Significance
Visceral leishmaniasis (VL), also known as kala-azar, is a major parasitic disease on the Indian subcontinent, with 85% of the disease incidence in India. As with malaria, indoor residual spraying (IRS) can be used to control and, ultimately, eliminate VL as a public health problem. However, in India, widespread resistance to dichlorodiphenyltrichloroethane (DDT), the insecticide used, combined with poor quality assurance (QA) of IRS and limited entomological surveillance, is hindering the VL elimination effort. Here, we present entomological abundance and insecticide resistance data and data arising from QA of IRS to explore these issues and establish an evidence base for improving the Indian VL elimination program. Unless these issues are addressed, the South Asian VL elimination effort will fail.
Keywords: leishmaniasis, elimination, DDT, IRS, India
Abstract
Indoor residual spraying (IRS) is used to control visceral leishmaniasis (VL) in India, but it is poorly quality assured. Quality assurance was performed in eight VL endemic districts in Bihar State, India, in 2014. Residual dichlorodiphenyltrichloroethane (DDT) was sampled from walls using Bostik tape discs, and DDT concentrations [grams of active ingredient per square meter (g ai/m2)] were determined using HPLC. Pre-IRS surveys were performed in three districts, and post-IRS surveys were performed in eight districts. A 20% threshold above and below the target spray of 1.0 g ai/m2 was defined as “in range.” The entomological assessments were made in four districts in IRS and non-IRS villages. Vector densities were measured: pre-IRS and 1 and 3 mo post-IRS. Insecticide susceptibility to 4% DDT and 0.05% deltamethrin WHO-impregnated papers was determined with wild-caught sand flies. The majority (329 of 360, 91.3%) of pre-IRS samples had residual DDT concentrations of <0.1 g ai/m2. The mean residual concentration of DDT post-IRS was 0.37 g ai/m2; 84.9% of walls were undersprayed, 7.4% were sprayed in range, and 7.6% were oversprayed. The abundance of sand flies in IRS and non-IRS villages was significantly different at 1 mo post-IRS only. Sand flies were highly resistant to DDT but susceptible to deltamethrin. The Stockholm Convention, ratified by India in 2006, calls for the complete phasing out of DDT as soon as practical, with limited use in the interim where no viable IRS alternatives exist. Given the poor quality of the DDT-based IRS, ready availability of pyrethroids, and susceptibility profile of Indian sand flies, the continued use of DDT in this IRS program is questionable.
Leishmaniases are diseases caused by protozoan parasites that are transmitted to humans through the bites of infected female sand flies. The visceral form of leishmaniasis (VL) is endemic on the Indian subcontinent and in East Africa and Brazil, and 90% of the estimated 200–400,000 annual cases come from people living in these countries (1).
An estimated 200 million people are at risk for VL, of whom 65 million live in India (2). The majority of Indian VL cases occur in the northeastern states, predominantly in Bihar (3, 4). The numbers of VL cases reported in India are based on passive case reporting, and therefore may be an underestimation (2, 5); however, regardless, advances in VL diagnostic tests and improved treatment modalities have ensured that the number of deaths due to VL in India has gradually decreased over the past 5 y (6).
VL in India is caused by Leishmania donovani and is only transmitted by the female sand fly, Phlebotomus argentipes; no animal reservoir exists (2, 7). During the period of 1953–1962, indoor residual spraying (IRS) undertaken by the Indian national malaria program using dichlorodiphenyltrichloroethane (DDT) at 1 grams of active ingredient per square meter (g ai/m2) for malaria control had the beneficial side effect of significantly reducing the number of VL cases in India. A similar effect on VL due to IRS for malaria control was also seen in Bangladesh from 1961–1970 (8). In India, VL did not reappear in areas such as Assam until the late 1970s (9). This inadvertent control of VL led to the adoption of IRS by the Indian VL elimination program as the main focus for P. argentipes control, and two periods of IRS using DDT at 1 g ai/m2 during 1977–1979 and 1992–1995 both saw sharp decreases in the number of VL cases reported (6).
In India, IRS is performed by spraying houses and animal shelters to control the endophilic and exophagic vector P. argentipes (10). Although the effect of IRS on P. argentipes is based on limited data (11–13), a cluster randomized trial performed in India, Bangladesh, and Nepal demonstrated that IRS using DDT reduced the indoor abundance of P. argentipes by 72.4% in intervention clusters compared with controls; this effect was greater than the effect of environmental modification or the use of long-lasting insecticide-treated nets (42.0% and 43.7%, respectively) (14). Older models also predict that IRS is capable of achieving VL elimination, provided that sand fly densities are reduced by 67% (15). Given that effective prevention through vector control and rapid diagnosis and treatment methodologies exist (7, 16), elimination of VL in this region should therefore be technically feasible. However, in order for the predicted outcomes of IRS to become a reality, the IRS itself must be of sufficient quality to achieve an impact (17, 18).
Strong regional political support for VL elimination was established through a tripartite agreement between Bangladesh, India, and Nepal that was signed in 2005 with the aim of reducing the annual incidence of VL and post–kala-azar dermal leishmaniasis (PKDL) to less than one case per 10,000 population at the subdistrict level by 2015 (19). This program was a four-phase process to interrupt transmission, enhance early diagnosis and treatment, and improve health education (20). The tripartite agreement also aimed to standardize IRS in the region, as recommended by the regional technical advisory group (RTAG) (21) (Table 1).
Table 1.
Schedule for the elimination of VL in India, Bangladesh, and Nepal
| Years | Phase |
| 2005–2007 | Preparatory phase: Establish IRS and build capacity |
| 2008–2013 | Attack phase: Intensify IRS, increase vector surveillance, improve early diagnosis and treatment, and introduce case surveillance |
| 2014–2016 | Consolidation phase: Limited IRS and case surveillance |
| 2016 onward | Maintenance phase: Surveillance and rapid response to prevent reintroduction |
Data are from ref. 21.
In India, the elimination effort is led by the Indian National Vector Borne Disease Control Programme (NVBDCP) and supported by a range of national research institutes and nongovernmental organizations. Following the successful completion of all four phases of the elimination plan, the WHO will be approached to review the status and provide a certificate of elimination (22).
A range of insecticides could be used for IRS. DDT, malathion, deltamethrin, cyfluthrin, alpha-cypermethrin, and lambda-cyhalothrin are registered and have been used for malaria IRS-based control activities in India (23). DDT is by far the most controversial of these insecticides, belonging to a group of persistent organic pollutants (POPs) that have received significant global attention due to their bioaccumulation properties, high toxicity, and ubiquitous exposure of humans and wildlife. Under the Stockholm Convention, agreed on in 2000 for “Protecting human health and the environment from POPs,” there is international agreement that DDT, although maintained temporarily for IRS for vector-borne disease control where other alternatives are not viable, should be totally phased out as soon as practical (24). India ratified the Stockholm Convention in 2006. Despite this fact, and the availability of a range of alternative chemicals, the decision was made to use DDT at 1.0 g ai/m2 for scaling up of VL elimination activities.
In the attack phase of the program, two rounds of IRS should be performed annually, the first in February/March and the second in May/June (19, 20). However, the IRS implementation was suboptimal in India and Nepal and did not start in Bangladesh until 2010 (25). Although Bangladesh and Nepal, where transmission is lower, have subsequently made significant gains (26), the Indian program remains in the attack phase.
The RTAG recommended that close monitoring of insecticide resistance should be performed as part of the drive toward elimination (21). Although the current data on sand fly resistance are limited, a review of resistance in the region clearly shows that DDT resistance in sand flies has been regularly reported in India since the 1990s (8). There are also sporadic reports of low levels of deltamethrin resistance in sand flies; however, because none of the studies applied the same standardized protocol, it is difficult to map overall trends in resistance prevalence.
Here, we present the first systematic analysis, to our knowledge, of the quality of the delivery of insecticide to wall surfaces in the Indian IRS program in eight districts in Bihar State and discuss the implications of the findings for the regional VL elimination program.
Results
Study Sites.
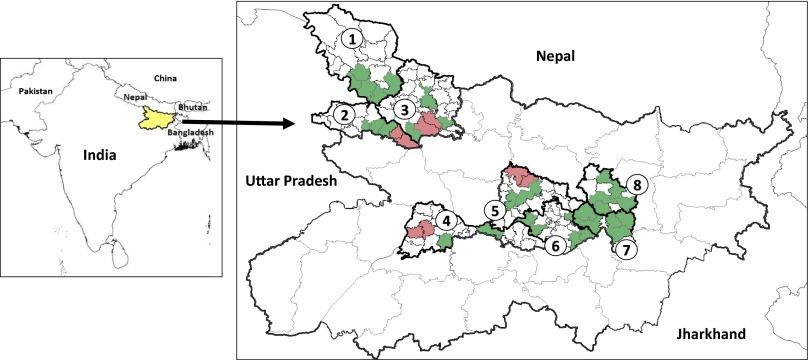
Eight districts were selected for IRS quality assurance (QA) surveys: Begusarai, Gopalganj, Khagaria, Paschim Champaran, Patna, Purbi Champaran, Saharsa, and Samastipur. Four of these districts, Gopalganj, Patna, Purbi Champaran, and Samastipur, were chosen for entomological assessment (Fig. 1). The post-IRS surveys covered 41 primary health centers (PHCs) across the eight study districts.
Fig. 1.
Map of the Indian subcontinent showing VL-endemic countries. VL-endemic countries are labeled. (Inset) Eight study districts in Bihar, where 1 = Paschim Champaran, 2 = Gopalganj, 3 = Purbi Champaran, 4 = Patna, 5 = Samastipur, 6 = Begusarai, 7 = Khagaria, and 8 = Saharsa. Smaller divisions within districts indicate study PHC boundaries; green indicates PHCs where only IRS QA was performed, and red indicates PHCs where IRS QA and entomological assessments were performed.
IRS QA.
Pre-IRS surveys.
In total, 360 IRS wall samples, corresponding to 90 households, were tested for DDT residue concentration. The majority of households (73 of 90, 81.1%) were from villages where the last IRS round had occurred within the year. The median number of days between the last spray and the sample collection date from these households was 108 d (interquartile range: 103–158 d).
The vast majority of samples (329 of 360, 91.3%) had DDT concentrations below 0.1 g ai/m2. The remainder had average household DDT residue levels of 0.24 g ai/m2 [95% confidence interval (CI): 0.15–0.32 g/m2].
Post-IRS surveys.
Overall, 560 households were sampled for DDT post-IRS, resulting in 2,228 samples. All samples were collected between 0 and 45 d postspraying. The median number of days between spraying and sample collection was 5 d. The vast majority of samples (90.1%) were collected 0–15 d postspraying.
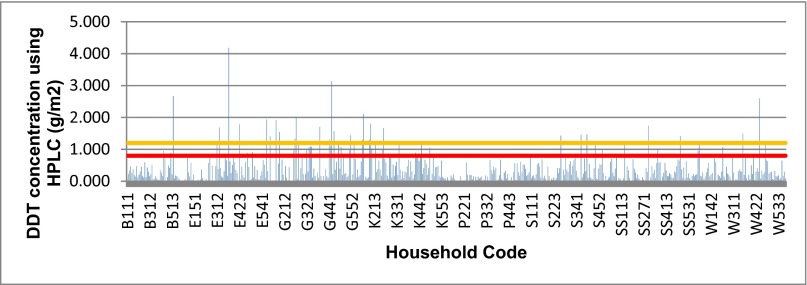
The average level of DDT found on walls post-IRS was 0.37 g ai/m2 (95% CI: 0.35–0.40). Overall, 84.9% (1,892 of 2,228) of walls were undersprayed (below 80% of target dose of 1 g ai/m2), 7.4% (166 of 2,228) of walls were within the target range (0.8–1.2 g/ai m2), and 7.6% (170 of 2,228) of walls were oversprayed (above 1.2 g ai/m2). Average household results showed 489 of 560 (87.3%) undersprayed houses, 41 of 560 (7.3%) houses on target, and 28 of 560 (5.0%) oversprayed houses (Fig. 2).
Fig. 2.
Average DDT concentrations on walls, per household, following the first round of IRS in 2014. DDT concentrations on walls in 560 households, using average household results. The orange line represents the upper limit of in-target quantification (1.2 g ai/m2), and the red line represents the lower limit of in-target quantification (0.8 g ai/m2). Bostik tape discs were used to remove the insecticide from walls, and all samples were measured using HPLC.
Four samples were taken from different positions in each household to investigate whether DDT was evenly distributed on the walls. Stratifying results by height showed almost identical average concentrations of DDT (high = 0.406 g ai/m2, medium = 0.378 g ai/m2, very low = 0.360 g ai/m2, and low = 0.346 g ai/m2; P = 0.16), indicating that the average quality of the IRS spraying between households was consistent. ANOVA within each household confirmed there were no significant differences (P = 0.32) in the amount of DDT distributed on walls.
When results were stratified by geographic location, it was evident that none of the study districts had DDT concentrations on the walls that were routinely within the target range set by the IRS program. Only one of 41 (2.4%) PHCs had an average concentration of DDT on walls that fell within the target range of 0.8–1.2 g ai/m2 (details are provided in SI Appendix). Stratifying data by village showed 8 of 187 (4.3%) of villages were on target, 5 of 187 (2.7%) villages were oversprayed, and 174 of 187 (93.0%) villages were undersprayed. The villages that were sprayed within the target range were evenly distributed across the eight study districts (Begusarai = 1, Purba Champaran = 1, Gopalganj = 1, Khagaria = 2, Patna = 0, Saharsa = 0, Samastipur = 2, and Paschim Champaran = 1, respectively).
When matched pre-IRS and post-IRS samples were compared using the Wilcoxon signed rank test (matched for household, wall position, and surface type), there was a 10-fold difference between the mean pre-IRS and post-IRS concentrations of DDT (P < 0.0001), although the concentrations of DDT were still well below the target dose post-IRS. Using the pre-IRS values to adjust the post-IRS values gave an average DDT residue on the walls from the spray round of 0.2 g ai/m2 compared with 0.37 g ai/m2.
Historical Insecticide Resistance Data.
India does not currently conduct routine programmatic monitoring of insecticide susceptibility levels in sand flies; therefore, comprehensive data were not available. A literature survey resulted in a total of 11 studies that met the inclusion criteria for basic resistance analysis, spanning the years 1978–2014. Data were only available for the insecticides DDT, deltamethrin, and malathion.
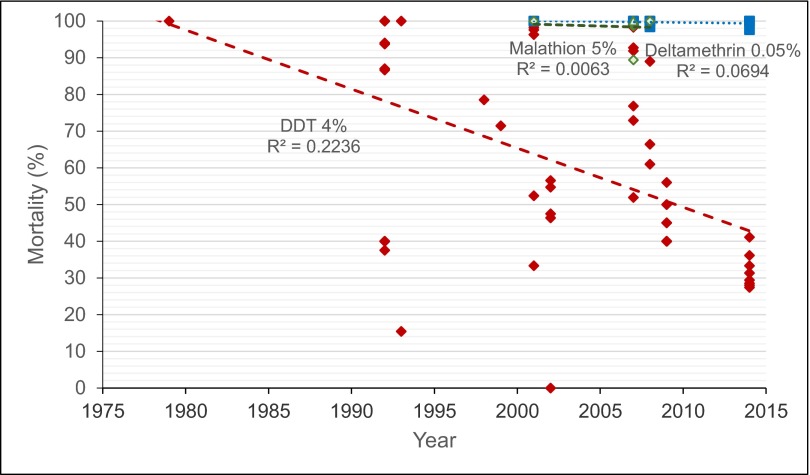
Analysis of combined data showed a decreasing trend in P. argentipes susceptibility to DDT in Bihar State over the past 22 y (Fig. 3). Resistance to malathion was identified in one of three studies, and decreased susceptibility to deltamethrin was identified in one in four studies; however, the number of studies identified overall was too low to draw broader conclusions regarding trends in resistance to these insecticides. Sand flies were susceptible to DDT in 1980, and resistance was first detected in 1993, a year after the third cycle of DDT use to prevent VL in India had started.
Fig. 3.
Trends in insecticide resistance in Bihar State, 1978–2014. Combined historical and current data show insecticide resistance to 4% DDT, 5% malathion, and 0.05% deltamethrin WHO-impregnated papers in P. argentipes. Red diamonds represent DDT susceptibility, green diamonds represent malathion, and blue squares represent deltamethrin. There is a general trend of decreasing susceptibility to DDT.
Insecticide Susceptibility Assays.
Susceptibility tests with wild-caught P. argentipes sand flies against 4% DDT WHO- impregnated papers in non-IRS and IRS villages showed corrected mortality ranges of 24.6–37.5% in control villages and 30.0–37.8% in IRS villages across the four districts sampled.
Due to the low numbers of sand flies collected, 0.05% deltamethrin susceptibility tests were only conducted in the non-IRS villages in Gopalganj and Patna districts. These results showed corrected mortality rates of 98.3% and 100%, respectively, consistent with previously published results (27).
Sand Fly Abundance.
Due to adverse weather conditions, no sand flies were collected during the pre-IRS surveys that were conducted in 48 houses across four districts of Bihar in February 2014. In post-IRS collections, a total of 1,073 sand flies [P. argentipes (n = 626), Phlebotomus sergentomyia (n = 412), and Phlebotomus papatasi (n = 35)] were collected over the four entomology study districts in non-IRS and IRS houses. In IRS villages, a total of 433 sand flies were caught, whereas in non-IRS villages, a total of 640 sand flies were collected.
P. argentipes abundance increased across all sentinel sites post-IRS; however, there was a significance difference seen in the number of sand flies collected in IRS and non-IRS villages during the 1-mo post-IRS collections (Table 2; P = 0.001). This difference was not observed in the 3-mo post-IRS collections (P = 1.0), indicating that IRS affected sand fly abundance for up to 4 wk immediately following spraying.
Table 2.
Sand fly abundance in IRS and non-IRS villages in four districts in Bihar State, India
| Total sand flies collected | ||||
| Time point | Collection months | IRS villages | Non-IRS villages | P |
| Pre-IRS | February | 0 | 0 | 1.0 |
| 1 mo post-IRS | May–August | 99 | 217 | 0.001 |
| 3 mo post-IRS | July–October | 122 | 123 | 1.0 |
Total numbers of sand flies collected in CDC light traps during entomology collections at time points pre-IRS and at 1 and 3 mo post-IRS. Significant differences between the IRS and non-IRS villages exist at 1 mo post-IRS only.
The highest per night/per trap abundance in 1-mo post-IRS collections was seen in the non-IRS sites of Gopalganj and Samastipur (5.5 and 5.0, respectively), and at 3 mo post-IRS, the highest per night/per trap abundance was seen in the IRS sites of Samastipur and Paschim Champaran (4.92 and 4.0, respectively). The mean 1-mo abundance in non-IRS sites was 4.34, and the mean 1-mo abundance in IRS sites was 2.02. The mean 3-mo abundance in non-IRS sites was 2.93, and the mean 3-mo abundance in IRS sites was 2.90. Aside from the 0.0 abundance seen in all sentinel sites during the pre-IRS surveys, the lowest abundance was seen in IRS sentinel sites in Patna, 3 mo post-IRS (1). This pattern of abundance concurs with previously observed seasonal sand fly distributions in Bihar, India, where the numbers of sandflies is naturally low during the winter months, followed by a peak in June through August, before naturally declining again toward the winter months (28).
Discussion
In order for IRS-based vector control to have the desired impact, an effective insecticide needs to be sprayed at a biologically effective concentration on the right structures at the right time. Timing is often driven by climate, which drives the annual changes in abundance of the insect vector population (29). Insecticide choice is a function of cost, formulation suitability, insecticide susceptibility status of the vector, and international and national regulatory approvals for use in public health (30, 31). Spray quality is measured by evenness of the spray coverage and proximity of the actual dosage achieved on the wall surface to the specified target concentration (22, 30, 31). Good IRS programs should have an appropriate monitoring and evaluation system in place that allows evidence-based insecticide choice and QA of the implementation of the spray program; guidelines from the WHO and WHO/Tropical Disease Research (TDR) are published (22).
It has been demonstrated historically in Bangladesh, India, and Nepal that IRS, but not long-lasting insecticide nets, is effective in reducing P. argentipes populations, and that this IRS subsequently reduces the number of cases of VL (8, 9, 14). This finding is also supported by recent collections of P. argentipes outdoors and associated with cattle sheds using CDC light traps (32), where a large proportion of the sand flies had fed on humans, suggesting endophilic and exophagic behaviors (32, 33). Therefore, if IRS is performed correctly and supported by case detection and treatment with effective drugs, such as miltefosine and amphotericin B (34, 35), elimination is feasible.
The effectiveness of the IRS program is dependent on the quality of the insecticide, formulation, and spray activities combined with the susceptibility status of the local sand fly populations. Because malaria transmission also occurs across much of the VL endemic region in India, a beneficial side effect of the VL activities should be a reduction in malaria prevalence if the insecticide chosen for VL control can also control the mosquito vectors. However, the current program in India has issues with both the quality of the IRS operations and the DDT susceptibility of both the local VL and malaria vectors. Despite these issues, VL case incidence has been falling, but whether this decline is linked to the limited impact of IRS or is part of the natural VL disease cycle in South Asia is unclear. However, the rate of decrease of VL cases following initiation of IRS in this phase is less than during the previous VL IRS campaigns (36–38), suggesting that the current IRS campaign has had less impact than previous iterations.
This paper presents the first large-scale detailed QA analysis of VL IRS undertaken by the established vector control program. The study covered eight districts in Bihar State, India, which has declared a target of VL elimination by 2015 (39). Our data indicate that the concentration of DDT delivered to walls during the most recent IRS spray round fell far short of the target concentration of 1.0 g ai/m2, achieving an average of 0.37 g ai/m2 across all eight study districts. There was little variation in DDT concentrations within households and across districts, indicating that the consistent underspraying is due to factors other than poorly performing individuals or spray teams. This finding is consistent with previous reports, which noted that only 16% of houses in Bihar showed uniform and complete spraying during IRS and coverage of only 53% (24, 37). Factors reducing the quality of the IRS included a sub-WHO specification formulation or expired insecticide (37) and rapid settling of the formulation compounded by the use of stirrup pumps. Previous operational attempts at QA of spraying involved visual inspections of powder residues on the walls. Although this information gives some indication of the evenness of formulation application, it is not adequate to assess the actual insecticide concentration delivered.
The length of residual action of DDT has been commonly referred to as over 6 mo (24). However, the results from our pre-IRS survey showed that after a median time of 3.5 mo, 91.3% of walls had a residual DDT level of less than 0.1 g ai/m2. This finding could be due to rapid degradation of the insecticide, quicker absorption into the walls than is commonly assumed, or low target concentrations being delivered to walls.
India is the only country that uses DDT at 1.0 g ai/m2, despite the WHO recommendation and utilization in other malaria endemic countries of 2.0 g ai/m2 (40). Monitoring of sand fly abundance in sprayed and unsprayed villages indicated that the current IRS activities only had a positive impact on reducing sand fly abundance for a period of ∼4 wk. Whether this poor efficacy is due entirely to underspraying of houses or to a combination of underspraying compounded by DDT-resistant vectors needs to be determined. Three previous DDT-based IRS campaigns reduced the cases of VL in India after 2–3 y of spraying (6). However, DDT resistance was only selected in the sand fly vector of VL in the second year of the third campaign.
No diagnostic dose has been determined directly for sand flies for DDT and deltamethrin insecticide susceptibility testing. The WHO diagnostic dosages established for malaria vectors have been used as a surrogate baseline for sand flies, but there is no solid evidence base for their adoption (41). Using the anopheline discriminating dosages, which are likely to be high for sand flies, DDT resistance has been detected by several groups in P. argentipes (27, 42–46); however, without established true diagnostic doses, these data can only be taken as indicative of resistance frequencies. It should also be noted that the WHO filter paper-based diagnostic dosage assay is not an accurate predictor of operational insecticide failure but should be used as an indicator that further assessment of continued operational efficacy is required.
Although true diagnostic doses are needed for P. argentipes, this assessment is problematic without a fully insecticide-susceptible strain. There is an urgent need to establish the susceptibility status of the Indian vector to a range of insecticides that could potentially be used as alternatives to DDT for IRS (47). Susceptibility to deltamethrin and organophosphates has been claimed (27); however, the numbers of insects tested in these studies are low. Our data, in line with other studies based on limited sand fly numbers, showed widespread resistance to DDT, which has obviously increased significantly since the last successful IRS campaign against VL in India, and susceptibility to deltamethrin (27, 46, 48). The major mosquito vector of malaria, Anopheles culicifacies, in the VL-endemic area is also highly DDT resistant (0–36% mortality on 4% DDT WHO-impregnated papers), although these mosquitos are susceptible or only weakly resistant to deltamethrin (76–100% mortality on 0.025% deltamethrin WHO-impregnated papers) (23). Hence, current VL IRS activities with DDT are likely to have a suboptimal impact on both VL and malaria control.
The Stockholm Convention limited the use of DDT as an insecticide other than for the purposes of public health (40). India is one of the few remaining countries that still uses DDT for vector control, and it is the only DDT manufacturer (40). The Government of India ratified the Stockholm Convention in 2006 and published its national implementation plan in 2011. Although India registered specific exemptions on DDT for acceptable vector control purposes until viable alternatives are found, the national plan underscores the commitment to phasing out DDT. This plan includes short-term priorities, due to be completed in 2011–2013, to improve the national policy and regulatory framework pertaining to POPs such as DDT, and then in the medium term (2012–2022) as the highest priorities to develop and promote alternatives to DDT and assist in identifying alternatives and formulating a strategy for phasing out DDT. Against this backdrop, with both VL and malaria vectors highly resistant to DDT, and alternatives such as pyrethroids readily available, to which the vectors remain susceptible, the continued use of DDT-based IRS is questionable.
Although this paper focuses on the quality of IRS and the insecticide susceptibility of the vector P. argentipes, these issues are not the only ones hindering the goal of elimination. In addition to issues with vector control, there may be a lack of diagnostic facilities at peripheral levels of the health system, resulting in delay in the treatment of VL and PKDL, which may act as human reservoirs. Drug stock-outs or drug quality may also be an issue in effective treatment (16).
This paper highlights a number of issues faced by the VL program in India. These issues must be addressed if India is to progress from the attack phase of the VL elimination plan to achieve the goal of regional elimination of VL as a public health problem.
Materials and Methods
QA Surveillance.
QA of IRS was performed in eight VL endemic districts in Bihar State that were scheduled to receive IRS in 2014. Districts were matched to those districts where external monitoring and evaluation of IRS were performed. Within districts, NVBDCP VL case incidence data from 2012 were used to identify the five PHCs with the highest incidence; these PHCs were duly selected for village and household sampling (38).
A modified version of the WHO TDR Monitoring and Evaluation Toolkit (49) for IRS study design was implemented. Five villages in each PHC were randomly selected using a random number generator in Microsoft Excel. In each village, three households were randomly selected for sampling. The sampling method was designed to ensure that isolated households had the same chance of being selected for sampling as houses in the center of a village.
Pre-IRS QA surveys to determine the level of residual DDT in houses in three districts were conducted in February 2014. Six PHCs (two per district) with the highest number of reported VL cases were chosen using 2012 NVBDCP case surveillance data to identify relevant PHCs as described above (nvbdcp.gov.in/kal10.html). Within each PHC, 15 houses were chosen from five randomly selected villages; 90 households were sampled in total.
Entomology Study Sites and Sample Collection.
Entomology sampling was conducted in four of eight (50%) of the study districts. Within each district, two PHCs with high VL case incidence in 2012 were selected for determining sand fly abundance and susceptibility data. A total of 96 sentinel sites were established across 16 villages (six sentinel houses per village).
Protocols for measuring impact on vector densities and insecticide susceptibility from the WHO/TDR Monitoring and Evaluation Toolkit (49) were used. Data were collected in February 2014 (pre-IRS), 1 mo post-IRS (May–July 2014), and 3 mo post-IRS (July–September 2014).
Surveys were conducted in villages receiving and not receiving IRS, which were randomly selected according to the 2014 Government of Bihar IRS microplan (nvbdcp.gov.in/kal10.html). Abundance data were collected using CDC light traps set up in six randomly selected houses over a period of one night (6:00 PM to 6:00 AM). The light traps were attached in the corner of the main bedroom and set up 15 cm away from the wall and 5 cm above ground. All sand flies were identified taxonomically based on morphological criteria from established taxonomic keys, where possible, to species level (50). A subsample of sand flies identified as P. argentipes was checked by PCR assay.
Sample Collection and Processing.
Samples to determine the concentration of DDT delivered to walls during IRS were collected from wall surfaces using 5-cm2 Bostik tape discs, as described by Russell et al. (51). Briefly, two discs per sample (10 cm2 in total) were placed onto the wall surface and rubbed firmly. Discs were removed from the wall surface using tweezers and placed on Whatman no. 1 filter paper, labeled, and stored at 4 °C. Samples were taken from each of the four walls in bedrooms at a different position per wall: high (4–6 feet from ground level, one sample), medium (2–4 feet from ground level, one sample), and low (0–2 feet from ground level, two samples).
The concentration of DDT present on wall surfaces was determined using HPLC. Solvent extraction was performed by an in-house method that used heptane. Samples equivalent to ∼10 cm2 were cut into pieces of ∼1 cm2. One milliliter of a heptane/1-propoanol mixture (9:1) containing 100 μg of the internal standard dicyclohexyl phthalate (DCP) was added, and samples were vortex-mixed for 2–3 min to extract p,p′-DDT. Extract (0.5 mL) was transferred to a clean glass tube and evaporated to dryness under nitrogen at 40 °C. One milliliter of methanol was added, and the mixture was centrifuged at 22,865 × g for 15 min.
HPLC analysis was performed by injection of 10-μL aliquots of extract on a reverse-phase Hypersil GOLD C18 column (75 Å, 250 × 4.6 mm, 5-μm particle size; Thermo Scientific) at 23–25 °C. A mobile phase of acetonitrile/water (93:7) was used at a flow rate of 1 mL⋅min−1 to separate DDT and DCP. The quantities of DDT and DCP were calculated from standard curves established by known concentrations of authenticated standards. Peaks were detected at 232 nm with the Ultimate 3000 UV detector (Dionex) and were analyzed with Dionex Chromeleon software. Final DDT content in grams per square meter was estimated using the following equation:
where A is p,p′-DDT in grams per square meter, B is p,p′-DDT in micrograms per milliliter obtained from HPLC, H is DDT extraction efficiency by heptane equal to 83.4, and D is the internal standard correction factor calculated from dividing the peak area of 100 μg/mL DCP by the DCP peak area obtained for unknown.
HPLC results were compared with the intended IRS target DDT concentration of 1.0 g ai/m2. A 20% cutoff threshold was used to classify results whereby a concentration of less than 0.8 g ai/m2 was considered an underspray, a range of 0.8–1.2 g ai/m2 was considered within the target range, and a concentration of greater than 1.2 g ai/m2 was considered an overspray.
Susceptibility Assays.
P. argentipes samples were collected inside houses, verandas, and cattle sheds, which are targeted for IRS, within IRS and non-IRS villages. Collected sand flies were exposed to DDT (4%) or deltamethrin (0.05%) WHO-impregnated papers (31, 41). Mortality was recorded after 24 h. Controls were performed for each test using the appropriate papers, and Abbott’s formula was applied where necessary.
Analysis of Historical Entomological Data.
A literature search for P. argentipes, insecticide resistance, and Bihar was conducted via the US National Library of Medicine (www.ncbi.nlm.nih.gov) and Google search. Historical data were collated using a decision support system database (52) adapted to VL. All references are given in SI Appendix. Only studies that had conformed to the standard WHO diagnostic assay protocol were included.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism version 6, Microsoft Excel 2010, and R version 3.1. A Fisher’s exact test was used to evaluate statistical significance, with significance set at <0.05. One-way ANOVA was used to analyze variance of DDT concentrations on walls within individual households. Linear mixed effect modeling in R was used to analyze differences in sampling heights. The Wilcoxon signed rank test was used to compare pre-IRS and post-IRS concentrations of DDT recovered from wall surfaces in matched households.
Ethics.
Ethical permission for the study was obtained from the Liverpool School of Tropical Medicine Research Ethics Committee (reference no. 14.028) and the Rajendra Memorial Research Institute for Medical Sciences Research Ethics Committee (date of approval: January 28, 2014).
Supplementary Material
Acknowledgments
We thank Dr. A. C. Dhariwal and Dr. R. K. Dasgupta (National Vector Borne Disease Control Programme) for facilitating this work. This work was funded by the Bill and Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507782112/-/DCSupplemental.
References
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Joshi A, et al. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45(2):105–111. [PubMed] [Google Scholar]
- 3.Alvar J, et al. WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narain JP, et al. Elimination of neglected tropical diseases in the South-East Asia Region of the World Health Organization. Bull World Health Organ. 2010;88(3):206–210. doi: 10.2471/BLT.09.072322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh VP, et al. Estimation of under-reporting of visceral leishmaniasis cases in Bihar, India. Am J Trop Med Hyg. 2010;82(1):9–11. doi: 10.4269/ajtmh.2010.09-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muniaraj M. The lost hope of elimination of Kala-azar (visceral leishmaniasis) by 2010 and cyclic occurrence of its outbreak in India, blame falls on vector control practices or co-infection with human immunodeficiency virus or therapeutic modalities? Trop Parasitol. 2014;4(1):10–19. doi: 10.4103/2229-5070.129143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh RK, Pandey HP, Sundar S. Visceral leishmaniasis (kala-azar): Challenges ahead. Indian J Med Res. 2006;123(3):331–344. [PubMed] [Google Scholar]
- 8.Ostyn B, et al. Vector control by insecticide-treated nets in the fight against visceral leishmaniasis in the Indian subcontinent, what is the evidence? Trop Medicine Int Health. 2008;13(8):1073–1085. doi: 10.1111/j.1365-3156.2008.02110.x. [DOI] [PubMed] [Google Scholar]
- 9.Joshi AB, et al. Elimination of visceral leishmaniasis in Nepal: Pipe-dreams and possibilities. Kathmandu Univ Med J (KUMJ) 2006;4(4):488–496. [PubMed] [Google Scholar]
- 10.Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae) Ann Trop Med Parasitol. 2001;95(2):197–202. doi: 10.1080/00034980120041071. [DOI] [PubMed] [Google Scholar]
- 11.Joshi RD, Rai RN. Impact of DDT spraying on populations of P. argentipes and P. papatasi in Varanasi district, Uttar Pradesh. J Commun Dis. 1994;26(1):56–58. [PubMed] [Google Scholar]
- 12.Kaul SM, Sharma RS, Dey KP, Rai RN, Verghese T. Impact of DDT indoor residual spraying on Phlebotomus argentipes in a kala-azar endemic village in eastern Uttar Pradesh. Bull World Health Organ. 1994;72(1):79–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhopadhyay AK, Hati AK, Chakraborty S, Saxena NB. Effect of DDT on Phlebotomus sandflies in Kala-Azar endemic foci in West Bengal. J Commun Dis. 1996;28(3):171–175. [PubMed] [Google Scholar]
- 14.Joshi AB, et al. Chemical and environmental vector control as a contribution to the elimination of visceral leishmaniasis on the Indian subcontinent: Cluster randomized controlled trials in Bangladesh, India and Nepal. BMC Med. 2009;7:54. doi: 10.1186/1741-7015-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stauch A, et al. Model-based investigations of different vector-related intervention strategies to eliminate visceral leishmaniasis on the Indian subcontinent. PLoS Negl Trop Dis. 2014;8(4):e2810. doi: 10.1371/journal.pntd.0002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh SP, et al. Options for active case detection of visceral leishmaniasis in endemic districts of India, Nepal and Bangladesh, comparing yield, feasibility and costs. PLoS Negl Trop Dis. 2011;5(2):e960. doi: 10.1371/journal.pntd.0000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhury R, et al. The Indian and Nepalese programmes of indoor residual spraying for the elimination of visceral leishmaniasis: Performance and effectiveness. Ann Trop Med Parasitol. 2011;105(1):31–35. doi: 10.1179/136485911X12899838683124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huda MM, et al. Toolkit for monitoring and evaluation of indoor residual spraying for visceral leishmaniasis control in the Indian subcontinent: Application and results. J Trop Med. 2011;2011:876742. doi: 10.1155/2011/876742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . Regional Strategic Framework for Elimination of Kala-Azar from the South-East Asia Region (2005–2015) WHO Regional Office for South-East Asia; New Delhi: 2005. [Google Scholar]
- 20.Picado A, et al. Phlebotomus argentipes seasonal patterns in India and Nepal. J Med Entomol. 2010;47(2):283–286. doi: 10.1603/me09175. [DOI] [PubMed] [Google Scholar]
- 21.Regional Technical Advisory Group on Kala-azar Elimination . Report on the First Meeting Manesar, Haryana, December 20–23, 2004. WHO Regional Office for South-East Asia; New Delhi: 2005. [Google Scholar]
- 22.WHO . Indicators for Monitoring and Evaluation of the Kala-Azar Elimination Programme. WHO Regional Office for South-East Asia; New Delhi: 2010. [Google Scholar]
- 23.Raghavendra K, et al. A note on the insecticide susceptibility status of principal malaria vector Anopheles culicifacies in four states of India. J Vector Borne Dis. 2014;51(3):230–234. [PubMed] [Google Scholar]
- 24.Van den Berg H . Stakeholders’ Meeting to Review the Interim Report for the Establishment of a Global Partnership to Develop Alternatives to DDT. United Nations Environment Programme; Geneva: 2008. Global status of DDT and its alternatives for use in vector control to prevent disease. [Google Scholar]
- 25.Picado A, Dash AP, Bhattacharya S, Boelaert M. Vector control interventions for visceral leishmaniasis elimination initiative in South Asia, 2005-2010. Indian J Med Res. 2012;136(1):22–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury R, et al. How far are we from visceral leishmaniasis elimination in Bangladesh? An assessment of epidemiological surveillance data. PLoS Negl Trop Dis. 2014;8(8):e3020. doi: 10.1371/journal.pntd.0003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh RK, Mittal PK, Dhiman RC. Insecticide susceptibility status of Phlebotomus argentipes, a vector of visceral leishmaniasis in different foci in three states of India. J Vector Borne Dis. 2012;49(4):254–257. [PubMed] [Google Scholar]
- 28.Malaviya P, et al. Exposure to Phlebotomus argentipes (Diptera, Psychodidae, Phlebotominae) sand flies in rural areas of Bihar, India: The role of housing conditions. PLoS ONE. 2014;9(9):e106771. doi: 10.1371/journal.pone.0106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO . Indoor Residual Spraying in an Operational Manual for IRS for Malaria Transmission and Elimination. WHO; Geneva: 2013. [Google Scholar]
- 30.WHO . Control of the Leishmaniases. WHO; Geneva: 1991. [Google Scholar]
- 31.WHO . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors. WHO; Geneva: 2013. [Google Scholar]
- 32.Poche D, Garlapati R, Ingenloff K, Remmers J, Poche R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2011;36(Suppl 1):S106–S117. doi: 10.1111/j.1948-7134.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 33.Poche RM, Garlapati R, Elnaiem DE, Perry D, Poche D. The role of Palmyra palm trees (Borassus flabellifer) and sand fly distribution in northeastern India. J Vector Ecol. 2012;37(1):148–153. doi: 10.1111/j.1948-7134.2012.00211.x. [DOI] [PubMed] [Google Scholar]
- 34.Thakur CP, Meenakshi Thakur AK, Thakur S. Newer strategies for the kala-azar elimination programme in India. Indian J Med Res. 2009;129(1):102–104. [PubMed] [Google Scholar]
- 35.Thakur CP. A new strategy for elimination of kala-azar from rural Bihar. Indian J Med Res. 2007;126(5):447–451. [PubMed] [Google Scholar]
- 36.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12(2):62–68. [PubMed] [Google Scholar]
- 37.NVBDCP . National Vector Borne Disease Control Programme: Joint Monitoring Mission Report. NVBDCP; Delhi: 2007. [Google Scholar]
- 38.NVBDCP . National Vector Borne Disease Control Programme Directorate General of Health Services Ministry of Health & Family Welfare. NVBDCP; Delhi: 2014. [Google Scholar]
- 39.WHO . Regional Strategic Framework for Elimination of Kala-azar from the South-East Asia Region India. WHO Regional Office for South-East Asia; New Delhi: 2012. [Google Scholar]
- 40.UNEP . DDT Information System. United Nations; Stockholm: 2009. [Google Scholar]
- 41.WHO . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. WHO; Geneva: 1998. [Google Scholar]
- 42.Basak B, Tandon N. Observations on susceptibility status of Phlebotomus argentipes to DDT in district South 24-Parganas, West Bengal. J Commun Dis. 1995;27(3):196–197. [PubMed] [Google Scholar]
- 43.Dhiman RC, Mittal PK. A note on susceptibility status of Phlebotomus papatasi (Scopoli) populations to insecticides. J Commun Dis. 2000;32(1):65–66. [PubMed] [Google Scholar]
- 44.Mukhopadhyay AK, Saxena NB, Narasimham MV. Susceptibility status of Phlebotomus argentipes to DDT in some kala-azar endemic areas of Bihar (India) Indian J Med Res. 1990;91:458–460. [PubMed] [Google Scholar]
- 45.Singh R, Das RK, Sharma SK. Resistance of sandflies to DDT in Kala-azar endemic districts of Bihar, India. Bull World Health Organ. 2001;79(8):793. [PMC free article] [PubMed] [Google Scholar]
- 46.Dinesh DS, et al. Insecticide susceptibility of Phlebotomus argentipes in visceral leishmaniasis endemic districts in India and Nepal. PLoS Negl Trop Dis. 2010;4(10):e859. doi: 10.1371/journal.pntd.0000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alexander B, et al. Susceptibility to chemical insecticides of two Brazilian populations of the visceral leishmaniasis vector Lutzomyia longipalpis (Diptera: Psychodidae) Trop Medicine Int Health. 2009;14(10):1272–1277. doi: 10.1111/j.1365-3156.2009.02371.x. [DOI] [PubMed] [Google Scholar]
- 48. WHO (2011) Elimination of Kala-zar: Report of the Fourth Meeting of the Regional Technical Advisory Group (RTAG) 12–14 July 2011, Kathmandu, Nepal (WHO Regional Office for South-East Asia, New Delhi) [Google Scholar]
- 49.WHO . Kala-Azar Elimination in Bangladesh, India, and Nepal. Regional Office for South-East Asia, WHO; New Delhi: 2010. Monitoring and evaluation tool kit for indoor residual spraying. [Google Scholar]
- 50. Kalra NL, Bang YM (1988) Manuals on Entomology in Visceral Leishmaniasis (WHO, New Delhi)
- 51.Russell TL, et al. Evaluating the feasibility of using insecticide quantification kits (IQK) for estimating cyanopyrethroid levels for indoor residual spraying in Vanuatu. Malar J. 2014;13:178. doi: 10.1186/1475-2875-13-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisen L, et al. Multi-disease data management system platform for vector-borne diseases. PLoS Negl Trop Dis. 2011;5(3):e1016. doi: 10.1371/journal.pntd.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.