Significance
Prions are self-propagating protein aggregates. We designed rational mutations in four nonprion proteins to examine possible mechanisms for how new prions could evolve. In each case, a small number of mutations were sufficient to cause the proteins to aggregate and, in two cases, to create prion activity. We likewise showed that simply creating tandem repeats of aggregation-prone segments within nonprion proteins can be sufficient to create prion activity, suggesting that such segment duplication may represent a mechanism for generation of new prion domains.
Keywords: yeast, prion, amyloid, Sup35
Abstract
Yeasts contain various protein-based genetic elements, termed prions, that result from the structural conversion of proteins into self-propagating amyloid forms. Most yeast prion proteins contain glutamine/asparagine (Q/N)-rich prion domains that drive prion activity. Here, we explore two mechanisms by which new prion domains could evolve. First, it has been proposed that mutation and natural selection will tend to result in proteins with aggregation propensities just low enough to function under physiological conditions and thus that a small number of mutations are often sufficient to cause aggregation. We hypothesized that if the ability to form prion aggregates was a sufficiently generic feature of Q/N-rich domains, many nonprion Q/N-rich domains might similarly have aggregation propensities on the edge of prion formation. Indeed, we tested four yeast Q/N-rich domains that had no detectable aggregation activity; in each case, a small number of rationally designed mutations were sufficient to cause the proteins to aggregate and, for two of the domains, to create prion activity. Second, oligopeptide repeats are found in multiple prion proteins, and expansion of these repeats increases prion activity. However, it is unclear whether the effects of repeat expansion are unique to these specific sequences or are a generic result of adding additional aggregation-prone segments into a protein domain. We found that within nonprion Q/N-rich domains, repeating aggregation-prone segments in tandem was sufficient to create prion activity. Duplication of DNA elements is a common source of genetic variation and may provide a simple mechanism to rapidly evolve prion activity.
Amyloid fibrils are ordered protein aggregates characterized by filamentous morphology and cross-β-sheet structure (1, 2). Amyloid fibril formation is associated with numerous human diseases, including Alzheimer’s disease and type II diabetes. Prions represent a subset of amyloid diseases in which the amyloid state is infectious (3). In addition to their role in disease, some prions and other amyloids appear to perform beneficial functions, such as acting as regulatory or structural elements (4, 5).
Saccharomyces cerevisiae has provided a useful model system for studying prions. Numerous amyloid-based prions have been identified in yeast (reviewed in refs. 6 and 7). One of the best characterized of these is [PSI+], which is the prion form of the translation termination factor Sup35 (8). Sup35 has three functionally distinct domains: an N-terminal glutamine/asparagine (Q/N)-rich intrinsically disordered prion-forming domain (PFD) that is required for prion aggregation; a C-terminal (C) functional domain that is necessary and sufficient for Sup35’s normal cellular function; and a highly charged middle (M) domain that is dispensable for both translation termination and prion activity, but stabilizes [PSI+] (9, 10).
Like the Sup35 PFD, most of the other yeast PFDs are Q/N rich; additionally, they tend to share other compositional features such as an underrepresentation of charged and hydrophobic amino acids (11). Similar prion-like domains (PrLDs) are common in eukaryotic genomes, and mutations in some of these have recently been linked to various degenerative disorders, including amyotrophic lateral sclerosis (reviewed in refs. 12 and 13). However, this set of compositional characteristics is not sufficient for prion-like activity. In one comprehensive study, Alberti et al. identified the 100 yeast peptide fragments with greatest compositional similarity to yeast PFDs (14). Each fragment was tested in four assays for prion-like activity: transient expression as a GFP fusion to measure the propensity to form foci, semidenaturing detergent agarose gel electrophoresis (SDD-AGE) to test for the formation of detergent-insoluble aggregates, in vitro monitoring of amyloid formation, and fusion to the Sup35 functional domain to assay prion activity. Although 18 of the fragments showed prion-like activity in all four assays, there was little correlation between similarity to known yeast PFDs and prion activity (14, 15).
We previously used a quantitative random mutagenesis approach to define the compositional requirements for prion activity and found that, despite their relative rarity in yeast PFDs, hydrophobic and aromatic residues strongly promote prion formation (15, 16). Unlike many amyloid-forming proteins that contain short, highly aggregation-prone segments, prion activity in the yeast PFDs appears to be more diffuse, with the prion-promoting residues distributed across larger, intrinsically disordered segments (15). Based on these data, we developed a prion aggregation prediction algorithm (PAPA) (17, 18), which is able to discriminate with reasonable accuracy between Q/N-rich domains with and without prion activity.
An obvious question is how proteins evolved these long, low-complexity disordered PFDs. One possibility is suggested by the “life on the edge” hypothesis of Tartaglia et al. (19). They proposed that there is evolutionary pressure to prevent protein aggregation, but that once a protein arrives at a sequence that does not aggregate under normal physiological conditions, there is little selective pressure to further reduce aggregation propensity. Because most mutations will tend to increase aggregation activity, random mutation will cause many proteins to exist very close to this aggregation threshold. Thus, if domains with prion-like composition evolve for reasons unrelated to prion formation, this theory predicts that a relatively small number of mutations should be sufficient to push them over the edge and into aggregation. If the ability to propagate as prions is a relatively common activity of Q/N-rich aggregates, then many nonprion Q/N-rich domains may likewise be just a few mutations away from prion activity. Indeed, functions independent of prion activity have been identified for some yeast PFDs (20–22), so it is possible that these functions preceded acquisition of the ability to form prions.
We previously proposed a second mechanism for how yeast PFDs could rapidly evolve (23). Oligopeptide repeat segments are found in multiple prion proteins, including PrP, Sup35, and Rnq1, and expansion of the PrP (24) or Sup35 (25) repeats increases prion activity. However, it is unclear whether the prion-promoting activity of these repeats is specific to these particular sequences. Although deletion of some or all of the repeats destabilizes [PSI+] (26), randomizing the order of the amino acids in the repeats does not prevent [PSI+] maintenance (23), suggesting that although the Sup35 repeat region is important for prion activity, the repeats per se are not. If prion activity is insensitive to the primary sequence of the repeats, then why are repeats so common in PFDs? We proposed that oligopeptide repeats may be common in PFDs simply because they provide a simple genetic mechanism for generating PFDs (23). Tandem repeats, both of single codons and larger oligopeptide segments, are common in eukaryotic genomes and can readily form due to errors in replication, repair, or recombination (27, 28). An easy way to generate the long low-complexity regions of consistent, modest prion activity that characterize yeast PFDs would be to create tandem repeats of short segments fitting these criteria.
We tested these two mechanisms for generating new PFDs by examining the mutations required to turn nonprion proteins into prions. Each mechanism makes specific predictions. The life on the edge model suggests that if a domain has amino acid composition similar to the yeast PFDs, but no detectable prion activity, it should require only a small number of mutations to generate prion-like activity. The repeat expansion model suggests that there is nothing particularly unique about the specific repeats found in yeast PFDs and that tandem duplications of any short segment with prion-like composition should be sufficient to generate prion-like activity. Strikingly, we find both of these predictions to be true.
Results
Targeted Mutations Increase in Vivo Aggregation.
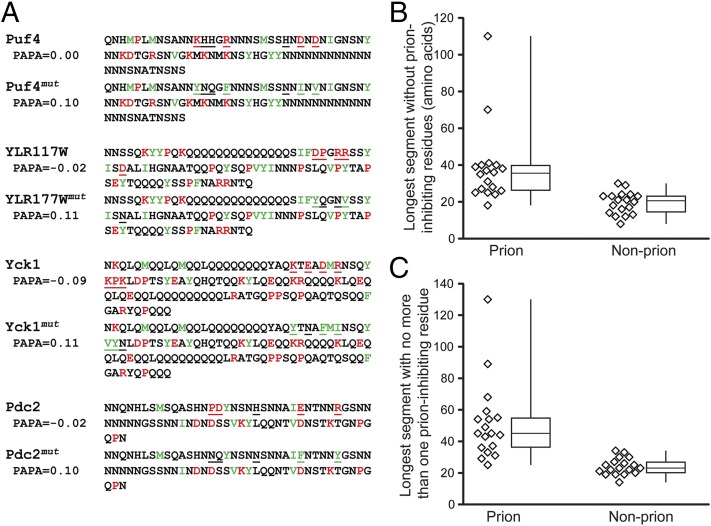
To test whether we could rationally design mutations to convert nonprion proteins into prions, we selected four PrLDs that were identified by Alberti et al. as having high compositional similarity to yeast prions, yet that showed no prion-like activity in four independent assays (14). The selected PrLDs are from Puf4, Pdc2, Yck1, and YLR177W (Fig. 1A). These PrLDs have PAPA scores ranging from −0.10 to 0.00. Because some studies suggest that Q and N residues have different effects on prion activity (29), two of the selected fragments are Q rich and two are N rich.
Fig. 1.
Design of prion-promoting mutations. (A) Sequences of the wild-type and mutant Puf4, YLR177W, Yck1, and Pdc2 PrLDs. Strongly prion-promoting amino acids (W, Y, F, V, I, and M) are indicated in green, whereas strongly prion-inhibiting amino acids (P, K, R, D, and E) are in red. Positions that were mutated are underlined. (B) PrLDs that do not show detectable prion activity tend to lack extended peptide stretches without prion-inhibiting residues. Alberti et al. (14) identified 100 yeast fragments with prion-like composition and tested each in four assays for prion-like activity. Shown are box-and-whiskers plots of the longest stretch without any prion-inhibiting residues for each of the proteins that showed prion-like activity in all four assays (Prion) and each of the proteins that failed all four assays (Nonprion). (C) Box-and-whiskers plot of the longest segments with no more than one prion-inhibiting residue.
Proline and charged residues strongly inhibit prion activity, whereas hydrophobic and aromatic residues promote prion activity (15). We hypothesized that the non-prion-forming PrLDs could be converted into prions by substituting inhibitory residues with either neutral or prion-promoting residues to increase the PrLD’s PAPA scores. Because strongly prion-promoting residues are relatively rare in PrLDs, even a small number of inhibitory residues can substantially reduce a PrLD’s prion activity (30, 31) and PAPA score. Consistent with this, contiguous stretches with few or no inhibitory residues are significantly underrepresented among nonprion Q/N-rich domains (Fig. 1 B and C). For example, among the 18 PrLDs shown by Alberti et al. (14) to lack any detectable prion-like activity, the longest contiguous stretch without a charged residue or proline is 30 aa; by contrast, of the 18 PrLDs that had prion-like activity in all four of the assays, 11 (61%) have stretches longer than 30 aa.
We therefore designed mutations to generate contiguous regions without intervening inhibitory residues. Because the two assays that we planned to use to test prion-like activity involved fusions to the C-terminal end of the PrLD, we concentrated the mutations on the N terminus. For each, we identified a contiguous stretch without an inhibitory residue and serially substituted adjacent inhibitory residues with a mixture of neutral (Q or N) or prion-promoting (F, Y, I, and V) residues until the PAPA score for the protein exceeded 0.10 (Fig. 1A).
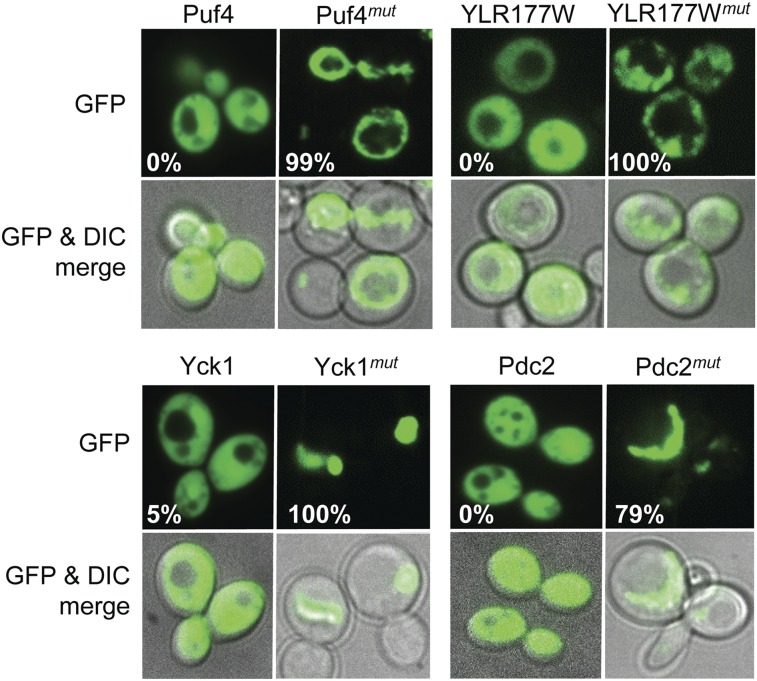
To test whether these mutations were sufficient to cause the PrLDs to form foci in yeast, we expressed the wild-type and mutant PrLDs as fusions with GFP (PrLD-GFP and PrLDmut-GFP, respectively). As previously reported (14), each wild-type PrLD-GFP fusion diffusely spread across the cytoplasm (Fig. 2). By contrast, each PrLDmut-GFP fusion formed foci or ring-like structures similar to those seen for known PFDs (Fig. 2).
Fig. 2.
Mutations in the PrLDs cause foci formation. Each of the wild-type and mutant PrLDs was fused to GFP and expressed from the GAL1 promoter. Cells were grown in galactose/raffinose dropout medium for 24 h and then visualized by confocal microscopy. The percentage of fluorescing cells with GFP foci (either rings or dots) is indicated; a minimum of 50 fluorescing cells were counted per construct.
Puf4 and YLR177W Mutants Support Formation of Stable Prions.
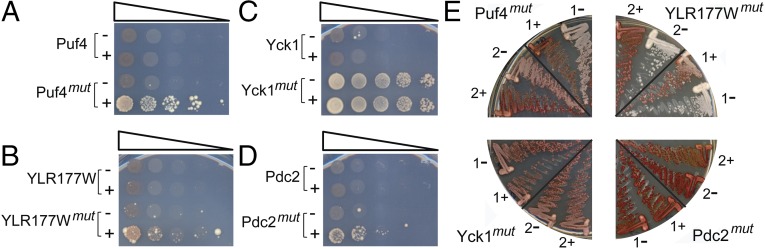
The ability to form foci is common to many nonprion proteins. As a more rigorous test of prion activity, we examined the ability of each PrLD to substitute for the Sup35 PFD in supporting prion formation. Fusions of each wild-type and mutant PrLD to the Sup35MC domain were expressed from the SUP35 promoter as the sole copy of SUP35 in the cell. Prion formation by the fusion proteins was detected by monitoring nonsense suppression of the ade2-1 allele. [psi−] cells are unable to grow in the absence of adenine and form red colonies in the presence of limiting adenine; [PSI+] cells are able to grow in the absence of adenine and form white or pink colonies in the presence of limiting adenine (32). [PSI+] formation is very rare when Sup35 is expressed at endogenous levels, but PFD overexpression increases [PSI+] formation by multiple orders of magnitude (33); this dependence on protein concentration is considered a hallmark of prion activity (8). Thus, each fusion was tested with and without overexpression of the matching PrLD.
The four wild-type PrLD-Sup35MC fusions formed only very rare Ade+ colonies, and transient PrLD overexpression had no detectable effect on Ade+ colony formation (Fig. 3), consistent with previous reports that these domains are unable to support prion activity (14). The PrLDmut-Sup35MC fusions showed more varied behavior. The Yck1mut-Sup35MC fusion was Ade+ even in the absence of PrLD overexpression (Fig. 3C), suggesting either that it was forming prions at a very high rate or that it had diminished activity, resulting in nonsense suppression. The other three PrLDmut-Sup35MC fusions showed clear prion-like behavior. Each formed only very rare Ade+ colonies when expressed at endogenous levels, but showed a substantial increase in Ade+ colony formation upon PrLDmut overexpression (Fig. 3).
Fig. 3.
Mutations in the PrLDs cause prion formation. (A–D) The wild-type and mutant PrLDs from Puf4 (A), YLR177W (B), Yck1 (C), and Pdc2 (D) were fused to the Sup35MC domain and expressed from the SUP35 promoter as the sole copy of Sup35 in the cell. Strains were transformed with either an empty vector (−) or a plasmid expressing the matching PrLD under control of the GAL1 promoter (+). Cells were grown in galactose/raffinose dropout medium for 3 d and then plated onto dextrose medium lacking adenine to select for [PSI+] cells. (E) For each mutant PrLD, to test for stability of the Ade+ phenotype, Ade+ colonies were streaked onto synthetic complete medium (−GdHCl) or synthetic complete medium supplemented with 4 mM guanidine HCl (+GdHCl). Cells were then restreaked onto YPD to test for loss of the Ade+ phenotype. Two prion isolates are shown for Puf4mut and YLR177Wmut; two representative isolates are shown for Pdc2mut and Yck1mut.
The Ade+ colonies formed by the Pdc2mut-Sup35MC fusion grew far slower on SC-Ade medium than is typical for [PSI+] cells, and all Ade+ isolates were unable to maintain the Ade+ phenotype without selection (Fig. 3E), suggesting that this fusion forms weak, poorly propagating prions. By contrast, for Puf4 the majority (18 of 28) of tested Ade+ isolates were able to stably maintain the Ade+ phenotype in the absence of selection; most of these showed a pink phenotype on limiting adenine, suggestive of a weak prion (Fig. 3E). All but one of these stable Ade+ isolates lost the Ade+ phenotype upon treatment with low concentrations of guanidine HCl (Fig. 3E). Guanidine HCl cures [PSI+] (34) by inhibiting the chaperone Hsp104 (35, 36). For YLR177W, most of the Ade+ isolates rapidly lost the Ade+ phenotype without selection, but a small subset (3 of 28) was able to stably maintain a strong Ade+ phenotype without selection; in each case, the Ade+ phenotype was curable by treatment with guanidine HCl (Fig. 3E). Thus, two of the four mutants were able to form stable, curable prions, whereas a third appears to form only unstable prions.
Controlling Prion Propensity.
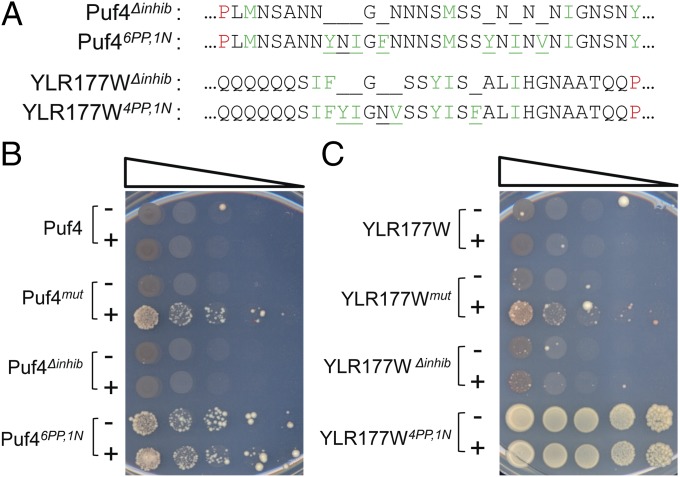
We next made a series of additional mutations in the YLR177W-Sup35MC and Puf4-Sup35MC fusions to more rigorously define the threshold for prion activity. The increase in prion activity in the original mutants was not simply due to the removal of inhibitory residues, as deletion of these inhibitory residues was not sufficient to turn the fusion proteins into prions (YLR177W∆inhib and Puf4∆inhib in Fig. 4).
Fig. 4.
Prion activity in the mutant PrLDs is sensitive to the number of prion-promoting amino acids. The mutated positions in Puf4mut and YLR177Wmut were either deleted (Puf4∆inhib and YLR177W∆inhib) or replaced with an increased ratio of prion-promoting to neutral amino acids (Puf46PP,1N and YLR177W4PP,1N). (A) Sequences of the mutants. Positions mutated are underlined. Blank spaces indicate deletions. (B and C) Ade+ colony formation assay for each of the mutant PrLDs fused to Sup35MC.
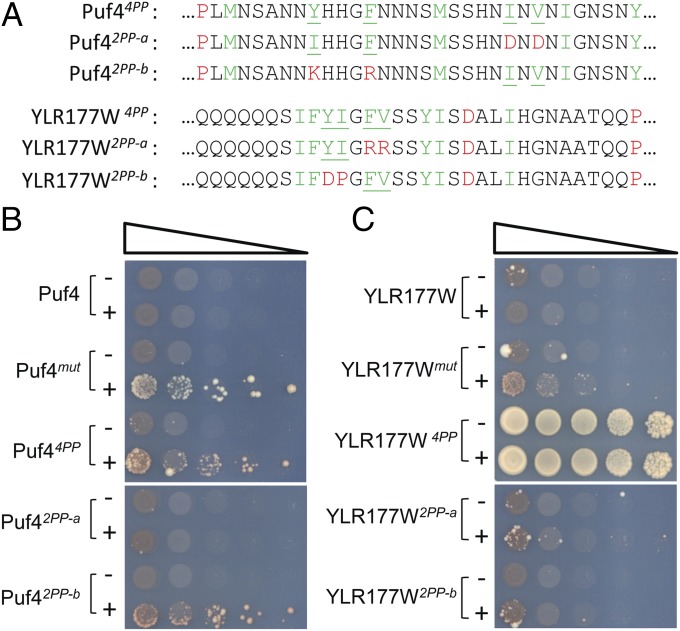
Prion activity could be further enhanced by replacing more of the prion-inhibiting residues with prion-promoting residues. For example, the original Puf4mut involved substitution of four strongly inhibitory charged residues and three moderately inhibitory histidines with four prion-promoting residues and three neutral residues. When the seven inhibitory residues were instead replaced with six prion-promoting residues and one neutral residue, the resulting construct (Puf46PP,1N-Sup35MC) efficiently formed Ade+ colonies even in the absence of overexpression, likely due to efficient prion-like aggregation (Fig. 4B). Likewise, the original YLR177W mutant had five inhibitory residues replaced with two prion-promoting residues and three neutral residues (Fig. 1A); changing this ratio to four prion-promoting residues and one neutral residue created a fusion (YLR177W4PP,1N-Sup35MC) with no detectable ade− state (Fig. 4C).
For Puf4, we were able to modestly reduce the number of mutations required for prion activity. Substituting only the four strongly inhibitory amino acids with prion-promoting amino acids (Puf44PP) was sufficient to create a construct with modest prion activity (Fig. 5 A and B). Interestingly, just substituting the last two of these residues (Puf42PP-b) was sufficient for substantial prion formation, whereas a construct with just the first two of these residues substituted (Puf42PP-a) showed no detectable prion formation (Fig. 5 A and B). For YLR177W, substituting four of the inhibitory residues with prion-promoting residues created a fusion (YLR177W4PP-Sup35MC) with no detectable ade− state (Fig. 5 A and C), but attempts to further reduce the number of mutations required for prion activity were unsuccessful.
Fig. 5.
Prion formation can be observed with as few as two mutations. (A) Sequences of mutants designed to test the minimal number of mutations required to create prion activity for the Puf4 and YLR177W PrLDs. (B and C) Ade+ colony formation assay for the Puf4 (B) and YLR177W (C) mutant PrLDs fused to Sup35MC.
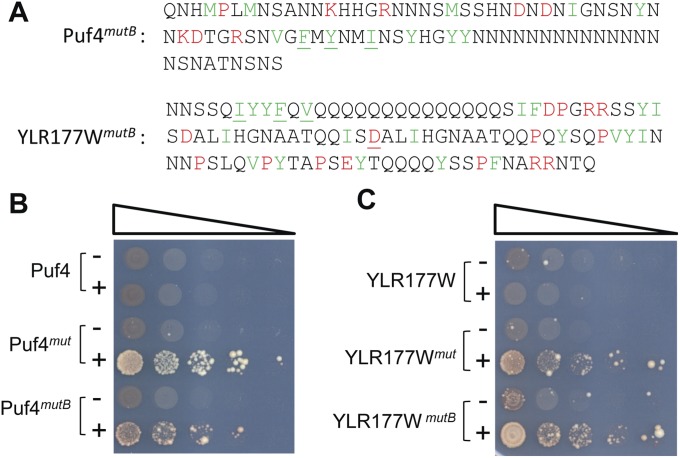
Finally, there appears to be nothing unique about the original set of mutations. For example, the original Puf4 mutant involved substitutions near the N terminus of the PrLD, but similar results were obtained with substitutions near the C terminus. Three lysines toward the C terminus break up a long segment without any other prion-inhibiting residues; substitution of these lysines with strongly prion-promoting residues was sufficient to create prion activity (Fig. S1, Puf4mut-B). Likewise, wild-type YLR177W PrLD contains a 17-aa stretch without any prion-inhibiting residues; YLR177Wmut was created by replacing the five inhibitory residues immediately after this stretch (Fig. 1A), but similar results were obtained when the three inhibitory residues before this stretch were replaced with prion-promoting residues (Fig. S1, YLR177Wmut-B).
Fig. S1.
Prion activity can be created by mutations at other positions. (A) Sequences of mutants targeting other regions of the Puf4 and YLR177W. (B and C) Ade+ colony formation assay for the Puf4 (B) and YLR177W (C) mutant PrLDs fused to Sup35MC.
Repeat Expansions to Create New Prion Proteins.
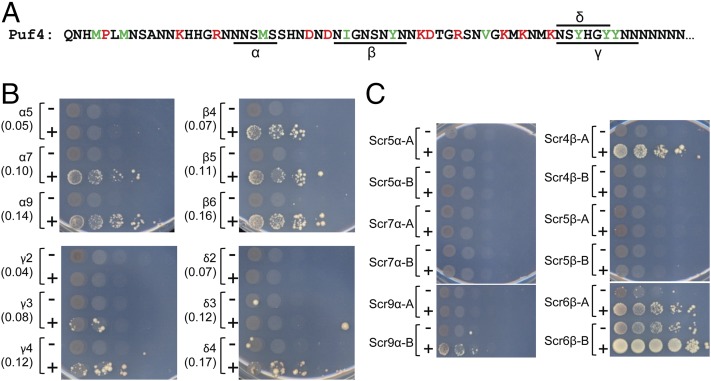
We hypothesized that another way to create long segments with modest prion propensity and few intervening prion-inhibiting residues would be to make tandem repeats of a short segment fitting this description. To test this hypothesis, we identified four short stretches in the Puf4 PrLD (indicated as α, β, γ, and δ in Fig. 6A) that lacked inhibitory residues. We avoided segments that were excessively Q/N rich, because it is already well established that glutamine expansions can promote aggregation activity. For each segment, we generated tandem repeat mutants designed to have PAPA scores of ∼0.05, 0.10, and 0.15. These mutants were tested as Sup35MC fusions.
Fig. 6.
Repeat expansions can create prion activity. (A) Short segments lacking any prion-inhibiting amino acids were identified in the Puf4 PrLD (indicated as α, β, γ, and δ in the PrLD sequence). (B) Versions of the Puf4 PrLD containing varying numbers of repeats of each segment were made. These repeat expansion mutant PrLDs were fused to Sup35MC and tested for prion activity. For each construct, the region duplicated and the copy number of the repeats are indicated. PAPA scores of each PrLD-Sup35MC fusion are indicated in parentheses. (C) For each of the repeat expansions of the α and β segments of Puf4, two scrambled versions were made in which the primary sequence of the full repeat region was randomized while keeping amino acid composition unchanged. Each PrLD containing scrambled repeats was fused to Sup35MC and tested for prion activity.
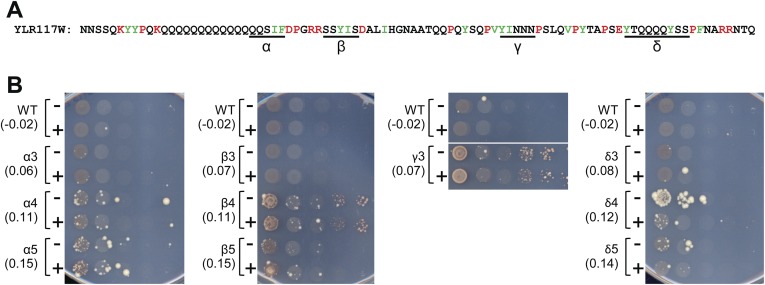
At all four positions, prion formation increased with progressively longer repeats, although the exact length threshold and degree of prion formation varied substantially among the different stretches (Fig. 6B). Interestingly, the results of similar experiments for YLR177W were less clear (Fig. S2). At each position, at least some of the expansions showed an increase in Ade+ colonies, but the length dependence was less linear and few of the segments showed the clear increase with overexpression that is generally observed for prion proteins.
Fig. S2.
Repeat expansions in the YLR177W PrLD. (A) Short segments lacking any prion-inhibiting amino acids were identified in the YLR177W PrLD (indicated as α, β, γ, and δ in the PrLD sequence). (B) Versions of the YLR177W PrLD containing varying numbers of repeats of each segment were made. These repeat expansion mutant PrLDs were fused to Sup35MC and tested for prion activity. For each construct, the region replicated and the copy number of the repeats are indicated. PAPA scores of each PrLD-Sup35MC fusion are indicated in parentheses. For the γ segment, four and five repeat versions were designed; however, cells expressing these fusion proteins as the sole copy of Sup35 were inviable. For the δ4 segment, it is unclear why fewer Ade+ colonies were seen upon PrLD overexpression. This may reflect toxicity of this overexpressed PrLD, particularly in prion-positive cells. Alternatively, the overexpressed PrLD may preferentially form structures that are poorly propagated.
There are two basic explanations for the length-dependent prion formation observed for the Puf4 expansions. First, repeated sequences may directly promote prion formation, for example by facilitating packing into the serpentine structures that individual PFD monomers are thought to adopt within prion fibers. Alternatively, the repeats may not promote prion formation per se, but instead may increase prion propensity simply by creating larger prion-prone regions. To distinguish between these two possibilities, we tested whether nonrepeat expansion would similarly promote prion formation. For the two repeat segments that showed clearest prion activity (α and β), we generated a parallel set of constructs where we scrambled the repeat elements (Fig. 6C). To reduce any bias created by subtle primary sequence effects, two scrambled versions were created at each length.
Interestingly, the data seem to suggest that both theories may be true to some degree (Fig. 6C). Some of the scrambled expansions did show prion activity, and generally more prion activity was seen at longer lengths. Overlaid on this general trend were clear primary sequence effects. At each length, there was variability between the two scrambled versions. And although there was a general trend toward longer constructs having more prion activity, there was one clear outlier: For segment β, although neither of the constructs containing five scrambled repeats showed prion activity, one of the constructs containing four scrambled repeats efficiently formed prions (Fig. 6C). Additionally, prion formation generally occurred at shorter repeat lengths for the nonscrambled constructs than for the scrambled constructs.
Collectively, these data demonstrate that duplication of segments to create tandem repeats is a viable mechanism for the creation of new prions, and although this effect may in part be due to the actual repeats, it is at least in part a simple result of generation of larger prion-prone segments.
Discussion
Domains that compositionally resemble yeast PFDs are common in eukaryotic genomes (13, 14). Although many of these have been shown to form either beneficial or pathogenic aggregates, a significant subset shows no detectable tendency to aggregate, even when overexpressed (14). Our results indicate that many of these nonaggregating domains may be just a few mutations away from aggregating under physiological conditions. Furthermore, although our mutants had two to seven point mutations, it is likely that a similar effect may be possible with fewer mutations. PAPA, which was used to design these mutations, considers only local amino acid composition. However, although amino acid composition clearly has a dominant effect in determining the aggregation propensity of PrLDs (15, 37), the exact positioning of prion-promoting mutations also has a significant effect (16). Therefore, it is likely that with better prediction abilities or more thorough screening, more efficient sets of mutations could be designed.
As expected, it was easier to design mutations to make the PrLDs aggregate than to make them form stable prions. Although all four of the original mutants formed foci when fused to GFP, and although all four were able to form Ade+ colonies when fused to Sup35MC, only two of the four showed the two stable states required to be truly considered a prion; the mutant Yck1 fusion lacked a stable Ade− state, whereas mutant Pdc2 fusion was unable to stably maintain its Ade+ state. Nevertheless, the fact that two of the four mutants formed stable prions highlights how generic the requirements for prion activity are. It also suggests a simple mechanism for evolving new prions; it seems that mutation and selection will push many PrLDs to the edge of aggregation, such that only a few mutations are required to confer prion activity. This may explain why single-point mutations in so many different PrLDs are sufficient to cause degenerative diseases like amyotrophic lateral sclerosis (13).
Our results also demonstrate that creation of tandem repeats could serve as an alternative mechanism for generating new PFDs. Duplication of DNA segments—ranging from short microsatellite mutations (38) to large copy number variants (39)—has emerged as a major source of evolutionary diversity, and tandem repeats are common across various organisms (28). Previous studies have clearly demonstrated that the Sup35 and PrP repeats can promote prion activity (24, 25, 40). However, it was unclear whether this was due to specific sequence/composition features of these repeats or simply a generic result of expanding a prion-prone segment. Our current results strongly argue for the second interpretation. All four of the tested Puf4 segments resulted in prion activity when repeated in sufficient numbers. These sequences were quite diverse; our only criteria for selection were the absence of strongly prion-inhibiting residues and modest Q/N content (the selected peptides ranged from 17% to 56% Q/N).
One unexpected result did emerge from the repeat expansion experiments. Although the scrambled repeats still showed prion activity for sufficiently long expansions, they did so at longer lengths than their corresponding nonscrambled variants. This result could be a coincidence, due to the limited sample sizes of the experiments, or it is possible that repeat sequences per se may exert subtle prion-promoting effects; for example, the regular spacing of prion-promoting/inhibiting residues in repeat elements may promote the formation of specific amyloid structures.
Finally, there are key caveats that must be considered with these experiments. The Sup35 fusion assay has the possibility of both false-positive and false-negative results (41). For example, because Sup35 is essential, a PrLD that is too effective at sequestering Sup35 may appear as a negative in the fusion assay. However, this seems unlikely to explain the failure of the four wild-type PrLDs to form prions, because each also failed to show aggregation activity in three other less stringent assays. A second possible source of false negatives is that the algorithm (14) used to select these domains was not necessarily perfect at defining the boundaries of PrLDs, so it is possible that one or more of our four PrLDs actually comes from a bona fide prion protein, in which imperfect selection of the PrLD resulted in a fragment without detectable aggregation activity. In particular, full-length Yck1 has been shown to stimulate [PSI+] formation when overexpressed (42), suggesting that the full-length protein may have some prion-like activity. Finally, regions outside of the Sup35 PFD can influence prion activity (12), so although we showed that a small number of mutations can confer prion activity on the Puf4 and YLR177W PrLDs, these domains may still not act as prions in their native context. Additionally, factors such as expression level, cellular localization, and binding partners likely all affect prion activity in ways that have not yet been fully defined. Thus, although our results show that many PrLDs may be just a few mutations away from supporting prion activity, more experiments will be required to determine how many of these domains exist in a context that is conducive to prion activity and to define these context requirements.
Materials and Methods
Strains and Media.
Standard yeast media and methods were used as previously described (43), except the YPD contained 0.5% yeast extract instead of the standard 1%. In all experiments, yeast were grown at 30 °C. All experiments were performed in S. cerevisiae strain YER632/pJ533 (16). This strain’s genotype is α kar1-1 SWQ5 ade2-1 his3 leu2 trp1 ura3 sup35::KanMx [psi−] [PIN+]; pJ533 expresses SUP35 from a URA3 plasmid as the sole copy of SUP35 in the cell.
Cloning of PrLDs.
To generate the PrLD-Sup35MC fusions, the Puf4, YLR177W, Yck1, and Pdc2 PrLDs were PCR amplified from strain YER632/pJ533, adding a start codon at the beginning of the PrLD (see Table S1 for a complete list of primer sequences). PCR products were reamplified with EDR236 and EDR1341 and then cotransformed with HindIII/BamHI-cut pJ526 (37) into yeast strain YER632/pJ533. Transformations were selected on SC-Leu and then transferred to 5-fluoroorotic acid plates to select for loss of pJ533. The resulting products were confirmed by DNA sequencing.
Table S1.
Oligonucleotides used in this study
| Name | Description | Sequence |
| EDR236 | Antisense primer to Sup35 | GAGACAAGCTTCAAAGTCTTCTTTGGTTTGGGAGCGGCCTGCTTTTGTTGCTTTTGAAAGTCGTTCAAAGACATACCTTGAGACTGTGGTTGGAAACCAG |
| EDR262 | Antisense primer binding in the Sup35 M domain | GCATCAGCACTGGTAACATTGG |
| EDR301 | Sense primer binding upstream of the Sup35 start site | CGTCACAGTGTTCGAGTCTG |
| EDR302 | Sense primer binding upstream of the Sup35 start site | GGCAGAATATCTGTCAACCACAC |
| EDR304 | Antisense primer binding in the Sup35 C domain | GTTTCGTACTCACCCTTTCTGG |
| EDR1084 | Antisense primer to build induction plasmids | CGATGCTACTCGAGTTTACATATCGTTAACAACTTCGTCATCCAC |
| EDR1924 | Antisense primer to build GFP fusions | GTCGATGCTACTCGAGTCGTTAACAACTTCGTCATCCACTTC |
| EDR1662 | Sense primer used to build Puf4-Sup35MC | CTGCCCACTAGCAACAATGTCTCAAAATCATATGCCGTTAATGAATAGCGCC |
| EDR1663 | Antisense primer used to build Puf4-Sup35MC | GAGACTGTGGTTGGAAACCAGCGCTGTTGCTGTTGGTAGCATTACTG |
| EDR1653 | Sense primer used to build YLR177W-Sup35MC | CTGCCCACTAGCAACAATGTCTAACAACAGCTCCCAAAAATACTATCCAC |
| EDR1654 | Antisense primer used to build YLR177W-Sup35MC | GAGACTGTGGTTGGAAACCAGCCTGGGTGTTGCGCCTAGCATTG |
| EDR1658 | Sense primer used to build Yck1-Sup35MC | CTGCCCACTAGCAACAATGTCTAACAAGCAGTTACAAATGCAGCAATTGC |
| EDR1659 | Antisense primer used to build Yck1-Sup35MC | GAGACTGTGGTTGGAAACCAGCTTGTTGTTGTGGTTGATAACGAGCG |
| EDR1666 | Sense primer used to build Pdc2-Sup35MC | CTGCCCACTAGCAACAATGTCTAATAATCAAAATCATTTAAGCATGTCACAAGCTAGCC |
| EDR1667 | Antisense primer used to build Pdc2-Sup35MC | GAGACTGTGGTTGGAAACCAGCATTTGGTTGTCCAGGGTTACCTG |
| EDR1773 | Sense primer to make YLR177W induction plasmids and GFP fusions | GAGCTACTGGATCCACAATGTCTAACAACAGCTCCCAAAAATACTATCC |
| EDR1774 | Sense primer to make Yck1 induction plasmids and GFP fusions | GAGCTACTGGATCCACAATGTCTAACAAGCAGTTACAAATGCAGCAATTGC |
| EDR1775 | Sense primer to make Puf4 induction plasmids and GFP fusions | GAGCTACTGGATCCACAATGTCTCAAAATCATATGCCGTTAATGAATAGCGCC |
| EDR1776 | Sense primer to make Pdc2 induction plasmids and GFP fusions | GAGCTACTGGATCCACAATGTCTAATAATCAAAATCATTTAAGCATGTCACAAGC |
| EDR1791 | Sense primer used to build Puf4mut | GCATGTCCAGCAACAACATCAATGTCAACATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR1792 | Antisense primer used to build Puf4mut | GACATTGATGTTGTTGCTGGACATGCTATTGTTATTGAAACCCTGATTGTAATTATTGGCGCTATTCATTAACGGCATATG |
| EDR1795 | Sense primer used to build YLR177Wmut | GCATCTTCTACCAGGGAAACGTCTCTTCCTATATTTCTAACGCGCTGATTCATGGCAATGC |
| EDR1796 | Antisense primer used to build YLR177Wmut | GAGACGTTTCCCTGGTAGAAGATGCTTTGTTGCTGTTGCTGCTGCTGCTGCTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR1797 | Sense primer used to build Yck1mut | GCTCAGTACACCAACGCCTTCATGATCAATTCTCAATATGTCTACAACCTAGACCCTACTTCTTATGAAGCTTAC |
| EDR1798 | Antisense primer used to build Yck1mut | CATGAAGGCGTTGGTGTACTGAGCATATTGCTGTTGCTGTTGCTGTTGTTGGAGCTGTTGCATTTGC |
| EDR1793 | Sense primer used to build Pdc2mut | GCCATCTTCAATACTAACAACTACGGCAGTAATAATAATAACAATAATAATGGTAGTAGTAATAATATTAATGATAATGATAGTAGCG |
| EDR1794 | Antisense primer used to build Pdc2mut | CTGCCGTAGTTGTTAGTATTGAAGATGGCATTGTTGCTATTATTACTGTTGTACTGGTTGTTGTGGCTAGCTTGTGACATG |
| EDR1998 | Sense primer used to build Puf4∆inhib | CATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR1999 | Antisense primer used to build Puf4∆inhib | GACCTGTGTCTTTATTGTTGTAATTAGAATTACCAATGTTATTATTACTTGACATGCTATTGTTATTACCATTATTGGCGCTATTCATTAACGGCATATG |
| EDR1857 | Sense primer used to build Puf46PP,1N | GCATGTCCAGCTACAACATCAATGTCAACATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR1858 | Antisense primer used to build Puf46PP,1N | GACATTGATGTTGTAGCTGGACATGCTATTGTTATTGAAACCAATATTGTAATTATTGGCGCTATTCATTAACGGCATATG |
| EDR2065 | Sense primer used to build YLR177W∆inhib | GCATCTTCGGATCTTCCTACATCTCTGCGCTGATTCATGGCAATGC |
| EDR2066 | Antisense primer used to build YLR177W∆inhib | GAGATGTAGGAAGATCCGAAGATGCTTTGTTGCTGTTGCTGCTGCTGCTGTTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR1849 | Sense primer used to build YLR177W4PP,1N | GCATCTTCTACATCGGAAACGTCTCTTCCTATATTTCTTTCGCGCTGATTCATGGCAATGC |
| EDR1850 | Antisense primer used to build YLR177W4PP,1N | GAGACGTTTCCGATGTAGAAGATGCTTTGTTGCTGTTGCTGCTGCTGCTGCTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR2002 | Sense primer used to build Puf44PP | CATGGTTTCAACAACAATAGCATGTCCAGTCATAATATTAATGTTAACATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR2003 | Antisense primer used to build Puf44PP | CTGGACATGCTATTGTTGTTGAAACCATGATGGTAATTATTGGCGCTATTCATTAACGGCATATG |
| EDR2010 | Sense primer used to build Puf42PP-a | CATGGTTTCAACAACAATAGCATGTCCAGTCATAATGACAATGACAACATTGG |
| EDR2003 | Antisense primer used to build Puf42PP-a | CTGGACATGCTATTGTTGTTGAAACCATGATGGTAATTATTGGCGCTATTCATTAACGGCATATG |
| EDR2011 | Sense primer used to build Puf42PP-b | GGTCGTAATAACAATAGCATGTCAAGTCACAATATTAATGTTAACATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR2012 | Antisense primer used to build Puf42PP-b | GTGACTTGACATGCTATTGTTATTACGACC |
| EDR2068 | Sense primer used to build YLR177W4PP | GTCTCTTCCTACATCAGCGATGCGCTGATTCATGGCAATGC |
| EDR2069 | Antisense primer used to build YLR177W4PP | GCATCGCTGATGTAGGAAGAGACGAATCCGATGTAAAAGATACTTTGTTGCTGTTGCTGCTGCTGCTGTTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR2071 | Sense primer used to build YLR177W2PP-a | GCAACAATCCATCTTCTACATCGGTAGAAGATCTTCCTATATTTCTGATGCGCTGATTCATGGCAATGC |
| EDR2072 | Antisense primer used to build YLR177W2PP-a | CCGATGTAGAAGATGGATTGTTGCTGCTGCTGCTGCTGCTGTTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR2068 | Sense primer used to build YLR177W2PP-b | GTCTCTTCCTACATCAGCGATGCGCTGATTCATGGCAATGC |
| EDR2070 | Antisense primer used to build YLR177W2PP-b | GCATCGCTGATGTAGGAAGAGACGAATCCCGGGTCAAAGATACTTTGTTGCTGTTGCTGCTGCTGCTGTTGCTGCTGCTGCTGCTGTTTCTGTGGATAG |
| EDR2008 | Sense primer used to build Puf4mut-b | GACACAGGTCGTTCTAACGTTGGTTTTATGTATAATATGATCAACTCCTATCACGGCTAC |
| EDR2009 | Antisense primer used to build Puf4mut-b | CCAACGTTAGAACGACCTGTGTC |
| EDR2073 | Sense primer used to build YLR177Wmut-b | GCTCCCAAATCTACTATTTCCAGGTCCAGCAGCAGCAGCAGCAACAGCAGCAGCAGCAACAGCAACAAAGCATCTTTGACC |
| EDR2074 | Antisense primer used to build YLR177Wmut-b | GACCTGGAAATAGTAGATTTGGGAGCTGTTGTTAGACATTTGTTGCTAG |
| EDR2014 | Sense primer used to build Pufα5 | GCATGTCAAACAACAGCATGTCCAGTCACAACGACAATGACAACATCGG |
| EDR2015 | Antisense primer used to build Pufα5 and Pufα7 | GGACATGCTGTTGTTTGACATGCTATTATTAGACATTGAGTTATTACTCATAGAATTATTTGACATGCTATTGTTATTACGACCATGATG |
| EDR2016 | Sense primer used to build Pufα7 | GCATGTCAAACAACAGCATGTCCAATAATTCTATGTCAAACAATAGTATGTCTAGTCACAACGACAATGACAACATCGG |
| EDR2017 | Sense primer used to build Pufα9 | CTCGATGAGCAACAACTCCATGTCAAACAATAGCATGTCAAGTCACAACGACAATGACAACATCGG |
| EDR2018 | Antisense primer to reamplify EDR304/2015 PCR, to make Pufα9 | CATGGAGTTGTTGCTCATCGAGTTATTAGACATTGAATTATTGGACATGCTGTTGTTTGACATGC |
| EDR2019 | Sense primer used to build Pufβ4 | CGAACTACAATAACATCGGAAACTCCAACTATAATAATATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR2020 | Antisense primer used to build Pufβ4, Pufβ5, and Pufβ6 | GGAGTTTCCGATGTTATTGTAGTTCGAATTGCCAATATTATTATAGTTACTATTTCCAATGTTGTCATTGTCATTATGACTTGACATGC |
| EDR2021 | Sense primer used to build Pufβ5 | CGAACTACAATAACATCGGAAACTCCAACTATAATAACATCGGCAATTCAAATTACAACAATATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR2022 | First of two sense primers used to build Pufβ6 | CCAACTACAACAATATCGGCAATAGCAATTATAACAATATTGGAAACTCTAACTACAACAATATTGGTAATTCTAATTACAACAATAAAGACACAGGTC |
| EDR2023 | Second of two sense primers used to build Pufβ6 | CGAACTACAATAACATCGGAAACTCCAACTACAACAATATCGGCAATAGC |
| EDR2024 | First of two sense primers used to build Pufγ2, Pufγ3, and Pufγ4 | CTCCTACCACGGCTATTACAACAATAATAATAATAATAATAATAATAACAATAATAATAATAACAGTAATGCTACCAACAGCAACAGCGCTGGTTTC |
| EDR2025 | Second of two sense primers used to build Pufγ2 | CATGAAGAACAGCTATCACGGTTACTATAATAACAACTCCTACCACGGCTATTACAAC |
| EDR2026 | Antisense primer used to build Pufγ2 and Pufγ3 | GTAACCGTGATAGCTGTTCTTCATGTTTTTCATTTTACCAACGTTAGAACGACCTGTG |
| EDR2027 | Second of two sense primers used to build Pufγ3 | CATGAAGAACAGCTATCACGGTTACTATAATAACAATTCTTATCATGGATATTATAATAACAACTCCTACCACGGCTATTACAAC |
| EDR2028 | Second of two sense primers used to build Pufγ4 | CCATGGGTACTACAACAACAATTCGTATCACGGCTATTATAATAACAACTCCTACCACGGCTATTACAAC |
| EDR2030 | Sense primer used to build Pufδ2, Pufδ3, and Pufδ4 | CAACTCCTACCACGGCTATTACAATAACAATAATAATAATAATAATAATAACAATAATAATAATAACAGTAATGCTACCAACAGCAACAGCGCTGGTTTC |
| EDR2031 | Antisense primer used to build Pufδ2 | GTAATAGCCGTGGTAGGAGTTGTATCCGTGATAACTGTTTTTCATATTTTTCATTTTACCAACG |
| EDR2032 | Antisense primer used to build Pufδ3 | GTAATAGCCGTGGTAGGAGTTGTAACCATGATATGAATTGTATCCGTGATAACTGTTTTTCATATTTTTCATTTTACCAACG |
| EDR2033 | Antisense primer used to build Pufδ4 | GTAATAGCCGTGGTAGGAGTTGTATCCATGATATGAATTATAACCATGGTAAGAATTGTATCCGTGATAACTGTTTTTCATATTTTTCATTTTACCAACG |
| EDR2034 | Antisense primer used to build YLRα3, YLRα4, and YLRα5 | GAAGATGGACTGCTGGAAAATCGACTGTTGCTGTTGTTGTTGTTGCTGTTGCTGTTGTTGCTGCTGTTTCTGTGGATAGTATTTTTGGGAG |
| EDR2035 | Sense primer used to build YLRα3 | GTCGATTTTCCAGCAGTCCATCTTCCAACAAAGCATCTTTGACCCGGGAAGAAGATC |
| EDR2036 | Sense primer used to build YLRα4 | GTCGATTTTCCAGCAGTCCATCTTCCAGCAATCTATATTCCAACAAAGCATCTTTGACCCGGGAAGAAGATC |
| EDR2037 | Sense primer used to build YLRα5 | GTCGATTTTCCAGCAGTCCATCTTCCAACAGTCAATTTTTCAGCAATCTATATTCCAACAAAGCATCTTTGACCCGGGAAGAAGATC |
| EDR2038 | Antisense primer used to build YLRβ3 and YLRβ4 | CTTCTTCCCGGGTCAAAGATGC |
| EDR2040 | Sense primer used to build YLRβ3 | GCATCTTTGACCCGGGAAGAAGATCTAGCTACATATCGTCAAGTTACATAAGCTCTTCCTATATTTCTGATGCGCTGATTC |
| EDR2041 | Sense primer used to build YLRβ4 | GCATCTTTGACCCGGGAAGAAGATCATCTTATATTAGTTCTAGCTACATATCGTCAAGTTACATAAGCTCTTCCTATATTTCTGATGCGCTGATTC |
| EDR2255 | Antisense primer used to build YLRβ5 | GCTTGACGAGATGTAGCTAGAGGAAATATAAGATGAACTAATGTATGAAGATCTTCTTCCCGGGTCAAAGATGC |
| EDR2256 | Sense primer used to build YLRβ5 | CCTCTAGCTACATCTCGTCAAGCTACATAAGCTCTTCCTATATTTCTGATGCGCTGATTC |
| EDR2042 | Antisense primer used to build YLRγ3 | GATGTATACAGGTTGCGAGTATTGTGGTTGC |
| EDR2043 | Sense primer used to build YLRγ3 | CCACAATACTCGCAACCTGTATACATCAATAATAATTATATAAACAATAACTATATTAACAACAACCCATCTTTGCAAGTACC |
| EDR2047 | Antisense primer to build YLRδ3, YLRδ4, and YLRδ5 | GTGTACTCGGAAGGAGCAGTGTATGG |
| EDR2048 | First of two sense primers to build YLRδ3, YLRδ4, and YLRδ5 | CAGCAGCAGTACTCCAGCTACACACAACAGCAACAATACAGTTCTTATACTCAACAGCAACAGTACTCGTCACCCTTC |
| EDR2049 | Second of two sense primers to build YLRδ3 | CCATACACTGCTCCTTCCGAGTACACTCAGCAGCAGCAGTACTCCAGCTAC |
| EDR2050 | Second of two sense primers to build YLRδ4 | CCATACACTGCTCCTTCCGAGTACACACAGCAACAACAGTATTCATCCTATACTCAACAGCAGCAGTACTCCAGCTAC |
| EDR2051 | Second of two sense primers to build YLRδ5 | CACTGCTCCTTCCGAGTACACACAACAGCAGCAATACTCTAGTTATACCCAGCAACAACAGTATTCATCCTATACTCAACAGCAGCAGTACTCCAGCTAC |
| EDR2230 | Antisense primer used to build Pufα5-ScrA | GAGCTCATCATCGAAGAGGAGTTCATATTTGAATTGTTATTAGAGGACATGCTGTTGTTGTTACGACC |
| EDR2231 | Sense primer used to build Pufα5-ScrA | GAACTCCTCTTCGATGATGAGCTCTAATTCTATGAACAATAGCCACAACGACAATGACAACATCG |
| EDR2232 | Antisense primer used to build Pufα5-ScrB | GGCTAGACATCATGGACGAAGAGTTATTGTTAGAATTCATCATGTTATTTGAATTATTAGATGAGGACATGCTGTTGTTGTTACGACC |
| EDR2233 | Sense primer used to build Pufα5-ScrB | CTCTTCGTCCATGATGTCTAGCCACAACGACAATGACAACATCG |
| EDR2234 | Antisense primer used to build Pufα7-ScrA | GGAGTTGTTCGAATTCATCATGCTGGAAGACATAGAATTGTTATTATTGTTCATAGAGGACATGCTGTTGTTGTTACGACC |
| EDR2235 | Sense primer used to build Pufα7-ScrA | CCAGCATGATGAATTCGAACAACTCCTCTAATTCAAATTCTAACATGTCAAATATGTCTAGCCACAACGACAATGACAACATCG |
| EDR2236 | Antisense primer used to build Pufα7-ScrB | GGAGCTCATCATGCTCGAGTTAGACATATTCATAGAATTATTGGACATGCTGTTGTTGTTACGACC |
| EDR2237 | Sense primer used to build Pufα7-ScrB | GTCTAACTCGAGCATGATGAGCTCCAATAACATGAACTCAAGTTCTAATTCTTCAAACAATATGAATAATTCTAGCCACAACGACAATGACAACATCG |
| EDR2238 | Antisense primer used to build Pufα9-ScrA | GTTCATCATCGAGTTGCTGTTGTTGGAATTAGATGAATTCATATTGTTTGAAGAATTCATACTATTCATAGAGGACATGCTGTTGTTGTTACGACC |
| EDR2239 | Sense primer used to build Pufα9-ScrA | CCAACAACAGCAACTCGATGATGAACAATAATTCTATGAATATGAGTTCTAACAACTCTTCTTCTTCTAATATGAGCCACAACGACAATGACAACATCG |
| EDR2240 | Antisense primer used to build Pufα9-ScrB | GTTGGACGAGCTCATCATGGAGTTATTATTATTATTAGAATTCATAGAACTATTAGACATTGAGTTAGACATATTGGACATGCTGTTGTTGTTACGACC |
| EDR2241 | Sense primer used to build Pufα9-ScrB | CTCCATGATGAGCTCGTCCAACAATTCAATGTCTAATAGTATGAACAACAATTCTATGTCTTCTAATAGCCACAACGACAATGACAACATCG |
| EDR2243 | Antisense primer used to build Pufβ4-ScrA | GCCTCCATTGTTGTAGTTGTAGCTGTTATTATTGTTATTTATATTACCATATGAAATAGAGTTGTAGTTGCTGTTGCCGATG |
| EDR2244 | Sense primer used to build Pufβ4-ScrA | CAGCTACAACTACAACAATGGAGGCAATAACAATATTAATAAAGACACAGGTCGTTCTAACGTTGG |
| EDR2245 | Antisense primer used to build Pufβ4-ScrB | GATGCCATTGTTGTTGCTGTTGTAGGAATAGTAGTTGTAGTTGCTGTTGCCGATG |
| EDR2246 | Sense primer used to build Pufβ4-ScrB | CCTACAACAGCAACAACAATGGCATCAATGGAAATAATAACATAAATTCAAATGGTATTAATAATAATAAAGACACAGGTCGTTCTAACGTTGG |
| EDR2247 | Antisense primer used to build Pufβ5-ScrA | GATGTTGCCGATGCTACCGTAGTTATTAATATTATTATAGTTATTATTGTTATAAGAATTGTTGTAGTTGCTGTTGCCGATG |
| EDR2248 | Sense primer used to build Pufβ5-ScrA | CTACGGTAGCATCGGCAACATCAATAATAACATTAGTTCAGGATATAATGGAAATAATAATAAAGACACAGGTCGTTCTAACGTTGG |
| EDR2249 | Antisense primer used to build Pufβ5-ScrB | CCGATGTTATTGATGTTGTTGGAGTTGCTATTATTATATCCATAAGAGTAAGAATTTCCACCGTTGTAGTTGCTGTTGCCGATG |
| EDR2250 | Sense primer used to build Pufβ5-ScrB | GCAACTCCAACAACATCAATAACATCGGTTATATAAACAATAATAACAATATTAACAATAATAATAAAGACACAGGTCGTTCTAACGTTGG |
| EDR2251 | Antisense primer used to build Pufβ6-ScrA | CCGCTGATATTGTTGTTGTAGATGTTGCCATAATTGTTATAATTATTATTGTTAGATATATTTGAATTATTGTTATTGTTGTAGTTGCTGTTGCCGATG |
| EDR2252 | Sense primer used to build Pufβ6-ScrA | GCAACATCTACAACAACAATATCAGCGGTTACAATTATGGAAACGGTTCTGGTATTTCAATTAATAATAATAATAAAGACACAGGTCGTTCTAACGTTGG |
| EDR2253 | Antisense primer used to build Pufβ6-ScrB | GATGTACGAGTTGATGTTGTTGCTGTTATTGTTTGAATAGCCATTTCCATTGTTACCAGAATTATTGTTTCCATTGTTGTAGTTGCTGTTGCCGATG |
| EDR2254 | Sense primer used to build Pufβ6-ScrB | CAGCAACAACATCAACTCGTACATCAATATAAATAACAATAATTATAATATTTATGGTAACTCTTATATTAATAAAGACACAGGTCGTTCTAACGTTGG |
All Puf4, YLR177W, Yck1, and Pdc2 PrLD mutants were generated by a two-step fusion-PCR method. First, the N-terminal portion of the PrLD-Sup35MC fusion was amplified with EDR302 and a mutant-specific primer, and the C-terminal portion of the fusion was amplified with EDR304 and a mutant-specific primer. For some of the repeat expansion mutants, either the N- or the C-terminal product was reamplified with EDR304 paired with an additional mutant-specific primer to finish adding repeats. Second, products of these N- and C-terminal reactions were combined and reamplified with EDR301 and EDR262. PCR products were cotransformed with AatII/HindIII-cut pJ526 into YER632/pJ533. Transformations were selected on SC-Leu and then transferred to FOA plates to select for loss of pJ533.
To generate induction plasmids, the NM domain of each mutant was amplified by PCR, using EDR1084 paired with a PrLD-specific primer. EDR1084 installs a stop codon and XhoI restriction site at the end of the M domain, whereas the mutant-specific primers installed a BamHI restriction site before the start codon. PCR products were digested with BamHI and XhoI and then inserted into BamHI/XhoI-cut pKT24, a TRP1 2-μm plasmid containing the GAL1 promoter (37). Ligation products were transformed into Escherichia coli and analyzed by DNA sequencing.
To generate the PrLD-GFP fusions, each PrLD-Sup35M domain was amplified with EDR1924 and a PrLD-specific primer. PCR products were digested with BamHI and XhoI and then inserted into BamHI/XhoI-cut pER760 (16).
[PSI+] Formation.
Prion formation assays were performed as previously described (16). Briefly, strains expressing each PrLD-Sup35MC fusion were transformed with either pKT24 or a derivative of pKT24 expressing the corresponding PrLD under control of the GAL1 promoter. Cells were grown for 3 d in galactose/raffinose dropout medium lacking tryptophan, and serial 10-fold dilutions were spotted onto SC-ade medium, which shuts off expression from the GAL1 promoter and selects for [PSI+] cells.
Acknowledgments
This work was supported by National Science Foundation Grant MCB-1023771 and National Institutes of Health Grant GM105991 (to E.D.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Y.O.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501072112/-/DCSupplemental.
References
- 1.Sipe JD, Cohen AS. Review: History of the amyloid fibril. J Struct Biol. 2000;130(2–3):88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 2.Kisilevsky R, Fraser PE. A beta amyloidogenesis: Unique, or variation on a systemic theme? Crit Rev Biochem Mol Biol. 1997;32(5):361–404. doi: 10.3109/10409239709082674. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 4.Byers JS, Jarosz DF. Pernicious pathogens or expedient elements of inheritance: The significance of yeast prions. PLoS Pathog. 2014;10(4):e1003992. doi: 10.1371/journal.ppat.1003992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid—from bacteria to humans. Trends Biochem Sci. 2007;32(5):217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191(4):1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickner RB, et al. Yeast prions: Structure, biology, and prion-handling systems. Microbiol Mol Biol Rev. 2015;79(1):1–17. doi: 10.1128/MMBR.00041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wickner RB. [URE3] as an altered URE2 protein: Evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264(5158):566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 9.Liu JJ, Sondheimer N, Lindquist SL. Changes in the middle region of Sup35 profoundly alter the nature of epigenetic inheritance for the yeast prion [PSI+] Proc Natl Acad Sci USA. 2002;99(Suppl 4):16446–16453. doi: 10.1073/pnas.252652099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ter-Avanesyan MD, Dagkesamanskaya AR, Kushnirov VV, Smirnov VN. The SUP35 omnipotent suppressor gene is involved in the maintenance of the non-Mendelian determinant [psi+] in the yeast Saccharomyces cerevisiae. Genetics. 1994;137(3):671–676. doi: 10.1093/genetics/137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Z. The complexity and implications of yeast prion domains. Prion. 2011;5(4):311–316. doi: 10.4161/pri.5.4.18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascarina SM, Ross ED. Yeast prions and human prion-like proteins: Sequence features and prediction methods. Cell Mol Life Sci. 2014;71(11):2047–2063. doi: 10.1007/s00018-013-1543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King OD, Gitler AD, Shorter J. The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 2012;1462:61–80. doi: 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137(1):146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toombs JA, McCarty BR, Ross ED. Compositional determinants of prion formation in yeast. Mol Cell Biol. 2010;30(1):319–332. doi: 10.1128/MCB.01140-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez Nelson AC, et al. Increasing prion propensity by hydrophobic insertion. PLoS ONE. 2014;9(2):e89286. doi: 10.1371/journal.pone.0089286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross ED, Maclea KS, Anderson C, Ben-Hur A. A bioinformatics method for identifying Q/N-rich prion-like domains in proteins. Methods Mol Biol. 2013;1017:219–228. doi: 10.1007/978-1-62703-438-8_16. [DOI] [PubMed] [Google Scholar]
- 18.Toombs JA, et al. De novo design of synthetic prion domains. Proc Natl Acad Sci USA. 2012;109(17):6519–6524. doi: 10.1073/pnas.1119366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartaglia GG, Pechmann S, Dobson CM, Vendruscolo M. Life on the edge: A link between gene expression levels and aggregation rates of human proteins. Trends Biochem Sci. 2007;32(5):204–206. doi: 10.1016/j.tibs.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Hosoda N, et al. Translation termination factor eRF3 mediates mRNA decay through the regulation of deadenylation. J Biol Chem. 2003;278(40):38287–38291. doi: 10.1074/jbc.C300300200. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Rayman JB, Kandel ER, Derkatch IL. Functional role of Tia1/Pub1 and Sup35 prion domains: Directing protein synthesis machinery to the tubulin cytoskeleton. Mol Cell. 2014;55(2):305–318. doi: 10.1016/j.molcel.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shewmaker F, Mull L, Nakayashiki T, Masison DC, Wickner RB. Ure2p function is enhanced by its prion domain in Saccharomyces cerevisiae. Genetics. 2007;176(3):1557–1565. doi: 10.1534/genetics.107.074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toombs JA, Liss NM, Cobble KR, Ben-Musa Z, Ross ED. [PSI+] maintenance is dependent on the composition, not primary sequence, of the oligopeptide repeat domain. PLoS ONE. 2011;6(7):e21953. doi: 10.1371/journal.pone.0021953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadsworth JD, Hill AF, Beck JA, Collinge J. Molecular and clinical classification of human prion disease. Br Med Bull. 2003;66:241–254. doi: 10.1093/bmb/66.1.241. [DOI] [PubMed] [Google Scholar]
- 25.Liu JJ, Lindquist S. Oligopeptide-repeat expansions modulate ‘protein-only’ inheritance in yeast. Nature. 1999;400(6744):573–576. doi: 10.1038/23048. [DOI] [PubMed] [Google Scholar]
- 26.Parham SN, Resende CG, Tuite MF. Oligopeptide repeats in the yeast protein Sup35p stabilize intermolecular prion interactions. EMBO J. 2001;20(9):2111–2119. doi: 10.1093/emboj/20.9.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kajava AV. Tandem repeats in proteins: From sequence to structure. J Struct Biol. 2012;179(3):279–288. doi: 10.1016/j.jsb.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 28.Katti MV, Sami-Subbu R, Ranjekar PK, Gupta VS. Amino acid repeat patterns in protein sequences: Their diversity and structural-functional implications. Protein Sci. 2000;9(6):1203–1209. doi: 10.1110/ps.9.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halfmann R, et al. Opposing effects of glutamine and asparagine govern prion formation by intrinsically disordered proteins. Mol Cell. 2011;43(1):72–84. doi: 10.1016/j.molcel.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DePace AH, Santoso A, Hillner P, Weissman JS. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell. 1998;93(7):1241–1252. doi: 10.1016/s0092-8674(00)81467-1. [DOI] [PubMed] [Google Scholar]
- 31.Doel SM, McCready SJ, Nierras CR, Cox BS. The dominant PNM2- mutation which eliminates the psi factor of Saccharomyces cerevisiae is the result of a missense mutation in the SUP35 gene. Genetics. 1994;137(3):659–670. doi: 10.1093/genetics/137.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox BS. PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity. 1965;20:505–521. [Google Scholar]
- 33.Kochneva-Pervukhova NV, Poznyakovski AI, Smirnov VN, Ter-Avanesyan MD. C-terminal truncation of the Sup35 protein increases the frequency of de novo generation of a prion-based [PSI+] determinant in Saccharomyces cerevisiae. Curr Genet. 1998;34(2):146–151. doi: 10.1007/s002940050379. [DOI] [PubMed] [Google Scholar]
- 34.Tuite MF, Mundy CR, Cox BS. Agents that cause a high frequency of genetic change from [psi+] to [psi-] in Saccharomyces cerevisiae. Genetics. 1981;98(4):691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira PC, Ness F, Edwards SR, Cox BS, Tuite MF. The elimination of the yeast [PSI+] prion by guanidine hydrochloride is the result of Hsp104 inactivation. Mol Microbiol. 2001;40(6):1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- 36.Jung G, Masison DC. Guanidine hydrochloride inhibits Hsp104 activity in vivo: A possible explanation for its effect in curing yeast prions. Curr Microbiol. 2001;43(1):7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- 37.Ross ED, Edskes HK, Terry MJ, Wickner RB. Primary sequence independence for prion formation. Proc Natl Acad Sci USA. 2005;102(36):12825–12830. doi: 10.1073/pnas.0506136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ellegren H. Microsatellite mutations in the germline: Implications for evolutionary inference. Trends Genet. 2000;16(12):551–558. doi: 10.1016/s0168-9525(00)02139-9. [DOI] [PubMed] [Google Scholar]
- 39.Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- 40.Tank EM, Harris DA, Desai AA, True HL. Prion protein repeat expansion results in increased aggregation and reveals phenotypic variability. Mol Cell Biol. 2007;27(15):5445–5455. doi: 10.1128/MCB.02127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacLea KS, Ross ED. Strategies for identifying new prions in yeast. Prion. 2011;5(4):263–268. doi: 10.4161/pri.5.4.17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derkatch IL, Bradley ME, Hong JY, Liebman SW. Prions affect the appearance of other prions: The story of [PIN(+)] Cell. 2001;106(2):171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 43.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]