If you go fishing and cast your lure out across a pond, settling it skillfully by the lily pads, you may see the water drop out from under your bait with a sucking whirlpool as you engage with the most popular sport fish in the world, the largemouth bass. Almost all popular recreational sport fish species (bass, salmon, trout, pike, grouper, snapper) feed by attacking their living prey with powerful suction, expanding their mouth and pharynx rapidly to suck the prey in before biting down and swallowing. This behavior is of great interest to scientists and recreational fishers alike because it is a dramatic, predatory event (Fig. 1) that is at the crux of survival for both predator and prey. It involves a complex system of bones and muscles to expand the head, is a widespread and ecologically successful strategy among aquatic animals, and is a major source of the excitement and allure of recreational fishing, one of the largest and most effective economic engines for environmental conservation of freshwater and marine habitats. In addition, suction feeding has been challenging to understand from the perspective of biomechanics (the study of how organisms work) because it is such a fast and complex behavior. In PNAS, Camp et al. (1) report a major advance in our understanding of how suction feeding works, using cutting-edge imaging technology and analyses of muscle mechanics to identify the source of the power that drives suction feeding in our feisty friend from the pond, the largemouth bass.
Fig. 1.
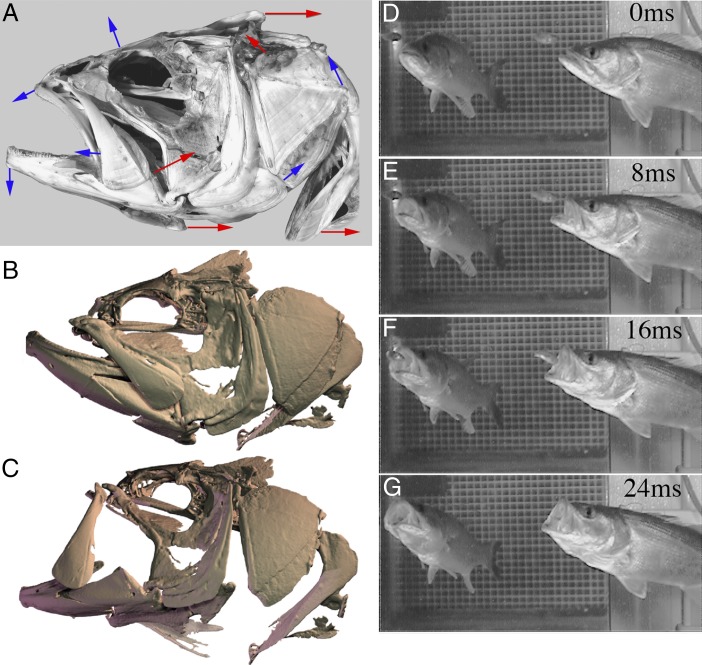
Skull morphology and strike mechanics of the large-mouth bass, Micropterus salmoides. (A) Skeletal morphology of the largemouth bass, with red arrows indicating primary muscle force vectors, and blue arrows showing movements of bones during the opening phase of suction feeding. (B and C) Rendered frames from an XROMM animation of a sample strike before the onset of expansion of the mouth (B) and just before peak gape (C). (D–G) Suction feeding in the largemouth bass Micropterus salmoides. Simultaneous lateral (Left) and ventral (Right) views show the role of lateral expansion in prey capture by suction. From time 0–8 ms cranial elevation and mouth opening occur before contact with the prey item. At 16 ms the maxilla is observed in anteriorly rotated position and expansion of the head and suction forces are near peak. At time 24 ms the prey is being sucked into the mouth, after which the jaws close on the food item within about 50 ms (or 1/20 of a second). Images B and C courtesy of Ariel Camp (Brown University, Providence, RI) and ref. 20.
Suction feeding is the primary mode of prey capture in fishes (2, 3), a method used to draw prey into the mouth by using the density of water as a tool for prey transport (Fig. 1). Suction feeding behavior has been the focus of numerous studies involving high-speed video analysis of kinematics (4, 5), electromyographic study of the motor patterns that drive suction feeding (6, 7), and application of a range of techniques, such as pressure transduction (8, 9), sonomicrometry (10), and digital particle imaging velocimetry (11). These studies have discovered the timing of different aspects of cranial kinesis involved in suction feeding, revealed the patterns of muscle contraction underlying suction generation, and measured the hydrodynamics, changing water pressure, and suction velocity during feeding. As a result, we have a detailed understanding of the morphology, behavior, and biomechanics of one of the fastest and most widespread feeding behaviors among vertebrates. However, the sources of power for this explosive behavior have remained poorly understood.
It takes a lot of power (force × velocity) to move water fast for suction feeding, and the source of that power is muscle contraction. Fish heads are packed with muscles for jaw opening and closing, rotating and flaring bones for breathing and feeding, and muscles in the pharynx for swallowing and controlling the gills. However, the key to powering large suction forces is high muscle power during jaw opening, and the biggest muscles in the head, the biting jaw adductor muscles, are for jaw closing and thus cannot power suction. So whence does suction power come? The intriguing answer, arrived at over years of research, mostly on largemouth bass, is that suction feeding is powered by the dorsal and ventral body muscles that have been co-opted from their swimming role to be used during the predatory strike.
Many studies of cranial functional morphology and electromyography in fishes (3) have measured cranial elevation powered by the epaxial muscles, in which the body muscles just behind the head cause the skull to rotate upward during feeding. The extent of this body muscle role was revealed by detailed analysis of multisite EMG recordings (12) showing that these modified anterior pointing arms of the complex musculature were highly active during feeding, and were active independent of other myomere components that function primarily in swimming. Tackling another piece of the puzzle, the ventral sternohyoideus muscle was examined (13) for dynamic patterns of activation and length change during feeding to show that it too was contributing power to suction feeding. In a key pair of papers, Andrew Carroll and colleagues (14, 15) used a combination of biomechanical modeling, muscle strain, suction pressure, and muscle contractile properties to compute the power required to generate rapid suction pressures, and experimentally measure the actual in vitro contractile power capacity of body muscle in the bass. These authors found that muscle power is variable across cranial muscles, that epaxialis body muscle had the highest power capacity measured, and yet the epaxialis was operating under such an intense loading regime for suction feeding, it was likely that maximal power production was constrained. The need for speed is critical in suction feeding, but this research showed a mismatch between the peak muscle power possible and the peak power actually observed during suction feeding. This synthesis of kinematic studies, muscle research, and new mathematical models (16) was converging on a relatively complete picture of suction feeding to inform both mechanistic and broader comparative studies of this important behavior, but one thing was still missing: a complete, high-speed, 3D view of how the system worked.
Camp et al. (1) advance our understanding of suction feeding significantly by measuring and reconstructing multiple aspects of the behavior in three dimensions using a technique called X-ray reconstruction of moving morphology (XROMM). This research used XROMM technology involving two high-speed X-ray video camera systems to view the internal motions of suction feeding with high accuracy in 3D. Static, 3D models of each bone in the skull were produced with computed tomography scans that can reconstruct the skeletal morphology in high resolution. The animation of this static model to render highly resolved accuracy of motion in 3D is achieved with marker-based XROMM, in which radio-opaque markers are surgically implanted into the bones to track their motion (17). The motion captured by these markers is then applied to the 3D computed tomographic models of the bones to animate the translations and rotations of each bone (Fig. 1 B and C). In a key step, the shortening of muscles and the volume of the mouth cavity can be resolved with high accuracy in synchrony with the skeletal motion by embedding markers along the muscle fibers and by creating a dynamic digital endocast using skeletal landmarks. The result is synchronous, high-speed, high-resolution, 3D tracking of muscle contraction, skull motion, and mouth cavity volume to provide an unprecedented view of this explosive feeding behavior.
Several important insights are gained from this research in regard to feeding behavior, the power sources for suction feeding, and musculoskeletal biomechanics. First, suction feeding involves coordinated motion of many skull components in less than a tenth of a second, and XROMM research has refined our understanding of translation and rotation of the neurocranium, suspensorium, pectoral girdle, hyoid, and jaws during feeding (18). This is important for understanding the basic mechanics of producing suction, as well as developing comparative approaches that can help us understand the evolution of suction strategies. For example, the first evolutionary exploration of jaws in fishes—the placoderms—are thought to have had extensive cranial elevation and likely used suction feeding 400 million y ago (19). Second, the muscle power for suction feeding is confirmed to be primarily from the body muscles, with significant contributions (up to 95% of total power) by the hypaxial and epaxial muscles to the overall power budget of feeding in bass (1, 14, 18). This is an intriguing result when we consider that largemouth bass and many other suction feeding predators swim toward the prey at the same time they initiate suction feeding behavior in the skull. Camp et al. (1) point out several questions that remain about these muscles, such as how they meet competing demands for performance in both locomotor and feeding behaviors, and the likely complexity of neural control necessary to partition these functions. Third, this work provides new insight into the transmission systems that operate in fish heads. Muscle power is transmitted through a complex series of levers and linkages during feeding (3) to achieve the rapid skull and jaw motions that happen at a considerable distance from the muscle itself, and this work (1) is enabling a novel 3D consideration of linkage biomechanics in fishes (20, 21). So, as we continue our hook-and-line battle with that big bass out at the pond, we reflect that exciting future research about suction feeding is emerging from this field at many levels, from mechanistic questions involving muscles, bones, and neuromechanics, to comparative and evolutionary questions across the spectacular diversity of suction feeding fishes.
Footnotes
The authors declare no conflict of interest.
See companion article on page 8690.
References
- 1.Camp AL, Roberts TJ, Brainerd EL. Swimming muscles power suction feeding in largemouth bass. Proc Natl Acad Sci USA. 2015;112:8690–8695. doi: 10.1073/pnas.1508055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferry-Graham LA, Lauder GV. Aquatic prey capture in ray-finned fishes: A century of progress and new directions. J Morphol. 2001;248(2):99–119. doi: 10.1002/jmor.1023. [DOI] [PubMed] [Google Scholar]
- 3.Westneat MW. 2006. Skull biomechanics and suction feeding in fishes. Fish Biomechanics. Fish Physiology. eds Shadwick RE, Lauder G (Academic, San Diego) pp 29–75.
- 4.Grobecker DB, Pietsch TW. High-speed cinematographic evidence for ultrafast feeding in antennariid anglerfishes. Science. 1979;205(4411):1161–1162. doi: 10.1126/science.205.4411.1161. [DOI] [PubMed] [Google Scholar]
- 5.Westneat MW, Wainwright PC. Feeding mechanism of Epibulus insidiator (Labridae: Teleostei): Evolution of a novel functional system. J Morphol. 1989;202(2):129–150. doi: 10.1002/jmor.1052020202. [DOI] [PubMed] [Google Scholar]
- 6.Alfaro ME, Janovetz J, Westneat MW. Motor control across trophic strategies: Muscle activity of biting and suction feeding fishes. Am Zool. 2001;41(6):1266–1279. [Google Scholar]
- 7.Grubich JR. Prey capture in actinopterygian fishes: A review of suction feeding motor patterns with new evidence from an elopomorph fish, Megalops atlanticus. Am Zool. 2001;41(6):1258–1265. [Google Scholar]
- 8.Muller M, Osse JWM. Hydrodynamics of suction feeding in fish. Trans Zool Soc Lond. 1984;37(2):51–135. [Google Scholar]
- 9.Carroll AM, Wainwright PC, Huskey SH, Collar DC, Turingan RG. Morphology predicts suction feeding performance in centrarchid fishes. J Exp Biol. 2004;207(Pt 22):3873–3881. doi: 10.1242/jeb.01227. [DOI] [PubMed] [Google Scholar]
- 10.Sanford CPJ, Wainwright PC. Use of sonomicrometry demonstrates the link between prey capture kinematics and suction pressure in largemouth bass. J Exp Biol. 2002;205(Pt 22):3445–3457. doi: 10.1242/jeb.205.22.3445. [DOI] [PubMed] [Google Scholar]
- 11.Ferry-Graham LA, Wainwright PC, Lauder GV. Quantification of flow during suction feeding in bluegill sunfish. Zoology (Jena) 2003;106(2):159–168. doi: 10.1078/0944-2006-00110. [DOI] [PubMed] [Google Scholar]
- 12.Thys T. Spatial variation in epaxial muscle activity during prey strike in largemouth bass (Micropterus salmoides) J Exp Biol. 1997;200(Pt 23):3021–3031. doi: 10.1242/jeb.200.23.3021. [DOI] [PubMed] [Google Scholar]
- 13.Carroll AM. Muscle activation and strain during suction feeding in the largemouth bass Micropterus salmoides. J Exp Biol. 2004;207(Pt 6):983–991. doi: 10.1242/jeb.00862. [DOI] [PubMed] [Google Scholar]
- 14.Carroll AM, Wainwright PC. Muscle function and power output during suction feeding in largemouth bass, Micropterus salmoides. Comp Biochem Physiol A Mol Integr Physiol. 2006;143(3):389–399. doi: 10.1016/j.cbpa.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin DJ, Carroll AM. In vitro estimates of power output by epaxial muscle during feeding in largemouth bass. Comp Biochem Physiol A Mol Integr Physiol. 2006;145(4):533–539. doi: 10.1016/j.cbpa.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright PC, Day SW. The forces exerted by aquatic suction feeders on their prey. J R Soc Interface. 2007;4(14):553–560. doi: 10.1098/rsif.2006.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brainerd EL, et al. X-ray reconstruction of moving morphology (XROMM): Precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol. 2010;313(5):262–279. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- 18.Camp AL, Brainerd EL. Role of axial muscles in powering mouth expansion during suction feeding in largemouth bass (Micropterus salmoides) J Exp Biol. 2014;217(Pt 8):1333–1345. doi: 10.1242/jeb.095810. [DOI] [PubMed] [Google Scholar]
- 19.Anderson PSL, Westneat MW. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biol Lett. 2007;3(1):76–79. doi: 10.1098/rsbl.2006.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camp AL, Brainerd EL. Reevaluating musculoskeletal linkages in suction-feeding fishes with X-Ray Reconstruction of Moving Morphology (XROMM) Integr Comp Biol. 2015;55(1):36–47. doi: 10.1093/icb/icv034. [DOI] [PubMed] [Google Scholar]
- 21.Olsen A. 2015 linkR: 3D Lever and Linkage Mechanism ModelingR package version 1.0.0. Available at cran.r-project.org/web/packages/linkR. Accessed June 1, 2015.