Significance
More than 50% of people now live in urban areas. By 2050 this proportion will be 70%. Urbanization is associated with increased levels of mental illness, but it’s not yet clear why. Through a controlled experiment, we investigated whether nature experience would influence rumination (repetitive thought focused on negative aspects of the self), a known risk factor for mental illness. Participants who went on a 90-min walk through a natural environment reported lower levels of rumination and showed reduced neural activity in an area of the brain linked to risk for mental illness compared with those who walked through an urban environment. These results suggest that accessible natural areas may be vital for mental health in our rapidly urbanizing world.
Keywords: environmental neuroscience, nature experience, rumination, psychological ecosystem services, emotion regulation
Abstract
Urbanization has many benefits, but it also is associated with increased levels of mental illness, including depression. It has been suggested that decreased nature experience may help to explain the link between urbanization and mental illness. This suggestion is supported by a growing body of correlational and experimental evidence, which raises a further question: what mechanism(s) link decreased nature experience to the development of mental illness? One such mechanism might be the impact of nature exposure on rumination, a maladaptive pattern of self-referential thought that is associated with heightened risk for depression and other mental illnesses. We show in healthy participants that a brief nature experience, a 90-min walk in a natural setting, decreases both self-reported rumination and neural activity in the subgenual prefrontal cortex (sgPFC), whereas a 90-min walk in an urban setting has no such effects on self-reported rumination or neural activity. In other studies, the sgPFC has been associated with a self-focused behavioral withdrawal linked to rumination in both depressed and healthy individuals. This study reveals a pathway by which nature experience may improve mental well-being and suggests that accessible natural areas within urban contexts may be a critical resource for mental health in our rapidly urbanizing world.
Never before has such a large percentage of humanity been so far removed from nature (1); more than 50% of people now live in urban areas, and by 2050, this proportion will be 70% (2). What are the potential mental health implications of this demographic shift? Although urbanization has many benefits, it is also associated with increased levels of mental illness, including anxiety disorders and depression (3–5). Causal mechanisms for this increased prevalence of mental illness are likely manifold and are not well understood (6, 7).
One aspect of urbanization that has attracted research attention in recent years is a corresponding decrease in nature experience (8, 9). Using a variety of methodologies, researchers have demonstrated affective and cognitive benefits of nature experience, thereby contributing to an evolving understanding of the types of psychological benefits of which humanity may be deprived as urbanization continues. Correlational findings show that growing up in rural vs. urban settings is associated with lesser stress responsivity (3). A recent longitudinal study, tracking the well-being and mental distress of more than 10,000 people over a period of nearly two decades demonstrates a significant positive effect of proximity to greenspace on well-being (9). This effect traces to living location within the same individuals as they moved closer or further from greenspace. Other correlational studies reveal that window views that include natural elements (compared with window views that do not) are associated with superior memory, attention, and impulse inhibition (10), as well as greater feelings of subjective well-being (11). These correlational findings are buttressed by experimental findings showing, for example, that nature experience (usually in urban greenspace) can improve memory and attention (12) and increase positive mood (13). Experimenters also have used psychophysiological methods to characterize the ways in which images and sounds of the natural environment lead to decreased stress and negative emotion after participants have been subjected to stressful stimuli (14, 15). Taken together, these and numerous other studies provide compelling evidence that nature experience may confer real psychological benefits.
Although this body of work is now substantial, there remains a fundamental yet unanswered question: by what mechanism(s) might nature experience buffer against the development of mental illness? One possible mechanism—and our focus here—is a decrease in rumination, a maladaptive pattern of self-referential thought that is associated with heightened risk for depression and other mental illnesses (16–18) and with activity in the subgenual prefrontal cortex (sgPFC) (19). The sgPFC has been shown to display increased activity during sadness (20) and the behavioral withdrawal and negative self-reflective processes tied to rumination in healthy (21) and depressed (22–24) individuals.
Rumination is a prolonged and often maladaptive attentional focus on the causes and consequences of emotions—most often, negative, self-relational emotions (25). This pattern of thought has been shown to predict the onset of depressive episodes (17), as well as other mental disorders (26). Positive or neutral distraction (vs. maladaptive distractions such as binge drinking of alcohol) has been shown to decrease rumination (27). To be effective in decreasing rumination, these positive or neutral distractions must be engrossing, to maintain the shift of attention onto the distracting stimuli (27). From this perspective, we aimed to observe whether a 90-min nature experience has the potential to decrease rumination. In addition to gathering self-report measures, we examined brain activity in the sgPFC, an area that has been shown to be particularly active during the type of maladaptive, self-reflective thought and behavioral withdrawal that occurs during rumination (19). This behavioral and neural evidence—when taken together—would provide convincing evidence for a change in rumination resultant from nature experience.
We quantified the impacts of a brief nature experience on rumination and neural activity in the sgPFC through a controlled experiment, comparing changes that occur in a 90-min nature walk to those in a 90-min urban walk. We hypothesized that we would observe decreased rumination and decreased neural activity in the sgPFC for urban residents who experienced a nature walk, whereas we would not observe such a decrease in those who experienced an urban walk. We obtained measures of individuals’ self-reported levels of rumination using the rumination portion of the Reflection Rumination Questionnaire (RRQ) (28). We documented activity in the sgPFC by using a neuroimaging method called arterial spin labeling (ASL, presented more fully in Methods), through which we measured regional cerebral blood flow (rCBF): the volume of cerebral blood passing through the region of interest. This technique can detect effects associated with longer-lasting psychological phenomena such as rumination, in contrast to momentary, reactive emotional responses such as a startle response (29).
Thirty-eight healthy participants took part in the study. Although rumination is often studied in the context of clinically depressed individuals, we studied participants with no history of mental disorder to broaden the applicability of our findings. Our sample comprised individuals residing in urban environments. We posited that these individuals, although currently healthy, would enter the study with a somewhat elevated level of rumination resulting from the ongoing and chronic stressors associated with urban experience, and their corresponding deprivation of regular contact with nature. We therefore hypothesized that a nature experience would reduce the baseline rumination levels of such participants, compared with those who had an urban experience.
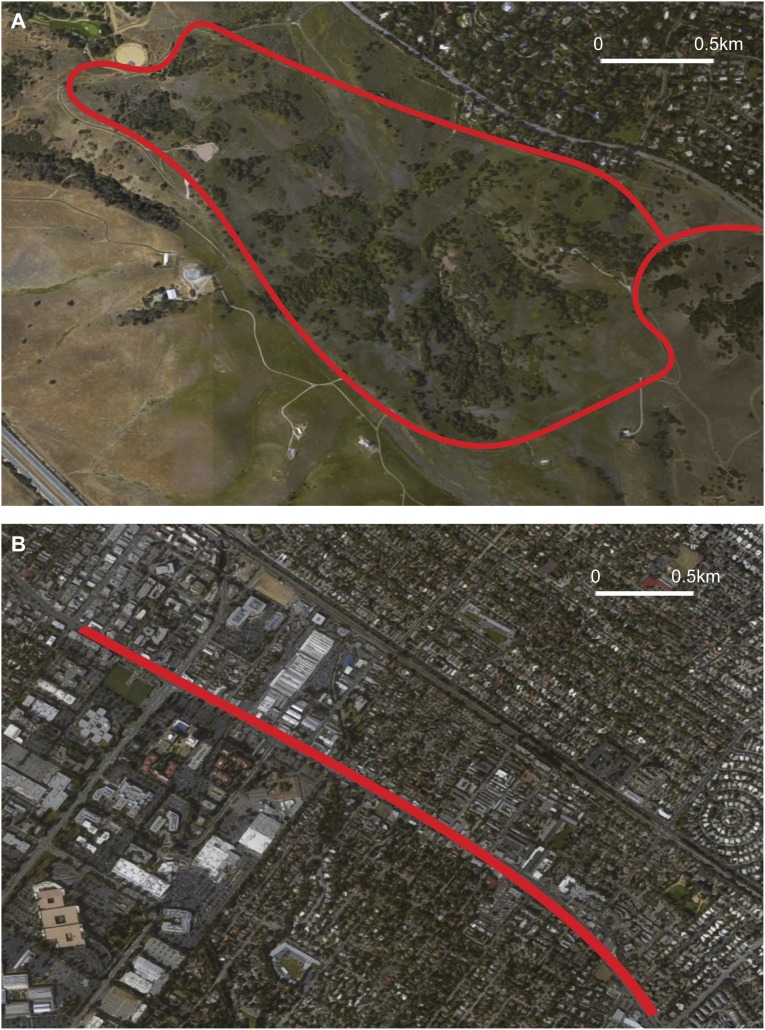
On arrival at our laboratory, each participant completed a self-report measure of rumination (RRQ) and underwent our scanning procedure. We then randomly assigned each participant to a 90-min walk in either a natural environment (19 participants) or urban environment (19 participants). The nature walk took place near Stanford University, in a greenspace comprising grassland with scattered oak trees and shrubs. The urban walk took place on the busiest thoroughfare in nearby Palo Alto (El Camino Real), a street with three to four lanes in each direction and a steady stream of traffic (Fig. S1). After the walk, each participant returned to the laboratory and provided a second, follow-up self-report of levels of rumination (RRQ) before undergoing a second resting-state ASL scan. Transportation to and from the walk was via a car ride of 15-min duration (for both walks). Participants were given a smartphone and told to take 10 photographs during their walk (Fig. S2). These photographs were used to verify that participants went on the walk. We also tracked the phone itself during the walk, as further verification that the correct route was taken by each participant.
Fig. S1.
Satellite maps of (A) the nature walk and (B) the urban walk. Both walks were a total of 5.3 km.
Fig. S2.
Sample photographs taken by participants during (A and B) the nature walk and (C and D) the urban walk.
Results
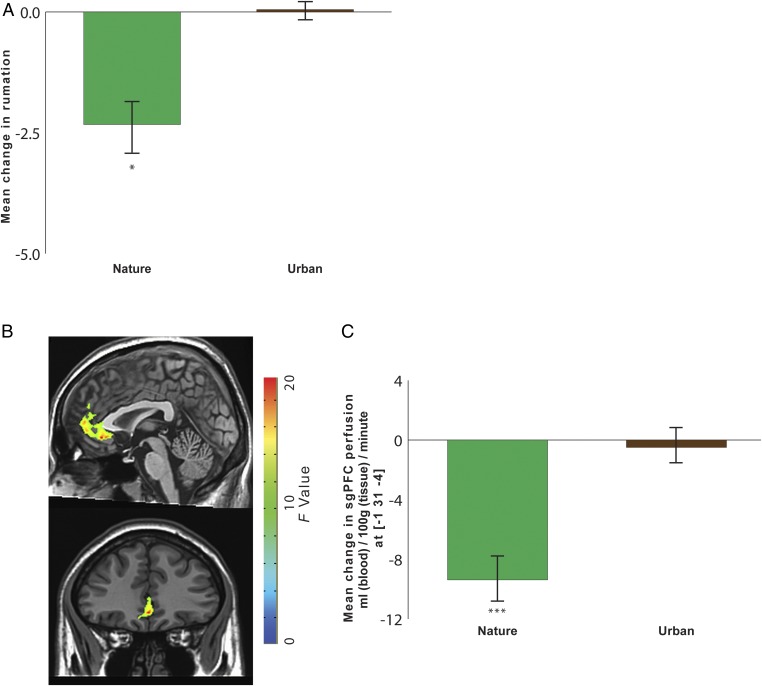
To analyze the impact of nature experience on self-reported rumination, we conducted a two-way ANOVA, with time as a within-subjects factor (before vs. after the walk) and environment as a between-subjects factor (nature walk vs. urban walk). This analysis revealed an interaction between time and environment [F(1,35) = 3.51, P = 0.07, = 0.09]. Consistent with our hypothesis, follow-up t tests indicated that our results were driven by a decrease in self-reported rumination for the nature group but not for the urban group (Fig. 1A). There was a simple effect of time for the nature group [t(17) = −2.69, P < 0.05, d = 0.34; mean change pre- to postwalk = −2.33, SE = 0.55; mean score prewalk = 35.39, SE = 1.60; mean score postwalk = 33.06, SE = 1.61], with decreases from pre- to postwalk. There was no such effect for the urban group (mean score prewalk = 30.11, SE = 2.61; mean score postwalk = 30.16, SE = 2.50).
Fig. 1.
The impact of nature experience on self-reported rumination and blood perfusion to the sgPFC. (A) Change in self-reported rumination (postwalk minus prewalk) for participants randomly assigned to take a 90-min walk either in a natural setting or in an urban setting. (B) A time-by-environment interaction in blood perfusion was evident in the sgPFC. F map of significant interactions at a threshold of P < 0.05, FWE corrected for multiple comparisons. (C) Change in blood perfusion (postwalk minus prewalk) for participants randomly assigned to take a 90-min walk either in a natural setting or in an urban setting. Error bars represent SE within subjects: *P < 0.05, ***P < 0.001.
To analyze the impact of nature experience on blood perfusion in the sgPFC, we conducted a similarly structured ANOVA with time as a within-subjects factor (before vs. after the walk) and environment as a between-subjects factor (nature walk vs. urban walk). Clusters reflecting a significant time-by-environment interaction were corrected for familywise error (FWE; voxelwise P = 0.05, cluster threshold = 1,713 mm3) for multiple comparisons across the whole brain. The sgPFC was the a priori area of interest in this study (Table S1 and Fig. S3 for whole brain analyses). All reported perfusion values are in units of milliliters of blood per 100 g tissue per minute. As hypothesized, sgPFC perfusion showed an interaction effect of time by environment, indicating an effect of the nature walk vs. the urban walk [Fig. 1B; F(1,29) = 23.41, P < 0.0001, = 0.45].
Table S1.
Nonhypothesized regions from whole-brain analysis showing time-by-environment interactions
| Talairach coordinates | ||||||
| Region | x | y | z | Voxels | F | P |
| Left middle occipital gyrus | −36 | −78 | 1 | 12,181 | 44.17 | <0.0001 |
| Right supramarginal gyrus | 55 | −45 | 27 | 7,264 | 30.78 | <0.0001 |
| Right superior temporal gyrus | 55 | 13 | −9 | 3,911 | 14.12 | <0.001 |
| Right medial frontal gyrus | 5 | 5 | 50 | 3,227 | 18.49 | <0.001 |
| Right posterior cingulate | 2 | −52 | 22 | 1,729 | 19.93 | <0.001 |
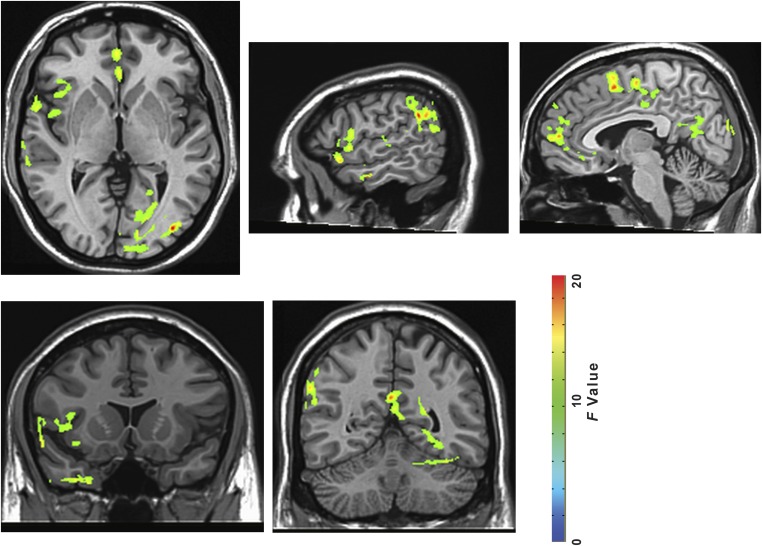
Coordinates refer to the location of peak activity in Talairach space; voxels are in 1-mm3 units and refer to the size of the cluster in which the interaction of time-by-environment was significant, P < 0.05, FWE corrected; F refers to the F-statistic of the interaction effect, with the corresponding P value. As we did not conduct behavioral assessments for further investigating effects in these regions of the brain, the behaviors and processes for which these activations are neural correlates remain a matter of speculation. Taken as a whole, however, the overall pattern of a decrease in activity in these areas in the nature vs. urban experience support our hypothesis. Although it is unclear why we would observe a difference in visual processing (occipital gyrus), differences in activation of other areas support the idea that nature experience results in a decrease in inner mentation. The supramarginal gyrus has been shown to be involved in sensory processing and social cognition, especially as it relates to perspective taking and perceptions regarding emotions (53–55). The superior temporal gyrus has been associated with auditory and language processing of self-directed, internal speech, and social cognition (56, 57). Differences between groups in perfusion to the medial frontal gyrus may result from a variety of causes, including changes in executive functioning, such as in formulating plans and cognitive models of the world (58). The observed interaction in the posterior cingulate is of most relevance to our primary findings, as this area of the brain has been associated with autobiographical processing (59).
Fig. S3.
All brain regions showing time-by-environment interaction (P < 0.05, FWE corrected). Slice coordinates from left to right: z = 1, x = 55, x = 5, y = 13, y = −52.
We investigated the composition of this interaction in the sgPFC by examining (for each participant and scan) cerebral blood flow estimates centered at the cluster peak. As predicted, follow-up t tests revealed that our results were driven by decreases in blood flow resulting from nature vs. urban experience. There was an effect of time for the nature group [t(15) = −6.89, P < 0.0001, d = 1.01] with decreases from pre- to postwalk in the nature group, but there was no effect of time for the urban group (Fig. 1C).
To assess whether the effects of the nature vs. urban experience on rCBF arose from different physiological effects of these walks (e.g., potential differences in the physical demands of each walk), heart rate and respiration rate were measured during the rCBF scans (physiology data for three of the participants were eliminated because sensors were loosened while participants were placed into the MRI scanner). Although the nature walk contained short sections of path with a higher slope gradient than the urban walk, and the cumulative elevation gain of the walks did differ, with more total gain in the nature walk than in the urban walk (Methods), total distances of the walks were equal; we observed no interaction effect on physiology (i.e., differential change in heart rate or respiration rate due to environment). Heart rate showed a main effect of time [F(1,26) = 5.21, P < 0.05, = 0.17; mean change pre- to postwalk = 5.51, SE = 2.37; mean score prewalk = 68.54, SE = 2.22; mean score postwalk = 74.05, SE = 3.88], but no interaction effect of time by environment [F(1,26) = 0.08, not significant]. Respiration rate showed neither a main effect of time nor a time-by-environment interaction (all P > 0.37). The lack of group by time effects for either physiological measure speaks against the possibility that observed behavioral and brain effects were due to residual differences in walk-related physiological activation.
Discussion
Our results indicate that nature experience reduced rumination and sgPFC activation. Participants who went on a 90-min nature walk showed reductions in self-reported rumination and decreases in sgPFC activity, whereas those who went on an urban walk did not show these effects. Given the documented link between rumination and risk for depression and other psychological illnesses, the reduction in rumination among those with the nature experience suggests one possible mechanism by which urbanization—which reduces opportunities for nature experience—may be linked to mental illness. This suggestion draws support from our finding that at a neurobiological level, nature experience led to decreases in sgPFC activity, a brain region that previously has been shown to be associated with a self-focused behavioral withdrawal linked to rumination in both depressed and healthy individuals.
These findings support the view that natural environments may confer psychological benefits to humans (30). In the literature on “restorative” environments (31), researchers have shown that individuals tend to select favorite environments as a means to transform negative psychological states to more positive ones. These areas tend to be natural environments, although not exclusively so. Natural environments with pleasing aesthetic qualities including open views (32) and lack of loud, distracting noises are often chosen as preferred restorative environments (30). Effects of these landscapes are captured in the Perceived Restorativeness Scale (33), and include those that engender somewhat effortless, “soft fascination”; the “sense of belonging”; and the “sense of being away.” This literature relates to our findings insofar as we may consider these preferred environments to engender the type of positive distraction that has been shown to decrease rumination and negative affect in depressed individuals (27). Specifically, our findings of decreased sgPFC activity in the nature group point to a possible causal mechanism for the affective benefits of nature experience.
Our findings may have relevance beyond the neural correlates of rumination. Activity in the sgPFC is also more broadly tied to behavioral withdrawal (19). Although we observed peak activity in the sgPFC, this significant cluster of voxels also includes the perigenual anterior cingulate cortex (pACC): a region that has been shown to display increased reactivity in individuals born in urban areas during social stress processing (3). Other forms of affective appraisal, emotion regulation, and reactivity to social hierarchies involve coordinated activity of this region with other areas of the brain, including the insula, ventral striatum, and amygdala (34). Decreased functional connectivity between the pACC and amygdala is found in schizophrenia (3) and bipolar disorder (35) and is a predictor of anxiety (34). Considered without the context provided by self-reports of rumination, sgPFC findings could be related to the neural processing of sadness (20), guilt, remorse, negative autobiographical narratives, or peer rejection (19, 36, 37). It is also possible that other psychological processes (e.g., stress or anxiety processing) or hormones (e.g., dopamine or oxytocin release) may mediate the affective benefits of nature experience. These possibilities provide a rich area for further study.
Our findings of the effects of a relatively brief nature experience suggest that feasible investments in access to natural environments could yield important benefits for the “mental capital” (38) of cities and nations. More research is needed to refine our understanding of the “production functions” of natural environments (39) for mental health benefits, clarifying both key characteristics of the environments and the duration, frequency, and types of experience that generate benefits (40). By accounting for these psychological ecosystem services (40), we can better assess the value that natural areas provide with respect to mental health, an essential issue given the significant contribution of depression and other mental illnesses to the global burden of disease (41).
As empirical understanding builds regarding the ways in which nature experience benefits human cognitive function and mood, we can move toward a more complete incorporation of these benefits into the paradigm of ecosystem services. Doing so will require new research on the ways in which these impacts vary with biophysical attributes of natural land- and seascapes, frequency and duration of nature experience, as well as characteristics and personality attributes of the individual. Already, some cities and nations are incorporating these benefits into urban design, treating proximity of buildings (especially schools) and public access to greenspace as important aspects of city planning that may influence stress, mental health, and even cognitive functioning (42–46). With deeper understanding, mental health benefits of nature can be incorporated into a wide array of initiatives and investments in sustainable cities and conservation (47–49). Understanding the mechanisms by which nature experience buffers against the negative repercussions of urban life (50) will help us better plan for an ever more urban world.
Methods
Ethics Statement.
The study was approved by the Stanford University Human Subjects Committee. Participants were paid $20/h to participate in the study and signed informed consent.
Participants.
Thirty-eight participants (18 female, total mean age = 26.6 y) with no current or past diagnosis of neurologic or psychiatric disorder were invited to participate in a study that measured affective and cognitive functioning before and after a walk. All participants lived and worked in urban parts of the San Francisco Bay Area. No reference was made to the type of environment they would experience during their walk. Participants had normal or corrected-to-normal vision and were not taking any psychotropic medications. Each participant was randomly assigned to either a nature walk (19 participants; 8 females, mean age = 25.9 y) or an urban walk (19 participants; 10 females, mean age = 27.2 y), and each underwent our scanning procedure before and after the walk. Groups did not differ by age [t(36) = 0.48, P > 0.1] or sex [χ2(1) = 0.475, P > 0.1]. Seven participants had to be eliminated before perfusion analysis due to excessive movement during scanning, leaving 31 participants (16 female, mean age = 26.4 y, all right-handed) for perfusion analysis. One participant was eliminated in analysis of self-reported rumination due to a decrease in rumination after nature experience that was 3 SDs below the mean.
Locations and Instructions for Walks.
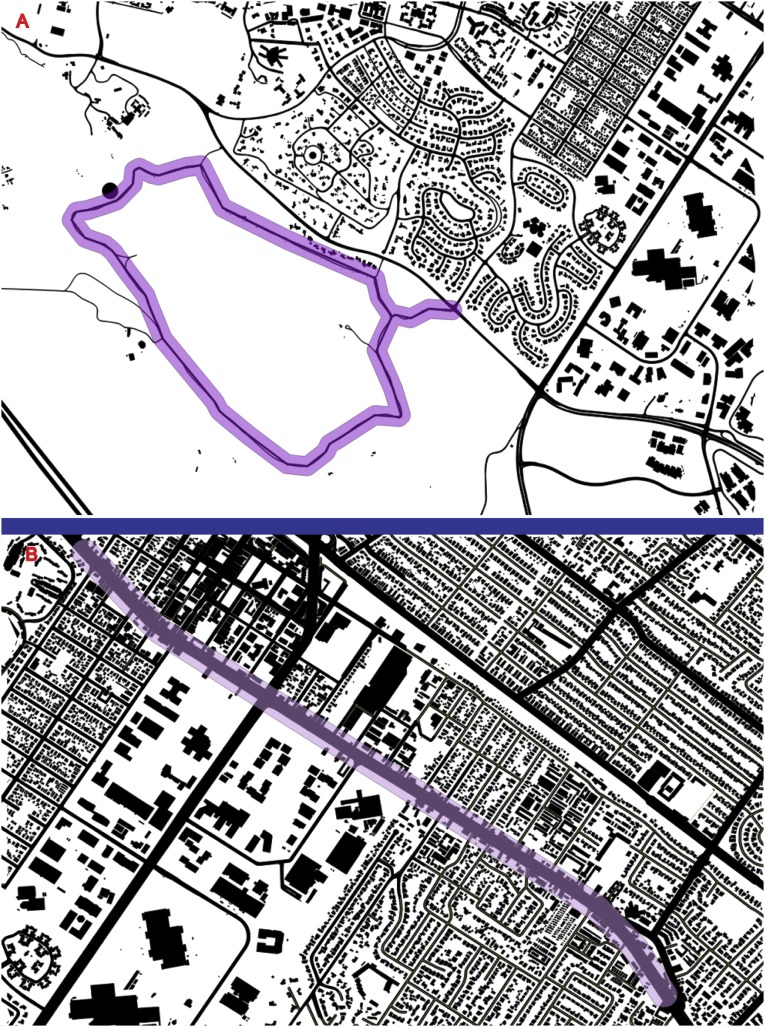
The nature walk took place in a greenspace near Stanford University spanning an area ∼60 m northwest of Junipero Serra Boulevard and extending away from the street in a 5.3-km loop, including a significant stretch that is far (>1 km) from the sounds and sights of the surrounding residential area. As one proxy for urbanicity, we measured the proportion of impervious surface (e.g., asphalt, buildings, sidewalks) within 50 m of the center of the walking path (Fig. S4). Ten percent of the area within 50 m of the center of the path comprised of impervious surface (primarily of the asphalt path). Cumulative elevation gain of this walk was 155 m. The natural environment of the greenspace comprises open California grassland with scattered oaks and native shrubs, abundant birds, and occasional mammals (ground squirrels and deer). Views include neighboring, scenic hills, and distant views of the San Francisco Bay, and the southern portion of the Bay Area (including Palo Alto and Mountain View to the south, and Menlo Park and Atherton to the north). No automobiles, bicycles, or dogs are permitted on the path through the greenspace.
Fig. S4.
Quantum GIS (QGIS) maps of (A) the nature walk and (B) the urban walk. Fifty-meter buffer zones around the paths are shown in purple. Buildings and roads were used to calculate percentage of this buffer that consisted of impervious surface. To calculate percent imperviousness for each environment, we used QGIS (www.qgis.org) to create a buffer zone of 50 m around each walking path (i.e., center of El Camino Real and the center of the Dish trail). We then used combined building footprint and road data to calculate the amount of impervious surface contained within the total area of each 50-m buffer zone. Area within the buffer was then coded in a binary fashion as impervious or nonimpervious, resulting in a percentage of total buffer area that could be classified as impervious.
The urban walk took place on the busiest thoroughfare in nearby Palo Alto (El Camino Real), a street with three to four lanes in each direction and a steady stream of traffic. Participants were instructed to walk down one side of the street in a southeasterly direction for 2.65 km, before turning around at a specific point marked on a map. This spot was chosen as the midpoint of the walk for the urban walk to match the nature walk with respect to total distance and exercise. Participants were instructed to cross the street at a pedestrian crosswalk/stoplight, and return on the other side of the street (to simulate the loop component of the nature walk and greatly reduce repeated encounters with the same environmental stimuli on the return portion of the walk), for a total distance of 5.3 km; 76% of the area within 50 m of the center of this section of El Camino was comprised of impervious surfaces (of roads and buildings) (Fig. S4). Cumulative elevation gain of this walk was 4 m. This stretch of road consists of a significant amount of noise from passing cars. Buildings are almost entirely single- to double-story units, primarily businesses (fast food establishments, cell phone stores, motels, etc.). Participants were instructed to remain on the sidewalk bordering the busy street and not to enter any buildings. Although this was the most urban area we could select for a walk that was a similar distance from the MRI facility as the nature walk, scattered trees were present on both sides of El Camino Real. Thus, our effects may represent a conservative estimate of effects of nature experience, as our urban group’s experience was not devoid of natural elements.
For both walks, participants were transported (2 km) to the starting point of the walk by car, individually, and went on the walk alone. They were given a smartphone with which they were instructed to take 10 photographs of whatever captured their attention. These instructions were given primarily to help hide the purpose of the study, as well as to provide confirmatory evidence that the participant completed the entire walk on returning to the start/end point. We also tracked the participants during their walks through the use of a tracking application installed on the phone, as further confirmatory evidence that they went on the assigned walks and did not stray from their instructed routes, stop at specific spots, or go inside of buildings. Per our tracking data and photographic evidence, all participants completed their walks as instructed.
Rumination.
Rumination was assessed using the RRQ (28). The RRQ is divided into two scales (rumination and reflection). In this study, only the rumination scale was used, as this was our dependent variable of interest. This scale consists of 12 items that measure ruminative tendencies (e.g., “My attention is often focused on aspects of myself I wish I’d stop thinking about”), each rated on a five-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Higher means of the sum of scores indicate higher degrees of rumination.
Image Acquisition and Reduction.
Scans were acquired using a 3-T General Electric MR750 Discovery Scanner at the Stanford Center for Cognitive and Neurobiological Imaging. The high-resolution T1-weighted MR images included 186 0.9-mm slices with an in-plane resolution of 0.898 mm2. High-resolution image acquisition was followed by a pulsed continuous ASL flow alternating inversion recovery (FAIR) sequence using a postlabel delay of 1,525 ms; TR = 4.674 s; TE = 10.968 ms; FOV = 240 × 240 mm; matrix size = 512 × 8; 38 axial slices; slice thickness = 3.2 mm; voxel dimensions 1.875 × 1.875 × 3.2 mm, one total measurement, for a total acquisition time of 4 min and 31 s.
We acquired perfusion weighted data and proton density maps and then combined information from those per the standard CBF flow equation quantification algorithm (51)
where T1b is T1 of blood and is assumed to be 1.6 s at 3 T. Partial saturation of the reference image (PD) is corrected for by using a T1t of 1.2 s (typical of gray matter). ST is saturation time (set to 2 s). is the partial coefficient that is set to the whole brain average (0.9). is overall efficiency (0.6), a combination of inversion efficiency (0.8) and background suppression efficiency (0.75). PLD is postlabeling delay used for the ASL sequence, and LT is the labeling duration (1. 5 s). PW is perfusion weighted (or raw difference) image. SPPW is the scaling factor of the PW sequence. NEXPW is the number excitation for PW images. A 500-μs Hanning pulse was used for the labeling pulse, and the labeling gradient during the pulse is 0.7 G/cm, with an average gradient of 0.07 G/cm. We used a 2-s saturation time for the reference image, which is a PD (and T1) weighted saturation recovery image. Background suppression was used with five inversion pulses.
This calculation rendered voxelwise quantitative maps reflecting milliliters of blood per 100 g tissue per minute (volume × time/mass). We coregistered ASL volumes to each individual’s anatomical scans and then performed a combined affine and nonlinear warping process of the anatomical data to standard (Talairach) space, using AFNI’s 3dQWarp. We then resampled the ASL data to a 1-mm3 voxel dimension. Following this, we applied the same warping parameters to the ASL data. Finally, to account for potential general changes in cerebral blood flow resulting from the distinct walks, we mean-normalized each participant’s regional CBF estimates relative to the mean CBF in gray matter. The same protocol was followed before and after each walk for every participant.
Our dependent variable was blood perfusion, measured as milliliters of blood per 100 g tissue per minute (volume × time/mass). We implemented an investigation of interaction of time-by-environment effects using AFNI’s 3dMVM (52). We then used t tests to further analyze data from our region of interest (sgPFC) that showed this interaction to better understand the composition of these effects.
Acknowledgments
We thank P. Kareiva and H. Tallis for comments on the manuscript and L. Bugatus, S. Kolarik, N. Le, B. Levy, S. Maples, S. McClure, C. Chambliss-Rudiger, J. Ryan, C. Shin, A. Swenson, C. Tan, M. Wibowo, and G. Young for research assistance. We also thank P. R. Ehrlich for many helpful discussions. We are grateful to members of the Stanford Center for Conservation Biology, the Stanford Psychophysiology Laboratory, and the Emmett Interdisciplinary Program in Environment and Resources (EIPER), and for funding from the Winslow Foundation, the George Rudolf Fellowship Fund, the Victoria and David Rogers Fund, the Mr. & Mrs. Dean A. McGee Fund, the Stanford Center for Cognitive and Neurobiological Imaging, and EIPER. G.N.B. was supported by the Stanford Graduate Fellowship Program in Science and Engineering (as a David and Lucille Packard Fellow) and the Stanford Interdisciplinary Graduate Fellowship Program (as a James and Nancy Kelso Fellow).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510459112/-/DCSupplemental.
References
- 1.Turner WR, Nakamura T, Dinetti M. Global urbanization and the separation of humans from nature. Bioscience. 2004;54(6):585–590. [Google Scholar]
- 2.Dye C. Health and urban living. Science. 2008;319(5864):766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- 3.Lederbogen F, et al. City living and urban upbringing affect neural social stress processing in humans. Nature. 2011;474(7352):498–501. doi: 10.1038/nature10190. [DOI] [PubMed] [Google Scholar]
- 4.Peen J, Schoevers RA, Beekman AT, Dekker J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand. 2010;121(2):84–93. doi: 10.1111/j.1600-0447.2009.01438.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang JL. Rural-urban differences in the prevalence of major depression and associated impairment. Soc Psychiatry Psychiatr Epidemiol. 2004;39(1):19–25. doi: 10.1007/s00127-004-0698-8. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin RD, Taha F. Global health benefits of being raised in a rural setting: Results from the National Comorbidity Survey. Psychiatry Clin Neurosci. 2014;68(6):395–403. doi: 10.1111/pcn.12144. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Park B-J, Ohira T, Kagawa T, Miyazaki Y. Acute effects of exposure to a traditional rural environment on urban dwellers: A crossover field study in terraced farmland. Int J Environ Res Public Health. 2015;12(2):1874–1893. doi: 10.3390/ijerph120201874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maller C, Townsend M, Pryor A, Brown P, St Leger L. Healthy nature healthy people: ‘Contact with nature’ as an upstream health promotion intervention for populations. Health Promot Int. 2006;21(1):45–54. doi: 10.1093/heapro/dai032. [DOI] [PubMed] [Google Scholar]
- 9.White MP, Alcock I, Wheeler BW, Depledge MH. Would you be happier living in a greener urban area? A fixed-effects analysis of panel data. Psychol Sci. 2013;24(6):920–928. doi: 10.1177/0956797612464659. [DOI] [PubMed] [Google Scholar]
- 10.Taylor AF, Kuo FE, Sullivan WC. Views of nature and self-discipline: evidence from inner city children. J Environ Psychol. 2002;22(1-2):49–63. [Google Scholar]
- 11.Kaplan R. The nature of the view from home. Environ Behav. 2001;33(4):507–542. [Google Scholar]
- 12.Berman MG, et al. Interacting with nature improves cognition and affect for individuals with depression. J Affect Disord. 2012;140(3):300–305. doi: 10.1016/j.jad.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartig T, Mitchell R, de Vries S, Frumkin H. Nature and health. Annu Rev Public Health. 2014;35:207–228. doi: 10.1146/annurev-publhealth-032013-182443. [DOI] [PubMed] [Google Scholar]
- 14.Ulrich R, et al. Stress recovery during exposure to natural and urban environments. J Environ Psychol. 1991;11(3):201–230. [Google Scholar]
- 15.Brown DK, Barton JL, Gladwell VF. Viewing nature scenes positively affects recovery of autonomic function following acute-mental stress. Environ Sci Technol. 2013;47(11):5562–5569. doi: 10.1021/es305019p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolen-Hoeksema S, Morrow J. Effects of rumination and distraction on naturally occurring depressed mood. Cogn Emotion. 1993;7(6):561–570. [Google Scholar]
- 17.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109(3):504–511. [PubMed] [Google Scholar]
- 18.Kuehner C, Weber I. Responses to depression in unipolar depressed patients: An investigation of Nolen-Hoeksema’s response styles theory. Psychol Med. 1999;29(6):1323–1333. doi: 10.1017/s0033291799001282. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience [published online February 24, 2015] Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayberg HS, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 21.Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biol Psychiatry. 2009;65(5):361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman MG, et al. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton JP, Glover GH, Hsu J-J, Johnson RF, Gotlib IH. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp. 2011;32(1):22–31. doi: 10.1002/hbm.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100(4):569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 26.Ghaznavi S, Deckersbach T. Rumination in bipolar disorder: Evidence for an unquiet mind. Biol Mood Anxiety Disord. 2012;2(2):1–11. doi: 10.1186/2045-5380-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 28.Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: Distinguishing rumination from reflection. J Pers Soc Psychol. 1999;76(2):284–304. doi: 10.1037//0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- 29.Detre JA, Rao H, Wang DJJ, Chen YF, Wang Z. Applications of arterial spin labeled MRI in the brain. J Magn Reson Imaging. 2012;35(5):1026–1037. doi: 10.1002/jmri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korpela KM, Hartig T, Kaiser FG, Fuhrer U. Restorative experience and self-regulation in favorite places. Environ Behav. 2001;33(4):572–589. [Google Scholar]
- 31.Hartig T, Staats H. Guest editors’ introduction: Restorative environments. J Environ Psychol. 2003;23(2):103–107. [Google Scholar]
- 32.Herzog TR. Assessing the restorative components of environments. J Environ Psychol. 2003;23(2):159–170. [Google Scholar]
- 33.Hartig T, Korpela K, Evans GW, Gärling T. A measure of restorative quality in environments. Housing. Theory Soc. 1997;14(4):175–194. [Google Scholar]
- 34.Pezawas L, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66(5):516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahn R, et al. The neural basis of human social values: Evidence from functional MRI. Cereb Cortex. 2009;19(2):276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masten CL, et al. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Dev Psychopathol. 2011;23(1):283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beddington J, et al. The mental wealth of nations. Nature. 2008;455(7216):1057–1060. doi: 10.1038/4551057a. [DOI] [PubMed] [Google Scholar]
- 39.Kareiva PK, Tallis H, Ricketts TH, Daily GC, Polasky S. Natural Capital: Theory & Practice of Mapping Ecosystem Services. Oxford Univ Press; Oxford, UK: 2011. [Google Scholar]
- 40.Bratman GN, Hamilton JP, Daily GC. The impacts of nature experience on human cognitive function and mental health. Ann N Y Acad Sci. 2012;1249(1):118–136. doi: 10.1111/j.1749-6632.2011.06400.x. [DOI] [PubMed] [Google Scholar]
- 41.Collins PY, et al. Scientific Advisory Board and the Executive Committee of the Grand Challenges on Global Mental Health Grand challenges in global mental health. Nature. 2011;475(7354):27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson LE. The relationship of urban design to human health and condition. Landsc Urban Plan. 2003;64(4):191–200. [Google Scholar]
- 43.van Dillen SME, de Vries S, Groenewegen PP, Spreeuwenberg P. Greenspace in urban neighbourhoods and residents’ health: Adding quality to quantity. J Epidemiol Community Health. 2012;66(6):e8. doi: 10.1136/jech.2009.104695. [DOI] [PubMed] [Google Scholar]
- 44.Ward Thompson C, et al. More green space is linked to less stress in deprived communities: Evidence from salivary cortisol patterns. Landsc Urban Plan. 2012;105(3):221–229. [Google Scholar]
- 45.Wu C-D, et al. Linking student performance in Massachusetts elementary schools with the “greenness” of school surroundings using remote sensing. PLoS ONE. 2014;9(10):e108548. doi: 10.1371/journal.pone.0108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzoulas K, et al. Promoting ecosystem and human health in urban areas using Green Infrastructure: A literature review. Landsc Urban Plan. 2007;81(3):167–178. [Google Scholar]
- 47.Martin-Breen P, Anderies JM. Resilience: A Literature Review. Rockefeller Foundation; New York: 2011. [Google Scholar]
- 48.Chiesura A. The role of urban parks for the sustainable city. Landsc Urban Plan. 2004;68(1):129–138. [Google Scholar]
- 49.Schreiber F, Kavanaugh L. ICLEI, 2014, Resilient Cities 2014: Congress Report. Holzforschung. 2014;50:1–97. [Google Scholar]
- 50.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012;15(5):663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 51.Alsop DC, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lawrence EJ, et al. The role of ‘shared representations’ in social perception and empathy: An fMRI study. Neuroimage. 2006;29(4):1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16(9):1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 55.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 56.Kumari V, et al. Functional MRI of verbal self-monitoring in schizophrenia: Performance and illness-specific effects. Schizophr Bull. 2010;36(4):740–755. doi: 10.1093/schbul/sbn148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bigler ED, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31(2):217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 58.Barbey AK, Krueger F, Grafman J. Structured event complexes in the medial prefrontal cortex support counterfactual representations for future planning. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1291–1300. doi: 10.1098/rstb.2008.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]