Significance
Extinction is a ubiquitous feature of biodiversity history, and although many lineages increase in diversity through time, most of them eventually decline and get replaced. Dinosaurs and mammals represent an extreme and iconic example of such replacement. Here we investigate the causes of the sequential wax and wane of three subfamilies in the dog family Canidae. Contrary to current expectation, we find that competition from phylogenetically distant, but ecologically similar, clades played a more substantial role in canid diversification than climate change and body size evolution. Our results provide novel quantitative evidence indicating that competition from multiple clades can actively drive the displacement and extinction of entire lineages.
Keywords: mammals, speciation, extinction, macroevolution, fossils
Abstract
The history of biodiversity is characterized by a continual replacement of branches in the tree of life. The rise and demise of these branches (clades) are ultimately determined by changes in speciation and extinction rates, often interpreted as a response to varying abiotic and biotic factors. However, understanding the relative importance of these factors remains a major challenge in evolutionary biology. Here we analyze the rich North American fossil record of the dog family Canidae and of other carnivores to tease apart the roles of competition, body size evolution, and climate change on the sequential replacement of three canid subfamilies (two of which have gone extinct). We develop a novel Bayesian analytic framework to show that competition from multiple carnivore clades successively drove the demise and replacement of the two extinct canid subfamilies by increasing their extinction rates and suppressing their speciation. Competitive effects have likely come from ecologically similar species from both canid and felid clades. These results imply that competition among entire clades, generally considered a rare process, can play a more substantial role than climate change and body size evolution in determining the sequential rise and decline of clades.
Biodiversity dynamics are determined by different speciation and extinction regimes that lead clades to successively rise, decline, and replace one another (1–4). Understanding the roles of biotic and abiotic factors in driving these deep time changes is a central focus in evolutionary biology (5). Several studies have suggested that climate changes played an overarching role in determining the fate of whole clades (refs. 6–8, but see ref. 9), but the wax and wane of clades could also be the result of biotic interactions, and in particular, competition for resources and predation (5, 10–12). Competition is usually assumed to occur mostly among closely related species, but it may also take place among species from different clades with similar ecology (13). At a macroevolutionary scale, the role of competition in clade replacement has been explained by two main biologic mechanisms: passive replacement and active displacement (14). In the case of passive replacement, an incumbent clade initially prevents a competing clade from radiating. The latter clade can only radiate after the incumbent clade declines because of extrinsic factors, such as climate change, freeing ecologic niche space. In contrast, active displacement occurs when the rise in diversity of a clade drives the decline of another clade by outcompeting it on limited resources. Most work suggests that passive replacement is the most common mechanism (5, 13, 15–17). The demise of nonavian dinosaurs by the Cretaceous–Paleogene meteorite impact around 66 million years ago (Ma) and the subsequent evolutionary and ecologic diversification of mammals provide an iconic example of passive replacement (18, 19). In contrast, an active clade replacement driven by competitive interactions is usually considered negligible at a geologic time scale (5, 10, 15), although theoretically possible and potentially important at local and short time scales (5, 10, 17, 20, 21).
The subfamilies within the dog family Canidae show the characteristic sequential clade replacement repeatedly seen in the deep history of biodiversity. Canids comprise three clades: the Hesperocyoninae and Borophaginae subfamilies, which are extinct, and the subfamily Caninae, which includes extinct and living species. Their diversification dynamics have been causally linked to the evolution of intrinsic properties, including increase in body size and an exclusively carnivorous diet (hypercarnivory) (22–24). The rich and well-identified fossil record of the family (23–25) offers a unique opportunity to study the processes and mechanisms of their diversification. Furthermore, all subfamilies originated in North America, and the two extinct subfamilies are geographically restricted to this continent, thus reducing the number of confounding effects (e.g., major differences in climatic conditions and biologic interactions) (26). Finally, the three subfamilies overlap in time and space with each other, but also with several other potential competitor clades (SI Appendix, Figs. S1–S8) (13).
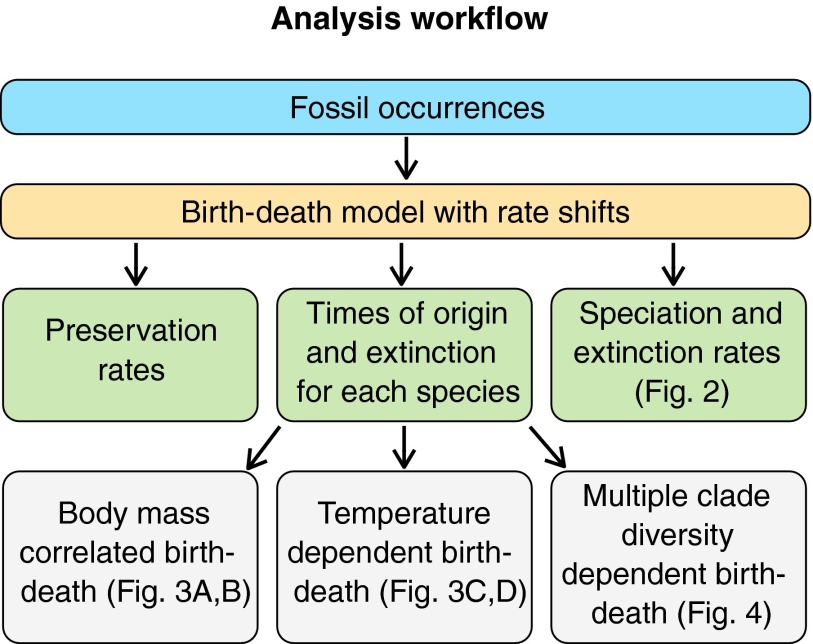
We analyzed ca. 1,500 fossil occurrences for 120 canid species, which span the entire existence of the family from c. 40 Ma to the present. Because competition could derive from other animals present in North America, we compiled data (744 occurrences and 115 species) for five additional carnivore families: Felidae (cats), Amphicyonidae (bear dogs), Nimravidae (false saber-toothed cats), Barbourofelidae, and Ursidae (bears). We develop a probabilistic framework (Methods and SI Appendix) to investigate whether and to which extent speciation and extinction rates responded to body mass evolution (which is also a good proxy of diet in canids) (23), climate change, and competition among multiple clades. The method implements process-based speciation and extinction models that fully incorporate the preservation process and the uncertainties associated with the age of each fossil occurrence (27). This framework allows us to simultaneously test the effect of the diversity of multiple cooccurring clades on speciation and extinction rates (diversity dependence) by quantitatively investigating the passive and active roles of competition [SI Appendix, Competition and positive interactions (MCDD model)]. The analytic workflow implemented here involves several steps and is summarized in Fig. 1.
Fig. 1.
Schematic representation of the analytic workflow developed here. The fossil occurrence data are first analyzed under a variable rates birth–death model, using PyRate (37), while jointly modeling the preservation process to estimate rates of speciation, extinction, and preservation. This step also estimates the times of origin and extinction of all species, which are used for the subsequent analyses under different birth–death models assessing the effect of body size evolution, climate change, and clade competition on speciation and extinction rates. Here, we performed 100 replicates of all analyses to account for uncertainties in the age of fossil occurrences [SI Appendix, Birth-death model with shifts (BDS)].
Results and Discussion
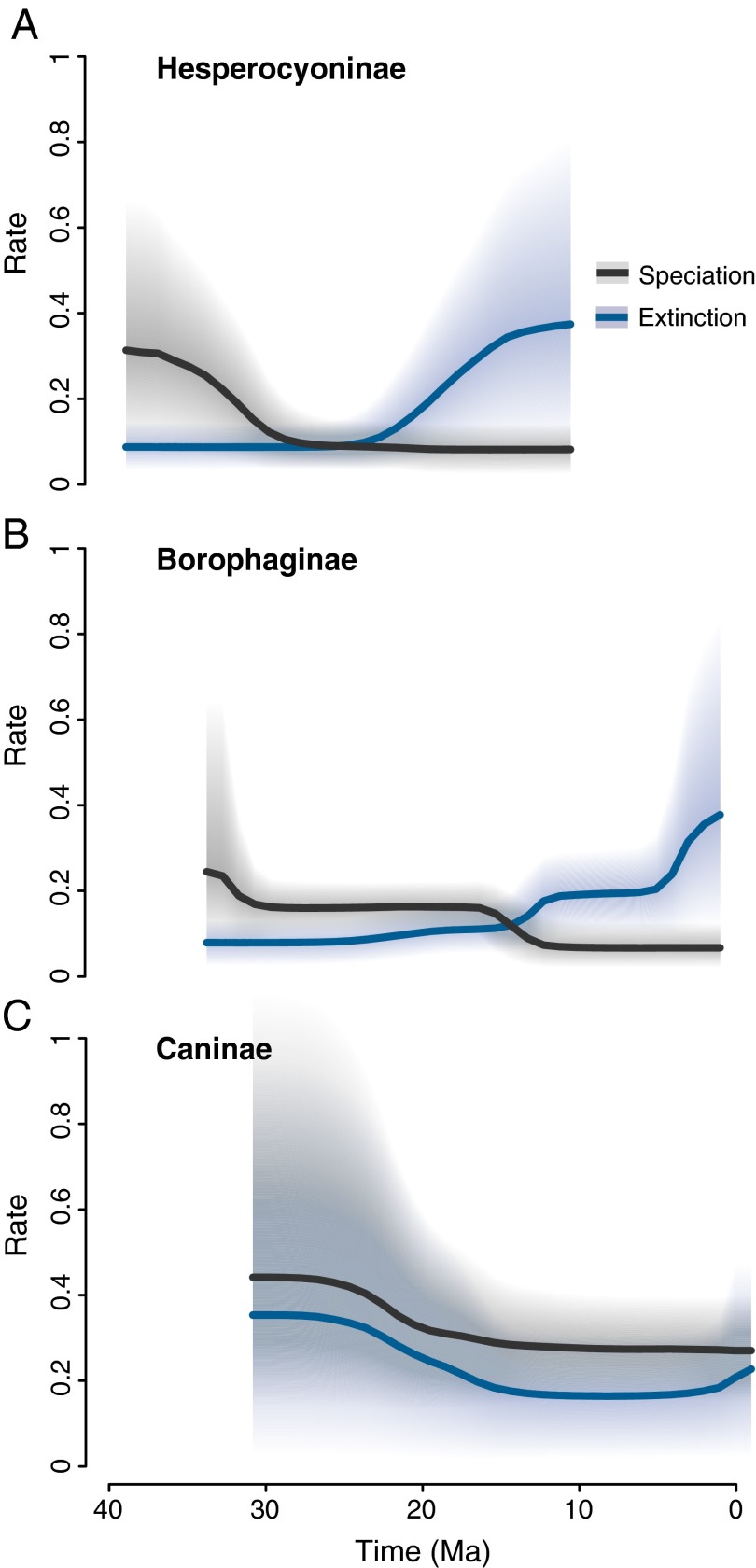
Our results indicate that the diversity dynamics of the extant subfamily Caninae conform to a constant birth–death process with a positive, albeit small, net diversification rate (Fig. 2A; SI Appendix, Table S2; diversification is defined as speciation minus extinction). In contrast, we found evidence for significant temporal changes in both speciation and extinction rates for the two extinct subfamilies Hesperocyoninae and Borophaginae (Fig. 2 and SI Appendix, Table S2). Therefore, our results support the idea that the demise of a clade is controlled not only by an increase in its extinction rate but also by a failure to originate (2). Several factors could explain these dynamics, and we used our Bayesian framework to test competing hypotheses.
Fig. 2.
Rates of speciation (black) and extinction (blue) through time for the three subfamilies of Canidae. (A) The diversification of Hesperocyoninae is characterized by a fourfold decrease in speciation rate c. 30 Ma, followed by an almost fivefold increase of extinction starting at c. 20 Ma. (B) In Borophaginae, speciation and extinction were constant until c. 15 Ma, when the speciation rate underwent a threefold drop and the extinction rate experienced a twofold increase, followed by another increase close to the time of the clade’s demise. (C) Caninae, the only canid subfamily with living species, followed a roughly constant rate birth–death model, with small but positive net diversification. Solid lines indicate mean rates, whereas shaded areas show the 95% credible intervals (95% CIs).
There is a trend toward the evolution of a larger body mass in canids (Fig. 3A; see also refs. 22 and 23), which has been recently interpreted as the result of adaptations to repeated evolutionary changes in diet (28). Previous work suggests that increasing size could negatively affect species longevity (23). In addition, larger body sizes in mammals usually correlate with higher extinction risk or other life history traits that have been shown to affect extinction (29). Last, previous analyses suggested that the trend toward larger body sizes in North American Canidae was produced by preferential origination of larger species, rather than changes in extinction related to body size (22). Despite these earlier indications, we find no evidence in our data that changes in body size per se directly led to changes in speciation and extinction rates (Fig. 3B and SI Appendix, Table S3). Body size could still indirectly correlate with diversification dynamics, as an increase in body size predisposes the evolution of hypercarnivory and feeding specialization (28), which in turn have been shown to increase extinction in canids (23).
Fig. 3.
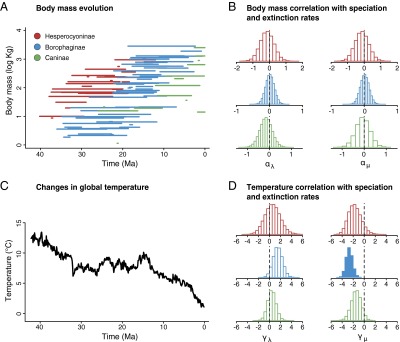
Correlations between diversification dynamics and two putative causes of rate variation: body mass evolution and climatic changes. (A) The evolution of body sizes in North American canids shows a temporal trend toward larger sizes. Each line shows the reconstructed life span of a species obtained from one of 100 replicated analyses. (B) Changes in body mass across species do not correlate with variations in speciation and extinction rates, as shown by the posterior estimate of the correlation parameters αλ and αμ (as they do not differ significantly from 0). (C) Relative global temperature curve, based on oxygen isotopes (30), used as a proxy for environmental changes. (D) Temperature variation does not correlate with speciation and extinction rates with the exception of a significant negative correlation with the extinction rate of Borophaginae. (B and D) Color codes similar to the legend on A. Histogram with filled color denotes significant correlation, based on the 95% CI.
We find evidence that climate and environmental changes [here approximated by a global temperature curve (30); Fig. 2C] correlate with changes in diversification rates only in Borophaginae (Fig. 3D and SI Appendix, Table S4), whereas no significant correlations emerged for the other two subfamilies (see also ref. 9 for similar results in North American mammals as a whole). Extinction rates are negatively correlated with temperature (SI Appendix, Table S4) in Borophaginae, potentially explaining the twofold increase in extinction rate at c. 15 Ma (Figs. 2B and 3D). This shift follows the mid-Miocene Climatic Optimum (c. 17–14 Ma), after which global temperatures began to decline (Fig. 3A). During this cooling period, North American vegetation experienced substantial changes, with the appearance and expansion of grass-dominated open habitats replacing Eocene and early Miocene forests (31). Such environments were possibly less favorable to the hunting behavior of late Borophaginae lineages, which had limited adaptations to running (11, 24).
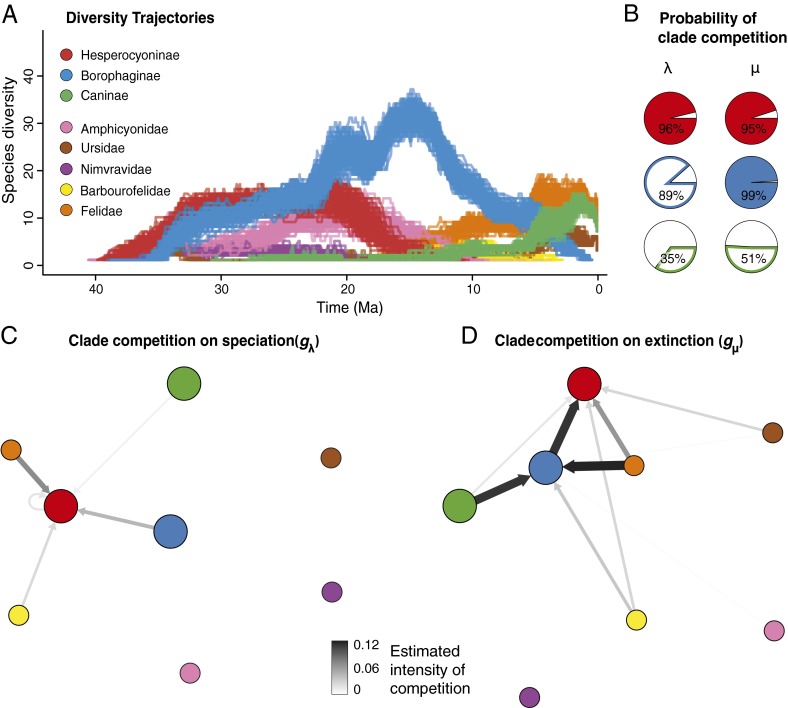
In contrast, our results provide very strong evidence that clade competition played a major role in the diversification dynamics and clade replacement in North American canids (Fig. 4 A and B). Although clade competition did not affect the diversification dynamics of Caninae (Fig. 4B and SI Appendix, Table S13), changes in speciation and/or extinction rates of the two extinct canid subfamilies are strongly correlated with diversity changes of multiple putative competitors (Fig. 4B and SI Appendix, Table S13).
Fig. 4.
Diversity trajectories and the effect of clade competition on the speciation and extinction rates of each canid subfamily. (A) The diversity trajectories of the three canid subfamilies and the additional potential competitor carnivore clades (only North American species included). Reconstructions of diversity trajectories are replicated 100 times to incorporate uncertainties around the age of the fossil occurrences. (B) Posterior probabilities that multiple clade competition affects speciation and extinction rates. Changes in speciation and extinction rates of Hesperocyoninae and changes in extinction rates of Borophaginae are significantly correlated (posterior probabilities greater than 0.95) with changes in the diversity of multiple competitor clades. (C) Network showing the competitive effect imposed by each individual clade on the other clades’ speciation rate. (D) Network showing the competitive effect imposed by each individual clade on the other clades’ extinction rate. (C and D) Each arrow indicates the intensity of competition (gλ, gμ) imposed by a given clade toward one of the canid subfamilies. This metric quantifies the proportion of rate change (decrease for speciation and increase for extinction) associated with the addition of one species of the competing clade. Only arrows associated with significant correlations (based on B) are shown. (B–D) Color code identification presented on A also applies to the other panels.
The increased extinction rate through time found in Hesperocyoninae is largely attributed to competition with Borophaginae (Fig. 4D). Although our analytic framework in its current implementation is unable to infer changes in the strength of competition through time [SI Appendix, Multiple Clade Diversity Dependence model (MCDD)], this competition was probably mostly a result of the late, larger, and exclusively carnivorous lineages of Borophaginae, rather than the earlier, smaller, and hypocarnivorous species. As Borophaginae diversified, they progressively increased their body size overlap with Hesperocyoninae (Fig. 3A) and eventually evolved hypercarnivory, similar to Hesperocyoninae (23, 24). This suggests an increase in ecologic similarity and stronger competition between the two clades toward the second half of the existence of Hesperocyoninae (24). This is confirmed by significantly higher extinction rates of Hesperocyoninae between 20 and 10 Ma (Fig. 2A), when they coexisted with similar, hypercarnivorous Borophaginae. The immigration of Felidae and Barbourofelidae from Eurasia (SI Appendix, Figs. S7 and S8) (11) intensified clade competition by both suppressing speciation rates and increasing extinction rates of Hesperocyoninae (Fig. 4 C and D). Because Felidae and Barbourofelidae only overlapped during a short time with Hesperocyoninae, the moderate competition effects inferred for those two Feliformia clades probably represent an effect toward the very end of the existence of Hesperocyoninae.
Although the changes in Borophaginae diversity clearly affected the speciation and extinction rates in Hesperocyoninae, our results show that neither speciation nor extinction rates in Borophaginae correlate with diversity changes in Hesperocyoninae (Fig. 4 C and D). Previous work suggested that the extinction of large Hesperocyoninae opened niches for animals of large body size and allowed the diversification of Borophaginae (22), but our results clearly suggest that the unidirectional interactions between Hesperocyoninae and Borophaginae conform to an active displacement driven by Borophaginae, rather than to a passive replacement (SI Appendix, Fig. S19 and Passive replacement and active displacement). The vacancy left by the demise of Hesperocyoninae did not result in an increased speciation rate (or decreased extinction rate) of Borophaginae (SI Appendix, Figs. S17 and S18), as would be expected if Hesperocyoninae were an incumbent clade (5, 13, 17) (SI Appendix, Passive replacement and active displacement). Instead, the diversification dynamics of Borophaginae were only affected by competition with clades that either originated or immigrated after their origin. Competition with Felidae, Caninae (most likely with the later hypercarnivorous species), and to a lesser extent, Barbourofelidae increased their extinction rate (Fig. 4D). Thus, active competition among multiple clades has played a substantial role also in the demise and evolutionary displacement of Borophaginae.
Previous criticisms against the active role of competition as a driver of clade displacement stem from the hypothesis that diversity is not limited (15). However, evidence suggests diversity equilibrium for North American mammals (26), and for carnivores at a global scale (32). Our results strongly indicate that competition among several clades of canids and other carnivores drove the changes in diversification rates and the replacement of entire clades. Previous evidence of clade replacement has also been shown among higher taxa (e.g., genus or family) in marine invertebrates for pairs of clades (10, 14), between a focal clade and a collection of clades (33), and between multiple “faunas” (34). Those studies investigated clade replacement by modeling how the diversity trajectories of multiple clades are coupled under competitive interactions (14, 33, 34) and laid out key conceptual foundations in the study of clade replacement. Our approach, through the explicit integration of competition in birth–death processes, allowed us to identify, quantify, and further explore properties of clade replacement that could not be assessed with previous methodologies. For example, we find that the competitive effects are not symmetric with respect to clade identity (e.g., between Hesperocyoninae and Borophaginae) and that competition may affect speciation and extinction rates differently. Similar birth–death processes have been developed in phylogenetic methods, but based on the idea that competition only occurs within clades (35). We show here that, at least for the two extinct canid subfamilies, diversity dependence within each subfamily is considerably smaller (Fig. 4 and SI Appendix, Fig. S16) than competition generated by multiple clades, which have either invaded North America with already similar ecologies (e.g., Felidae) or undergone similar evolution toward larger body size and similar ecology (e.g., Borophaginae and Caninae). Competition among multiple clades involving distantly related, but ecologically interacting, species may therefore be a much more common and important mechanism driving biodiversity changes than previously recognized.
Methods
Fossil Data Sets.
We compiled a data set of all fossil occurrences for North American Canidae retrieved from the Paleobiology database (paleobiodb.org; accessed between January and March 2014) and split the data into three subsets based on taxonomic assignment to the subfamilies Hesperocyoninae, Borophaginae, and Caninae. Body size information for extinct Canidae (estimated from the length of the first lower molar) was retrieved from the Paleobiology database, accessed through the web-based portal Fossilworks (fossilworks.org) and from Tedford et al. (25). For extant canid species, we used estimates from Smith et al. (36), using the specific estimate for North America when available. We also compiled fossil data for five additional carnivore clades for competition analyses: Amphicyonidae (extinct) and Ursidae from the Caniformia suborder, and Nimravidae (extinct), Barbourofelidae (extinct), and Felidae from the Feliformia suborder. These clades were chosen as the main carnivore lineages that are or have been potentially competing for resources with Canidae species, as suggested by (at least partial) similarities in their diet (11, 13), the fact that they possess or have evolved toward large body sizes, their temporal and spatial overlap (SI Appendix, Figs. S1–S8), and previous hypotheses (13, 22). We included in the final data sets only fossil occurrences found in North America and identified to a species level. We incorporated the uncertainties associated with the age of the fossil occurrences by replicating 100 times all the analyses described here, after resampling the fossil ages from the respective temporal ranges (SI Appendix, Fossil data sets).
Analysis of the Fossil Data.
We carried out the analyses of the fossil data sets using a Bayesian framework recently developed, tested, and validated on both simulated and empiric data sets (27). We modeled fossil occurrences as the result of two processes: preservation, which includes the fossilization and sampling of organisms, and speciation and extinction, which generate and shape species diversity. In an initial set of analyses, we used the original implementation of the approach (27) to analyze the fossil data sets under a birth–death model with time-varying rates. For each clade, we estimated the parameters of the preservation process (the expected number of fossil occurrences per lineage per million years), the times of speciation and extinction of each species, and the speciation and extinction rates and their variation through time [Fig. 2 and SI Appendix, Figs. S10–S12, and Birth-death model with shifts (BDS)]. We used the estimated times of speciation and extinction of all species to carry out the diversification analyses outlined here [SI Appendix, Birth-death model with shifts (BDS)]. All methods shown here are implemented within the open source program PyRate (37).
Body Mass Correlated Diversification.
We tested whether the diversification dynamics of canid clades may be linked with changes in body mass, using the Covar birth–death model (37). Under this model, changes in speciation and extinction rates correlate with changes in (log-transformed) body mass through the correlation parameters (αλ, αμ), which are estimated from the data. The birth–death rates are therefore transformed on a lineage-specific basis, rather than through time. Thus, α > 0 indicates a positive correlation between the trait value and the birth–death rates, α < 0 indicates a negative correlation, and α ∼ 0 indicates no correlation [SI Appendix, Body mass correlated diversification (Covar model)].
Temperature-Dependent Diversification.
We used a birth–death model with time-varying rates to test for a correlation between speciation and extinction rates in canids and changes in global temperature through time. The temperature data were derived from stable isotope proxies (30). The model is based on the temperature-dependent birth–death model originally described in a phylogenetic framework (38). The parameters γλ and γμ quantify the correlation between changes in birth–death rates and temperature changes. Thus, γ > 0 indicates positive correlation between the trait value and the birth–death rates, and γ < 0 indicates negative correlation [SI Appendix, Temperature dependent BD model (BDT)].
Competition Among Clades.
To assess the effect of competition on the diversification of canids, we developed a model in which speciation and extinction rates are linearly correlated with the diversity trajectory of a clade. Under competitive interactions, increasing species diversity has the effect of suppressing the speciation rates and/or increasing the extinction rates. Such birth–death models are generally referred to as diversity-dependent models (e.g., ref. 35). Although in phylogenetics, diversity dependence is typically tested within a single clade, our model allows for competition to take place not only among the species of a given clade but also among species that are not closely related but share similar ecology. Therefore, we extended the diversity-dependent birth–death model to assess the effects of competition within and between clades. We named this model Multiple Clade Diversity Dependence and used Bayesian variable selection [SI Appendix, Multiple Clade Diversity Dependence model (MCDD)] to jointly analyze all clades and estimate the baseline speciation and extinction rates for each clade, the marginal probability of competition for each clade, and competition parameters that quantify the intensity of the diversity dependence between each pair of clades [Fig. 4 and SI Appendix, Figs. S16–S18 and Multiple Clade Diversity Dependence model (MCDD) and Competition and positive interactions (MCDD model)]. Each competition parameter expresses a diversity dependence relationship between the diversity of a clade and the speciation or extinction rates of the other clade. Thus, the model is able to infer directionality of the reciprocal interactions between two clades. Even though we do not expect positive interactions to be present among carnivore clades, we incorporated this possibility in our model to increase its flexibility and usefulness for other systems. Increasing diversity of a clade can therefore correlate with higher speciation rates or lower extinction rates in another clade [SI Appendix, Multiple Clade Diversity Dependence model (MCDD) and Tables S5–S13]. We tested the performance of the Multiple Clade Diversity Dependence model through the analysis of simulated data sets and found that our approach is very robust against spurious diversity dependence effects (“false positives”; SI Appendix, Robustness of the MCDD model).
Supplementary Material
Acknowledgments
We thank C. M. Janis, N. Lartillot, P. Guimarães, M. Pires, and A. Kostikova for discussion and the editors and two anonymous reviewers for constructive suggestions. Analyses were run at the high-performance computing center Vital-IT of the Swiss Institute of Bioinformatics (Lausanne, Switzerland). Funding was provided from Carl Tryggers stiftelse and Wenner-Gren foundation (D.S.); from the Swedish Research Council (B0569601), the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013, ERC Grant Agreement 331024), and a Wallenberg Academy Fellowship (to A.A.); from the Swiss National Science Foundation (CR32I3_143768 to N.S.); and from Fundação de Amparo à Pesquisa do Estado de São Paulo (Grant 2012/04072-3) and Universidade de São Paulo (T.B.Q.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502803112/-/DCSupplemental.
References
- 1.Gilinsky NL, Bambach RK. Asymmetrical patterns of origination and extinction in higher taxa. Paleobiology. 1987;13(4):427–445. [Google Scholar]
- 2.Quental TB, Marshall CR. How the Red Queen drives terrestrial mammals to extinction. Science. 2013;341(6143):290–292. doi: 10.1126/science.1239431. [DOI] [PubMed] [Google Scholar]
- 3.Sepkoski JJ. A factor analytic description of the Phanerozoic marine fossil record. Paleobiology. 1981;7(1):36–53. [Google Scholar]
- 4.Van Valen L. A theory of origination and extinction. Evol Theory. 1985;7:133–142. [Google Scholar]
- 5.Benton MJ. The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science. 2009;323(5915):728–732. doi: 10.1126/science.1157719. [DOI] [PubMed] [Google Scholar]
- 6.Janis CM. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu Rev Ecol Syst. 1993;24(1):467–500. [Google Scholar]
- 7.Figueirido B, Janis CM, Pérez-Claros JA, De Renzi M, Palmqvist P. Cenozoic climate change influences mammalian evolutionary dynamics. Proc Natl Acad Sci USA. 2012;109(3):722–727. doi: 10.1073/pnas.1110246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortelius M, et al. Evolution of Neogene mammals in Eurasia: Environmental forcing and biotic interactions. Annu Rev Earth Planet Sci. 2014;42(1):579–604. [Google Scholar]
- 9.Alroy J, Koch PL, Zachos JC. Global climate change and North American mammalian evolution. Paleobiology. 2000;26(4):259–288. [Google Scholar]
- 10.Sepkoski JJ, Jr, McKinney FK, Lidgard S. Competitive displacement among post-Paleozoic cyclostome and cheilostome bryozoans. Paleobiology. 2000;26(1):7–18. doi: 10.1666/0094-8373(2000)026<0007:cdappc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Van Valkenburgh B. Deja vu: The evolution of feeding morphologies in the Carnivora. Integr Comp Biol. 2007;47(1):147–163. doi: 10.1093/icb/icm016. [DOI] [PubMed] [Google Scholar]
- 12.Vermeij GJ. Evolution and Escalation: An Ecological History of Life. Princeton University Press; Princeton, NJ: 1987. [Google Scholar]
- 13.Van Valkenburgh B. Major patterns in the history of carnivorous mammals. Annu Rev Earth Planet Sci. 1999;27:463–493. [Google Scholar]
- 14.Sepkoski JJ. Competition in macroevolution: The double wedge revisited. In: Jablonski D, Erwin DH, Lipps JH, editors. Evolutionary Paleobiology. University of Chicago Press; Chicago, IL: 1996. pp. 211–255. [Google Scholar]
- 15.Benton M. On the nonprevalence of competitive replacement in the evolution of tetrapods. In: Jablonski D, Erwin DH, Lipps JH, editors. Evolutionary Biology. University of Chicago Press; Chicago, IL: 1996. [Google Scholar]
- 16.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science. 2008;321(5895):1485–1488. doi: 10.1126/science.1161833. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig M, McCord R. Incumbent replacement: Evidence for long-term evolutionary progress. Paleobiology. 1991;17(2):202–213. [Google Scholar]
- 18.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48(1):107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 19.Archibald JD. Extinction and Radiation: How the Fall of the Dinosaurs Led to the Rise of the Mammals. Johns Hopkins University Press; Baltimore, MD: 2011. [Google Scholar]
- 20.Benson RBJ, Frigot RA, Goswami A, Andres B, Butler RJ. Competition and constraint drove Cope’s rule in the evolution of giant flying reptiles. Nat Commun. 2014;5:3567. doi: 10.1038/ncomms4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Valen L, Sloan RE. The extinction of the multituberculates. Syst Zool. 1966;15(4):261–278. [Google Scholar]
- 22.Finarelli JA. Mechanisms behind active trends in body size evolution of the Canidae (Carnivora: Mammalia) Am Nat. 2007;170(6):876–885. doi: 10.1086/522846. [DOI] [PubMed] [Google Scholar]
- 23.Van Valkenburgh B, Wang X, Damuth J. Cope’s rule, hypercarnivory, and extinction in North American canids. Science. 2004;306(5693):101–104. doi: 10.1126/science.1102417. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Tedford RH, Antón M. The Dog Family, Canidae, and their Evolutionary History. Columbia University Press; New York: 2008. [Google Scholar]
- 25.Tedford RH, Wang XM, Taylor BE. Phylogenetic systematics of the North American fossil Caninae (Carnivora: Canidae) Bull Am Mus Nat Hist. 2009;325:1–218. [Google Scholar]
- 26.Alroy J. Constant extinction, constrained diversification, and uncoordinated stasis in North American mammals. Palaeogeogr Palaeoclimatol Palaeoecol. 1996;127(1-4):285–311. [Google Scholar]
- 27.Silvestro D, Schnitzler J, Liow LH, Antonelli A, Salamin N. Bayesian estimation of speciation and extinction from incomplete fossil occurrence data. Syst Biol. 2014;63(3):349–367. doi: 10.1093/sysbio/syu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slater GJ. Iterative adaptive radiations of fossil canids show no evidence for diversity-dependent trait evolution. Proc Natl Acad Sci USA. 2015;112(16):4897–4902. doi: 10.1073/pnas.1403666111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardillo M, et al. Multiple causes of high extinction risk in large mammal species. Science. 2005;309(5738):1239–1241. doi: 10.1126/science.1116030. [DOI] [PubMed] [Google Scholar]
- 30.Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451(7176):279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- 31.Stromberg CAE. Evolution of grasses and grassland ecosystems. Annu Rev Earth Planet Sci. 2011;39:517–544. [Google Scholar]
- 32.Liow LH, Finarelli JA. A dynamic global equilibrium in carnivoran diversification over 20 million years. Proc Biol Sci. 2014;281(1778):20132312. doi: 10.1098/rspb.2013.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AI, Sepkoski JJ., Jr Modeling bivalve diversification: The effect of interaction on a macroevolutionary system. Paleobiology. 1988;14(4):364–369. doi: 10.1017/s0094837300012100. [DOI] [PubMed] [Google Scholar]
- 34.Sepkoski JJ. A kinetic-model of Phanerozoic taxonomic diversity. III. Post-Paleozoic families and mass extinctions. Paleobiology. 1984;10(2):246–267. [Google Scholar]
- 35.Etienne RS, et al. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Proc Biol Sci. 2012;279(1732):1300–1309. doi: 10.1098/rspb.2011.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith FA, et al. Body mass of late Quaternary mammals. Ecology. 2003;84(12):3403. [Google Scholar]
- 37.Silvestro D, Salamin N, Schnitzler J. PyRate: A new program to estimate speciation and extinction rates from incomplete fossil data. Methods Ecol Evol. 2014;5(10):1126–1131. [Google Scholar]
- 38.Condamine FL, Rolland J, Morlon H. Macroevolutionary perspectives to environmental change. Ecol Lett. 2013;16(Suppl 1):72–85. doi: 10.1111/ele.12062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.