Significance
The benefits of breast milk in neonatal development are well characterized. However, very little is known about the essential nutrient components in breast milk that are critical for neonatal development and how these nutrients are maintained at adequate levels in breast milk. The present work demonstrates that the intestine is an essential sensor of systemic iron demand during lactation. During high iron demand from lactation, hypoxia-inducible factor-2α–mediated increase in maternal intestinal iron absorption is essential to maintain milk iron levels. This work demonstrates the significant role of maternal intestinal iron absorption in postnatal iron homeostasis of newborns and provides a therapeutic target to maintain iron homeostasis during pregnancy and lactation in anemic patients who are refractory to iron supplementation.
Keywords: iron homeostasis, anemia, HIF-2α, pregnancy, lactation
Abstract
The mechanisms that are essential for the maintenance of nutrient status in breast milk are unclear. Our data demonstrate that the intestine via hypoxia-inducible factor (HIF)-2α is an essential regulatory mechanism for maintaining the quality of breast milk. During lactation, intestinal HIF-2α is highly increased, leading to an adaptive induction of apical and basolateral iron transport genes. Disruption of intestinal HIF-2α (but not HIF-1α) or the downstream target gene divalent metal transporter (DMT)-1 in lactating mothers did not alter systemic iron homeostasis in the mothers, but led to anemia, decreased growth, and truncal alopecia in pups which was restored following weaning. Moreover, pups born from mothers with a disruption of intestinal HIF-2α led to long-term cognitive defects. Cross-fostering experiments and micronutrient profiling of breast milk demonstrated that the defects observed were due to decreased maternal iron delivery via milk. Increasing intestinal iron absorption by activation of HIF-2α or parenteral administration of iron-dextran in HIF-2α knockout mothers ameliorated anemia and restored neonatal development and adult cognitive functions. The present work details the importance of breast milk iron in neonatal development and uncovers an unexpected molecular mechanism for the regulation of nutritional status of breast milk through intestinal HIF-2α.
Iron is the most abundant trace element involved in numerous biological processes such as oxygen delivery, mitochondrial respiration, and metabolism. Under normal circumstances, dietary iron provides 1–3% of the systemic iron requirement, due to an efficient recycling of iron from senesced red blood cells (1). During conditions of high iron demand, iron homeostasis relies on intestinal iron absorption (2, 3). A major systemic regulator of intestinal iron absorption is the liver-derived peptide hormone hepcidin. Hepcidin expression is regulated by systemic iron levels and in turn can regulate the only known iron exporter ferroportin (FPN) (4). This adaptive mechanism is essential for systemic iron homeostasis as evidenced in patients with iron-related disorders exhibiting decreased hepcidin expression or function (5). Moreover, mouse models with disruption of hepcidin expression, intestine specific disruption of FPN, or mice with a specific mutation of FPN with decreased hepcidin binding results in robust iron-related disorders, detailing the importance of the hepcidin–FPN axis in iron homeostasis (6–8).
During pregnancy, the systemic iron requirement increase by 10-fold to support placental and fetal growth (9). Fetal and neonatal iron deficiency results in decreased growth, immunological dysfunction, anemia, and irreversible cognitive defects (10). Iron supplementation is highly recommended to prevent iron deficiency anemia during pregnancy (11). The increase in iron demand during pregnancy is met by an adaptive decrease in maternal hepcidin levels leading to enhanced iron absorption. This is a highly conserved process observed in humans and animal models (12–14). However, hepcidin levels are restored after parturition or early lactation period during which the maternal iron requirement is still at its peak (15, 16). This suggests alternative mechanisms exist during late pregnancy and lactation that allow efficient intestinal iron absorption independent of hepcidin levels.
In addition to hepcidin, the intestinal oxygen-sensing pathway is important in iron homeostasis through cell-autonomous regulation of iron absorptive genes in the intestine (2, 3, 17, 18). The oxygen-sensitive transcription factor hypoxia inducible factor (HIF)-2α has been well characterized as a key transcription factor that promotes intestinal iron absorption by regulating the expression of ferric reductase (DcytB), divalent metal transporter 1 (DMT-1) and FPN in the intestine (17–21). The expression of DcytB, DMT-1, and FPN are highly induced in the intestine during pregnancy and lactation (16, 22). However, it is not known whether HIF-2α is involved in the regulation of maternal iron homeostasis during pregnancy and lactation, the two physiological states of high iron demand. Disruption of HIF-2α in pregnant females resulted in only moderate anemia during pregnancy and lactation, and no significant differences in their litters at birth were observed. Intriguingly, pups nursing from mothers with an intestinal disruption of HIF-2α resulted in progressive anemia and retarded growth and the pups had long-term cognitive defects. Further studies demonstrated that the HIF-2α is essential in maintaining the quality of breast milk through intestinal iron absorption. The contribution of fetal iron reserves and breast milk in neonatal iron homeostasis and health was unresolved due to lack of appropriate animal models (23–25). Our findings indicate a previously unknown and essential role for intestinal HIF-2α in the postnatal growth and development of pups via maternal breast milk.
Results
Disruption of Maternal Intestinal HIF-2α Results in Neonatal Anemia.
Previous work from our laboratory and others have demonstrated that intestinal HIF-2α is essential for the adaptive increase in iron absorption during conditions of high iron demand (17, 19, 20). Maternal iron demand is significantly increased during pregnancy and lactation to support the growth and development of the fetus and neonates (26, 27). It is not known whether intestinal HIF-2α is involved in the regulation of maternal iron homeostasis during pregnancy and lactation. To determine if HIF-2α is essential for maternal and neonatal iron homeostasis, hematological parameters in pregnant and lactating Hif-2αIntKO mice and their offspring were assessed. No significant difference was found in serum or liver iron in pregnant and lactating Hif-2αIntKO mice (Fig. S1 A and B). However, complete blood count (CBC) analysis revealed a moderate but significant decrease in the RBC, hemoglobin (Hb), and hematocrit (HCT) levels during pregnancy as well as 1 d postpartum in Hif-2αIntKO mice (Fig. S1C). Maternal anemia is associated with stillbirths, decreased birth weight, and anemia in the offspring (28). The moderate anemia in Hif-2αIntKO mice during pregnancy did not affect litter size in Hif-2αIntKO mice monitored over a 6-mo period (Fig. S2A). In addition, no difference in the birth weight or CBC parameters were observed in pups born to Hif-2αIntKO mice at 1 d old (Fig. 1A and Fig. S2B). To assess the role of maternal HIF-2α on postnatal growth, body weight was monitored in pups born to Hif-2αF/F and Hif-2αIntKO mothers. The growth (body weight gain) was significantly delayed until weaning in pups nursing from Hif-2αIntKO mothers compared with pups nursing from Hif-2αF/F dams (Fig. 1B). The body weight of pups from Hif-2αF/F and Hif-2αIntKO dams were similar at 45 d, when placed on a regular diet following weaning at 3 wk of age (Fig. 1B). The pups nursing from Hif-2αIntKO dams also displayed truncal hair loss (Fig. 1C). Truncal hair loss persisted until weaning and the pups gained their hair coat when placed on standard chow diet. Decreased growth rate and truncal alopecia are signs of anemia in mice (29). To investigate whether pups nursing from Hif-2αIntKO mothers develop anemia, CBC analysis was performed in pups at 10, 20, 30, and 45 d postpartum. RBC, hemoglobin (Hb), hematocrit (HCT), and mean corpuscular volume (MCV) were significantly decreased in pups nursing from Hif-2αIntKO mothers until 3 wk after weaning (Fig. 1D and Fig. S2C). However, no signs of anemia were observed between the groups in 45-d-old pups, when placed on a regular diet following weaning at day 21. Interestingly, anemia in the pups was independent of pup genotype, suggesting that intestinal HIF-2α in the pups is dispensable for iron absorption from milk. We further assessed whether maternal HIF-1α is essential for neonatal iron homeostasis using mice with an intestine-specific disruption of HIF-1α (Hif-1αIntKO). CBC analysis revealed no signs of anemia in pups nursing from Hif-1αIntKO mothers (Fig. 1E). In addition, hepatocyte disruption of HIF-1α or HIF-2α in the mothers did not cause anemia in their offspring (Fig. S3 A and B). Collectively, these data suggest that maternal HIF-2α specifically in the intestine is critical for proper neonatal development.
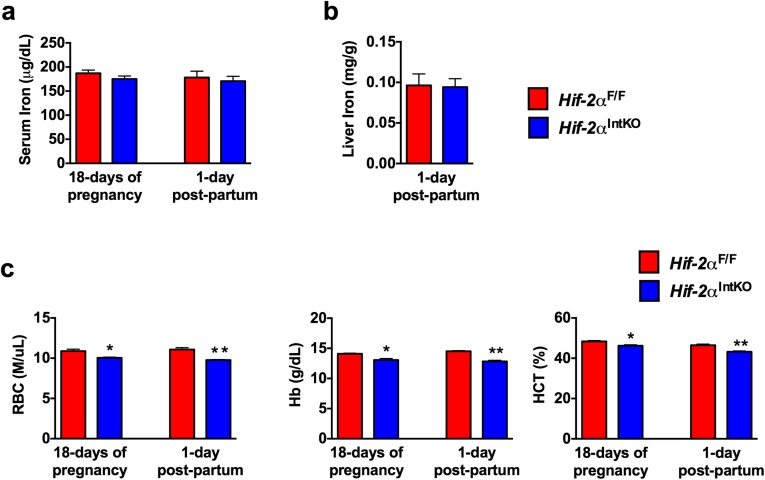
Fig. S1.
Disruption of intestinal HIF-2α results in maternal anemia. (A) Serum iron of HIF-2αF/F and HIF-2αIntKO mice at day 18 of pregnancy and at 1 d postpartum. (B) Liver iron of HIF-2αF/F and HIF-2αIntKO mice at 1 day postpartum. (C) Red blood cells (RBCs), hemoglobin (Hb), and hematocrit (HCT) levels of HIF-2αF/F and HIF-2αIntKO mice at day 18 of pregnancy and at 1 d postpartum. Each bar represents the mean value ± SEM *P < 0.05 vs. HIF-2αF/F.
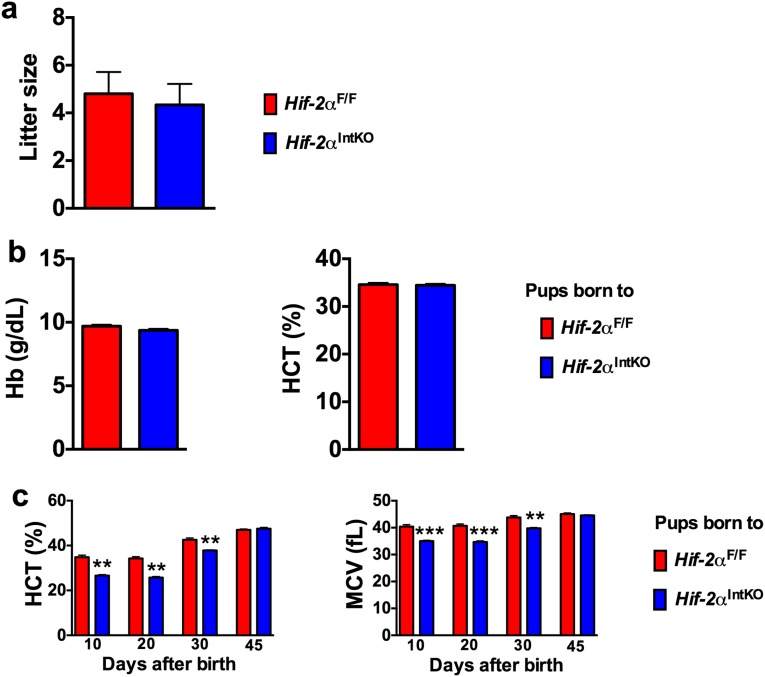
Fig. S2.
Disruption of maternal HIF-2α in intestine does not affect litter size or neonatal iron homeostasis at birth. (A) Average litter size monitored in HIF-2αF/F or HIF-2αIntKO dams for 6 mo. (B) Hb and HCT values of HIF-2αF/F and HIF-2αIntKO pups at day 1 after birth. (C) HCT and MCV assessed in the pups from Hif-2αF/F and Hif-2αIntKO mothers before weaning (10 and 20 d) and after weaning (30 and 45 d). Pups were placed on a regular chow diet after weaning. Each bar represents the mean value ± SEM *P < 0.05 vs. Hif-2αF/F; **P < 0.01 vs. Hif-2αF/F; ***P < 0.001 vs. Hif-2αF/F.
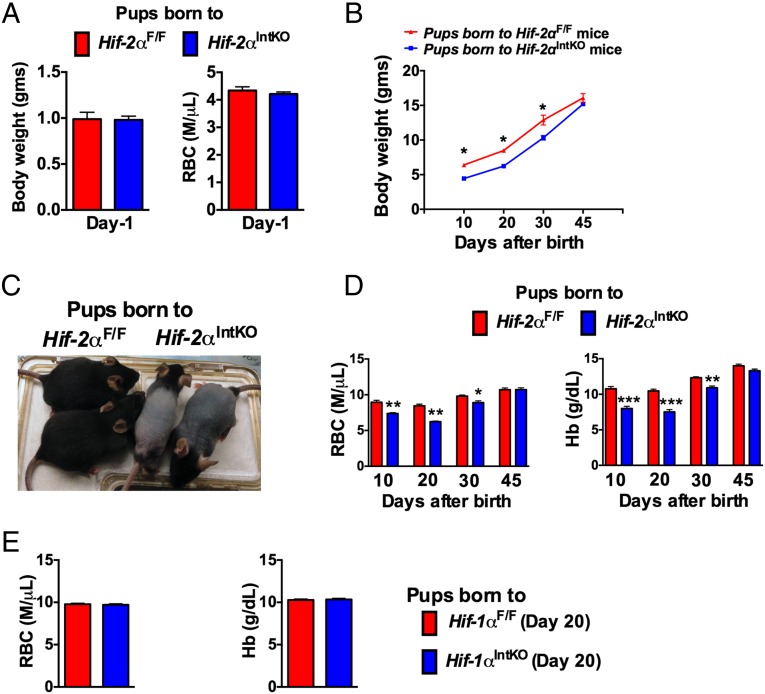
Fig. 1.
Pups nursing from mice with an intestinal disruption of HIF-2α exhibit neonatal anemia. (A) Body weight and RBC count of 1-d-old Hif-2αF/F and Hif-2αIntKO pups. (B) Body weight monitored in Hif-2αF/F and Hif-2αIntKO pups. (C) Appearance of pups nursing from Hif-2αIntKO or Hif-2αF/F mothers at the time of weaning. (D) RBC and hemoglobin (Hb) assessed in the pups from Hif-2αF/F and Hif-2αIntKO mothers before weaning (10 and 20 d) and after weaning (30 and 45 d). Pups were placed on a regular chow diet after weaning. Each bar represents the mean value ± SEM *P < 0.05 vs. HIF-2αF/F; **P < 0.01 vs. HIF-2αF/F; ***P < 0.001 vs. HIF-2αF/F. (E) CBC analyses performed in 20-d-old pups born to HIF-1αIntKO dams. Four to six litters per panel were assessed for each genotype.
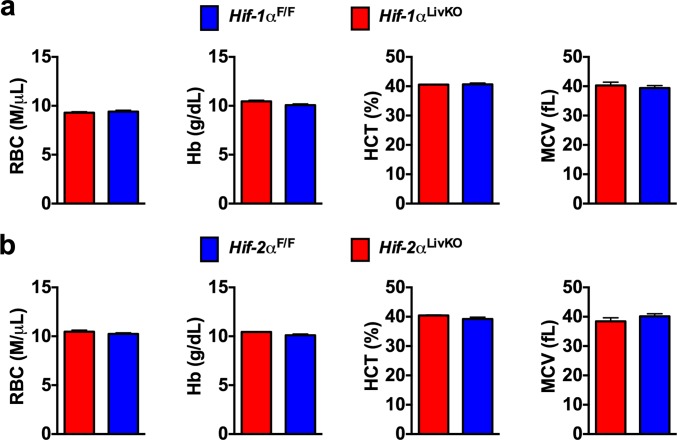
Fig. S3.
Maternal HIF-1α or HIF-2α in the liver is not required for neonatal iron homeostasis. CBC analyses of 20-d-old pups born to (A) Hif-1αLivKO dams and (B) Hif-2αLivKO dams.
Iron Stores at Birth Are Inadequate to Support Neonatal Iron Demand.
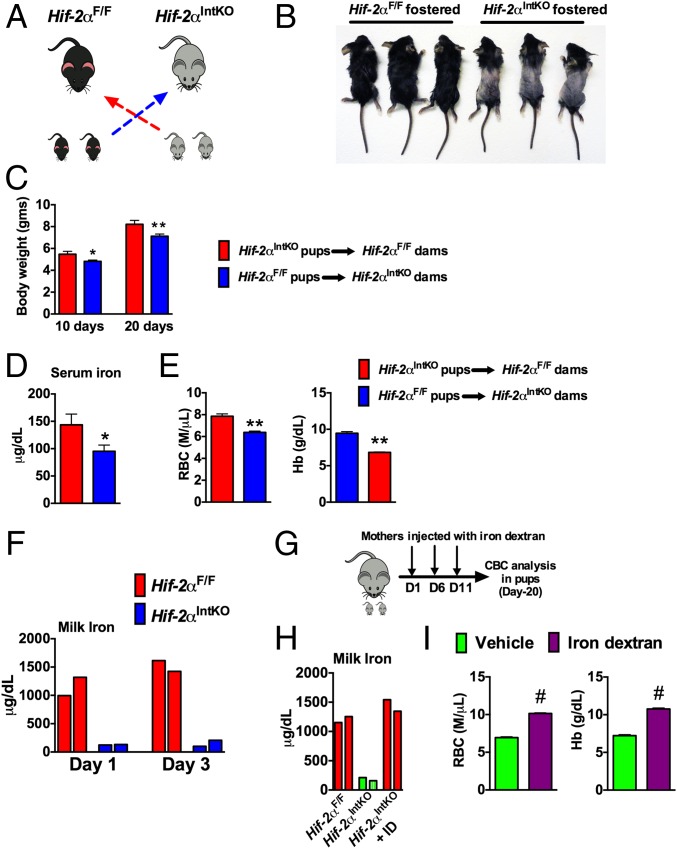
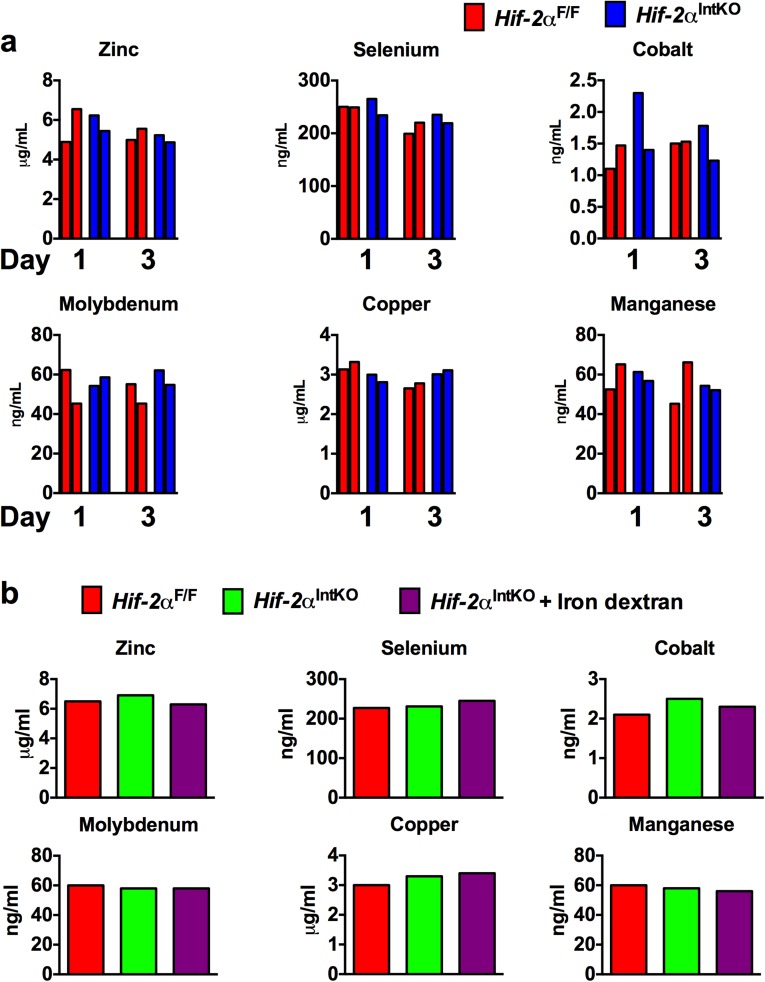
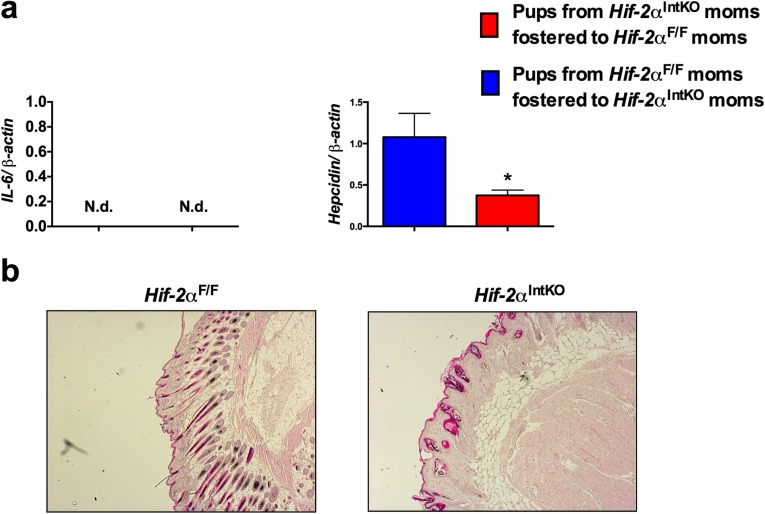
It has been proposed that newborns are born with adequate iron reserves in the liver and spleen to meet iron demands early in their growth and development (30, 31). To investigate whether decreased iron stores led to anemia in Hif-2αIntKO pups, cross-fostering experiments were performed immediately after birth (Fig. 2A). Cross-fostering pups born to Hif-2αF/F dams with Hif-2αIntKO mothers resulted in truncal alopecia and decreased growth rate (Fig. 2 B and C), but not in pups born to Hif-2αIntKO mothers cross-fostered with Hif-2αF/F dams. In addition, serum iron levels were significantly decreased in pups cross-fostered with Hif-2αIntKO dams (Fig. 2D), which is accompanied by a significant decrease in RBC parameters (Fig. 2E). Inductively coupled plasma mass spectrometry (ICP-MS) was performed in pooled breast milk from five lactating Hif-2αF/F and Hif-2αIntKO mothers in two independent cohorts at day 1 and day 3 postpartum for detailed micronutrient analysis. Iron content in the milk was lower in Hif-2αIntKO mice compared with Hif-2αF/F mice (Fig. 2F). No significant change was observed for other micronutrients in the milk of Hif-2αIntKO mice (Fig. S4A). To definitively determine if neonatal anemia is caused by dysregulated maternal iron homeostasis, Hif-2αIntKO mothers were injected with iron dextran intraperitoneally starting on day 1 after parturition (Fig. 2G). ICP-MS in the milk revealed a significant increase in milk iron content in iron dextran-injected Hif-2αIntKO mice assessed at 3 d postpartum (after one injection of iron dextran) (Fig. 2H); however, no significant change was observed for other micronutrients in the milk of iron-supplemented Hif-2αIntKO mice (Fig. S4B). Iron supplementation ameliorated anemia in pups nursing from Hif-2αIntKO mothers compared with vehicle-treated Hif-2αIntKO mothers (Fig. 2I). Previous work has demonstrated inflammatory lipids in the milk as a potential cause for retarded growth and alopecia in neonates (32). However, Il6 mRNA levels were undetectable in both the groups and the expression of hepcidin was significantly decreased in the livers of pups fostered to Hif-2αIntKO mothers (Fig. S5A). The skin showed decreased active hair follicles in Hif-2αIntKO pups without any signs of inflammation (Fig. S5B). Collectively, these data suggest that maternal HIF-2α is essential to maintain iron homeostasis in lactating mothers.
Fig. 2.
Maternal HIF-2α in the intestine is essential during lactation for the growth and development of nursing pups. (A) Experimental design in which pups born to Hif-2αIntKO and Hif-2αF/F dams were cross-fostered immediately after birth (day 1). (B) Appearance of 20-d-old pups cross-fostered to Hif-2αF/F or Hif-2αIntKO dams. (C) Body weight monitored at 10 and 20 d after cross-fostering. (D) Serum iron and (E) CBC analysis of 20-d-old pups cross-fostered to Hif-2αF/F or Hif-2αIntKO dams. Five to six litters were assessed per genotype. (F) Milk iron assessed by ICP-MS from Hif-2αF/F and Hif-2αIntKO mothers at day 1 and day 3 postpartum and each bar represents analysis in pooled milk from five mice. (G) Experimental design of the iron rescue experiment. (H) Milk iron assessed by ICP-MS in Hif-2αF/F, Hif-2αIntKO, and iron dextran-injected Hif-2αIntKO at day 3 after parturition. Each bar represents analysis in pooled milk from five mice. (I) CBC analysis of 20-d-old pups nursing from Hif-2αIntKO mothers injected intraperitoneally with either vehicle or iron dextran immediately after parturition. Five to six litters were assessed per genotype. With the exception of the pooled milk analysis, each bar represents the mean value ± SEM *P < 0.05 vs. Hif-2αF/F; **P < 0.01 vs. Hif-2αF/F. #P < 0.001 vs. vehicle.
Fig. S4.
Disruption of maternal HIF-2α in intestine affects the micronutrient levels in milk. (A) Mineral analysis in the milk from Hif-2αF/F and Hif-2αIntKO mothers at day 1 and day 3 postpartum using inductively coupled plasma mass spectrometry. (B) ICP-MS performed in the milk from Hif-2αF/F, Hif-2αIntKO, and iron dextran-injected Hif-2αIntKO at day 3 after parturition. Each bar represents analysis in pooled milk from five mice.
Fig. S5.
Truncal alopecia is due to anemia but not mediated by local or systemic inflammation. (A) qPCR analysis of hepcidin and IL-6 in the livers of 20-d-old pups cross-fostered to either Hif-2αF/F or Hif-2αIntKO dams. Each bar represents the mean value ± SEM *P < 0.05 vs. Hif-2αF/F. ND, not detectable. (B) H&E staining of skin from 20-d-old pups born to Hif-2αF/F or Hif-2αIntKO dams.
Neonatal Iron Stores Are Rapidly Depleted in the Absence of Intact Intestinal HIF-2α Signaling in Lactating Mothers.
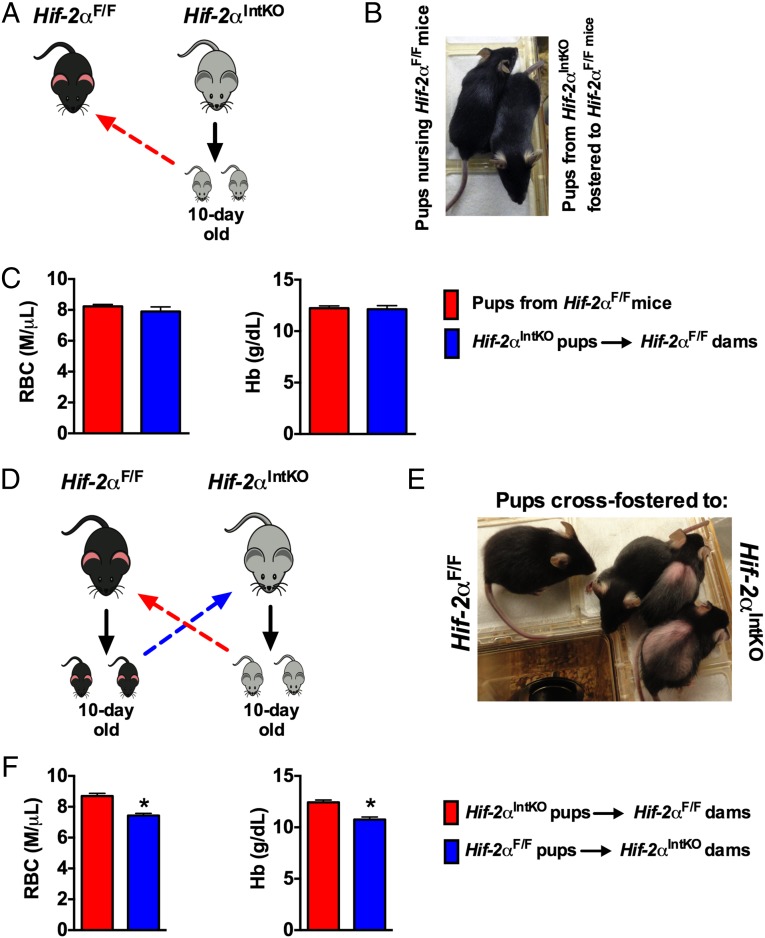
To further demonstrate that neonatal anemia is reversible if maternal iron homeostasis during lactation is intact, 10-d-old pups born to Hif-2αIntKO mothers were fostered to Hif-2αF/F mothers (Fig. 3A). Fostering to Hif-2αF/F mothers restored the hair coat (Fig. 3B) and ameliorated anemia in pups born to Hif-2αIntKO mothers (Fig. 3C). In addition, 10-d-old pups born to Hif-2αF/F or Hif-2αIntKO mothers were cross-fostered (Fig. 3D). This resulted in severe hair loss and anemia in pups born to Hif-2αF/F mothers and fostered to Hif-2αIntKO mothers, whereas reciprocal fostering completely restored the hair loss and the anemia (Fig. 3 E and F). These data emphasize that neonatal iron stores are rapidly depleted and maternal HIF-2α is essential to prevent anemia in nursing pups.
Fig. 3.
Delayed cross-fostering experiments demonstrate the rapid manner by which maternal HIF-2α regulates neonatal iron homeostasis. (A) Experimental design in which 10-d-old pups born to HIF-2αF/F dams were fostered to HIF-2αIntKO dams for an additional 11 d (until weaning). (B) Photographs of pups fostered to HIF-2αF/F dams. (C) CBC analyses in HIF-2αIntKO pups fostered to HIF-2αF/F dams for 11 d. (D) Experimental design in which 10-d-old pups born to HIF-2αIntKO dams were fostered to HIF-2αF/F dams for an additional 11 d (until weaning). (E) Photographs of pups at 11 d after fostering to HIF-2αF/F dams. (F) CBC analyses in HIF-2αIntKO pups fostered to HIF-2αF/F dams for 11 d. Each bar represents the mean value ± SEM *P < 0.01 vs. HIF-2αF/F. Three to five litters were assessed per cross-fostering experiment.
Intestinal DMT-1 Is Essential in the Mother for Adequate Iron Delivery to Suckling Pups and in the Pups for Adequate Dietary Iron Absorption.
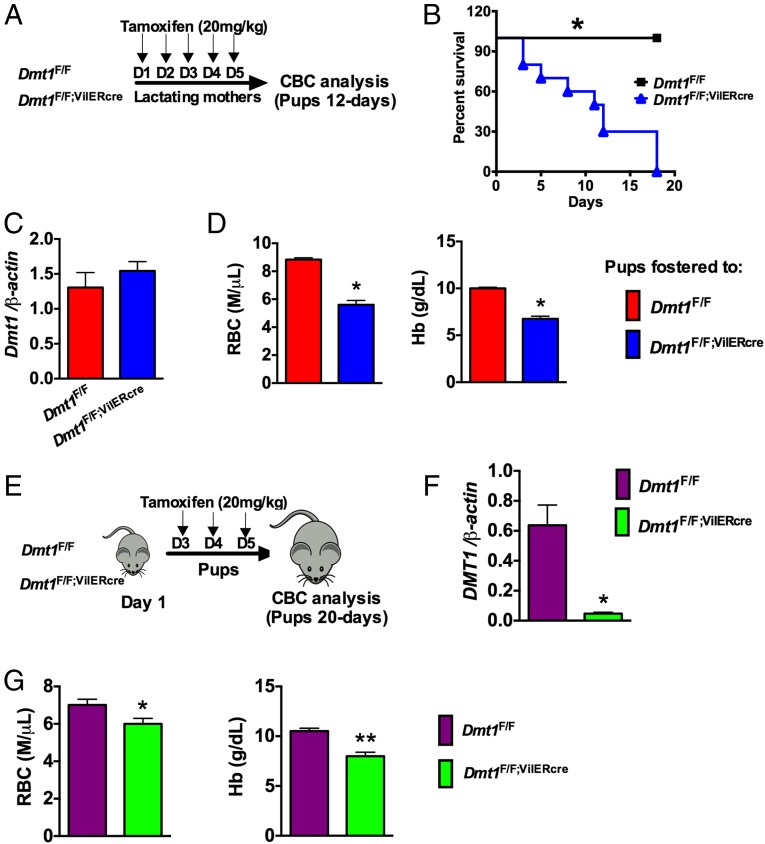
The iron transporter, divalent metal transported-1 (DMT-1) is expressed on the apical surface of the intestine and is required for maintaining systemic iron homeostasis (33). Dmt-1 expression is regulated by systemic iron demand through a HIF-2α–dependent mechanism (17, 18). To investigate if DMT-1 is the critical downstream target of HIF-2α in the regulation of maternal iron homeostasis, mice with a tamoxifen-inducible intestinal epithelial disruption of DMT-1 (Dmt1F/F;VilERcre) were assessed. Dmt1F/F or Dmt1F/F;VilERcre mothers were treated with tamoxifen 1 d following parturition. We observed a significant increase in pup mortality and neonatal anemia in pups nursing from mothers with an intestinal disruption of DMT-1 (Dmt1F/F;VilERcre) (Fig. 4 A and B). Injecting the mothers with tamoxifen did not affect the expression of intestinal Dmt-1 in the pups, demonstrating that tamoxifen did not excrete into the breast milk (Fig. 4C) and disruption of maternal DMT-1 expression resulted in significant decrease in RBC and Hb (Fig. 4D). To assess if DMT-1 expression in neonatal intestine is essential for iron homeostasis during the nursing period, 3-d-old Dmt1F/F;VilERcre and Dmt1F/F pups were treated with tamoxifen (Fig. 4E), and Dmt1 gene expression (Fig. 4F) and CBC analysis (Fig. 4G) were assessed at the time of weaning. Treatment of Dmt1F/F;VilERcre pups with tamoxifen resulted in a significant decrease in intestinal Dmt1 (Fig. 4F), which is associated with severe anemia as observed by significant decreases in RBC and Hb (Fig. 4G). Together, these data demonstrate that intestinal iron absorption via DMT-1 is critical for adequate levels of iron in breast milk and intestinal iron absorption from breast milk.
Fig. 4.
Intestinal iron absorption via DMT-1 is critical for maternal and neonatal iron homeostasis. (A) Experimental design in which Dmt-1F/F and Dmt-1F/F;VilERcre mothers were injected with 20 mg/kg tamoxifen on 5 consecutive days starting at day 1 postpartum and pups were assessed at day 12 after birth. (B) Survival curve of the pups fostered to tamoxifen-injected Dmt-1F/F and Dmt-1F/F;VilERcre mothers. (C) pPCR analysis demonstrating that intestinal DMT-1 expression in Dmt-1F/F;VilERcre pups is not affected by administration of tamoxifen to the mothers. (D) CBC analysis of pups nursing from tamoxifen-treated Dmt-1F/F and Dmt-1F/F;VilERcre mothers. Four to five litters were assessed per genotype. (E) Experimental design in which Dmt-1F/F and Dmt-1F/F;VilERcre pups were injected with 20 mg/kg tamoxifen on 3 consecutive days starting at day 3 after birth. (F) qPCR analysis for Dmt-1 in the duodenum of Dmt-1F/F and Dmt-1F/F;VilERcre pups that were treated with tamoxifen. (G) CBC analysis of Dmt-1F/F and Dmt-1F/F;VilERcre pups treated with tamoxifen. Six to eight pups were assessed per genotype. Gene expression was normalized to β-actin and each bar represents the mean value ± SEM *P < 0.05 vs. DMT-1F/F; **P < 0.01 vs. DMT-1F/F.
Maternal Iron Deficiency Impairs Cognitive Function in Nursing Pups.
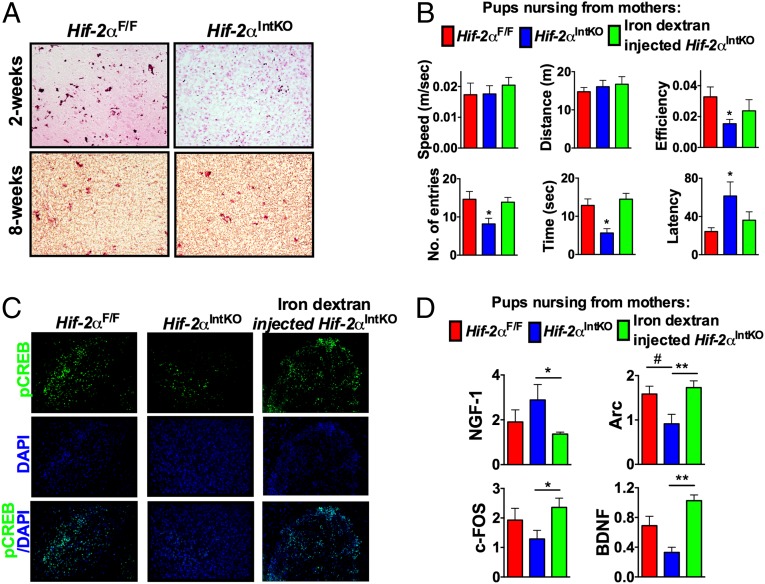
Perinatal iron deficiency in neonates has been consistently associated with cognitive dysfunction in infants and young adults (34, 35). Due to a lack of animal models of perinatal anemia, prior studies have been unable to determine if iron deficiency predisposes neonates to neuronal dysfunction. Hif-2αIntKO mice provide an ideal model that could delineate whether iron deficiency in milk due to maternal anemia contributes to long-term cognitive defects in neonates. Brain iron assessed using enhanced Prussian blue staining revealed an intense iron staining in the hippocampus of the Hif-2αF/F pups at 2 wk of age (Fig. 5A). Iron levels were significantly decreased in the hippocampus of pups nursing from Hif-2αIntKO mothers, whereas no difference in iron staining was observed between the groups at 2 mo of age (Fig. 5A). High brain iron levels during the neonatal period are essential to promote neurogenesis and neurobehavioral function in mice (36, 37). Therefore, to test the long-term effects of neonatal iron deficiency on cognitive function, a novel object recognition test was performed in mice that were weaned from Hif-2αF/F, Hif-2αIntKO, or iron dextran-treated Hif-2αIntKO mothers at day 21 and maintained on chow diet until 8 wk. This age was assessed as the pups born from Hif-2αIntKO mothers do not show any differences with respect to CBC and body weight in comparison with pups from Hif-2αF/F breeders (Fig. 1 D and E). The motor activity measured by the average speed and distance traveled by the mice during the experiment was not significantly affected in Hif-2αIntKO pups (Fig. 5B). However, measures of cognitive function such as number of entries to novel object zone, time spent with novel object, and latency to novel object were significantly affected in pups born from Hif-2αIntKO mothers, suggesting cognitive defects (Fig. 5B). Moreover, the cognitive defects were completely restored in pups nursing from Hif-2αIntKO mothers supplemented with iron during lactation (Fig. 5B). The hippocampus is responsible for the spaciotemporal recognition and cohesive memory of novel objects with respect to environmental cues (38). Neurogenesis especially in the hippocampus continues even after birth to establish hippocampal circuitry and plasticity (39). Evidence suggests that activation of cAMP response element binding (CREB) signaling plays a key role in brain development by regulating the expression of various genes and growth factors involved in neuronal plasticity, synapse formation, neurogenesis, cognition, and long-term memory consolidation (40, 41). Consistent with decreased brain iron levels and impaired cognitive function, immunostaining analysis revealed decreased levels of phospho-CREB in the CA3 region of hippocampus, which is responsible for long-term spaciotemporal memory in mice (Fig. 5C) (40, 41). Further analysis revealed no difference in the expression of nerve growth factor (NGF) (Fig. 5D); however, the CREB target gene Arc is significantly decreased in the hippocampus (Fig. 5D). In addition, a trend toward decreased expression of c-Fos and Bdnf in the hippocampus of pups nursing from Hif-2αIntKO mothers was also noted (Fig. 5D). Iron supplementation of Hif-2αIntKO mothers during lactation completely restored pCREB signaling and expression of CREB target genes in the hippocampus of their offspring (Fig. 5 C and D). Collectively, the data demonstrate that breast milk iron is essential for long-term cognitive function in the neonates.
Fig. 5.
Neonatal anemia in Hif-2αIntKO pups leads to long-term cognitive defects. (A) Brain iron staining performed using enhanced Prussian blue stain in 2-wk-old and 8-wk-old pups born to Hif-2αF/F and Hif-2αIntKO mothers. The 8-wk-old pups were nursing from Hif-2αF/F and Hif-2αIntKO mothers until weaning at 21 d, following which the pups were placed on a regular chow diet. (B) Cognitive test performed using ANY-Maze in 8-wk-old pups born to Hif-2αF/F, Hif-2αIntKO, or iron dextran-injected Hif-2αIntKO mothers. (C) Immunostaining for phospho-CREB in the CA3 region of hippocampus. (D) qPCR analysis of nerve growth factor-1 (NGF-1), activity-regulated cytoskeleton-associated protein (Arc), c-FOS, and brain-derived neurotrophic factor (BDNF) in the hippocampus of 8-wk-old mice born to Hif-2αF/F, Hif-2αIntKO, or iron dextran-injected Hif-2αIntKO mothers. Expression was normalized to β-actin and each bar represents the mean value ± SEM #P < 0.05 vs. HIF-2αF/F; *P < 0.05 vs. HIF-2αIntKO; **P < 0.01 vs. HIF-2αIntKO. Seven to nine litters were assessed per genotype.
Intestinal Sensing of Iron Status and Up-Regulation of Genes Participating in Iron Absorption Meet Iron Demands of Lactation.
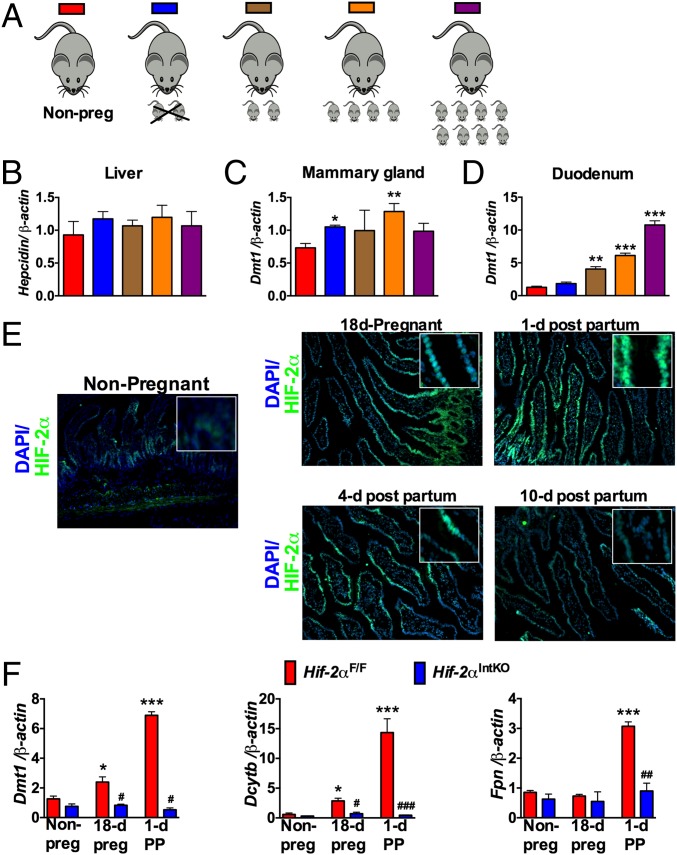
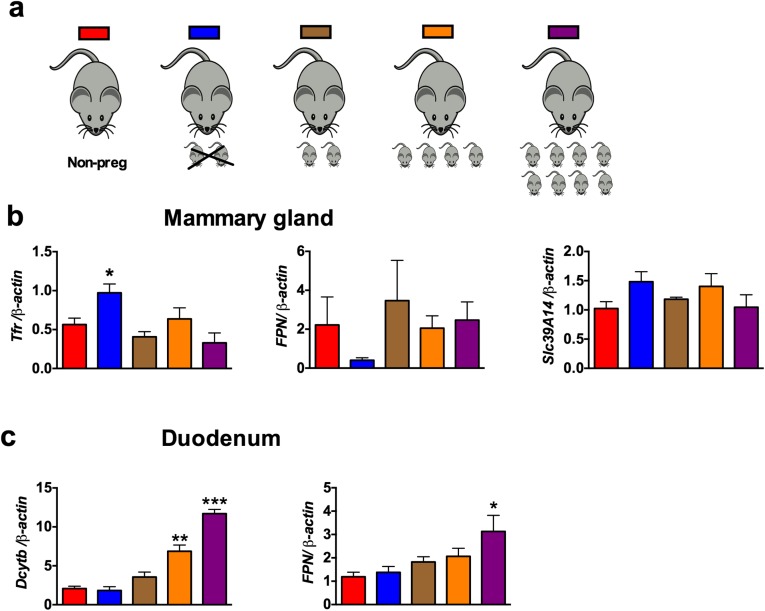
The data thus far demonstrate a critical role of intestine in the regulation of milk iron content. However, it is not clear if the intestine responds to systemic iron demand during lactation. Liver as well as local regulation in the mammary gland may also be important. Therefore, to test the central role of the intestine in maternal iron homeostasis, the expression of iron transport genes was assessed in the liver, mammary gland, and duodenum of nonpregnant females, mothers from which pups were removed immediately after parturition or mothers that were allowed to nurse with a set number of pups for 36 h (Fig. 6A). High iron demand due to an increase in nursing pups did not significantly affect the mRNA levels of liver hepcidin (Fig. 6B) or mammary gland iron transport genes such as Dmt1, transferrin receptor (Tfr1), Fpn, and recently identified iron transporter Slc39a14 (42) (Fig. 6C and Fig. S6). However, mRNA levels of Dmt1 and Dcytb were significantly increased in the maternal intestine by increasing the numbers of nursing pups, compared with nonpregnant mice and mice in which pups were removed immediately after parturition (Fig. 6D and Fig. S6). A conserved response in both humans and animals is a transient decrease in RBC levels and CBC parameters late in pregnancy (26). To assess if this moderate and transient anemia during pregnancy and lactation is associated with an increase in intestinal HIF-2α, immunostaining was performed in the intestine of pregnant and lactating mice. Intense HIF-2α staining was observed in the intestinal epithelial cells during pregnancy and lactation (Fig. 6E). Consistent with the increase in HIF-2α, the mRNA levels of iron absorption genes, Dmt1, Dcytb, and Fpn were significantly induced in the duodenum of Hif-2αF/F mice during pregnancy and 1 d postpartum, but the increase in iron absorption genes was completely abrogated in Hif-2αIntKO mice (Fig. 6F). Taken together, the data suggest that the intestine responds to iron demands during the perinatal and lactation periods through HIF-2α–mediated increase in iron absorption genes.
Fig. 6.
Intestinal HIF-2α and iron absorptive genes are induced in response to systemic iron demand during lactation. (A) Schematics where nonpregnant females, mothers with pups removed for 3 d, or lactating mothers fostered with zero, two, four, and eight pups for 3 d were assessed. (B) qPCR analysis of Hepc in the liver, Dmt-1 in the (C) mammary gland, and (D) duodenum of nonpregnant females and mothers where the pups were removed or mothers nursing a different number of pups. Expression was normalized to β-actin and each bar represents the mean value ± SEM *P < 0.05 vs. nonpregnant females; **P < 0.01 vs. nonpregnant females; ***P < 0.001 vs. nonpregnant females. Four to six mice were assessed per group. (E) Immunostaining for HIF-2α in the small intestine of nonpregnant, pregnant (18 d), and lactating mothers (at day 1, 4, and 10 postpartum). Inset shows higher magnification. (F) qPCR analysis of DMT-1, Dcytb, and FPN in the small intestine of Hif-2αF/F and Hif-2αIntKO mice during gestation and 1 d postpartum. Expression was normalized to β-actin and each bar represents the mean value ± SEM *P < 0.05, ***P < 0.001 vs. nonpregnant; #P < 0.05 vs. HIF-2αF/F; ##P < 0.01 vs. HIF-2αF/F; and ###P < 0.001 vs. HIF-2αF/F. Five to eight female mice were assessed for each panel.
Fig. S6.
Intestine responds to systemic iron demand during lactation. (A) Schematics where nonpregnant females, mothers with pups removed for 3 d, or lactating mothers fostered with zero, two, four, and eight pups for 3 d were assessed. (B) qPCR analysis for Transferin receptor (Tfr), Fpn1, and Slc9A14 in the mammary gland of nonpregnant and lactating mothers with pups removed or nursing two, four, and eight pups for 3 d. (C) qPCR analysis for dcytb and Fpn1 in the duodenum of nonpregnant and lactating mothers with pups removed or nursing two, four, and eight pups for 3 d. Expression was normalized to β-actin and each bar represents the mean value ± SEM. *P < 0.05 vs. nonpregnant females; **P < 0.01 vs. nonpregnant females; ***P < 0.001 vs. nonpregnant females. Four to six mice were assessed per group.
Activation of Intestinal HIF-2α Signaling Increases Milk Iron During Lactation.
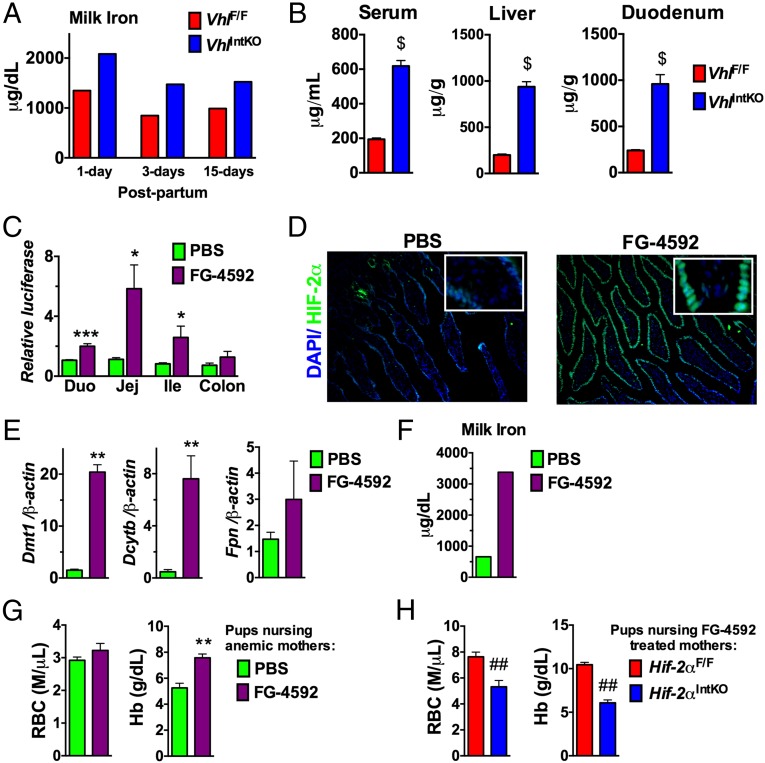
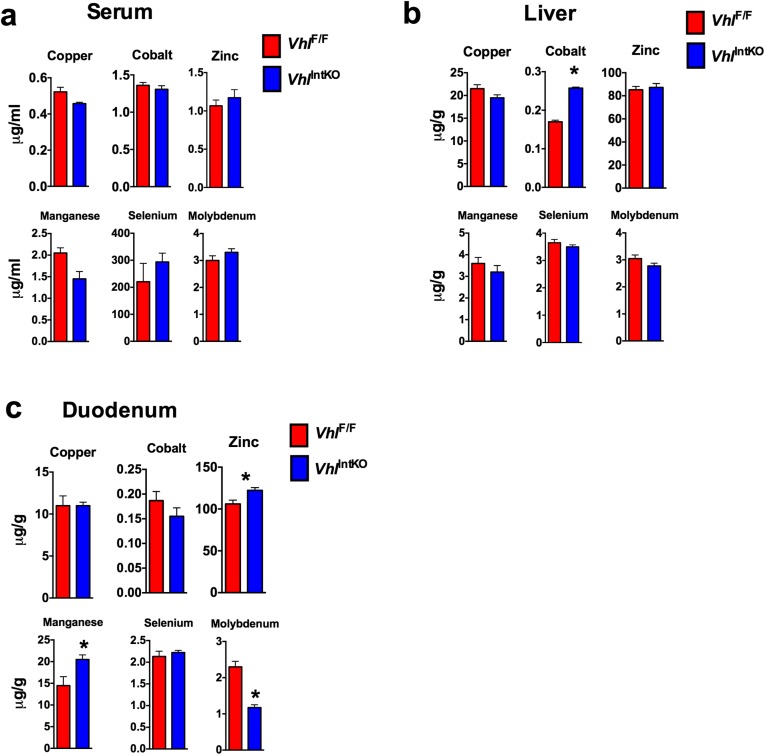
To demonstrate if intestinal HIF-2α is sufficient in driving breast milk iron content, genetic and pharmacological approaches to activate HIF signaling were assessed. The Von Hippel-Lindau (VHL) tumor suppressor protein is the critical regulator of HIF degradation. Disruption of VHL in the intestine stabilizes HIF-2α and increases iron absorption (17, 20). Stabilization of HIF significantly increased the iron content of milk in VhlIntKO mice (Fig. 7A). In addition, iron levels in the serum, liver, and duodenum were significantly increased in VhlIntKO mice (Fig. 7B). Micronutrient profiling using ICP-MS revealed iron was the major metal regulated by intestinal HIFs; however, marginal yet significant changes were also observed in other micronutrients (Fig. S7). To test whether activation of HIF-2α by pharmacological intervention could improve milk iron, lactating mothers were treated with a HIF prolyl hydroxylase (PHD) inhibitor, FG-4592. Currently FG-4592 is under phase III clinical trial to treat anemia in patients with chronic renal failure (43). Whether FG-4592 could stabilize intestinal HIF signaling was assessed using highly sensitive HIF reporter mice. The reporter mice have an oxygen-dependent degradation domain (ODD) of HIF fused with the luciferase gene, thus factors that stabilize HIFs will increase the luciferase expression in the target tissue (21). Treatment with FG-4592 significantly increased the luciferase activity in the duodenum, jejunum, and ileum but not in the colon, suggesting stabilization of HIF in those tissues (Fig. 7C). In addition, immunostaining revealed an intense nuclear staining of HIF-2α in the intestine of mice treated with FG-4592 (Fig. 7D). Stabilization of intestinal HIF-2α by FG-4592 resulted in a significant increase in the expression of Dmt1 and Dcytb, but not Fpn (Fig. 7E). The increase in iron absorption genes correlated with a threefold increase in milk iron content by FG-4592 treatment (Fig. 7F). Mice are resistant to iron-deficiency anemia and long-term iron deprivation in C57BL/6 mice led to reduced litter size and high rate of stillbirths. Similarly, phenylhydrazine (PHZ)-induced anemia also resulted in a significant loss in litters in the C57BL/6 mice. Therefore, to test whether FG-4592 could improve neonatal iron homeostasis in anemic mothers, the FVB/NJ strain was used. Female breeders were first treated with PHZ to induce anemia and then placed immediately on iron-deficient diets. Expected litter sizes were observed following treatment, however 15-d-old pups had marked anemia (Fig. 7G). Mothers at day 1 following parturition were gavaged with FG-4592 and CBC analysis was assessed in the pups. FG-4592 treatment of anemic mothers significantly ameliorated anemia in nursing pups as assessed by CBC analysis (Fig. 7G). Although RBC levels were not significantly improved, there was an increase in Hb, HCT (PBS, 15.40 ± 0.62 vs. FG-4592, 18.5 ± 1.67; P < 0.07) and MCV (PBS, 52.77 ± 0.38 vs. FG-4592, 60.50 ± 1.76; P < 0.001) (Fig. 7G). To rule out the systemic effects of FG-4592 in iron homeostasis, Hif-2αIntKO mothers were assessed similarly. CBC analysis revealed no improvement in the anemia in the pups nursing from FG-4592 Hif-2αIntKO mothers (Fig. 7H). Taken together, these data demonstrate that increasing intestinal HIF-2α by genetic and pharmacological approaches increases milk iron content during lactation and improves iron homeostasis in nursing pups.
Fig. 7.
Activation of intestinal HIF-2α improves milk iron levels and ameliorates anemia in nursing pups. (A) Milk iron was assessed at 1, 3, and 15 d postpartum in mice with intestine-specific disruption of VHL (VhlIntKO). Each bar represents analysis in pooled milk from five mice. (B) Iron content assessed in the serum, liver, and duodenum of VhlIntKO mice at day 3 postpartum. Four to five mice per genotype were assessed. (C) Luciferase assay performed in the duodenum, jejunum, ileum, and colon of ODD-Luc mice 1 h after oral administration of FG-4592 at a dose of 60 mg/kg body weight. Luciferase values were normalized to protein content. Four to five mice per genotype were assessed. (D) Immunostaining for HIF-2α in the small intestine. Inset shows higher magnification. Four to five mice per genotype were assessed. (E) qPCR analysis of iron absorption genes in mice treated with one dose of 60 mg/kg body weight FG-4592. Expression was normalized to β-actin. Four to five mice per treatment group were assessed. (F) Milk iron assessed in mice treated with four doses of 60 mg/kg body weight FG-4592 on alternate days. Each bar represents analysis in pooled milk from five mice. (G) CBC analysis of pups nursing from PHZ-induced anemic mice that were treated with FG-4592. (H) CBC analysis of pups nursing from Hif-2αF/F or Hif-2αIntKO mice that were treated with four doses of 60 mg/kg body weight FG-4592 on alternate days. Three to four litters were assessed per genotype or treatment. With the exception of the pooled milk analysis, each bar represents the mean value ± SEM $P < 0.001 vs. VhlF/F *P < 0.05 vs. PBS; **P < 0.01 vs. PBS; ***P < 0.001 vs. PBS; ##P < 0.01 vs. Hif-2αF/F.
Fig. S7.
Activation of maternal HIF-2α in intestine affects the micronutrient levels in various tissues. Mineral analysis performed in the (A) serum, (B) liver, and (C) duodenum of VhlIntKO mothers postpartum using inductively coupled plasma mass spectrometry.
Discussion
The beneficial effects of breastfeeding in neonatal development have been well characterized. Breastfeeding is recommended for the first 6 mo of life and is the main source of nutrients for neonates. However, very little is known about the mechanisms that are needed to sense and maintain nutrient levels in breast milk. The present work demonstrates an important role of the intestine through activation of HIF-2α in sensing maternal iron requirements during lactation and increasing an adaptive iron absorptive response, which helps to maintain adequate levels of milk iron. Moreover, the role of breast milk iron in neonatal development was hitherto unclear and now the present work clearly demonstrates an essential role of maternal iron delivery via milk in postnatal development.
Iron deficiency during pregnancy has several negative impacts on pregnant females including maternal deaths associated with anemia (28). Iron absorptive genes increase during pregnancy to maintain maternal iron homeostasis (16, 44). Intestinal HIF-2α plays a key role in the adaptive increase in iron absorption during high iron demands (2, 3). However, mice with disruption of intestinal HIF-2α exhibited only moderate anemia during the perinatal period. These data are consistent with previous work that demonstrates a decrease in maternal hepcidin as the major mechanism by which iron demands are met during pregnancy (14).
At the time of parturition and immediately after delivery, there is an increase in iron demand due to a loss of iron through bleeding during delivery and to supply iron to the mammary gland for milk iron. During the perinatal period, hepcidin levels are very low; therefore, hepcidin-independent mechanisms are required to avoid acute iron deficiencies and maintain maternal iron homeostasis. HIF-2α–dependent increase in maternal intestinal iron absorption serves as a major mechanism to maintain systemic iron homeostasis and consequently milk iron content during lactation. Micronutrient profiling revealed a robust decrease in iron content of breast milk in mice with an intestinal disruption of HIF-2α. Although iron levels have been shown to be important in regulation of other micronutrients, no significant change was observed in any other metals assessed (45). DMT-1, FPN, and TFR-1 are expressed in the mammary gland and transport iron to milk. Consistent with prior studies (23, 46), high iron demand during lactation has no profound effect on the expression of iron transporters in the mammary gland or liver hepcidin. Indeed, serum hepcidin levels return to normal levels immediately after delivery (15), where intestinal HIF-2α signaling uncouples a repressive effect of hepcidin on intestinal iron absorption. The present work shows that intestinal HIF-2α is not only a critical regulator, but also an essential sensor of maternal iron demand during lactation and essential to maintain adequate levels of iron in breast milk by enhancing the expression of intestinal iron absorption genes. The mechanism by which HIF-2α is robustly induced during lactation is not currently clear. In both human and animal studies, a transient anemia is associated with late pregnancy and during lactation (26). An important consideration not explored in this study is the role of iron regulatory protein (IRP)-1 in regulating HIF-2α expression during late pregnancy and lactation (47–49). Future work will focus on defining the precise mechanism.
Maternal iron deficiency has major impacts on fetal and neonatal growth, irreversible cognitive defects, anemia, and immune disorders (10). The significance of iron supply from breast milk in neonatal iron homeostasis is highly controversial (30). It is not clear if neonatal health issues that arise from maternal iron deficiency are strictly mediated by fetal iron deficiency (decreased iron stores at birth) or postnatal iron deficiency (decreased iron supply from milk). Premature introduction of cow’s milk that is poor in bioavailable iron results in iron deficiency anemia and cognitive defects in infants (50, 51). These data suggest that continuous iron supply is necessary during early neonatal growth and development. Neonatal anemia and long-term cognitive defects associated with maternal iron deficiency during pregnancy could be recapitulated in pups from Hif-2αIntKO mothers, providing direct evidence that breast milk iron is essential in proper postnatal development.
The anemic phenotype of the pups nursing from Hif-2αIntKO mothers could be recapitulated by disruption of DMT-1 in intestinal epithelial cells of lactating mothers. Currently cognitive testing could not be performed due to decreased survival in pups nursing from DMT-1 disrupted mothers. This is an unexpected find because pups born from Hif-2αIntKO mothers do not show reduced survival. The decrease in survival could be attributed to a decrease in milk production by tamoxifen, in addition to a decrease in maternal iron absorption (52). These mice also provide an ideal system to understand if apical iron transport in the intestine is critical in neonates for adequate iron absorption during the suckling period. Disrupting DMT-1 expression in the intestine of pups led to a rapid development of anemia at weaning age, suggesting that DMT-1 is essential in neonatal iron absorption. This phenotype is distinct from that of intestinal disruption of HIF-2α in pups, where there was a negligible role in iron homeostasis. Similarly, in adult mice HIF-2α is not critical to maintain basal DMT-1 expression (17, 20). However, two rodent models of spontaneous loss-of-function DMT-1 mutation have been characterized, the microcytic anemia mice and the Belgrade rats. Although neonatal intestinal iron absorption has not been clearly characterized in the microcytic anemia mice, Belgrade rat newborns efficiently absorb iron (46, 53–55). It is possible that Belgrade rats have established a compensatory mechanism to maintain iron absorption, whereas those compensatory mechanisms are not established following acute disruption in pups. Future studies will focus on understanding how intestinal DMT-1 expression in pups impacts iron homeostasis and CBC parameters.
Iron supply during the nursing period is essential for brain iron levels. Failure to accumulate iron results in irreversible low iron levels in brain and associated motor, behavioral, and cognitive defects in adulthood, independent of improvement in peripheral hematological parameters in rodents and primates (56, 57). Cognitive defects persisted in pups nursing from Hif-2αIntKO mothers even after restoration of hematological parameters by feeding a regular diet after weaning. Iron dextran injection in lactating Hif-2αIntKO mothers corrected anemia and cognitive function in their offspring. The data demonstrates that maternal iron homeostasis during the nursing period is critical in the neurodevelopment of pups. Defective dendrite development, impaired neurogenesis, and loss of neuronal plasticity in the hippocampus are involved in anemia-related neurological disorders in rodents (58). Iron has a pleiotropic role in neuronal development. Iron-induced reactive oxygen species play a key role in neuronal development and plasticity by activating CaM kinase and CREB signaling pathways (59). CREB signaling in the CA3 region of hippocampus is critical for cognitive function (40, 41). Iron deficiency and cognitive defects in pups nursing from Hif-2αIntKO mothers is associated with decreased CREB signaling in the CA3 region of hippocampus.
Maternal iron homeostasis is essential both during both pregnancy and breastfeeding periods to avoid anemia and developmental defects in pups. The present work clearly demonstrates that the adaptive increase in iron absorption mediated by HIF-2α is adequate to meet the daily iron requirements of lactating mothers. The rapid postnatal growth including brain development increases iron demand in pups, which could not be adequately supplied from the neonatal iron stores. Therefore, postnatal iron supply from mothers is critical for rodent growth and development. However, in humans, entire brain development occurs before birth, and iron requirement is mainly to meet iron demand from erythropoietic activity in newborns. Therefore, cognitive defects, decreased memory function, and brain-related defects in newborns could be contributed by anemia, rather than low iron levels in the brain (35). Indeed, numerous studies have shown strong association between anemia and behavioral defects in infants (24). Moreover, infants nursing from anemic mothers display signs of anemia (30), further demonstrating an intricate dependency of neonatal iron homeostasis on maternal iron homeostasis in humans. Although the multiparous nature of mice considerably increases iron requirement compared with humans, importance of maternal iron homeostasis in infant growth and development cannot be discounted in humans. Currently, there are no postpartum recommendations available for iron supplementation during breastfeeding in humans. Numerous factors such as age, inflammation, gastrointestinal disease, and obesity have profound effects on maternal iron absorption. In some cases of obesity, iron supplementation does not restore iron-deficiency anemia due to increased levels of hepcidin (60, 61). Similarly, iron supplementation is not effective in the treatment of anemia in iron refractory iron deficiency anemia (IRIDA) (62). Therefore, alternative strategies aimed at increasing maternal dietary iron absorption will be highly beneficial in patients who are refractory to iron supplementation. The present work provides evidence that the PHD inhibitor FG-4592 that is currently under phase III clinical trial for treatment of anemia could effectively stabilize intestinal HIF-2α and improve milk iron levels and ameliorate neonatal anemia (43).
In this work, for the first time to our knowledge we demonstrate a molecular mechanism in which maternal HIF-2α plays a central role in milk quality and neonatal iron homeostasis. The World Health Organization recommendations of 30–60 mg of oral iron supplementation is only through the duration of pregnancy, and the present work demonstrates that neonatal iron stores are inadequate during early neonatal growth and intact maternal iron homeostasis is critical throughout the lactation period. Targeting intestinal HIF-2α could be an effective therapy to maintain iron homeostasis and ameliorate iron deficiency during pregnancy and lactation, especially in anemic patients who are refractory to iron supplementation due to dysregulated hepcidin expression.
Materials and Methods
Animals and Diets.
Hif-2αF/F, Hif-2αIntKO, Hif-1αF/F, Hif-1αIntKO, Hif-1αLivKO, Hif-2αLivKO, Dmt-1F/F, Dmt-1F/F;VilERcre, and ODD-luc mice were described previously (17, 21, 33). All mice were fed ad libitum and kept in a 12-h dark/light cycle. Mice were fed with either breeder diet or standard chow diet (Research Diets). All animal studies were carried out in accordance with Association for Assessment and Accreditation of Laboratory Animal Care International guidelines and approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Animal Treatments.
Hif-2αF/F and Hif-2αIntKO breeder cages were set at the same time and monitored for pregnancy. Numbers of nursing pups were kept the same between the groups. In case of small litter size, pups from other breeders were fostered to maintain even number of pups between the groups and excess pups were killed immediately. For cross-fostering experiments, equal numbers of pups were switched between Hif-2αF/F and Hif-2αIntKO mothers immediately after birth or 10 d after birth, depending on the experimental design. Body weight and hair coat of the pups were monitored throughout the study. All pups were weaned at day 21.
For iron dextran injection, Hif-2αIntKO mothers were injected s.c. with 100 μg iron dextran on the day of parturition and repeated twice in 5-d intervals. For PHD inhibitor treatment, four doses of FG-4592 (Cayman Chemicals) were administered orally at 60 mg/kg body weight to mothers immediately after delivery on alternate days. FG-4592 was dissolved in DMSO (1 mg/μL) and gavaged after diluting with PBS. To induce anemia, 2-mo-old female FVB/NJ mice were injected with PHZ at 60 mg/kg body weight for 2 consecutive days and set up as breeder with age-matched males. For collecting milk from the mothers, pups were separated from the mothers for 4 h and then mothers were anesthetized using avertin. Mothers were injected intraperitoneally with oxytocin (1 unit/kg body weight) and 10 min after oxytocin injection, milk was collected manually using Pasteur pipettes from all of the teats. To procure adequate amount of milk for ICP-MS, milk collected from 5 mice was pooled together for further analysis.
Complete Blood Count Analysis.
Complete blood count analysis was performed as previously described (21). Briefly, mice were exsanguinated by retroorbital bleeding using heparinized capillary tubes, the blood was collected in heparinized-EGTA tubes, and CBC analysis was done immediately using the University of Michigan Lab Animal Medicine (ULAM) Pathology core. In 1-d-old pups, blood was collected using heparinized capillary tubes immediately after decapitation. ULAM typically performs CBC analysis with 50 μL of blood.
Nonheme Iron Assay.
Nonheme iron in serum or tissue was assessed as previously described (21). Assays using milk were performed in pooled milk from at least three lactating mothers. ICP-MS analysis for minerals in serum, duodenum, and liver was performed using the core facility at Michigan State University.
Real-Time Quantitative PCR.
RNA extraction, reverse transcription, and quantitative PCR (qPCR) were described previously (20). The primers used in the study are listed in Table S1.
Table S1.
qPCR primers
| Primers | Sequence |
| DMT-1-F | TGTTTGATTGCATTGGGTCTG |
| DMT-1-R | CGCTCAGCAGGACTTTCGAG |
| Dcytb-F | CATCCTCGCCATCATCTC |
| Dcytb-R | GGCATTGCCTCCATTTAGCTG |
| FPN-F | ATGGGAACTGTGGCCTTCAC |
| FPN-R | TCCAGGCATGAATACGGAGA |
| NGF-F | GGAGCGCATCGAGTTTTGG |
| NGF-R | CCTCACTGCGGCCAGTATAG |
| Arc-F | GCTGAGCTCTGCTCTTCTTCA |
| Arc-R | GGTGAGCTGAAGCCACAAAT |
| c-Fos-F | GATGTTCTCGGGTTTCAACG |
| c-Fos-R | GGAGAAGGAGTCGGCTGG |
| BDNF-F | GCCTTCATGCAACCGAAGTA |
| BDNF-R | TGAGTCTCCAGGACAGCAAA |
| Tfr-F | GGAAGACTCTGCTTTGCAGCTAT |
| Tfr-R | GCCCAGGTAGCCCATCATGA |
| Slc39a14-F | CTGGTCAAACTGAGCCAACA |
| Slc39a14-R | CCCTGGATAGTGAGGCTGC |
Immunostaining.
OCT-embedded frozen tissues were sectioned (6 μm) and fixed with PBS-buffered formalin for 15 min and permeabilized for 10 min using 0.05% Triton X-100. Sections were blocked in 5% (wt/vol) skim milk in TBST for 30 min at room temperature and probed with polyclonal rabbit anti–HIF-2α antibody (Novus) or pCREB antibody (Cell Signaling Technologies) overnight at 4 °C.
Enhanced Prussian Blue Staining.
Enhanced Prussian blue staining was performed in 6-μm frozen sections as previously described (21).
Novel Object Recognition Test.
For cognitive test, pups from Hif-2αF/F, Hif-2αIntKO, or iron dextran Hif-2αIntKO mothers were weaned at day 21 and placed on a regular chow diet until 2 mo of age. Before the experimental procedure, pups were accustomed for 2 d in a transparent Perspex chamber containing two objects of similar size and shape, placed on opposite sides. During the test phase, one of the objects was replaced with a novel object of similar volume but different shape. Then the mice were introduced into the arena for 5 min to explore. The recognition pattern of the novel and familiar objects was recorded for individual mice using ANY-Maze software for 5 min.
Statistical Analysis.
Results are expressed as mean ± SEM. Significance among multiple groups was tested using one-way analysis of variance followed by Dunnett’s post hoc comparisons and significance between two groups was calculated by independent t test. P < 0.05 was considered significant.
Acknowledgments
This work was supported by NIH Grants CA148828 and DK095201 (to Y.M.S.), the University of Michigan’s Gastrointestinal Peptide Research Center (P30 DK034933), Diabetes Research and Training Center (P30 DK20572), and Gastrointestinal Oncology SPORE (CA130810). S.K.R. was supported by a postdoctoral fellowship from American Heart Association (15POST22650034).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504891112/-/DCSupplemental.
References
- 1.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341(26):1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 2.Mastrogiannaki M, Matak P, Peyssonnaux C. The gut in iron homeostasis: Role of HIF-2 under normal and pathological conditions. Blood. 2013;122(6):885–892. doi: 10.1182/blood-2012-11-427765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah YM, Xie L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology. 2014;146(3):630–642. doi: 10.1053/j.gastro.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 6.Altamura S, et al. Resistance of ferroportin to hepcidin binding causes exocrine pancreatic failure and fatal iron overload. Cell Metab. 2014;20(2):359–367. doi: 10.1016/j.cmet.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Donovan A, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lesbordes-Brion JC, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–1405. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 9. Anonymous (1998) Recommendations to prevent and control iron deficiency in the United States. Recommendations and Reports: Morbidity and Mortality Weekly Report (Centers for Disease Control, Atlanta), 47(RR-3):1–29. [PubMed]
- 10.Cao C, O’Brien KO. Pregnancy and iron homeostasis: An update. Nutr Rev. 2013;71(1):35–51. doi: 10.1111/j.1753-4887.2012.00550.x. [DOI] [PubMed] [Google Scholar]
- 11.Milman N. Oral iron prophylaxis in pregnancy: Not too little and not too much! J Pregnancy. 2012;2012:514345. doi: 10.1155/2012/514345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett JF, Whittaker PG, Williams JG, Lind T. Absorption of non-haem iron from food during normal pregnancy. BMJ. 1994;309(6947):79–82. doi: 10.1136/bmj.309.6947.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehu M, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol. 2010;85(4):345–352. doi: 10.1111/j.1600-0609.2010.01479.x. [DOI] [PubMed] [Google Scholar]
- 14.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6(8):3062–3083. doi: 10.3390/nu6083062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyarmati B, et al. Serum maternal hepcidin levels 3 days after delivery are higher compared to those measured at parturition. J Obstet Gynaecol Res. 2011;37(11):1620–1624. doi: 10.1111/j.1447-0756.2011.01586.x. [DOI] [PubMed] [Google Scholar]
- 16.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;53(5):655–660. doi: 10.1136/gut.2003.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9(2):152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastrogiannaki M, et al. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson ER, Xue X, Shah YM. Intestinal hypoxia-inducible factor-2alpha (HIF-2alpha) is critical for efficient erythropoiesis. J Biol Chem. 2011;286(22):19533–19540. doi: 10.1074/jbc.M111.238667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor M, et al. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology. 2011;140(7):2044–2055. doi: 10.1053/j.gastro.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson ER, et al. Intestinal HIF2α promotes tissue-iron accumulation in disorders of iron overload with anemia. Proc Natl Acad Sci USA. 2013;110(50):E4922–E4930. doi: 10.1073/pnas.1314197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinder D, Oates PS, Thomas C, Sadleir J, Morgan EH. Localisation of divalent metal transporter 1 (DMT1) to the microvillus membrane of rat duodenal enterocytes in iron deficiency, but to hepatocytes in iron overload. Gut. 2000;46(2):270–276. doi: 10.1136/gut.46.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anaokar SG, Garry PJ. Effects of maternal iron nutrition during lactation on milk iron and rat neonatal iron status. Am J Clin Nutr. 1981;34(8):1505–1512. doi: 10.1093/ajcn/34.8.1505. [DOI] [PubMed] [Google Scholar]
- 24.Lozoff B, et al. 2006. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev 64(5 Pt 2):S34–43; discussion S72–S91.
- 25.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12(1):54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rugh R, Somogyi C. The effect of pregnancy on peripheral blood in the mouse. Biol Bull. 1969;136(3):454–460. doi: 10.2307/1539687. [DOI] [PubMed] [Google Scholar]
- 27.Hubbard AC, et al. Effect of dietary iron on fetal growth in pregnant mice. Comp Med. 2013;63(2):127–135. [PMC free article] [PubMed] [Google Scholar]
- 28.Brabin BJ, Hakimi M, Pelletier D. 2001. An analysis of anemia and pregnancy-related maternal mortality. J Nutr 131(2S-2):604S–614S; discussion 614S–615S. [DOI] [PubMed]
- 29.Du X, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaparro CM. Setting the stage for child health and development: Prevention of iron deficiency in early infancy. J Nutr. 2008;138(12):2529–2533. doi: 10.1093/jn/138.12.2529. [DOI] [PubMed] [Google Scholar]
- 31.Dallman PR, Siimes MA, Stekel A. Iron deficiency in infancy and childhood. Am J Clin Nutr. 1980;33(1):86–118. doi: 10.1093/ajcn/33.1.86. [DOI] [PubMed] [Google Scholar]
- 32.Wan Y, et al. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21(15):1895–1908. doi: 10.1101/gad.1567207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunshin H, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115(5):1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter RC, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126(2):e427–e434. doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Congdon EL, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes-Hampton GP, et al. Changing iron content of the mouse brain during development. Metallomics. 2012;4(8):761–770. doi: 10.1039/c2mt20086d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakrabarti M, Barlas MN, McCormick SP, Lindahl LS, Lindahl PA. Kinetics of iron import into developing mouse organs determined by a pup-swapping method. J Biol Chem. 2015;290(1):520–528. doi: 10.1074/jbc.M114.606731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Florian C, Mons N, Roullet P. CREB antisense oligodeoxynucleotide administration into the dorsal hippocampal CA3 region impairs long- but not short-term spatial memory in mice. Learn Mem. 2006;13(4):465–472. doi: 10.1101/lm.249306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourtchuladze R, et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 41.Tully T, Bourtchouladze R, Scott R, Tallman J. Targeting the CREB pathway for memory enhancers. Nat Rev Drug Discov. 2003;2(4):267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 42.Nam H, et al. ZIP14 and DMT1 in the liver, pancreas, and heart are differentially regulated by iron deficiency and overload: Implications for tissue iron uptake in iron-related disorders. Haematologica. 2013;98(7):1049–1057. doi: 10.3324/haematol.2012.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beuck S, Schänzer W, Thevis M. Hypoxia-inducible factor stabilizers and other small-molecule erythropoiesis-stimulating agents in current and preventive doping analysis. Drug Test Anal. 2012;4(11):830–845. doi: 10.1002/dta.390. [DOI] [PubMed] [Google Scholar]
- 44.Batey RG, Gallagher ND. Role of the placenta in intestinal absorption of iron in pregnant rats. Gastroenterology. 1977;72(2):255–259. [PubMed] [Google Scholar]
- 45.Gulec S, Collins JF. Molecular mediators governing iron-copper interactions. Annu Rev Nutr. 2014;34:95–116. doi: 10.1146/annurev-nutr-071812-161215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leong WI, Bowlus CL, Tallkvist J, Lönnerdal B. Iron supplementation during infancy—effects on expression of iron transporters, iron absorption, and iron utilization in rat pups. Am J Clin Nutr. 2003;78(6):1203–1211. doi: 10.1093/ajcn/78.6.1203. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14(5):420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 48.Anderson SA, et al. The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 2013;17(2):282–290. doi: 10.1016/j.cmet.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosh MC, et al. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α. Cell Metab. 2013;17(2):271–281. doi: 10.1016/j.cmet.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Male C, et al. Euro-Growth Iron Study Group Prevalence of iron deficiency in 12-mo-old infants from 11 European areas and influence of dietary factors on iron status (Euro-Growth study) Acta Paediatr. 2001;90(5):492–498. doi: 10.1080/080352501750197601. [DOI] [PubMed] [Google Scholar]
- 51.Anderson A, Burggren A. Cognitive and neurodevelopmental benefits of extended formula-feeding in infants: Re: Deoni et al. 2013. Neuroimage. 2014;100:706–709. doi: 10.1016/j.neuroimage.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Masala A, et al. Inhibition of lactation and inhibition of prolactin release after mechanical breast stimulation in puerperal women given tamoxifen or placebo. Br J Obstet Gynaecol. 1978;85(2):134–137. doi: 10.1111/j.1471-0528.1978.tb10467.x. [DOI] [PubMed] [Google Scholar]
- 53.Thompson K, Molina RM, Donaghey T, Brain JD, Wessling-Resnick M. Iron absorption by Belgrade rat pups during lactation. Am J Physiol Gastrointest Liver Physiol. 2007;293(3):G640–G644. doi: 10.1152/ajpgi.00153.2007. [DOI] [PubMed] [Google Scholar]
- 54.Canonne-Hergaux F, et al. The Nramp2/DMT1 iron transporter is induced in the duodenum of microcytic anemia mk mice but is not properly targeted to the intestinal brush border. Blood. 2000;96(12):3964–3970. [PubMed] [Google Scholar]
- 55.Fleming MD, et al. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet. 1997;16(4):383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 56.Felt BT, Lozoff B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J Nutr. 1996;126(3):693–701. doi: 10.1093/jn/126.3.693. [DOI] [PubMed] [Google Scholar]
- 57.Patton SM, Coe CL, Lubach GR, Connor JR. Quantitative proteomic analyses of cerebrospinal fluid using iTRAQ in a primate model of iron deficiency anemia. Dev Neurosci. 2012;34(4):354–365. doi: 10.1159/000341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Georgieff MK. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36(Pt 6):1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muñoz P. Iron-mediated redox modulation in neural plasticity. Commun Integr Biol. 2012;5(2):166–168. doi: 10.4161/cib.18710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baumgartner J, et al. Overweight impairs efficacy of iron supplementation in iron-deficient South African children: A randomized controlled intervention. Int J Obes (Lond) 2013;37(1):24–30. doi: 10.1038/ijo.2012.145. [DOI] [PubMed] [Google Scholar]
- 61.Tussing-Humphreys LM, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring) 2010;18(7):1449–1456. doi: 10.1038/oby.2009.319. [DOI] [PubMed] [Google Scholar]
- 62.Heeney MM, Finberg KE. Iron-refractory iron deficiency anemia (IRIDA) Hematol Oncol Clin North Am. 2014;28(4):637–652, v. doi: 10.1016/j.hoc.2014.04.009. [DOI] [PubMed] [Google Scholar]