Significance
The dilution effect hypothesis suggests that diverse ecological communities limit disease spread via several mechanisms. Therefore, biodiversity losses could worsen epidemics that harm humans and wildlife. However, there is contentious debate over whether the hypothesis applies broadly, especially for parasites that infect humans. We address this fundamental question with a formal meta-analysis of >200 assessments relating biodiversity to disease in >60 host–parasite systems. We find overwhelming evidence of dilution, which is independent of host density, study design, and type and specialization of parasites. A second analysis identified similar effects of diversity in plant–herbivore systems. Thus, biodiversity generally decreases parasitism and herbivory. Consequently, human-induced declines in biodiversity could increase human and wildlife diseases and decrease crop and forest production.
Keywords: biodiversity, parasitism, dilution effect, associational resistance, meta-analysis
Abstract
Infectious diseases of humans, wildlife, and domesticated species are increasing worldwide, driving the need to understand the mechanisms that shape outbreaks. Simultaneously, human activities are drastically reducing biodiversity. These concurrent patterns have prompted repeated suggestions that biodiversity and disease are linked. For example, the dilution effect hypothesis posits that these patterns are causally related; diverse host communities inhibit the spread of parasites via several mechanisms, such as by regulating populations of susceptible hosts or interfering with parasite transmission. However, the generality of the dilution effect hypothesis remains controversial, especially for zoonotic diseases of humans. Here we provide broad evidence that host diversity inhibits parasite abundance using a meta-analysis of 202 effect sizes on 61 parasite species. The magnitude of these effects was independent of host density, study design, and type and specialization of parasites, indicating that dilution was robust across all ecological contexts examined. However, the magnitude of dilution was more closely related to the frequency, rather than density, of focal host species. Importantly, observational studies overwhelmingly documented dilution effects, and there was also significant evidence for dilution effects of zoonotic parasites of humans. Thus, dilution effects occur commonly in nature, and they may modulate human disease risk. A second analysis identified similar effects of diversity in plant–herbivore systems. Thus, although there can be exceptions, our results indicate that biodiversity generally decreases parasitism and herbivory. Consequently, anthropogenic declines in biodiversity could increase human and wildlife diseases and decrease crop and forest production.
Human activities are dramatically reducing biodiversity (1), and the frequency and severity of infectious disease outbreaks in human, wildlife, and domesticated species are increasing (2–5). These concurrent patterns have prompted suggestions that biodiversity and the spread of diseases may be causally linked. For example, the dilution effect hypothesis proposes that diverse host communities inhibit the abundance of parasites through several mechanisms, such as regulating populations of susceptible hosts or interfering with the transmission process (6–8). Thus, diverse communities may inhibit the proliferation of parasites, thereby promoting the stability of ecological communities and ecosystem services (e.g., nutrient cycling, carbon sequestration, and natural product production) (9).
Understanding the generality of these dilution effects is crucial for projections of future disease outbreaks, which can threaten human health, species conservation, and ecosystem services (3, 9). If biodiversity generally inhibits parasites, then human-driven biodiversity loss could exacerbate disease risk for humans and wildlife. Biodiversity conservation might then limit the abundance of many parasites of wildlife and humans (10–12). However, if parasites are unaffected or even stimulated by host biodiversity, then human-mediated biodiversity loss might decrease the risk of outbreaks, and disease management approaches based on biodiversity could backfire (13).
There is support for dilution effects and the key underlying mechanisms in some systems (8, 14–16). Despite this, the generality of the dilution effect hypothesis remains contentiously debated (13, 17, 18). For example, parasite dynamics may be driven by the identity of the particular species present, rather than diversity per se (17). In addition, some undisturbed habitats (e.g., intact forests) can contain higher densities of parasites or vectors than disturbed sites (13, 18). However, such comparisons can inappropriately equate habitat disturbance with biodiversity loss while ignoring other confounding factors (19).
We addressed the generality of the dilution effect hypothesis with a formal meta-analysis. We searched the published literature for all available data sources, including experimental and observational studies of human and wildlife diseases, to rigorously assess the generality of this phenomenon. We estimated the effect of biodiversity on parasite abundance using the Hedges’ g effect size (thus negative values indicate dilution effects) and used a multilevel model to include nonindependence among effect sizes that arise from the same parasite species or experiments. Last, we compared the evidence for dilution effects with the evidence for associational resistance, an analogous hypothesis that posits that plant diversity inhibits the abundance of herbivores via mechanisms similar to those hypothesized to drive dilution effects (20). If both of these natural enemies are inhibited by host/plant diversity, then biodiversity might limit the growth and potential impact of natural enemies generally (e.g., predators, parasitoids, etc.), increasing the production and stability of diverse communities (9, 21).
Results and Discussion
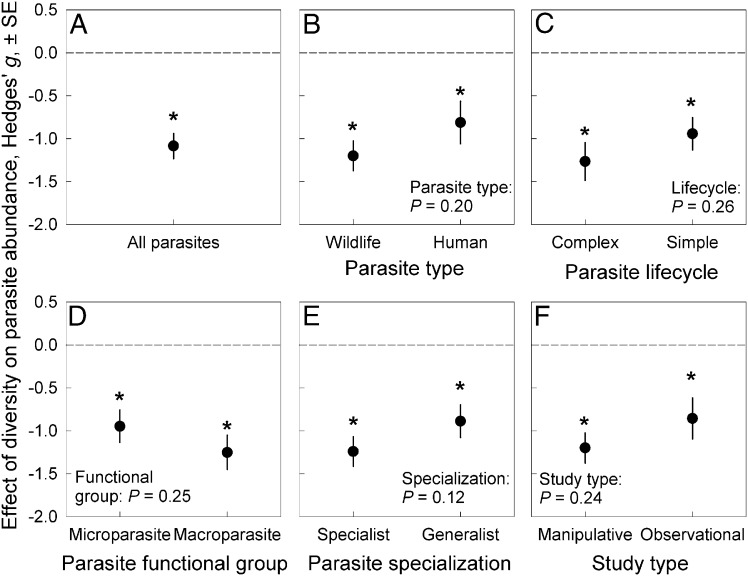
First, we tested whether host diversity generally inhibits the abundance of parasites (e.g., infection prevalence for microparasites, mean parasite load for macroparasites, density of infected vectors for vector-borne parasites, or percent diseased tissue for plant parasites). Our literature search on biodiversity and parasitism yielded 202 effect sizes from 61 parasite species (Dataset S1). Our dataset contained 47 species that exclusively infect wildlife hosts and 14 species that also infect humans. Most (168) effect size estimates arose from manipulative experiments, and 34 were derived from field observations. Species richness of the host community (including the focal host species and co-occurring or added species), the dominant index of biodiversity, ranged from 1 to 32 in experiments and 1 to 757 in observational studies. Overall, the average Hedges’ g for the effect of host diversity on parasite abundance was g = –1.08 (±0.15 SE; P < 0.0001; Fig. 1A). Thus, the mean relationship between biodiversity and parasite abundance is significantly negative. Further, the magnitude of this effect size represents strong evidence for the dilution effect across host–parasite systems based on traditional categorizations of Hedges’ g (22).
Fig. 1.
Results of the meta-analysis of the generality of the dilution effect hypothesis. (A) Overall, there was a strong negative relationship between host diversity and parasite abundance. (B–F) The strength of dilution effects did not differ between (B) parasites that infect only wildlife and those that infect humans, (C) parasites with complex and simple lifecycles, (D) microparasites and macroparasites, (E) specialist and generalist parasites, or (F) manipulative and observational studies. Despite this lack of differences between groups, all groups exhibited significant evidence for the dilution effect [asterisks indicate significant (P < 0.05) differences from zero]. Error bars represent ± SE.
Second, we tested whether several ecological factors could explain variation in the effect of host diversity on parasite abundance. Specifically, we tested whether the strength of dilution effects differed between (i) parasites that infect humans vs. wildlife, (ii) macro- vs. microparasites, (iii) parasites with complex vs. direct lifecycles, (iv) observational vs. manipulative studies, and (v) specialist parasites vs. those able to infect the multiple host species present. We then conducted a model selection analysis allowing for all possible combinations of main effects and two-way interactions among these factors. We found no evidence that the magnitude of dilution depended on any of these factors (Fig. 1 B–F) or their interactions (Table S1). Thus, dilution effects were generally robust across all ecological contexts examined.
Table S1.
Results of the model selection analysis for factors that could modulate the effect of biodiversity on parasite spread in the meta-analysis
| Rank | Terms*,† | Parameters‡ | AICc | ΔAICc | Model weight |
| 1 | Specialist | 5 | 620.1 | 0 | 0.055 |
| 2 | Design*Lifecycle | 7 | 620.1 | 0.03 | 0.054 |
| 3 | (Intercept) | 4 | 620.2 | 0.12 | 0.052 |
| 4 | Human | 5 | 620.7 | 0.66 | 0.040 |
| 5 | Specialist + Lifecycle | 6 | 620.8 | 0.73 | 0.038 |
| 6 | Specialist*Lifecycle | 7 | 621.1 | 0.99 | 0.034 |
| 7 | Functional_group | 5 | 621.1 | 1.02 | 0.033 |
| 8 | Design*Specialist + Design*Lifecycle | 9 | 621.2 | 1.1 | 0.032 |
| 9 | Human + Lifecycle | 6 | 621.3 | 1.2 | 0.030 |
| 10 | Specialist + Human | 6 | 621.6 | 1.6 | 0.025 |
| 11 | Design*Lifecycle + Human | 8 | 621.7 | 1.6 | 0.024 |
| 12 | Specialist + Functional_group | 6 | 621.9 | 1.8 | 0.021 |
| 13 | Design + Specialist | 6 | 622.1 | 2.0 | 0.020 |
| 14 | Design*Lifecycle + Functional_group | 8 | 622.1 | 2.1 | 0.020 |
| 15 | Design*Specialist | 7 | 622.1 | 2.6 | 0.020 |
| 16 | Specialist + Human + Lifecycle | 7 | 622.2 | 2.1 | 0.019 |
| 17 | Specialist*Lifecycle + Human | 8 | 622.7 | 2.6 | 0.015 |
| 18 | Specialist*Human | 7 | 622.7 | 2.7 | 0.015 |
| 19 | Design + Specialist + Lifecycle | 7 | 622.8 | 2.7 | 0.014 |
| 20 | Human*Lifecycle | 7 | 622.8 | 2.8 | 0.014 |
AICc, Akaike information criterion.
Description of model terms: Design, manipulative vs. observational; Functional_group, microparasite vs. macroparasite; Human, infects humans vs. infects only wildlife; Lifecycle, simple vs. complex; Specialist, specialist vs. generalist.
An interaction term, for example, Specialist*Design, indicates that the main effects and the interaction are present in the model. Thus, an interaction term contributes three parameters to a model.
All models also contain three variance components (for parasite species, experiment, and effect size). Thus, the “intercept-only” model contains four parameters, the fewest among all models under consideration.
Although the strength of dilution effects did not differ among any of the contrasts that we tested (Fig. 1 B–F), each group yielded strong evidence for the dilution effect in these univariate contrasts. Two critical results emerged. First, observational studies yield a strongly negative mean effect of biodiversity on disease. Thus, dilution is commonly observed in natural systems. Second, there is strong evidence for the dilution effect for parasites that infect only wildlife and those that also infect humans (vector-borne and non–vector-borne zoonotic parasites; Fig. 1B). This suggests that human-induced biodiversity declines may generally increase the abundance of zoonotic and vector-borne parasites and consequently increase human disease risk. Therefore, biodiversity conservation may generally provide a concomitant benefit of reducing human disease risk. This result contradicts an earlier meta-analysis that suggested an idiosyncratic effect of biodiversity on human parasites (23). However, the previous meta-analysis only included field studies of six zoonotic human parasites and thus incorporated many fewer effect sizes and parasite species. Consequently, the authors of this previous meta-analysis acknowledged low statistical power to detect a relationship between biodiversity and human disease risk (19). Given that this prior meta-analysis focused only on very few human parasites, its results should not be extrapolated broadly.
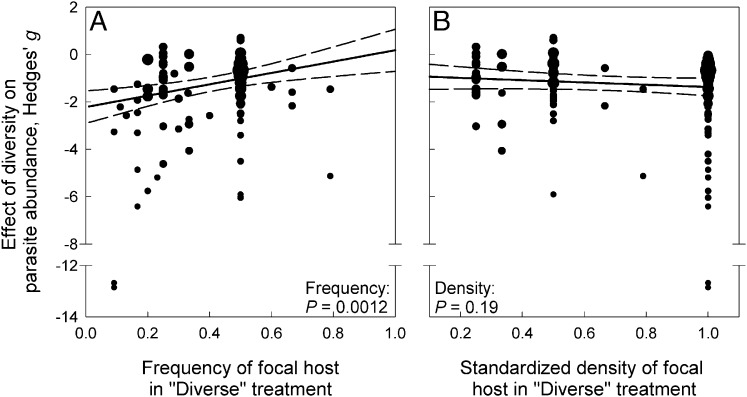
Compared with single-species populations, diverse communities can reduce the frequency (i.e., relative abundance or proportion) and/or density of focal hosts. Therefore, we next tested whether the strength of dilution effects in manipulative experiments was related to focal host frequency or density. We used a subset of our database (118 effect sizes from 19 parasite species) that included all experiments that manipulated the density or frequency of focal hosts in monospecific “control” treatments contrasted with one or more “diverse” treatments to test whether focal host density or frequency could explain variation in dilution effects. We predicted that dilution effects would be largest when focal hosts were reduced to low frequencies or densities. Indeed, dilution effects were significantly stronger at low host frequencies (Fig. 2A). However, the strength of dilution was unrelated to the standardized density of focal hosts (Fig. 2B). Thus, diverse communities seem to most strongly inhibit parasites when they reduce the frequency, rather than density, of key host species. Consequently, biodiversity management programs may most efficiently reduce disease risk by minimizing the frequency of highly competent hosts even without reducing the absolute density of such hosts.
Fig. 2.
Results of the metaregressions relating the strength of diversity to the (A) frequency and (B) density of focal host individuals in the experimental communities. (A) Dilution effects were significantly stronger (i.e., more negative Hedges’ g) when the frequency of focal hosts was lower in the diverse treatments. (B) In contrast, the strength of dilution was unrelated to the density of focal hosts. Solid lines indicate the fit of a multiple linear regression model while holding the other factor constant at its mean value. Dashed lines indicate the 95% confidence interval for these model fits.
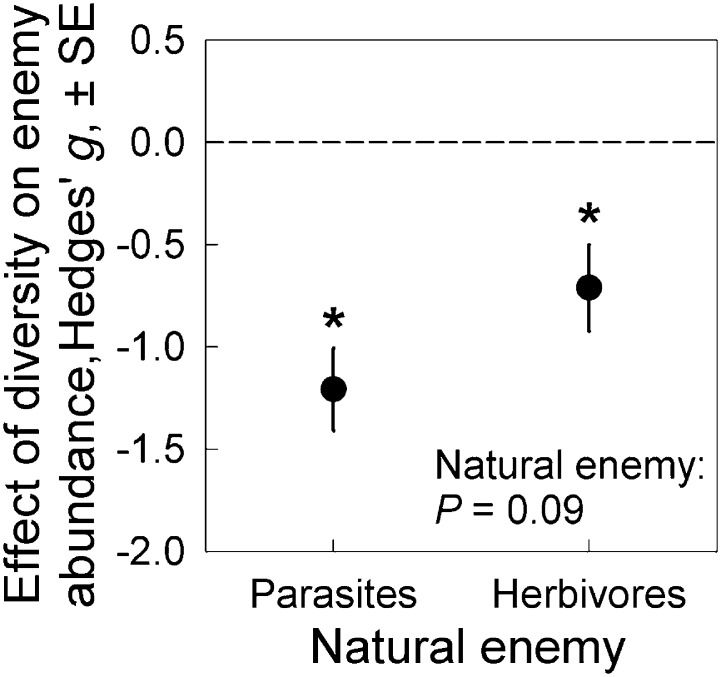
Finally, we incorporated effect sizes from two recent meta-analyses related to the associational resistance hypothesis in plant–herbivore systems (20, 24) to test whether the strength of dilution effects differed between herbivores and parasites. We independently confirmed 136 effect sizes from 39 herbivore taxa (reports on 32 individual species and 7 broader taxonomic/functional groups; Dataset S2). The average effect sizes for parasites and herbivores were g = −1.21 (±0.20 SE; P < 0.001) and −0.71 (±0.21 SE; P = 0.001), respectively (Fig. 3). There was no difference in the strength of dilution effects for parasites and associational resistance for herbivores (P = 0.09). Thus, there is broad evidence that biodiversity inhibits the abundance of both types of natural enemies. Similar inhibitory effects of host/prey diversity might then be likely for other types of natural enemies, such as predators or parasitoids.
Fig. 3.
Results of the meta-analysis of dilution effects in host–parasite and plant–herbivore systems. Both natural enemy types were significantly inhibited by host diversity [asterisks indicate significant (P < 0.05) differences from zero]. Further, there was no significant difference in the strength of dilution effects between parasites and herbivores. Error bars represent ± SE.
Our meta-analysis revealed strong, widespread inhibition of both parasites and herbivores by diverse host communities (Fig. 3). However, biodiversity does not inhibit every enemy in every system. As an example, in a grassland biodiversity experiment, most foliar parasites significantly decreased with increasing plant diversity, but a fungal leaf spot on one plant species increased with diversity (25). There could be general ecological scenarios in which positive diversity–disease relationships might arise. For example, during succession or restoration, host species must establish in a community before their parasites can invade (26). More broadly, theoretical models suggest that the effect of biodiversity on disease depends on the traits of hosts, parasites, and additional species (27, 28). However, our meta-analysis detected dilution effects robustly across variation in study design, resistance traits of diluting hosts, host range of parasites (human vs. wildlife), and parasite lifecycle and type (macro- vs. microparasite; Fig. 1). Thus, although there can be exceptions, our results support the generality of the dilution effect hypothesis.
Our results highlight the need to move beyond debates over the generality of the dilution effect and toward a mechanistic, predictive framework for biodiversity–disease interactions. Whereas our meta-analysis suggests that the magnitude of dilution is generally related to the frequency, rather than density, of focal host individuals (Fig. 2), it was not designed to directly assess the specific mechanisms driving dilution or amplification. Thus, we call for increased focus on the mechanisms causing diversity–disease patterns. For example, nonfocal host species can inhibit disease spread by regulating host populations via competition or predation, interfering with the transmission process, or altering host behavior (6, 8). Theoretical models can generally delineate which mechanisms can promote or inhibit disease (27, 28), but experimental tests that assess the relative importance of these mechanisms, their generality across disease systems, and their dependence on temporal and spatial scales remain scarce. These open issues represent increasingly important intersections among ecology, conservation science, and epidemiology.
We also call for more ecologically relevant experimental tests of the dilution effect. Our meta-analysis included 168 effect sizes from manipulative experiments relevant to the dilution effect. A slight majority, 89 (53%), of these effect sizes compare infection risk for a single focal host species with risk in the presence of one additional species. Such studies risk confounding the effect of species diversity with that of the particular species added, a criticism of the dilution effect literature (17). However, several manipulative studies in our meta-analysis directly tested the relationship between biodiversity and parasite abundance over larger gradients of diversity [44 of these 168 effect sizes (26%) used regression-based designs, including numerous combinations of 1–32 species communities]. Therefore, we assessed the robustness of empirical evidence for the dilution effect by reanalyzing only those 44 empirical studies that spanned a gradient of diversity. These empirical studies strongly support the dilution effect hypothesis (g = −0.75 ± 0.13 SE; P < 0.0001). Importantly, these regression-based studies yield effect sizes that are interpretable as standardized slopes relating parasite abundance to host diversity along these broader gradients. Hence, the significantly negative effect sizes represent a broad negative effect of host diversity on parasite abundance across realistic diversity gradients. Thus, support for the dilution effect hypothesis does not rest on comparisons between single- and two-species communities. The continued use of more realistic, species-rich communities will greatly enhance the ecological relevance of manipulative tests of the dilution effect (25), especially if they are assembled (or disassembled) in ecologically realistic sequences (14).
Understanding the impact of biodiversity on outbreaks of natural enemies is critical because human activities are dramatically reducing biodiversity (1) and outbreaks are increasing (2–5). This meta-analysis provides broad quantitative evidence that diverse communities inhibit the proliferation of parasites and herbivores, two major classes of natural enemies, in both experiments and field surveys. These broad patterns suggest that further human-mediated reductions in biodiversity could exacerbate pest outbreaks and impair human health, species conservation, and the stability and function of ecosystems. However, these results also suggest that biodiversity conservation may yield a promising strategy to minimize pest outbreaks and mitigate these consequences. Nonetheless, a greater understanding of the mechanisms underlying dilution effects is still needed to maximize the chances of success for control programs designed for specific parasites or herbivores.
Materials and Methods
Data Compilation.
We sought to analyze all studies that examined the relationship between host diversity and the abundance of parasites [defined functionally (29)]. We located studies in the Web of Science by searching for several combinations of search terms: parasite, pathogen, diversity, richness, evenness, dilution effect, and decoy effect (the final search was conducted in October 2014). We identified additional papers by searching the literature-cited sections of these articles, as well as those of reviews and meta-analyses of related topics (10, 13, 23). We included studies that performed surveys or experiments in the field as well as laboratory or mesocosm experiments.
Selection Criteria and Data Collection.
We only included studies that presented a measure of parasite abundance and a measure of host biodiversity. Specifically, we only included studies that reported infection prevalence, mean parasite load, density of infected vectors, or percent diseased tissue because these quantities are the most relevant metrics of disease risk for microparasites, macroparasites, vector-borne parasites, and plant parasites, respectively. Overwhelmingly, host biodiversity was reported as species richness, but occasionally a measure of evenness was reported. For experimental studies, species richness includes all taxa added by the experimenters. In observational studies, species richness corresponds to a focal taxonomic or functional group of host species as defined in the primary study (e.g., herbaceous plants, trees, birds, or small mammals).
We extracted data from text and tables manually and from figures using Plot Digitizer version 2.6.6 (plotdigitizer.sourceforge.net). In addition, we recorded other data relating to the biology or methodology of each study. For all studies, we recorded parasite and host taxa, type of parasite (infecting only wildlife or also infecting humans), focal host species, associated species (i.e., additional species whose presence may dilute or amplify parasite abundance, operationally defined as “potential diluters”), the diversity (e.g., richness) in the treatments (or in the field survey), parasite functional group (macroparasite vs. microparasite), parasite lifecycle (complex vs. direct), parasite specialization (infecting only a focal host vs. capable of also infecting the associated, potential diluter species), and study design (manipulative vs. observational). For manipulative experiments that compared individual monospecific control treatments with one or more diverse treatments, we also recorded the frequency and standardized density of individuals of the focal host species in the diverse treatments. We defined standardized density as the density of focal hosts in a diverse treatment divided by their density in the monospecific control treatment. Some of these frequency and density data were unavailable for some effect sizes, which we omitted from that particular analysis.
Effect Sizes.
Meta-analyses must be performed using a common measure of the relationship in question for all included studies, an effect size (30). We used Hedges’ g statistic as our measure of effect size. Most of the experiments in our analysis compared enemy abundance between a monospecific control treatment and one or more diverse treatments. For these studies, we directly calculated Hedges’ g statistic, a standardized measure of effect size:
| [1] |
where is the mean enemy abundance in the diverse treatment, is the mean abundance in the control treatment, s is the pooled SD, and J is a small-sample correction factor that reduces bias (30). When experiments or field surveys related diversity to disease using linear regression, we converted the correlation coefficient to g using standard equations (30). In a few cases, we refit linear regressions to datasets when the original publications only reported nonlinear regressions. Negative values of g indicate reduced parasite abundance in diverse host communities, consistent with the dilution effect hypothesis. In contrast, positive values represent a positive relationship between host diversity and disease.
Meta-Analytical Models.
We analyzed these data with a multilevel random-effects model (28). Our compilation of effect sizes showed a clear hierarchical structure; there were multiple effect sizes for some experiments and some parasite species. Ignoring the nonindependence among effect sizes induced by this structure exaggerates the information content of the primary data, leading to biased results and increased risk of type I error (28–30). We accounted for the nonindependence among some effect sizes by specifying additional random effects for enemy species and experiments as well as the estimated sampling covariance between effect sizes that shared some experimental units (e.g., when two diverse treatments within a study could be compared against the same control). We conducted this analysis using the rma.mv function in the metafor package in the R statistical computing language (31). We did not use funnel plots or rank correlation tests to assess publication bias in this dataset, because these analyses are invalidated by the presence of underlying heterogeneity among effect sizes, for example, random effects of species and experiments described above (32).
Using the model described above, we first tested for the overall relationship between host diversity and parasite abundance using the entire parasite database. Next, we tested for context dependence in the magnitude of dilution effects. We conducted a model selection analysis in which we fit all possible combinations and two-way interactions among five binary factors that might explain variation in the effects of diversity on parasite abundance: parasite functional group (macroparasite vs. microparasite), parasite lifecycle (complex vs. direct), parasite specialization (infecting only a focal host vs. capable of also infecting the associated potential diluter species), study design (manipulative vs. observational), and parasite type (infects humans vs. infects only wildlife). We omitted a small number of the possible models because they could not be fit due to multicollinearity among some predictors. We obtained the top 20 models (according to the corrected Akaike information criterion) from this set using a genetic algorithm implemented with the glmulti package in R (33).
We then tested whether the effect of biodiversity on parasites was related to the frequency or density of focal hosts in diverse communities. Using a subset of the parasite database, manipulative experiments that reported the density and frequency of focal hosts in control and diverse treatments, we fit a metaregression model containing both terms and their interaction but removed the interaction term because it was nonsignificant (P = 0.35). We plotted the predicted values from the resulting metaregression against the observed effect size estimates and rescaled the size of each data point according to its sampling variance.
We also assessed the robustness of empirical evidence for the dilution effect hypothesis by reanalyzing only those 44 effect sizes that arose from empirical studies that spanned a gradient of host diversity using a regression-based design. Using this subset of the data, we fit a meta-analysis model as above with an intercept only to characterize the mean effect size from these 44 studies.
Comparison with Herbivores.
We independently confirmed 136 effect size estimates on 39 herbivore taxa relating herbivore abundance to plant biodiversity in experimental or observational plots from two recent meta-analyses of the associational resistance hypothesis (10, 21). In 32 cases, herbivore taxa were reported as individual species, and in 7 cases they were reported as larger taxonomic/functional groups (e.g., leaf rollers, lepidopterans, or insects). We tested for differences in dilution effects in parasite and herbivore systems using a multilevel random-effects model with a single fixed factor (natural enemy: parasite vs. herbivore) and random effects and estimates of sampling variance/covariance as described above.
Supplementary Material
Acknowledgments
We thank Marc Lajeunesse for statistical advice and acknowledge funding from the National Science Foundation (Grant EF-1241889 to J.R.R. and Predoctoral Fellowship 1144244 to C.N.O.), National Institutes of Health (Grant R01GM109499 to J.R.R. and Postdoctoral Fellowship F32AI112255 to D.J.C.), US Department of Agriculture (Grants NRI 2006-01370 and 2009-35102-0543 to J.R.R.), and US Environmental Protection Agency (Grant CAREER 83518801 to J.R.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 8523.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506279112/-/DCSupplemental.
References
- 1.Mace GM, Masundire H, Baillie JEM. Biodiversity. In: Hassan R, Scholes R, Ash N, editors. Ecosystems and Human Well-Being: Current State and Trends. Vol 1. Island; Washington, DC: 2005. pp. 77–122. [Google Scholar]
- 2.Harvell D, et al. The rising tide of ocean diseases: Unsolved problems and research priorities. Front Ecol Environ. 2004;2(7):375–382. [Google Scholar]
- 3.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafferty K, Porter J, Ford S. Are diseases increasing in the ocean? Annu Rev Ecol Evol Syst. 2004;35:31–54. [Google Scholar]
- 5.Mas-Coma S, Valero MA, Bargues MD. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet Parasitol. 2009;163(4):264–280. doi: 10.1016/j.vetpar.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Johnson PTJ, Thieltges DW. Diversity, decoys and the dilution effect: How ecological communities affect disease risk. J Exp Biol. 2010;213(6):961–970. doi: 10.1242/jeb.037721. [DOI] [PubMed] [Google Scholar]
- 7.Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Annu Rev Ecol Evol Syst. 2012;43:157–182. [Google Scholar]
- 8.Keesing F, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468(7324):647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd IL, Freer-Smith PH, Gilligan CA, Godfray HCJ. The consequence of tree pests and diseases for ecosystem services. Science. 2013;342(6160):1235773. doi: 10.1126/science.1235773. [DOI] [PubMed] [Google Scholar]
- 10.Cardinale BJ, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486(7401):59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- 11.Ostfeld R, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool. 2000;78(12):2061–2078. [Google Scholar]
- 12.Ostfeld RS, Keesing F. Biodiversity and disease risk: The case of Lyme disease. Conserv Biol. 2000;14(3):722–728. [Google Scholar]
- 13.Wood CL, et al. Does biodiversity protect humans against infectious disease? Ecology. 2014;95(4):817–832. doi: 10.1890/13-1041.1. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PTJ, Preston DL, Hoverman JT, Richgels KLD. Biodiversity decreases disease through predictable changes in host community competence. Nature. 2013;494(7436):230–233. doi: 10.1038/nature11883. [DOI] [PubMed] [Google Scholar]
- 15.Venesky MD, Liu X, Sauer EL, Rohr JR. Linking manipulative experiments to field data to test the dilution effect. J Anim Ecol. 2013;83(3):557–565. doi: 10.1111/1365-2656.12159. [DOI] [PubMed] [Google Scholar]
- 16.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100(2):567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randolph SE, Dobson ADM. Pangloss revisited: A critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology. 2012;139(7):847–863. doi: 10.1017/S0031182012000200. [DOI] [PubMed] [Google Scholar]
- 18.Wood CL, Lafferty KD. Biodiversity and disease: A synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol Evol. 2013;28(4):239–247. doi: 10.1016/j.tree.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Ostfeld RS, Keesing F. Straw men don’t get Lyme disease: Response to Wood and Lafferty. Trends Ecol Evol. 2013;28(9):502–503. doi: 10.1016/j.tree.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa P, et al. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu Rev Ecol Evol Syst. 2009;40:1–20. [Google Scholar]
- 21.Letourneau DK, et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol Appl. 2011;21(1):9–21. doi: 10.1890/09-2026.1. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 23.Salkeld DJ, Padgett KA, Jones JH. A meta-analysis suggesting that the relationship between biodiversity and risk of zoonotic pathogen transmission is idiosyncratic. Ecol Lett. 2013;16(5):679–686. doi: 10.1111/ele.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jactel H, Brockerhoff EG. Tree diversity reduces herbivory by forest insects. Ecol Lett. 2007;10(9):835–848. doi: 10.1111/j.1461-0248.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell CE, Tilman D, Groth JV. Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology. 2002;83(6):1713–1726. [Google Scholar]
- 26.Huspeni TC, Lafferty KD. Using larval trematodes that parasitize snails to evaluate a saltmarsh restoration project. Ecol Appl. 2004;14(3):795–804. [Google Scholar]
- 27.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9(4):485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 28.Rohr JR, et al. Predator diversity, intraguild predation, and indirect effects drive parasite transmission. Proc Natl Acad Sci USA. 2015;112(10):3008–3013. doi: 10.1073/pnas.1415971112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafferty KD, Kuris AM. Trophic strategies, animal diversity and body size. Trends Ecol Evol. 2002;17(11):507–513. [Google Scholar]
- 30.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. John Wiley & Sons; Hoboken, NJ: 2011. [Google Scholar]
- 31.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 32.Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calcagno V, de Mazancourt C. glmulti: An R package for easy automated model selection with (generalized) linear models. J Stat Softw. 2010;34(12):1–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.