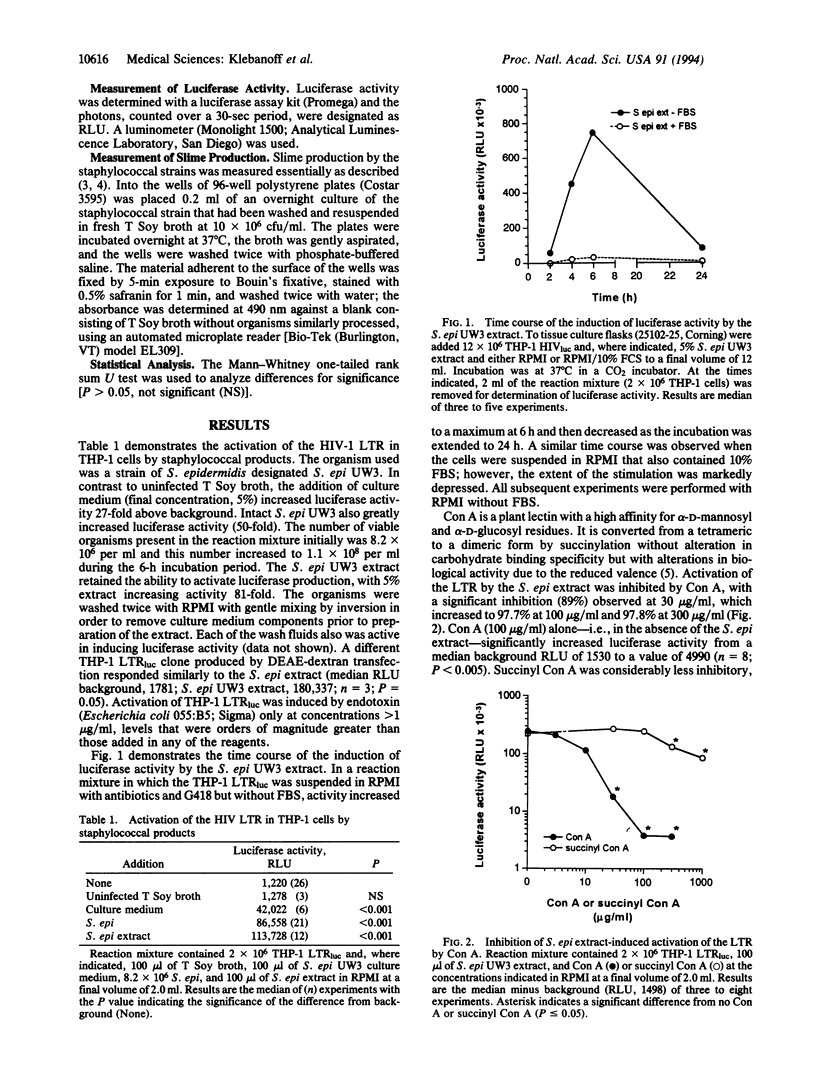
Abstract
Staphylococcal strains can release a factor that strongly activates the human immunodeficiency virus type 1 (HIV-1) long terminal repeat (LTR) in THP-1 cells transfected with the HIV-1 LTR-driven luciferase reporter gene (THP-1 LTRluc). The factor is present in the overnight culture fluid and is readily released from the organisms into aqueous medium by vigorous mixing. Staphylococcal extracellular material is a complex mixture of polysaccharide and protein containing peptidoglycan and teichoic acid, released in part by cell wall turnover. The importance of the carbohydrate component is emphasized by concanavalin A (Con A) inhibition of staphylococcal product-induced LTR activation but not of activation by phorbol 12-myristate 13-acetate or tumor necrosis factor. The effect of Con A was decreased or abolished by sugars in the order methyl alpha-D-mannopyranoside > methyl alpha-D-glucopyranoside > mannose > glucose = fructose > N-acetylglucosamine. Wheat germ agglutinin was less inhibitory than Con A; in this instance N-acetylglucosamine decreased inhibition, whereas methyl alpha-D-mannopyranoside or methyl alpha-D-glucopyranoside did not. The induction of luciferase activity in THP-1 LTRluc by the staphylococcal extracellular product also was inhibited by fetal bovine and normal human serum. A comparison of 31 staphylococcal isolates (9 Staphylococcus aureus, 11 Staphylococcus epidermidis, 2 Staphylococcus haemolyticus, 4 Staphylococcus hominis, 2 Staphylococcus capitis, 2 Staphylococcus warneri, 1 Staphylococcus saprophyticus) revealed wide variation in LTR activating activity that did not correlate closely with slime production. Our findings, using induction of luciferase in THP-1 LTRluc as a model for upregulation of HIV infection, raise the possibility that staphylococci, as well as certain other microorganisms, release carbohydrate-containing exopolymers, which can activate the HIV-1 LTR, thus influencing progression of HIV infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Coapes H. E. The interaction of concanavalin A with teichoic acids and bacterial walls. Biochem J. 1971 Jul;123(4):665–667. doi: 10.1042/bj1230665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battan R., Raviglione M. C., Wallace J., Cort S., Boyle J. F., Taranta A. S. aureus nasal carriage among homosexual men with and without HIV infection. Am J Infect Control. 1991 Apr;19(2):98–100. doi: 10.1016/0196-6553(91)90046-f. [DOI] [PubMed] [Google Scholar]
- Blümel P., Uecker W., Giesbrecht P. Zero order kinetics of cell wall turnover in Staphylococcus aureus. Arch Microbiol. 1979 May;121(2):103–110. doi: 10.1007/BF00689972. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Modulation of HIV-enhancer activity by heterologous agents: a minireview. Gene. 1991 May 30;101(2):165–170. doi: 10.1016/0378-1119(91)90407-3. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of HIV-1 gene expression. FASEB J. 1991 Jul;5(10):2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Doyle R. J., Chaloupka J., Vinter V. Turnover of cell walls in microorganisms. Microbiol Rev. 1988 Dec;52(4):554–567. doi: 10.1128/mr.52.4.554-567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Galbraith L., Wilkinson B. J., Wilkinson S. G. Staphylococcal slime: a cautionary tale. J Clin Microbiol. 1990 Jun;28(6):1292–1296. doi: 10.1128/jcm.28.6.1292-1296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Collins C., Hastings J. G., White P. J. Radiochemical assay to measure the biofilm produced by coagulase-negative staphylococci on solid surfaces and its use to quantitate the effects of various antibacterial compounds on the formation of the biofilm. J Med Microbiol. 1992 Jul;37(1):62–69. doi: 10.1099/00222615-37-1-62. [DOI] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Comparison of cell-wall teichoic acid with high-molecular-weight extracellular slime material from Staphylococcus epidermidis. J Med Microbiol. 1992 Dec;37(6):368–375. doi: 10.1099/00222615-37-6-368. [DOI] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis. 1991 Mar;163(3):534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- Hussain M., Wilcox M. H., White P. J. The slime of coagulase-negative staphylococci: biochemistry and relation to adherence. FEMS Microbiol Rev. 1993 Apr;10(3-4):191–207. doi: 10.1111/j.1574-6968.1993.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Gellermann H., Chambers H. Staphylococcus aureus bacteremia and recurrent staphylococcal infection in patients with acquired immunodeficiency syndrome and AIDS-related complex. Am J Med. 1988 Aug;85(2):172–176. doi: 10.1016/s0002-9343(88)80337-1. [DOI] [PubMed] [Google Scholar]
- Jarløv J. O., Hansen J. E., Rosdahl V. T., Espersen F. The typing of Staphylococcus epidermidis by a lectin-binding assay. J Med Microbiol. 1992 Sep;37(3):195–200. doi: 10.1099/00222615-37-3-195. [DOI] [PubMed] [Google Scholar]
- Jones J. W. Interaction of coagulase negative staphylococci with lectins. J Clin Pathol. 1993 Aug;46(8):761–763. doi: 10.1136/jcp.46.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. J., White D. A., Fels A. O. The incidence and significance of Staphylococcus aureus in respiratory cultures from patients infected with the human immunodeficiency virus. Am Rev Respir Dis. 1990 Jan;141(1):89–93. doi: 10.1164/ajrccm/141.1.89. [DOI] [PubMed] [Google Scholar]
- Ludwicka A., Uhlenbruck G., Peters G., Seng P. N., Gray E. D., Jeljaszewicz J., Pulverer G. Investigation on extracellular slime substance produced by Staphylococcus epidermidis. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Dec;258(2-3):256–267. doi: 10.1016/s0176-6724(84)80043-7. [DOI] [PubMed] [Google Scholar]
- Pfaller M., Davenport D., Bale M., Barrett M., Koontz F., Massanari R. M. Development of the quantitative micro-test for slime production by coagulase-negative staphylococci. Eur J Clin Microbiol Infect Dis. 1988 Feb;7(1):30–33. doi: 10.1007/BF01962167. [DOI] [PubMed] [Google Scholar]
- Raviglione M. C., Battan R., Pablos-Mendez A., Aceves-Casillas P., Mullen M. P., Taranta A. Infections associated with Hickman catheters in patients with acquired immunodeficiency syndrome. Am J Med. 1989 Jun;86(6 Pt 2):780–786. doi: 10.1016/0002-9343(89)90473-7. [DOI] [PubMed] [Google Scholar]
- Raviglione M. C., Mariuz P., Pablos-Mendez A., Battan R., Ottuso P., Taranta A. High Staphylococcus aureus nasal carriage rate in patients with acquired immunodeficiency syndrome or AIDS-related complex. Am J Infect Control. 1990 Apr;18(2):64–69. doi: 10.1016/0196-6553(90)90083-5. [DOI] [PubMed] [Google Scholar]
- Reeder W. J., Ekstedt R. D. Study of the interaction of concanavalin A with staphylocccal teichoic acids. J Immunol. 1971 Feb;106(2):334–340. [PubMed] [Google Scholar]
- Weinke T., Schiller R., Fehrenbach F. J., Pohle H. D. Association between Staphylococcus aureus nasopharyngeal colonization and septicemia in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1992 Nov;11(11):985–989. doi: 10.1007/BF01967787. [DOI] [PubMed] [Google Scholar]
- Wong W., Chatterjee A. N., Young F. E. Regulation of bacterial cell walls: correlation between autolytic activity and cell wall turnover in Staphylococcus aureus. J Bacteriol. 1978 May;134(2):555–561. doi: 10.1128/jb.134.2.555-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



