Significance
Brucella abortus is an intracellular bacterial pathogen that inflicts a significant health burden on both humans and their livestock on a global scale. We demonstrate that an essential regulatory system controls the growth and morphology of B. abortus, and that this system is required for survival inside mammalian host cells. Using experimental and computational tools of structural biology, we further define how the protein components of this regulatory pathway interact at the atomic scale. Our results provide evidence for multiple, asymmetric modes of binding between essential pathway proteins that control transcription. The multimodal molecular interactions we observe provide evidence for new layers of allosteric control of this conserved gene regulatory system.
Keywords: Brucella abortus, two-component system, cell cycle, ChpT, CtrA
Abstract
We have functionally and structurally defined an essential protein phosphorelay that regulates expression of genes required for growth, division, and intracellular survival of the global zoonotic pathogen Brucella abortus. Our study delineates phosphoryl transfer through this molecular pathway, which initiates from the sensor kinase CckA and proceeds through the ChpT phosphotransferase to two regulatory substrates: CtrA and CpdR. Genetic perturbation of this system results in defects in cell growth and division site selection, and a specific viability deficit inside human phagocytic cells. Thus, proper control of B. abortus division site polarity is necessary for survival in the intracellular niche. We further define the structural foundations of signaling from the central phosphotransferase, ChpT, to its response regulator substrate, CtrA, and provide evidence that there are at least two modes of interaction between ChpT and CtrA, only one of which is competent to catalyze phosphoryltransfer. The structure and dynamics of the active site on each side of the ChpT homodimer are distinct, supporting a model in which quaternary structure of the 2:2 ChpT–CtrA complex enforces an asymmetric mechanism of phosphoryl transfer between ChpT and CtrA. Our study provides mechanistic understanding, from the cellular to the atomic scale, of a conserved transcriptional regulatory system that controls the cellular and infection biology of B. abortus. More generally, our results provide insight into the structural basis of two-component signal transduction, which is broadly conserved in bacteria, plants, and fungi.
Brucellosis, caused by Brucella spp., is among the most common zoonotic diseases worldwide (1). These intracellular pathogens are estimated to cause at least 500,000 new human infections each year and, in areas of Africa, Asia, and South America, inflict significant agricultural losses due to decreased livestock production (2, 3). Survival of Brucella within mammals is linked to their ability to infect and survive inside professional phagocytic cells (2). If left untreated in human hosts, Brucella eventually spread to multiple tissue types, which can lead to a range of debilitating chronic sequelae including reticuloendothelial, cardiovascular, gastrointestinal, and neurological damage.
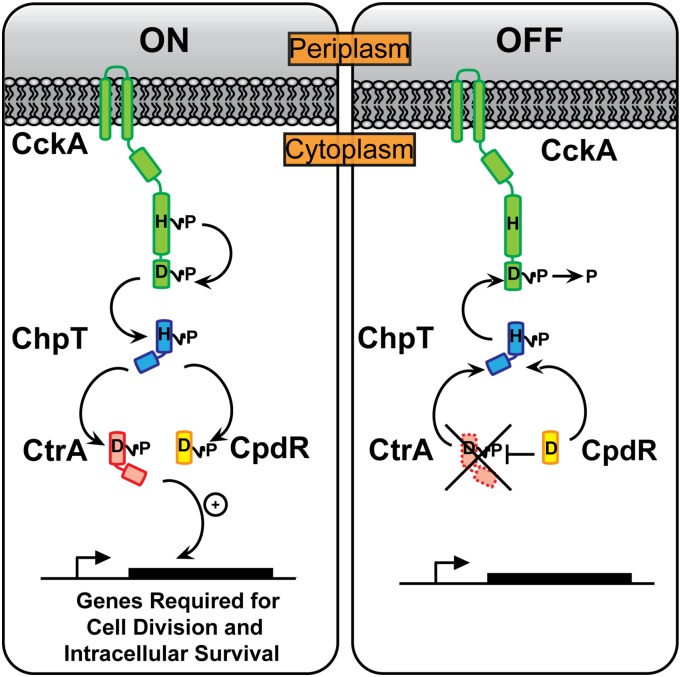
Brucella are members of the α-proteobacteria, a diverse class of Gram-negative species adapted for growth across a range of environmental conditions including plant surfaces and roots, aquatic and soil ecosystems, and the interior of mammalian cells (4, 5). Among the central regulatory systems controlling the α-proteobacterial cell cycle is a multistep phosphorelay composed of four proteins: (i) the hybrid sensor histidine kinase (HK) CckA, (ii) the histidine phosphotransferase ChpT, (iii) the DNA-binding response regulator CtrA, and (iv) the phospho-receiver protein CpdR (Fig. 1). Our current understanding of this conserved regulatory system is based largely on studies of the related aquatic bacterium Caulobacter crescentus (6, 7). In Caulobacter, autophosphorylated CckA transfers phosphoryl groups to a conserved histidine on ChpT. ChpT∼P subsequently transfers phosphoryl groups to either CpdR or CtrA (8). CtrA is a regulator of cell cycle and developmental gene transcription; its activity is controlled by phosphorylation and by proteolysis. Specifically, CtrA is active as a transcription factor when phosphorylated (CtrA∼P); CtrA protein is stabilized in the cell by CpdR∼P and is proteolyzed when CpdR is in its unphosphorylated form (9). Thus, ChpT regulates CtrA-dependent transcription both by phosphorylating CtrA and controlling CtrA protein stability via CpdR.
Fig. 1.
Model of the CckA–ChpT–CtrA–CpdR phosphorelay. The HK CckA (green) autophosphorylates on a conserved His (H) residue and transfers a phosphoryl group to a conserved Asp (D) residue on its C-terminal REC domain. CckA∼P transfers phosphoryl groups to the ChpT phosphotransferase (blue), which can subsequently transfer phosphoryl groups to the REC domains of CtrA (red) and CpdR (yellow). CtrA∼P is a DNA-binding response regulator that modulates transcription of genes controlling cell polarity, division, and intracellular survival in mammalian macrophages. CpdR controls steady-state levels of CtrA in the B. abortus cell.
The genes encoding the CckA–ChpT–CtrA–CpdR regulatory system have been identified in several α-proteobacteria. However, there is notable diversity in the transcriptional output of this system across the clade (10–13), which likely reflects the breadth of niches inhabited by these species (14). The function of this system in Brucella abortus, which is capable of infecting, growing, and replicating inside mammalian cells, is poorly understood, although previous studies of Brucella CtrA have revealed a possible role in the control of cell division (15). It is further known that genetic perturbation of the DNA methyltransferase CcrM, which is directly regulated by CtrA, results in a virulence defect (16).
In this study, we define the full set of genes encoding the B. abortus CckA–ChpT–CtrA–CpdR phosphorelay and characterize molecular and structural requirements of signaling through this conserved pathway. These four proteins comprise an essential phosphorelay that controls B. abortus cell growth, division, and infection biology. Expression of conditional mutant alleles of these genes results in defects in growth and division site selection, yielding cells with branched morphology and altered DNA content. Although genetic perturbation of this pathway has no effect on cell survival in vitro, or entry during a macrophage infection, pathway mutants exhibit significantly reduced survival inside human cells. These data support a model in which the CckA–ChpT–CtrA–CpdR proteins constitute a regulatory system that controls B. abortus cell development and intracellular survival. We have further extended our functional analysis of this regulatory system to the molecular scale and determined the structures of B. abortus ChpT to 1.7-Å resolution and ChpT bound to the receiver domain of CtrA (ChpT–CtrAREC) to 2.7-Å resolution. The ChpT structure reveals a symmetrical homodimer with an HK-like fold. Unlike classic HKs, ChpT does not bind ATP but efficiently and specifically transfers phosphoryl groups from the CckA kinase to the receiver domains of both CtrA and CpdR. The ChpT–CtrAREC crystal structure reveals an asymmetric protein complex that defines a phosphotransferase-receiver interface in molecular detail and provides insight into the mechanism by which ChpT regulates CtrA activity. Our study illuminates, on multiple scales, mechanisms by which a conserved signaling pathway controls the developmental and infection biology of a bacterial intracellular pathogen.
Results
B. abortus CckA–ChpT–CtrA–CpdR Constitute a Specific Phosphorelay.
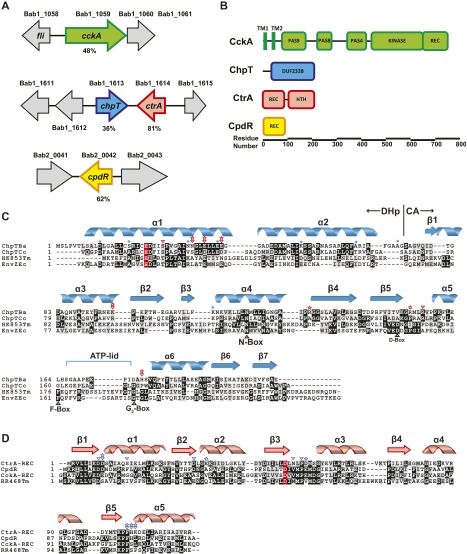
We used sequence-based approaches via the MiST2 database (17) to identify putative orthologs of CckA, ChpT, CtrA, and CpdR in B. abortus. Amino acid sequence identity of the putative CckA (Bab1_1059; 65%), ChpT (Bab1_1613; 36%), CtrA (Bab1_1614; 81%), and CpdR (Bab2_0042; 74%) proteins to the C. crescentus orthologs (Fig. S1) suggested that B. abortus encodes a fully intact CckA–ChpT–CtrA–CpdR phosphorelay. However, overall sequence identity of B. abortus Bab1_1613 to bona fide ChpT proteins of other α-proteobacteria was low and did not clearly distinguish Bab1_1613 as a ChpT ortholog. To test our prediction that these four genes constitute a unified signaling pathway in B. abortus, we expressed and purified each of these proteins. B. abortus CckA (Bab1_1059) is predicted to contain a transmembrane domain at its N terminus, so we generated a construct to express a soluble CckA fragment (amino acids 554–946) that contains only the HK and C-terminal receiver (REC) domains. This truncated CckA yielded an active kinase capable of autophosphorylation in the presence of excess ATP (Fig. 2A). Half-maximal autophosphorylation was observed within 5 min; signal for phospho-CckA (CckA∼P) saturated within 30 min (Fig. 2A) and is consistent with the autophosphorylation profile of other bacterial HKs (18–20).
Fig. S1.
(A) Genome context of cckA (green), chpT (blue), ctrA (red), and cpdR (yellow) in B. abortus strain 2308. Locus numbers are indicated above each gene. Percent amino acid sequence identity to C. crescentus orthologs is indicated below each gene. (B) Domain organization of B. abortus CckA, ChpT, CtrA, and CpdR proteins. All proteins are drawn to scale. (C) C. crescentus and B. abortus ChpT phosphotransferase proteins aligned with the sequences of HKs E. coli EnvZ and T. maritima HK853. Residues involved in protein dimerization (blue circles) and interaction with CtrA(C) (small red arrow) and CtrA(D) (small red star) are indicated. The conserved histidine phosphorylation site is highlighted red. (D) Alignment of B. abortus CtrA, CpdR, CckA, and T. maritima RR468 REC domains. Site of aspartyl phosphorylation is highlighted red. Residues that interact with ChpT(A) (small blue arrow) and ChpT(B) (small blue star) are indicated. All secondary structure is indicated above the sequence (spiral, helix; horizontal arrow, beta strand).
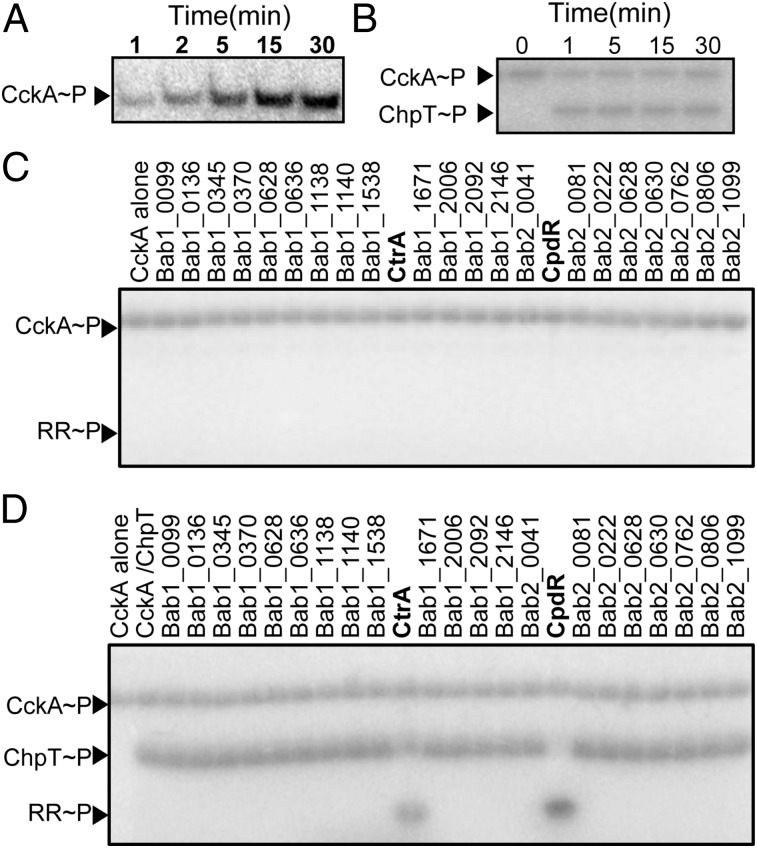
Fig. 2.
CckA–ChpT–CtrA–CpdR proteins constitute a phosphorelay system in vitro. (A) Autoradiograph of CckA autophosphorylation from 1 to 30 min in the presence of [γ-32P]ATP. (B) Autoradiograph of phosphotransfer from CckA∼P to ChpT measured from 0 to 30 min in the presence of [γ-32P]ATP. CckA was permitted to autophosphorylate for 30 min before incubation with ChpT. (C and D) Phosphoryl transfer from CckA∼P was assayed for 15 s against all 23 B. abortus response regulators in the (C) absence or (D) presence of ChpT.
To test whether B. abortus CckA∼P transfers a phosphoryl group to the predicted ChpT protein (Bab1_1613), we incubated CckA∼P with ChpT for periods of 1–30 min. A clear ChpT∼P band appeared within 1 min (Fig. 2B). The in vitro phosphotransfer kinetics provides evidence that CckA and ChpT form a cognate signaling interaction in vivo (21). We next sought to identify other possible phosphorylation substrates of CckA∼P using the approach known as phosphotransfer profiling (22). Because REC domains are the preferred substrates for bacterial HKs (23), we generated constructs to express the soluble REC domains from each of the 23 response regulator proteins encoded within the B. abortus genome. Incubation of CckA∼P with each REC domain for 15 s showed no evidence of phosphotransfer (Fig. 2C). However, the addition of ChpT to each of these 23 phosphotransfer reactions resulted in rapid phosphorylation of two substrates: CpdR and CtrA. We conclude that phosphotransfer from B. abortus CckA to the REC domains of CpdR and CtrA is specific and requires the ChpT protein. Our results provide biochemical support for our prediction that these B. abortus genes constitute a bona fide regulatory phosphorelay, and that this gene set is orthologous to the cckA–chpT–cpdR–ctrA systems defined in related α-proteobacteria (8, 22).
Genes Encoding CckA–ChpT–CtrA–CpdR Phosphorelay Control B. abortus Cell Division.
To investigate the function of the CckA–ChpT–CtrA–CpdR phosphorelay in vivo we attempted to delete these genes from their chromosomal loci in B. abortus. We were unable to delete or disrupt cckA, chpT, or ctrA, but were able to generate a strain harboring a chromosomal in-frame deletion of cpdR. We could only delete the chromosomal copies of chpT or cckA when expressing a complementing copy of these genes in trans from their native promoters (Table S1). Because CckA and ChpT are required to phosphorylate CtrA in vitro (Fig. 2), our inability to delete these genes in the absence of a complementing copy is consistent with previous reports that CtrA is essential in B. abortus (15) and several other α-proteobacteria (24–27). Thus, our data provide evidence that the CckA–ChpT–CtrA phosphorelay is essential in B. abortus. To further test the effects of phosphorelay perturbation on B. abortus biology we generated strains encoding conditional alleles of these genes, which we discuss below.
Table S1.
Deletion statistics for the essential B. abortus chpT and cckA genes
| Strain | Results of chpT deletion | ||
| WT | sacB inactivation | ΔchpT | |
| 2308 | 81 | 17 | 0 |
| 2308 PchpT-chpT | 57 | 6 | 8 |
| Results of cckA deletion | |||
| WT | sacB inactivation | ΔcckA | |
| 2308 | 58 | 42 | 0 |
| 2308 PcckA-cckA | 41 | 37 | 21 |
Gene deletion by double-crossover recombination, using sacB counter selection on sucrose, was attempted in WT B. abortus strain 2308 in the absence (n = 98 total colonies screened) and presence (n = 71 total colonies screened) of a complementing copy of chpT on a replicating plasmid (PchpT-chpT). Deletion of cckA was attempted on WT B. abortus strain 2308 in the absence (n = 100 total colonies screened) and presence (n = 99 total colonies screened) of a complementing copy of cckA on a replicating plasmid (PcckA-cckA). The table reports the number of colonies identified as WT revertants after sucrose counterselection (WT), the number of colonies with random sacB inactivation, and the number of colonies harboring an in-frame deletion of chpT (ΔchpT) or cckA (ΔcckA). We only identified colonies harboring an in-frame deletion when the complementing copy of chpT or cckA was present.
We first constructed a B. abortus strain in which the WT copy of ctrA was replaced with the ctrA(V148F) allele, a known temperature-sensitive allele of C. crescentus ctrA (28). We reasoned this analogous substitution in B. abortus CtrA would confer a similar temperature-sensitive phenotype given the high sequence similarity between these proteins (81%) (Fig. S1). Indeed, B. abortus expressing ctrA(V148F) from the native ctrA locus displays no growth defect at 30 °C but fails to replicate when grown at 37 °C (see Fig. 4D). Because we could not generate a B. abortus chpT null mutant, we sought to disrupt signaling through the CckA–ChpT–CtrA–CpdR pathway by transforming B. abortus with an inducible chpT overexpression plasmid (chpT++). Given that unphosphorylated CpdR regulates steady-state CtrA levels in other α-proteobacteria (9, 29), we also generated strains carrying either WT cpdR [cpdR(WT)++] or an allele of cpdR that cannot be phosphorylated [cpdR(D52A)++], which we expressed from a lac-inducible promoter on a replicating plasmid.
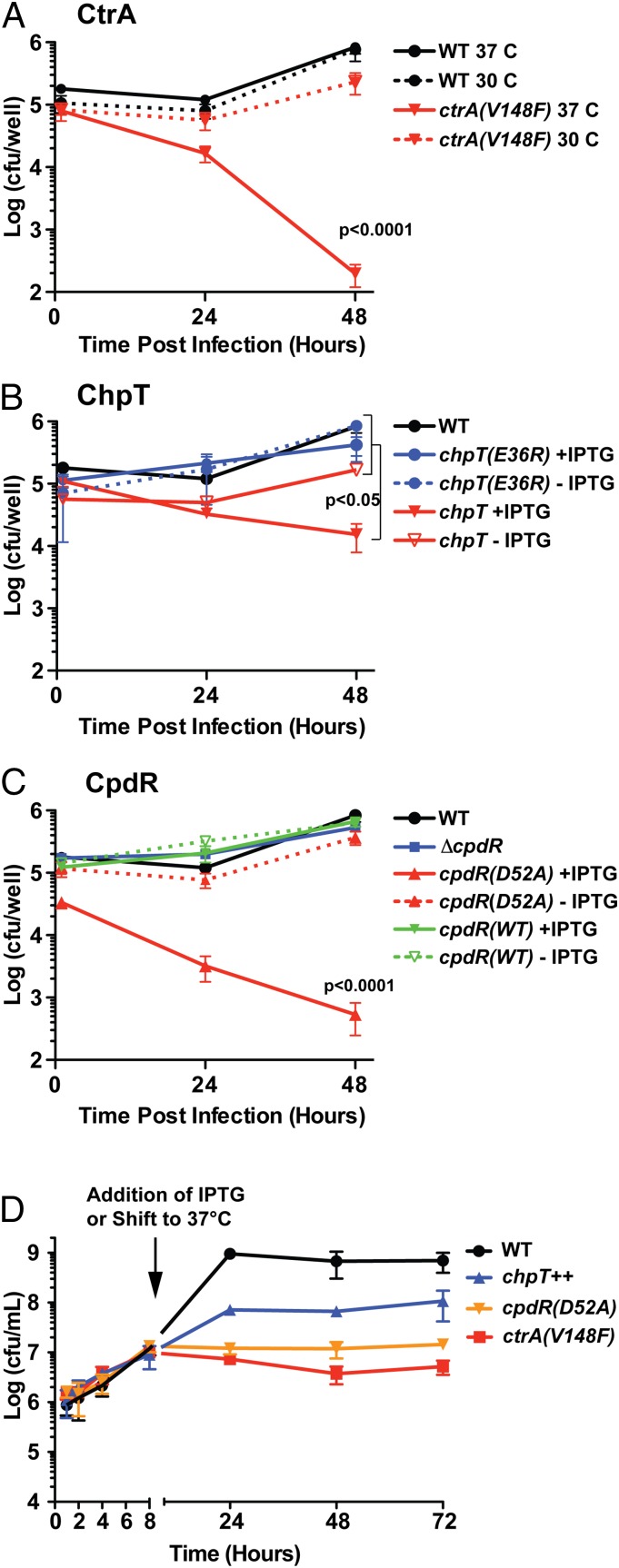
Fig. 4.
The CckA–ChpT–CtrA–CpdR phosphorelay regulates intracellular replication, and survival of WT and mutant B. abortus strains in terminally differentiated THP-1 macrophages. (A) Enumeration (cfu per well) of the intracellular ctrA(V148F) temperature-sensitive strain recovered from THP-1 cells cultured at the nonpermissive (37 °C) and permissive (30 °C) temperatures. (B) Enumeration of intracellular B. abortus overexpressing chpT or (C) cpdR recovered from THP-1. (D) Growth and survival of WT and mutant B. abortus strains cultured axenically over 48 h in complex medium. Cultures were first grown for 8 h under conditions in which mutant alleles were not activated/induced [30 °C for ctrA(V148F) and without IPTG for cpdR(WT)++ and cpdR(D52A)++ strains]. Cultures were then shifted to inducing conditions [37 °C for ctrA(V148F) and adding IPTG to cpdR(WT)++ and cpdR(D52A)++]. Numbers indicate B. abortus viable cfu per milliliter of medium, recovered at time points through this culture procedure. Data points represent mean cfu of three independent samples ± SEM.
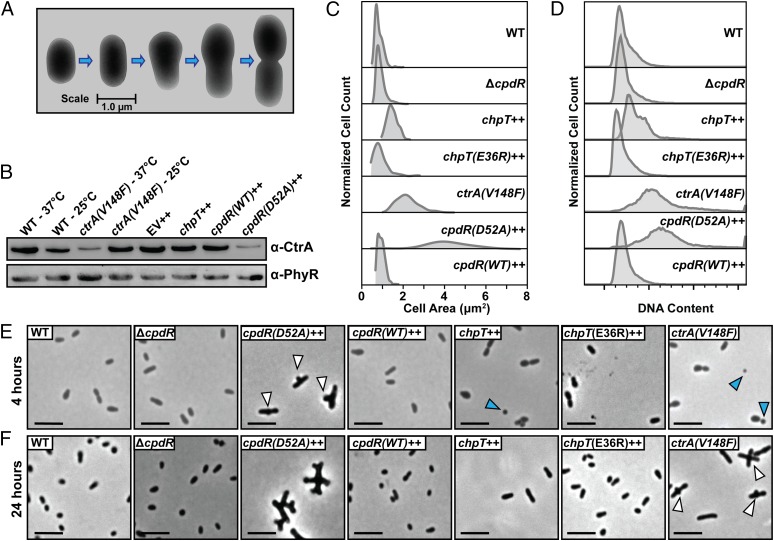
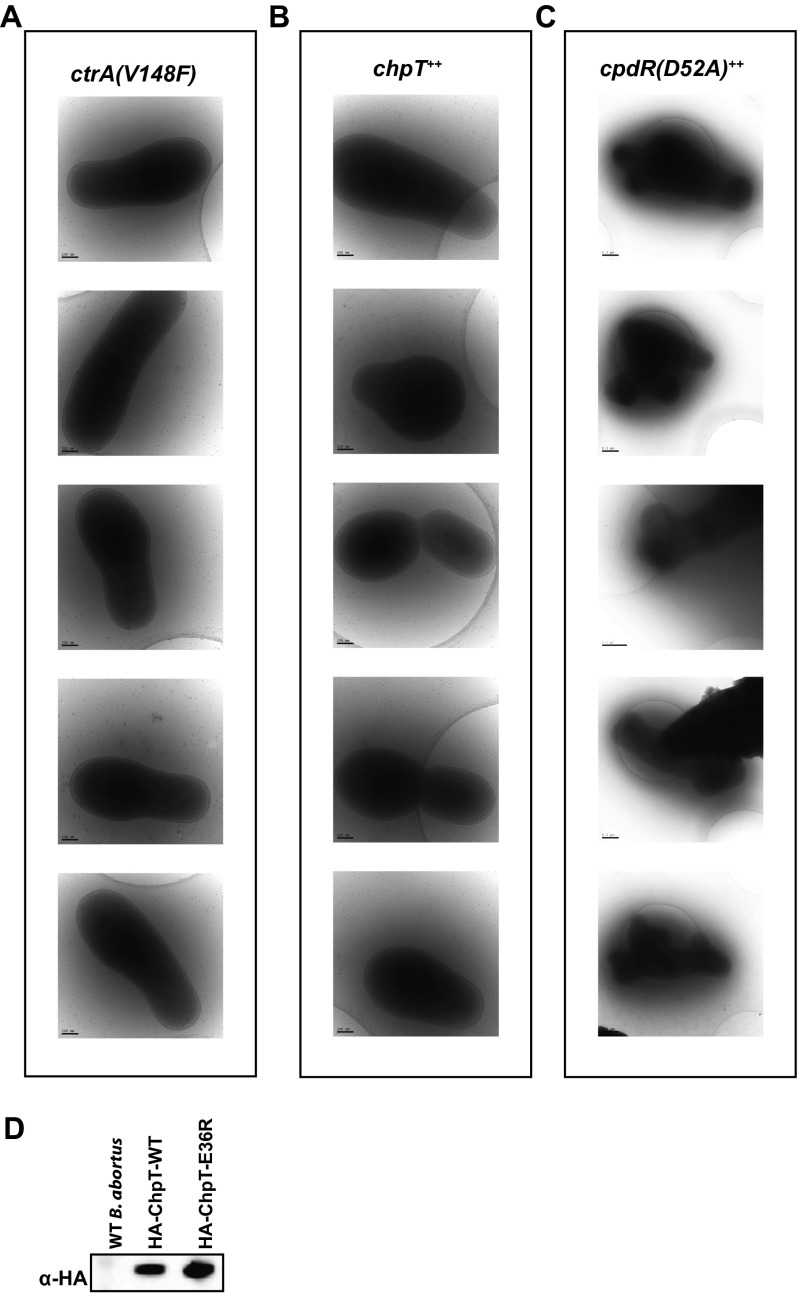
To assess the effects of expressing these conditional alleles, we cultured WT and mutant B. abortus strains to midlogarithmic phase and analyzed cell morphology by cryo-electron microscopy (cryoEM) and light microscopy (LM). WT B. abortus cells grown to log phase in complex medium clearly occupied multiple stages of the cell cycle and exhibited hallmarks of cell growth and division described for the order Rhizobiales of α-proteobacteria (30). Specifically, predivisional B. abortus cells exhibit features of polar cell growth in which a narrower, rod-shaped cell emerges from the pole of a wider, rounder cell (Fig. 3A). In contrast, ctrA(V148F) mutant cells cultured for either 4 or 24 h at the nonpermissive temperature (37 °C) were abnormally elongated; by 24 h we observed defects in cell divison, with apparent budding from the midcell position (Fig. 3 E and F and Fig. S2A). CtrA(V148F) protein is still present in the cell after growth at 37 °C for 24 h, although at slightly decreased levels (Fig. 3B). Induction of chpT expression (chpT++) by the addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 and 24 h increased the proportion of elongated cells; we further observed the presence of minicells in these cultures (Fig. 3 E and F and Fig. S2B).
Fig. 3.
The CckA–ChpT–CtrA–CpdR phosphorelay regulates B. abortus cell division. (A) CryoEM images of fixed WT B. abortus cells captured at stages through a typical cell cycle. (B) Western blot showing steady-state CtrA protein levels 24 h after shift to the nonpermissive temperature [37 °C for ctrA(V148F)] or addition of IPTG [chpT++, cpdR(WT)++, and cpdR(D52A)++]. (C) Total cell area distributions of populations of B. abortus cells (n ≈ 300) after growth for 24 h under conditions in which conditional alleles are induced/activated. (D) DNA content of WT and mutant B. abortus cells (n = 20,000) measured by propidium iodide staining followed by flow cytometry. Light micrographs of WT and mutant B. abortus strains taken after (E) 4-h and (F) 24-h cultivation in complex medium at 37 °C, containing IPTG. Blue arrows mark apparent B. abortus minicells; white arrows mark cells with disrupted cell growth and division polarity. (Scale bars, 2 μm.)
Fig. S2.
Cryoelectron micrographs of B. abortus (A) ctrA(V148F), (B) chpT++, and (C) cpdR(D52A)++ overexpression strains. (D) Western blot of HA-tagged ChpT and ChpT(E36R) proteins from B. abortus cell lysate resolved by SDS/PAGE. WT B. abortus cell lysate was run and blotted as a negative control.
Finally, we measured the effects of cpdR allele expression on B. abortus cell morphology and on steady-state CtrA levels. Because unphosphorylated CpdR activates proteolysis of CtrA in C. crescentus (29), we tested whether unphosphorylated CpdR affects steady-state levels of CtrA in B. abortus. After inducing cpdR(WT)++ or cpdR(D52A)++ expression with IPTG for 24 h, we observed a significant reduction in CtrA protein in the strain expressing cpdR(D52A)++, which is missing the site of aspartyl phosphorylation. Expression of cpdR(WT)++ had no effect on steady-state CtrA levels assessed by Western blot (Fig. 3B). This result is consistent with a model in which unphosphorylated CpdR destabilizes CtrA in B. abortus. We further analyzed cpdR(WT)++ and cpdR(D52A)++ overexpression strains by cryoEM and LM and observed large elongated cells with apparent budding at the midcell after 4 h of cpdR(D52A)++ induction (Fig. 3E); after 24 h of induction we observed large B. abortus cells that were highly branched, which is consistent with a defect in proper cell division (Fig. 3F and Fig. S2). Overexpression of cpdR(WT)++ or deletion of cpdR (ΔcpdR) did not result in a gross defect in cell morphology (Fig. 3).
CryoEM and LM images of a small number of cells provide evidence that perturbation of the CckA–ChpT–CtrA–CpdR pathway affects cell growth and division. We next imaged a large number of fixed WT and mutant B. abortus cells, extracted the 2D cell contours, and analyzed these cell contours to determine 2D cell area (31). This analysis permitted us to more thoroughly quantify variation in cell area across all strains. After 24 h of growth at 37 °C in complex medium (adding IPTG where applicable) the mean cell area of WT B. abortus was 0.67 ± 0.19 μm2. However, mean cell areas of ctrA(V148F) (2.1 ± 0.57 μm2), chpT++ (1.1 ± 0.26 μm2), and cpdR(D52A)++ (3.9 ± 1.4 μm2) were significantly increased (one-way ANOVA; Dunnett’s post test; P < 0.0001) compared with WT. The ΔcpdR (0.75 ± 0.22 μm2) and cpdR(WT)++ (0.79 ± 0.19 μm2) strains exhibited less increase in cell area relative to WT but were still significantly larger than WT (P < 0.001). We further tested whether the DNA content of these mutant strains differed from WT B. abortus by staining DNA with propidium iodide and analyzing DNA content using flow cytometry. The ctrA(V148F), chpT++, and cpdR(D52A)++ strains all have highly increased DNA content relative to WT (Fig. 3D), consistent with the gross cell division defects of these mutants.
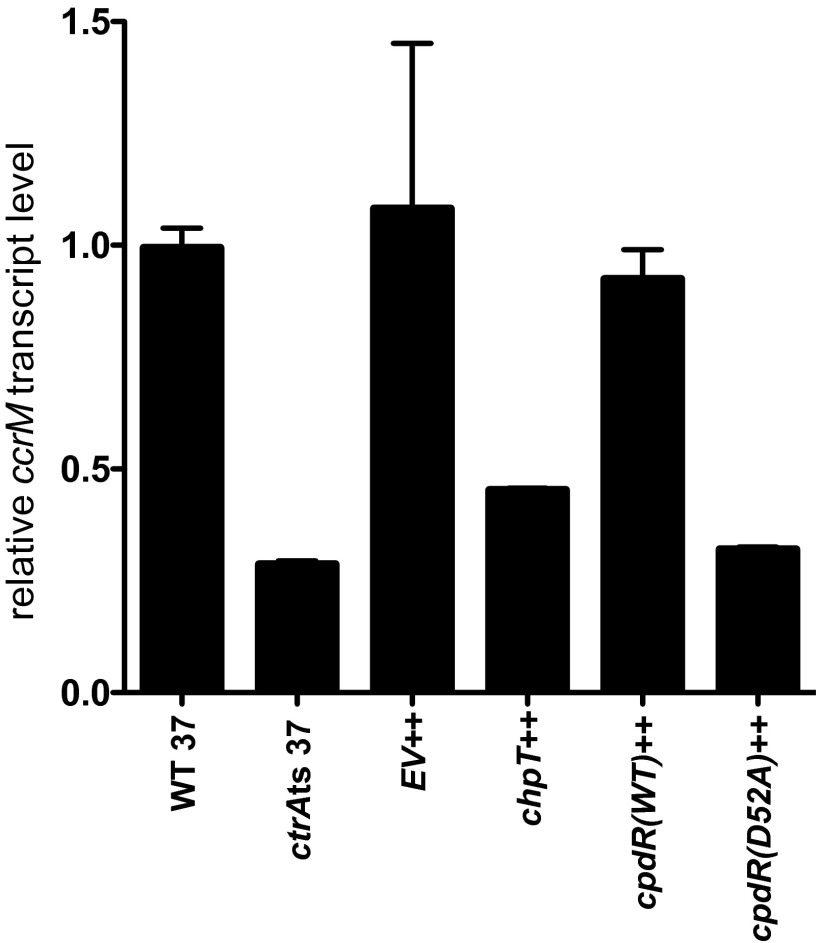
To assess the impact that CckA–ChpT–CtrA–CpdR pathway perturbation has on CtrA-dependent transcription, we quantified transcript levels of the CtrA-regulated gene ccrM (15), by quantitative reverse-transcription PCR (qRT-PCR). After 4 h of growth at the nonpermissive temperature or addition of IPTG, ctrA(V148F), chpT++, and cpdR(D52A)++ strains have significantly decreased ccrM transcript abundance compared with WT B. abortus or an empty vector control (Fig. S3). We conclude that these genetic perturbations reduce CtrA-dependent transcription in B. abortus. Overall, our data support a model in which phosphorelay through the CckA–ChpT–CtrA–CpdR pathway regulates B. abortus processes that determine cell growth, cell cycle, and cell division via control of CtrA-dependent transcription.
Fig. S3.
qRT-PCR analysis of ccrM transcript levels in WT, ctrA(V148F), chpT++, EV++ (empty vector control), cpdR(WT)++, and cpdR(D52A)++ backgrounds. ccrM transcript levels in each independent strain sample (n = 3) are normalized to the WT mean, which is set to a value of 1.
The CckA–ChpT–CtrA–CpdR Pathway Is Required for Intracellular Survival of B. abortus in Human Macrophages.
A natural niche of B. abortus is the interior of mammalian cells. We assessed the effect CckA–ChpT–CtrA–CpdR pathway perturbation has on entry, replication, and survival inside terminally differentiated THP-1 macrophages. Before infection, overnight cultures of WT and conditional B. abortus mutant strains were grown under noninducing conditions in rich medium. Conditional alleles were then activated/induced by culturing for 4 h at 37 °C with IPTG (where indicated) before infecting macrophages. WT and mutant B. abortus cells were added to THP-1 cells at a multiplicity of infection (MOI) of 100 cfu per macrophage. The initial 1 h post infection (hpi) time point in these experiments generally reflects B. abortus entry. No statistically significant differences were observed at this time point, indicating there is no defect in macrophage entry of any mutant strain (Fig. 4 A–C). In contrast, there is a significant reduction in intracellular B. abortus replication and/or survival in the ctrA(V148F), chpT++, and cpdR(D52A)++ strains relative to WT and uninduced controls at 48 hpi. The number of ctrA(V148F) cells isolated from THP-1 (at 37 °C) is ∼1 log lower at 24 hpi and 3.5 logs lower than WT B. abortus at 48 hpi (Fig. 4A). ctrA(V148F) infections performed at the permissive temperature (30 °C) revealed no significant decrease in cfu at 48 hpi compared with WT cultured under equivalent conditions. Overexpression of chpT++ resulted in a 1.0 log decrease in the number of cells recovered from THP-1 compared with uninduced and empty vector controls at 48 hpi (Fig. 4B). The most severe intracellular defect was observed in the strain overexpressing cpdR(D52A)++: We observed a 1.5 log decrease in cells recovered from THP-1 at 24 hpi and a 3.5 log reduction of recovered cells at 48 hpi relative to the uninduced control. There was no difference in recovery of B. abortus expressing cpdR(WT)++ or the ΔcpdR in-frame deletion strain relative to WT (Fig. 4).
We next tested whether mutant attenuation observed in macrophages is due to a general loss of B. abortus viability, or whether CckA–ChpT–CtrA–CpdR pathway perturbation results in a replication/survival defect that is specific to the intracellular niche. We first cultured B. abortus ctrA(V148F), chpT++, and cpdR(D52A)++ conditional mutants under noninducing conditions in liquid growth medium for 8 h, enumerating bacteria over this growth period. At 8 h (or 1 × 107 cfu/mL) we shifted to the restrictive temperature (37 °C) and induced expression of conditional alleles with IPTG; we chose to shift cells at this point because this was the approximate density of cells used for macrophage infections. We enumerated cfu of these induced/activated B. abortus mutants at similar time points assayed in our THP-1 macrophage infection experiments (ref. 24, 48 h). Upon induction/activation, the ctrA(V148F) and cpdR(D52A)++ mutants were completely inhibited in replication whereas the chpT++ mutant replicated, but at a lower rate than WT. In contrast to what we observed in macrophages, neither the ctrA(V148F), chpT++, nor cpdR(D52A)++ strains lost viability under these conditions (over 48 h) (Fig. 4D). We conclude the reduced numbers of ctrA(V148F), chpT++, and cpdR(D52A)++ cells recovered from THP-1 reflects a specific requirement for the CckA–ChpT–CtrA–CpdR pathway for survival inside macrophages. We further conclude that this signaling pathway does not generally control features of the cell required for B. abortus entry into mammalian macrophages.
ChpT: A Histidine Phosphotransferase with an HK-Like Structure.
Having established the importance of the CckA–ChpT–CtrA–CpdR system in B. abortus cellular and infection biology, we next sought to characterize the structural basis of phosphotransfer through this conserved pathway. To this end, we purified, crystallized, and solved the structure of the ChpT phosphotransferase (PDB ID code 4QPK). ChpT formed tetragonal crystals of space group P43 (a = b = 70.84, c = 87.01 Å) that diffracted to 1.7-Å resolution; we phased the ChpT crystal structure by molecular replacement using a model based on structures of a C. crescentus homolog (32, 33). B. abortus ChpT was refined to Rwork = 0.164 and Rfree = 0.188. Crystallographic data and refinement statistics are summarized in Table S2.
Table S2.
Crystallographic data and refinement statistics
| Statistics | ChpT | ChpT–CtrA |
| Data collection statistics | ||
| Resolution range, Å | 35.42–1.66 (1.719–1.66) | 17.88–2.742 (2.839–2.742) |
| Unique reflections | 50,679 (5,049) | 32,556 (3,179) |
| Rmerge | 0.043 | 0.086 |
| I/σI | 27.66 (2.68) | 23.30 (2.52) |
| Redundancy | 7.5 | 11.8 |
| Completeness, % | 99.96 (100.00) | 99.80 (98.03) |
| Refinement statistics | ||
| Space group | P 43 | P 3221 |
| Unit cell | 70.84 70.84 87.01 (90 90 90) | 124.951 124.951 136.332 (90 90 120) |
| Rfree reflections | 3,925 | 2,007 |
| Rwork | 0.1624 (0.2455) | 0.1753 (0.2337) |
| Rfree | 0.1906 (0.2620) | 0.2216 (0.2875) |
| Average B-factor | 27.3 | 65.4 |
| RMS (bonds) | 0.007 | 0.009 |
| RMS (angles) | 1.11 | 1.22 |
| Ramachandran analysis | ||
| Favored, % | 98.3 | 98.0 |
| Allowed, % | 1.7 | 1.8 |
| Outliers, % | 0.0 | 0.2 |
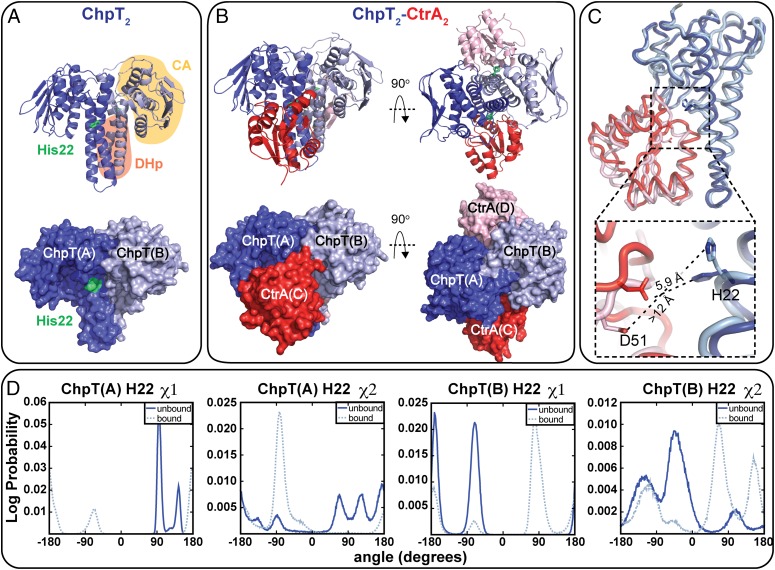
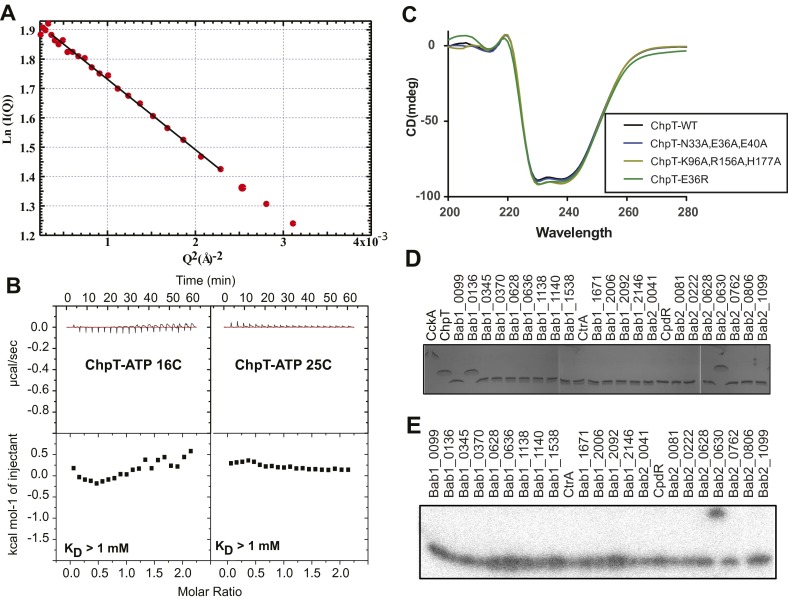
The crystallographic asymmetric unit contains two ChpT molecules, organized as a symmetric dimer. The structure is similar to HKs (34–36) and other histidine phosphotransfer (Hpt) proteins (32, 33, 37). Each ChpT monomer is composed of an N-terminal dimerization and histidine phosphotransfer (DHp) domain and a C-terminal domain that is structurally similar to the catalytic and ATP-binding (CA) domain found in HKs. A least-squares fit of the two monomers to each other shows that they are highly similar (rmsd = 0.33 Å on Cα). Within the structure, the DHp domains from each monomer dimerize to form a four-helix bundle (Fig. 5A). Small angle X-ray scattering (SAXS) data collected on ChpT in solution revealed a particle with radius of gyration (Rg) of 26.4 ± 0.4 Å at 15 μM concentration (Fig. S4A); this is consistent with Rg calculated (38) from the ChpT crystal structure (24.0 Å), providing evidence that ChpT is also a dimer in solution.
Fig. 5.
Molecular structures of B. abortus ChpT and ChpT– CtrAREC complex. (A) Ribbon (Top) and surface views (Bottom) of homodimeric B. abortus ChpT at 1.7-Å resolution (PDB ID code 4QPK). One ChpT monomer is drawn in dark blue and one in light blue. The conserved site of ChpT phosphorylation, His22, is highlighted green; DHp (highlighted pink) and CA-like domains (highlighted yellow) of ChpT are labeled. (B) Ribbon (Top) and surface views (Bottom) of the 2:2 ChpT-CtrAREC complex at 2.7-Å resolution (PDB ID code 4QPJ); diagram illustrates two CtrA receiver domain (REC) monomers (in red and pink) bound to a central homodimer of ChpT (ChpT monomers in dark blue and light blue). (C) Least-squares Cα fit of the two halves of ChpT–CtrAREC complex: ChpT(A) dark blue, ChpT(B) light blue, CtrAREC(C) dark red, and CtrAREC(D) pink. Distances between ChpT(A)–CtrA(C) and ChpT(B)–CtrA(D) phosphoryltransfer residues (ChpT H22 and CtrA D51) are labeled in the expanded box. (D) Log probability of ChpT(A) and ChpT(B) H22 χ1 and χ2 rotamer angle occupancy in the ChpT (unbound; solid blue) and in the ChpT2–CtrA2 complex structures (bound; light dotted blue) based on MD simulations (Materials and Methods); distributions represent Gaussian fluctuations of H22 conformation about a local equilibrium state.
Fig. S4.
(A) Guinier fit of B. abortus ChpT SAXS data. (B) ITC assay of ChpT titrated with ATP. Isotherms at 16 °C and 25 °C indicate no apparent binding (>1 mM equilibrium affinity) between ChpT and ATP. (C) CD spectra of purified WT ChpT and mutant alleles of ChpT. (D) Coomassie-stained SDS polyacrylamide gel of purified proteins used in Fig. 2. (E) [32P]acetyl phosphate labeling of purified B. abortus receiver (REC) domains.
Although ChpT has strong similarity to classic HKs at the level of tertiary and quaternary structure, the pseudo-CA domain lacks regions required for ATP binding including the D-box, F-Box, and G-Box; ChpT also has a truncated ATP lid (Fig. S1C). ChpT retains the conserved N-box, which is known to bind a divalent cation (39), but we do not observe electron density consistent with ions or solvent in this region of the structure. Isothermal titration calorimetry (ITC) experiments demonstrate that ChpT, unlike multiple classes of HKs (40, 41), is unable to bind ATP (Fig. S4B). Based on these data, we conclude that B. abortus ChpT is unable to catalyze ATP hydrolysis and functions solely as a phosphotransferase (Fig. 2).
Structural Asymmetry in the ChpT–CtrA Signaling Complex.
We have demonstrated that B. abortus ChpT is the central phosphotransferase in a pathway that begins with CckA∼P and terminates at the receiver domains of CtrA and CpdR (Fig. 2). To further define the molecular basis of signaling through the CckA–ChpT–CtrA–CpdR system, we solved the structure of ChpT bound to the receiver domain of CtrA (CtrAREC) (PDB ID code 4QPJ). Both ChpT and CtrAREC were purified separately and combined at an equimolar ratio before screening for crystals. The complex formed crystals of space group P3221 (cell dimensions a = 124.95, b = c = 136.33 Å) that diffracted to 2.7-Å resolution. We solved the structure of the ChpT–CtrAREC complex by molecular replacement using the ChpT structure as a search model; the solution provided sufficient phase information to build models for the bound CtrAREC domains. The structure was refined to Rwork = 0.175 and Rfree = 0.222; all crystallographic data are summarized in Table S2.
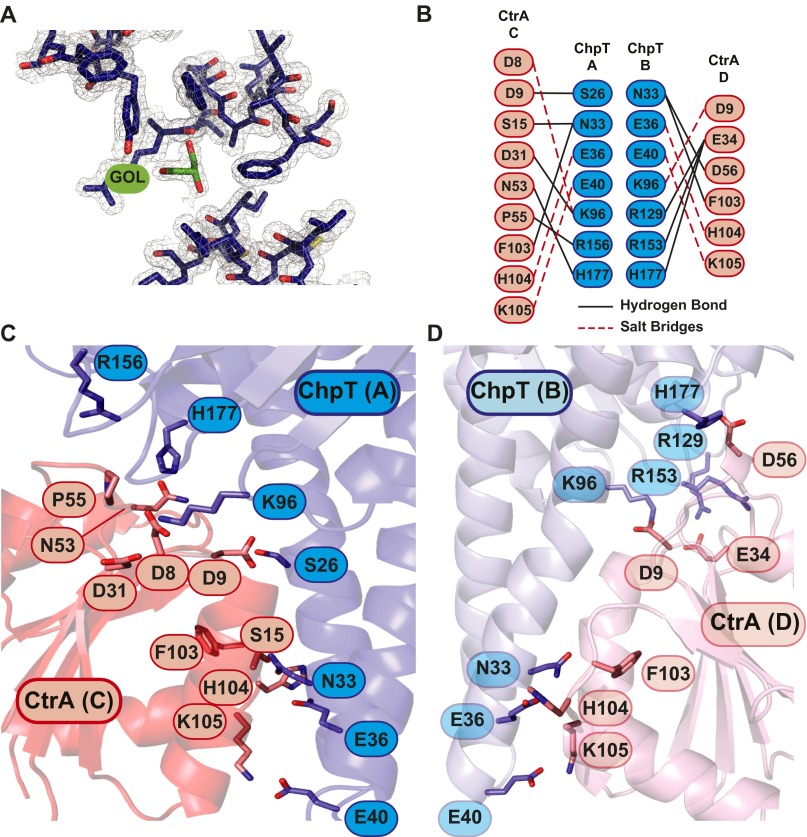
The structure reveals two unphosphorylated CtrAREC monomers positioned against opposing DHp domains of the ChpT dimer, forming a 2:2 complex (ChpT2–CtrA2) (Fig. 5B). We hereafter annotate the two phosphotransferase monomers as ChpT(A) and ChpT(B); the two REC domains are annotated CtrA(C) and CtrA(D). The overall structure of ChpT in complex with CtrAREC is largely unchanged relative to the isolated structure of ChpT (rmsd of 0.62 Å on Cα). This stands in contrast to Thermotoga maritima HK853, which undergoes a large conformational change about the DHp-CA domain linker upon binding to its receiver substrate RR468 (42). Unlike HK853 bound to RR468, or Spo0B phosphotransferase bound to Spo0F (43), the complex is not fully symmetrical. Notably, the side-chain orientation of the ChpT phosphorylation sites (H22) show marked asymmetry between ChpT(A) and ChpT(B). In ChpT(A), the εN atom of H22 is positioned 5.9 Å away and is aligned with the δO atom of CtrA(C) phosphoacceptor residue D51 (Fig. 5C). This measured distance is consistent with defined His–Asp (εN–δO) distances in the Spo0B–Spo0F (43) and the YPD1–SLN1 (44) phosphotransfer complexes and is ∼2 Å shorter than the corresponding εN–δO distances in the unmodified HK853–RR468 complex (42). In terms of His–Asp distance, ChpT(A) thus seems poised for facile phosphotransfer to CtrA(C) without the need for significant conformational change in either ChpT or CtrA. On the opposing side of the ChpT dimer, the side chains of ChpT(B) H22 and CtrA(D) D51 are in different conformations (Fig. 5C): The εN atom of H22 is positioned >12 Å away and is not aligned with the D51 δO atom of CtrA(D). This half of the ChpT–CtrA complex is therefore not competent to catalyze His–Asp phosphotransfer without a significant change in protein conformation. Overall, the absence of large-scale conformational change in ChpT upon receiver binding provides evidence that the structural mechanism of phosphotransfer from ChpT to its substrates is distinct from HK-receiver phosphotransfer defined in the T. maritima HK853–RR468 system, because large structural changes are observed in HK853 upon RR468 binding. ChpT H22 and CtrA D51 asymmetry, as it may relate to phosphotransfer, is discussed in a later section of this manuscript.
An analysis of the CtrA structure across each half of the ChpT dimer reveals additional asymmetric features of the signaling complex. Both CtrA molecules retain the standard αβα sandwich fold observed in other REC domain structures (23). However, an alignment of CtrA(C) to CtrA(D) revealed a higher Cα rmsd (1.6 Å) than observed in ChpT(A)–ChpT(B) alignments. Approximately half the intermolecular interactions within the ChpT–CtrA complex occur through the α1 helix of ChpT and the β1–α1, β3–α3, and β5–α5 loops of CtrA; these contacts are consistent with known HK–REC interfaces (45). Five ChpT residues that interact with CtrA (N33, E36, E40, K96, and H177) are observed on each half of the dimer, but there are significant differences in how CtrA(C) and CtrA(D) contact ChpT. In particular, a set of hydrogen bonds between E34 of CtrA(D) helix α2 and residues R129, R153, and H177 of ChpT(B) are unique to that interface (Fig. 5C). There is clear asymmetry in the ChpT–CtrA contact surface on either side of the complex: Contact between ChpT(A) and CtrA(C) is more extensive than ChpT(B) and CtrA(D) (935 Å2 versus 829 Å2 buried surface area). These structural data predict that the phosphotransfer “competent” half of the complex [i.e., ChpT(A) to CtrA(C)] may form a higher-affinity interface than ChpT(B) to CtrA(D). Indeed, calculations based on total solvation energies of isolated and interfacing structure (46) suggest an approximate 4.5 kcal/mol difference in free energy between the two ChpT–CtrA interfaces.
Conformational Asymmetry and Dynamics of the ChpT Phosphorylation Site.
The crystal structure of ChpT bound to CtrA raised the question of whether conformational and dynamical asymmetry of the ChpT phosphorylation site (H22) was a genuine feature of this signaling complex. To begin to address this question, we performed molecular dynamics (MD) simulations on our experimental structures of the ChpT dimer (1,800 ns) and the ChpT2–CtrA2 heterotetramer (900 ns) in a fully atomistic system with explicit solvent. These simulations were aimed at testing whether the dynamics of ChpT H22 in the isolated ChpT structure and in the ChpT–CtrA complex are inherently asymmetric across the twofold ChpT dimer axis.
As described above, our crystal structure of ChpT–CtrA revealed two distinct conformations of the ChpT H22. MD simulations show that H22 stably occupies multiple χ1/χ2 rotamer angles on chains A and B, both when bound and unbound to CtrA (Fig. 5D). When not bound to CtrA, ChpT(A) H22 evinces substantial heterogeneity: Five stable rotameric states are apparent. The rotamer conformations occupied by H22 differ in each ChpT monomer both when bound and not bound to CtrA. These results provide evidence that the significant asymmetry we observe in conformations of ChpT H22 in our complex crystal structure is not an artifact of crystallization, but rather reflects true asymmetry in protein conformational dynamics at this site. We conclude that small differences in side-chain and backbone structure, likely enforced by ChpT–ChpT and ChpT–CtrA interaction at the quaternary level, affect the energy landscape of H22 conformational transitions. This in turn affects the primary rotamer conformations that H22 samples on each side of the ChpT dimer.
Structural and Functional Analysis of ChpT Phosphoryltransfer.
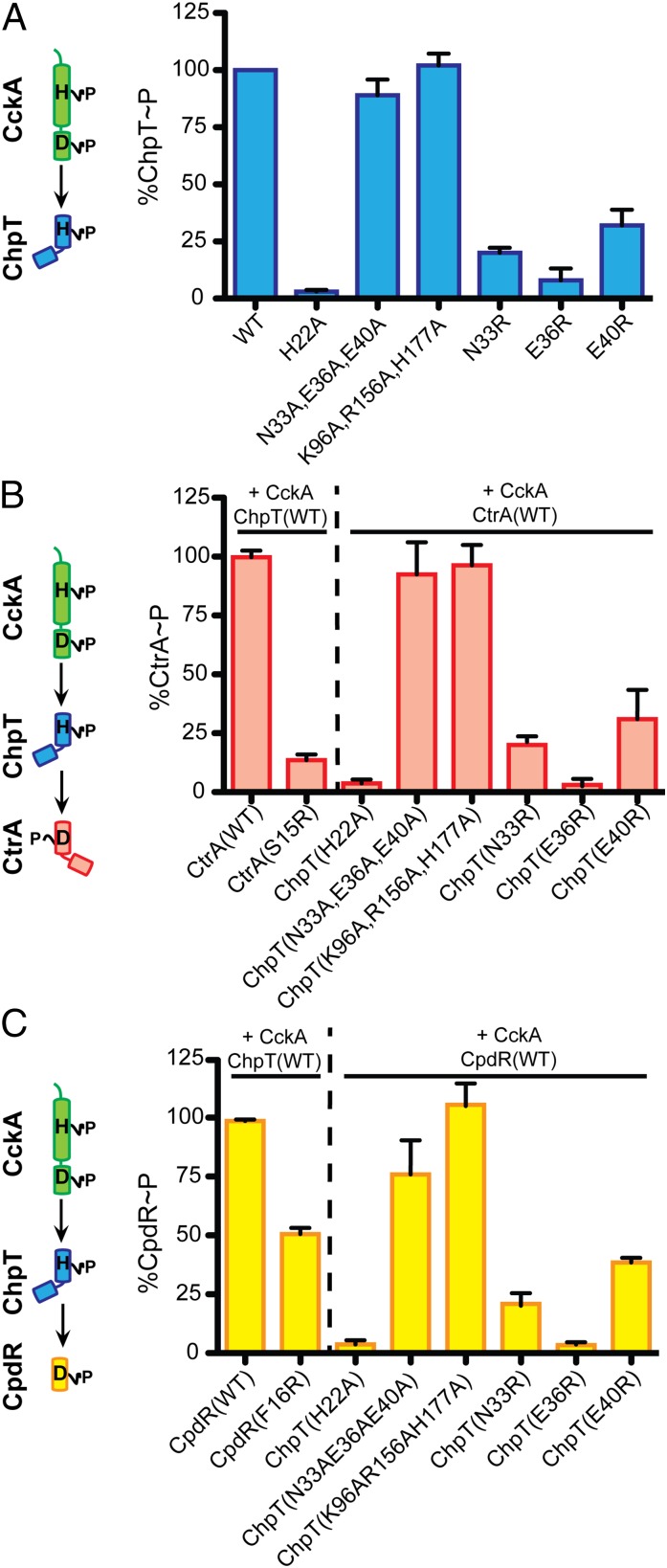
The experimental crystal structures of B. abortus ChpT and the 2:2 ChpT–CtrAREC complex provide a foundation for understanding the molecular basis of phosphoryltransfer through the CckA–ChpT–CtrA–CpdR system. Using these data, we constructed point mutations in components of the pathway to test predictions of our structural models in vitro. We initially tested whether ChpT residues observed to interact with CtrA in our crystal structure are required for the initial phosphotransfer step in this pathway (i.e., CckA to ChpT). In our experimental structure, three residues within the DHp domain of ChpT (N33, E36, and E40) and three residues within the CA domain (K96, R153, and H177) interact extensively with CtrA in the asymmetric ChpT2–CtrA2 complex (Fig. 5B). We generated and purified ChpT proteins with combinations of alanine mutations in the CtrA-interacting residues of the DHp and CA domains. We then assayed these mutant proteins for their ability to receive phosphoryl groups from equimolar CckA∼P over the course of a 5-min reaction. Neither the ChpT(N33A,E36A,E40A) nor ChpT(K96A,R156A,H177A) triple point mutant proteins exhibited a measurable defect in phosphotransfer with CckA (Fig. 6A). Although these residues make direct CtrA contact in the crystal structure, our results suggest that this interface is robust to truncation of larger side chains; loss of contact residues permitted near WT levels of phosphotransfer. Alternatively, different residues in ChpT could be required for interaction with CckA–REC and CtrA. We further generated ChpT(N33R), ChpT(E36R), and ChpT(E40R) mutant proteins. Addition of these single, larger, charged residues to the interaction interface reduced phosphotransfer ∼4–10 fold (Fig. 6A). Defects in phosphotransfer were not a result of a large-scale unfolding or disruption of ChpT structure as assayed by CD spectroscopy of purified ChpT mutant proteins (Fig. S4C).
Fig. 6.
A structural analysis of phosphoryltransfer. (A) Phosphoryltransfer from phospho-CckA (CckA∼P) to an equimolar concentration of WT and mutant ChpT (ChpT point mutations labeled on axis). (B) Phosphoryltransfer from CckA∼P to CtrA(WT) and CtrA(S15R) proteins in the presence of WT or mutant ChpT proteins. (C) Phosphoryltransfer from CckA∼P to CpdR(WT) and CpdR(F16R) proteins in the presence of WT or mutant ChpT proteins. All phosphotransfer reactions are normalized to phosphotransfer reactions performed with WT controls (100%); mean ± SEM is shown for three independent replicates.
We further investigated whether mutations in this region of ChpT are important for phosphotransfer to CtrA and CpdR. Not surprisingly, both the ChpT(N33A,E36A,E40A) and ChpT(K96A,R156A,H177A) mutant proteins exhibited only modest defects in phosphotransfer to both CtrA and CpdR (Fig. 6 B and C). However, ChpT(N33R), ChpT(E36R), and ChpT(E40R) mutants displayed severe defects in phosphotransfer to CtrA and CpdR; the ChpT(E36R) mutant protein displayed the greatest defect in phosphotransfer as indicated by ∼95% reduction of CtrA∼P and CpdR∼P levels relative to ChpT(WT). Finally, we sought to test the role of an important residue of CtrA/CpdR helix α1 in phosphotransfer with ChpT. Helix α1 residue S15 of CtrA interacts directly with ChpT(A) in our crystal structure (Fig. 5) and is important for HK–receiver interactions in a number of different systems (42, 47, 48). We substituted CtrA(S15) and the homologous position in CpdR(F16) to arginine and assayed CckA–ChpT-dependent phosphorylation. These mutations resulted in an approximate 50% reduction in the level of CpdR∼P and a ∼90% reduction in CtrA∼P (Fig. 6 B and C).
To further assay the functional significance of a molecular interaction defined in our crystal structures, we expressed the chpT(E36R) mutant allele in B. abortus cells (Fig. 3). Although overexpression of WT chpT (chpT++) results in a defect in cell morphology, DNA content, and intracellular survival, overexpression of chpT(E36R) does not have a statistically significant effect on any of these phenotypes (Figs. 3 and 4). This result is consistent with the inability of chpT(E36R) to facilitate phosphoryltransfer in vitro (Fig. 6). As a control, we confirmed that WT ChpT and the ChpT(E36R) alleles were expressed at similar levels in the B. abortus cell (Fig. S2D).
Discussion
Protein phosphorelays regulate many important processes in bacteria, including asymmetric cell division, sporulation, and multicellular development (49, 50). The conserved CckA–ChpT–CtrA–CpdR phosphorelay controls transcription of a diverse set of genes involved in multiple aspects of α-proteobacterial cellular biology (8, 10, 14). To date, the identities and functions of the genes encoding this pathway in the intracellular pathogen B. abortus had remained largely undefined. We have identified and biochemically reconstituted the complete B. abortus CckA–ChpT–CtrA–CpdR system in vitro and provide evidence that it comprises an essential and specific phosphorelay that regulates replication, cell division, and survival in the intracellular niche. ChpT is central in this pathway and shuttles phosphoryl groups between the HK CckA and two receiver substrates, CtrA and CpdR. CtrA is a classical DNA-binding response regulator, whereas CpdR is a single domain receiver that regulates steady-state CtrA levels in the cell (Fig. 3). Our data support a model in which the regulatory topology of this pathway is conserved between B. abortus and C. crescentus, two α-proteobacterial species that inhabit widely different environmental niches and have distinct cellular features.
We have discovered that B. abortus mutants defective in signaling through this pathway have no deficiency in entry into a human macrophage cell line but exhibit reduced intracellular survival over a 48-h timescale (Fig. 4). This survival defect is specific to the intracellular niche because expression of conditional ctrA(V148F), chpT++, and cpdR(D52A)++ alleles that strongly perturb the phosphorelay does not result in cell death in axenic culture (Fig. 4D). This result raises the question of what specific genes are under transcriptional control of the CckA–ChpT–CtrA–CpdR system, and which of these genes are required for survival of B. abortus inside mammalian cells. A previous study has identified a CtrA binding site in front of the cell division protein ftsE (15), which is consistent with observed cell division defects. However, there are certainly other direct targets of this essential response regulator, many of which are likely to be important for the intracellular lifestyle of B. abortus. Like the PhoPQ two-component system of Salmonella, this phosphorelay may control cell surface remodeling during infection, which is known to be important for intracellular survival (51).
The transcriptional output of conserved phosphorelays like the CckA–ChpT–CtrA–CpdR system (14) or more simple, archetypal two-component systems like FixL–FixJ (52) are often tailored to the unique physiologies of the species in which they are encoded. We note that consensus CtrA binding sites, based on published B. abortus CtrA targets (15), are present in front of several known B. abortus virulence factors including superoxide dismutase (sodC) and flagellar regulatory gene ftcR (53, 54). Defining genes in the Brucella CtrA regulon that uniquely control its function as a facultative intracellular pathogen is an important area of future investigation.
The structure of ChpT is related to classic HKs and is distinct from phosphotransferase proteins like Ypd1, which lack a CA-like domain (44). It is likely that ChpT arose through HK gene duplication and subsequently evolved to function solely as a phosphotransferase. Indeed, the ChpT CA domain does not bind ATP, supporting a model in which ChpT structure and function have diverged from an ancestral HK (32). This raises the question of what function ChpT–CA plays if it does not bind nucleotide. It is possible that the ChpT–CA domain is conserved because it has an important structural role in binding to receiver domain substrates. Certainly, the crystal structure of ChpT bound to CtrAREC reveals several direct interactions between CtrAREC and ChpT–CA (Fig. 5 and Fig. S5 B, C, and D) that are related to those in structurally defined HK–REC systems (42). The ChpT–CA domain may simply enhance stability of ChpT–REC complexes and facilitate efficient phosphotransfer. Alternatively, ChpT–CA may confer additional regulatory capacity on the system. For example, the binding of different nucleotide forms by the CA domain can modulate both kinase and phosphatase activities of HKs (18, 55, 56). Although ChpT does not bind ATP, there may be other small molecule ligands that can serve to regulate interaction of ChpT with its receiver substrates. The clear presence of a glycerol molecule in the electron density maps of the ChpT–CA binding pocket (Fig. S5A) demonstrates that CA has the capacity to accommodate small molecules and suggests that small-molecule binding to ChpT–CA could allow for the integration of additional signals into this important pathway.
Fig. S5.
(A) Simulated annealing composite omit map (contoured at ±2σ) of a region surrounding a glycerol molecule (GOL; colored green) in the binding pocket of the ChpT CA domain. (B) Interaction map of polar contacts between ChpT monomers (A and B; blue) and CtrAREC domains (C and D; red), hydrogen bonds (black lines), and salt bridges (dotted red lines). Magnified view of ChpT–CtrA complex structure showing (C) ChpT(A)–CtrA(C) and (D) ChpT(B)–CtrA(D) interacting residues.
The experimental structure of the ChpT–CtrA heterotetramer revealed three residues in the DHp-like domain (N33, E36, and E40) and three residues in the CA-like domain of ChpT (K96, R153, and H177) that interact with CtrA on each half of the ChpT homodimer (Fig. 5B). These ChpT residues correspond to the so-called specificity residues that govern specific HK-to-response regulator (RR) phosphoryltransfer (48, 57). Our structure thus provides evidence that the ChpT phosphotransferase interacts with its cognate substrates in a manner that is structurally similar to classic HK–RR interactions. Alanine mutagenesis showed that the ChpT–RR structural interface is robust to multiple substitutions under the tested in vitro conditions. This result is consistent with mutagenesis studies of the kinases CrdS from Myxococcus xanthus and EnvZ from Escherichia coli, in which substitution of select CrdS and EnvZ specificity residues had minimal impact on phosphoryltransfer to their cognate RRs in vitro (58, 59). However, it may be the case that these substitutions affect phosphoryltransfer specificity in vitro or signaling in B. abortus cells.
A notable structural feature reported in this study is asymmetry in the positions of the ChpT phosphorylation site (H22) and the CtrA phosphoryl receiver residue (D51) (Fig. 5 C and D). H22 occupies identical rotamer conformations in the structure of ChpT alone but two distinct rotamer conformations on each side of the ChpT homodimer in the 2:2 ChpT–CtrA complex. On one side of the complex, H22 is in line with D51 of CtrA, which is well-positioned to receive a phosphoryl group. On the opposite side of the complex, both H22 and D51 adopt different rotamer conformations and would not be able to undergo phosphotransfer without a large change in H22 and D51 side-chain conformation. The half of the complex that is positioned to undergo phosphotransfer has a larger ChpT–CtrA contact surface than the half that is incompatible with phosphotransfer. These structural data suggest that the ChpT dimer has at least two distinct interaction modes with CtrA substrate: one that is competent to catalyze phosphotransfer and one that buries less ChpT–REC surface area and is structurally incompetent for phosphotransfer. Although the asymmetry of ChpT–CtrA interaction observed in our structure may be a function of embedment in the crystal lattice, long-time-scale MD simulations presented herein support a model in which the histidine phosphorylation sites on each half of the ChpT dimer have distinct structural and dynamical properties, particularly in the context of the 2:2 ChpT–CtrA complex (Fig. 5 B–D and Fig. S5). Thus, at any instant only one side of the ChpT–CtrA complex may be competent to undergo a His–Asp phosphotransfer reaction. Our structures and simulations do not directly address the effects of adding phosphoryl groups to H22 or D51. The affects of phosphoryl addition to H22 or D51 on protein structure and dynamics are a topic of ongoing investigation.
We note that structural asymmetry has been reported for other HKs. Thus, the structural and dynamical asymmetry we observe across the central twofold axis of the 2:2 ChpT–CtrA heterodimer may reflect a general (but not necessarily universal) feature of two-component phosphorelays. Indeed, it is established that the autophosphorylation equilibria of each subunit of dimeric E. coli NtrB differ by nearly two orders of magnitude (60). The structures of HKs HK853 (34), DesK (61), YF1 (62), VicK (36), and CpxA (35) adopt different tertiary structures in the frame of the central HK dimer axis. Structures of VicK, YF1, and CpxA in particular show highly asymmetric positioning of the CA domain: One CA is proximal to the site of histidine phosphorylation and the second CA domain is distal. Moreover, recent work on T. maritima HK853 provides structural evidence for asymmetry in the histidine autokinase reaction (63), even though the ATP-binding CA domains occupy symmetrical positions in the unphosphorylated structure (34). Although ChpT is not a bona fide HK, our study provides evidence that this protein has retained some structural features common to other two-component His–Asp phosphorelay systems and may thus serve as an excellent model to investigate the structural and dynamical mechanisms that underpin function of these complex regulatory systems.
Materials and Methods
Bacterial Culture and Strain Construction.
All strains and primers used in this study are detailed in Tables S3 and S4. E. coli strains were constructed using routine cloning techniques. All B. abortus strains were maintained and constructed using previously described techniques (64) under biosafety level 3 (BSL3) conditions per Centers for Disease Control and Prevention (CDC) rules and regulations governing the use of select agents. Further details on strain generation and maintenance are provided in Supporting Information.
Table S3.
Strains used in this study
| FC no. | E. coli strain | Notes |
| 2271 | Rosetta / pET28a-RR0099 | Expresses soluble REC domain - Bab1_0099 |
| 2272 | Rosetta / pET28a-RR0136 | Expresses soluble REC domain - Bab1_0136 |
| 2273 | Rosetta/pET28a-RR0345 | Expresses soluble REC domain - Bab1_0345 |
| 2274 | Rosetta/pET28a-RR0370 | Expresses soluble REC domain - Bab1_0370 |
| 2275 | Rosetta/pET28a-RR0628 | Expresses soluble REC domain - Bab1_0628 |
| 2276 | Rosetta/pET28a-RR0636 | Expresses soluble REC domain - Bab1_0636 |
| 2277 | Rosetta/pET28a-RR1138 | Expresses soluble REC domain - Bab1_1138 |
| 2278 | Rosetta/pET28a-RR1140 | Expresses soluble REC domain - Bab1_1140 |
| 2279 | Rosetta/pET28a-RR1538 | Expresses soluble REC domain - Bab1_1538 |
| 2280 | Rosetta/pET28a-RR1614 | Expresses soluble REC domain - ctra - Bab1_1614 |
| 2281 | Rosetta/pET28a-RR1671 | Expresses soluble REC domain - Bab1_1671 |
| 2282 | Rosetta/pET28a-RR2006 | Expresses soluble REC domain - Bab1_2006 |
| 2283 | Rosetta/pET28a-RR2092 | Expresses soluble REC domain - Bab1_2092 |
| 2284 | Rosetta/pET28a-RR2146 | Expresses soluble REC domain - Bab1_2146 |
| 2285 | Rosetta/pET28a-RR0041 | Expresses soluble REC domain - cpdr - Bab2_0041 |
| 2286 | Rosetta/pET28a-RR0042 | Expresses soluble REC domain - Bab2_0042 |
| 2287 | Rosetta/pET28a-RR0081 | Expresses soluble REC domain - Bab2_0081 |
| 2288 | Rosetta/pET28a-RR0222 | Expresses soluble REC domain - Bab2_0222 |
| 2289 | Rosetta/pET28a-RR0628 | Expresses soluble REC domain - Bab2_0628 |
| 2290 | Rosetta/pET28a-RR0630 | Expresses soluble REC domain - Bab2_0630 |
| 2291 | Rosetta/pET28a-RR0762 | Expresses soluble REC domain - Bab2_0762 |
| 2292 | Rosetta/pET28a-RR0806 | Expresses soluble REC domain - Bab2_0806 |
| 2293 | Rosetta/pET28a-RR1099 | Expresses soluble REC domain - Bab2_1099 |
| 2294 | Rosetta/pET28a-chpT | Expresses full-length chpt - Bab1_1613 |
| 2295 | Rosetta/pET28a-chpT(N33A,E36A,E40A) | Site-directed mutant of FC2294 |
| 2296 | Rosetta/pET28a-chpT(K96A,R156A,H177A) | Site-directed mutant of FC2294 |
| 2297 | TOP10/pNPTS138::ctrA(V148F) | For making V148F mutant in Brucela |
| 2298 | Rosetta/pET28a-chpT(N33R) | Site-directed mutant of FC2294 |
| 2299 | Rosetta/pET28a-chpT(E36R) | Site-directed mutant of FC2294 |
| 2300 | Rosetta/pET28a-chpT(E40R) | Site-directed mutant of FC2294 |
| 2301 | Rosetta/pET28a-ctrA(S15R) | Site-directed mutant of FC2280 |
| 2302 | Rosetta/pET28a-cpdR(F16R) | Site-directed mutant of FC2285 |
| 2303 | Rosetta/pET28a-cckAsoluble | Expresses soluble fragment of ccka |
| CBA no. | Brucella strain | |
| 21 | B. abortus 2308 | WT Brucella strain |
| 25 | 2308 pSRK- | WT Brucella with empty pSRK plasmid |
| 113 | 2308 pSRK-chpT | WT Brucella expressing chpT-WT from pSRK |
| 114 | 2308 pSRK-chpT(E36R) | WT Brucella expressing chpT(E36R) from pSRK |
| 115 | 2308 ctrA(V148F) | Temperature-sensitive allele of ctrA |
| 116 | 2308 pSRK-cpdR(WT) | WT Brucella expressing cpdR-WT |
| 117 | 2308 pSRK-cpdR(D52A) | WT Brucella expressing cpdR-D52A |
“FC” and “CBA” refer to S.C. laboratory strain prefixes.
Table S4.
Primers used in this study
| Name | Sequence 5′→3′ | Notes |
| RR0099-BamHI F | AAGGATCCTTGATGAACGATCTCGAACATAGG | BAB1_0099; for cloning BamHI/HindIII fragment into pET28a for overexpression; FL protein |
| RR0099-HindIII R | AAAGCTTTCAGGCGACGCTGGATGA | |
| RR0136 - BamHI - F | GGATCCATGGAAGACTTGAAGCA | BAB1_0136 - for cloning BamHI/HindIII fragment into pET28a for overexpression |
| RR0136 - HindIII - R | AAGCTTTCAGCGCGGCGCGCGCT | |
| RR0345 - BamHI- F | GGATCCATGCATTTCATTATCGC | BAB1_0345 - for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–121 |
| RR0345 - HindIII - R | AAGCTTtcaGCCGGCAAGCACCGCACCCA | |
| RR0370 - BamHI - F | GGATCCATGACGAATGAATGCAA | BAB1_0370; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–126 |
| RR0370 - HindIII - R | AAGCTTtcaGCCGGAGGCGACGGCAT | |
| RR0628 - BamHI - F | GGATCCTTGCGTATCCTGATTGTTGA | BAB1_0628; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–120 |
| RR0628 - HindIII - R | AAGCTTtcaGGCAGCACGGCGGATCA | |
| RR0636 - BamHI - F | GGATCCATGAAAATTCTCGTTATC | BAB1_0636; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–118 |
| RR0636 - HindIII - R | AAGCTTtcaGCGGCGCTGCAAGACCTCGACAC | |
| RR1138 - BamHI - F | GGATCCATGGCGGCCGATATTCT | BAB1_1138; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–120 |
| RR1138 - HindIII - R | AAGCTTtcaGGTTTCCAGCGCCCGTT | |
| RR1140 - BamHI - F | GGATCCATGATTGCAGGGCGTCCGA | BAB1_1140; for cloning BamHI/HindIII fragment into pET28a for overexpression: amino acids 1–120 |
| RR1140 - HindIII - R | AAGCTTtcaTTCCGAGAGCGCGCGCC | |
| RR1538 - BamHI - F | GGATCCATGGGATCGAAAGACGC | BAB1_1538; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–137 |
| RR1538 - HindIII - R | AAGCTTtcaACGGCGCAGGATATTGT | |
| RR1614 - BamHI -F | GGATCCATGCGCGTCCTTTTGAT | BAB1_1614; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–118 |
| RR1614 - XhoI - R | CTCGAGtcaGCGGCGGACGATCGCATGGATACGG | |
| RR1671 - BamHI - F | GGATCCCGTCTGATGATCATTGAGGATG | BAB1_1671; for cloning BamHI/XhoI fragment into pET28a for overexpression; amino acids 141–264 |
| RR1671 - XhoI - R | CTCGAGTCAGGCAGCAACTTGCGA | |
| RR2006 - BamHI - F | GGATCCATGACAGGCCGTACTAT | BAB1_2006; for cloning BamHI/XhoI fragment into pET28a for overexpression; amino acids 1–122 |
| RR2006 - XhoI - R | CTCGAGtcaCTGGCGCAACTGCGCGC | |
| RR2092 - BamHI - F | GGATCCATGAAGGAAGCTTCGGC | BAB1_2092; for cloning BamHI/XhoI fragment into pET28a for overexpression; amino acids 1–128 |
| RR2092 - XhoI - R | CTCGAGtcaGCGCGCCGCCACGCGGC | |
| RR2146 - BamHI - F | GGATCCATGTCCCAAGTACCCCGA | BAB1_2146; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–124 |
| RR2146 - HindIII - R | AAGCTTtcaCGCCTTGACGCGGGCAA | |
| RR0041 - BamHI - F | GGATCCATGAACTGCGCATATGA | BAB2_0041; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–126 |
| RR0041- HindIII - R | AAGCTTtcaTGTCCGGATCGCCTGTT | |
| RR0042- BamHI - F | GGATCCATGAAGAGAATCCTTCTAGCTGA | BAB2_0042; for cloning BamHI/HindIII fragment into pET28a for overexpression; FL protein |
| RR0042 - HindIII - R | AAGCTTTCAGGCGGCGATCAGCA | |
| RR0081 - BamHI -F | GGATCCATGGCAACGCGCATTCT | BAB2_0081; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–121 |
| RR0081 - HindIII - R | AAGCTTtcaGAGTTTCAACACATTTGC | |
| RR0222 - BamHI - F | GGATCCATGAGAATTATCCTCAT | BAB2_0222; for cloning BamHI/HindIII fragment into pET28a for overexpression |
| RR0222- HindIII - R | AAGCTTtcaGCGCCGCGCTACCGCGT | |
| RR0628 - BamHI -F | GGATCCATGACGAAAAGCGTAAT | BAB2_0628; for cloning BamHI/HindIII fragment into pET28a for overexpression; FL protein |
| RR0628 - HindIII - R | AAGCTTCTAGGCATCGCCCAGAT | |
| RR0630 - BamHI - F | GGATCCATGACAGCAAGGATTCTCGTCG | BAB2_0630: for cloning BamHI/XhoI fragment into pET28a for overexpression: For amplification of both REC domains together |
| RR0630 - XhoI - R | CTCGAGtcaGCGCTTGATCTGTGTGC | |
| RR0762 - BamHI - F | GGATCCTTGAAAGAAGACGCCCACATTC | BAB2_0762; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–121 |
| RR0762 - HindIII - R | AAGCTTtcaACGGCGAAGCACGGCGC | |
| RR0806 - BamHI - F | GGATCCATGAATATTTCTCCACA | BAB2_0806; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–157 |
| RR0806 - HindIII - R | AAGCTTtcaGCCAGCCATGGCAAGCCGA | |
| RR1099 - BamHI - F | GGATCCATGATTGTTGTCGTTGACGACA | BAB2_1099; for cloning BamHI/HindIII fragment into pET28a for overexpression; amino acids 1–106 |
| RR1099 - HindIII - R | AAGCTTtcaGCGGACATGAACGGGCTT | |
| Hpt1613 - BamHI - F | GGATCCATGTCCTTGCCCGTTAC | Bab1_1613, ChpT; for cloning BamHI/HindIII fragment into pET28a for overexpression; expresses entire protein |
| Hpt1613 - Hindiii - R | AAGCTTTCATTCCGCGGAAAAGACGATAT | |
| dHpt1613-Up-BamHI-F | GGATCCAGCGATGAAGGAGACAAACAGA | For deletion of B. abortus Hpt 1613 |
| dHpt1613-Up-R | ccgcggaCAAGGACATGGCGCTTT | |
| dHpt1613-Dn-F | ATGTCCTTGTCCGCGGAATGAATTGA | |
| dHpt1613-Dn-SalI-R | AAGTCGACACGATTATATGACCAAG | |
| CckA-kinase only-BamHI-F | ATATGGATCCGCGCTGGAAAACCAGAT | For cloning BamHI/HindIII fragment into pET28a for overexpression; for soluble form of CckA |
| CckA-FL-HindIII-R | ATATAAGCTTTCAATCCTGTTTCTCCAG | |
| ChpT-F-NdeI-Brucella-o/E | TATACATATGTCCTTGCCCGTTAC | Site-directed mutagenesis primers |
| ChpT-R-KpnI-Brucella-o/E | ATATGGTACCTCATTCCGCGGAAAAGACGATAT | |
| ChpT-E40A-R | catcggcgccacctgcttccagcaattcc | |
| ChpT-E40A-F | ggaattgctggaagcaggtggcgccgatg | |
| ChpT-N33A,E36A-R | cttcttccagcaatgccagaccagcattgatcgcaccgaccggt | |
| ChpT-N33A,E36A-F | accggtcggtgcgatcaatgctggtctggcattgctggaagaag | |
| ChpT-K96A-R | aggtgaattccggcgcttcgttcctgaaatattcagtggcga | |
| ChpT-K96A-F | tcgccactgaatatttcaggaacgaagcgccggaattcacct | |
| ChpT-R156A-R | cggcggcacggctagcatccggcctttgac | |
| ChpT-R156A-F | gtcaaaggccggatgctagccgtgccgccg | |
| ChpT-H177A-R | gggctgtacggaagcggcgtcgatgggc | |
| ChpT-H177A-F | gcccatcgacgccgcttccgtacagcc | |
| chpT-R-HA+XbaI | tctagaTCAAGCGTAATCTGGAACATCGTATGGGTATTCCGCGGAAAAGACGATAT | |
| CtrA-V148F-R | ccggtcagatgaaagcgctggcctgcg | |
| CtrA-V148F-F | cgcaggccagcgctttcatctgaccgg | |
| ChpT-N33R-R | gcaattccagacccctattgatcgcaccgaccggtgag | |
| ChpT-N33R-F | ctcaccggtcggtgcgatcaataggggtctggaattgc | |
| ChpT-E36R-R | gccaccttcttccagcaatctcagaccattattgatcgca | |
| ChpT-E36R-F | tgcgatcaataatggtctgagattgctggaagaaggtggc | |
| ChpT-E40R-R | atcggcgccacctctttccagcaattccagaccattattg | |
| ChpT-E40R-F | caataatggtctggaattgctggaaagaggtggcgccgat | |
| ctrA-s15R-R | ttgagcatcaactcaatcctctgtgcgatagcact | |
| ctrA-s15R-F | agtgctatcgcacagaggattgagttgatgctcaa | |
| cpdR-F16R-R | ccagcgcttttacgaggcgacggcgcatatcgttatcg | |
| cpdR-F16R-F | cgataacgatatgcgccgtcgcctcgtaaaagcgctgg | |
| rplK_qPCR_F | CCGGTGACCTACTTCCTCAA | Housekeeping control for qPCR |
| rplK_qPCR_R | ATCGAACGGGCAGAACCT | |
| ccrM_qPCR_F | GGCTCGACTCCATCATCAAA | For qPCR to analyze B. abortus ccrM (bab1_0516). Yields 109-bp PCR product |
| ccrM_qPCR_R | CCGCCAAGCTGGAGATTATAG |
In Vitro Kinase and Phosphotransfer Assays.
Genes encoding proteins to be overexpressed were cloned into pET28a (Novagen), expressed in Rosetta E. coli (DE3), and purified using an N-terminal His6-tag. All kinase and phosphotransfer reactions were performed following previously published methods (20, 21, 65). For complete protocols see Supporting Information.
Crystallization of ChpT and the ChpT–CtrA Complex.
All crystallization conditions used the hanging drop vapor diffusion technique. The structure of ChpT alone was obtained by mixing CckAsoluble (20 mg⋅mL−1 final) and ChpT (4.7 mg⋅mL −1 final) and seeding using a horse hair in the following crystallization buffer: 0.2 M ammonium sulfate, 0.1 M sodium acetate pH 5.5, and 10% (wt/vol) PEG 2000 MME. The ChpT2–CtrA2 complex was obtained by mixing ChpT (8.4 mg⋅mL −1) and CtrA (5.3 mg⋅mL −1) after concentration using the following crystallization buffer: 0.1 M Hepes pH 7.0 and 8% (wt/vol) PEG 8000. After mixing 1.5 μL of protein solution and 1.5 μL of crystallization buffer against 500 μL of crystallization buffer in the well, all crystals grew for 7 d and were mounted after soaking for 1 min in crystallization buffer supplemented with 25% (vol/vol) glycerol.
Crystallographic Data Collection, Processing, Phasing, and Refinement.
All diffraction data were collected on beamline 21-ID-F (Life Sciences Collaborative Access Team, Advanced Photon Source) and reduced using the HKL 2000 software suite. All structures were solved by molecular replacement in PHENIX (66) using models that were initially based off C. crescentus ChpT (33). Model building and refinement was conducted using Coot and PHENIX, accessed through the SBGrid consortium (67). Coordinates of the B. abortus ChpT (PDB ID code 4QPK) and the 2:2 ChpT–CtrA complex (PDB ID code 4QPJ) have been deposited in the Protein Data Bank.
Biophysical Analyses of ChpT Structure.
Analyses of ChpT by CD spectroscopy, ITC, and SAXS were performed using previously published methods (59, 68, 69) and are described in detail in Supporting Information. SAXS data on ChpT were collected at the Advanced Photon Source beamline 18-ID (BioCAT).
Molecular Dynamics Simulations.
Fully atomistic models of ChpT, CtrA, and the ChpT–CtrA complex were constructed based on the crystallographic data presented in this study. The simulations were run for 1,851, 1,000, and 886 ns, respectively. Further details are provided in Supporting Information.
Imaging and Analysis of Brucella Cells.
Before analysis by LM, flow cytometry, and CryoEM, B. abortus cells were fixed following established BSL3 B. abortus protocols at the University of Chicago Ricketts Laboratory. All infection assays using differentiated THP-1 macrophages used an MOI of 100. All results presented are the mean ± SEM. Details of sample preparation and analysis are outlined in Supporting Information.
SI Materials and Methods
Bacterial Culture and Strain Construction.
All strains used in this study are detailed in Table S3. E. coli strains used for cloning were grown in LB (37 °C) supplemented with 50 μg⋅mL−1 kanamycin (Kan) or 100 μg⋅mL−1 ampicillin when required. All strains were constructed using standard cloning techniques. Primers are listed in Table S4. Site-directed mutagenesis was performed using the PCR-sewing method. All constructs were verified by DNA sequencing.
All B. abortus strains were grown on either Schaedler agar (Difco) supplemented with 5% defibrinated bovine blood (SBA) or Brucella broth (Difco) and grown at 37 °C supplemented with 5% CO2 [with the exception of the ctrA(V148F) temperature-sensitive strain, which was grown at 30 °C]. When needed, for maintenance of plasmids, B. abortus strains were supplemented with 50 μg⋅mL−1 Kan to maintain selection. All studies on live B. abortus strains were performed at the University of Chicago Howard T. Ricketts Laboratory under BSL3 conditions per CDC rules and regulations.
Overexpression of chpT and cpdR(WT) and cpdR(D52A) was performed by cloning into the replicating, IPTG-inducible vector pSRK (70). Expression was induced by the addition of 2 mM IPTG.
Purification of His6-Tagged Proteins Expressed in E. coli.
Genes encoding proteins to be overexpressed were cloned into pET28a (Novagen) and expressed in Rosetta E. coli(DE3). All proteins used in the kinase and phosphotransfer assays were purified using the following method: For each strain, a 10-mL overnight culture was used to inoculate a 250-mL culture of Terrific Broth supplemented with Kan50 and grown (37 °C, 220 rpm) until OD600 reached 0.7–0.8. Cultures were then induced with 1 mM IPTG and grown for 4–6 h at 20 °C, before being pelleted by centrifugation in a Sorvall F10S-6 × 500y rotor at 6,000 × g for 10 min. Cell pellets were stored at −20 °C until purification. For purification, cell pellets were thawed and suspended in 10 mL of lysis buffer [25 mM Tris pH 7.6, 125 mM NaCl, 10 mM imidazole, 1% (vol/vol) Triton X-100, 5 μg⋅mL−1 DNaseI, 50 μg⋅mL−1 PMSF, and 3 mg⋅mL−1 lysozyme]. Samples were incubated for an hour and pelleted to remove insoluble cell debris. Lysates were added to a column containing 4 mL of Chelating Sepharose FF (GE) charged with Ni2+ and equilibrated with column buffer (25 mM Tris pH 7.6, 125 mM NaCl, and 10 mM imidazole). CckA and REC domain proteins were dialyzed overnight at 4 °C against storage buffer (25 mM Tris pH 7.6, 125 mM NaCl, and 50% glycerol) and stored at −20 °C. After purification ChpT proteins were dialyzed against 25 mM Tris pH 7.6 and 125 mM NaCl and aliquots were snap-frozen in liquid nitrogen and stored at −80 °C.
For protein crystallography 50 mL of overnight E. coli cultures were added to 2 L of LB, grown with shaking (37 °C, 220 rpm) until OD600 reached 0.6, and induced with 1 mM IPTG. After 4 h, cells were pelleted as above and stored at −20 °C until purification. For purification, cells were thawed and resuspended in 50 mL buffer A (25 mM Tris pH 7.6 and 125 mM NaCl) and lysed by three passages through a French pressure cell. Cells were clarified by a 20,000 × g spin and lysates were loaded onto a column loaded with 10 mL chelating resin listed above, equilibrated in buffer A. Resin was washed five times with three column volumes of column buffer and eluted with elution buffer (25 mM Tris pH 7.6, 125 mM NaCl, and 500 mM Imidazole). All proteins were dialyzed against 2 L of dialysis buffer (25 mM Tris pH 7.6 and 125 mM NaCl), concentrated using 3-kDa (molecular weight cut-off, MWCO) centrifugal filters (Amicon; Millipore). All proteins were assayed for purity by 14% SDS/PAGE.
In Vitro Kinase and Phosphotransfer Assays.
All kinase and phosphotransfer reactions were carried out in 1× kinase buffer (10× kinase buffer, 250 mM Tris pH 7.6, 500 mM KCl, 10 mM MgCl2, 10 mM MnCl2, 10 mM CaCl2, and 10 mM DTT) (59). Reactions were incubated at room temperature and were initiated by addition of an ATP mix (25 μM ATP and 0.3 μM [γ-32P]ATP). At the indicated times, aliquots were removed and stopped by addition to an equal volume of SDS-loading buffer. In phosphotransfer reactions, CckA was allowed to autophosphorylate for 30 min before addition to ChpT and or CpdR/CtrA. All proteins were used at a final concentration of 5 μM. Samples were resolved by 15% SDS/PAGE. The dye front, which contains unincorporated ATP, was removed and gels were subsequently exposed for 2–4 h on a phosphor screen before visualization using a FX Pro Plus Molecular Imager (Bio-Rad).
Phosphotransfer profiling experiments were performed following previously published methods (20, 21). Briefly, CckA was allowed to autophosphorylate for 30 min and incubated with either ChpT or buffer for 5 min before proceeding with phosphotransfer reactions. CckA or CckA–ChpT solutions were incubated with each REC domain for 15 s before reactions were stopped by addition of an equal volume of SDS/PAGE buffer. The final concentration of proteins in these profiling experiments was 2.5 μM CckA, 6.6 μM ChpT, and 12.5 μM of each REC domain. AcP labeling of REC domains was performed as previously described (20, 59). Reactions were resolved by running on a Precast Criterion 18% SDS/PAGE gel (Bio-Rad) and visualized as above.
Crystallization of ChpT and the ChpT–CtrA Complex.
Proteins were purified following the methods described above. All crystallization conditions used the hanging drop vapor diffusion technique. The final concentration of proteins used for screening for the ChpT–CtrA complex were 8.4 mg⋅mL−1 ChpT and 5.3 mg⋅mL−1 CtrA. The structure of ChpT alone was obtained by mixing CckAsoluble (20 mg⋅mL −1 final) and ChpT (4.7 mg⋅mL−1 final) in solution after purification and concentration. Initial screening was performed in a 96-well plate format using a Mosquito robot (TTP Labtech), which mixed 0.1 μL of protein solution and 0.1 μL of the crystallization solution and subsequently equilibrated this drop against 75 μL of crystallization buffer. Commercial crystallization kits were used for initial crystallization screening (Nextel Suites; Qiagen). After manual refinement in 24-well plates (Hampton Research) the best crystals for ChpT–CtrA were obtained in the following crystallization buffer at 19 °C: 0.1 M Hepes pH 7.0 and 8% (wt/vol) PEG 8000. The best crystals for ChpT alone were obtained after seeding using a horse hair in the following crystallization buffer: 0.2 M ammonium sulfate, 0.1 M sodium acetate pH 5.5, and 10% (wt/vol) PEG 2000 MME. For each crystallization condition, manual drops were set up by adding 1.5 μL of protein solution and 1.5 μL of crystallization buffer against 500 μL of crystallization buffer in the well. Crystals grew for 7 d and were mounted after soaking for 1 min in crystallization buffer supplemented with 25% (vol/vol) glycerol.
Crystallographic Data Collection, Processing, Phasing, and Refinement.
All diffraction data were collected on beamline 21-ID-F (Life Sciences Collaborative Access Team, Advanced Photon Source) using a MAR Mosaic 225 detector. Datasets were obtained from frozen, loop-mounted single crystals at 100 K using an oscillation range of 1° and reduced using the HKL 2000 software suite.
All structures were solved using by molecular replacement in PHENIX (66) using models that were initially based off the structure of C. crescentus ChpT (33). All model building and refinements were conducted using Coot and PHENIX, which are maintained by the SBGrid consortium (67). Coordinates of the B. abortus ChpT (PDB ID code 4QPK) and the 2:2 ChpT–CtrA complex (PDB ID code 4QPJ) have been deposited in the Protein Data Bank.
CD Spectroscopy.
Protein samples for CD spectroscopy were purified as described above, dialyzed overnight in CD buffer (25 mM Na phosphate pH 7.6 and 50 mM NaCl) and diluted to a final concentration of 25 μM in CD buffer. Samples were analyzed using a 1 mM cuvette on an Aviv 202 CD spectrometer. Data shown are the average of three separate spectral scans.
ChpT–ATP Binding Measurements Using ITC.
Proteins for ITC analysis were purified as detailed above and dialyzed overnight against ITC buffer (25 mM Tris pH 7.6, 125 mM NaCl, and 1 mM MgCl2) and concentrated using Amicon Ultra 3k MWCO spin columns. Samples were subsequently purified further using a HiPrep 26/60 Sephacryl S-200 (GE) gel-filtration column equilibrated in ITC buffer and again dialyzed overnight against ITC buffer.
All binding measurements were performed using a MicroCal200 iTC (GE). All solutions and samples were extensively degassed. The cell of the MicroCal200 iTC was filled with ChpT at concentrations of 200 μM (monomer). ChpT protein concentrations were determined using the calculated extinction coefficient of ChpT. The syringe was filled with 1 mM ATP (Sigma-Aldrich) resuspended in ITC buffer. Titrations were initially performed at 25 °C with constant stirring. To confirm that the absence of observed binding between ChpT and ATP was not due to high-affinity binding reactions with ΔH values near 0, we performed separate ITC experiments at 16 °C (where there was also no apparent binding).
MD Simulations.
Fully atomistic models of ChpT, CtrA, and the ChpT–CtrA complex were constructed based on the crystallographic data presented in this study. Missing side chains were added to the models using a least-squares fit to the backbone atoms when necessary. All simulations were run with the Gromacs MD package, version 4.6.5 (71). Each structure was solvated in TIP3 water with Na+ and Cl− counter ions to achieve a neutral periodic cell at 150 mM concentration. The Amber99sb-ILDN forcefield was used (72). The systems contained 95,606, 20,830, and 157,620 atoms, respectively. Each system was energy-minimized using a gradient descent method to remove high-energy contacts between the protein and the solvent and relax any steric clashes. All bonds were replaced by constraints using the LINCS algorithm (73). To equilibrate the systems, 10-ns simulations in the NPT ensemble were performed at 300 K, using the Parrinello–Rahman barostat at a pressure of 1 bar (74). Samples were collected from simulations of the NVT ensemble, which was maintained by a Nosé–Hoover thermostat with a reference temperature of 300 K. The simulations were run for 1,851, 1,000, and 886 ns, respectively. A 5-fs time step was used in all production simulations. Electrostatics were evaluated every four time steps using the particle mesh Ewald method (75).
SAXS.
SAXS data on ChpT were collected at the Advanced Photon Source beamline 18-ID (BioCAT, Argonne National Laboratory). Purified ChpT was suspended in 25 mM Tris pH 7.6 and 125 mM NaCl at a concentration of 15 μM. Data were collected using an Aviex CCD detector and analyzed in IgorPro.
LM and CryoEM of B. abortus.
Brucella cells were grown in rich media for the indicated time and fixed with 4% (vol/vol) paraformaldehyde in PBS for 30 min. Samples were washed two times in PBS and suspended in EM imaging buffer (20 mM Tris pH 7.6, 50 mM glucose, and 10 mM EDTA) and samples were confirmed to contain no viable cells, following established BSL3 B. abortus protocols at the University of Chicago Ricketts Laboratory. Phase-contrast images of cells were collected using a Leica DM 5000B microscope with an HCX PL APO 63×/1.4 N.A. Ph3 objective. Images were acquired with a mounted Orca-ER digital camera (Hamamatsu) controlled by the Image-Pro software suite (Media Cybernetics).
From visualization by cryoEM fixed cells were mixed with BSA-treated 10 nm colloidal gold solution to avoid aggregation (76). Three to four microliters of a cell culture–gold solution mixture were applied to glow discharged copper Quantifoil grids (Quantifoil Micro Tools). The grids were blotted either manually or automatically using the Vitrobot (FEI) and plunged into a liquid ethane/propane mixture (76, 77). The grids were kept in liquid nitrogen until they were loaded into a Gatan 626 cryoholder and examined on an FEI T12 electron microscope at 120 keV.
Analysis of Brucella Cell Shape and DNA Content.
Cells analyzed by LM and flow cytometery were first grown to the indicated time in rich media, fixed, and removed from BSL3 containment as detailed above. Phase-contrast images were obtained as detailed as above and uniformly thresholded using ImageJ (78). Cell contours were extracted and cell area analyzed using the open source Celltool application (31). The following numbers of cells were analyzed for cell shape analysis: WT (n = 611), ΔcpdR (n = 1,395), ctrA(V148F) (n = 216), chpT++ (n = 140), chpT(E36R)++ (n = 261), cpdR(WT)++ (n = 321), and cpdR(D52A)++ (n = 113).
Flow cytometery to measure DNA content was performed on a BD FACSAria II cytometer. Propidium iodide was added (10 μg/mL final) to 1 mL of fixed cells before analysis by flow cytometery. Data for 20,000 individual cells were collected and analyzed using the FlowJo software suite (FlowJo Enterprise).
THP1 Macrophage Infection Assays.
Human monocytic THP-1 cells (ATCC TIB-202) were maintained by culture in RPMI-1640 supplemented with 10% (vol/vol) heat-inactivated FBS and 2 mM l-glutamine. For monocytic differentiation of THP-1 cells, 1 × 105 cells in RPMI supplemented with phorbol ester 12-O-tetradecanoylphorbol-13-acetate (40 μg⋅mL−1) were added to a each well of 96-well plate, and cells were grown for 24–48 h before proceeding with infections. All THP-1 cells were grown at 37 °C with 5% (vol/vol) atmospheric CO2.
For infections, B. abortus cells were resuspended in warmed RPMI and added at an MOI of 100 cfu per macrophage cell. To synchronize infections after the addition of Brucella cells, plates were centrifuged at 200 × g for 5 min. After a 30-min incubation, media was removed and replaced with RPMI supplemented with 100 μg⋅mL−1 gentamycin to kill extracellular bacteria. Afterward, samples were incubated for 1 h, after which monolayers were washed with PBS and THP-1 cells were lysed by addition of 0.01% Triton X-100. Serial dilutions were prepared and viable cells were determined by plating on TSA plates. For longer time points, the RPMI was removed and replaced with RPMI supplemented with 50 μg⋅mL−1 gentamycin. Additional samples were lysed as above and serial dilutions plated. All THP-1 assays were performed in triplicate using independent B. abortus cultures. The results presented illustrate the mean ± the SEM. For THP-1 cell infections at the ctrA(V148F) permissive growth temperature of 30 °C, THP-1 cells were initially prepared as above, but after infections cells were grown at 30 °C with 5% (vol/vol) atmospheric CO2.
Western Blot Analysis of CtrA Levels.
For Western blot analysis, overnight cultures of B. abortus were diluted to OD600 of 0.1 and cultured for 4 h after shift to the nonpermissive growth condition or addition of IPTG as indicated. Five milliliters of cultures were spun down and resuspended in 4× SDS-loading buffer treated with DNaseI and 15 μL was resolved by SDS/PAGE. Primary CtrA polyclonal antiserum was a gift from Peter Chien, University of Massachusetts, Amherst, MA, and Brucella α-PhyR polyclonal antiserum was used as a loading control. α-Rabbit antibody was used as a secondary, which was conjugated to Alexa 488 (Life Technologies).
RNA Isolation and qRT-PCR.
RNA was extracted following a previously published method (65) after 4 h of growth at the nonpermissive temperature or IPTG addition in rich medium (starting at an OD600 = 0.1). cDNA was generated using the SuperScript III cDNA kit (Invitrogen) and qPCR was carried out using a SYBR Green PCR kit (Life Technologies). Relative quantification of the ccrM transcript was calculated using the 2−ΔΔCt method, using rplK as the reference gene.
Acknowledgments
We thank Aretha Fiebig and members of the S.C. laboratory for discussions and guidance during the preparation of this manuscript and Elena Solomaha and Ryan Duggan for technical assistance. This project has been funded in whole or in part with federal funds from NIH–National Institute of Allergy and Infectious Diseases Grants U19 AI107792 and R01 AI107159 (to S.C.). J.W.W. is supported by NIH Ruth Kirschstein Postdoctoral Fellowship F32 GM109661. Funding for LS-CAT Sector 21 was provided by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor Grant 085P1000817. Small angle X-ray scattering at Advanced Photon Source–BioCAT is supported by NIH Grant P41 GM103622.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4QPK and 4QPJ).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503118112/-/DCSupplemental.
References
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 2.Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–541. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- 3.Dean AS, et al. Clinical manifestations of human brucellosis: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1929. doi: 10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batut J, Andersson SG, O’Callaghan D. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat Rev Microbiol. 2004;2(12):933–945. doi: 10.1038/nrmicro1044. [DOI] [PubMed] [Google Scholar]
- 5.Philippot L, et al. The ecological coherence of high bacterial taxonomic ranks. Nat Rev Microbiol. 2010;8(7):523–529. doi: 10.1038/nrmicro2367. [DOI] [PubMed] [Google Scholar]
- 6.McAdams HH, Shapiro L. System-level design of bacterial cell cycle control. FEBS Lett. 2009;583(24):3984–3991. doi: 10.1016/j.febslet.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsokos CG, Laub MT. Polarity and cell fate asymmetry in Caulobacter crescentus. Curr Opin Microbiol. 2012;15(6):744–750. doi: 10.1016/j.mib.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biondi EG, et al. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444(7121):899–904. doi: 10.1038/nature05321. [DOI] [PubMed] [Google Scholar]
- 9.Iniesta AA, McGrath PT, Reisenauer A, McAdams HH, Shapiro L. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc Natl Acad Sci USA. 2006;103(29):10935–10940. doi: 10.1073/pnas.0604554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laub MT, Chen SL, Shapiro L, McAdams HH. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci USA. 2002;99(7):4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung MM, Brimacombe CA, Beatty JT. Transcriptional regulation of the Rhodobacter capsulatus response regulator CtrA. Microbiology. 2013;159(Pt 1):96–106. doi: 10.1099/mic.0.062349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, et al. The CtrA phosphorelay integrates differentiation and communication in the marine alphaproteobacterium Dinoroseobacter shibae. BMC Genomics. 2014;15:130. doi: 10.1186/1471-2164-15-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zan J, Heindl JE, Liu Y, Fuqua C, Hill RT. The CckA-ChpT-CtrA phosphorelay system is regulated by quorum sensing and controls flagellar motility in the marine sponge symbiont Ruegeria sp. KLH11. PLoS ONE. 2013;8(6):e66346. doi: 10.1371/journal.pone.0066346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Nisco NJ, Abo RP, Wu CM, Penterman J, Walker GC. Global analysis of cell cycle gene expression of the legume symbiont Sinorhizobium meliloti. Proc Natl Acad Sci USA. 2014;111(9):3217–3224. doi: 10.1073/pnas.1400421111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellefontaine AF, et al. Plasticity of a transcriptional regulation network among alpha-proteobacteria is supported by the identification of CtrA targets in Brucella abortus. Mol Microbiol. 2002;43(4):945–960. doi: 10.1046/j.1365-2958.2002.02777.x. [DOI] [PubMed] [Google Scholar]
- 16.Robertson GT, et al. The Brucella abortus CcrM DNA methyltransferase is essential for viability, and its overexpression attenuates intracellular replication in murine macrophages. J Bacteriol. 2000;182(12):3482–3489. doi: 10.1128/jb.182.12.3482-3489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich LE, Zhulin IB. The MiST2 database: A comprehensive genomics resource on microbial signal transduction. Nucleic Acids Res. 2010;38(Database issue):D401–D407. doi: 10.1093/nar/gkp940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutu AD, Wayne KJ, Sham LT, Winkler ME. Kinetic characterization of the WalRKSpn (VicRK) two-component system of Streptococcus pneumoniae: Dependence of WalKSpn (VicK) phosphatase activity on its PAS domain. J Bacteriol. 2010;192(9):2346–2358. doi: 10.1128/JB.01690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett JW, Kirby JR. CrdS and CrdA comprise a two-component system that is cooperatively regulated by the Che3 chemosensory system in Myxococcus xanthus. MBio. 2011;2(4):e00110–e00111. doi: 10.1128/mBio.00110-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willett JW, et al. Specificity residues determine binding affinity for two-component signal transduction systems. MBio. 2013;4(6):e00420–e13. doi: 10.1128/mBio.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT. Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: A system-level analysis. PLoS Biol. 2005;3(10):e334. doi: 10.1371/journal.pbio.0030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laub MT, Biondi EG, Skerker JM. Phosphotransfer profiling: Systematic mapping of two-component signal transduction pathways and phosphorelays. Methods Enzymol. 2007;423:531–548. doi: 10.1016/S0076-6879(07)23026-5. [DOI] [PubMed] [Google Scholar]
- 23.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 24.Barnett MJ, Hung DY, Reisenauer A, Shapiro L, Long SR. A homolog of the CtrA cell cycle regulator is present and essential in Sinorhizobium meliloti. J Bacteriol. 2001;183(10):3204–3210. doi: 10.1128/JB.183.10.3204-3210.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brilli M, et al. The diversity and evolution of cell cycle regulation in alpha-proteobacteria: A comparative genomic analysis. BMC Syst Biol. 2010;4:52. doi: 10.1186/1752-0509-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Heindl JE, Fuqua C. Coordination of division and development influences complex multicellular behavior in Agrobacterium tumefaciens. PLoS ONE. 2013;8(2):e56682. doi: 10.1371/journal.pone.0056682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quon KC, Marczynski GT, Shapiro L. Cell cycle control by an essential bacterial two-component signal transduction protein. Cell. 1996;84(1):83–93. doi: 10.1016/s0092-8674(00)80995-2. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs C, Domian IJ, Maddock JR, Shapiro L. Cell cycle-dependent polar localization of an essential bacterial histidine kinase that controls DNA replication and cell division. Cell. 1999;97(1):111–120. doi: 10.1016/s0092-8674(00)80719-9. [DOI] [PubMed] [Google Scholar]
- 29.Smith SC, et al. Cell cycle-dependent adaptor complex for ClpXP-mediated proteolysis directly integrates phosphorylation and second messenger signals. Proc Natl Acad Sci USA. 2014;111(39):14229–14234. doi: 10.1073/pnas.1407862111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown PJB, et al. Polar growth in the Alphaproteobacterial order Rhizobiales. Proc Natl Acad Sci USA. 2012;109(5):1697–1701. doi: 10.1073/pnas.1114476109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pincus Z, Theriot JA. Comparison of quantitative methods for cell-shape analysis. J Microsc. 2007;227(Pt 2):140–156. doi: 10.1111/j.1365-2818.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 32.Blair JA, et al. Branched signal wiring of an essential bacterial cell-cycle phosphotransfer protein. Structure. 2013;21(9):1590–1601. doi: 10.1016/j.str.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fioravanti A, et al. Structural insights into ChpT, an essential dimeric histidine phosphotransferase regulating the cell cycle in Caulobacter crescentus. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68(Pt 9):1025–1029. doi: 10.1107/S1744309112033064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005;24(24):4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mechaly AE, Sassoon N, Betton JM, Alzari PM. Segmental helical motions and dynamical asymmetry modulate histidine kinase autophosphorylation. PLoS Biol. 2014;12(1):e1001776. doi: 10.1371/journal.pbio.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, et al. Mechanistic insights revealed by the crystal structure of a histidine kinase with signal transducer and sensor domains. PLoS Biol. 2013;11(2):e1001493. doi: 10.1371/journal.pbio.1001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Varughese KI, Madhusudan, Zhou XZ, Whiteley JM, Hoch JA (1998) Formation of a novel four-helix bundle and molecular recognition sites by dimerization of a response regulator phosphotransferase. Mol Cell 2(4):485–493. [DOI] [PubMed]
- 38.Svergun D, Barberato C, Koch MHJ. CRYSOL - A program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J Appl Cryst. 1995;28:768–773. [Google Scholar]
- 39.Dutta R, Yoshida T, Inouye M. The critical role of the conserved Thr247 residue in the functioning of the osmosensor EnvZ, a histidine Kinase/Phosphatase, in Escherichia coli. J Biol Chem. 2000;275(49):38645–38653. doi: 10.1074/jbc.M005872200. [DOI] [PubMed] [Google Scholar]
- 40.Alexandre MT, et al. Electronic and protein structural dynamics of a photosensory histidine kinase. Biochemistry. 2010;49(23):4752–4759. doi: 10.1021/bi100527a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilwes AM, Quezada CM, Croal LR, Crane BR, Simon MI. Nucleotide binding by the histidine kinase CheA. Nat Struct Biol. 2001;8(4):353–360. doi: 10.1038/86243. [DOI] [PubMed] [Google Scholar]
- 42.Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139(2):325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 43.Varughese KI, Tsigelny I, Zhao H. The crystal structure of beryllofluoride Spo0F in complex with the phosphotransferase Spo0B represents a phosphotransfer pretransition state. J Bacteriol. 2006;188(13):4970–4977. doi: 10.1128/JB.00160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Q, Porter SW, West AH. The yeast YPD1/SLN1 complex: Insights into molecular recognition in two-component signaling systems. Structure. 2003;11(12):1569–1581. doi: 10.1016/j.str.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Capra EJ, Laub MT. Evolution of two-component signal transduction systems. Annu Rev Microbiol. 2012;66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 48.Skerker JM, et al. Rewiring the specificity of two-component signal transduction systems. Cell. 2008;133(6):1043–1054. doi: 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroos L. The Bacillus and Myxococcus developmental networks and their transcriptional regulators. Annu Rev Genet. 2007;41:13–39. doi: 10.1146/annurev.genet.41.110306.130400. [DOI] [PubMed] [Google Scholar]
- 50.Shapiro L, McAdams HH, Losick R. Why and how bacteria localize proteins. Science. 2009;326(5957):1225–1228. doi: 10.1126/science.1175685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo L, et al. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1997;276(5310):250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 52.Crosson S, McGrath PT, Stephens C, McAdams HH, Shapiro L. Conserved modular design of an oxygen sensory/signaling network with species-specific output. Proc Natl Acad Sci USA. 2005;102(22):8018–8023. doi: 10.1073/pnas.0503022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee JM, et al. The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect Immun. 2005;73(5):2873–2880. doi: 10.1128/IAI.73.5.2873-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Léonard S, et al. FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae. J Bacteriol. 2007;189(1):131–141. doi: 10.1128/JB.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Igo MM, Ninfa AJ, Stock JB, Silhavy TJ. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 56.Jiang P, Atkinson MR, Srisawat C, Sun Q, Ninfa AJ. Functional dissection of the dimerization and enzymatic activities of Escherichia coli nitrogen regulator II and their regulation by the PII protein. Biochemistry. 2000;39(44):13433–13449. doi: 10.1021/bi000794u. [DOI] [PubMed] [Google Scholar]
- 57.Podgornaia AI, Laub MT. Determinants of specificity in two-component signal transduction. Curr Opin Microbiol. 2013;16(2):156–162. doi: 10.1016/j.mib.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Capra EJ, et al. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6(11):e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willett JW, Kirby JR. Genetic and biochemical dissection of a HisKA domain identifies residues required exclusively for kinase and phosphatase activities. PLoS Genet. 2012;8(11):e1003084. doi: 10.1371/journal.pgen.1003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang P, Peliska JA, Ninfa AJ. Asymmetry in the autophosphorylation of the two-component regulatory system transmitter protein nitrogen regulator II of Escherichia coli. Biochemistry. 2000;39(17):5057–5065. doi: 10.1021/bi992921w. [DOI] [PubMed] [Google Scholar]
- 61.Albanesi D, et al. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci USA. 2009;106(38):16185–16190. doi: 10.1073/pnas.0906699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diensthuber RP, Bommer M, Gleichmann T, Möglich A. Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure. 2013;21(7):1127–1136. doi: 10.1016/j.str.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 63.Casino P, Miguel-Romero L, Marina A. Visualizing autophosphorylation in histidine kinases. Nat Commun. 2014;5:3258. doi: 10.1038/ncomms4258. [DOI] [PubMed] [Google Scholar]
- 64.Kim HS, Caswell CC, Foreman R, Roop RM, 2nd, Crosson S. The Brucella abortus general stress response system regulates chronic mammalian infection and is controlled by phosphorylation and proteolysis. J Biol Chem. 2013;288(19):13906–13916. doi: 10.1074/jbc.M113.459305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HS, Willett JW, Jain-Gupta N, Fiebig A, Crosson S. The Brucella abortus virulence regulator, LovhK, is a sensor kinase in the general stress response signalling pathway. Mol Microbiol. 2014;94(4):913–925. doi: 10.1111/mmi.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morin A, et al. Collaboration gets the most out of software. eLife. 2013;2:e01456. doi: 10.7554/eLife.01456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Herrou J, Crosson S. myo-inositol and D-ribose ligand discrimination in an ABC periplasmic binding protein. J Bacteriol. 2013;195(10):2379–2388. doi: 10.1128/JB.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrou J, Rotskoff G, Luo Y, Roux B, Crosson S. Structural basis of a protein partner switch that regulates the general stress response of α-proteobacteria. Proc Natl Acad Sci USA. 2012;109(21):E1415–E1423. doi: 10.1073/pnas.1116887109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan SR, Gaines J, Roop RM, 2nd, Farrand SK. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl Environ Microbiol. 2008;74(16):5053–5062. doi: 10.1128/AEM.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pronk S, et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29(7):845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindorff-Larsen K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78(8):1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: A linear constraint solver for molecular simulations. J Comput Chem. 1997;18:1463–1472. [Google Scholar]
- 74.Parrinello M, Rahman A. Polymorphic transitions in single crystals: A new molecular dynamics method. J Appl Phys. 1981;52:7182–7190. [Google Scholar]
- 75.Darden T, York D, Pedersen L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 76.Iancu CV, et al. Electron cryotomography sample preparation using the Vitrobot. Nat Protoc. 2006;1(6):2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- 77.Tivol WF, Briegel A, Jensen GJ. An improved cryogen for plunge freezing. Microsc Microanal. 2008;14(5):375–379. doi: 10.1017/S1431927608080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]