Significance
Reading is a critical linguistic skill, but understanding of its cognitive and neural bases is incomplete. Using functional MRI, we found reading-related activation in two areas of anterior temporal cortex, an area not previously associated with reading. Activation profiles of these sites were consistent with the predictions of computational reading models that ascribe a key role to semantic knowledge in reading words with irregular spellings. We also found individual differences in the reading neural network that were predicted by an independent measure of semantic reliance in reading. Such individual differences have been hypothesized on theoretical grounds to explain variation in reading abilities among neurodegenerative patients. Here we provide the first direct evidence, to our knowledge, for their existence in the healthy brain.
Keywords: triangle model, anterior temporal lobe, semantic reliance, reading, surface dyslexia
Abstract
The goal of cognitive neuroscience is to integrate cognitive models with knowledge about underlying neural machinery. This significant challenge was explored in relation to word reading, where sophisticated computational-cognitive models exist but have made limited contact with neural data. Using distortion-corrected functional MRI and dynamic causal modeling, we investigated the interactions between brain regions dedicated to orthographic, semantic, and phonological processing while participants read words aloud. We found that the lateral anterior temporal lobe exhibited increased activation when participants read words with irregular spellings. This area is implicated in semantic processing but has not previously been considered part of the reading network. We also found meaningful individual differences in the activation of this region: Activity was predicted by an independent measure of the degree to which participants use semantic knowledge to read. These characteristics are predicted by the connectionist Triangle Model of reading and indicate a key role for semantic knowledge in reading aloud. Premotor regions associated with phonological processing displayed the reverse characteristics. Changes in the functional connectivity of the reading network during irregular word reading also were consistent with semantic recruitment. These data support the view that reading aloud is underpinned by the joint operation of two neural pathways. They reveal that (i) the ATL is an important element of the ventral semantic pathway and (ii) the division of labor between the two routes varies according to both the properties of the words being read and individual differences in the degree to which participants rely on each route.
Cognitive neuroscience offers the exciting prospect of understanding the neural basis of cognitive behaviors, a goal which requires integration of sophisticated cognitive models with knowledge about the underlying neural machinery. As a key cognitive skill, reading provides an advanced test case. Computationally implemented theories present detailed accounts of how reading is accomplished at a cognitive level (1–4). To achieve a full understanding of reading and its impairment in neurological disorders, these cognitive theories must be integrated with information about the neural basis of the reading system. However, although various studies have explored the neural basis of reading (5), the level of integration between cognitive and neural models remains limited. In the present study, we used an improved functional MRI (fMRI) protocol to test the specific predictions of an influential computational model of word reading: the connectionist “Triangle Model” (2). We were able to map specific elements of this cognitive model onto different cortical regions, thereby providing a direct link between cognitive theorizing and neural implementation. Specifically, we provide insights into the division of labor between semantic and phonological processes in supporting reading aloud, which has been a long-standing source of controversy among cognitive models (6, 7). Moreover, we demonstrate that this division of labor is jointly influenced by variation in the properties of words being read and by individual differences in the neurocognitive architecture across participants and that these individual differences can be predicted using an independent behavioral measure of reading style.
Cognitive and neural models of single-word reading agree that multiple pathways contribute to reading aloud (1, 2, 8–10). These pathways consist of a route that maps directly from orthographic forms to phonological representations (allowing us, for example, to pronounce novel letter strings such as “flumpt”) and an indirect route mediated by word-level lexical or semantic knowledge. The exact function of these pathways remains a source of active debate, much of it focused on the role of semantic (word meaning) knowledge in reading aloud. Dual-route models hold that all words can be read either via grapheme-to-phoneme rules or through access to orthographic and phonological lexica that are distinct from knowledge of word meaning (1, 10). In contrast, the Triangle Model proposes that semantic knowledge plays an integral part in pronouncing words correctly (2, 6, 11). The cornerstone of this second approach is the view that, because reading is, in evolutionary terms, a recently developed skill, it is unlikely to be served by a dedicated neural architecture. Instead, it is underpinned by the phylogenetically more mature primary systems of vision, phonology, and semantics (9). This idea has been instantiated in several connectionist computational models known as Triangle Models because they comprise a direct pathway from visual (or orthographic) to phonological representations (here termed the “O→P pathway”) and an indirect pathway in which the translation from orthography to phonology is mediated through access to semantic knowledge (2–4, 12). In such models, the direct O→P pathway becomes sensitive to the statistical regularities in the mapping from spelling to sound. It therefore is efficient in computing the pronunciations of words with typical spelling-to-sound mappings (e.g., “mint”) but is less successful for words with exceptional spelling-to-sound correspondences, such as “pint” (2, 13). The indirect, semantically mediated pathway (O→S→P) provides additional support for reading, which is especially important for exception words that are poorly served by the O→P pathway.
The Triangle Model assumes that each of the primary systems of vision, phonology, and semantics makes distinct contributions to reading. This position is supported by neuropsychological investigations of patients with acquired dyslexias, in which damage to visual, phonological, and semantic systems are associated with distinct patterns of reading deficit. The visually based reading deficit sometimes termed “pure alexia” is associated with damage to the left ventral occipitotemporal cortex (14, 15), an area that is ubiquitously activated for tasks involving orthographic processing (8, 16). Critically, such patients also have visual deficits for nonorthographic stimuli such as checkerboards and faces (15, 17, 18), supporting the primary systems view that this brain region is a more general visual-processing area. In a similar vein, patients with phonological dyslexia have concomitant phonological deficits in tasks that do not involve written words (19–21). Lesion–symptom mapping studies indicate that these patients’ reading deficits are associated with damage to frontal and temporal perisylvian cortex (22). The involvement of premotor cortex in such patients is of particular interest, because this region is robustly activated when healthy individuals read aloud in the scanner (23). This area is strongly associated with phonological processing and in the computation of phonological output representations (24, 25). These findings reinforce the notion that reading aloud draws on a core phonological system that also is recruited by nonreading tasks.
Involvement of the semantic system in reading aloud has proved the most controversial element of the Triangle Model. Semantic involvement is supported by the study of surface dyslexia, a reading disorder characterized by poor reading of exception words. This pattern is strongly associated with semantic dementia (SD), a disorder in which patients suffer from a progressive, selective deterioration in semantic knowledge (26). Both reading deficits and semantic deficits in this group have been linked to atrophy centered on the left anterior temporal lobe (22, 27, 28), suggesting that this area makes a critical contribution both to semantic knowledge and to the reading of exception words. However, although these studies provide persuasive evidence for the co-occurrence of semantic impairment and exception word reading deficits, they provide limited data regarding the exact neuroanatomical locus of such effects. A number of recent neuroimaging studies have highlighted the involvement of the ventral anterior temporal lobe (ATL), i.e., the inferior temporal and fusiform gyri, in semantic processing of verbal and nonverbal stimuli (29–31) in an area that coincides with the peak neural correlate of semantic impairment in patients with SD (32). The ATL is rarely activated in studies of word reading. One intriguing exception is a study in which participants were trained to associate pseudowords with particular meanings (33). Following this training, activation in the lateral ATL was observed when participants read the pseudowords aloud, suggesting that linking of novel pseudowords with meanings triggered engagement of the ATL. In general, however, there is scant neuroimaging evidence for the involvement of the ATL in the reading of familiar words, as various authors have noted (8, 34–36). As a consequence, the ATL is rarely included in models of the neural basis of reading (5, 36).
There are two possible interpretations of these results. The first is that the ATL and, by extension, the semantic representations supported by this region, are not involved in the process of converting print to sound. Indeed, some have claimed that exception-word reading deficits in patients with SD are caused instead by mild atrophy in the posterior temporal lobe (7, 37). [There are, however, cases of surface dyslexia in SD without evidence of disruption to posterior temporal regions; see Woollams et al. (38).] The alternative argument, which we propose and test in this paper, is that the ATL does make a critical contribution to reading aloud and that the paucity of neuroimaging evidence for this contribution is the result of a number of technical and methodological factors. When these factors are addressed, we demonstrate that ATL activation is present and that it conforms to the predictions of the Triangle Model.
It is well known that various methodological factors can reduce the probability of ATL activations being observed in neuroimaging studies. These include use of a restricted field of view that excludes the inferior parts of the temporal lobe from image acquisition and the use of low-level baselines that do not adequately control for automatic activation of the semantic system during passive conditions (39). In addition, gradient-echo fMRI is susceptible to magnetic field inhomogeneities that cause signal dropout and distortion in the orbitofrontal and ventral temporal cortices, including the ATL (40). One technique that combats this problem involves spin-echo (SE) imaging combined with processing that corrects for the spatial distortion incurred in problem areas (41). A number of studies using this distortion-corrected fMRI have found activation in the ventral and lateral ATLs for semantic decisions made in response to written and spoken words, pictures, and environmental sounds (29, 30, 42). To our knowledge, the present study is the first to use this technique to investigate reading aloud.
In addition to general challenges in imaging the ATL, there are two issues that are specific to reading studies. The first is that it is not straightforward to predict which words should elicit the greatest activation in regions dedicated to semantic processing. Based on the Triangle Model and the poor exception word reading of patients with SD, one might expect the semantically mediated reading route to be more active when participants read exception words (6). In contrast, other authors have argued that reading any word elicits automatic activation of its meaning and that this activation occurs independently of any functional role in accessing the appropriate phonological form (36). This view predicts similar levels of activation for regular and exception words, with activation influenced instead by psycholinguistic variables such as frequency and imageability that index the strength of semantic representation.
The second issue concerns individual differences in the degree to which people require semantic activation to compute the pronunciations of exception words. It has been observed that similar levels of semantic impairment in SD can result in varying degrees of impairment in exception word reading (6). In a few cases, patients even may present initially with normal exception word reading despite having measurable semantic impairment. Based on these findings, it has been proposed that individuals vary in their reliance on the semantic pathway, with Triangle Model simulations used to demonstrate that this variation in semantic reliance theoretically could account for the variability observed in SD (4, 6, 11). Individuals with an efficient and flexible direct pathway need little semantic support, whereas those with a less efficient direct pathway will need to fall back on semantic activation for harder words with atypical spelling–sound correspondences. The Triangle Model therefore predicts that the size of an individual’s consistency effect (i.e., the difference in reading performance between words with consistent versus inconsistent mappings) indexes the degree to which they use semantic knowledge when reading aloud. We refer to this quantity as a reader’s level of semantic reliance (SR). One important prediction arising from this research is that the level of ATL activation during reading varies systematically across individuals as a function of the individual’s SR. This between-subject variability may be an additional factor contributing to the failure to find ATL activations in previous studies. In this study, we used a behavioral measure of SR to model this variation explicitly, for the first time to our knowledge.
In summary, the Triangle Model predicts that reading aloud should engage regions of the ATL involved in semantic processing but the existing neuroimaging literature provides little support for this claim, hampering efforts to integrate the cognitive model with neural data. Importantly, the absence of evidence for ATL involvement in word reading may be a result of (i) factors in imaging protocols that reduce the likelihood of observing activation in the ATL and (ii) failure to take into account the degree to which ATL activation in reading may be modulated by both word-level and subject-level variability. To maximize sensitivity to ATL activation in the present study, we used a distortion-corrected SE fMRI protocol with full brain field-of-view and an active baseline (see Fig. S1 for signal-to-noise map). We also tested for the effects of word-level factors as well as subject-level variation in SR. Although word-level variation is commonly investigated in reading studies, this is the first study to our knowledge to test for changes in activation relating to individual differences in SR. Our investigation proceeded in two stages. First, we investigated the effects of frequency and spelling-to-sound regularity on activation in regions involved in orthographic, phonological, and semantic processing. Dynamic causal modeling was used to explore the interactions between these areas. In the second part of the study, we tested the hypothesis that individual differences in SR could explain variation in ATL activation. We adopted a triangulation technique for identifying areas involved in the semantic reading pathway. The Triangle Model predicts that activation associated with semantically mediated reading is influenced by the type of word being read (exception > regular) and by the degree to which individual participants make use of this pathway (high SR > low SR). We therefore sought regions that exhibited a conjunction of these independent word-based and subject-based effects.
Fig. S1.
Temporal signal-to-noise map, averaged across all participants.
Results
Behavioral Results.
During scanning, participants read aloud single words that varied in frequency and spelling-to-sound regularity. Accuracy and response times (RTs) are reported in Table 1. Accuracy of word responses was analyzed in 2 × 2 ANOVA, which confirmed the expected main effects of frequency [F(1,25) = 59.8, P < 0.001] and regularity [F(1,25) = 39.1, P < 0.001] and an interaction [F(1,25) = 44.3, P < 0.001]. Similar effects were found when RTs were analyzed [frequency: F(1,25) = 70.6, P < 0.001; regularity: F(1,25) = 10.4, P = 0.003; interaction: F(1,25) = 15.7, P = 0.001]. Hence, the participants as a group exhibited the well-established pattern of performance in single-word reading, in which exception words are read less efficiently than regular words, particularly if they are low in frequency.
Table 1.
Mean accuracy and RT in each condition
| HF regular | HF exception | LF regular | LF exception | Baseline | |
| Accuracy, % | 99.9 (4.4) | 99.2 (1.4) | 99.3 (1.5) | 91.1 (6.3) | 100 (0) |
| RT, ms | 695 (85) | 698 (85) | 733 (96) | 777 (112) | 815 (126) |
Standard deviations in parentheses.
Whole-Brain Analysis of Reading vs. Baseline.
We performed a whole-brain analysis to identify areas involved in word reading. This analysis revealed a network of regions commonly identified in reading studies, including posterior temporal, inferior parietal, and premotor regions (Fig. 1; peak coordinates are reported in Table S1). Importantly, we also observed activation on the ventral surface of the ATL (anterior fusiform/inferior temporal gyrus). Signal in this area typically is heavily attenuated by dropout; however, as in the present investigation, recent studies using distortion-corrected fMRI have implicated this region in a range of semantic tasks (29, 30), providing initial support for the involvement of ATL semantic regions in reading aloud.
Fig. 1.
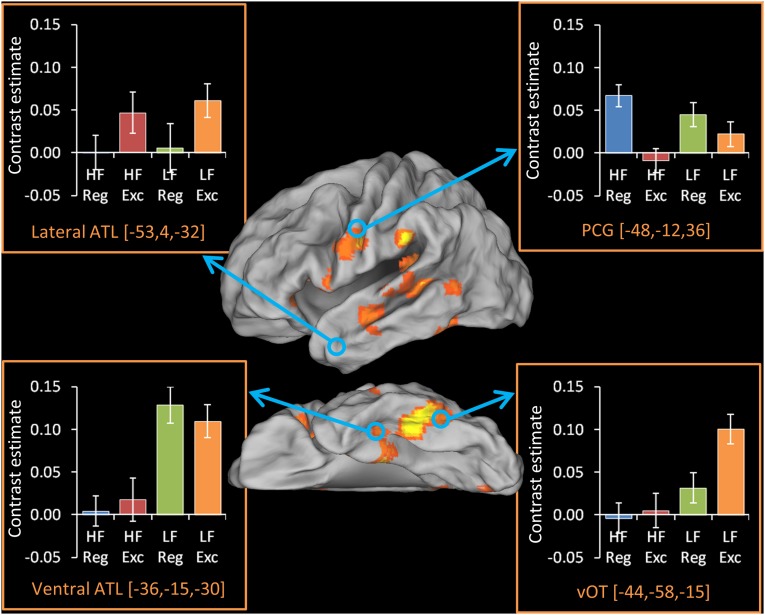
Activations for each word type in ROIs. The activation map indicates areas activated for contrast of all word types over baseline, for 26 participants, thresholded at a cluster-corrected level of P < 0.05 (see Methods for details). Bar charts indicate the contrast estimate for each word type over baseline within four a priori ROIs. Error bars indicate one SEM. Exc, exception; Reg, regular.
Table S1.
MNI peak coordinates for reading over baseline
| Hemisphere | Location | Extent in voxels | Z | MNI coordinates | ||
| x | y | z | ||||
| Left | Pars opercularis | 816 | 4.06 | −40 | 8 | 14 |
| Precentral gyrus | 3.76 | −50 | −12 | 20 | ||
| Anterior insula | 3.74 | −32 | 12 | 12 | ||
| Midfusiform | 599 | 4.36 | −42 | −36 | −26 | |
| PHG | 3.68 | −22 | −20 | −24 | ||
| Anterior Fusiform | 3.11 | −40 | −18 | −30 | ||
| pMTG | 304 | 4.05 | −46 | −46 | 4 | |
| pMTG | 3.00 | −52 | −58 | 4 | ||
| pSTS | 2.70 | −66 | −36 | 8 | ||
| Mid-MTG | 230 | 3.39 | −52 | −14 | −18 | |
| Mid-MTG | 3.37 | −46 | −8 | −18 | ||
| Cingulate gyrus | 144 | 3.92 | −12 | −14 | 44 | |
| Cingulate gyrus | 2.69 | −16 | −28 | 46 | ||
| PHG | 119 | 2.84 | −30 | 2 | −28 | |
| Temporal pole | 2.67 | −38 | 4 | −32 | ||
| Pars orbitalis | 100 | 3.17 | −42 | 28 | −6 | |
| Pars triangularis | 2.43 | −54 | 28 | 2 | ||
| Anterior cingulate | 85 | 3.43 | −6 | 20 | 30 | |
| Anterior cingulate | 3.10 | −2 | 28 | 28 | ||
| Posterior cingulate | 77 | 2.89 | −2 | −56 | 16 | |
| Posterior cingulate | 2.38 | −4 | −54 | 24 | ||
| SMG | 76 | 3.96 | −64 | −36 | 32 | |
| Medial occipital cortex | 73 | 3.75 | −24 | −52 | 4 | |
| Posterior insula | 62 | 3.18 | −38 | −34 | 16 | |
| Lingual gyrus | 62 | 3.09 | −2 | −80 | −10 | |
| Right | Pars orbitalis | 761 | 4.60 | 32 | 32 | −16 |
| Temporal pole | 4.40 | 40 | 16 | −30 | ||
| Temporal pole | 3.64 | 28 | 20 | −28 | ||
| Posterior insula | 209 | 3.66 | 36 | 0 | 16 | |
| Operculum | 2.95 | 44 | −12 | 22 | ||
| Operculum | 2.94 | 50 | −8 | 18 | ||
| Precentral gyrus | 190 | 3.68 | 56 | −6 | 28 | |
| Precentral gyrus | 3.13 | 54 | 2 | 26 | ||
| Precentral gyrus | 2.94 | 44 | −6 | 36 | ||
| Medial occipital cortex | 171 | 4.54 | 30 | −50 | 4 | |
| Medial occipital cortex | 3.59 | 32 | −42 | 0 | ||
| Medial frontal cortex | 140 | 3.43 | 12 | 52 | 12 | |
| Medial frontal cortex | 3.33 | 4 | 50 | 10 | ||
| Cingulate gyrus | 133 | 3.72 | 14 | −2 | 44 | |
| Cingulate gyrus | 3.18 | 14 | 14 | 38 | ||
| Cingulate gyrus | 2.47 | 8 | 2 | 34 | ||
| Anterior cingulate | 127 | 3.12 | 12 | 40 | 26 | |
| Superior frontal gyrus | 2.57 | 14 | 38 | 40 | ||
| Posterior cingulate | 124 | 3.05 | 14 | −36 | 0 | |
| Thalamus | 2.81 | 18 | −28 | −2 | ||
| Hippocampus | 114 | 3.69 | 20 | −6 | −18 | |
| Hippocampus | 2.91 | 32 | −20 | −16 | ||
| PHG | 2.77 | 28 | −16 | −24 | ||
| Postcentral gyrus | 94 | 4.34 | 30 | −26 | 48 | |
| Postcentral gyrus | 2.67 | 32 | −30 | 60 | ||
| Orbitofrontal cortex | 92 | 3.37 | 2 | 32 | −28 | |
| Orbitofrontal cortex | 2.47 | 2 | 48 | −28 | ||
| STG | 76 | 3.50 | 66 | −6 | 2 | |
| Superior parietal lobe | 61 | 4.04 | 34 | −42 | 62 | |
| Mid-MTG | 59 | 3.24 | 66 | −6 | −16 | |
| Anterior MTG | 2.48 | 62 | 6 | −22 | ||
ITG, inferior temporal gyrus; MTG, middle temporal gyrus; PHG, parahippocampal gyrus; pMTG, posterior middle temporal gyrus; pSTS, posterior superior temporal sulcus; SMG, supramarginal gyrus; STG, superior temporal gyrus.
Effects of Word-Level Factors.
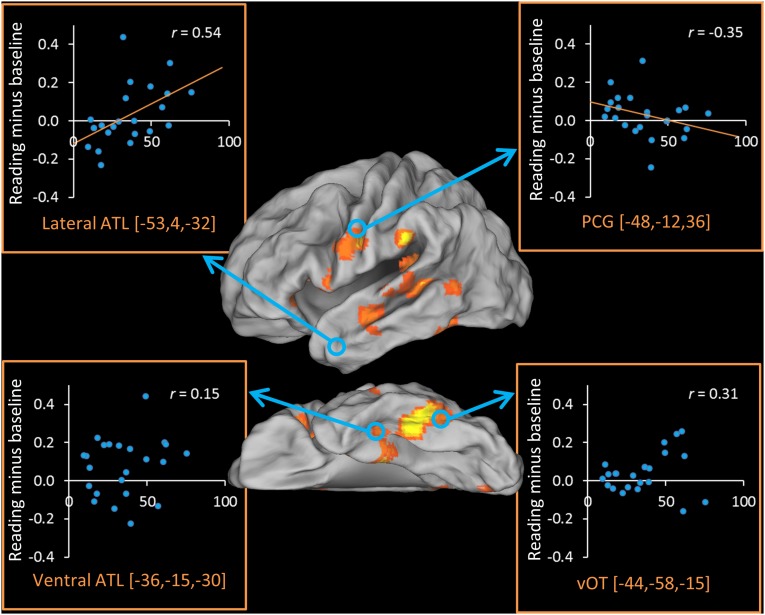
We next examined sensitivity to word-level factors in four a priori regions of interest (ROIs) selected based on their involvement in orthographic, phonological, and semantic processes (Fig. 1). Effects of frequency and regularity in each region were assessed with a series of 2 × 2 ANOVAs (Table 2). The ventral occipitotemporal cortex (vOT) responded more strongly to low-frequency (LF) than to high-frequency (HF) words and to exception words than to regular words. In the precentral gyrus (PCG), activation was greater for regular words than for exception words. In the lateral ATL, the opposite pattern was observed, although this difference fell short of statistical significance (P = 0.1) for the group as a whole. [The nonsignificance of this result stems from the fact that, as described later, participants with low SR did not activate this area at all and therefore were unlikely to show an effect of word type. When the four participants with the least SR were excluded from the analysis, the effect of regularity became significant in this region (P = 0.03).] The ventral ATL (vATL) displayed a different pattern, activating to an equal extent for regular and exception words but displaying greater activation for LF than for HF words. Further analysis confirmed that the two ATL regions displayed divergent profiles: When the two sites were compared directly in 2 × 2 × 2 ANOVA, there was a significant interaction between frequency and site [F(1,25) = 11.9, P = 0.002] and a marginal interaction between regularity and site [F(1,25) = 3.78, P = 0.06]. This result suggests a dissociation in function between these different ATL subregions, which we consider further in Discussion.
Table 2.
Effects of frequency and regularity in ROIs
| ROI | Effect of frequency | Effect of regularity |
| vOT | F(1,25) = 13.0, P = 0.001 | F(1,25) = 2.77, P = 0.11 |
| PCG | F(1,25) = 0.14, P = 0.71 | F(1,25) = 12.4, P = 0.002 |
| vATL | F(1,25) = 13.6, P = 0.001 | F(1,25) = 0.02, P = 0.91 |
| Lateral ATL | F(1,25) = 0.16, P = 0.69 | F(1,25) = 2.88, P = 0.10 |
Significant effects are shown in bold. No regions displayed a significant frequency × regularity interaction.
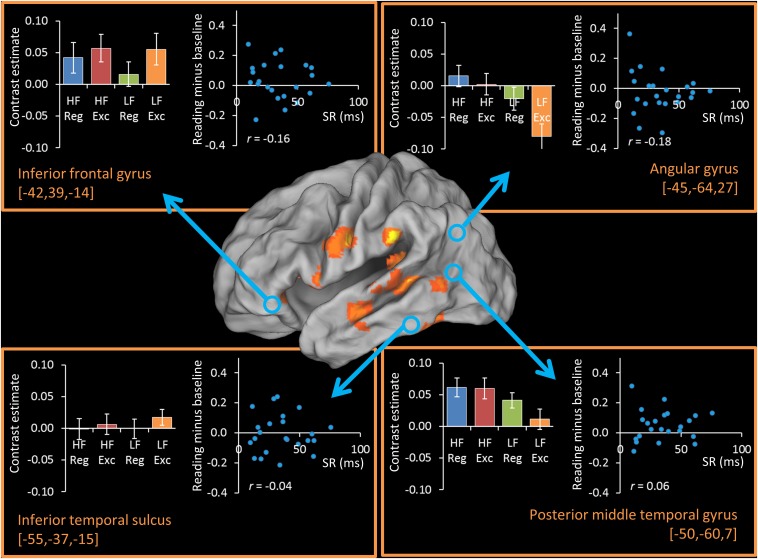
We also examined four additional regions linked to semantic processing outside the ATL: the inferior frontal gyrus, angular gyrus, posterior middle temporal gyrus, and inferior temporal sulcus. Results from these ROI analyses are presented in Fig. S2. None of these regions showed a significant effect of spelling–sound regularity (Table S2).
Fig. S2.
Activations and correlations with SR in additional semantic ROIs. The activation map indicates areas activated for contrast of all word types over baseline, for 26 participants, thresholded at a cluster-corrected level of P < 0.05.
Table S2.
Effects of frequency and regularity in additional semantic ROIs
| ROI | Effect of frequency | Effect of regularity |
| Inferior frontal gyrus | F(1,25) = 0.33, P = 0.57 | F(1,25) = 1.14, P = 0.30 |
| Angular gyrus | F(1,25) = 8.09, P = 0.009 | F(1,25) = 3.71, P = 0.07 |
| Posterior middle temporal gyrus | F(1,25) = 3.57, P = 0.07 | F(1,25) = 1.10, P = 0.31 |
| Inferior temporal sulcus | F(1,25) = 0.13, P = 0.72 | F(1,25) = 1.05, P = 0.32 |
Significant effects are shown in bold. No regions displayed a significant frequency × regularity interaction.
Dynamic Causal Modeling.
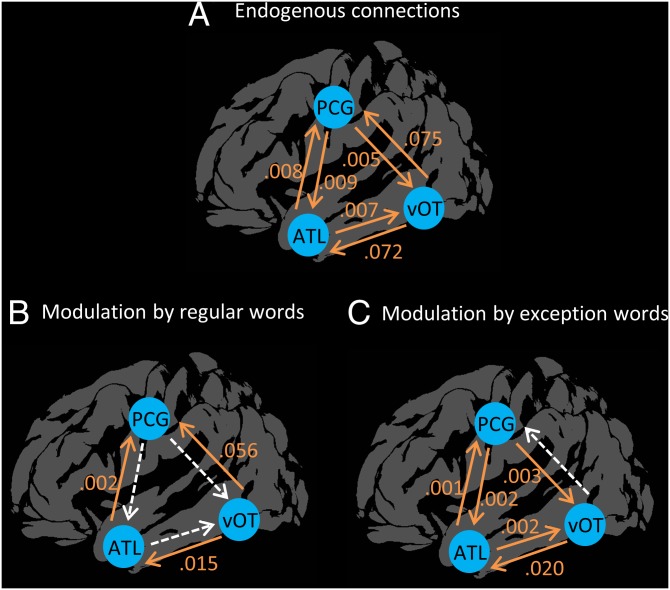
We next explored functional interactions between orthographic, semantic, and phonological regions. The Triangle Model predicts greater direct interaction between orthographic and phonological areas for regular words and greater functional connectivity via the semantic pathway for exception words. These predictions were tested in dynamic causal modeling (DCM) that modeled connections between the vOT, PCG, and lateral ATL. Endogenous connections between these regions, in the absence of experimental manipulation, were all positive (Fig. 2A). Importantly, we assessed changes in functional connectivity when participants read regular and exception words. Modulation of the functional connectivity in response to each word type is presented in Fig. 2. When participants read regular words, there was a significant increase in functional connection strength from the vOT to the ATL and PCG and from the ATL to the PCG. The pattern of connectivity was different during exception word reading. vOT→ATL and ATL→PCG connections again were significantly modulated, and additional feedback connections to the ATL and vOT were observed. Crucially, there was no modulation of the direct vOT→PCG functional connection when participants read exception words. The modulation of the vOT→PCG parameter was compared directly between word types using a paired-samples t test, which confirmed that the functional connection was modulated more strongly by regular words than by exception words [t (25) = 3.16, P = 0.004]. This functional connectivity analysis therefore supports the Triangle Model’s prediction that direct interaction between orthographic and phonological processing regions is strongest when participants read regular words.
Fig. 2.
Functional interactions between regions and their modulation by regular and exception word, as revealed by DCM. (A) Endogenous connections between regions in the absence of stimuli. (B and C) Changes in functional connectivity during word reading. Positive values indicate increases in connectivity upon stimulus presentation. Statistically significant modulations are shown in orange.
Effect of Subject-Level Variation in SR.
Twenty-four participants completed a behavioral test outside the scanner that provided an index of the degree to which they rely on semantic knowledge to read exception words (based on the difference in reading performance for reading low-imageability words with consistent vs. inconsistent spelling–sound mappings). Fig. 3 shows the correlation across participants between the SR index and contrast estimates in each of our ROIs. We found a strong positive correlation between activation in the lateral ATL and SR (P = 0.01), supporting the idea that this area supports semantically mediated reading. In fact, participants with the lowest SR did not activate this area at all. Activation in vATL was not correlated with SR. There also was a weak negative correlation between SR and activation in the PCG (one-tailed P = 0.05). Individuals with high SR activated this area to a lesser extent, indicating that the greater involvement of semantic processing regions was offset by reduced activation in phonological regions.
Fig. 3.
Correlations between reading activation and semantic reliance in each ROI. Reading activation in each ROI is plotted against the behavioral measure of SR. Data were available for 24 participants.
We also examined correlations in our additional regions associated with semantic processing outside the ATL. As shown in Fig. S2, activity in these regions was not correlated with SR.
Whole-Brain Triangulation of the Semantic Contribution.
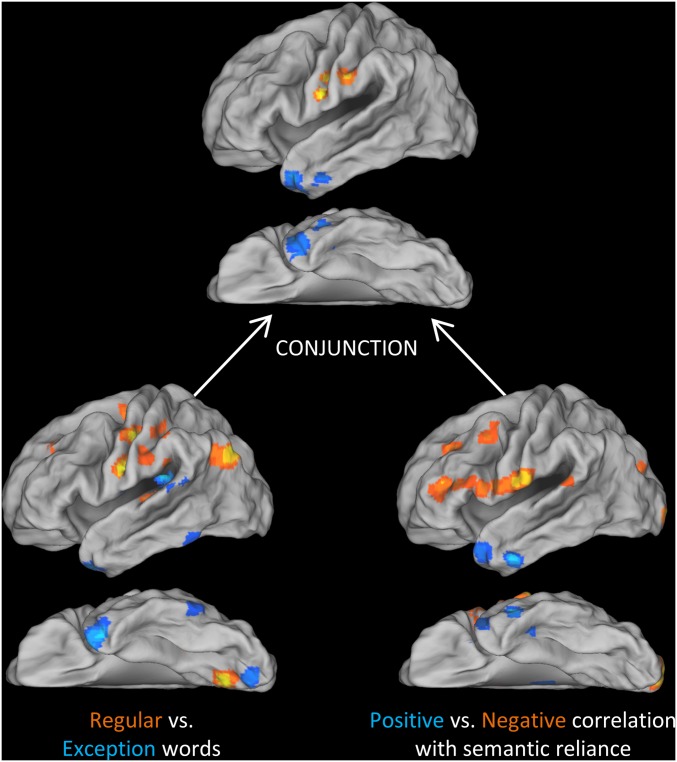
Finally, we performed whole-brain conjunction analyses to test whether any regions outside our ROIs showed the combination of an exception > regular effect and a positive correlation between reading activation and SR, as identified in the lateral ATL ROI. We also tested for the reverse conjunction. The results of the individual tests and their conjunctions are shown in Fig. 4. Peak coordinates for the conjunctions are reported in Table 3 (coordinates for individual contrasts are given in Tables S3 and S4). The semantic conjunction identified two clusters in the lateral ATL, with no effects elsewhere in the brain. The reverse conjunction identified two clusters in the PCG, the larger of which extended into postcentral gyrus. Again, no effects were found elsewhere. This triangulation analysis supports our earlier ROI-based approach in two ways. First, it indicates that the a priori ROIs for semantic and phonological regions were close to the key centers of activity in our data. Second, it confirms that the conjunction of word- and subject-level effects was specific to these regions.
Fig. 4.
Whole-brain conjunction analysis of word-level and subject-level effects.
Table 3.
MNI coordinates for conjunction analyses of regularity and SR
| Analysis | Location | Extent in voxels | Z | MNI coordinates | ||
| x | y | z | ||||
| Exc > reg ∩ positive semantic reliance | Anterior MTG and temporal pole | 96 | 2.21 | −48 | 14 | −34 |
| 2.16 | −40 | 10 | −42 | |||
| 1.80 | −34 | 18 | −42 | |||
| Anterior ITG | 52 | 2.16 | −52 | −6 | −32 | |
| 1.71 | −42 | −8 | −32 | |||
| Reg > exc ∩ negative semantic reliance | Precentral and postcentral gyri | 95 | 2.54 | −50 | −6 | 32 |
| 2.28 | −60 | −22 | 34 | |||
| Precentral gyrus | 70 | 2.66 | −58 | −6 | 20 | |
| 2.12 | −50 | −2 | 20 | |||
Exc, exception; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; Reg, regular.
Table S3.
MNI peak coordinates for contrast of regular vs. exception words
| Hemisphere | Location | Extent in voxels | Z | MNI coordinates | ||
| x | y | z | ||||
| Regular > exception | ||||||
| Left | Postcentral gyrus | 408 | 4.37 | −46 | −22 | 48 |
| Intraparietal sulcus | 3.31 | −54 | −34 | 50 | ||
| Precentral gyrus | 3.10 | −36 | −14 | 50 | ||
| Posterior angular gyrus | 313 | 3.79 | −38 | −78 | 38 | |
| Angular gyrus | 3.15 | −44 | −64 | 38 | ||
| Precentral gyrus | 274 | 4.03 | −54 | −6 | 22 | |
| Heschl's gyrus | 127 | 3.99 | −48 | −18 | 4 | |
| Heschl's gyrus | 3.09 | −38 | −26 | 2 | ||
| STG | 2.99 | −52 | −30 | 8 | ||
| Dorsal precentral gyrus | 125 | 3.14 | −22 | −12 | 62 | |
| Dorsal precentral gyrus | 3.00 | −20 | −20 | 62 | ||
| SMG | 120 | 2.93 | −60 | −40 | 28 | |
| SMG | 2.82 | −60 | −24 | 32 | ||
| Superior frontal gyrus | 89 | 3.46 | −18 | 28 | 40 | |
| Lingual gyrus | 70 | 3.08 | −30 | −50 | −2 | |
| Lingual gyrus | 2.63 | −34 | −60 | −6 | ||
| Right | Precuneus | 1863 | 3.74 | 12 | −54 | 58 |
| Angular gyrus | 3.66 | 52 | −70 | 32 | ||
| Precuneus | 3.65 | 0 | −64 | 48 | ||
| Precentral gyrus | 175 | 3.64 | 58 | −4 | 18 | |
| Postcentral gyrus | 3.28 | 56 | −10 | 24 | ||
| SMG | 2.72 | 62 | −16 | 26 | ||
| SMG | 122 | 3.34 | 64 | −38 | 38 | |
| SMG | 2.74 | 60 | −32 | 30 | ||
| Temporoparietal junction | 2.56 | 54 | −32 | 22 | ||
| Supplementary motor area | 93 | 3.32 | 6 | −20 | 64 | |
| Insula | 88 | 3.03 | 34 | 4 | 4 | |
| Putamen | 2.71 | 28 | −4 | 6 | ||
| Posterior insula | 2.65 | 38 | −12 | 8 | ||
| Middle frontal gyrus | 75 | 3.64 | 34 | 54 | 8 | |
| Anterior insula | 68 | 3.09 | 32 | 6 | −12 | |
| Insula | 2.82 | 38 | −8 | −10 | ||
| Bilateral | Lingual gyrus | 419 | 4.16 | −10 | −72 | −2 |
| Lingual gyrus | 3.65 | 4 | −70 | −4 | ||
| Lingual gyrus | 3.40 | −22 | −76 | −2 | ||
| Exception > regular | ||||||
| Left | Temporal pole | 200 | 4.07 | −38 | 12 | −42 |
| Temporal pole | 3.76 | −28 | 18 | −38 | ||
| Anterior ITG | 2.76 | −48 | 8 | −46 | ||
| Operculum | 120 | 4.07 | −38 | −34 | 22 | |
| Posterior ITG | 102 | 2.96 | −52 | −62 | −24 | |
| Posterior ITG | 2.76 | −54 | −50 | −22 | ||
| Operculum | 89 | 3.26 | −48 | −4 | 10 | |
| Insula | 2.89 | −42 | −2 | 16 | ||
| Pars opercularis | 2.59 | −48 | 10 | 6 | ||
| Posterior STG | 65 | 2.89 | −68 | −40 | 14 | |
| Posterior STG | 2.76 | −64 | −50 | 16 | ||
| Right | Pars orbitalis | 469 | 4.06 | 40 | 40 | −16 |
| Pars orbitalis | 3.69 | 28 | 28 | −12 | ||
| Pars orbitalis | 3.52 | 28 | 48 | −8 | ||
| Medial fusiform | 165 | 3.55 | 22 | −2 | −44 | |
| PHG | 3.04 | 24 | −18 | −42 | ||
| Pars opercularis | 122 | 3.54 | 44 | 18 | 14 | |
| Posterior fusiform | 108 | 3.14 | 38 | −68 | −22 | |
| Posterior fusiform | 2.97 | 26 | −74 | −22 | ||
| Calcarine cortex | 107 | 4.32 | 12 | −72 | 16 | |
| Caudate | 105 | 3.51 | 14 | 14 | 20 | |
| Caudate | 3.48 | 18 | 2 | 24 | ||
| Operculum | 98 | 3.71 | 34 | −18 | 20 | |
| Cingulate gyrus | 79 | 2.98 | 10 | −16 | 50 | |
| Cingulate gyrus | 2.94 | 16 | −20 | 46 | ||
| Middle occipital gyrus | 61 | 2.99 | 26 | −80 | 16 | |
| Bilateral | Calcarine cortex | 306 | 3.58 | 0 | −82 | −16 |
| Cerebellum | 3.48 | −12 | −90 | −28 | ||
| Cerebellum | 3.15 | 12 | −82 | −24 | ||
| Cingulate gyrus | 227 | 3.45 | 8 | 6 | 46 | |
| Cingulate gyrus | 2.88 | 12 | −2 | 42 | ||
| Cingulate gyrus | 2.60 | −8 | 6 | 46 | ||
| Cingulate gyrus | 116 | 3.19 | 6 | −22 | 28 | |
| Cingulate gyrus | 2.99 | 2 | 0 | 26 | ||
| Cingulate gyrus | 2.97 | −2 | −12 | 26 | ||
ITG, inferior temporal gyrus; PHG, parahippocampal gyrus; SMG, supramarginal gyrus; STG, superior temporal gyrus.
Table S4.
MNI peak coordinates for correlation with SR
| Hemisphere | Location | Extent in voxels | Z | MNI coordinates | ||
| x | y | z | ||||
| Positive correlation with semantic reliance | ||||||
| Left | Anterior ITG | 133 | 3.78 | −50 | −4 | −34 |
| Anterior ITG | 2.90 | −42 | −12 | −28 | ||
| Lingual gyrus | 121 | 4.22 | −22 | −66 | 4 | |
| Anterior MTG | 105 | 3.04 | −48 | 16 | −34 | |
| Temporal pole | 2.49 | −36 | 22 | −28 | ||
| Thalamus | 95 | 3.06 | −12 | −26 | 14 | |
| Posterior cingulate | 68 | 4.11 | −14 | −48 | 30 | |
| Posterior cingulate | 2.50 | −14 | −40 | 36 | ||
| Right | Precuneus | 227 | 3.90 | 12 | −60 | 58 |
| Superior parietal | 3.40 | 30 | −54 | 58 | ||
| Superior parietal | 3.40 | 28 | −34 | 58 | ||
| Negative correlation with semantic reliance | ||||||
| Left | Postcentral gyrus | 400 | 3.44 | −66 | −12 | 18 |
| Pars opercularis | 3.29 | −58 | 12 | 12 | ||
| Precentral gyrus | 3.27 | −62 | −6 | 14 | ||
| Striate cortex | 170 | 3.64 | −8 | −104 | −6 | |
| Striate cortex | 3.53 | −18 | −104 | −6 | ||
| Pars triangularis | 146 | 3.31 | −38 | 40 | 12 | |
| Pars triangularis | 2.84 | −44 | 44 | 8 | ||
| Pars triangularis | 2.48 | −42 | 38 | 0 | ||
| Temporoparietal junction | 102 | 3.12 | −52 | −40 | 18 | |
| MFG | 96 | 3.08 | −30 | 32 | 36 | |
| Middle occipital | 64 | 4.07 | −32 | −90 | 30 | |
| Anterior cingulate | 64 | 2.88 | −10 | 44 | 0 | |
| MFG | 59 | 2.90 | −44 | 12 | 46 | |
| MFG | 2.61 | −36 | 6 | 54 | ||
| Right | MFG | 88 | 3.06 | 24 | 40 | 34 |
| Bilateral | Striatum | 107 | 2.86 | −2 | 2 | −2 |
| Striatum | 2.79 | 8 | −2 | −2 | ||
ITG, inferior temporal gyrus; MFG, middle frontal gyrus.
Discussion
Reading is a critical skill that has been studied from both cognitive and neural perspectives. Here, we integrated these two approaches by using fMRI to investigate the neural basis of an implemented computational model of word reading: the Triangle Model (2). We focused particularly on the role of the ATL, a key site for the representation of semantic knowledge, in reading. The potential contribution of this area has been overlooked in the neuroimaging literature; however, the Triangle Model predicts that the semantic representations supported by this area are critically involved in reading, particularly (i) for words with exceptional spelling-to-sound mappings and (ii) in individuals who rely heavily on semantic information to read these words. We identified a site in the lateral ATL that uniquely demonstrated both these characteristics. Furthermore, areas of premotor cortex involved in phonological processing demonstrated the reverse pattern, indicating a division of labor between direct and semantically mediated pathways for converting written words to speech sounds. These findings necessitate a reconsideration of the neural network for reading aloud and indicate that individual differences in reading style can have major and theoretically meaningful effects on the configuration of this network.
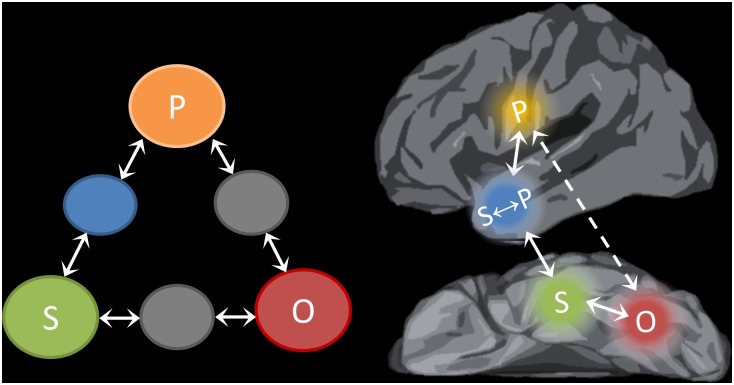
Fig. 5 illustrates how elements of the Triangle Model of reading aloud might map onto the neural regions investigated in this study. The network outlined here is broadly consistent with the established notion of distinct dorsal and ventral pathways for reading (5, 8, 43, 44). The vOT cortex is strongly implicated in the visual analysis of words and letters (8, 16) and therefore is linked to the orthographic processing units of the Triangle Model. This region displayed less activation for higher-frequency words, as is consistent with reduced processing demands for words with highly familiar orthographic forms. In addition (and consistent with the DCM results), some researchers have suggested that this region summates feedback from semantic and phonological regions, and this function is why its activation mirrors the behavioral RT pattern (45). PCG is critically involved in phonological processing (24) and has been linked specifically with articulatory-motor phonological codes required for speech production (25, 46). This area therefore maps to the phonological component of the Triangle Model. According to the model, the “direct” pathway between these regions is sensitive to statistical regularities in the mapping between spelling and sound and is well-suited to reading words that conform to these patterns. In line with this view, our DCM analysis revealed strong functional connectivity between the vOT and PCG when participants read words with regular spelling-to-sound mappings but not when they read exception words. However, this functional connection does not rule out the involvement of other regions; in particular, regions of the inferior parietal cortex also may be involved in the direct mapping from orthography to phonology (5, 35, 36). Like the PCG, these regions exhibited greater activation for regular words.
Fig. 5.
Schematic of the Triangle Model (Left) and its possible mapping onto the neural regions investigated in this study (Right). Highlighted neural regions are those investigated in the present study. Other regions also may contribute to reading aloud. The O→P pathway is shown as a dashed line because the exact white-matter connections that support this functional connection are currently unknown. O, orthography; P, phonology; S, semantics.
Our main focus was on the potential role of the ATL in semantically mediated reading. We identified two distinct ATL regions that have not hitherto been considered parts of the reading network. These regions had distinct activation profiles, consistent with different elements of the Triangle Model. First, we found robust activation of the vATL during reading of both regular and exception words. Activations for written words are commonly found in the mid-fusiform gyrus (typically between y = −35 and y = −50) (36, 43). Although we did observe activation in this area, the vATL activation we refer to here was more anterior still, at y = −18. This region is severely affected by signal dropout in standard fMRI, perhaps explaining why most previous studies have not observed activation here. However, PET studies and recent distortion-corrected fMRI studies indicate that this is a key site for the representation of conceptual knowledge (30, 31). Because the great majority of experience with written words involves reading for meaning (3), it is likely that this core semantic knowledge is activated automatically whenever written words are processed (36).
The vATL displayed uniform activation for regular and exception words, and its activation was not predicted by the individual’s level of SR in reading, supporting the idea that its activation reflects automatic retrieval of word meaning, irrespective of whether this information plays a functional role in access to phonology. We therefore have linked the vATL with the semantic representations in the Triangle Model. These representations develop as the ATL learns to map between the various sources of information (e.g., visual, auditory, verbal) that contribute to understanding of a concept (4, 47). As a consequence, they are able to capture supramodal semantic structure that extends beyond a single modality (48).
The second ATL region lay on the lateral surface of the temporal lobe. Wilson et al. (49) recently found that this region was activated when participants read exception words but not when they read pseudowords. The present study reveals that it responds more strongly to exception words than to regular words and is activated more strongly by individuals with high SR. The considerable between-subject variability in this area may explain why lateral ATL activation has not been observed in most previous studies of reading aloud. The activation profile of this region suggests that it plays an intermediary role in mapping between word meaning and speech sounds (50). According to the Triangle Model, semantic knowledge makes a critical contribution to the reading of exception words, whose irregular pronunciations are poorly served by the direct O→P pathway. As a consequence, although semantic representations in the vATL are activated whenever written words are processed, the interaction between these and phonological regions, mediated by the lateral ATL, is increased for exception words. In addition, the intermediary function of the lateral ATL is up-regulated in individuals who rely heavily on semantic activation to support exception word reading.
The structural connectivity of the lateral ATL is well-suited to an intermediary role between semantic and phonological representation. In addition to reciprocal connections with the vATL (51), the lateral ATL is connected directly to prefrontal and premotor cortices via the extreme capsule complex and with phonological processing areas in the posterior superior temporal gyrus via the middle longitudinal fasciculus (51, 52). Neuropsychological studies and neuroanatomically constrained computational models also implicate the lateral ATL in semantically mediated speech production (50, 53). This role is consistent with the view that (i) as a result of the convergence of multiple white-matter tracts and connections, the ATL region as a whole acts as a representational system and (ii) there are graded specializations in different parts of this system, as a consequence of systematic variations in structural connectivity across the region (54, 55).
These findings have important implications for models of the neural basis of reading. Functional neuroimaging studies have long distinguished between a dorsal reading pathway, typically assumed to include the posterior vOT, supramarginal gyrus, PCG, and posterior inferior prefrontal cortex, and a ventral reading pathway, comprising the anterior portion of vOT and the ventral and anterior parts of inferior prefrontal cortex (5, 8, 36, 43, 44). The present results indicate that the ATL contains important and hitherto unrecognized elements of the ventral pathway and also begin to reconcile the neuroimaging literature on reading aloud with the striking deficits in exception word reading observed in patients with SD (6, 35). The ventrolateral ATL region is the centerpoint of atrophy in SD (27), suggesting that this region is implicated in exception word reading. However, the lack of corroboratory evidence from neuroimaging studies has led to speculation that more subtle damage to posterior temporal cortex is the cause of these patients’ reading deficits (7, 37). In contrast to these claims, the lateral ATL region we have identified falls squarely within the typical region of atrophy in SD, providing converging evidence that this anterior region plays an important role in computing the pronunciations of exception words.
A second major contribution is the finding that individual differences in SR predicted the degree to which individuals activated both semantic and phonological regions when reading aloud. A growing literature indicates substantial variability in the functional architecture of the reading network across individuals (56, 57). This conclusion is perhaps not surprising, given that reading is a relatively late-acquired skill and individuals vary greatly in their level of reading ability, in their exposure to print (58), and in the nature of the reading instruction they receive. A number of studies have reported structural and functional correlations with reading skill (59–62). For example, Welcome and Joanisse (59) asked participants to make orthographic, phonological, and semantic judgments to pairs of written words. In line with our findings, the left PCG was selectively activated by phonological judgments. The semantic task activated the posterior temporal, inferior parietal, and prefrontal regions. No ATL activation was observed, perhaps reflecting the technical challenges of measuring activity in this region (see the Introduction). These authors also investigated how reading-related activation (collapsed over orthographic, phonological, and semantic judgments) was predicted by measures of reading ability, revealing a complex pattern of increases and decreases in activity as a function of reading skill.
Our approach differs from these in that the SR measure used here is not an index of general reading skill; rather, it is a measure of the degree to which semantic knowledge contributes to exception word reading. According to the Triangle Model, this measure indexes the division of labor between the direct and semantic reading routes (6, 11). Readers with high SR are assumed to activate the semantic pathway strongly when reading exception words, whereas those with lower SR are thought to favor the direct pathway. In line with this view, we found that greater SR was associated with both increases and decreases in activation in different parts of the reading network. Readers with high SR tended to activate the lateral ATL more than those with low SR, but the reverse was true for activation in the PCG. Thus, our study indicates a division of labor between the anterior temporal semantic regions and the prefrontal phonological regions that varies across subjects.
Another recent fMRI study suggests that similar divisions of labor exist across other brain areas. Seghier et al. (56) identified two networks whose activation during reading varied across individuals. They found that subjects with slow exception word reading were more likely to activate a network comprising the anterior vOT, left inferior frontal cortex, medial frontal gyrus, and supramarginal gyrus. ATL activation was not observed by Seghier et al. perhaps for the technical reasons described above; however, this area has strong anatomical connections with the inferior prefrontal and occipitotemporal cortex and thus is likely to form part of this ventral network.
Although the present study was concerned with the functional reading network, these functional effects are likely to be underpinned by differences in structural connectivity between reading-related regions. Graves et al. (63) investigated how individual differences in reading style relate to the volumes of key white-matter tracts in the left hemisphere. Their individual difference measure (the size of the imageability effect computed over words varying along a number of other dimensions, including spelling–sound consistency) was used to index semantic involvement in reading aloud. This measure was positively related to the volume of two white-matter tracts, corresponding to the inferior and middle longitudinal fasciculi (ILF and MLF), which course through the temporal lobe and converge in the anterior temporal region (64). Participants showing greater semantic involvement tended to have greater volume in both tracts. The fascinating implication linking cognitive function and white-matter neuroanatomy (hodology) is that these tracts play an important functional role in semantically mediated reading by connecting distinct processing regions within the semantic reading route. Each tract projects to large but primarily nonoverlapping swathes of temporal, parietal, and occipital cortex. The only shared gray-matter terminations of the ILF and MLF are those found at their convergence within the ATL.
The critical next step is to establish which of these connected regions is functionally responsible for the individual differences in semantic reliance. Graves et al. (63) hypothesized that connections between the posterior temporal and inferior parietal sites were critical. Our functional neuroimaging data are consistent with Graves et al.’s hodological findings but support a different interpretation, namely that the ATL, and not the posterior regions, provides the semantic contribution to reading aloud. Our results suggest that the ILF and MLF are implicated in semantically mediated reading because they connect posterior regions with the anterior temporal cortex. This alternative hypothesis is, of course, consistent with the convergent evidence for the importance of the ATL in semantic representation and the fact that patients with SD, with semantic impairment contingent on ATL-centered atrophy, exhibit acquired surface dyslexia.
Finally, we briefly consider why individuals differ in the degree to which they use the semantic vs. direct routes in reading aloud. Dilkina et al. (4) proposed two factors that may influence this difference: the amount of the individual’s reading experience and the relative capacity of the two routes (11). In their connectionist simulations, greater reading experience resulted in the development of a more efficient O→P pathway, so that most words could be read with minimal contribution from semantic knowledge. Similarly, when the capacity of the direct pathway was increased by providing it with more processing units, the contribution of the semantic route was reduced. One possibility, therefore, is that highly semantic readers adopt greater reliance on semantic representations to compensate for poorer orthographic or phonological processing skills. This account is consistent with our individual difference effects, whereby readers with high SR demonstrate greater activation in an ATL semantic site but less activity in the PCG phonological area. Future research will need to adopt a developmental perspective to reveal the origins of these individual variations in the neurocognitive reading system.
Methods
Participants.
Twenty-seven healthy, right-handed participants took part (11 male; mean age, 25y; range, 20–39 y). Data from one participant were discarded because of image artifacts. All participants were native English speakers with no history of neurological or psychiatric disorders and with normal or corrected-to-normal vision. None reported any difficulty with reading. The study was approved by the UK National Research Ethics Service Greater Manchester West research ethics committee and informed consent was obtained from all participants.
In-Scanner Task.
Participants read aloud 180 words taken from the Cambridge surface reading list (65). We selected these stimuli because they have proven highly reliable in identifying surface dyslexia in patients with SD (6). The words in the surface list are divided into four conditions based on an orthogonal manipulation of frequency and regularity. Further details of the stimulus characteristics are provided in SI Details of the In-Scanner Reading Task.
All words were presented individually for reading aloud. Each trial began with a fixation cross, presented in the center of the screen for 500 ms and followed by a single word presented in black text (Arial font) on a white background. Each word was presented for a total of 2,200 ms. Participants were instructed to read the words aloud as quickly and accurately as possible. As a baseline task, participants were presented with strings of Greek letters of between three and five characters in length. They were instructed to say “ok” upon seeing each string.
Stimuli were presented in a blocked design using Eprime software. Each block consisted of five trials (duration, 13.5 s) taken from one of the four word conditions or from the baseline condition. Blocks were presented in a fixed, pseudorandom order. After every five blocks of task, there was 13.5-s rest block during which the screen remained blank.
Verbal responses were captured using an MRI-compatible microphone manufactured by Optoacoustics and were digitally recorded for later analysis. To minimize head movement, participants were asked to speak without moving their jaw (i.e., with teeth together) and practiced this speaking before entering the scanner.
Imaging Acquisition and Preprocessing.
We used an SE imaging sequence combined with a postacquisition distortion correction, which greatly improves signal quality in the ventral temporal lobes (41). Standard preprocessing steps and statistical analyses were performed in SPM8. Full details of the imaging protocol and temporal signal-to-noise maps are provided in SI Image Acquisition and Preprocessing.
Following preprocessing, data were treated with a high-pass filter with a cutoff of 160 s, and images were masked to restrict analysis to cerebral gray-matter voxels and were analyzed using a general linear model. At the individual subject level, each of the five stimulus conditions was modeled with a separate regressor (HF regular, HF exception, LF regular, LF exception, and Greek character baseline). Blocks were modeled with a boxcar function convolved with the canonical hemodynamic response function. Motion parameters were entered into the model as covariates of no interest.
Whole-Brain Analysis of Reading vs. Baseline.
To identify regions making a general contribution to reading across word types, we contrasted all word reading with the baseline task. A voxel-height threshold of P < 0.01 was adopted for all whole-brain analyses. To control for multiple comparisons, a minimum cluster size was determined using a Monte Carlo analysis (66). This modeled the entire smoothed image volume, assumed an individual voxel type-1 error rate of 0.01, and ran 1,000 simulations to determine the minimum cluster size associated with a corrected P < 0.05. The cluster threshold obtained using this method was 57 voxels.
Effects of Word-Level Factors.
We next investigated effects of stimulus type in left-hemisphere ROIs associated with orthographic, phonological, and semantic processes. For each region, we created a sphere with a 10-mm radius centered on coordinates obtained from previous studies. Orthographic and phonological regions were identified using published meta-analyses of reading studies. The vOT is strongly associated with orthographic processing. We used the mean MNI vOT coordinates (−44, −58, −15) from a meta-analysis of 35 reading studies (8). Phonological processes, both in word reading and in language tasks more generally, are strongly associated with the PCG. This region was not included in the Jobard et al. meta-analysis (8), so we selected peak MNI coordinates (−48, −12, 36) from Turkeltaub et al.’s (23) meta-analysis of 11 reading studies.
Semantic processing depends critically on the ATL, but this region has rarely been identified in studies of single-word reading. To investigate this region, we therefore obtained two sets of coordinates from previous neuroimaging and neurostimulation studies of semantic processing. PET and distortion-corrected fMRI studies have consistently identified activation in the vATL for meaningful stimuli across multiple modalities (30, 31, 67). We took coordinates on the border of the anterior fusiform and inferior temporal gyri (MNI coordinates −36, −15, −30) from an fMRI study in which participants made synonym judgments to written words (29). In parallel, transcranial magnetic stimulation has been used to probe the functions of the lateral ATL (68, 69). To investigate this area, we selected coordinates in the anterior middle temporal gyrus (MNI coordinates −53, 4, −32) that were the stimulation target in the aforementioned studies.
Our main focus in this study was on the ATL, because evidence from patients with SD suggests that this region supports the semantic contribution to reading aloud. In a secondary analysis, however, we investigated four other regions associated with semantic processing: the inferior frontal gyrus, angular gyrus, posterior middle temporal gyrus, and inferior temporal sulcus. Full details of these sites are provided in SI Additional Semantic ROIs Outside the ATL.
In each ROI, the Marsbar toolbox (70) was used to obtain contrast estimates for each participant for each of the reading conditions relative to baseline. Condition effects within and between ROIs then were assessed using ANOVA.
DCM.
DCM is a method for assessing the neuronal interactions between specified brain regions and the modulation of these interactions under different experimental conditions (71). We used this technique to assess interactions between neural regions associated with orthographic, semantic, and phonological processes, as defined above. We selected the lateral ATL site as our semantic region because this site showed the expected pattern of greater activation for exception words than for regular words. For each participant, we estimated parameters for a single DCM model with the following properties:
-
i)
Orthographic (vOT), semantic (ATL), and phonological (PCG) regions were assumed to have bidirectional endogenous (fixed) connections with one another.
-
ii)
The strength of each of these endogenous connections was allowed to vary upon presentation of regular and exception words. This design yielded two modulation parameters for each connection: its change in strength (relative to rest) when regular words were presented and its change when exception words were presented.
-
iii)
External input to the model was applied at the vOT region, because this region is strongly connected with the primary visual cortex. Input corresponded to presentation of words or baseline character strings, because both elicited activation in vOT.
Further details of the DCM are provided in SI Further Details of DCM Analyses.
Effect of Subject-Level Variation in SR.
To investigate individual differences in SR, on a separate day to the imaging 24 of the 26 participants completed a short word reading test (see SI Outside-Scanner Test of SR During Reading and Table S5 for full details). The measure of SR was based on the difference in reading performance for reading low-imageability words with consistent vs. inconsistent spelling–sound mappings. Low-imageability words have intrinsically weak semantic representations (72), so individuals who rely heavily on semantic activation in reading find it difficult to read these words, particularly when they have exceptional pronunciations that are not well served by the direct O→P pathway. In contrast, people who rely on the O→P pathway to read all words show small effects of consistency. In other words, the size of the consistency effect is taken as a measure of the degree to which an individual relies on semantic activation to read words with atypical mappings. This conclusion is in line with connectionist triangle models, which show increasing consistency effects as the division of labor in reading is shifted toward the semantic (O→S→P) pathway and away from the direct (O→P) pathway (4, 11). We assessed whether the SR measure was correlated with activation (for reading all words vs. baseline) in our four a priori ROIs and included this variable in a whole-brain analysis as detailed below.
Table S5.
Psycholinguistic properties of words in the SR test
| Property | Regular | Exception | ||
| High imageability | Low imageability | High imageability | Low imageability | |
| Imageability | 552.55 (47.23) | 381.60 (60.19) | 552.58 (53.68) | 380.6 (46.44) |
| Kucera–Francis frequency | 80.63 (143.47) | 87.20 (155.85) | 82.55 (153.35) | 85.4 (116.07) |
| Letter length | 4.35 (0.74) | 4.60 (0.59) | 4.43 (0.84) | 4.53 (0.78) |
| Phonemic length | 3.30 (0.52) | 3.45 (0.64) | 3.30 (0.72) | 3.28 (0.68) |
| Body neighbors | 11.90 (6.53) | 12.08 (6.56) | 10.38 (6.52) | 13.08 (8.56) |
| No. of friends | 11.90 (6.53) | 12.08 (6.56) | 3.28 (3.08) | 4.5 (4.62) |
| Summed friend frequency | 677.80 (1331.97) | 659.28 (837.87) | 325.70 (465.15) | 535.95 (818.56) |
| No. of enemies | 0.00 (0.00) | 0.00 (0.00) | 7.10 (4.47) | 8.58 (5.05) |
| Summed enemy frequency | 0.00 (0.00) | 0.00 (0.00) | 1,356.40 (3347.08) | 1,059.28 (1288.23) |
| Type consistency ratio | 1.00 (0.00) | 1.00 (0.00) | 0.32 (0.15) | 0.33 (0.15) |
| Token consistency ratio | 1.00 (0.00) | 1.00 (0.00) | 0.39 (0.30) | 0.37 (0.31) |
Triangulation of the Semantic Contribution in the Whole Brain.
Connectionist models predict that regions involved in the semantic reading pathway should be influenced both by the spelling-to-sound regularity of the word and by the reader’s degree of SR. We therefore used a triangulation approach, seeking voxels that displayed a conjunction of these effects. A contrast of reading vs. baseline was computed for each participant, and the resulting maps were entered into a second-level model that included the SR measure as a between-subjects covariate. This method allowed us to identify voxels in which reading activation was correlated with SR. To ensure that reading speed could not influence these results, we included each participant’s mean in-scanner reading RT as a second covariate in the model. In a separate model, we contrasted activation elicited by regular vs. exception words (with no covariates).
A conjunction analysis was performed to identify voxels that were more active for exception words than for regular words and whose activity was positively correlated with SR across subjects. We performed a second conjunction to test for the reverse pattern. Each conjunction was assessed against the conjunction null hypothesis (73) to identify voxels that were significantly active in both contrasts. Because the conjunction test is inherently conservative (74), a voxel threshold of P < 0.05 was adopted for these analyses, with a minimum cluster size of 50 voxels.
SI Details of the In-Scanner Reading Task
Participants read aloud 180 words taken from the Cambridge surface reading list (65). HF words had a mean frequency of 824 counts per million (SD = 1,283) in the CELEX database (75), whereas LF words had a mean frequency of 7.2 (SD = 8). Spelling-to-sound regularity was assessed by calculating the feed-forward consistency for each word (76). Regular words had a mean consistency of 0.89 (SD = 0.22), and exception words had a mean consistency of 0.52 (SD = 0.32). All words were monosyllabic and were between two and six letters in length.
HF and LF words also differed in their semantic characteristics. HF words were significantly less imageable than LF words (356 vs. 448; t = 4.2, P < 0.001), meaning that they were less likely to elicit strong semantic activations (because low-imageability words have weaker semantic representations; see refs. 3 and 72). Furthermore, the HF set included a number of pronouns (e.g., “your”), conjunctions (“while”), number words (“nine”), adverbs (“much”), auxiliary verbs (“did”), and light verbs (“goes”). These words, which comprised 38% of the HF set, convey little meaning when presented in isolation and so would not be expected to engage the semantic system strongly. In contrast, the LF words were all content-bearing nouns, verbs, and adjectives.
SI Image Acquisition and Preprocessing
Images were acquired on a 3-T Philips Achieva scanner using an eight-element SENSE head coil with a sense factor of 2.5. The SE echo planar imaging (EPI) sequence included 31 slices covering the whole brain with echo time (TE) = 70 ms, time to repetition (TR) = 3,200 ms, flip angle = 90°, 96 × 96 matrix, reconstructed in-plane resolution 2.5 × 2.5 mm, and slice thickness 4.0 mm. In total, 224 images were acquired in a single run of 12 min. Following the standard method for distortion-corrected SE fMRI (41), the images were acquired with a single-direction k space traversal and a left–right phase encoding direction. In addition, a brief pre-scan was acquired, consisting of 10 volumes of dual-direction k space traversal SE EPI scans. This method gave 10 pairs of images matching the functional time series but with distortions in both phase-encoding directions (10 left–right and 10 right–left). These scans were used in the distortion correction procedure. In addition, a high-resolution T1-weighted 3D turbo field echo inversion recovery image was acquired (TR = 8,400 ms, TE =3.9 ms, flip angle 8°, 256 × 205 matrix reconstructed to 256 × 256, reconstructed resolution 0.938 × 0.938 mm, slice thickness of 0.9 mm, SENSE factor = 2.5) with 160 slices covering the whole brain.
The spatial remapping correction was computed using the method reported by Embleton et al. (41). In the first step, each image from the main functional time-series was registered to the mean of the prescan images using a six-parameter rigid-body transformation in SPM8. Subsequently, a spatial transformation matrix was calculated from the prescan images consisting of the spatial remapping necessary to correct the distortion. This transformation then was applied to each of the 224 coregistered functional images.
Next, the motion and distortion-corrected images for each participant first were coregistered to their T1 structural scan. Spatial normalization of the T1 scans into MNI space was computed using DARTEL (77), and the resulting transformation was applied to the functional images, which were resampled to a voxel size of 2 × 2 × 2 mm and smoothed with an 8-mm FWHM Gaussian kernel. At this point, temporal signal-to-noise (TSNR) maps were generated for each participant by dividing the mean signal in each voxel by its SD (78). The mean TSNR map across all participants is shown in Fig. S1. The TSNR exceeded 60 in ventral temporal regions. Unlike gradient-echo fMRI, which shows a pronounced drop in TSNR in ventral temporal regions relative to the rest of the brain, the TSNR in the ventral temporal lobes was comparable to that in frontal and superior temporal regions.
SI Additional Semantic ROIs Outside the ATL
As detailed in the main text, we hypothesized that the ATL plays a critical in semantically mediated reading. However, to test whether semantic effects were specific to this region, we conducted secondary ROI analyses on other areas linked with semantic processing. These areas were as follows:
-
i)
Inferior frontal gyrus. This area is associated with controlled semantic processing (79) and also is implicated in the ventral reading route (5). Coordinates for this region were taken from a recent meta-analysis of controlled semantic processing (80) and corresponded to the most anterior and ventral portion of this region (Brodmann area 47).
-
ii)
Angular gyrus. This area is thought by some to support semantic representation (81), although other functions also have been proposed (82). Graves et al. (63) have proposed that this region provides semantic constraints on the phonological system during reading, particularly of connected text. Coordinates for this region were taken from Boukrina and Graves (62), who used the same ROIs in their study as Graves et al. (63).
-
iii)
Posterior middle temporal gyrus. This area is frequently activated in studies of semantic processing and also has been linked to controlled semantic processing (79). However, Graves et al. (63) have suggested that its principal role in reading is to map directly between orthography and phonology. Coordinates were taken from Boukrina and Graves (62).
-
iv)
Inferior temporal sulcus. This term refers to the mid- to posterior section of the inferolateral temporal lobe. This area also has been proposed as a candidate region for the semantic contribution to reading aloud (63). Coordinates were taken from Boukrina and Graves (62).
ROIs were generated by creating spheres of 10-mm radius in the left hemisphere around the peak coordinates in each case. Data from these ROIs were subjected to the same analyses as the primary ROIs, as described in the main text.
SI Further Details of DCM Analyses
Analyses were performed using DCM10, including modeling of slice timings, within SPM8. The first stage in the analysis involved extracting the time-course of activation for each region in each participant. Regions were based on coordinates from previous studies as described earlier. To extract the signal for each participant, we created a sphere with a 10-mm radius around the coordinates and searched within this volume for the voxel with the maximum t-value for the contrast of reading over rest. Signal then was extracted from a sphere with a 5-mm radius centered on this voxel (principal eigenvector, adjusted for effects of interest). This process was repeated for all three regions in each participant. The DCM model was estimated separately for each participant, resulting in six endogenous parameter estimates and 12 modulatory parameter estimates each. These estimates were subjected to second-level random effects analysis, in which one-sample t tests were conducted to determine which parameters differed significantly from zero across the group. Because multiple tests were conducted, a false-discovery rate–corrected threshold of P < 0.05 was adopted.
SI Outside-Scanner Test of SR During Reading
The 160 words included in the test consisted of a factorial manipulation of spelling–sound consistency and imageability, with 40 monosyllabic items per cell. Items consisted of quartets across the conditions that were matched in terms of their initial phoneme. All the regular words were perfectly consistent in that they had orthographic bodies that always were pronounced in the same way (83, 84), meaning that they all had a type and token consistency ratio (friends/friends − enemies) of 1 (76). The exception words all had orthographic bodies that were pronounced with a different rime in at least one other word, and the summed frequency of their body pronunciation was less than half the total in the corpus, yielding a token consistency ratio of less than 0.5.
The properties of the stimuli are displayed in Table S1 and were analyzed using a series of 2 × 2 (consistency × imageability) ANOVAs. Imageability ratings showed the expected effect of imageability [F(1,156) = 431.84, P < 0.0005] with no effect of consistency and no interaction [F(1,156) <0.004, P > 0.951]. No significant differences across conditions were observed for Kucera–Francis (85) frequency [F(1,156) <0.043, P > 0.835]; number of letters [F(1,156) <2.21, P > 0.139]; number of phonemes [F(1,156) <0.739, P > 0.391]; and orthographic body neighborhood size ([F(1,156) <1.64, P > 0.202]. Number of friends and summed frequency of friends showed main effects of consistency [F(1,156) = 90.05, P < 0.0005; F(1,156) = 2.77, P = 0.098] with no reliable effects of imageability or any interaction [F(1,156) <0.673, P > 0.413]. Number of enemies and summed frequency of enemies also showed the expected main effects of consistency [F(1,156) = 215.90, P < 0.0005; F(1,156) =18.15, P < 0.0005] with no effects of imageability or any interaction [F(1,156) <0.191, P > 0.169]. Finally, the type and token consistency ratio showed significant main effects of consistency [F(1,156) = 1643.14, P < 0.0005; F(1,156) = 329.29, P < 0.0005] but no main effect of imageability and no interaction [F(1,156) <0.072, P > 0.789].
Participants read aloud all 160 words, each presented individually for a maximum of 2 s. RTs were recorded automatically with a voice-triggered microphone. The measure of semantic reliance that we correlated with neural effects consisted of the performance difference between low-imageability regular and exception words. Because reading effects can manifest either in terms of increased RT or decreased accuracy, we computed inverse efficiency scores that combined the two by penalizing RTs in lower-accuracy conditions (86). We then subtracted the low-imageability exception score from the low-imageability regular score to give a semantic reliance value for each participant.
Acknowledgments
This research was supported by Medical Research Council Programme Grant MR/J004146/1 (to M.A.L.R.), a Manchester Mental Health Social Care Trust Fellowship (to P.H.), a Leverhulme Trust Research Fellowship (to A.M.W.), and Wellcome Trust Institutional Strategic Support Fund Award 097820 to the University of Manchester.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502032112/-/DCSupplemental.
References
- 1.Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- 2.Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychol Rev. 1996;103(1):56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- 3.Harm MW, Seidenberg MS. Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychol Rev. 2004;111(3):662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- 4.Dilkina K, McClelland JL, Plaut DC. A single-system account of semantic and lexical deficits in five semantic dementia patients. Cogn Neuropsychol. 2008;25(2):136–164. doi: 10.1080/02643290701723948. [DOI] [PubMed] [Google Scholar]
- 5.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woollams AM, Ralph MA, Plaut DC, Patterson K. SD-squared: On the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114(2):316–339. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]
- 7.Coltheart M, Tree JJ, Saunders SJ. Computational modeling of reading in semantic dementia: Comment on Woollams, Lambon Ralph, Plaut, and Patterson (2007) Psychol Rev. 2010;117(1):256–271, discussion 271–272. doi: 10.1037/a0015948. [DOI] [PubMed] [Google Scholar]
- 8.Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- 9.Patterson K, Ralph MA. Selective disorders of reading? Curr Opin Neurobiol. 1999;9(2):235–239. doi: 10.1016/s0959-4388(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 10.Perry C, Ziegler JC, Zorzi M. Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychol Rev. 2007;114(2):273–315. doi: 10.1037/0033-295X.114.2.273. [DOI] [PubMed] [Google Scholar]
- 11.Plaut DC. Structure and function in the lexical system: Insights from distributed models of word reading and lexical decision. Lang Cogn Process. 1997;12:765–805. [Google Scholar]
- 12.Welbourne SR, Woollams AM, Crisp J, Ralph MA. The role of plasticity-related functional reorganization in the explanation of central dyslexias. Cogn Neuropsychol. 2011;28(2):65–108. doi: 10.1080/02643294.2011.621937. [DOI] [PubMed] [Google Scholar]
- 13.Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989;96(4):523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- 14.Leff AP, Spitsyna G, Plant GT, Wise RJ. Structural anatomy of pure and hemianopic alexia. J Neurol Neurosurg Psychiatry. 2006;77:1004–1007. doi: 10.1136/jnnp.2005.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DJ, et al. Efficient visual object and word recognition relies on high spatial frequency coding in the left posterior fusiform gyrus: Evidence from a case-series of patients with ventral occipito-temporal cortex damage. Cereb Cortex. 2013;23(11):2568–2580. doi: 10.1093/cercor/bhs224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woollams AM, Silani G, Okada K, Patterson K, Price CJ. Word or word-like? Dissociating orthographic typicality from lexicality in the left occipito-temporal cortex. J Cogn Neurosci. 2011;23(4):992–1002. doi: 10.1162/jocn.2010.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrmann M, Plaut DC. Bilateral hemispheric processing of words and faces: Evidence from word impairments in prosopagnosia and face impairments in pure alexia. Cereb Cortex. 2014;24(4):1102–1118. doi: 10.1093/cercor/bhs390. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DJ, et al. Processing deficits for familiar and novel faces in patients with left posterior fusiform lesions. Cortex 2015 doi: 10.1016/j.cortex.2015.02.003. , 10.1016/j.cortex.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woollams AM, Patterson K. The consequences of progressive phonological impairment for reading aloud. Neuropsychologia. 2012;50(14):3469–3477. doi: 10.1016/j.neuropsychologia.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Patterson K, Marcel A. In: Phonological ALEXIA or PHONOLOGICAL Alexia? Analytic Approaches to Human Cognition. Alegria J, Holender D, de Morais J, Monique R, editors. North-Holland; Oxford, UK: 1992. pp. 259–274. [Google Scholar]
- 21.Crisp J, Lambon Ralph MA. Unlocking the nature of the phonological-deep dyslexia continuum: The keys to reading aloud are in phonology and semantics. J Cogn Neurosci. 2006;18(3):348–362. doi: 10.1162/089892906775990543. [DOI] [PubMed] [Google Scholar]
- 22.Henry ML, Beeson PM, Alexander GE, Rapcsak SZ. Written language impairments in primary progressive aphasia: A reflection of damage to central semantic and phonological processes. J Cogn Neurosci. 2012;24(2):261–275. doi: 10.1162/jocn_a_00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 24.Vigneau M, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz MF, Faseyitan O, Kim J, Coslett HB. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135(Pt 12):3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodges JR, Patterson K. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol. 2007;6(11):1004–1014. doi: 10.1016/S1474-4422(07)70266-1. [DOI] [PubMed] [Google Scholar]
- 27.Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer’s disease and semantic dementia. Neuroimage. 2006;30(3):1010–1020. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Brambati SM, Ogar J, Neuhaus J, Miller BL, Gorno-Tempini ML. Reading disorders in primary progressive aphasia: A behavioral and neuroimaging study. Neuropsychologia. 2009;47(8-9):1893–1900. doi: 10.1016/j.neuropsychologia.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Binney RJ, Embleton KV, Jefferies E, Parker GJM, Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and Semantic dementia. Cereb Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- 30.Visser M, Lambon Ralph MA. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. J Cogn Neurosci. 2011;23(10):3121–3131. doi: 10.1162/jocn_a_00007. [DOI] [PubMed] [Google Scholar]
- 31.Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. J Neurosci. 2006;26(28):7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mion M, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133(11):3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- 33.Sandak R, et al. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cogn Affect Behav Neurosci. 2004;4(1):67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Price CJ, Mechelli A. Reading and reading disturbance. Curr Opin Neurobiol. 2005;15(2):231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SM, et al. The neural basis of surface dyslexia in semantic dementia. Brain. 2009;132(Pt 1):71–86. doi: 10.1093/brain/awn300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor JSH, Rastle K, Davis MH. Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull. 2013;139(4):766–791. doi: 10.1037/a0030266. [DOI] [PubMed] [Google Scholar]
- 37.Noble K, Glosser G, Grossman M. Oral reading in dementia. Brain Lang. 2000;74(1):48–69. doi: 10.1006/brln.2000.2330. [DOI] [PubMed] [Google Scholar]
- 38.Woollams AM, Lambon Ralph MA, Plaut DC, Patterson K. SD-Squared Revisited: Reply to Coltheart, Tree, and Saunders (2010) Psychological Review. 2010;117(1):273–281. doi: 10.1037/a0017641. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Embleton KV, Jefferies E, Parker GJM, Ralph MA. The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48(6):1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Ojemann JG, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 41.Embleton KV, Haroon HA, Morris DM, Ralph MA, Parker GJM. Distortion correction for diffusion-weighted MRI tractography and fMRI in the temporal lobes. Hum Brain Mapp. 2010;31(10):1570–1587. doi: 10.1002/hbm.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffman P, Binney RJ, Lambon Ralph MA. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 2015;63:250–266. doi: 10.1016/j.cortex.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mechelli A, et al. Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci. 2005;17(11):1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- 44.Pugh KR, et al. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Ment Retard Dev Disabil Res Rev. 2000;6(3):207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 45.Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13(2):135–145. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers TT, et al. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychol Rev. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 48.Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci USA. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson MA, et al. The role of the left anterior temporal lobe in exception word reading: Reconciling patient and neuroimaging findings. Neuroimage. 2012;60(4):2000–2007. doi: 10.1016/j.neuroimage.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72(2):385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Binney RJ, Parker GJM, Lambon Ralph MA. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion-weighted imaging probabilistic tractography. J Cogn Neurosci. 2012;24(10):1998–2014. doi: 10.1162/jocn_a_00263. [DOI] [PubMed] [Google Scholar]
- 52.Fan L, et al. Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb Cortex. 2014;24(12):3365–3378. doi: 10.1093/cercor/bht196. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz MF, et al. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(Pt 12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Plaut DC. Graded modality-specific specialisation in semantics: A computational account of optic aphasia. Cogn Neuropsychol. 2002;19(7):603–639. doi: 10.1080/02643290244000112. [DOI] [PubMed] [Google Scholar]
- 55.Rice GE, Lambon Ralph MA, Hoffman P. The roles of left versus right anterior temporal lobes in conceptual knowledge: An ALE meta-analysis of 97 functional neuroimaging studies. Cereb Cortex, in press. [DOI] [PMC free article] [PubMed]
- 56.Seghier ML, Lee HL, Schofield T, Ellis CL, Price CJ. Inter-subject variability in the use of two different neuronal networks for reading aloud familiar words. Neuroimage. 2008;42(3):1226–1236. doi: 10.1016/j.neuroimage.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kherif F, Josse G, Seghier ML, Price CJ. The main sources of intersubject variability in neuronal activation for reading aloud. J Cogn Neurosci. 2009;21(4):654–668. doi: 10.1162/jocn.2009.21084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stanovich KE, Cunningham AE. Studying the consequences of literacy within a literate society: The cognitive correlates of print exposure. Mem Cognit. 1992;20(1):51–68. doi: 10.3758/bf03208254. [DOI] [PubMed] [Google Scholar]
- 59.Welcome SE, Joanisse MF. Individual differences in skilled adult readers reveal dissociable patterns of neural activity associated with component processes of reading. Brain Lang. 2012;120(3):360–371. doi: 10.1016/j.bandl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 60.Klingberg T, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 61.Jobard G, Vigneau M, Simon G, Tzourio-Mazoyer N. The weight of skill Interindividual variability of reading related brain activation patterns in fluent readers. J Neurolinguist. 2011;24(1):113–132. [Google Scholar]
- 62.Boukrina O, Graves WW. Neural networks underlying contributions from semantics in reading aloud. Front Hum Neurosci. 2013;7:518. doi: 10.3389/fnhum.2013.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Graves WW, et al. Anatomy is strategy: Skilled reading differences associated with structural connectivity differences in the reading network. Brain Lang. 2014;133:1–13. doi: 10.1016/j.bandl.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gloor P. The Temporal Lobe and the Limbic System. Oxford Univ Press; Oxford, UK: 1997. [Google Scholar]
- 65.Patterson K, Hodges JR. Deterioration of word meaning: Implications for reading. Neuropsychologia. 1992;30(12):1025–1040. doi: 10.1016/0028-3932(92)90096-5. [DOI] [PubMed] [Google Scholar]
- 66.Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17(1):75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 67.Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional anatomy of a common semantic system for words and pictures. Nature. 1996;383(6597):254–256. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- 68.Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: Convergent evidence from rTMS. Cereb Cortex. 2009;19(4):832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- 69.Pobric G, Jefferies E, Ralph MA. Anterior temporal lobes mediate semantic representation: Mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci USA. 2007;104(50):20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brett M, Anton J-L, Valabregue R, Poline J-B. 2002. Region of interest analysis using an SPM toolbox in 8th International Conference on Functional Mapping of the Human Brain (Sendai, Japan)
- 71.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 72.Plaut DC, Shallice T. Deep dyslexia: A case study in connectionist neuropsychology. Cogn Neuropsychol. 1993;10:377–500. [Google Scholar]
- 73.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Baayen RH, Piepenbrock R, van Rijn H. The CELEX Lexical Database (CD-ROM) Linguistic Data Consortium, University of Pennsylvania; Philadelphia: 1993. [Google Scholar]
- 76.Ziegler JC, Stone GO, Jacobs AM. What is the pronunciation for -ough and the spelling for vertical bar u vertical bar? A database for computing feedforward and feedback consistency in English. Behav Res Methods Instrum Comput. 1997;29(4):600–618. [Google Scholar]
- 77.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Murphy K, Bodurka J, Bandettini PA. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. Neuroimage. 2007;34(2):565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jefferies E. The neural basis of semantic cognition: Converging evidence from neuropsychology, neuroimaging and TMS. Cortex. 2013;49(3):611–625. doi: 10.1016/j.cortex.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- 81.Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex 2014 doi: 10.1093/cercor/bhu198. , in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jared D. Spelling-sound consistency and regularity effects in word naming. J Mem Lang. 2002;46(4):723–750. [Google Scholar]
- 84.Jared D. Spelling-sound consistency affects the naming of high-frequency words. J Mem Lang. 1997;36(4):505–529. [Google Scholar]
- 85.Kucera H, Francis WN. Computational Analysis of Present-Day American English. Brown Univ Press; Providence, RI: 1967. [Google Scholar]
- 86.Townsend JT, Ashby FG. Methods of modeling capacity in simple processing systems. In: Castellan J, Restle F, editors. Cognitive Theory. Vol 3. Erlbaum; Hillsdale, NJ: 1978. pp. 200–239. [Google Scholar]