrophils were stimulated with 20 µM calcium ionophore at 37 °C for 15 min before the microparticles were isolated and quantified.
Statistics.
Data are expressed as mean ± SEM. Comparisons used the Student’s t test (unpaired and two tailed). P < 0.05 was considered to be significant.
Acknowledgments
This work was supported by Grants HL085607 and HL034363 from the National Institutes of Health, WT103744MA from the Wellcome Trust, and HR11-42 from the Oklahoma Center for Advancement of Science and Technology.
Footnotes
Conflict of interest statement: R.P.M. has equity interest in Selexys Pharmaceuticals Corporation.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507712112/-/DCSupplemental.
References
- 1.Simons K, Gerl MJ. Revitalizing membrane rafts: New tools and insights. Nat Rev Mol Cell Biol. 2010;11(10):688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- 2.Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 3.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141(4):929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horejsi V, Hrdinka M. Membrane microdomains in immunoreceptor signaling. FEBS Lett. 2014;588(15):2392–2397. doi: 10.1016/j.febslet.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Levental I, Grzybek M, Simons K. Greasing their way: Lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49(30):6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 6.Potter BA, Hughey RP, Weisz OA. Role of N- and O-glycans in polarized biosynthetic sorting. Am J Physiol Cell Physiol. 2006;290(1):C1–C10. doi: 10.1152/ajpcell.00333.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 8.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lokeshwar VB, Bourguignon LY. Post-translational protein modification and expression of ankyrin-binding site(s) in GP85 (Pgp-1/CD44) and its biosynthetic precursors during T-lymphoma membrane biosynthesis. J Biol Chem. 1991;266(27):17983–17989. [PubMed] [Google Scholar]
- 11.Han H, et al. Comprehensive characterization of the N-glycosylation status of CD44s by use of multiple mass spectrometry-based techniques. Anal Bioanal Chem. 2012;404(2):373–388. doi: 10.1007/s00216-012-6167-4. [DOI] [PubMed] [Google Scholar]
- 12.McEver RP. Selectins: Lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14(5):581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 13.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201(8):1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yago T, et al. Core 1-derived O-glycans are essential E-selectin ligands on neutrophils. Proc Natl Acad Sci USA. 2010;107(20):9204–9209. doi: 10.1073/pnas.1003110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, et al. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J Biol Chem. 1996;271(11):6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- 16.Ellies LG, et al. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9(6):881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 17.Ju T, Brewer K, D’Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002;277(1):178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 18.Del Conde I, Shrimpton CN, Thiagarajan P, López JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 19.Miner JJ, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112(5):2035–2045. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neame SJ, Uff CR, Sheikh H, Wheatley SC, Isacke CM. CD44 exhibits a cell type dependent interaction with Triton X-100 insoluble, lipid rich, plasma membrane domains. J Cell Sci. 1995;108(Pt 9):3127–3135. doi: 10.1242/jcs.108.9.3127. [DOI] [PubMed] [Google Scholar]
- 21.Millán J, Montoya MC, Sancho D, Sánchez-Madrid F, Alonso MA. Lipid rafts mediate biosynthetic transport to the T lymphocyte uropod subdomain and are necessary for uropod integrity and function. Blood. 2002;99(3):978–984. doi: 10.1182/blood.v99.3.978. [DOI] [PubMed] [Google Scholar]
- 22.Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281(45):34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yago T, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling. Blood. 2010;116(3):485–494. doi: 10.1182/blood-2009-12-259556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced α(L)β(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26(6):773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarbock A, et al. PSGL-1 engagement by E-selectin signals through Src kinase Fgr and ITAM adapters DAP12 and FcR gamma to induce slow leukocyte rolling. J Exp Med. 2008;205(10):2339–2347. doi: 10.1084/jem.20072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hrachovinová I, et al. Interaction of P-selectin and PSGL-1 generates microparticles that correct hemostasis in a mouse model of hemophilia A. Nat Med. 2003;9(8):1020–1025. doi: 10.1038/nm899. [DOI] [PubMed] [Google Scholar]
- 27.Bruehl RE, et al. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leukoc Biol. 1997;61(4):489–499. doi: 10.1002/jlb.61.4.489. [DOI] [PubMed] [Google Scholar]
- 28.Alonso-Lebrero JL, et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95(7):2413–2419. [PubMed] [Google Scholar]
- 29.Serrador JM, et al. CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts. Blood. 1998;91(12):4632–4644. [PubMed] [Google Scholar]
- 30.Serrador JM, et al. A juxta-membrane amino acid sequence of P-selectin glycoprotein ligand-1 is involved in moesin binding and ezrin/radixin/moesin-directed targeting at the trailing edge of migrating lymphocytes. Eur J Immunol. 2002;32(6):1560–1566. doi: 10.1002/1521-4141(200206)32:6<1560::AID-IMMU1560>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Rossy J, Schlicht D, Engelhardt B, Niggli V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS ONE. 2009;4(4):e5403. doi: 10.1371/journal.pone.0005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135(6 Pt 1):1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauer S, et al. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J. 2000;19(14):3556–3564. doi: 10.1093/emboj/19.14.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramachandran V, et al. Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc Natl Acad Sci USA. 1999;96(24):13771–13776. doi: 10.1073/pnas.96.24.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassall DG, Graham A. Changes in free cholesterol content, measured by filipin fluorescence and flow cytometry, correlate with changes in cholesterol biosynthesis in THP-1 macrophages. Cytometry. 1995;21(4):352–362. doi: 10.1002/cyto.990210407. [DOI] [PubMed] [Google Scholar]
- 36.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lefort CT, Ley K. Neutrophil arrest by LFA-1 activation. Front Immunol. 2012;3:157. doi: 10.3389/fimmu.2012.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sánchez-Madrid F, Serrador JM. Bringing up the rear: Defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10(5):353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 39.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark MC, Baum LG. T cells modulate glycans on CD43 and CD45 during development and activation, signal regulation, and survival. Ann N Y Acad Sci. 2012;1253:58–67. doi: 10.1111/j.1749-6632.2011.06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Q, et al. PILRα and PILRβ have a siglec fold and provide the basis of binding to sialic acid. Proc Natl Acad Sci USA. 2014;111(22):8221–8226. doi: 10.1073/pnas.1320716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMillan SJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood. 2013;121(11):2084–2094. doi: 10.1182/blood-2012-08-449983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Shiratori I, Uehori J, Ikawa M, Arase H. Neutrophil infiltration during inflammation is regulated by PILRα via modulation of integrin activation. Nat Immunol. 2013;14(1):34–40. doi: 10.1038/ni.2456. [DOI] [PubMed] [Google Scholar]
- 45.Brinkman-Van der Linden EC, et al. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol. 2003;23(12):4199–4206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuroki K, et al. Structural basis for simultaneous recognition of an O-glycan and its attached peptide of mucin family by immune receptor PILRα. Proc Natl Acad Sci USA. 2014;111(24):8877–8882. doi: 10.1073/pnas.1324105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: Light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28(13):5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- 48.Rose MC, Voter WA, Sage H, Brown CF, Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984;259(5):3167–3172. [PubMed] [Google Scholar]
- 49.Alfalah M, et al. O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr Biol. 1999;9(11):593–596. doi: 10.1016/s0960-9822(99)80263-2. [DOI] [PubMed] [Google Scholar]
- 50.Castillon GA, Michon L, Watanabe R. Apical sorting of lysoGPI-anchored proteins occurs independent of association with detergent-resistant membranes but dependent on their N-glycosylation. Mol Biol Cell. 2013;24(12):2021–2033. doi: 10.1091/mbc.E13-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto M, et al. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175(12):8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 52.Alcaide P, et al. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127(8):1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 53.Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2012;110(2):356–369. doi: 10.1161/CIRCRESAHA.110.233403. [DOI] [PubMed] [Google Scholar]
- 54.Affentranger S, Martinelli S, Hahn J, Rossy J, Niggli V. Dynamic reorganization of flotillins in chemokine-stimulated human T-lymphocytes. BMC Cell Biol. 2011;12:28. doi: 10.1186/1471-2121-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ludwig A, et al. Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol. 2010;191(4):771–781. doi: 10.1083/jcb.201005140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinelli S, et al. Ezrin/Radixin/Moesin proteins and flotillins cooperate to promote uropod formation in T cells. Front Immunol. 2013;4:84. doi: 10.3389/fimmu.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baumann T, Affentranger S, Niggli V. Analysis of close associations of uropod-associated proteins in human T-cells using the proximity ligation assay. PeerJ. 2013;1:e186. doi: 10.7717/peerj.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34(4):1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 59.Fu J, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118(11):3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia L, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109(7):939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meng F, Lowell CA. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med. 1997;185(9):1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao B, et al. Signal-dependent slow leukocyte rolling does not require cytoskeletal anchorage of P-selectin glycoprotein ligand-1 (PSGL-1) or integrin αLβ2. J Biol Chem. 2012;287(23):19585–19598. doi: 10.1074/jbc.M112.361519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Setiadi H, McEver RP. Clustering endothelial E-selectin in clathrin-coated pits and lipid rafts enhances leukocyte adhesion under flow. Blood. 2008;111(4):1989–1998. doi: 10.1182/blood-2007-09-113423. [DOI] [PMC free article] [PubMed] [Google Scholar]