Significance
Posttraumatic stress disorder is characterized by a resistance to extinction learning and dysregulated signaling of the neurotransmitter norepinephrine. Previous research suggested the prelimbic and infralimbic subdivisions of the medial prefrontal cortex (mPFC) regulate fear expression and suppression, respectively. However, noradrenergic signaling in response to psychological stress may disrupt mPFC function, contributing to extinction deficits. Here we show, for the first time to our knowledge, that footshock stress dysregulates mPFC spike firing; this can be stabilized by propranolol, a β-noradrenergic receptor blocking drug, which in turn facilitates extinction when it normally fails. These findings suggest that propranolol may be a particularly effective adjunct to behavioral therapy soon after trauma, when stress is high, at least in part by normalizing prefrontal cortical function.
Keywords: fear, extinction, propranolol, prefrontal cortex, rat
Abstract
Stress-induced impairments in extinction learning are believed to sustain posttraumatic stress disorder (PTSD). Noradrenergic signaling may contribute to extinction impairments by modulating medial prefrontal cortex (mPFC) circuits involved in fear regulation. Here we demonstrate that aversive fear conditioning rapidly and persistently alters spontaneous single-unit activity in the prelimbic and infralimbic subdivisions of the mPFC in behaving rats. These conditioning-induced changes in mPFC firing were mitigated by systemic administration of propranolol (10 mg/kg, i.p.), a β-noradrenergic receptor antagonist. Moreover, propranolol administration dampened the stress-induced impairment in extinction observed when extinction training is delivered shortly after fear conditioning. These findings suggest that β-adrenoceptors mediate stress-induced changes in mPFC spike firing that contribute to extinction impairments. Propranolol may be a helpful adjunct to behavioral therapy for PTSD, particularly in patients who have recently experienced trauma.
Individuals exposed to extreme psychological stress, such as combat-related trauma or sexual abuse, are at risk for developing anxiety and trauma disorders, including posttraumatic stress disorder (PTSD). Although the etiology of PTSD is complex, it is widely believed that associative learning processes, including Pavlovian fear conditioning, contribute to its genesis. Moreover, an inability to suppress or extinguish fear memories may sustain pathologically high levels of fear in patients with PTSD years after the trauma (1–6). A variety of clinical interventions to facilitate fear extinction in patients with PTSD are currently being explored, although effective treatment for many afflicted individuals remains elusive (7, 8).
One promising therapeutic target for facilitating extinction in PTSD patients is the noradrenergic system. Norepinephrine (NE) not only plays an important role in mood and arousal, but also in the encoding, retrieval, and reconsolidation of emotional memories (9–13). Endogenous NE signaling is elevated in PTSD and drugs that block NE receptors are already being used with some success to either prevent or treat PTSD, including its symptoms of hyperarousal and nightmares (14–18). Clinically effective noradrenergic drugs include the α1-adrenoceptor antagonist, prazosin, and the β1/β2-adrenoceptor antagonist, propranolol (17, 19–21). The neural mechanisms underlying the efficacy of these drugs remain poorly understood.
It has previously been suggested that stress-induced changes in prefrontal cortical structure and function observed in animal models may contribute to the extinction deficits observed in patients with PTSD (22–24). Given the abundant literature implicating NE signaling in prefrontal cortical function (25, 26), it is conceivable that stress-induced elevations in prefrontal NE release (27–29) contribute to extinction impairments associated with PTSD. The medial prefrontal cortex (mPFC), comprising the prelimbic (PL) and infralimbic (IL) subdivisions in rodents, plays a key role in the regulation of emotional behavior in both humans and rats (30, 31). Previous studies have suggested that PL plays an important role in fear expression, whereas IL is preferentially involved in fear extinction (32–34). Stress-induced alterations in the balance of neuronal activity in PL and IL as a consequence of noradrenergic hyperarousal might therefore contribute to extinction impairments and the maintenance of PTSD. To address this question, we combine in vivo microelectrode recording in freely moving rats with behavioral and pharmacological manipulations to determine whether β-noradrenergic receptors mediate stress-induced alterations in mPFC neuronal activity. Further, we examine whether systemic propranolol treatment, given immediately after an aversive experience, rescues stress-induced impairments in fear extinction.
Results
Propranolol Stabilizes Medial Prefrontal Activity After Footshock Stress.
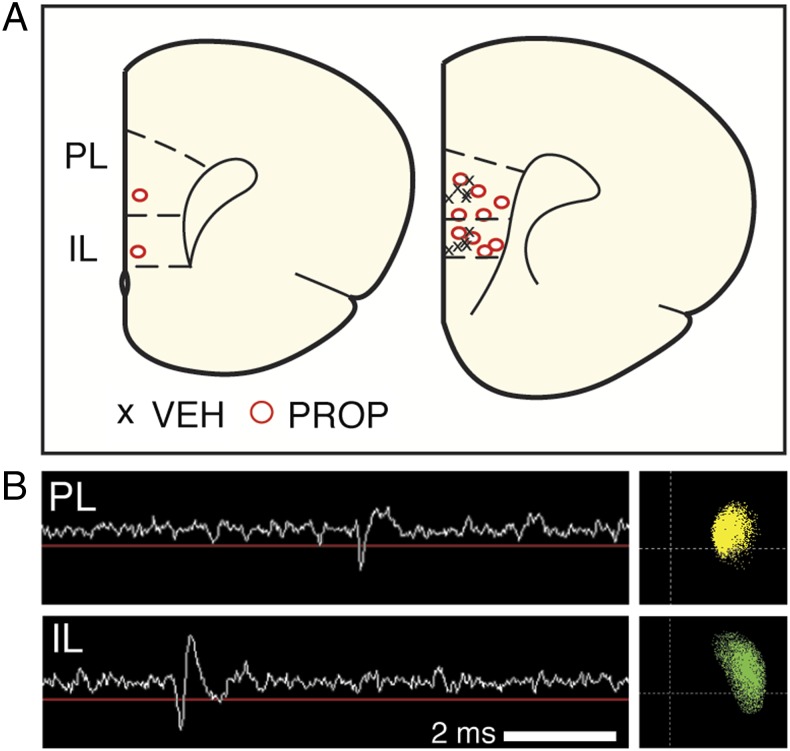
To investigate the effect of systemic noradrenergic blockade on stress-induced changes in the mPFC, we performed single-unit recordings in freely moving rats (Materials and Methods). Animals were first surgically implanted with a 16-channel microelectrode array (Innovative Neurophysiology) that spanned both PL and IL (eight wires in each) in the right hemisphere of each animal. Array placements in each rat are shown in Fig. 1A. The recording sites varied somewhat in their mediolateral (i.e., laminar) or dorsoventral position within PL and IL across rats. However, there were no significant differences in single-unit firing or bursting as a function of mediolateral or dorsoventral position within these brain regions. Representative single-unit waveforms and their corresponding clusters from PL and IL are shown in Fig. 1B.
Fig. 1.
In vivo mPFC recordings in freely moving rats. (A) Histological localization of the center of each electrode array in the mPFC; each array targeted both PL (eight wires) and IL (eight wires). Right hemisphere, coronal sections represent (left to right) coordinates +3.2 and +2.7 relative to bregma in the anteroposterior plane. Six rats received propranolol (PROP) treatment, and five received vehicle (VEH) treatment. In one of the propranolol rats, recordings were obtained only from PL. (B) Example voltage trace from an electrode in PL (Upper) and IL (Lower), showing an action potential and its corresponding principal component scatter plot.
After a 1-wk recovery period, rats were transported to the recording chamber for the first of two recording sessions. During the first recording session, the animals received a standard Pavlovian fear conditioning procedure after systemic administration of either vehicle or propranolol (10 mg/kg, i.p.); the second session served as a retention test for conditioned fear. For both recording sessions, rats were connected with a flexible headstage cable to a multichannel OmniPlex recording system (Plexon), and their freezing behavior was monitored inside a standard conditioning chamber using a load-cell transducer and amplifier (35). We recorded from a total of 220 mPFC neurons on day 1 [vehicle (VEH)-PL, n = 52; VEH-IL, n = 34; propranolol (PROP)-PL, n = 85; PROP-IL, n = 49] and 185 neurons on day 2 (VEH-PL, n = 47; VEH-IL, n = 34; PROP-PL, n = 63; PROP-IL, n = 41). Although some of the units recorded on day 2 may have been the same as those recorded on day 1, we did not assume that they were and treated them as a separate population of neurons. The baseline firing rates of the units recorded on day 1 (mean ± SEM; 3-min predrug baseline) were as follows: VEH-PL = 5.15 ± 0.78 Hz (range: 0.26–26.21 Hz); VEH-IL = 5.59 ± 0.88 Hz (range: 1.39–21.13 Hz); PROP-PL = 7.52 ± 0.75 Hz (range: 0.28–41.09 Hz); PROP-IL = 8.60 ± 1.41 Hz (range: 0.46–55.90 Hz). Although 23 cells had firing rates (>15 Hz; 10% of the sample) typical of those observed in inhibitory interneurons, it was not clear that these cells reflected a different population when various rate and waveform parameters were examined and they were consequently included in all analyses.
Drug administration before Pavlovian fear conditioning on day 1 (Fig. 2A) did not significantly alter the spontaneous firing rate of PL or IL neurons. Although firing rates decreased slightly in all animals over the preconditioning recording period, this decrease was similar in vehicle- and propranolol-treated rats [main effect of time, F(2,432) = 15.57, P < 0.01; time × drug interaction, not significant; time × drug × brain region interaction, not significant]; this contrasts with a previous report that found a significant decrease in PL firing with propranolol (36). Average firing rates in the final 3-min block of the postinjection period were as follows: VEH-PL = 4.73 ± 0.71 Hz; VEH-IL = 4.79 ± 0.92 Hz; PROP-PL = 7.17 ± 0.75 Hz; and PROP-IL = 7.36 ± 1.14 Hz.
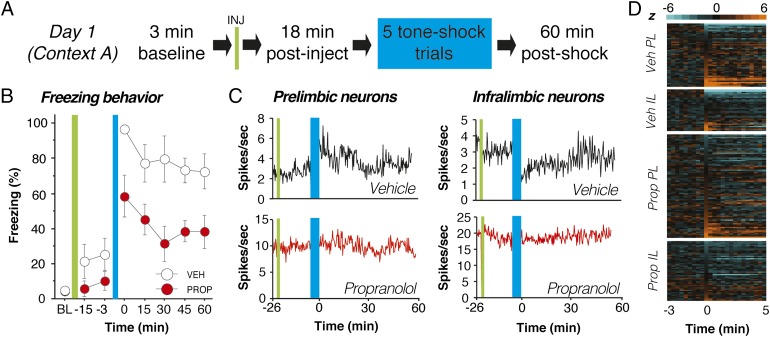
Fig. 2.
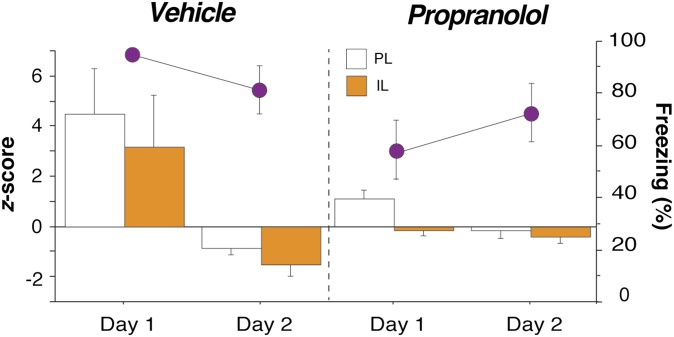
Propranolol stabilizes single-unit firing in mPFC neurons after footshock stress. (A) Day 1 experimental design. (B) Propranolol-treated rats (red circles) exhibited reduced freezing throughout the day 1 recording session relative to vehicle-treated controls (white circles). (C) Four representative histograms (20-s bins) showing spontaneous firing rate from neurons recorded in PL (Left) and IL (Right). Fear conditioning (blue bar) altered the firing rate of the PL and IL neurons obtained from vehicle-treated rats (black traces, Upper) and propranolol administration mitigated this effect (red traces, Lower). (D) Normalized firing rate heat maps showing postconditioning increases (light orange) and decreases (light blue) relative to baseline (preconditioning) firing rate (black) for all of the units recorded in each group and brain region. Only the 3 min before conditioning and the first 5 min after conditioning are shown for clarity. In both PL and IL, single units in vehicle-treated rats exhibited increases or decreases in firing rate after conditioning, and propranolol treatment mitigated these effects. Injection (INJ) is denoted by green vertical bar; conditioning (tone-shock pairings) is denoted by blue vertical bar. Data during the conditioning period were not recorded. All values are means ± SEM for freezing.
Eighteen minutes after drug administration, the rats received five pairings of an innocuous auditory conditioned stimulus (CS; 2 s, 2 kHz, 80 dB) with an aversive footshock unconditioned stimulus (US; 0.5 s, 1.0 mA); trials were separated by a 1-min intertrial interval (ITI). Fear conditioning was followed by a 60-min stimulus-free period during which the neural and behavioral effects of conditioning were recorded. Not surprisingly, fear conditioning yielded robust increases in freezing behavior in vehicle-treated rats. Interestingly, postshock freezing was significantly blunted by propranolol treatment [main effect of drug, F(1,9) = 22.97, P < 0.01; Fig. 2B]. Correspondingly, fear conditioning produced dramatic changes in the spontaneous firing rate of PL and IL neurons and these conditioning-induced changes in firing rate were dampened in propranolol-treated rats. Fig. 2C shows firing rate histograms for representative single units in PL and IL from vehicle- and propranolol-treated rats. In vehicle-treated rats, fear conditioning produced substantial changes in the firing rate in both PL and IL; these changes were minimal in single units from propranolol-treated rats. This effect is particularly evident in heat maps illustrating the normalized firing rate in the entire population of single units recorded on day 1 (Fig. 2D). A much greater proportion of neurons recorded in vehicle-treated rats exhibited either markedly enhanced (light orange) or suppressed (light blue) firing rates soon after fear conditioning (t > 0) relative to units recorded in propranolol-treated rats.
To assess group differences in spontaneous firing rate, we generated average firing rate histograms for all of the units recorded in each area and treatment condition (Fig. 3). In vehicle-treated rats, fear conditioning massively, but transiently, increased the spontaneous firing rate among mPFC neurons in the minutes following fear conditioning. These firing rate changes were mitigated by propranolol-treatment in both PL [Fig. 3A; drug × time interaction, F(178,24030) = 4.36, P < 0.01] and IL [Fig. 3B; drug × time interaction, F(178,14418) = 2.82, P < 0.01]. For example, propranolol significantly attenuated conditioning-related increases in PL in the first immediate postshock period [Fig. 3A, Inset; t(135) = 2.26, P < 0.05] and IL [Fig. 3B, Inset; t(81) = 1.98, P = 0.05] firing. In addition to the rapid increases in IL and PL firing rate after conditioning, IL neurons exhibited a sustained decrease in spontaneous firing that persisted (on average) for roughly 30 min after conditioning. This decreased firing period corresponds to a time window within which rats are resistant to extinction (23) and suggests that shock-induced depression of IL activity may, at least in part, account for this stress-induced immediate extinction deficit (IED).
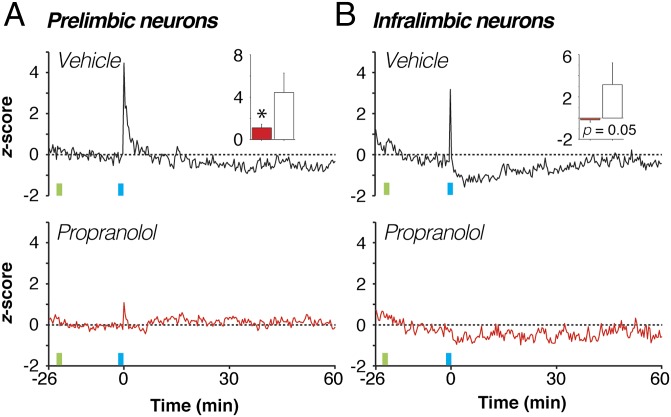
Fig. 3.
Propranolol stabilizes single-unit firing in the population of mPFC neurons. Spontaneous firing rates were averaged across all neurons and normalized to the preconditioning baseline for each brain region and treatment group. Fear conditioning (blue vertical bar) induced a dramatic increase in average spontaneous firing rate in PL neurons from vehicle-treated rats [A, black trace; Inset shows first 20-s postshock bin, comparing vehicle (white bar) with drug (red bar)] that was mitigated by propranolol treatment (A, red trace). Conditioning induced a weaker postshock increase in spontaneous firing in IL neurons from vehicle-treated rats (B, black trace; Inset shows first 20-s postshock bin) and produced an enduring suppression of this activity. Propranolol treatment (B, red trace) counteracted both types of firing rate changes in IL. Injection (INJ) is denoted by green vertical bar; conditioning (tone-shock pairings) is denoted by blue vertical bar. Data during the conditioning period were not recorded. *P < 0.05 vs. vehicle. All values are means (±SEM for insets).
Bursting of mPFC neurons has been implicated in both extinction learning (23, 36) and the IED (23). We therefore examined whether burst firing in PL and IL was modulated by footshock stress and noradrenergic blockade. Although normalized burst firing mirrored the patterns observed for overall firing rate (Fig. S1), propranolol treatment did not reliably alter shock-induced changes in burst firing in the immediate postshock period in PL neurons. This finding does not, however, rule out the possibility that shock-induced changes in spontaneous firing in mPFC are responsible for the IED.
Fig. S1.
Propranolol stabilizes single-unit bursting in the mPFC after footshock stress. (A and B) Spontaneous bursting rates averaged across all neurons and normalized to the preconditioning baseline for each brain region and treatment group. Fear conditioning (blue vertical bar) induced an increase in average spontaneous bursting rate in PL neurons from vehicle-treated rats (A, black trace; Inset shows first 20-s postshock bin in vehicle- and propranolol-treated rats) that was mitigated by propranolol treatment (A, red trace). Inset graph reveals no drug-induced difference in PL bursting, unlike for day 1 firing rate; red bar indicates propranolol and white bar indicates vehicle. Conditioning also induced a postshock increase in spontaneous bursting in IL neurons from vehicle-treated rats (B, black trace; Inset shows first 20-s postshock bin comparing vehicle and drug) and produced an enduring suppression of this activity. Propranolol treatment (B, red trace) mitigated this effect. Injection (INJ) is denoted by green vertical bar; conditioning (tone-shock pairings) is denoted by blue vertical bar. Data collected during the injection and conditioning periods were not recorded. All values are means (±SEM in insets).
Propranolol Mitigates Shock-Induced Increases and Decreases in mPFC Firing Rate.
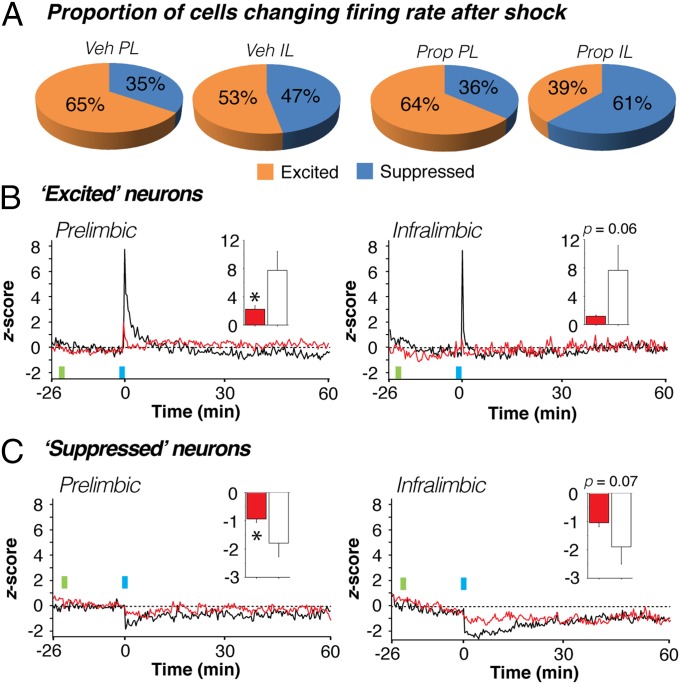
Fear conditioning induced robust changes in the average spontaneous firing rate of mPFC neurons. Nonetheless, individual single units exhibited considerable diversity in their firing rate after fear conditioning (Fig. 2D). We were interested in whether propranolol altered the proportion of neurons that increased or decreased their firing rate after conditioning and whether the magnitude of the firing rate changes in these populations differed in the treatment conditions. We therefore categorized units in both PL and IL according to their response bias in the immediate postshock interval (i.e., the 20-s period after the final tone-shock pairing), as well as during a 20-s period at the end of the 60-min postshock recording session.
There were no significant differences in the proportion of neurons increasing or decreasing their firing rates in vehicle- compared with propranolol-treated rats at either the immediate (Fig. 4A) or remote time points (Fig. S2). However, despite increasing their firing rates after conditioning, the majority of single units in both PL and IL exhibited a suppression of firing 60 min after conditioning (Fig. S2). This increase in the proportion of neurons with suppressed firing rates across the recording session was observed in both the PL [χ2(1) = 3.90, P < 0.05] and IL [χ2(1) = 4.98, P < 0.05] of vehicle-treated rats. In propranolol-treated rats, this effect was only observed in PL neurons [χ2(1) = 10.38, P < 0.01], insofar as IL neurons were already more likely to be suppressed immediately after conditioning. Overall, these data indicate that the tendency for many neurons to show a transient increase in firing rate in the immediate postshock period gave way to suppression in rate by the end of the 60-min postshock period.
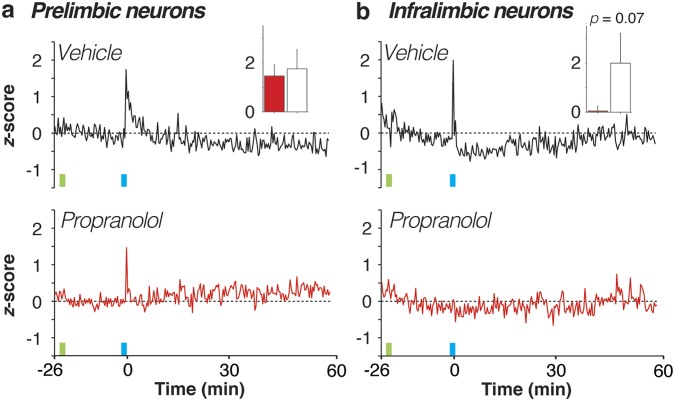
Fig. 4.
Propranolol stabilizes both increases and decreases in mPFC firing rate. (A) Proportion of neurons in each of the four groups exhibiting immediate postconditioning (first 20-s bin) increases (z > 0, excited) or decreases (z < 0, suppressed) in spontaneous firing rate. Regardless of drug treatment, PL neurons tended to increase their firing rate after shock more so than IL neurons. (B and C) Normalized firing rate histograms for PL (Left) and IL (Right) neurons that were excited (B) or suppressed (C) in the immediate postshock period in vehicle- (black traces) and propranolol-treated (red traces) rats. Inset graphs show the values for the first 20-s postshock bin in vehicle- (white bars) and propranolol-treated (red bars) rats. Injection (INJ) is denoted by green vertical bar; conditioning (tone-shock pairings) is denoted by blue vertical bar. Data during the conditioning period were not recorded. *P < 0.05 vs. vehicle. All values are means (±SEM in insets).
Fig. S2.
Single-unit firing rates tend to be suppressed after 60 min. At the 60-min time point of day 1, the proportion of neurons with suppressed firing was significantly increased relative to the immediate postshock period in all of the regions and conditions, except in IL neurons from propranolol-treated rats.
Although the proportion of neurons showing immediate postshock changes in firing was similar in vehicle- and propranolol-treated rats, there were considerable differences in the firing rate of these neurons (Fig. 4 B and C). Excited PL neurons in vehicle-treated rats that exhibited immediate postshock increases in firing rate showed a large but transient increase in firing in the postconditioning period; this effect was counteracted by propranolol treatment [drug × time interaction, F(178,15308) = 5.68, P < 0.01; Fig. 4B, Left]. Indeed, shock-induced increases in firing in the first immediate postshock bin were significantly attenuated by propranolol treatment [t(86) = 2.56, P < 0.05; Fig. 4B, Left, Inset]. Similarly, excited IL neurons exhibited a transient increase in firing in the postconditioning period that was attenuated in propranolol-treated rats [drug × time interaction, F(178,6230) = 2.71, P < 0.01; Fig. 4B, Right]. However, this effect only approached statistical significance in the first postshock bin [t(35) = 1.92, P = 0.06; Fig. 4B, Right, Inset].
Conditioning-induced decreases in firing were also sensitive to propranolol treatment (Fig. 4C). In vehicle-treated rats, suppressed PL neurons that exhibited immediate postshock decreases in rate showed a decrease in firing in the postconditioning period that was mitigated in propranolol-treated rats [drug × time interaction, F(178,8366) = 1.33, P < 0.01; Fig. 4C, Left]. Firing during the immediate postshock bin was significantly greater in propranolol-treated rats [t(47) = 2.19, P < 0.05; Fig. 4C, Left, Inset]. Similarly, propranolol treatment limited the magnitude of firing rate suppression in IL neurons [drug × time interaction, F(178,7832) = 2.90, P < 0.01; Fig. 4C, Right], although this only approached significance in the first immediate postshock bin [t(44) = 1.84, P = 0.07; Fig. 4C, Right, Inset]. In summary, noradrenergic blockade stabilizes fear conditioning-induced changes in mPFC firing rate by limiting both increases and decreases in spontaneous firing rate.
Expression of Conditional Freezing Is Not Sufficient to Increase mPFC Firing Rate.
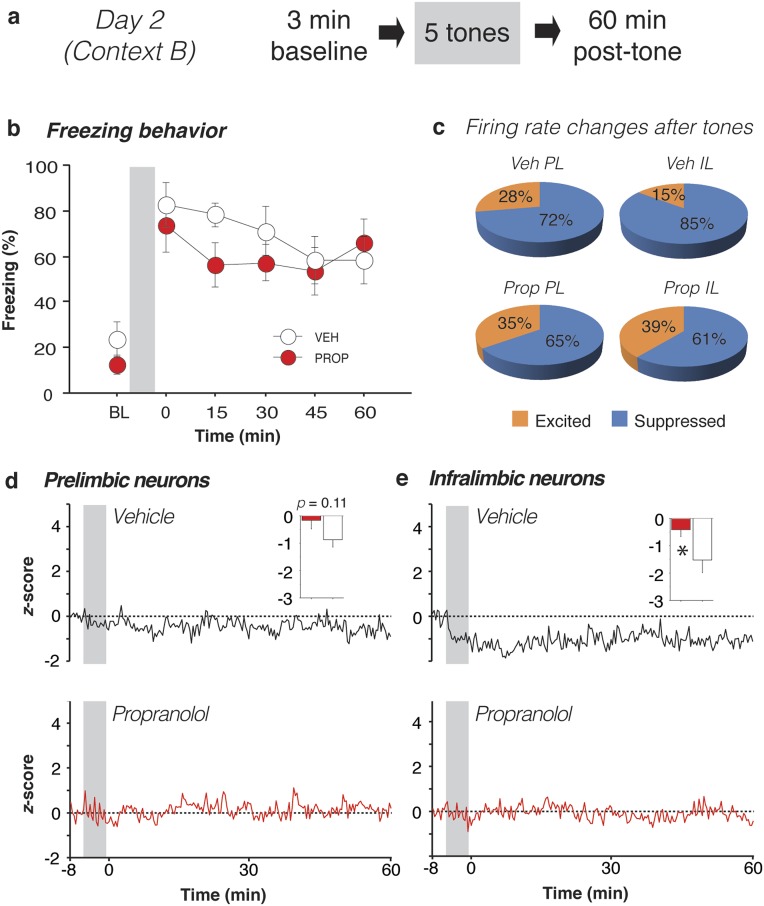
Twenty-four hours after the first recording session, a second session (day 2) was conducted to examine whether the neural changes observed immediately after aversive conditioning also occurred during the expression of fear to the CS. To this end, rats were returned to the recording chamber, which was modified to create a context that was distinct from that used for conditioning (Fig. S3; see Materials and Methods for details). After a 3-min baseline period, rats received five CS-alone trials (i.e., without footshocks; 1-min ITI), and a subsequent 60-min stimulus-free period. Both groups of rats exhibited high levels of conditioned freezing in the 3-min period immediately after presentation of the tones, indicating that systemic administration of propranolol before fear conditioning the previous day did not prevent the acquisition of conditioned fear (Fig. S3). There was no significant effect of preconditioning drug treatment on day 1 on conditioned freezing behavior on day 2 (Fs < 1).
Fig. S3.
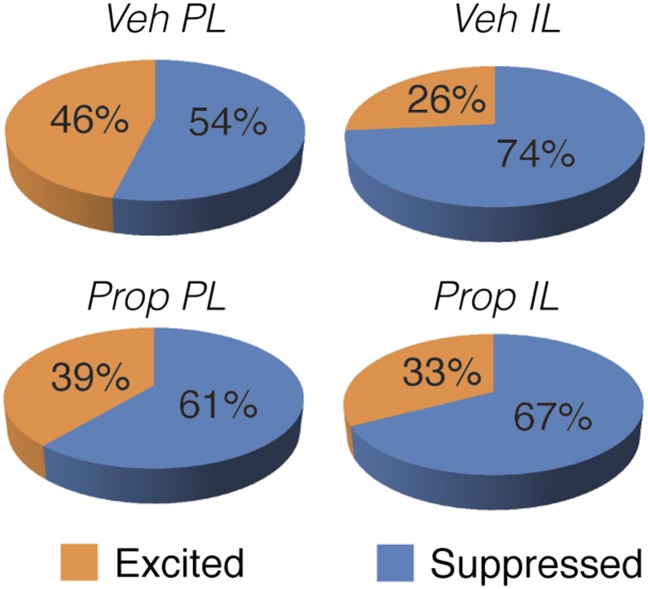
Propranolol stabilizes single-unit firing in IL after CS delivery. (A) Day 2 experimental design. (B) Propranolol-treated rats (red circles) did not exhibit altered freezing relative to vehicle-treated rats (white circles) when averaged across the entire session. (C) After CS presentation, the majority of mPFC neurons in vehicle-treated rats showed decreases in firing rate, and this was most pronounced in IL neurons. A significantly greater proportion of IL neurons was suppressed in the vehicle- compared with propranolol-treated animals. (D and E) Unlike day 1, the normalized firing rate of PL neurons in both vehicle- and propranolol-treated rats exhibited little change after presentation of the aversive CS. Interestingly, IL firing rate was depressed by CS presentations, and this effect was mitigated by propranolol. Insets show normalized firing in the first 20-s bin after the last CS for neurons from vehicle- (white bars) and propranolol-treated rats (red bars). Gray bars denote the CS period. *P < 0.05 vs. vehicle. All error bars indicate mean ± SEM.
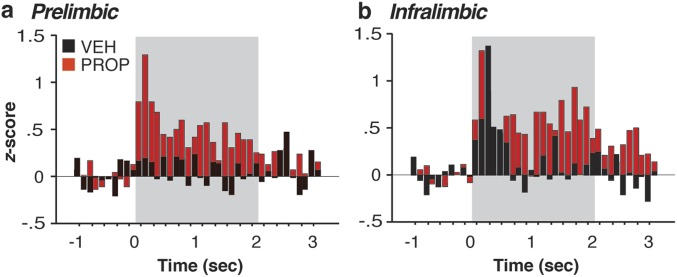
Importantly, CS presentations on day 2 produced qualitatively different changes in spontaneous firing rate relative to day 1 despite yielding high levels of conditioned freezing behavior (Fig. S3). After CS presentation, the majority of mPFC neurons in rats that had been treated with vehicle the previous day showed decreases in firing rate (Fig. S3C), and this was most pronounced in IL neurons. A significantly greater proportion of IL neurons was suppressed in the vehicle- compared with propranolol-treated animals [χ2(1) = 5.45, P < 0.05]. This pattern was also reflected in the normalized firing rates across the recording session (Fig. S3 D and E). Spontaneous firing tended to decrease in both PL and IL after CS presentation, and propranolol administration before fear conditioning on day 1 reliably dampened this effect in IL [drug × time interaction, F(179,13067) = 2.25, P < 0.01]. This latter effect was particularly robust in the 20-s period immediately after delivery of the last tone [t(73) = 2.14, P < 0.05; Fig. S3E, Inset]. Similar to day 1, neuronal bursting largely mirrored the spontaneous firing rate data. In addition to influencing spontaneous firing rate on day 2, propranolol administration before conditioning on day 1 significantly affected CS-evoked firing during the tone presentations themselves (Fig. S4).
Fig. S4.
CS-evoked responses in the mPFC are modulated by propranolol. On day 2, CS-evoked activity was significantly different between vehicle- and propranolol-treated rats. Perievent time histograms (100-ms bins) computed on average normalized firing rate (post-CS firing normalized to 1-s pre-CS baseline) across the five CS presentations revealed that both PL (A) and IL (B) neurons exhibited increased firing due to prior propranolol treatment [PL, drug × time interaction, F(29,2958) = 2.52, P < 0.01; IL, F(29,2117) = 1.67, P < 0.05].
The fact that CS presentations on day 2 evoked robust freezing behavior but minimally altered spontaneous firing in mPFC suggests that a transition from a low fear state to a high fear state is not responsible for the firing rate changes observed on day 1. Fig. 5 illustrates this observation by plotting freezing behavior and normalized firing rate across the two recording sessions. Although the levels of freezing were similar immediately after either CS-US (day 1) or CS-alone (day 2) trials on each day, changes in neuronal activity were markedly different. On day 1, vehicle-treated rats showed a postshock increase in spontaneous firing rate relative to propranolol-treated animals [main effect of drug, F(1,216) = 8.41, P < 0.01]. This result contrasted with day 2, where vehicle rats showed suppression of firing that was counteracted by propranolol [main effect of drug, F(1,181) = 7.18, P < 0.01]. Thus, on both days, propranolol treatment mitigated changes in post-CS firing that were evident in vehicle-treated rats.
Fig. 5.
Freezing behavior does not alter mPFC firing rate. Freezing behavior in both vehicle-treated (Left) and propranolol-treated (Right) rats was not markedly different during the immediate postshock period on day 1 compared with the immediate post-CS period on day 2 [values represent the average freezing immediately following the last US (day 1) or CS (day 2)]. Despite similarities in day 1 and day 2 freezing, normalized firing rate during the first 20 s following the last US (day 1) or CS (day 2) was dramatically elevated in both IL and PL on day 1 relative to day 2 in vehicle-treated rats (Left). This effect was mitigated by propranolol treatment (Right). Hence, marked differences in firing rate in the mPFC cannot be attributed to freezing behavior per se, because both posttrial periods (on day 1 and day 2) yielded similar and high levels of freezing. All values are means ± SEM.
Propranolol Facilitates Extinction Under Stress.
We previously established that extinction fails when given soon after footshock, when levels of acute psychological stress are high; that is, rats exhibit an IED when extinction trials are delivered soon after fear conditioning (23, 37–39; but also see refs. 40 and 41). It is notable that we now show that fear conditioning is followed by a lasting suppression of IL firing (Fig. 3B), a time at which CS-alone trials are ineffective at supporting long-term extinction (37–39). We previously suggested that the IED results from a high state of fear that interferes with mPFC function. Given that systemic propranolol reduces shock-induced freezing and stabilizes mPFC neural activity, we tested whether it would mitigate the IED.
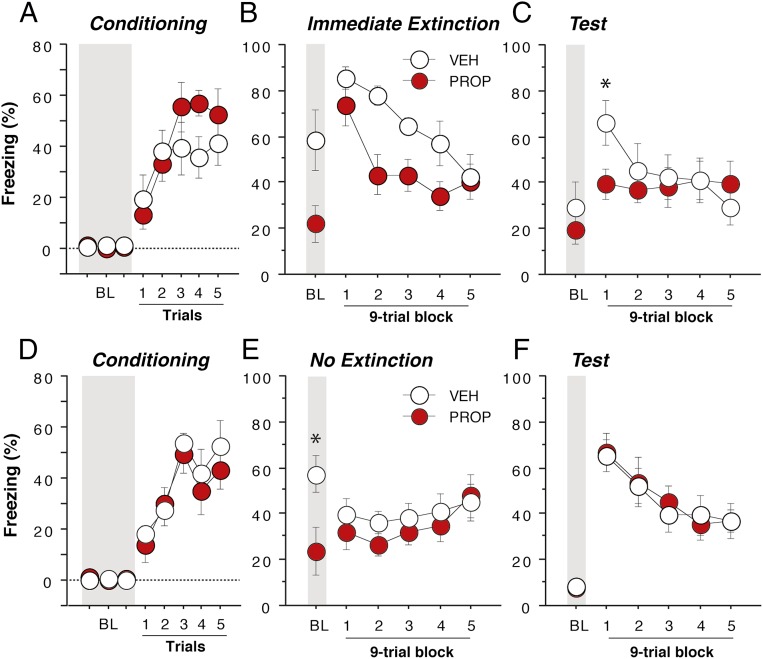
In this experiment, rats were systemically administered either vehicle (n = 7) or propranolol (n = 7) immediately after fear conditioning; an immediate extinction session consisting of 45 presentations of the CS (1-min ITI) was conducted 30 min after conditioning. All rats were then given a retrieval test 48 h after extinction using identical procedures to those used during the extinction session (i.e., 45 CS-alone test trials). Both groups acquired fear conditioning, and there were no differences in conditioned freezing before drug treatment [Fig. 6A; F(1,12) < 1]. However, propranolol treatment before the immediate extinction session significantly reduced both pretrial (BL) and within-session freezing during the extinction trials [Fig. 6B; main effect of drug, F(1,12) = 8.40, P < 0.05] of the immediate extinction session. Importantly, propranolol facilitated the acquisition of long-term extinction; freezing in propranolol-treated rats was significantly lower during the first nine-trial block of the retention test [Fig. 6C, drug × time interaction, F(5,60) = 3.27, P < 0.05]. Thus, propranolol treatment before the immediate extinction procedure limited the spontaneous recovery of fear that characterizes the IED in vehicle-treated rats and promoted the retention of extinction under conditions of high psychological stress that normally impair extinction learning. Of course, in testing the effects of propranolol on the IED, it is possible that propranolol administered immediately after fear conditioning reduced conditioned freezing by impairing the consolidation of the fear memory, rather than facilitating extinction. To address this possibility, we conducted an additional experiment in which rats received vehicle (n = 8) or propranolol (n = 8) immediately after conditioning but did not receive extinction trials (Fig. 6 D–F). As before, both groups acquired fear conditioning and exhibited similar levels of conditioned freezing before drug treatment [Fig. 6D; main effect of group, F(1,14) < 1]. Thirty minutes after conditioning, the rats were returned the conditioning chambers (context B), but no extinction trials were delivered. Similar to the previous IED experiment, propranolol-treated rats exhibited reduced freezing early in the session [Fig. 6E; drug × time interaction, F(5,70) = 2.88, P < 0.05]. Importantly, during the fear recall test 48 h after conditioning, there was no difference in conditioned freezing between the groups [Fig. 6F; Fs < 1]. This finding indicates that postconditioning propranolol did not impair consolidation of the fear memory, an effect that is consistent with a previous report (10). These data support the view that postconditioning propranolol facilitates immediate extinction by reducing postshock fear and stabilizing mPFC firing.
Fig. 6.
Propranolol mitigates the immediate extinction deficit. Propranolol administration immediately after fear conditioning (A) reduced both baseline (BL) and freezing during the immediate extinction session (B, 30 min after conditioning). Propranolol facilitated the recall of extinction during a retention test conducted 48 h after extinction (vehicle, n = 7; propranolol, n = 7) (C). This effect on freezing was not due to impaired consolidation of the fear conditioning memory. Rats that were administered propranolol immediately after conditioning (D), but not extinguished exhibited reductions in freezing early in the no-extinction session (E, context exposure only) and high levels of freezing during the retention test that did not differ from vehicle-treated controls (vehicle, n = 8; propranolol, n = 8) (F). Gray bars denote the 3-min baseline (BL) period before delivery of conditioning or extinction trials. *P < 0.05 vs. propranolol. All values are means ± SEM.
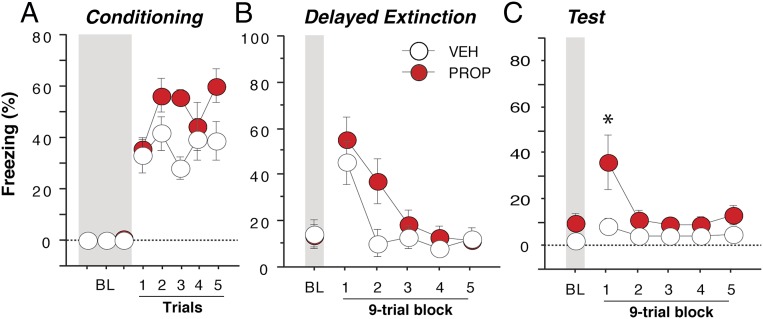
If propranolol facilitates extinction learning under conditions of high noradrenergic arousal, how might it affect learning when the psychological stress level is presumably lower? To address this question, we conducted another experiment in which rats received vehicle or propranolol (n = 8 per group) before delayed extinction (24 h after conditioning). As shown in Fig. 7, freezing behavior before the first CS trial during the extinction session was low (Fig. 7B) in both vehicle- and propranolol-treated rats. This finding indicates that delayed extinction limits the high levels of sensitized fear observed with the immediate extinction procedure (Fig. 6 B and E). In addition, propranolol administration did not influence either the expression of fear or within-session extinction [Fig. 7B; Fs < 2], consistent with the suggestion that basal noradrenergic arousal must be high in order for propranolol to limit freezing. However, propranolol-treated rats exhibited impairments in extinction recall during the retention test 24 h after extinction. Specifically, rats in the propranolol group exhibited greater levels of freezing during the first block of nine trials of the session relative to vehicle-treated rats [Fig. 7C; drug × time interaction, F(5,70) = 2.84, P < 0.05]. Thus, propranolol given before delayed extinction impaired, rather than enhanced, learning during this putatively lower state of psychological stress.
Fig. 7.
Propranolol impairs delayed extinction. In contrast to its effects on immediate extinction, propranolol given 30 min before a delayed extinction session, which took place 24 h after conditioning (A) did not significantly alter within-session extinction (B). Moreover, propranolol-treated rats exhibited a deficit in extinction recall 24 h after extinction (C) (vehicle, n = 8; propranolol, n = 8). Gray bars denote the 3-min baseline (BL) period before delivery of conditioning or extinction trials. *P < 0.05 vs. vehicle. All values are means ± SEM.
Discussion
Stress-induced extinction deficits, including the IED, have been posited to arise from dysregulation of mPFC function (23, 39, 42). In support of this hypothesis, the present experiments reveal that footshock stress accompanying fear conditioning produces dramatic changes in both the firing rate and bursting profile of single units recorded in PL and IL. Systemic β-adrenergic blockade by propranolol counteracts these effects by maintaining a relative balance in PL and IL neural activity after the footshock stressor. In addition, propranolol administration rescued the IED, suggesting that noradrenergic stabilization of mPFC activity buffers against the deleterious effects of stress on extinction learning. This previously unidentified finding has important implications for understanding how β-noradrenergic interventions minimize the deleterious effects of marked psychological stress or trauma and improve psychotherapeutic outcomes (17, 18). Moreover, these data suggest that propranolol may be particularly effective in facilitating fear reduction when prevailing stress at the onset of extinction training is high. Thus, the timing of propranolol administration may be critical to maximizing its therapeutic efficacy in the treatment of stress- or trauma-related disorders such as PTSD.
It has previously been suggested that PL and IL have opposing roles in the regulation of fear (23, 32–34, 43). The present data lend some support to this view insofar as the expression of freezing behavior after conditioning was associated with distinct patterns of firing among simultaneously recorded neurons in PL and IL. Immediately after the last fear conditioning trial on day 1, PL neurons (on average) exhibited a massive, but transient increase in spontaneous firing rate. In contrast, IL neurons (on average) exhibited a weaker increase in firing rate, followed by a sustained decrease in firing that persisted for much of the recording session. Hence, a shift in the balance of PL and IL activity accompanied both the induction and maintenance of freezing behavior in the aftermath of conditioning. However, it is important to note that sustained decreases in IL firing, rather than sustained increases in PL firing, were associated with the maintenance of freezing behavior. This observation suggests that regulation of IL-mediated inhibition of amygdala excitability, for example, is not only involved in the expression of extinction (44), but also the expression of conditioned fear. Consistent with the sustained changes in firing rate we observed after footshock, a study of restraint stress in rats found that a population of mPFC neurons showed an increase in firing rate that persisted for over 2 h after the stressor (45).
Surprisingly, fear-related changes in mPFC firing were qualitatively different after fear conditioning than after the presentation of fear CSs. Neurons in both IL and PL exhibited much more dramatic changes in spontaneous firing immediately after CS-US pairings on day 1 than after presentation of the CS alone on day 2, despite similar (and nearly asymptotic) levels of freezing behavior in each session, particularly in vehicle-treated rats. These results indicate that it is not high levels of fear per se that correlate with changes in mPFC neuronal firing (23, 24, 32–34), but rather the emotional context in which that fear is experienced (46, 47). Specifically, our results suggest that mPFC firing is particularly sensitive to the acute effects of the footshock US, possibly reflecting unconditioned components of fear in the immediate aftermath of shock exposure. Experiments examining the consequences of footshock delivery immediately on placement in the recording chamber (i.e., an immediate shock procedure that yields little freezing) would help to resolve this issue (48, 49). Collectively, our results reveal that, whereas recent exposure to footshock strongly modulates neuronal firing in mPFC, exposure to the CS alone does not. It should also be noted that within-session spontaneous firing rates in PL and IL did not correlate with ongoing freezing behavior (and by inference, fear state). For example, firing rates in both PL and IL largely returned to baseline by the end of the first recording session, despite the fact that freezing behavior remained markedly elevated relative to the preshock baseline. This finding suggests that circuits other than mPFC mediate the sustained freezing behavior we observed, although PL and IL may initiate or otherwise contribute to this effect.
Because β-adrenergic receptors have previously been implicated in stress-induced modulation of prefrontal function (50), we next examined whether systemic propranolol administration affected shock-induced changes in PL and IL firing. We found that propranolol administration before fear conditioning stabilized spontaneous activity in PL and IL after footshock, dampening the magnitude of shock-induced spike firing changes observed among single-units in each area. Specifically, propranolol both attenuated the immediate postshock increases in firing rate in PL, as well as the decreases in IL firing that accompanied the expression of fear during the remainder of the session. This observation suggests that propranolol may reduce fear after conditioning, at least in part, by stabilizing neuronal firing in PL and IL in the aftermath of footshock.
Indeed, the stabilizing effect of propranolol on PL and IL spike firing may underlie the facilitation of extinction that we observed behaviorally when this drug was given before immediate extinction. That is, propranolol administered immediately after fear conditioning reduced the expression of freezing behavior during the immediate extinction session and facilitated lasting extinction. This effect was not due to an effect of propranolol on fear memory consolidation (10) as the drug had no effect on conditioned freezing in animals that did not undergo extinction. Similar to our results, Quirk and colleagues observed decreases in freezing behavior after systemic propranolol administered before an extinction session that was conducted 24 h after conditioning (36). Interestingly, however, they found no lasting effect of propranolol on extinction under these conditions (36) and, in a related study, they reported extinction impairments after intra-IL propranolol infusion (51). We suggest that the disparities in these results are related to the timing of extinction and propranolol administration relative to fear conditioning. Specifically, propranolol administration soon after conditioning facilitates immediate extinction by dampening shock-induced noradrenergic arousal (27–29, 52), whereas propranolol administration long after conditioning, when noradrenergic arousal is low, impairs extinction learning by reducing adrenergic transmission below optimal levels (25). This latter hypothesis is consistent with the present data showing that propranolol administered before delayed extinction actually impairs learning. Collectively, our data suggest that propranolol administered during stress stabilizes PL and IL activity and facilitates extinction learning.
Of course, a critical question is whether the β-adrenergic receptors mediating the effects of systemic propranolol are located in the mPFC or in other brain regions that regulate the mPFC including the locus coeruleus (LC) and basolateral amygdala (BLA) (53). Consistent with the former possibility, IL infusion of propranolol has been reported to influence extinction recall (51); however, it is not known whether this manipulation facilitates immediate extinction. Alternatively, noradrenergic modulation of BLA excitability (53) may influence mPFC firing to regulate extinction. Consistent with this possibility, it has been found that induction of inflammatory pain decreases mPFC firing, a change that was mediated by hyperexcitability in the BLA (54). Indeed, other stressors have also been reported to modulate mPFC through the amygdala (55), and the BLA regulates fear and extinction through its long-range projections to mPFC (56). Ultimately, stress-induced NE release from LC terminals, which has been broadly implicated in the regulation of memory and emotion (53), may influence mPFC spike firing either directly or through indirect modulatory circuits.
The present experiments have critical implications for developing pharmacotherapeutic interventions for anxiety- and trauma-related disorders in humans. For example, a commonly used, albeit controversial approach to prevent PTSD is so-called psychological debriefing, in which behavioral therapy is given soon after exposure to a traumatic event (57–59). Administration of noradrenergic pharmacological agents, such as propranolol, soon after trauma could enhance the effectiveness of debriefing or other early interventions by modulating prefrontal cortical activity as we have described here. Consistent with this, it has been reported that propranolol treatment within days of trauma in humans reduces the incidence of PTSD (21). Moreover, propranolol administered after trauma reactivation in patients with PTSD has a therapeutic effect on physiological responding to traumatic imagery weeks after the pharmacological intervention (17, 18). Together, these studies suggest that propranolol administration may be particularly effective when trauma-related arousal is high (i.e., soon after trauma and after trauma reactivation). The present data suggest that the efficacy of propranolol under these conditions would be greatly enhanced by concurrent exposure therapy.
In summary, exposure to footshock stress initiates pronounced signaling changes in mPFC and freezing behavior and β-noradrenergic blockade by propranolol mitigates these effects. Collectively, these findings shed light on prefrontal executive control of fear-related behavior while also suggesting that propranolol treatment may enhance behavioral debriefing aimed at preventing PTSD development after recent exposure to trauma.
Materials and Methods
Subjects.
Adult male Long–Evans Blue Spruce rats (weighing 200–224 g; 50–57 d old) were obtained from a commercial supplier (Harlan Sprague–Dawley). On arrival and throughout the experiments, these experimentally naïve rats were individually housed in cages within a temperature- and humidity-controlled vivarium and kept on a 14:10-h light/dark cycle (lights on at 7:00 AM) with ad libitum access to food and water. All experiments took place in the daytime during the light phase. Rats were handled for ∼30 s a day for 5 d before any behavioral testing or surgical procedures were carried out to habituate them to the experimenter. The number of rats used in each experiment is stated in the figure legends. All experiments were conducted at Texas A&M University with full approval from its Animal Care and Use Committee.
Drugs.
d,l-Propranolol hydrochloride was obtained from a commercial supplier (Sigma-Aldrich). The drug was dissolved in distilled water (5 mg/mL) and injected systemically (10 mg/kg, i.p.) in a volume of 2 mL/kg.
In Vivo Electrophysiology.
Twelve rats (vehicle, n = 6; propranolol, n = 6) were used for the electrophysiological experiments; one rat in the vehicle group died before completing the experiment leaving five rats in that group. Rats were assigned to each drug condition such that each condition was alternated across the experiment. For implantation of the recording array, rats were anesthetized with isoflurane (5% induction, 2% maintenance) and secured in a stereotaxic apparatus (Kopf Instruments). The scalp was incised and retracted; three burr holes were drilled for anchor and ground screws. A portion of the skull overlying the mPFC was removed to allow for microelectrode implantation. The rat was implanted with a 16-channel microelectrode array (Innovative Neurophysiology) targeting both the PL (eight wires) and IL (eight wires) subdivisions of the mPFC in the right hemisphere. The 2 × 8 wire microarray was constructed from two rows of 50-µm-diameter tungsten wires of two different lengths (PL, 6.9 mm; IL, 8.0 mm); wires in each row and the rows themselves were spaced 200 µm apart. The array was positioned with its long axis parallel to the anterior-posterior plane. The coordinates for the centermost wires of the array were as follows: PL, +2.7 mm AP, +0.55 mm ML, −4.0 mm DV and IL, +2.7 mm AP, +0.35 mm ML, −5.1 mm DV (relative to bregma at skull surface). The mediolateral offset (200 µm) between the PL and IL electrode rows minimized damage to the overlying cortex during array implantation. Also, the slightly more medial coordinate of the IL wires, relative to the PL ones, accommodates the slightly thinner IL cortex, allowing recordings in similar layers in the two brain areas within a given rat. The array was secured to the skull with dental acrylic and one week was allowed for recovery before in vivo recordings began.
A standard rodent conditioning chamber (30 × 24 × 21 cm; Med Associates) housed in a sound-attenuating cabinet was modified to allow for electrophysiological recordings. The chamber consisted of two aluminum sides, a Plexiglas rear wall, and a hinged Plexiglas door. The grid floor contained 19 stainless steel rods (4 mm diameter) spaced 1.5 cm apart (center-to-center). Rods were connected to a shock source and solid-state grid scrambler (Med Associates) for the delivery of footshocks. A loudspeaker mounted on the outside of a grating in one aluminum wall was used to play auditory tones. Locomotor activity was transduced by a load cell under the floor of the chamber, and the output of the load cell was recorded by an OmniPlex recording system (Plexon). The experimenters were not blind to drug treatment group, but all behavioral and neural activity was recorded automatically.
Single-unit recordings occurred over 2 d in two distinct contexts. On day 1, the rats were transported to the recording room in a black box, connected to the headstage cable, and placed in the recording chamber. The chamber was cleaned with 1% ammonium hydroxide to provide a distinct olfactory cue, a black pan containing a thin layer of the same solution was placed under the grid floor, and the room was illuminated with ambient red lights (context A). After a 3-min stimulus-free baseline period, the rat was briefly removed from the chamber and injected with either propranolol (10 mg/kg, i.p) or vehicle (distilled water) and then immediately returned to the recording chamber. Neural and behavioral data were not recorded during injection (∼1 min) due to the electrical noise associated with handling the rat. Twenty minutes after the injection (36), five tone (2 s, 80 dB, 2 kHz)–footshock (0.5 s, 1 mA) trials were delivered (shock onset occurred at tone offset) with a 1-min ITI. Behavioral and neuronal data were not recorded during the conditioning period due to the electrical noise associated with shock delivery; recordings commenced immediately after the last footshock. The recording session continued for 60 min after the last footshock, after which the rat was returned to its home cage.
On day 2, the transport and recording contexts were altered to reduce generalization of fear from the conditioning session to the test session. The rat was transported in a white box. The recording chamber was cleaned with 1% acetic acid to provide a distinct olfactory cue, a white pan containing a thin layer of the same solution was placed under the grid floor, the grid floor was covered with a transparent rubber mat, the back wall was covered with alternating black and white stripes, and the room was illuminated with ambient fluorescent lights (context B). After a 3-min stimulus-free baseline period, the rat was presented with five tone-alone trials (1-min ITI; all tone parameters were the same as on day 1); the rat remained in the chamber for 60 min after the final tone, and behavioral and neuronal data were recorded throughout the session.
Extracellular single-unit activity was recorded using a multichannel neurophysiological recording system (OmniPlex; Plexon). Wideband signals recorded on each channel were referenced to one of the recording wires (resulting in 15 channels of activity per rat), amplified (8,000×), digitized (40 kHz), and saved on a PC for offline sorting and analysis. The recording reference wire was located in PL and was randomly selected to optimize the quality of the recordings. After high-pass filtering the signal at 600 Hz, waveforms were sorted manually using 2D principal component analysis (Offline Sorter; Plexon). Only well-isolated units were used in the analysis. If two units with similar waveforms and identical time stamps for their action potentials appeared on adjacent electrodes, only one unit was used. Sorted waveforms and their timestamps were then imported to NeuroExplorer (Nex Technologies) for analysis.
The analysis of neuronal activity focused on spontaneous single-unit firing and bursting during each recording session; CS-evoked activity was also analyzed on day 2. To compute firing rate histograms, spike rates were binned (20 s) and normalized (z-scores) to control for differences in baseline firing rate. On day 1, firing rate was normalized to the entire preconditioning period (3-min preinjection and 20-min postinjection periods). On day 2, the data were normalized to the 3-min baseline period before CS presentations. For the burst analyses, a burst was defined as two spikes with an interspike interval of <25 ms followed by a third spike within 50 ms of the second spike (60); bursts could continue if additional spikes occurred within 50-ms intervals of one another.
IED and Delayed Extinction Experiments.
Sixteen adult Long–Evans (Blue Spruce) rats served as subjects. All behavioral training was conducted in two adjacent rooms, each containing eight identical conditioning chambers (same dimensions as for the in vivo recordings). Video cameras mounted above the behavioral chambers were used to monitor the animals during each session. Each chamber rested on a load cell platform that transduced locomotor activity (Med Associates). Load cell activity was digitized (Threshold Activity Software; Med Associates) and transformed as previously described to measure freezing behavior. Rats received three phases of training. They first received fear conditioning (context A, room 1) followed 30 min later by an immediate extinction session (context B, room 2). An extinction retrieval test (context B, room 2) was conducted 48 h after extinction. Contexts had distinct olfactory and visual cues, similar to those described above.
Fear conditioning consisted of a 3-min stimulus-free baseline period, followed by five tone (10 s, 80 dB, 2 kHz)–shock (2 s, 1 mA) pairings (1 min ITI); the rats remained in the chambers 3 min after the last trial. Immediately after conditioning, half the rats received systemic administration of propranolol (n = 8, 10 mg/kg, i.p.) and the other half received vehicle (n = 8, distilled water); after injection, they were returned to their home cages in the vivarium. Thirty minutes after fear conditioning, the rats were returned to a novel room and context (context B) and presented with 45 tone-alone trials (1-min ITI; same tone parameters as during fear conditioning) after a 3-min baseline. All rats were given a subsequent extinction retrieval session (retention test; context B; same tone parameters as before) 48 h after conditioning to assess long-term extinction memory. One rat from each group exhibited levels of conditioned freezing during the retention test that was ±2 SD from the group mean; these statistical outliers were excluded from the analysis.
In a second behavioral experiment (no extinction) with 16 adult Long–Evans rats, we examined whether postconditioning propranolol treatment interferes with fear memory consolidation. The experiment was identical to that described in preceding IED experiment, except that CS-alone trials were not delivered 30 min after conditioning, although rats were still placed in the extinction context. Rats did receive a retention test 48 h later, in which the CSs were administered.
A third experiment examined delayed extinction (16 adult Long–Evans rats), in which the extinction session took place 24 h after fear conditioning and propranolol or vehicle was given 30 min before extinction. A retention test was then given 24 h after extinction.
Histology.
After the completion of experiments, recording rats were overdosed with pentobarbital, and electrolytic lesions (80 µA, 10 s; A365 stimulus isolator; World Precision Instruments) were generated through six of the recording wires to mark the location of the recording array in the medial prefrontal cortex. The rats were then perfused transcardially with 0.9% saline followed by 10% formalin (10% formaldehyde by volume, diluted in physiological saline). Brains were extracted from the skull and postfixed in a 10% formalin solution for 24 h followed by 10% formalin/30% sucrose solution [30% sucrose (wt/vol), diluted in 10% formalin solution] where they remained for a minimum of 48 h. After the postfix period, brains were sectioned (50 µm) on a cryostat (−20 °C), mounted on subbed microscope slides, and stained with thionin (0.25%) to visualize electrode placements.
Statistics.
Data were analyzed with conventional parametric statistics (StatView; SAS Institute). Two-way ANOVA and repeated-measures ANOVA were used to assess general main effects and interactions (α = 0.05). Unpaired Student's two-tailed t tests were also used for pairwise comparisons of means. Results are shown as means ± SEMs.
Acknowledgments
This work was supported by National Institutes of Health Grant R01MH065961.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500682112/-/DCSupplemental.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Weston CSE. Posttraumatic stress disorder: A theoretical model of the hyperarousal subtype. Front Psychiatry. 2014;5:37. doi: 10.3389/fpsyt.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom Med. 2006;68(2):307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 4.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45(9):2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Jovanovic T, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oznur T, et al. Is transcranial magnetic stimulation effective in treatment-resistant combat related posttraumatic stress disorder? Neurosciences (Riyadh) 2014;19(1):29–32. [PubMed] [Google Scholar]
- 9.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 10.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129(2):267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiol Learn Mem. 2011;96(2):263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 12.van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joëls M. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiol Learn Mem. 2010;93(1):56–65. doi: 10.1016/j.nlm.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Bos MGN, Beckers T, Kindt M. The effects of noradrenergic blockade on extinction in humans. Biol Psychol. 2012;89(3):598–605. doi: 10.1016/j.biopsycho.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Southwick SM, et al. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54(8):749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 15.Southwick SM, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 16.Pervanidou P, Chrousos GP. Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149–160. doi: 10.1016/S0079-6123(10)82005-9. [DOI] [PubMed] [Google Scholar]
- 17.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42(6):503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, et al. Trauma reactivation plus propranolol is associated with durably low physiological responding during subsequent script-driven traumatic imagery. Can J Psychiatry. 2014;59(4):228–232. doi: 10.1177/070674371405900408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koola MM, Varghese SP, Fawcett JA. High-dose prazosin for the treatment of post-traumatic stress disorder. Ther Adv Psychopharmacol. 2014;4(1):43–47. doi: 10.1177/2045125313500982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Writer BW, Meyer EG, Schillerstrom JE. Prazosin for military combat-related PTSD nightmares: A critical review. J Neuropsychiatry Clin Neurosci. 2014;26(1):24–33. doi: 10.1176/appi.neuropsych.13010006. [DOI] [PubMed] [Google Scholar]
- 21.Vaiva G, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 22.Farrell MR, Sengelaub DR, Wellman CL. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol Behav. 2013;122:208–215. doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS ONE. 2010;5(8):e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzgerald PJ, et al. Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiol Learn Mem. 2014;113:69–81. doi: 10.1016/j.nlm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64(3):619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 28.Dazzi L, Seu E, Cherchi G, Biggio G. Chronic administration of the SSRI fluvoxamine markedly and selectively reduces the sensitivity of cortical serotonergic neurons to footshock stress. Eur Neuropsychopharmacol. 2005;15(3):283–290. doi: 10.1016/j.euroneuro.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem. 1994;63(2):575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- 30.Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol Learn Mem. 2010;94(2):240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Rive MM, et al. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37(10 Pt 2):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13(6):728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27(4):840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29(26):8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J Neurosci. 1998;18(8):3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65(10):887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci USA. 2006;103(47):18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CH, Maren S. Early extinction after fear conditioning yields a context-independent and short-term suppression of conditional freezing in rats. Learn Mem. 2009;16(1):62–68. doi: 10.1101/lm.1085009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maren S. Nature and causes of the immediate extinction deficit: A brief review. Neurobiol Learn Mem. 2014;113:19–24. doi: 10.1016/j.nlm.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13(2):216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norrholm SD, et al. Timing of extinction relative to acquisition: A parametric analysis of fear extinction in humans. Behav Neurosci. 2008;122(5):1016–1030. doi: 10.1037/a0012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26(21):5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16(8):486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson ME, Moghaddam B. Distinct patterns of plasticity in prefrontal cortex neurons that encode slow and fast responses to stress. Eur J Neurosci. 2006;24(6):1702–1710. doi: 10.1111/j.1460-9568.2006.05054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goosens KA, Hobin JA, Maren S. Auditory-evoked spike firing in the lateral amygdala and Pavlovian fear conditioning: Mnemonic code or fear bias? Neuron. 2003;40(5):1013–1022. doi: 10.1016/s0896-6273(03)00728-1. [DOI] [PubMed] [Google Scholar]
- 47.Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23(23):8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15(4):177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 49.Lattal KM, Abel T. An immediate-shock freezing deficit with discrete cues: A possible role for unconditioned stimulus processing mechanisms. J Exp Psychol Anim Behav Process. 2001;27(4):394–406. [PubMed] [Google Scholar]
- 50.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther. 2007;113(3):523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28(2):369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66(3):253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 53.McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36(7):1750–1762. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji G, et al. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30(15):5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23(11):4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senn V, et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81(2):428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Deahl M. Psychological debriefing: Controversy and challenge. Aust N Z J Psychiatry. 2000;34(6):929–939. doi: 10.1080/000486700267. [DOI] [PubMed] [Google Scholar]
- 58.Mansdorf IJ. Psychological interventions following terrorist attacks. Br Med Bull. 2008;88(1):7–22. doi: 10.1093/bmb/ldn041. [DOI] [PubMed] [Google Scholar]
- 59.Forneris CA, et al. Interventions to prevent post-traumatic stress disorder: A systematic review. Am J Prev Med. 2013;44(6):635–650. doi: 10.1016/j.amepre.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]