Abstract
Background
Chorioamnionitis (CA) is associated with premature delivery and bronchopulmonary dysplasia (BPD). We hypothesize that preterm infants exposed to CA have reduced suppressive regulatory T cells (Treg) and increased non-regulatory T cell pro-inflammatory cytokines, increasing risk for BPD.
Objective
To evaluate cord blood CD4+ T cell regulatory phenotype and pro-inflammatory cytokine production in CA and BPD groups.
Study Design
Cord blood mononuclear cells from infants (GA ≤32 weeks), with or without placental histological evidence of CA (hChorio), were analyzed by flow cytometry. Clinical information was collected by retrospective chart review. Numbers of putative Treg (CD4+FoxP3+CD25+CD127Dim), CD4+ non-Tregs, and CD4+ T cell intracellular cytokine content following in vitro stimulation were compared with CA status and oxygen requirement at 36 weeks postmenstrual age.
Result
Absolute Treg numbers were not different in CA and non-CA exposed samples. However, the infants who developed BPD had a significant decrease in Treg and non-regulatory T cell numbers. Greater IL-6 production was observed in hCA group.
Conclusion
A pro-inflammatory CD4+ T cell status is noted in CA and BPD but the later disease is also associated with decrease in Tregs, suggesting that the development of BPD is marked by distinct inflammatory changes from those of CA exposed infants.
Keywords: Cord blood mononuclear cell, Chorioamnionitis, Bronchopulmonary dysplasia, CD4+ T cells, IL-6
1. Introduction
Preterm infants less than 32 weeks are now surviving in increased numbers[1]. However, long-term morbidities continue to be observed in the survivors. Chronic lung injury (bronchopulmonary dysplasia, BPD) remains highly problematic in prematurely born infants and continues to affect children into adolescence and adulthood[2]. Even very low birth weight (VLBW) infants without BPD are more likely to have wheezing and require pulmonary interventions than term infants [3-6]. Inflammatory responses to infections, oxygen, mechanical ventilation and other stimuli found in the NICU setting are, at least in part, responsible for BPD [7, 8]. An important question is how are these inflammatory responses regulated, and how can they be modified or controlled to improve outcomes for the premature infant? This study explores a potential control point deficiency: an insufficient suppression of inflammatory responses in premature infants due to abnormal behaviors of CD4+ T lymphocytes.
CD4+ T lymphocytes play an important role in both pro-inflammatory and anti-inflammatory responses. CD4+ regulatory T cells (Tregs) are widely accepted as an important component of the immune system. Tregs are generally found to have an immune suppressive effect and are considered an indispensable part of immune homeostasis, allowing adequate, but reducing harmful, inflammatory responses. The role of Tregs in the establishment and maintenance of a T helper cell balance needed to prevent inflammatory disease of the newborn remains unclear. The available data have implicated abnormalities in Tregs in the development of inflammatory, allergic and autoimmune diseases in humans and animals [9-11]. In a recent study, a comparison of cord blood T cell subsets reported increased Tregs in preterm infants as compared to term infants, though suppressive function was not assessed [12]. The authors also describe a decreased capacity of preterm cord blood cells to produce IFN-γ production relative to term and that there was an increased capacity of these cells to produce IFN-γ as subjects aged to 16 months [12]. Another publication reports that cord blood CD25+ CD4+ T cells are capable of suppressing effector cell proliferation though they produce less IL-10 than adult Tregs [13]. Low Treg counts were identified in inflammatory and autoimmune diseases in animals [14]. Tregs have been proposed to play an important role in allergic diseases, viral infections and RSV bronchiolitis by providing anti-inflammatory responses and protective immunity. These diseases are significantly associated with morbidity in preterm infants [9, 11, 15, 16].
Chorioamnionitis (CA), or acute placental inflammation, detected by histology (hChorio) has been observed in 87% of premature births <27 weeks gestation and can occur without rupture of membranes [17, 18]. CA is a known cause of prematurity and may play a role in the pathogenesis of lung disease within the first month after birth and in the pathogenesis of BPD when more mature [19-21]. Acute hChorio is identified as primarily neutrophilic infiltration into the cord and chorio-amnion membranes, suggesting an inflammatory environment that has been associated with increased inflammatory cytokines in tracheal aspirates of these subjects [21-23]. A recent study on rhesus monkeys showed that intraamniotic administration of IL-1β induces hChorio and lung inflammation. It transiently decreased the Tregs and increased IL-17A production in lymphoid organs [24-26]. In other chronic inflammatory states, increased IL-6 production by CD4+ non-regulatory T cells (non-Tregs) has been reported [27-29]. It is possible that the pro-inflammatory state observed in CA infants results from or causes reduced CD4+ cell numbers and/or function that in turn contribute to self-injurious inflammation in these infants.
An extensive literature review demonstrated no studies to date that specifically examine the characteristics of Tregs from the cord blood of preterm CA-exposed infants or how they relate to development of inflammatory lung disease.
In order to address these questions, we developed a retrospective cohort study of cord blood mononuclear cells (CBMC) isolated from premature infants ≤ 32 weeks gestational age to characterize CD4+ T cells. Using surface and intracellular markers consistent with Tregs and analysis by flow cytometry, we determined the frequency of cord blood CD4+ T cells and cytokine producing CD4+ T cells from premature infants with and without CA. We then determined if this pattern of immune dysregulation at birth is also associated with later development of BPD.
2. Materials and methods
2.1. Ethics statement
Written consent was given for each study participant from a legal guardian in accordance with the Research Subject Review Board at the University of Rochester (URMC RSRB protocol #s 29824 and 37933) and at the Children's Hospital of Buffalo (#(61) 2707).
2.2. Subjects and samples
Cord blood samples were collected immediately after placental discard. Patients with congenital malformations or syndromes, maternal insulin dependent diabetes and maternal autoimmune disease were excluded due to potential effects on the developing immune system. Parents were approached within 60 days after delivery for permission to use the cord blood for research in combination with collection of maternal and infant electronic medical record data, per RSRB protocol.A total of 40 preterm infants with gestational age ≤ 32 weeks with hChorio (n=20) and without hChorio (n=20) determined by placental pathology were matched for this study.Using 18-color flow cytometry, cord blood mononuclear cell (CBMC) samples were analyzed for absolute Treg number, as well as for selected cytokine producing non-Tregs and Tregs. Maternal and neonatal clinical data were collected by retrospective chart review. The demographic and clinical data was compared with the matched cases and controls in the cohort.
2.3. Preparation of cord blood samples
2.3.1. Umbilical cord mononuclear cell isolation
Cord blood (0.5-20 cc) was collected into heparinized (heparin) tubes by direct draw from umbilical vessels according to standard operating procedure (SOP) developed and active in the Pryhuber and Human Immunology Center Laboratories. CBMC were isolated within 24 hours of blood draw to optimize viability and minimize post-draw phenotypic changes, as previously published [30]. CBMC were isolated, counted, re-suspended in 90% FBS plus 10% DMSO, frozen in Mr. Frosty (Thermo-Fisher Scientific, Waltham, MA) rate-controlled containers and preserved in liquid nitrogen vapor phase prior to analysis.
2.3.2. Cell culture conditions
CBMC vials, frozen at approximately 5xe6 per ml and 5xe6 per vial, were individually thawed. The cells were washed with RPMI 1640 culture media supplemented with 10% fetal bovine serum, L-glutamine, penicillin and streptomycin two times. Following an overnight rest at 37°C in 5% CO2, half of the cells were stimulated with phorbol 12-myristate 13-acetate (PMA, 50 μg/ml) plus ionomycin (1 μmol) and monensin (1 μmol) (PMA&iono&mon) for 4.5 hours. The remaining cells were cultured in the presence of monensin alone as un-stimulated control.
2.3.3. Cell staining process
Following the overnight rest and stimulation periods, surface and intracellular antigen staining was completed to quantify the frequency of Tregs, non-Tregs and cytokine production. Non-specific antibody binding was blocked in 2% normal mouse serum (Sigma-Aldrich, St. Louis, MO; catalog #M5905) in PBS for 10 minutes at room temperature (r.t.). The CBMC were then recovered by centrifugation at 300xg at °C for 10 minutes. The cells were then washed in PBS and stained with LIVE/DEAD Fixable Aqua Dead Cell Stain (Invitrogen, Grand Isle, NY; catalog # L34957) for 15 minutes at r.t.
Following a wash in PBS&1% Bovine Serum Albumin (BSA),, cells were incubated with the following anti-human antibodies: CD19 (HIB19) Pacific Blue (Biolegend, San Diego, CA; catalog # 302223), CD235a (HI264) Pacific Blue (Biolegend #349107), CD8 (RPA-T8) Pacific Blue (Biolegend #301026), CD14 (HCD14) Pacific Blue (Biolegend #325615), CD4 (OKT4) BV650 (Biolegend #317435), CD127 (A019D5) PE-Cy5 (Biolegend #351324), CD69 (FN50) PerCPCy5.5 (Biolegend #310925), and CD3 (HIT3a) APC-Cy7 (Biolegend #300317) for 80 minutes on ice. Following a PBS&1%BSA wash, cells were fixed and permeabilized using the FoxP3 /Transcription Factor Staining Buffer Set (e-Bioscience #00-5523-00) for 30 minutes on ice. Cells were then spun at 1000xg at °C for 10 minutes followed by a wash with permeabilization buffer. Following a spin at 1000xg for 10 min at °C, cells were incubated with a cocktail of anti-human IL-6 (MQ2-13A5) FITC (Biolegend #501103), IL-10 (JES3-19F1) PE (Biolegend #506804), IFN-γ (4S.B3) BV570 (Biolegend #502533), FoxP3 (236A/E7) Alexa Fluor 647 (e-Bioscience, San Diego, CA; catalog #51-4777-41), IL-17A (BL168) BV711 (Biolegend #512327) and TNF-α (MAb11) PE-Cy7 (Biolegend #502929), which were diluted in permeabilization buffer, for 150 minutes in the dark and on ice. Cells were then washed using permeabilization buffer and resuspended in PBS&1%BSA. The fluorescent antibody stained cell events were detected on an LSR 18 color flow cytometer and data was analyzed using FlowJo software (Tree Star, Ashland, OR).
2.3.4. Gating scheme to identify CD4+ regulatory T cell and CD4+ non-regulatory T cell populations
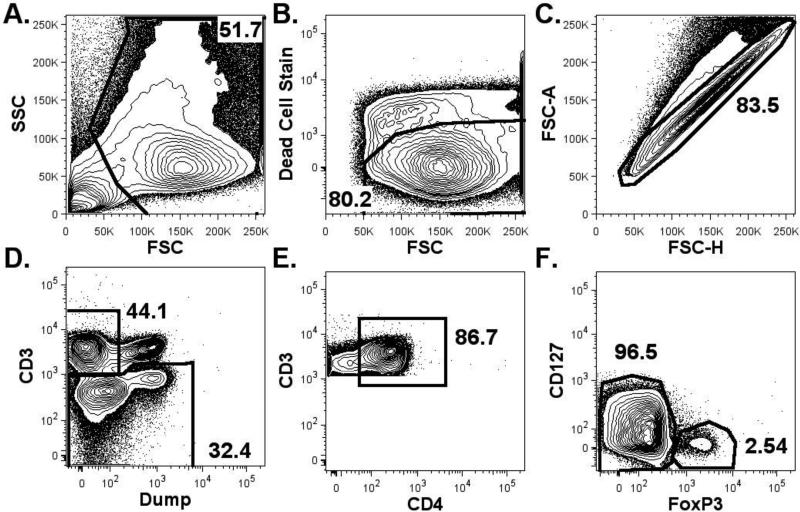
Cellular phenotyping data obtained from flow cytometry was analyzed to determine the frequencies of cell populations. First, cellular debris, dead cells and cell aggregates were excluded to identify the cell population studied. CD4+ T cells were then identified by placing a gate on the CD3pos&CD4pos&dump negative population (CD235a, CD19, CD14, CD8 neg). The CD4+ T cells were further categorized as Tregs (FoxP3pos CD127low) and non-Tregs (FoxP3neg CD127high/int) (Figure 1).
Figure 1. Representative Gating Scheme to Identify Regulatory and Non-regulatory CD4+ T Cells by Flow Cytometry.
First, cellular debris was excluded by examining side scatter (SSC) and forward scatter (FCS) in order to identify the cell population (A; 51.7%). Next, a gate was placed on viable cells (B; retained 80.2%). A gate was then placed to include only cells that were not aggregated (C; retained 83.5%). A gate was then placed on the CD3pos&/dump negative cells (D; 44.1%) in order to identify non-CD8+ T cells. The CD4+ T cells were then identified (86.6%) and further sub-classified as either Tregs (FoxP3pos CD127low (2.54%)) or as non-Tregs (FoxP3neg CD127high/int (96.5%) (E). “Dump” = CD19, CD14, CD8.
2.3.5. Detection of intracellular cytokines
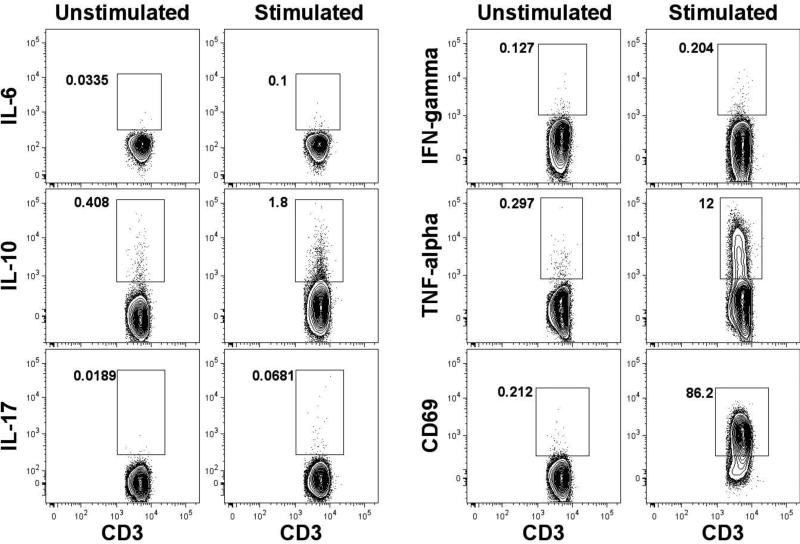
Half of the CBMC samples were stimulated with PMA & Ionomycin to induce the production of intracellular cytokines from non-Treg and Treg populations and half were un-stimulated. The stimulated samples were then compared with un-stimulated samples by flow cytometry. Representative data are shown (Figure 4).
Figure 4. Detection of intracellular cytokine production by non-regulatory T cells.
Umbilical cord blood cells were stimulated using PMA/ionomycin or left un-stimulated, both in the presence of monensin. The flow cytometery gating strategy illustrated in Figure 1 was used to identify the non-T regulatory cells (Dead-Dump- CD3+CD4+CD127+FoxP3-). A gate was then placed to identify the frequency of cytokine producing or activated (CD69+) T cells. Representative data are shown from matched stimulated and un-stimulated patient samples.
2.3.6. Calculation of CD4+ regulatory T cells in cord blood
For each cord blood specimen, the number of viable CBMCs recovered was determined by hemacytometer count using trypan blue as a dead cell marker. The number of CBMCs per ml of blood was then calculated. The frequency of Tregs and non-Tregs in each sample was determined as the product of the frequencies of cells in each of the following gates: dump negative x CD4+ x CD127low&FoxP3+ and dump negative x CD4+ x CD127high&FoxP3-, respectively The frequency of Tregs and non-Tregs was multiplied by the CBMCs per ml isolated prior to cryopreservation to obtain the absolute number of cells per ml of CBMC.
2.4. Statistical Analysis
Statistical analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, North Carolina). Patient clinical factors such as APGAR scores, gestational age, birth weight, antenatal steroids and number of surfactant doses received were compared between study groups using two sample t test for continuous variables and Chi square test or Fisher's exact test for categorical variables. Exact Mantel-Haenszel test was used to compare ordinal variables such as BPD. Absolute numbers of putative Tregs (CD4+FoxP3+CD127Dim), non-Tregs, and CD4+ T cell intracellular cytokine content were summarized by CA and BPD groups with descriptive statistics such as mean and standard deviation.
Since the outcome measures are highly skewed and data transformations (such as logarithm transformation) failed to improve the normality of data distributions, we decided to evaluate the mean differences between CA and BPD groups using Generalized Estimating Equations (GEE) [31]. This approach forgoes the distribution assumption, providing consistent and robust estimate by specifying marginal mean effects on the outcome variable. No multiple comparisons justification was made in this analysis.
3. Results
3.1 Characteristics of the study population
The 40 cord blood samples from premature infants ≤32 weeks gestational age were equally divided by study design into 2 groups on the basis of histological evidence of CA (hChorio). BPD status at 36 weeks of corrected gestational age was also defined for each patient using the National Institute of Health (NIH) 2001 Workshop definition [32]. Classification of BPD status based on this NIH Workshop definition was defined for each subject and secondary analysis was performed using patient demographics. Patient demographics are reported as mean ± SD for continuous variables and frequencies for categorical variables based on hChorio and BPD status (Table 1).
Table 1.
Patient Characteristics of Chorioamnionitis and Bronchopulmonary Dysplasia Positive and Negative Subjects.
| hChorio | BPD | |||||
|---|---|---|---|---|---|---|
| positive (n=20) | negative (n=20) | p-value | positive (n=17) | negative (n=23) | p-value | |
| GA (wks)† | 28.7 ± 2.1 | 30.0 ± 2.1 | 0.06 | 27.5 ± 1.9 | 30.6 ± 1.3 | <0.01 |
| Birth weight (gm)† | 1262.5 ± 344.3 | 1353.9 ± 385.3 | 0.28 | 1052.1 ± 315.0 | 1497.5 ± 272.1 | <0.01 |
| Antenatal steroids (%) | 100 | 92.86 | >0.99 | 94 | 96 | 0.83 |
| Received surfactant (%) | 95 | 95 | >0.99 | 82 | 26 | <0.01 |
| Rupture of membranes >18hrs (%) | 75 | 25 | <0.01 | 59 | 39 | 0.22 |
| Clinical chorio (cChorio) (%) | 45 | 15 | 0.04 | 47 | 17 | 0.04 |
| Apgar scores at 5 mins† | 8.0 ± 1.1 | 7.6 ± 2.0 | 0.48 | 6.9 ± 1.6 | 8.4 ± 1.2 | <0.01 |
| Total number of ventilation days† | 4.7 ± 12.1 | 3.5 ± 8.2 | 0.71 | 8.5 ± 14.6 | 0.9 ± 1.9 | 0.05 |
| Total number of respiratory support days† | 34.8 ± 33.4 | 22.7 ± 23.3 | 0.19 | 55.7 ± 25.9 | 8.8 ± 6.2 | <0.01 |
| Mild BPD % (n)4 | 35 (7) | 20 (4) | 0.20 | 64.7 (11) | 0 | |
| Mod BPD % (n)4 | 20 (4) | 10 (2) | 0.20 | 35.3 (6) | 0 | |
| Severe BPD (n)4 | 0 | 0 | 0 | 0 | ||
| Length of hospital stay in days† | 62.8 ± 31.1 | 47.0 ± 20.5 | 0.06 | 78.1 ± 25.5 | 37.7 ± 10.8 | <0.01 |
Mean ± SD.
P-values are from t-test or Chi-square or Fisher exact test.
National Institute of Child Health and Human Development (NICHD) 2001 Consensus Definition.
There was no significant difference between hChorio positive and negative groups with respect to APGAR scores, gestational age, birth weight, antenatal steroids and number of surfactant doses received. Prolonged rupture of membranes (> 18 hours) was more common in hChorio positive group as compared to hChorio negative group (P < 0.01). Clinical chorio (cChorio), obstetrician-defined using American College of Obstetricians and Gynecologists (ACOG) guidelines, which presents as the presence of a fever plus two additional features including uterine tachycardia, abnormal amniotic fluid, and uterine tenderness [33-35]. cChorio occurred in only 45% of those with hChorio, but this was significantly higher than in the 15% observed in the hChorio negative group (P=0.04). The overall prevalence of cChorio in our cohort was only 30%.
We extracted information on respiratory support required by the subjects (Table 1). A trend toward increased total number of ventilation days, respiratory support days and length of stay is noted in the hChorio positive group, suggesting a correlation with in utero inflammation, though the comparisons did not reach statistical significance. A trend toward increased BPD (35% mild BPD and 20% moderate BPD vs. 20% mild BPD and 10% moderate BPD) was observed in hChorio positive group as compared to hChorio negative group, but this did not reach statistical significance (p=0.20). None of the infants developed severe BPD in our cohort.
No significant demographic differences were found between BPD and non-BPD groups for the categories of antenatal steroids, rupture of membranes (> 18 hours) and total number of ventilation days. For all other variables, a statistically significant difference was found (P < 0.01; Table 1).
3.1.1. Comparison of absolute CD4+ regulatory T cells in chorioamnionitis group
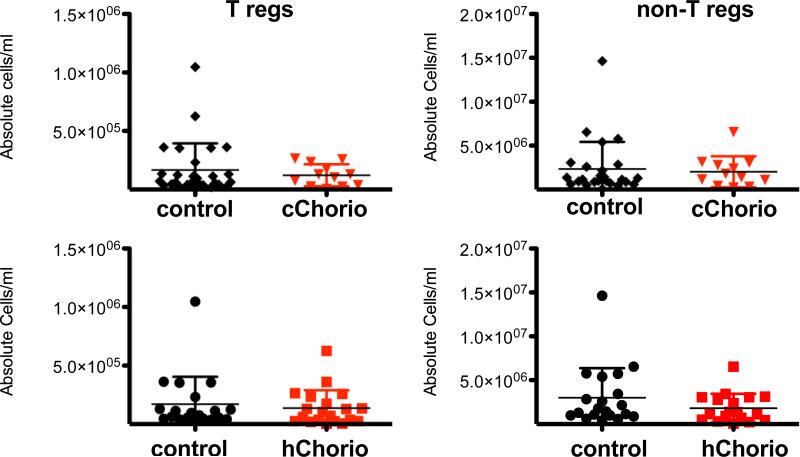
Tregs were identified as described in the methods section (Figure 1). The absolute Treg cell number per milliliter of cord blood in both hChorio and cChorio groups were calculated and compared (Figure 2). No significant difference in Treg numbers or non-Tregs was observed in either hChorio or cChorio subjects as compared to controls. The frequency of CD4+ T cells that were FoxP3+&CD127low was 5.74±1.50% for hChorio subjects versus 5.83±2.35% in controls (mean±S.D.), and 5.70±1.60% in cChorio versus 6.00±2.70% in non-cChorio subjects (data not shown), and revealed no significant differences between CA groups with matched controls.
Figure 2. Absolute CD4+ T cell numbers in histological & clinical Chorio are similar to controls.
The absolute number of CD4+ T regulatory cells and non-regulatory cells per milliliter of cord blood was calculated for each patient and graphed. Patients were grouped into cChorio (top; inverted red triangles) or hChorio (bottom; red squares) and controls. Means ± SD are shown. P= 0.58 and 0.36 for Tregs in hChorio and cChorio versus controls, respectively. P= 0.12 and 0.48 for non-Tregs in hChorio and cChorio versus controls, respectively.
3.1.2. Comparison of absolute CD4+ T cells in bronchopulmonary dysplasia groups
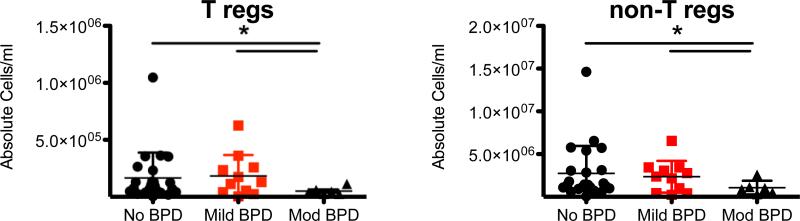
To determine if BPD is associated with changes in CD4+ T cell counts, the absolute Treg and non-Treg cell numbers between BPD (mild & moderate) and normal subjects were compared (Figure 3). No significant differences were observed in CD4+ T cell counts between the control and mild BPD groups. However, the average absolute number of Treg and non-Treg cells in moderate BPD patients was significantly lower when compared to subjects with no BPD or mild BPD (P=0.01 and 0.01 respectively for Tregs and P=0.02 and 0.04 for non-Tregs). The frequencies of Tregs from the CD4+ T cell population was 6.74±1.82% in non-BPD subjects, 6.73±2.39% in mild BPD subjects, and 5.22±1.60% in moderate BPD subjects (data not shown). GEE analysis of these groups revealed that the frequency of Tregs in the moderate BPD group was not significant (P=0.0765). Thus, fewer umbilical cord CD4+ T cells were associated with infants who later developed moderate BPD.
Figure 3. Fewer absolute CD4+ T cells in cord blood of subjects who develop moderate BPD patients versus control and mild BPD.
The absolute number of T regulatory cells (left) and non-regulatory CD4+ T cells (right) from each subject cord blood sample was calculated and graphed according to BPD status. (Means ±SD; p- values are derived from GEE estimation; * represents a statistically significant difference. Control vs. Mod BPD (p =0.01), Mild BPD vs. Mod BPD (p = 0.01) and control vs. Mild BPD (0.80) for T regulatory cells. Control vs. Mod BPD (p =0.02), Mild BPD vs. Mod BPD (p = 0.04) and control vs. Mild BPD (0.64) for non-regulatory T cells.
3.1.3. Comparison of cytokine producing cells in chorioamnionitis group
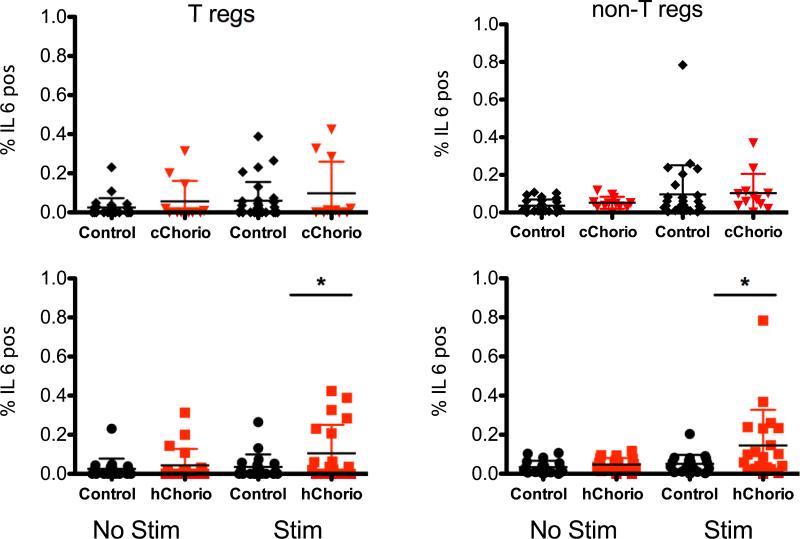
Cytokine production from PMA&iono&mon stimulated and un-stimulated cord blood non-Treg lymphocytes was detected using intracellular cytokine staining followed by flow cytometery. Representative data are shown in Figure 4. Typically, cells that were positive for cytokine were also positive for CD69. The viability of the CBMCs between the hChorio and control group was compared and found to be significantly different for both stimulated and unstimulated conditions (P=0.0452 and P=0.0195, respectively). The frequency of cytokine producing CD4+ T cells from the hChorio and control groups was compared (Table 2). While the overall percentage of CD4+ T cells expressing IL-6 was low as predicted for CD4+ T cells, IL-6 production was significantly higher in non-Tregs from hChorio positive subjects (P=0.02) as compared to hChorio negative controls (Fig 5). This difference remained significant upon controlling for gestational age and length of stay in the hospital (P=0.0484). No difference in other measured cytokine production between groups was observed.
Table 2.
CD4+ Non-Treg Cytokine Producing Cells in Histological Chorio (hChorio) GEE (Generalized Estimating Equation) method.
| hChorio – (n=20) | hChorio + (n=20) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | Mean | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.04 | 0.03 | 0.05 | 0.03 | 0.25 |
| S | 0.05 | 0.05 | 0.15 | 0.18 | 0.02 | |
| IL-10 | U | 1.03 | 1.44 | 0.83 | 1.33 | 0.64 |
| S | 1.68 | 2.93 | 1.30 | 1.61 | 0.60 | |
| IL-17 | U | 0.04 | 0.08 | 0.02 | 0.04 | 0.41 |
| S | 0.14 | 0.37 | 0.08 | 0.14 | 0.45 | |
| IFN-γ | U | 0.12 | 0.24 | 0.12 | 0.37 | 0.98 |
| S | 0.10 | 0.22 | 0.16 | 0.32 | 0.48 | |
| TNF-α | U | 0.14 | 0.16 | 0.36 | 1.01 | 0.32 |
| S | 8.62 | 4.61 | 8.77 | 5.64 | 0.92 | |
Figure 5. Increased frequency of IL-6 producing CD4+ T cells in hChorio, but not in cChorio subjects.
T regulatory cells (T regs) and non regulatory T cells (non-T regs) were identified using flow cytometry and the frequency of IL-6 positive cells was determined from stimulated and unstimulated samples. Data are graphed as mean ± SD. * indicates statistical significance by GEE test (p=0.02).
Cytokine production from stimulated and un-stimulated cord blood Treg lymphocytes among hChorio groups was also compared (Table 3). Frequencies of IL-6-producing CD4+ Tregs were higher in cord blood samples from the hChorio group as compared to the non-hChorio group (P=0.02), which remained significant when controlling for gestational age and length of stay in the hospital (P=0.0274). There was no difference in other cytokine frequencies between the two groups.
Table 3.
CD4+ Treg Cytokine Producing Cells in Histological Chorio (hChorio) GEE (Generalized Estimating Equation) method.
| hChorio – (n=20) | hChorio + (n=20) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | Mean | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.03 | 0.05 | 0.04 | 0.08 | 0.41 |
| S | 0.03 | 0.06 | 0.14 | 0.20 | 0.02 | |
| IL-10 | U | 0.75 | 1.27 | 1.02 | 2.28 | 0.64 |
| S | 1.13 | 2.51 | 0.96 | 1.42 | 0.79 | |
| IL-17 | U | 0.03 | 0.06 | 0.12 | 0.41 | 0.32 |
| S | 0.08 | 0.25 | 0.58 | 2.48 | 0.36 | |
| IFN-γ | U | 0.17 | 0.45 | 0.57 | 2.03 | 0.39 |
| S | 0.09 | 0.20 | 0.10 | 0.30 | 0.93 | |
| TNF-α | U | 0.11 | 0.22 | 0.10 | 0.32 | 0.96 |
| S | 0.47 | 0.75 | 0.52 | 1.00 | 0.86 | |
We then tested whether there was a difference in the frequency of cytokine-producing cord blood non-Tregs in maternal cChorio exposed infants versus controls (Table 4). No significant difference was found in cell viability between cChorio and controls for both stimulated and unstimulated conditions (P=0.2022 and P=0.4222, respectively). The frequencies of IL-6 producing Treg and non-Treg cells from stimulated and unstimulated cells are shown in Figure 5. The difference in IL-6, which was seen between hChorio groups, was not present when compared between cChorio groups. Clinical CA, however, was marked by a trend toward increase in TNF- α versus non-cChorio controls (Table 4). Interestingly, when gestational age and length of stay in the hospital were taken into consideration a significant difference was observed in the percentage TNF- α expressing non-Tregs (P=0.0354). The frequency of cytokine positive cells from the Treg population in the cChorio group revealed very low frequencies of cytokine production (Table 5). Furthermore, no significant difference in the frequency of cytokine producing cells versus control was observed between cChorio subjects and controls (Table 5).
Table 4.
CD4+ Non-Treg Cytokine Producing Cells in Clinical Chorio (cChorio) GEE (Generalized Estimating Equation) method.
| cChorio – (n=28) | cChorio+ (n=12) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | Mean | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.04 | 0.03 | 0.05 | 0.03 | 0.20 |
| S | 0.10 | 0.15 | 0.10 | 0.10 | 0.89 | |
| IL-10 | U | 0.89 | 1.22 | 1.00 | 1.71 | 0.84 |
| S | 1.55 | 2.48 | 1.35 | 2.08 | 0.79 | |
| IL-17 | U | 0.03 | 0.07 | 0.03 | 0.06 | 0.81 |
| S | 0.12 | 0.31 | 0.10 | 0.17 | 0.82 | |
| IFN-γ | U | 0.09 | 0.21 | 0.18 | 0.47 | 0.50 |
| S | 0.09 | 0.19 | 0.24 | 0.40 | 0.19 | |
| TNF-α | U | 0.13 | 0.14 | 0.53 | 1.30 | 0.27 |
| S | 7.66 | 4.55 | 11.12 | 5.63 | 0.05 | |
Table 5.
CD4+ Treg Cytokine Producing Cells in Clinical Chorio (cChorio) GEE (Generalized Estimating Equation) method.
| cChorio – (n=28) | cChorio+ (n=12) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | Mean | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.03 | 0.05 | 0.06 | 0.10 | 0.29 |
| S | 0.08 | 0.16 | 0.09 | 0.16 | 0.88 | |
| IL-10 | U | 0.65 | 1.08 | 1.44 | 2.91 | 0.34 |
| S | 1.12 | 2.11 | 0.86 | 1.84 | 0.68 | |
| IL-17 | U | 0.09 | 0.35 | 0.04 | 0.10 | 0.46 |
| S | 0.06 | 0.21 | 0.97 | 3.19 | 0.30 | |
| IFN-γ | U | 0.14 | 0.39 | 0.91 | 2.61 | 0.29 |
| S | 0.07 | 0.17 | 0.16 | 0.38 | 0.41 | |
| TNF-α | U | 0.08 | 0.19 | 0.16 | 0.40 | 0.48 |
| S | 0.39 | 0.64 | 0.74 | 1.25 | 0.33 | |
3.1.4. Comparison of cytokine producing cells in bronchopulmonary dysplasia
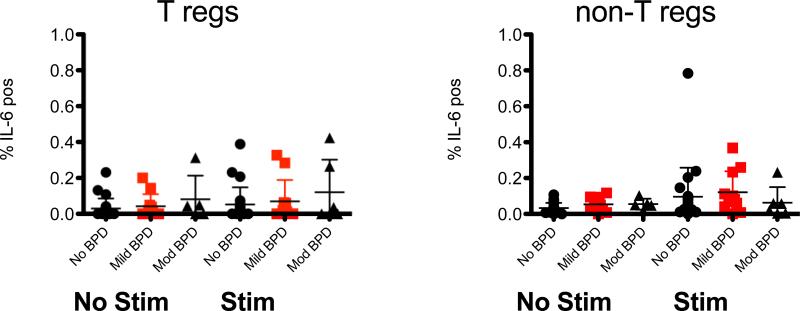
In our cohort of 40 patients, 17 patients developed BPD. Eleven were classified as mild BPD and 6 as moderate BPD (Table 1). Cell viability was compared between BPD and non-BPD groups for both stimulated and unstimulated conditions, and no differences were observed (P=0.7374 and P=0.925, respectively). We compared cytokine production from the Tregs and non-Treg lymphocytes between BPD vs. non-BPD patients (Table 6). Unlike the association between hChorio and elevated IL-6, a correlation between elevated IL-6 and BPD was not found in this limited cohort (Fig 6 ). If the mild and moderate BPD groups were combined, there was a modest but significant (p=0.04) increase in IL-6+ cells between no BPD and BPD groups seen directly ex vivo in unstimulated groups (Table 6). However, this difference was no longer significant (P=0.2195) upon controlling for gestational age and length of stay in the hospital. Furthermore, upon stimulation in vitro, there was no significant difference in IL-6 production between no BPD and combined BPD patients. In the stimulated Treg population (Table 7), IFN-γ producing cells trended higher in BPD patients versus normal controls (P=0.07).
Table 6.
CD4+ Non-Treg Cytokine Producing Cells in BPD vs Non-BPD GEE (Generalized Estimating Equation) method.
| BPD – (n=23) | BPD + (n=17) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | Mean | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.03 | 0.03 | 0.05 | 0.03 | 0.04 |
| S | 0.10 | 0.16 | 0.10 | 0.11 | 0.91 | |
| IL-10 | U | 0.84 | 1.21 | 1.05 | 1.60 | 0.66 |
| S | 0.90 | 1.07 | 2.29 | 3.26 | 0.08 | |
| IL-17 | U | 0.03 | 0.06 | 0.04 | 0.06 | 0.63 |
| S | 0.09 | 0.23 | 0.15 | 0.33 | 0.53 | |
| IFN-γ | U | 0.06 | 0.10 | 0.21 | 0.46 | 0.18 |
| S | 0.08 | 0.17 | 0.21 | 0.36 | 0.17 | |
| TNF-α | U | 0.11 | 0.11 | 0.46 | 1.12 | 0.20 |
| S | 7.71 | 4.86 | 10.04 | 5.22 | 0.14 | |
Figure 6. No difference in IL-6 producing CD4+ T cells in BPD subjects.
T regulatory cells (T regs) and non regulatory T cells (non-T regs) were identified using flow cytometry and the frequencty of IL-6 positive cells was determined from stimulated and unstimulated samples from subjects with No BPD and those with Mild or Moderate disease. Data are graphed as mean ± SD.
Table 7.
CD4+ Treg Cytokine Producing Cells in BPD vs Non-BPD GEE (Generalized Estimating Equation) method.
| BPD – (n=23) | BPD + (n=17) | |||||
|---|---|---|---|---|---|---|
| % | Un-stimulated (U) Stimulated (S) | M e a n | Std Dev | Mean | Std Dev | p-value (GEE) |
| IL-6 | U | 0.02 | 0.05 | 0.05 | 0.09 | 0.25 |
| S | 0.06 | 0.10 | 0.13 | 0.21 | 0.17 | |
| IL-10 | U | 0.58 | 0.96 | 1.34 | 2.62 | 0.25 |
| S | 0.56 | 0.42 | 1.69 | 2.98 | 0.11 | |
| IL-17 | U | 0.11 | 0.38 | 0.03 | 0.07 | 0.29 |
| S | 0.02 | 0.04 | 0.75 | 2.68 | 0.25 | |
| IFN-γ | U | 0.10 | 0.38 | 0.77 | 2.26 | 0.22 |
| S | 0.03 | 0.08 | 0.19 | 0.36 | 0.07 | |
| TNF-α | U | 0.13 | 0.32 | 0.07 | 0.17 | 0.48 |
| S | 0.38 | 0.59 | 0.66 | 1.15 | 0.34 | |
4. Discussion
This study addresses the phenotypic and functional consequences of in utero inflammation on CD4+ T cells and their relationship to the development of BPD. We hypothesized that premature infants with previous exposure to CA would have increased inflammatory cytokine-positive CD4+ T cell frequencies and decreased numbers of Tregs. We further hypothesized that the observed pro-inflammatory bias at birth would correlate with diagnosis of BPD by 36 weeks CGA. To test our hypotheses, we measured frequencies of pro-inflammatory cytokine-positive CD4+ T cells (IL-6, IL-17, TNF-α, IFN-γ) and anti-inflammatory CD4+ T cells (IL-10 and Tregs) from umbilical cord blood in subjects exposed and not exposed to CA. We observed a statistically significant increase in the frequency of IL-6+ CD4+ T cells from hChorio exposed infants versus controls. The CD4+ T cell characteristics varied with the basis of the CA diagnosis, with a trend toward higher TNF-α+ CD4+ T cells in cChorio patients versus control. Thus, CD4+ T cells express distinct cytokines between the two groups of CA exposed infants.
Inconsistency between clinical and histological diagnosis of CA has been previously reported; only about 60% of placentas from cChorio cases were found to have histological evidence of inflammation [36]. Non-inflammatory causes of clinical symptoms suggestive of cChorio, such as epidural anesthesia, can explain a clinical diagnosis without pathological evidence of disease. Likewise, early in utero infection or non-infectious etiologies may cause placental inflammation but not result in maternal or fetal symptoms. That different T lymphocyte cytokine responses were detected in cChorio versus hChorio may be due to different pathogenic mechanisms, timing or severity of illness involved. Thus, our data suggest that symptomatic, maternal inflammation might be associated with an increased activation state of the fetal CD4+ T cells. However, a study with larger cohorts that would generate higher power and correct for potential confounders, including fetal and maternal inflammatory responses, would be necessary to further examine the effect of CA on the inflammatory state of the fetal immune system.
The correlation among CA, fetal systemic inflammatory response [23, 37] and elevated plasma IL-6 is well documented. Consistent with this observation, Yoon et al. reported increased IL-6 in the amniotic fluid of preterm babies less than 33 weeks gestation and who later developed BPD [38]. We now report that CD4+ T cells from infants exposed to hChorio express IL-6 at a higher frequency than controls. Although there was a significant difference found in cell viability of total CBMCs between hChorio and control groups for stimulated and unstimulated cells, we are confident that the difference in IL-6 production is independent of cell viability in part because of our parallel observations of increased IL-6 production by the CD4+ cells in the BPD group where equivalent cell viabilities were observed. Additionally, this difference in viability in the hChorio group is likely not affecting the CD4+ T cell inflammatory response since there was no increase in TNF-α production. In contrast, the cChorio group showed an increase in TNF-α expression but had no differences in cell viability. Evidence in the literature suggests that expression of IL-6 by CD4+ T cells is pathogenic. For instance, IL-6 production by CD4+ T cells has been associated with bone loss in a gingivitis model in mice [28]. Additionally, patients with the autoimmune disorder neuromyelitis optica, which targets optic neurons, also exhibit IL-6 production by CD4+ T cells [29]. This is also true for patients with relapsing/remitting multiple sclerosis [27]. It is possible that a fetal inflammatory response characterized by CA stimulates T cell effector function that potentiates perinatal inflammatory diseases in premature infants, such as BPD.
Very preterm infants are prone to long-lasting respiratory morbidities, and those who were diagnosed with BPD are most at risk [39]. Several inflammatory factors have been related to the development of lung damage. Oxidative species and inflammatory cells have been proposed to mediate damage in lung disease seen in infants [7]. Additionally, high circulating levels of IL-6 in cord blood has been implicated as a predictor of babies who will develop BPD [10]. Chronic stimulation of T cells can also lead to alterations in the pattern of cytokine production in T cells and their eventual deletion [40]. This is illustrated in T cells from patients with relapsing/remitting multiple sclerosis, which produce high levels of IL-6 in vitro with only anti-CD28 stimulation [27]. Evidence exists that chronic activation of CD4+ T cells from chronic Hepatitis B patients leads to higher expression of surface IL-6 receptor [41]. This provides evidence that with chronic stimulation, CD4+ T cells may not only produce IL-6, but their cytokine receptor expression pattern may be altered by the presence of other circulating cytokines. Furthermore, it may be that early exposure to higher IL-6, as is found with hChorio, interferes with normal neonatal immune suppressive mechanisms, including Treg function, thereby enabling immunopathology. Consistent with this concept, we found that non-T cell (CD3 negative) CBMCs from subjects who were hChorio positive expressed a significantly greater frequency of IL-6 in both stimulated and unstimulated conditions (P=0.0314 and P=0.0474, respectively; data not shown). This difference in IL-6 production was not observed in cChorio or BPD groups (data not shown).
Our cohort included only 11 patients who were exposed to CA who then developed BPD. The size of our study did not allow sufficient power to examine the activation of T cells with respect to combinations of CA and BPD. As there is an increased risk of BPD in CA-exposed infants [7, 8], a study including bigger sample size, more CA-exposed subjects with BPD and controlling for potential confounders (GA and birth weight etc) would be important in correlating the CD4+ T cell phenotype in CA patients who develop BPD versus those who do not. Additional studies directly investigating the relationship between IL-6, Treg homeostasis/function and BPD are indicated.
Several lines of evidence suggest that altered immune responses in preterm infants leads to respiratory morbidity into childhood. Infants who were diagnosed with BPD are more likely to be hospitalized due to RSV infection into childhood [42]. Another report shows that high levels of IL-6 production by umbilical cord mononuclear cells is predictive of a more severe RSV infection into childhood [43]. Considering that increased severity of RSV infection has been associated with BPD in preterm infants, an understanding how CD4+ T cell development is altered in preterm infants is of critical importance [42, 44, 45].
In our study, subjects who developed moderate BPD had significantly fewer CD4+ T cells per milliliter of blood versus no BPD or mild BPD groups, and there was a slight decrease in the frequency of FoxP3+CD127low CD4+ T cells in the subjects who developed moderate BPD versus the non-BPD group (P=0.0765). In mouse studies, Tregs were shown to be essential in limiting lung damage following RSV infection [12, 39, 46], thus illustrating their ability to limit pulmonary pathology following viral infection. Dysregulation of Treg function has been noted in many other diseases [9-11]. For instance, Tregs from diabetic patients have been reported to produce pro-inflammatory cytokines [47]. Furthermore, in vitro Tregs from inflamed joints of patients with rheumatological disease have reduced function due to exposure to TNF-α and IL-6, which was reversed when these cytokines were neutralized [48]. Our study reports that a higher frequency of Tregs produce IL-6 in the hChorio group versus controls, which may indicate a suppressive functional impairment. Additional studies that directly test Treg suppressive capacity in cord blood and its association with later BPD development will be informative and could provide an early diagnostic for BPD risk.
Increased pro-inflammatory potential and reduced CD4+ T cell number before birth may tilt the balance towards enhanced inflammation that promotes the development of BPD in those infants. The fact that no decrease in CD4+ T cells in either of the Chorio groups is of interest as this result suggests that BPD is unique in the lymphopenia with respect to the CD4+ T cell pool. Furthermore, we observe an increase in the frequency of TNF-α producing CD4+ T cells in patients with cChorio when confounding factors are considered, which further implies differing alterations in the immune system of preterm subjects who are exposed to inflammation in utero versus those who develop lung BPD.
It is also possible that CD4+ T cells localize to inflamed lung tissue in neonates who develop moderate BPD, which could be the consequence of increased sequestration in the lungs due to altered homing. Precedent for altered Treg homing in mucosal tissue was seen in preterm infants who developed necrotizing enterocolitis (NEC) and exhibited a defect in Treg recruitment within the intestine [49]. A mouse model of NEC demonstrated transfer of Tregs attenuated the disease [50], which further illustrates a role for these cells in limiting tissue pathology. Alternatively, it is also possible that infants who develop moderate BPD could exhibit a state of overall lymphopenia, which could lead to an increase in activated T cells [51][52, 53].
In summary, we report an increased frequency of CD4+ T cells expressing IL-6 in subjects exposed to hChorio versus controls, which was not the case for individuals in the cChorio group. Furthermore, there was a decrease in the number of circulating CD4+ T cells in subjects who developed BPD. Our study offers insight into early changes in the CD4+ cell population in babies exposed to CA and those who go on to develop BPD. Our results are directly relevant to understanding how early modulation of CD4+ T cell function in preterm infants may contribute to lung pathology later in life.
Acknowledgements
The financial support of this study was provided by NIHT32HD057821, NHLBIU01HL101813, HHSN272201200005C (Respiratory Pathogens Research Center) and Department of Pediatrics at University of Rochester Medical Center, NY, USA. We thank Michael Sacilowski, Aimee Horan, Elizabeth Werner, Tanya Scalise, and Deanna Maffett for their work on this project. We would like to thank all patients and their guardians for helping out to do this research.
Abbreviations
- BSA
Bovine serum albumin
- BPD
Bronchopulmonary dysplasia
- non-Treg
CD4+ Non-regulatory T cells
- Treg
CD4+ Regulatory T cells
- CA
Chorioamnionitis
- cChorio
Clinical chorioamnionitis
- CBMC
Cord blood mononuclear cell
- CGA
Corrected gestational age
- GEE
Generalized Estimating Equation
- hChorio
Histological chorioamnionitis
- iono
Ionomycin
- mon
Monensin
- BPD36
Oxygen requirement at 36 weeks postmenstrual age
- PMA
Phorbol 12-myristate 13-acetate
- VLBW
Very low birth weight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Fanaroff AA, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147, e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Wong PM, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32(2):321–8. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi E, et al. Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;155(1):149–55. doi: 10.1164/ajrccm.155.1.9001304. [DOI] [PubMed] [Google Scholar]
- 4.Chan KN, et al. Lung function in children of low birth weight. Arch Dis Child. 1989;64(9):1284–93. doi: 10.1136/adc.64.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy JD, et al. Effects of birthweight and oxygen supplementation on lung function in late childhood in children of very low birth weight. Pediatr Pulmonol. 2000;30(1):32–40. doi: 10.1002/1099-0496(200007)30:1<32::aid-ppul6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Sell M, et al. One-year follow-up of 273 infants with birth weights of 700 to 1100 grams after prophylactic treatment of respiratory distress syndrome with synthetic surfactant or air placebo. American Exosurf Neonatal Study Group I. J Pediatr. 1995;126(5 Pt 2):S20–5. doi: 10.1016/s0022-3476(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 7.Jobe AH, Ikegami M. Prevention of bronchopulmonary dysplasia. Curr Opin Pediatr. 2001;13(2):124–9. doi: 10.1097/00008480-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Gigley JP, et al. T cell exhaustion in protozoan disease. Trends Parasitol. 2012;28(9):377–84. doi: 10.1016/j.pt.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DC, et al. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol. 2010;84(17):8790–8. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murai M, et al. Regulatory T-cell stability and plasticity in mucosal and systemic immune systems. Mucosal Immunol. 2010;3(5):443–9. doi: 10.1038/mi.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbari O, et al. Role of regulatory T cells in allergy and asthma. Curr Opin Immunol. 2003;15(6):627–33. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Dirix V, Vermeulen F, Mascart F. Maturation of CD4+ regulatory T lymphocytes and of cytokine secretions in infants born prematurely. J Clin Immunol. 2013;33(6):1126–33. doi: 10.1007/s10875-013-9911-4. [DOI] [PubMed] [Google Scholar]
- 13.Takahata Y, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32(7):622–9. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Liu MF, et al. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J Immunol. 2004;59(2):198–202. doi: 10.1111/j.0300-9475.2004.01370.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 17.Viscardi RM, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–17. doi: 10.1203/01.pdr.0000127015.60185.8a. [DOI] [PubMed] [Google Scholar]
- 18.Hartling L, Liang Y, Lacaze-Masmonteil T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2012;97(1):F8–F17. doi: 10.1136/adc.2010.210187. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 20.Stevens TP, et al. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Pediatr Pulmonol. 2010;45(4):371–9. doi: 10.1002/ppul.21199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Marter LJ, et al. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140(2):171–6. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 22.Aghai ZH, et al. Impact of histological chorioamnionitis on tracheal aspirate cytokines in premature infants. Am J Perinatol. 2012;29(7):567–72. doi: 10.1055/s-0032-1311980. [DOI] [PubMed] [Google Scholar]
- 23.Watterberg KL, et al. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97(2):210–5. [PubMed] [Google Scholar]
- 24.Kallapur SG, et al. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol. 2013;191(3):1102–9. doi: 10.4049/jimmunol.1300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfs TG, et al. IL-1alpha mediated chorioamnionitis induces depletion of FoxP3+ cells and ileal inflammation in the ovine fetal gut. PLoS One. 2011;6(3):e18355. doi: 10.1371/journal.pone.0018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong H, Zhou C, Qi G. Proportional changes of CD4+CD25+Foxp3+ regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin Invest Med. 2010;33(6):E422. doi: 10.25011/cim.v33i6.14594. [DOI] [PubMed] [Google Scholar]
- 27.Camperio C, et al. CD28 ligation in the absence of TCR stimulation up- regulates IL-17A and pro-inflammatory cytokines in relapsing-remitting multiple sclerosis T lymphocytes. Immunol Lett. 2014;158(1-2):134–42. doi: 10.1016/j.imlet.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Baker PJ, et al. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67(6):2804–9. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linhares UC, et al. The ex vivo production of IL-6 and IL-21 by CD4+ T cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol. 2013;33(1):179–89. doi: 10.1007/s10875-012-9780-2. [DOI] [PubMed] [Google Scholar]
- 30.Scheible K, et al. Stability of T cell phenotype and functional assays following heparinized umbilical cord blood collection. Cytometry A. 2012;81(11):937–49. doi: 10.1002/cyto.a.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; 1994. [Google Scholar]
- 32.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 33.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol. 2010;37(2):339–54. doi: 10.1016/j.clp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newton ER. Chorioamnionitis and intraamniotic infection. Clin Obstet Gynecol. 1993;36(4):795–808. doi: 10.1097/00003081-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg MB, et al. A first look at chorioamnionitis management practice variation among US obstetricians. Infect Dis Obstet Gynecol. 2012:628362. doi: 10.1155/2012/628362. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smulian JC, et al. Clinical chorioamnionitis and histologic placental inflammation. Obstet Gynecol. 1999;94(6):1000–5. doi: 10.1016/s0029-7844(99)00416-0. [DOI] [PubMed] [Google Scholar]
- 37.Gotsch F, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 38.Yoon BH, et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor- alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1997;177(4):825–30. doi: 10.1016/s0002-9378(97)70276-x. [DOI] [PubMed] [Google Scholar]
- 39.Durant LR, et al. Regulatory T cells prevent Th2 immune responses and pulmonary eosinophilia during respiratory syncytial virus infection in mice. J Virol. 2013;87(20):10946–54. doi: 10.1128/JVI.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129(4):474–81. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang F, et al. Elevated IL-6 receptor expression on CD4+ T cells contributes to the increased Th17 responses in patients with chronic hepatitis B. Virol J. 2011;8:270. doi: 10.1186/1743-422X-8-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyce TG, et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr. 2000;137(6):865–70. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 43.Juntti H, et al. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J Allergy Clin Immunol. 2009;124(1):52–58. e1–2. doi: 10.1016/j.jaci.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Carbonell-Estrany X, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J. 2000;19(7):592–7. doi: 10.1097/00006454-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143(5 Suppl):S133–41. doi: 10.1067/s0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 46.Luciano AA, et al. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One. 2011;6(2):e16698. doi: 10.1371/journal.pone.0016698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughson A, et al. Uncoupling of proliferation and cytokines from suppression within the CD4+CD25+Foxp3+ T-cell compartment in the 1st year of human type 1 diabetes. Diabetes. 2011;60(8):2125–33. doi: 10.2337/db10-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrath J, et al. The inflammatory milieu in the rheumatic joint reduces regulatory T-cell function. Eur J Immunol. 2011;41(8):2279–90. doi: 10.1002/eji.201041004. [DOI] [PubMed] [Google Scholar]
- 49.Weitkamp JH, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62(1):73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dingle BM, et al. FoxP3(+) regulatory T cells attenuate experimental necrotizing enterocolitis. PLoS One. 2013;8(12):e82963. doi: 10.1371/journal.pone.0082963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ge Q, et al. Naive to memory T-cell differentiation during homeostasis-driven proliferation. Microbes Infect. 2002;4(5):555–8. doi: 10.1016/s1286-4579(02)01572-1. [DOI] [PubMed] [Google Scholar]
- 52.Sheu TT, et al. Premature CD4+ T cell aging and its contribution to lymphopenia-induced proliferation of memory cells in autoimmune-prone non- obese diabetic mice. PLoS One. 2014;9(2):e89379. doi: 10.1371/journal.pone.0089379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sauce D, et al. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol. 2012;189(12):5541–8. doi: 10.4049/jimmunol.1201235. [DOI] [PubMed] [Google Scholar]