Significance
Ray-finned fishes are the most diverse and ecologically dominant group of vertebrates on the planet. Previous molecular phylogenies and paleontological studies have shown that modern ray-finned fishes (crown teleosts) radiated sometime in the Late Cretaceous or early Paleogene. Our data suggest that crown teleosts came into their current dominant ecological role in pelagic ecosystems immediately following the Cretaceous−Paleogene mass extinction 66 million years ago by filling newly vacated ecological niches and marking the beginning of an “age of ray-finned fishes.” Our study is, to our knowledge, the first geographically comprehensive, high-resolution study of marine vertebrate communities across the extinction.
Keywords: Cretaceous−Paleogene boundary, ichthyoliths, fossil fish, age of fishes, mass extinction
Abstract
Ray-finned fishes (Actinopterygii) comprise nearly half of all modern vertebrate diversity, and are an ecologically and numerically dominant megafauna in most aquatic environments. Crown teleost fishes diversified relatively recently, during the Late Cretaceous and early Paleogene, although the exact timing and cause of their radiation and rise to ecological dominance is poorly constrained. Here we use microfossil teeth and shark dermal scales (ichthyoliths) preserved in deep-sea sediments to study the changes in the pelagic fish community in the latest Cretaceous and early Paleogene. We find that the Cretaceous−Paleogene (K/Pg) extinction event marked a profound change in the structure of ichthyolith communities around the globe: Whereas shark denticles outnumber ray-finned fish teeth in Cretaceous deep-sea sediments around the world, there is a dramatic increase in the proportion of ray-finned fish teeth to shark denticles in the Paleocene. There is also an increase in size and numerical abundance of ray-finned fish teeth at the boundary. These changes are sustained through at least the first 24 million years of the Cenozoic. This new fish community structure began at the K/Pg mass extinction, suggesting the extinction event played an important role in initiating the modern “age of fishes.”
Ray-finned fishes are a dominant and exceptionally diverse member of modern pelagic ecosystems; however, both the fossil record and molecular clocks suggest that the vast majority of living ray-finned fishes developed only recently, during the last 100 million years (1–3). It has been proposed that the explosion in actinoptygerian diversity in the Late Mesozoic and Early Cenozoic represents a new “age of fishes” in contrast to the initial diversification of fish clades in the Devonian (2, 3). However, the mechanisms and timing of this Mesozoic−Cenozoic radiation and rise to dominance by ray-finned fishes are not well constrained in current molecular phylogenies or from the relatively sparse fossil record. While the Cretaceous−Paleogene (K/Pg) mass extinction occurred ∼66 million years ago (Ma), in the middle of this radiation, there is little clear phylogenetic evidence linking any changes in fish diversity directly to this event (1), although a recent phylogenetic study on pelagic fish families suggested that open ocean fishes radiated during the early Paleogene following the extinction (4).
The K/Pg extinction had a dramatic effect on open ocean marine ecosystems (5–7), although the severity of the extinction varied around the globe (7–9). Major groups at both the base and top of the food web were decimated (5, 6, 10). While the traditional model of mass extinction due to primary productivity collapse (11) has been generally discredited due to the continued productivity of select consumer groups (12, 13), it is likely that upheaval among primary producers reverberated up the food web to cause extinctions at higher trophic levels. In the open ocean, calcifying plankton such as foraminifera and calcareous nannofossils suffered >90% species-level extinctions (9, 14). These changes in the structure of the base of the food web likely helped to cause the extinctions of pelagic consumers such as ammonites and marine reptiles (10). The trophic link between the plankton and large consumers in pelagic ecosystems is small pelagic fish, which would be expected to be similarly decimated by changes in food web structure. However, recent work has shown that while there was a collapse of small pelagic fish production in the Tethys Sea, in the Pacific Ocean, these midlevel consumers maintained Cretaceous-like or higher levels of production in the earliest Danian (15).
Changes in abundance do not tell the whole story of how pelagic fishes responded to the extinction event. Indeed, despite dramatic levels of extinction, a few species of planktonic foraminifera thrived in the postextinction oceans, reaching abundances in the ∼500,000 y following the event that far exceed those of typical high-diversity Cretaceous assemblages (7). This foraminifer response shows that taxonomic diversity and biological production can be decoupled in postdisaster ecosystems like those of the earliest Danian. Fishes are highly diverse and occupy a range of ecological niches, from the smallest plankton feeders through predatory sharks. This means that different groups could exhibit differential responses to the extinction (16). Work on well-preserved body fossils has found that there was a selective extinction of shallow marine predatory fishes at the K/Pg extinction, and a radiation during the early Cenozoic (17, 18). Additionally, a low level of extinction (<33%) of sharks and rays has been inferred across the event (19, 20). However, the magnitude of pelagic fish extinction is poorly known, although a relatively modest ∼12% extinction has been documented for fish tooth morphotypes between the Late Cretaceous and the early Paleocene (21).
Here we use ichthyoliths, the isolated teeth and dermal scales (denticles) of sharks and ray-finned fishes found in deep-sea sediments, to investigate the response of sharks and fishes to the K/Pg extinction. Calcium phosphate ichthyoliths are found in nearly all marine sediments, even red clays (22), where other microfossils have been dissolved by corrosive bottom water conditions. Therefore, ichthyoliths are relatively unaffected by the preservation biases typically found in other microfossil groups. Teeth and denticles are reasonably common, with 10s to 100s found in a few grams of sediment, allowing studies of the fish community rather than isolated individuals. The abundance of ichthyoliths also allows for high-temporal resolution sampling similar to other microfossils. The well-resolved ichthyolith records stand in sharp contrast to those for the comparatively rare body fossil record, and can provide a complimentary analysis of abrupt biotic events such as mass extinctions or transient climate changes. In addition, the abundance, assemblage, and morphological composition of ichthyoliths record the productivity and biodiversity of the pelagic fish community.
We investigate how the pelagic fish community responded to the K/Pg extinction at six deep-sea sites in the Pacific, Atlantic, and Tethys Oceans. We use ichthyolith community metrics, including the relative abundance of microfossils from sharks and ray-finned fishes, and the size structure of the tooth assemblage to assess the changes in the pelagic fish community across the K/Pg mass extinction around the world. This represents, to our knowledge, the first geographically comprehensive, high-resolution study of pelagic marine vertebrate communities across the extinction.
Results
The K/Pg Boundary was identified in each site based on the global iridium anomaly layer, as well as the presence of tektites, impact ejecta, and slump deposits associated with the impact horizon (23–29). Site-specific chronologies were developed based on cyclostratigraphy, cobalt accumulation rates, strontium isotopes, biostratigraphy, and magnetostratigraphy, depending on the lithology at each site (see Materials and Methods and SI Materials and Methods for additional details).
Ichthyoliths are fundamentally divided into two broad taxonomic groups: teeth, which can belong to ray-finned fish as well as sharks, and dermal denticles, the tooth-like placoid scales that cover nearly all sharks and rays. We looked at the relative abundance of actinopterygian fishes to sharks before and after the extinction event, as interpreted by the relative abundance of teeth to shark denticles in an assemblage of microfossils retained on a 106-μm sieve. A tooth/denticle ratio of >1 means actinopterygian fish teeth dominated the >106-μm ichthyolith assemblage, while a ratio of <1 means shark denticles dominated the >106-μm ichthyolith assemblage. It is worthwhile to note that this metric considers only the numerical abundance of microfossils at a constant size fraction (>106 μm) and not individuals or biomass of these groups.
Actinopterygian fish typically have two distinct sets of teeth, oral teeth, which are found in the jaw, and the far more abundant but significantly smaller pharyngeal teeth. The >106 μm fraction generally contains mostly oral teeth, while smaller fractions are dominated by pharyngeal teeth and tooth fragments. Rates of tooth loss and regeneration of actinopterygian fishes are poorly constrained and vary with taxon, although teeth are replaced continuously throughout the life of the individual (30). However, in at least some taxa, many teeth are resorbed, rather than shed, during tooth replacement, so the number of teeth in the sedimentary record is likely an underrepresentation of teeth produced (30, 31).
Sharks can have 2–3 orders of magnitude more denticles—which scale numerically with body surface area—than sharks or ray-finned fish have oral or pharyngeal teeth. This means that the absolute value of the ratio of teeth to denticles in the >106-μm size fraction, considered in this study, is not the true ratio of ray-finned fish versus shark biomass or numerical abundance. However, when considering the ratio of teeth to denticles at a constant size fraction, the metric allows for consistent comparison between assemblages, and can be interpreted as changes in relative abundances of sharks and ray-finned fish. We consider the >106 μm fraction in this study as a uniform metric across all sample sites but note that the abundance of smaller teeth is correlated with the abundance of larger teeth, and the absolute value of the tooth/denticle ratio increases at smaller size fractions.
An additional consideration is that teeth in the >106 μm fraction likely include some shark teeth as well as those of ray-finned fishes. We note that shark teeth are often flattened, triangular forms with multiple cusps at the base and a cutting edge that may be ornamented with serrations. Such teeth represent <1% of the total tooth ichthyoliths in our >106 μm samples, and were not present in a majority of assemblages. Indeed, despite constant tooth replacement, sharks will still produce several orders of magnitude more denticles in a lifetime than teeth, depending on their body size. Hence, it is unsurprising that shark teeth are rare in our assemblages, compared with the abundance of denticles. Numerical simulations show that the presence of a few shark teeth in our tooth samples does not significantly bias our results (please see SI Materials and Methods for more information).
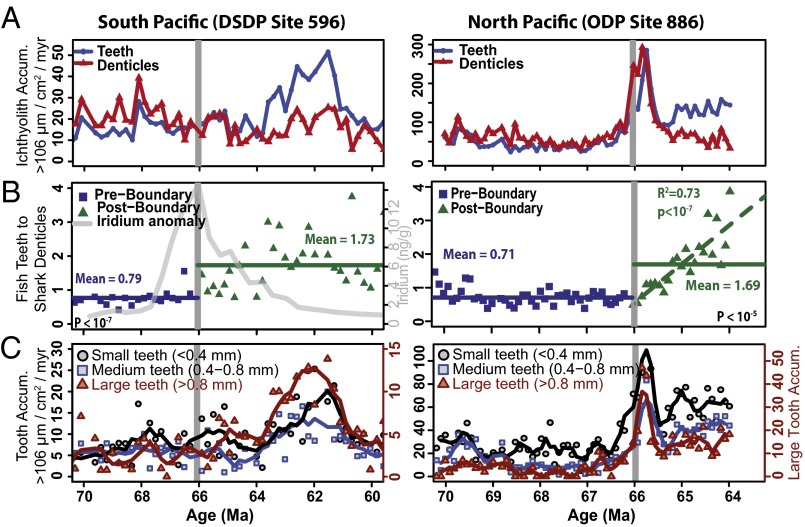
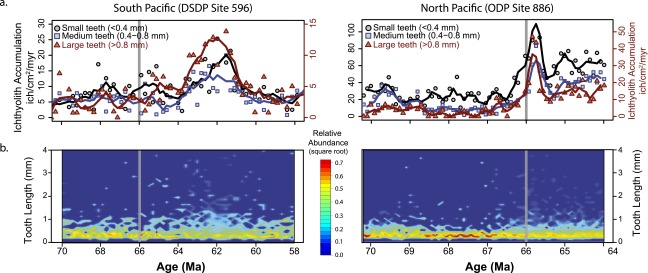
We find that in the Cretaceous Pacific Ocean, the accumulation of actinopterygian teeth is consistently lower than the accumulation of shark denticles, suggesting that sharks were more dominant in the Cretaceous pelagic vertebrate community than they were in the Paleocene (Fig. 1A). The tooth to denticle ratio in the Cretaceous was ∼0.71:1 in the North Pacific [Ocean Drilling Program (ODP) Site 886] Maastrichtian, and 0.76:1 in the South Pacific [Deep Sea Drilling Project (DSDP) Site 596]. The accumulation rate of teeth increases notably after the K/Pg extinction, leading to a tooth/denticle ratio of 1:1 at both Pacific locations in the first 500,000 y of the Paleocene before increasing toward 2:1 by the mid-Paleocene (Fig. 1B). This change is significant at both sites (two-sided t test, P < 0.0001). The community change occurs at the K/Pg boundary, and cannot be explained by background variability, since the tooth/denticle ratio is nearly constant in the Cretaceous (Fig. 1B). The main reason for the increase in the tooth/denticle ratio is the increased accumulation of ray-finned fish teeth in the Paleocene, and it does not represent a large decline in sharks (Fig. 1A).
Fig. 1.
South Pacific [Left; DSDP Site 596 (38)] and North Pacific [Right; ODP Site 886 (25)] across the Cretaceous−Paleogene boundary (horizontal gray line). (A) Ichthyolith accumulation. Blue circles are teeth, interpreted as ray-finned fish, and red triangles are denticles, interpreted as sharks and rays. Units are in ichthyoliths per square centimeter per million years. (B) Ratio of fish teeth to shark denticles. Solid lines are mean values for Cretaceous (blue) and Paleocene (green). The dotted green line (Right) is a regression fit to the Paleocene dataset. Iridium anomaly data are from ref. 23. (C) Accumulation rates of three size classes of fish teeth. Small teeth (black circles) have maximum length of <0.4 mm, medium teeth (blue squares) are 0.4–0.8 mm, and large teeth (red triangles) are >0.8 mm. Large tooth accumulation is on the secondary axis and is scaled to twice that of the small and medium teeth to show pattern. Solid lines are three-point running means.
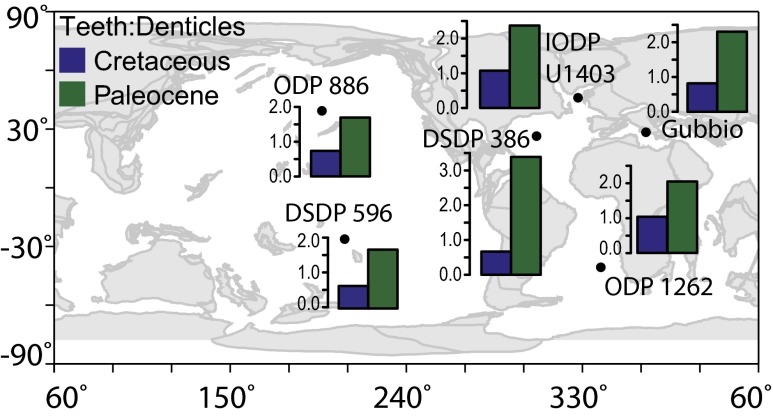
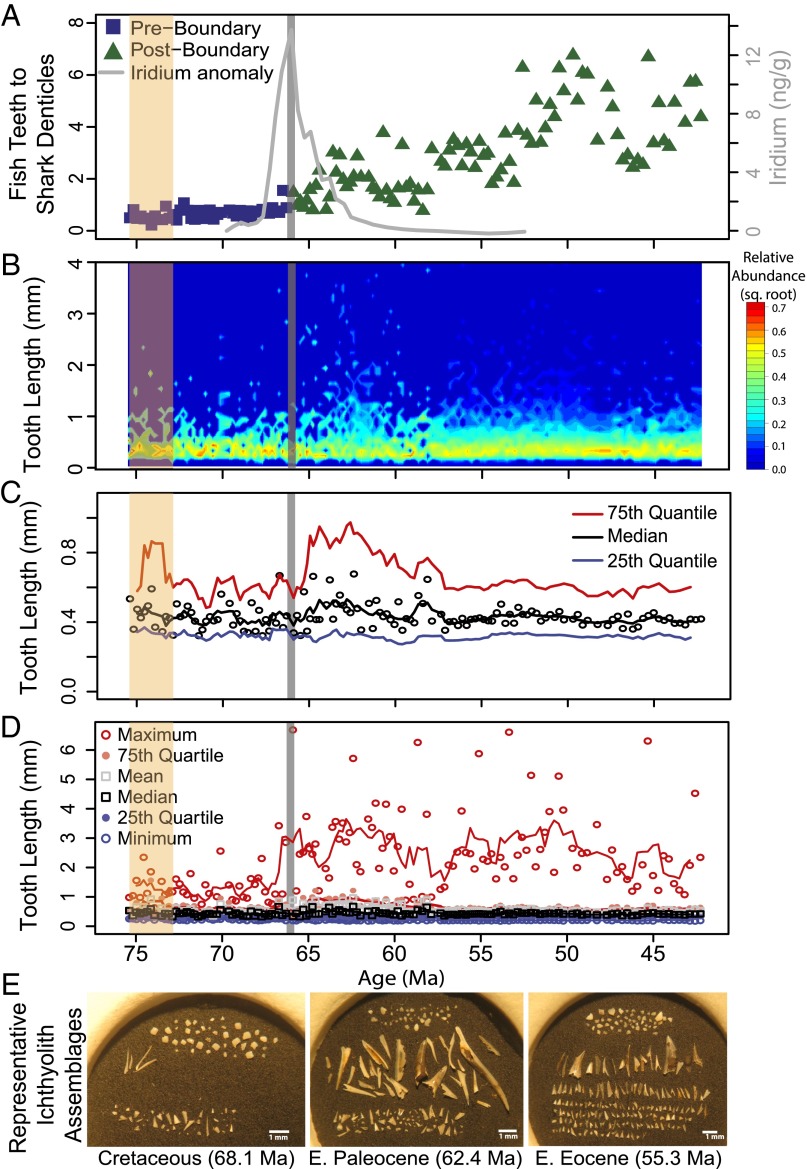
While low ichthyolith abundances > 106 μm in the Atlantic [International Ocean Discovery Program (IODP) U1403, DSDP 386, and ODP 1262] and Tethys Sea (Gubbio) preclude similar time series analysis, grouped assemblages of ichthyoliths from the latest Cretaceous and earliest Paleocene show the same pattern as in the Pacific, with a preextinction tooth/denticle ratio between 0.7 and 1, and a postextinction ratio between 2 and 3.5 (Fig. 2). These global results suggest that the relative abundance of ray-finned fish in marine vertebrate assemblages increased dramatically at the extinction all over the world. Notably, these changes in the tooth/denticle ratio occur despite local decreases in ichthyolith accumulation in the Tethys Sea and Atlantic sites (15). In the South Pacific, we find that the ratio of ray-finned fish teeth to shark dermal denticles in the assemblage continues to rise into the Eocene, from values of ∼2:1 in the earliest Paleocene to values of seven teeth per denticle in the Eocene (Fig. 3A). Hence, the increase in tooth/denticle ratio is initiated at the K/Pg boundary and continues to increase, at least in the South Pacific, for at least 24 million years after the extinction.
Fig. 2.
Paleomap showing the ratio of fish teeth to shark denticles from the Cretaceous (blue) and Paleocene (green) from six sites around the world’s ocean. Paleomap image created from the Ocean Drilling Stratigraphic Network Plate Tectonic Reconstruction Service. All histograms are plotted on the same vertical axis.
Fig. 3.
An extended record from the South Pacific Ocean (DSDP Site 596) of various ichthyolith community metrics; K/Pg boundary is vertical gray line. (A) Ratio of fish teeth to shark denticles. (B) Square root of relative abundance of ichthyolith size classes. Reds and yellows denote higher relative abundance at that tooth length, while blues denote relatively rare. (C) Black circles are individual assemblage tooth length medians, and solid lines are five-point running averages, with median (black), 25th quartile (blue), and 75th quartile (red). (D) Full size structure of tooth assemblages; solid lines are five-point running means. Maximum tooth size shown as open red circles. (E) Representative photos of ichthyolith assemblages > 106 μm from the Cretaceous, Paleocene, and Eocene from DSDP Site 596. Denticles (shark scales) are at the top of each assemblage, large teeth are in the middle, and smaller teeth are at the bottom of each photograph. Note the high abundance of “large” teeth in the Paleocene. Tan shading represents region of low tooth abundance. (Scale bar, 1 mm.)
The size structure and accumulation rate of the tooth assemblages also changed at the K/Pg boundary in the Pacific Ocean. Tooth size was measured as the longest dimension through the centroid of each tooth (Feret’s Diameter) > 106 μm using the open source image processing program ImageJ (32). Shark denticles were excluded from this analysis, as many denticles are preserved as fragments. Both Cretaceous and Paleocene tooth assemblages are dominated by small teeth, with lengths of <0.8 mm, and a median size of ∼0.43 mm (Fig. 1C). In the early Paleocene, the largest increases in tooth accumulation and relative abundance occur in the largest tooth size fraction (>0.8 mm; Fig. 1C). This increase in the abundance of large teeth begins at the K/Pg extinction in both Pacific sites, but it is most apparent in the South Pacific starting at 64 Ma and lasting until 58–59 Ma (Figs. 1C and 3B). A Cretaceous-like size structure is partly restored after 58 Ma in the South Pacific (Fig. 3B), despite an increase in the overall abundance of teeth in each sample. This suggests that the high abundance of large teeth in the early Paleocene is due to a change in community structure and is not just an effect of having more teeth in a given sample and therefore preserving more of the rare, larger teeth. In the North Pacific, all tooth size classes see increases in their accumulation rate, but there is no unusual increase in the largest teeth relative to smaller teeth (Fig. 1C). However, the North Pacific record is only preserved to 64 Ma, about the time that the large teeth become particularly prominent in the South Pacific.
While the median tooth length in the early Paleocene does not differ significantly from that of the latest Cretaceous (∼0.43 mm or 430 μm), the 75th quartile tooth length in each assemblage increases from ∼0.6 mm to 0.9 mm during the early Paleocene, suggesting that the largest teeth got larger and more abundant, without much change among the remainder of the tooth assemblage (Fig. 3C). Additionally, the maximum tooth size of a given assemblage tripled at the K/Pg boundary, from an average of 1 mm (maximum of 2.5 mm) in the Cretaceous to an average of 3 mm (maximum of >6 mm) in the Paleocene and Early Eocene. Large teeth are present in nearly every sample following the extinction for at least 24 Ma, suggesting a permanent change in the range of fish tooth size following the extinction (Fig. 3D).
SI Materials and Methods
Sample Preparation.
Excepting the samples from Gubbio, Italy, which were collected from the Contessa Highway outcrop north of the town (40), all samples were obtained through the International Ocean Discovery Program. To isolate the ichthyoliths, which are relatively rare in marine sediments, a series of methods were used based on the lithology. Before processing, all samples were dried at 50 °C, sometimes taking months, as in the case of red clays, to achieve a constant weight. Samples were processed with the goal of concentrating the ichthyoliths as much as possible without being destructive to the fossils.
Carbonate ooze samples (ODP Site 1262, IODP Site U1403) were disaggregated in deionized (DI) water and then dissolved using 5–10% acetic acid. The amount of acid varied per site, but ranged from 600 mL to 1,000 mL of 5–10% acid per 20-g sample. Samples were left in acid until they stopped reacting, about 2–4 h, and then washed over a 38-μm sieve using DI water. Red clays (DSDP Sites 386 and 596 and ODP Site 886) were simply disaggregated in DI water. Samples were then washed over a 38-μm sieve. The limestone samples from Gubbio were processed by first manually breaking the samples into ∼1-cm pieces with a hammer. Samples were then soaked in 10% acid bath for 24-h periods and then washed through 150-μm and 38-μm sieves. Material > 150 μm was returned to the acid bath, while <150-μm material was decanted onto Whatman P5 filter paper. Limestone samples (Gubbio) took between 4 and 12 wash cycles to completely disaggregate. Ichthyoliths were then manually picked out of the coarse fraction of the residue (>106 μm) and mounted on micropaleontology slides and classified into denticles and teeth, corresponding to sharks and ray-finned fish, respectively. Although diagnostic shark teeth are present, they typically account for <1% of teeth in the >106 μm assemblages (and significantly less at the smaller size fractions).
Ichthyolith Assemblage Analysis.
Images of ichthyolith assemblages from ODP Site 886 and DSDP Site 596 were processed and analyzed using ImageJ (32) to count and measure the size and shape of the ichthyoliths. This was done by converting photographs into black and white threshold-based images of the ichthyolith assemblages and then measuring the resulting particles using the “analyze particles” tool in ImageJ. The other sites considered in this study had small numbers of large (>106 μm) ichthyoliths, and were not sufficiently abundant enough to look at the size structure of the tooth assemblage. For these, we grouped individual samples into Cretaceous preboundary and Paleocene postboundary groups to compare the relative abundance of teeth and denticles. A summary data table is presented in Table S1.
Table S1.
Global ichthyolith assemblage ratios used for Fig. 2
| Cretaceous | Paleocene | |||||||||
| Site | Age, Ma | No. of samples | Teeth > 106 μm | Denticles > 106 μm | Ratio | Age, Ma | No. of samples | Teeth > 106 μm | Denticles > 106 μm | Ratio |
| DSDP 386 | Maastricht | 9 | 18 | 27 | 0.67 | Danian | 10 | 122 | 36 | 3.39 |
| DSDP 596 | 76–66 | 42 | 605 | 935 | 0.65 | 66–56 | 36 | 1,051 | 631 | 1.67 |
| ODP 8861 | 70.2–661 | 431 | 1,0021 | 1,4031 | 0.711 | 66–63.81 | 231 | 1,5921 | 1,2171 | 1.311 |
| ODP 8862 | 70.2–662 | 432 | 1,0022 | 1,4032 | 0.732 | 66–63.82 | 232 | 1,5922 | 1,2172 | 1.692 |
| ODP 8863 | 70.2–663 | 433 | 1,0023 | 1,4033 | 0.713 | 65.6–63.83 | 203 | 1,1573 | 6533 | 1.773 |
| ODP 8864 | 70.2–664 | 434 | 1,0024 | 1,4034 | 0.714 | 66–63.84 | 234 | 1,374.54 | 9354 | 1.474 |
| ODP 1262 | 66.9–66 | 45 | 52 | 50 | 1.04 | 66–62.1 | 117 | 447 | 218 | 2.05 |
| IODP 1403 | 66.4–66 | 21 | 29 | 27 | 1.07 | 66–64.7 | 121 | 121 | 51 | 2.37 |
| Gubibo | 66.3–66 | 10 | 63 | 77 | 0.82 | 66–62.9 | 26 | 320 | 139 | 2.30 |
Different metrics for ODP Site 886 are explained in SI Materials and Methods, with superscript referring to the method used to calculate each ratio.
For ODP Site 886, where the K/Pg boundary ichthyolith counts were very high, the tooth/denticle metric was substantially biased by these samples, and the average ratio for the time interval of the Paleocene (66–63.8 Ma for ODP Site 886) was significantly different from the ratio calculated by binning the samples as was done for the other sites. The tooth/denticle ratio did not appear to be biased by unusual ichthyolith abundance for the other sites, regardless of sample size. We suspect that there may have been sedimentary changes at site ODP 886 during the K/Pg interval. For ODP Site 886, we report four methods to account for this bias: method 1, by binning all samples from the post K/Pg interval, as in other sites; method 2, by averaging the ratios reported for each assemblage; method 3, by removing the five samples with abnormally high numbers of ichthyoliths found immediately adjacent to the K/Pg boundary, which dominated the signal; and method 4, by reducing the relative importance of these five samples by halving their numerical abundances, bringing them to a more comparable level to the other samples considered in the study, before calculating as for option 1. We note that compared with the other sites in our study, all of these estimates for the Paleocene are likely artificially low at ODP Site 886, since it accounts for the least amount of time in the Paleocene. Given the increasing trend of the tooth/denticle ratio during the first 2 million years of the Paleocene in the North Pacific (Fig. 1B) and the long-term increasing trend from the South Pacific (Fig. 3B), it is likely that the ratio in the North Pacific also increased through the Paleocene. On Fig. 2, we present the tooth/denticle value calculated by option 2, the mean ratio for all 23 Paleocene samples from Site 886 as a compromise between these biases.
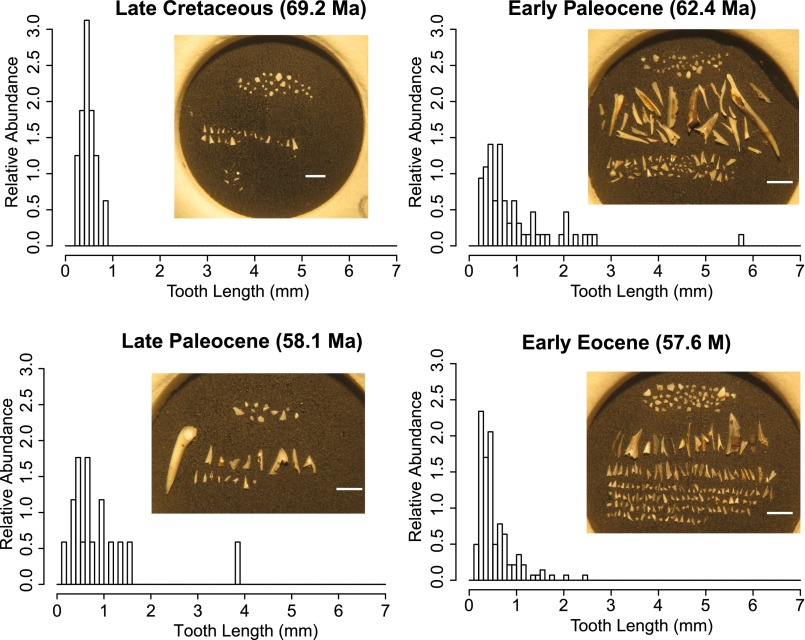
The size structure of each tooth assemblage was analyzed using R statistical package. Many ichthyolith samples have clay mineral or oxide clumps in them that retain small teeth on the 106-μm sieve, and are picked out for completion. We simulated a 106-μm sieve to exclude these small teeth that would not have otherwise been retained by removing all teeth with at least two maximum dimensions < 106 μm before analyzing the tooth assemblages. A histogram with equal bins (0.1 mm increments from 0 mm to 7 mm) was generated for each assemblage, and the relative abundance of each bin was considered in our analyses of the size structure across the K/Pg extinction (Figs. S1 and S2). We find that the patterns observed in the South Pacific (DSDP Site 596), of an increase in relative abundance of large teeth and a decrease in relative abundance of smaller teeth, is present in ODP Site 886 (Fig. S2).
Fig. S1.
Representative tooth-size histograms and ichthyolith assemblages from the South Pacific (DSDP Site 596). Histograms represent relative abundance of each tooth size class from the Late Cretaceous to the early Eocene. Note that the relative abundance of small teeth (<1 mm) decreases from the Late Cretaceous to the early Paleocene, and again from the late Paleocene to the early Eocene. The presence (and abundance) of large teeth (>1 mm) increases at the K/Pg boundary; into the early Eocene, small teeth become more abundant, and the large teeth are still present. (White scale bar, 1 mm.)
Fig. S2.
Size structure of ichthyoliths at South Pacific (DSDP Site 596) and North Pacific (ODP Site 886). (A) Accumulation of small (black circles, <0.4 mm), medium (blue squares, 0.4–0.8 mm), and large (red triangles, >0.8 mm) teeth. The red axis is scaled to 2× the small and medium teeth accumulation, to account for the relatively low numbers of large teeth present. Solid lines are three-point running means. (B) Relative abundance (square root) of tooth sizes across the K/Pg boundary. Note the decrease in relative abundance (from yellow to green at Site 596 and from red to yellow at Site 886) of the mode tooth size at both sites that is accompanied by an increase in the presence and abundance of larger teeth (from dark blue to light blue) in the early Paleocene.
In the case of tooth fragments, where it was apparent that the tooth length was not compromised by the fragmentation, the tooth was considered in the analysis. Accumulation rates of tooth size structure were calculated using sediment accumulation rate on three tooth size classes: small (<0.4 mm maximum length), medium (0.4–0.8 mm), and large (>0.8 mm).
Treatment of Shark Teeth.
While our manuscript generally treats denticles as sourced from sharks and triangular teeth as derived from ray-finned fish, we acknowledge that shark teeth are occasionally present in some our samples, especially in the larger size fractions, which can slightly bias the interpretation of the ratio of teeth to denticles reported in the manuscript. The only sites with any obvious shark teeth were DSDP 596 and ODP 886. The other sites considered in this study (DSDP 386, ODP 1262, U1403, and Gubbio) had small numbers of teeth > 106 μm to begin with, and revealed no shark-like teeth. We note that shark teeth are generally larger and more solid than the ray-finned fish teeth. They often have multiple cusps at the base, and often have an edge that is somewhat pointed, serrated, or otherwise indicative of slicing. Please see Fig. S1 for an example of a shark tooth in a Paleocene assemblage.
For DSDP Site 596 and ODP Site 886, there are zero to two shark teeth in any given sample assemblage, with >50% of samples not having any obvious shark teeth. Our Cretaceous samples generally have significantly fewer teeth and denticles than those of the Paleocene, and the samples in the Eocene are the largest. The presence of a shark tooth in a sample would cause the ratio to decrease considerably more in the Cretaceous than it would in the Paleocene or Eocene, and therefore push the relative abundance of sharks even higher in the Cretaceous, while not causing a major change to the larger samples of the Paleocene or Eocene.
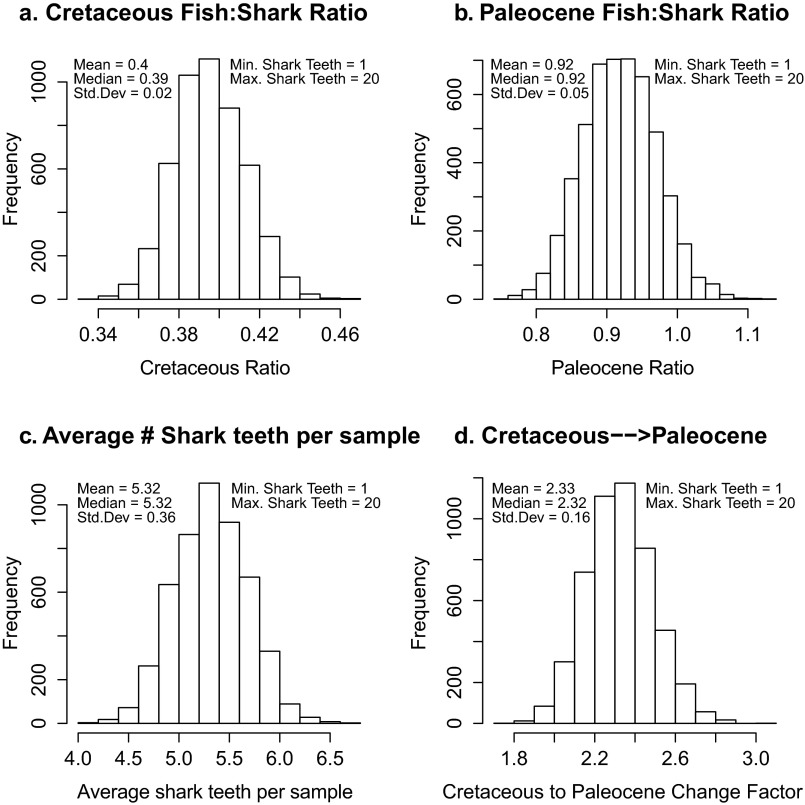
To quantify the effect of shark teeth in our assemblages, we performed a series of bootstrap analyses, assuming a particular abundance of shark teeth present in the samples from DSDP Site 596, and recalculated the Cretaceous and Paleocene ratios based on these numbers. The simulations were carried out using R Statistical Package. For simulations 3–7, the number of shark teeth per for each sample set in the time series was randomly chosen from a uniform integer distribution, and the resulting pre- and post-K/Pg ratios were calculated. Each simulation was run with 5,000 bootstrap replicates. In our simulated calculations, if a shark tooth was present, it was removed from the tooth part of the assemblage, and instead counted as part of the shark (denticle) assemblage. These simulations (except for scenarios 1 and 2) are deliberately significantly more extreme than the visual check of our samples suggests, allowing for a greater range of potential shark teeth in the assemblages, and test the robustness of the sample set and conclusions.
Simulations are as follows: simulation 1, 1% of teeth (rounded up) or at least one tooth per sample are shark teeth; simulation 2, 5% of teeth (rounded up) or at least one tooth per sample are shark teeth; simulation 3, a minimum of 0 and a maximum of 10 teeth (up to 50%) of each sample are shark teeth; simulation 4, a minimum of 1 and a maximum of 10 teeth (up to 50%) of each sample are shark teeth; simulation 5, a minimum of 0 and a maximum of 20 teeth (up to 50%) of each sample are shark teeth; simulation 6, a minimum of 1 and a maximum of 20 teeth (up to 50%) of each sample are shark teeth; and simulation 7, a minimum of 1 and a maximum of 40 teeth (up to 50%) of each sample are shark teeth.
The results from these experiments are summarized in Table S2, and the bootstrapped histograms from simulation 6 are shown in Fig. S3. For all simulations, the ratio of Paleocene actinopterygian to shark fossils is twice or more than the ratio in the Cretaceous. Additionally, none of our simulations shows any instances where the Paleocene ratio is less than the Cretaceous (change factor is <1). While the absolute value of the ratio is, by definition, sensitive to a number of variables, including the number of shark teeth (as well as the size fraction considered in the analysis), the overall conclusion, that the ratio of ray-finned fish versus shark fossils increases by a factor of two from the Cretaceous to the Paleocene, is robust to the presence of shark teeth.
Table S2.
Results from simulated presence of shark teeth
| Scenario | Cretaceous ratio | Paleocene ratio | Change factor | Change factor SD | Simulated shark teeth per sample |
| No shark teeth | 0.65 | 1.67 | 2.57 | n/a | 0 |
| 1: 1% or at least 1 | 0.58 | 1.52 | 2.62 | n/a | 1 |
| 2: 5% or at least 1 | 0.58 | 1.44 | 2.48 | n/a | 1.27 |
| 3: Min = 0, max = 10 | 0.43 | 1.12 | 2.60 | 0.16 | 3.93 |
| 4: Min = 1, max = 10 | 0.4 | 1.07 | 2.66 | 0.15 | 4.43 |
| 5: Min = 0, max = 20 | 0.42 | 0.96 | 2.26 | 0.18 | 4.83 |
| 6: Min = 1, max = 20 | 0.4 | 0.92 | 2.33 | 0.16 | 5.32 |
| 7: Min = 1, max = 40 | 0.4 | 0.85 | 2.16 | 0.18 | 5.71 |
Fig. S3.
Results of bootstrapped shark tooth simulation 6, showing the spread of ratios calculated for each of 5,000 simulations. (A) Cretaceous ratios in each simulation. (B) Paleocene ratios in each simulation. (C) Average number of shark teeth present in each assemblage for each simulation. (D) Factor of change between the Cretaceous ratio and the Paleocene ratio calculated in each simulation. Note that there is no value < 1, so the ratio observed in the Paleocene is always greater than the ratio observed in the Cretaceous.
Age Models and Time Series Analysis.
DSDP Site 386.
DSDP Site 386 is an Atlantic red clay site off Bermuda Rise at 4,793 m water depth (41). Due to the lack of microfossils, the age constraints are poor at the site; however, there is a distinct K/Pg boundary slump deposit (27). Thirty-gram samples were taken from the 2 m below and above the boundary to assess the fish community before and after the impact and extinction event. Due to the low abundance of >106-μm ichthyoliths in the core, samples were grouped as pre- and post-K/Pg boundary for analysis.
DSDP Site 596.
DSDP Site 596 is a red clay site from the South Pacific Gyre at 5,711 m water depth (42). Five- to 12-gram sediment samples were processed at 5-cm intervals (∼200-ky resolution) from 75.1 Ma to 42.2 Ma. The K/Pg boundary is identifiable by a prominent iridium anomaly (see Fig. 1B), and an age model developed based on the accumulation of cobalt in marine sediments was used to calculate ichthyolith accumulation rate during the study interval (23, 38). The chronology was shifted to a K/Pg boundary age of 66.04 Ma after GTS2012 (39).
ODP Site 886.
ODP Site 886C is a red clay site from the North Pacific Gyre, at 5,713 m water depth (43). The age model used to calculate ichthyolith accumulation is based on strontium stratigraphy of fish teeth and tied to the K/Pg boundary using the GTS2012 age of 66.04 Ma (39) by an iridium anomaly (25). Five- to 15-gram samples of red clay were processed at 5 cm intervals (∼100-ky resolution) from 70.0 Ma to 63.9 Ma. Unfortunately, a hiatus in the core precluded further analysis into the Paleocene.
ODP Site 1262.
ODP Site 1262 is from Walvis Ridge in the South Atlantic at 4,759 m water depth and is mostly carbonate and clay (44). Twenty-gram carbonate samples were processed at 20 cm intervals (∼20-ky resolution) from 66.8 Ma to 62.1 Ma with the cyclostratigraphic age model (29) tied to the GTS 2012 K/Pg Boundary age of 66.04 Ma (39). Due to the low numbers of large teeth (>106 μm), samples were clumped into pre- and post-K/Pg extinction for analysis.
IODP Site U1403.
IODP Site U1403 is a carbonate ooze site drilled at 4,956 m water depth off of Newfoundland in the North Atlantic Ocean (26). Twenty-gram carbonate samples were processed every 20 cm (∼30-ky resolution) from 66.4 Ma and 64.7 Ma. Preboundary and postboundary assemblages were considered based on an impact horizon layer identified in the shipboard site report (26).
Gubbio, Italy.
One-hundred-gram samples of limestone were processed from the Scalia Rosa formation on the Contessa Road Outcrop at Gubbio, Italy (28, 37). Samples were taken at 10- to 40-cm intervals across the K/Pg boundary between 66.3 Ma and 62.9 Ma based on a biostratigraphic age model (45) updated to GTS2012 dates (39). Samples were grouped into preboundary and postboundary assemblages for analysis.
Discussion
Our data show that the pelagic marine vertebrate community was profoundly affected by the K/Pg mass extinction. During the Late Cretaceous, dermal denticles make up over half of every >106-μm ichthyolith assemblage, and there is very low variability in assemblage composition (Fig. 1B), suggesting that the shift was not simply a result of a background trend that began during the latest Cretaceous. After the K/Pg boundary, teeth dominate the ichthyolith assemblages and become 2 to 3 times as abundant as denticles—a trend that is not reversed within the first 24 million years of the Cenozoic (Figs. 2 and 3). This change in the tooth/denticle ratio occurs at the peak iridium anomaly marking the boundary, even in the face of likely sediment mixing in slowly accumulating red clay sediments (Fig. 1B), implying that the Chicxulub impact was the driver for the ecological change (24). The increase in teeth relative to denticles is unlikely to reflect an artifact of misattribution of shark teeth to ray-finned fish teeth, since the absolute abundance of shark dermal denticles is nearly unchanged through the study time period and denticles are produced in vastly larger numbers than teeth in modern elasmobranchs, overwhelming any signal of elasmobranch teeth.
We interpret the change in the ratio of teeth to denticles in ichthyolith assemblages as an increase in the ecological importance of the ray-finned fishes that dominate the modern pelagic open ocean. We might expect that an increase in the population size of ray-finned fish would lead to an increase in sharks, since sharks rely on the biomass of lower trophic levels, commonly assumed to be ray-finned fish. However, the absolute abundance of shark fossils is nearly unchanged, or even decreases following the extinction (Fig. 1A), even as the ray-finned teeth increase, suggesting that increased ray-finned fish populations did not power an increase in the sharks. Instead, sharks appear to have remained stuck at similar abundances in the Paleocene as they had in the Cretaceous, suggesting that they were unable to exploit newly opened niches after the extinction, or that they traded niches to maintain an overall constant level of abundance.
Since ray-finned fish teeth are rare in the Cretaceous relative to shark denticles, fish may have been kept at low levels of abundance due to predation or were ecologically outcompeted in Cretaceous pelagic systems. The rapid increase in tooth abundance, but not in shark denticles, at the extinction suggests that the ray-finned fish seized the opportunity to diversify and colonize newly vacated niches in the open ocean that had been previously unavailable to them. Perhaps competition or predation by ammonites and squids or other rarely fossilized groups suppressed ray-finned fish populations in the Cretaceous, allowing fish to be ecologically released by the extinction of the ammonites during the K/Pg event. At least some species of ammonite were likely planktivores, perhaps competing directly with many pelagic fish groups for trophic resources (33). In other cases, fish may have been prey for cephalopods, analogous to the ravages of the modern Humboldt squid, Dosidicus, in midwater fishes with the expansion of the oxygen minimum zone in the eastern Pacific (34). Finally, the increase of ray-finned fish teeth in our assemblages may be due to a change in the rate of tooth loss in fishes rather than an increase in the number of ray-finned fish individuals from the Cretaceous to the Paleogene. There are examples of fish increasing their tooth production through changes in gene regulation, such as certain species of stickleback fish having more teeth in freshwater systems than in brackish or marine waters (35). However, the increase in tooth size and change in community structure suggest that this mechanism alone is unlikely to account for all of the changes seen in the ichthyolith community at the K/Pg boundary and maintained through the Paleocene and Eocene.
While the ratio of ray-finned fish to shark ichthyoliths appears to be relatively stable around the globe during the Cretaceous, the Paleocene ratios are more varied (Fig. 2). The productivity of pelagic ecosystems is known to have fallen abruptly in the Atlantic and Tethys Oceans while remaining relatively unchanged in the Pacific (7, 8, 12, 15). Hence, geographic variation in the fish-to-shark ratio may reflect spatial differences in dynamics of primary producers and export production in the post-K/Pg pelagic marine ecosystems (7, 8, 12) that could have supported different groups and abundances of fish in different regions (15). This is consistent with geographically heterogeneous patterns of recovery seen in other pelagic marine lineages (7).
The Paleocene increase in the size and accumulation rate of the largest teeth strongly suggests that the K/Pg event initiated a wholesale change in the fish community. Fish tooth size does not necessarily scale allometrically with body size, especially in the open ocean and deep sea: Some very large fish have only small teeth, while other very small fish have much larger teeth; hence we cannot directly interpret tooth size as a direct indication of body size. However, we suggest that the size structure of the ichthyolith assemblage does reflect the range of ecological niche space taken up by the fishes present in the system. Therefore, we infer that the large range of tooth sizes in the Paleocene indicates an expansion of the collective range of habitats and ecologies that fishes were able to exploit, somewhat analogous with the postextinction increase in the size diversity of Cenozoic mammals (36).
In addition to the increase in maximum tooth size, there is also a temporary increase in the accumulation rate, length, and relative abundance of the largest teeth (75th quartile or larger). The peak in accumulation rate and size of this largest group of teeth lasts only from the K/Pg boundary until about 60 Ma. The prominence of large teeth in the South Pacific suggests that ray-finned fishes explored a novel community structure for the first 6–7 million years of the Paleocene, in which large teeth were unusually abundant compared with those in Cretaceous and later Paleocene assemblages. The overall size structure of tooth assemblages in the later Paleocene and Eocene shows a lower relative abundance of large teeth. However, the constant presence of at least a few teeth in each sample that are significantly larger than the largest Cretaceous teeth (Fig. 3D) suggests that the decrease in relative abundance of large teeth represents a “filling in” of ecological niche space between the smallest and largest tooth sizes rather than a disappearance of large-toothed fishes in later Paleocene and Eocene fish assemblages.
A major advantage of our deep-sea ichthyolith records is that they can allow assessment of variability in marine vertebrate assemblages up to and across major events. Our Pacific records show that the latest Cretaceous vertebrate community was very stable, both in terms of the ratio of fish to sharks and the size structure of the tooth community (Fig. 3). Indeed, our South Pacific record of tooth/denticle ratios and tooth size “flatlines” for the last 10 million years of the Cretaceous. The stability of Cretaceous assemblages suggests that the changes observed at the K/Pg boundary and early Paleocene were abrupt, likely caused by the extinction event, and are not part of a background trend or the result of random chance. This change in fish community structure appears to have been caused by the Chicxulub impact (24) rather than being a long-term response to a prolonged period of volcanism during the Latest Cretaceous. Indeed, in parallel with the mammals on land (36), the K/Pg extinction event appears to have initiated major changes in the marine vertebrate community that lead to the great diversification and ecological rise to dominance of the ray-finned fishes in our oceans today.
Conclusions
While there were relatively low levels of extinction of pelagic fish at the K/Pg boundary, we find that the extinction event marked an ecological turning point for the pelagic marine vertebrates. Most open ocean, gyre-inhabiting, Cretaceous fishes were likely small and relatively rare—like the terrestrial mammals of their time—compared with their counterparts of the Paleocene and Eocene. In the Paleogene, ecological changes such as the increase in relative abundance of ray-finned fish compared with sharks and the persistence of large-toothed fishes are both permanent changes in the fish community that were initiated by the extinction. The presence of a novel “disaster fauna” of large-toothed fishes, at least in the South Pacific, suggests that rapid evolution occurred in the pelagic fish community following the extinction (4), before a more delayed filling in of niche space occurred in the later Paleocene and Eocene. The extinction event changed the fundamental ecosystem structure of the pelagic marine vertebrate community, allowing the ray-finned fishes to rapidly diversify in the early Cenozoic pelagic oceans. The extreme stability of Cretaceous ichthyolith accumulation rates, assemblage structure, and tooth sizes suggests that, without the extinction, it is unlikely that the system would have reset so dramatically in favor of ray-finned fishes. The K/Pg extinction appears to have been a major driver in the rise of ray-finned fishes and the reason that they are dominant in the open oceans today.
Materials and Methods
Sample Preparation.
Samples were obtained from Integrated Ocean Drilling Program (now International Ocean Discovery Program, IODP), ODP, and Deep Sea Drilling Project sites, from the North Pacific (ODP Site 886), South Pacific (DSDP Site 596), North Atlantic (IODP Site U1403), Central Atlantic (DSDP Site 386), and South Atlantic (ODP Site 1262). Gubbio samples were obtained from the limestone outcrop on Contessa Highway north of Gubbio, Italy (37). DSDP Sites 386 and 596 and ODP Site 886 are red clay, while ODP Site 1262 and IODP Site U1403 are primarily carbonate ooze. Sample processing varied based on lithology (see SI Materials and Methods). All samples were dried to constant weight in a 50 °C oven. Ichthyoliths were isolated from the samples by disaggregation with deionized water in the case of clays, and by dissolution in weak (5–10%) acetic acid in the case of carbonates. All particles > 38 μm were retained, and ichthyoliths > 106 μm were hand-picked from the residue using a dissection microscope and archived in cardboard micropaleontology slides using gum tragacanth glue to hold in place.
Ichthyolith Assemblage Analysis.
Ichthyoliths were classified into denticles (shark scales) and teeth (assumed to be mostly ray-finned fish teeth, <1% shark teeth; see SI Materials and Methods for more discussion). All Pacific assemblages were photographed using a Canon Powershot S5 IS microscope-mounted camera. The resulting image was processed and analyzed using ImageJ (32) to count and measure the size and shape of the ichthyoliths. The other sites considered in this study were simply grouped into “preboundary” and “postboundary” assemblages for analysis. Size structure analysis was restricted to teeth, since about 20–40% of the denticles were partially fragmented.
Age Models and Accumulation Rates.
While ichthyolith assemblage metrics are independent of age model beyond preboundary and postboundary, ichthyolith accumulation rate (reported as ichthyoliths per square centimeter per million years) is, by definition, age-model dependent, since the calculation depends on sedimentation rates and accurate age datums. The age model for DSDP Site 596 is based on a cobalt accumulation rate stratigraphy (38), and tied to the K/Pg boundary by a prominent iridium anomaly at that site (23). The age model has been shifted to hang on the Geologic Time Scale 2012 (GTS2012) K/Pg boundary age of 66.04 Ma (39). The ODP Site 886 age model is based on a compilation of radiolarian biostratigraphy and strontium isotope stratigraphy, and is also tied to the K/Pg boundary by an iridium anomaly (25) and given the GTS2012 age of 66.04 Ma. For the other sites, where accumulation rate is not considered, samples were grouped into preboundary and postboundary, based on prominent impact horizons in each location.
Acknowledgments
All DSDP/ODP/IODP samples were generously provided through the International Ocean Discovery Program. We thank L. Levin, P. Hastings, and two anonymous reviewers for insightful comments on this manuscript. E.C.S. is supported by a National Science Foundation Graduate Research Fellowship. R.D.N. is supported by the National Science Foundation-Ocean Sciences. Field collection of Gubbio samples was made possible by a field grant from the American Philosophical Society’s Lewis and Clark Fund for Astrobiology Field Work to E.C.S. in 2012.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Supplementary data are available at http://dx.doi.org/10.1594/PANGAEA.846789.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504985112/-/DCSupplemental.
References
- 1.Near TJ, et al. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc Natl Acad Sci USA. 2013;110(31):12738–12743. doi: 10.1073/pnas.1304661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Near TJ, et al. Resolution of ray-finned fish phylogeny and timing of diversification. Proc Natl Acad Sci USA. 2012;109(34):13698–13703. doi: 10.1073/pnas.1206625109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman M, Sallan LC. Five hundred million years of extinction and recovery: A Phanerozoic survey of large‐scale diversity patterns in fishes. Palaeontology. 2012;55(4):707–742. [Google Scholar]
- 4.Miya M, et al. Evolutionary origin of the Scombridae (tunas and mackerels): Members of a paleogene adaptive radiation with 14 other pelagic fish families. PLoS ONE. 2013;8(9):e73535. doi: 10.1371/journal.pone.0073535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coxall HK, D'Hondt S, Zachos JC. Pelagic evolution and environmental recovery after the Cretaceous-Paleogene mass extinction. Geology. 2006;34(4):297–300. [Google Scholar]
- 6.D'Hondt S. Consequences of the Cretaceous/Paleogene mass extinction for marine ecosystems. Annu Rev Ecol Evol Syst. 2005;36:295–317. [Google Scholar]
- 7.Hull PM, Norris RD, Bralower TJ, Schueth JD. A role for chance in marine recovery from the end-Cretaceous extinction. Nat Geosci. 2011;4(12):856–860. [Google Scholar]
- 8.Hull PM, Norris RD. Diverse patterns of ocean export productivity change across the Cretaceous-Paleogene boundary: New insights from biogenic barium. Paleoceanography. 2011;26(3):PA3205. [Google Scholar]
- 9.Jiang SJ, Bralower TJ, Patzkowsky ME, Kump LR, Schueth JD. Geographic controls on nannoplankton extinction across the Cretaceous/Palaeogene boundary. Nat Geosci. 2010;3(4):280–285. [Google Scholar]
- 10.Ward PD, Kennedy WJ, Macleod KG, Mount JF. Ammonite and inoceramid bivalve extinction patterns in Cretaceous Tertiary boundary sections of the Biscay region (southwestern France, northern Spain) Geology. 1991;19(12):1181–1184. [Google Scholar]
- 11.Hsu KJ, McKenzie JA. 1985. A “Strangelove” ocean in the earliest Tertiary. The Carbon Cycle and Atmospheric CO: Natural Variations Archean to Present, Geophysical Monograph Series, eds Sundquist ET, Broecker WS (Am Geophys Union, Washington, DC) Vol 32, pp 487–492.
- 12.Alegret L, Thomas E, Lohmann KC. End-Cretaceous marine mass extinction not caused by productivity collapse. Proc Natl Acad Sci USA. 2012;109(3):728–732. doi: 10.1073/pnas.1110601109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sepúlveda J, Wendler JE, Summons RE, Hinrichs KU. Rapid resurgence of marine productivity after the Cretaceous-Paleogene mass extinction. Science. 2009;326(5949):129–132. doi: 10.1126/science.1176233. [DOI] [PubMed] [Google Scholar]
- 14.Smit J. 1982. Extinction and evolution of planktonic foraminifera after a major impact at the Cretaceous/Tertiary boundary. Geol Soc Am Spec Pap 190:329–352.
- 15.Sibert EC, Hull PM, Norris RD. Resilience of Pacific pelagic fish across the Cretaceous/Palaeogene mass extinction. Nat Geosci. 2014;7(9):667–670. [Google Scholar]
- 16.Cavin L. In: Effects of the Cretaceous-Tertiary Boundary Event on Bony Fishes. Geological and Biological Effects of Impact Events, Impact Studies. Buffetaut E, Koeberl C, editors. Springer, Berlin; 2002. pp. 141–158. [Google Scholar]
- 17.Friedman M. Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proc Natl Acad Sci USA. 2009;106(13):5218–5223. doi: 10.1073/pnas.0808468106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman M. 2010. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proc R Soc B 277(1688):1675−1683.
- 19.Kriwet J, Benton MJ. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous-Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;214(3):181–194. [Google Scholar]
- 20.Guinot G, Adnet S, Cappetta H. An analytical approach for estimating fossil record and diversification events in sharks, skates and rays. PLoS ONE. 2012;7(9):e44632. doi: 10.1371/journal.pone.0044632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle PS, Riedel WR. 1979. Ichthyoliths: Present Status of Taxonomy and Stratigraphy of Microscopic Fish Skeletal Debris (Scripps Inst Oceanogr, La Jolla, CA)
- 22.Doyle PS, Riedel WR. 1985. Cenozoic and Late Cretaceous Ichthyoliths (Cambridge Univ Press, Cambridge, UK)
- 23.Zhou L, Kyte FT, Bohor BF. Cretaceous/Tertiary boundary of DSDP Site 596, South Pacific. Geology. 1991;19(7):694–697. [Google Scholar]
- 24.Schulte P, et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science. 2010;327(5970):1214–1218. doi: 10.1126/science.1177265. [DOI] [PubMed] [Google Scholar]
- 25.Snoeckx H, Rea D, Jones C, Ingram B. 1995. Eolian and silica deposition in the central North Pacific: Results from Sites 885/886. Proc Ocean Drill Program Sci Results 145:219−230.
- 26.Norris RD, et al. 2014. Site U1403. Proceedings of the Integrated Ocean Drilling Program (Integ Ocean Drill Program Intl, Tokyo), Vol 342, p 98.
- 27.Norris RD, Firth J, Blusztajn JS, Ravizza G. Mass failure of the North Atlantic margin triggered by the Cretaceous-Paleogene bolide impact. Geology. 2000;28(12):1119–1122. [Google Scholar]
- 28.Alvarez LW, Alvarez W, Asaro F, Michel HV. Extraterrestrial cause for the cretaceous-tertiary extinction. Science. 1980;208(4448):1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 29.Westerhold T, et al. Astronomical calibration of the Paleocene time. Palaeogeogr Palaeoclimatol Palaeoecol. 2008;257(4):377–403. [Google Scholar]
- 30.Trapani J, Schaefer S. Position of developing replacement teeth in teleosts. Copeia. 2001;2001(1):35–51. [Google Scholar]
- 31.Bemis WE, Giuliano A, McGuire B. Structure, attachment, replacement and growth of teeth in bluefish, Pomatomus saltatrix (Linnaeus, 1776), a teleost with deeply socketed teeth. Zoology (Jena) 2005;108(4):317–327. doi: 10.1016/j.zool.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Rasband WS. 1997−2014. ImageJ (US Nat Inst Health, Bethesda, MD)
- 33.Kruta I, Landman N, Rouget I, Cecca F, Tafforeau P. The role of ammonites in the Mesozoic marine food web revealed by jaw preservation. Science. 2011;331(6013):70–72. doi: 10.1126/science.1198793. [DOI] [PubMed] [Google Scholar]
- 34.Zeidberg LD, Robison BH. Invasive range expansion by the Humboldt squid, Dosidicus gigas, in the eastern North Pacific. Proc Natl Acad Sci USA. 2007;104(31):12948–12950. doi: 10.1073/pnas.0702043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleves PA, et al. Evolved tooth gain in sticklebacks is associated with a cis-regulatory allele of Bmp6. Proc Natl Acad Sci USA. 2014;111(38):13912–13917. doi: 10.1073/pnas.1407567111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48(1):107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez W, et al. Upper Cretaceous-Paleocene magnetic stratigraphy at Gubbio, Italy. 5. Type section for Late Cretaceous-Paleocene geomagnetic reversal time scale. Geol Soc Am Bull. 1977;88(3):383–388. [Google Scholar]
- 38.Zhou L, Kyte FT. Sedimentation history of the South Pacific pelagic clay province over the last 85 million years inferred from the geochemistry of Deep Sea Drilling Project Hole 596. Paleoceanography. 1992;7(4):441–465. [Google Scholar]
- 39.Gradstein FM, Ogg JG, Schmitz M, Ogg GM. 2012. The Geologic Time Scale (Elsevier, Amsterdam)
- 40.Arthur MA, Fischer AG. Upper Cretaceous-Paleocene magnetic stratigraphy at Gubbio, Italy. 1. Lithostratigraphy and sedimentology. Geol Soc Am Bull. 1977;88(3):367–371. [Google Scholar]
- 41.Tucholke BE, et al. Site 386: Fracture valley sedimentation on the central Bermuda Rise. Initial Rep Deep Sea Drill Proj. 1979;43:195–321. [Google Scholar]
- 42.Menard HW, et al. Site 596: Hydraulic piston coring in an area of low surface productivity in the Southwest Pacific. Initial Rep Deep Sea Drill Proj. 1987;91:245–267. [Google Scholar]
- 43.Rea DK, et al. Sites 885/886. Proc Ocean Drill Program Initial Rep. 1993;145:303–334. [Google Scholar]
- 44.Zachos JC, et al. Site 1262. Proc Ocean Drill Program Initial Rep. 2004;208:1, 92. [Google Scholar]
- 45.Silva IP. Upper Cretaceous–Paleocene magnetic stratigraphy at Gubbio, Italy II. Biostratigraphy. Geol Soc Am Bull. 1977;88(3):371–374. [Google Scholar]