Abstract
Background
Brain-derived neurotrophic factor (BDNF) plays an important role in Alzheimer's disease (AD) and other neurodegenerative disorders. BDNF function is adversely affected by amyloid beta in AD. BDNF levels in brain and peripheral tissues are lower in patients with AD and mild cognitive impairment (MCI) than in controls. Here we examined the association between plasma levels of BDNF and amyloid deposition in the brain measured with Pittsburgh Compound B (PiB).
Method
Our data set consisted of 18 AD, 56 MCI, and 3 normal control Alzheimer's Disease Neuroimaging Initiative-1 (ADNI1) subjects with available [11C] PiB and peripheral blood protein data. Magnetic resonance imaging (MRI)-coregistered positron emission tomography data were smoothed with a 15 mm kernel and mapped onto three-dimensional (3D) hemispheric models using the warping deformations computed in cortical pattern matching of the associated MRI scans. We applied linear regression to examine in 3D the associations between BDNF and PiB standard uptake value ratio, while adjusting for age and sex. We used permutation statistics thresholded at P < .01 for multiple comparisons correction.
Results
Plasma BDNF levels showed significant negative associations with left greater than right amyloid burden in the lateral temporal, inferior parietal, inferior frontal, anterior and posterior cingulate, and orbitofrontal regions (left Pcorrected = .03).
Conclusions
As hypothesized, lower plasma levels of BDNF were significantly associated with widespread brain amyloidosis.
Keywords: Brain-derived neurotrophic factor, Pittsburgh Compound B, PiB, Alzheimer's disease, Mild cognitive impairment, Amyloid
1. Introduction
Alzheimer's disease (AD), the most common neurodegenerative disorder, currently affects an estimated 5.2 million Americans and this number is expected to climb to 13.8 million in 2050 [1]. With the cost of care of AD projected to increase from $203 million in 2013 to $1.1 trillion in 2050 [1], the search for reliable disease modification strategies such as novel therapeutic and behavioral modifications has intensified. Several behavioral modification strategies, including exercise, participation in brain stimulating activities, and dietary changes, have been shown to have a beneficial effect on the brain and cognitive function through the upregulation of neurotrophic factors such as the brain-derived neurotrophic factor (BDNF) [2], [3], [4].
BDNF is the most widely distributed neurotrophin in the central nervous system (CNS). It mediates neuronal differentiation, proliferation, and survival, regulates synaptic function, facilitates brain plasticity, and modulates hippocampal long-term potentiation, learning, and memory formation [4], [5], [6], [7]. Multiple lines of evidence connect amyloid beta (Aβ) pathology, BDNF, and cognitive performance. Experimental intrahippocampal injections of Aβ reduce BDNF levels in the frontal cortex of rats [8]. Addition of Aβ to astrocyte culture seems to trigger increased BDNF production in astrocytes [9]. Lower BDNF levels measured in brain [10], [11], [12], [13], cerebrospinal fluid [14], and peripheral blood [5], [15], [16], [17], [18], [19] are seen in subjects with AD and mild cognitive impairment (MCI) relative to controls. One study even suggests an early compensatory increase in plasma BDNF levels followed by late decline in AD [15]. Decreased plasma BDNF levels correlate with worse cognition in amnestic MCI [18] and AD [15], [17]. Plasma BDNF levels seem to be also affected by age. However, cognitively normal elderly with more pronounced decreases in serum BDNF levels perform worse in the memory domain [20], [21].
The protective effects of BDNF against AD seem to be at least mediated in part by its stimulating effect on the nonamyloidogenic cleavage of the amyloid precursor protein (APP) and the subsequent release of the neurotrophic secreted fragment of APP [5], [22]. Both a therapeutic and preventative role for BDNF have been proposed, as pretreatment with BDNF seems to protect against Aβ toxicity, whereas post-treatment administration seems to restore neuronal function both in vitro and in murine experiments [23], [24].
Aβ accumulation in the brain is a key hallmark of AD. Brain amyloidosis visualized with positron emission tomography (PET) is widely recognized as the earliest imaging biomarker for AD [25]. Given BDNF's role in AD, its bidirectional functional links with Aβ and the well-documented decline in BDNF levels in brain and peripheral tissue in AD, we hypothesized that BDNF protein levels in plasma would negatively correlate with amyloid burden in the brains of subjects from the Alzheimer's Disease Neuroimaging Initiative (ADNI).
2. Methods
2.1. Subjects
Data used in preparing this article were obtained from the ADNI database (http://adni.loni.usc.edu). ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and nonprofit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure clinical progression in MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, and to lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W. Weiner MD, VA Medical Center and University of California—San Francisco. ADNI is the result of efforts of many coinvestigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the US and Canada. The initial goal of ADNI was to recruit 800 adults, aged 55 to 90 years, to participate in the research—approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years. For up-to-date information see www.adni-info.org.
The clinical description of the ADNI cohort has been previously published [26]. Diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association criteria [27]. AD subjects were required to have Mini-Mental State Examination (MMSE) [28] scores between 20 and 26 and a Clinical Dementia Rating scale (CDR) [29] score of 0.5 to 1 at baseline. Qualifying MCI subjects had memory complaints but no significant functional impairment, scored between 24 and 30 on the MMSE, had a global CDR score of 0.5, a CDR memory score of 0.5 or greater, and objective memory impairment on Wechsler Memory Scale—Logical Memory II test [30]. Normal control (NC) subjects had MMSE scores between 24 and 30, a global CDR of 0 and did not meet criteria for MCI and AD. Subjects were excluded if they refused or were unable to undergo MRI, had other neurological disorders, active depression, or history of psychiatric diagnosis, alcohol, or substance dependence within the past 2 years, less than 6 years of education, or were not fluent in English or Spanish. The full list of inclusion/exclusion criteria may be accessed on pages 23 to 29 of the online ADNI protocol (see http://www.adni-info.org/Scientists/ADNIScientistsHome.aspx). Written informed consent was obtained from all participants.
The subset of 18 AD, 56 MCI, and 3 cognitively normal ADNI subjects who received [11C]-Pittsburgh Compound B (PiB)-PET scans and provided peripheral blood protein data were included in this study.
2.2. MRI imaging acquisition and preprocessing
All ADNI subjects underwent serial 1.5 T MRI imaging on scanners from one of three manufacturers: GE Healthcare, Philips Medical Systems, or Siemens Medical Solutions [31]. At each visit, two T1-weighted MRI scans were acquired with a sagittal three-dimensional (3D) sequence for each subject. The image with a higher signal-to-noise ratio was selected by the ADNI MRI quality control center at the Mayo Clinic (Rochester, MN, USA) [31]. The scans were reconstructed with a 256 × 256 matrix and a voxel size of 0.9375 × 0.9375 × 1.2 mm3 in the x-, y-, and z-dimensions [31]. Additional image corrections included 3D Gradwarp correction for geometric distortions because of gradient nonlinearity [32], “B1-correction” for image intensity nonuniformity [31] and “N3” bias field correction for reducing intensity inhomogeneity [33]. Both raw and corrected image files are freely available for download at http://adni.loni.usc.edu.
MRI scans were registered with a nine-parameter (three translations, three rotations, three scales) transformation [34] to the International Consortium for Brain Mapping template [35] and corrected for image nonuniformities using a regularized tricubic B-spline approach [36]. The brains were automatically skull stripped with Brainsuite and all volumes were manually edited for mislabeled brain and nonbrain regions. After 3D hemispheric reconstruction, 38 sulci per hemisphere were traced and averaged across subjects. The cortical surfaces were parameterized, flattened, and warped to align all subjects to a respective average sulcal representation. 3D hemispheric mesh models for each subject were created.
2.3. PiB-PET acquisition and preprocessing
PiB-PET data collection was started as an “add on” project and enrolled 103 subjects across 14 ADNI sites [37]. This study included a subset of subjects with both [11C]-PiB-PET scans and peripheral blood protein data: 18 AD, 56 MCI, and 3 cognitively normal subjects. All scanning sites passed rigorous PET scanner certification using a Hoffman 3D brain phantom. Detailed PET protocol information can be found at http://www.adni-info.org/scientists/ADNIStudyProcedures.aspx but are briefly described later. Subjects were injected with 15 ± 1.5 mCi of PiB over 10 to 20 seconds. They underwent a dynamic, 3D scan consisting of four 30-second frames beginning approximately 50 minutes postinjection. PiB-PET scans were corrected using measured attenuation and reconstructed using scanner-specific parameters. All PET image files were assessed for artifacts and motion. Next, PET scans were coregistered, averaged, and smoothed with a scanner-specific filter function to produce images of a uniform isotropic resolution of 8-mm full-width at half-maximum (for details see http://adni.loni.usc.edu/wp-content/uploads/2010/09/PET_PIB_Tech_Procedures_Manual_Suppl_v1.3.pdf). The smoothed coregistered PiB-PET scans were downloaded from the ADNI repository from the Laboratory of Neuroimaging (https://ida.loni.usc.edu/login.jsp) for further processing. These scans were then coregistered to the MRI scan temporally closest to the PiB visit, smoothed with a 15-mm kernel and convected onto the 3D hemispheric mesh models for each subject.
2.4. Plasma protein biomarkers
Fasting blood samples were collected in two potassiumethylene diamine tetraacetic acid (K2EDTA) tubes at the baseline visit and centrifuged at room temperature for 15 minutes at 3000 rpm (http://www.adni-info.org/scientists/ADNIStudyProcedures.aspx#). The plasma portion was aliquoted into plastic transfer tubes, stored at −80°C, and shipped to Myriad RBM for evaluation on a 190-analyte multiplex immunoassay panel (Human Discovery MAP version 1.0; Myriad RBM) and a commercially available platform (Luminex 100; Luminex Corporation) [38]. BDNF met the quality control criteria for subsequent statistical analysis (http://adni.loni.usc.edu/wp-content/uploads/2010/11/BC_Plasma_Proteomics_Data_Primer.pdf). Plasma BNDF levels were log10 transformed.
2.5. Statistical analysis
We used one-way analyses of variance (ANOVAs) with post-hoc Bonferroni correction to examine differences in age, education, BDNF plasma levels, and MMSE score. A chi-squared test was used to assess for differences in sex distribution. We used Pearson's correlation statistic to examine the relationship between plasma BDNF levels and PiB standard uptake value ratio with cognitive function (MMSE, Rey's Auditory Verbal Learning Test-delayed recall [RAVLT-DR] and Clinical Dementia Rating Scale-sum of boxes [CDR-SOB]).
We applied linear regression to examine in 3D the associations between BDNF and PiB SUVR, while adjusting for age and sex since both have been previously shown to affect BDNF levels in peripheral blood [20], [39]. We used permutation statistics thresholded at P < .01 for multiple comparisons correction. Significance and beta coefficient maps were created.
3. Results
The results from the Bonferroni-corrected ANOVA and chi-squared comparisons of demographic variables can be seen in Table 1. MMSE score significantly differed between the three groups, with the NC group having the highest scores and the AD group having the lowest scores. There were no significant differences in age, education, plasma BDNF levels, or sex distribution between the three groups. PiB SUVR showed significant associations with MMSE (r = −0.26, P = .021), RAVLT-DR (r = −0.23, P = .048) but not with CDR-SOB. BDNF did not show significant associations with any of the cognitive variables.
Table 1.
Demographic comparisons of NC, MCI, and AD subjects
| Variable (SD) | NC (N = 3) | MCI (N = 56) | AD (N = 18) | P-value, ANOVA or chi-square |
|---|---|---|---|---|
| Age, yr | 70.2 (8.2) | 75.6 (7.9) | 74.3 (7.9) | .46 |
| Gender, F:M | 2:1 | 25:31 | 7:11 | .66 |
| Education, yr | 14.3 (3.2) | 16.4 (2.8) | 14.9 (2.9) | .12 |
| MMSE | 28.7 (0.6) | 27.1 (2.2) | 22.1 (3.0) | <.001 |
| BDNF | 0.38 (0.51) | 0.28 (0.38) | 0.40 (0.42) | .51 |
NOTE. Significant P values in bold show group differences.
Abbreviations: NC, normal control; MCI, mild cognitive impairment; AD, Alzheimer's disease; SD, standard deviation; ANOVA, analysis of variance; F:M, female:male; MMSE, Mini-Mental State Examination; BDNF, brain-derived neurotrophic factor.
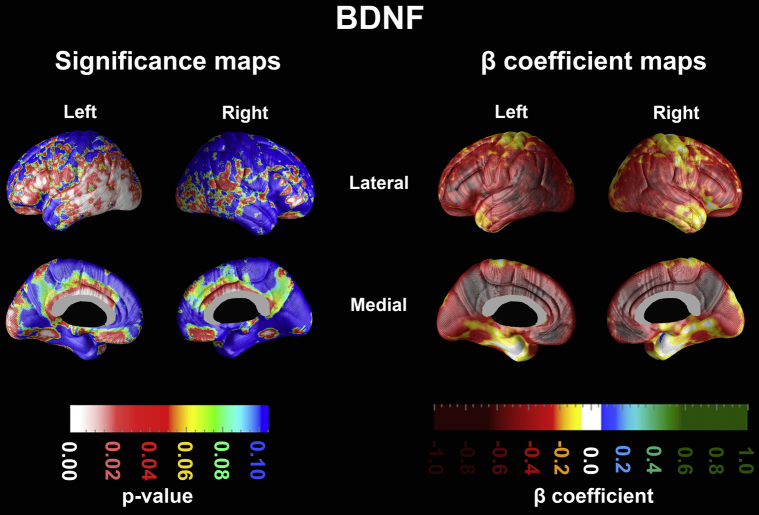
Our linear regression analyses in 3D indicated that plasma BDNF levels exhibited significant negative associations with amyloid burden in the lateral temporal, inferior parietal, inferior frontal, anterior and posterior cingulate, and orbitofrontal regions that were more prominent on the left (left Pcorrected = .03) than the right (right Pcorrected = .11) (Fig. 1). The results remained unchanged after excluding the three cognitively normal subjects (maps not shown).
Fig. 1.
Significance and beta coefficient maps showing the association between plasma brain-derived neurotrophic factor (BDNF) and amyloid burden in the brain measured with Pittsburgh Compound B (PiB).
4. Discussion
As hypothesized, we found a significant negative association between plasma BDNF levels and PiB binding in the brains of our ADNI1 subcohort. This is to our knowledge the first study that has examined this association. Our findings are a logical extension of prior work by others, who have consistently reported lower plasma BDNF levels in patients with AD and amnestic MCI [15], [16], [17], [19]. There is mounting evidence that the downregulation of BDNF results in a wide range of deleterious effects such as impairments in synaptic plasticity, hippocampal long-term potentiation, and learning and memory, as well as increased cleavage of APP to the toxic Aβ species [5], [6], [22], [23], [40].
The associations between plasma BDNF and PIB SUVR reached significance on the left but not the right. Although the precise reason for this asymmetry remains uncertain the most reasonable explanation is the greater SUVR measurement noise on the right compared with the left. There could be several plausible reasons for this. Our group has previously reported greater right-sided cortical atrophy most pronounced in the lateral temporal and inferior frontal cortices of subjects with amnestic MCI and AD [41]. If present in this sample such underlying atrophy could inadvertently result in asymmetric SUVR estimates. Asymmetric tracer uptake in the scalp [42] or white matter [43], especially in the settings of asymmetric white matter hyperintensity burden [44] can likewise affect the accuracy of the SUVR estimates and result in the observed hemispheric differences. Last but not least, a combination of these factors could also be at play. Recently, amyloid-positive individuals were shown to have greater white matter uptake relative to their amyloid negative counterparts [45]. Thus, in the MCI and AD stages SUVR could be influenced by asymmetric white matter uptake due to asymmetry in white matter hyperintensities in addition to disease- and white matter hyperintensity-associated asymmetric cortical atrophy.
Several strengths and limitations of this study should be acknowledged. ADNI is a large, national multisite longitudinal study collecting clinical, cognitive, imaging, and biochemical data using standardized and uniform protocols with stringent quality control. One of the limitations of ADNI lies in its strict exclusion criteria that models AD clinical trial methodology standards, which resulted in the enrollment of a study cohort with a lower prevalence of comorbidities than the general population. As such all ADNI findings require further corroboration in population-based settings. A limitation specific to our study is the small sample size dictated by the number of participants who provided both PiB scans and plasma protein data. Studying BDNF in peripheral blood might not be reflective of BDNF's fate in the CNS. BDNF crosses the blood-brain barrier in both directions. Thus, at least part of the circulating BDNF may originate from the CNS [39], [46]. Despite this, the preponderance of prior evidence describing a distinct peripheral blood BDNF signature in MCI [18], [19] and AD [15], [16], [17] patients is consistent with our findings and further supports the potential use of plasma BDNF as an AD biomarker.
Despite these limitations, our study documents a strong association between plasma BDNF levels and PiB binding in the brain, suggesting that plasma BDNF levels, either alone or in combination of other peripheral blood markers, may represent a peripheral signature of amyloid pathology in the brain. In addition, given the links between BDNF levels, AD pathology [8], [9], and cognitive decline [15], [17], [18], [20], [21] one should consider including this biomarker measure not only in clinical studies of dietary and exercise interventions that have already demonstrated an effect on BDNF, but also for clinical trials of therapies aimed at decreasing Aβ production.
Research in context.
-
1.
Systematic review: Brain-derived neurotrophic factor (BDNF), the most widely distributed neurotrophin in the central nervous system (CNS), has been shown to play a role in Alzheimer's disease (AD) and cognitive decline. The aim of our study was to examine the relationship between plasma BDNF levels and brain amyloid. To conduct our literature review, we searched the PubMed database for original research and review articles examining the association between BDNF, brain amyloid, and cognitive performance.
-
2.
Interpretation: Multiple studies connect amyloid beta pathology, BDNF, and cognitive performance. In our study, we found decreased plasma BDNF levels to associate with widespread brain amyloidosis suggesting that plasma BDNF levels may represent a peripheral signature of amyloid pathology in the brain.
-
3.
Future directions: Although BDNF crosses the blood-brain barrier in both directions it is not clear what fraction of the circulating BDNF originates from the CNS. Future studies examining this relationship would help clarify if plasma BDNF can be developed as a surrogate biomarker for observational studies and clinical trials.
Acknowledgments
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer's Association; Alzheimer's Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
The authors thank the members of the ADNI Imaging Core for their contributions to the image preprocessing, the members of the ADNI Biomarker Core for the CSF biomarker analyses and the investigators at the University of Pittsburgh for the PIB SUVR analyses.
The analyses reported in this manuscript were funded by the Easton Consortium for Alzheimer's Drug Discovery and Biomarker Development, NIA R01 AG040770, NIA K02 AG048240 and NIA P50 AG16570.
References
- 1.Thies W., Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Cotman C.W., Berchtold N.C. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 3.Bos I., De Boever P., Emmerechts J., Buekers J., Vanoirbeek J., Meeusen R. Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal Toxicol. 2012;24:676–686. doi: 10.3109/08958378.2012.714004. [DOI] [PubMed] [Google Scholar]
- 4.Mattson M.P., Chan S.L., Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- 5.Diniz B.S., Teixeira A.L. Brain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyond. Neuromolecular Med. 2011;13:217–222. doi: 10.1007/s12017-011-8154-x. [DOI] [PubMed] [Google Scholar]
- 6.Poo M.M. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen R., Marcussen A.B., Wörtwein G., Knudsen G.M., Aznar S. Abeta(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT(2A) levels. Exp Neurol. 2008;210:164–171. doi: 10.1016/j.expneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Kimura N., Takahashi M., Tashiro T., Terao K. Amyloid beta up-regulates brain-derived neurotrophic factor production from astrocytes: rescue from amyloid beta-related neuritic degeneration. J Neurosci Res. 2006;84:782–789. doi: 10.1002/jnr.20984. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer I., Marín C., Rey M.J., Ribalta T., Goutan E., Blanco R. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hock C., Heese K., Hulette C., Rosenberg C., Otten U. Region-specific neurotrophin imbalances in Alzheimer disease: decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch Neurol. 2000;57:846–851. doi: 10.1001/archneur.57.6.846. [DOI] [PubMed] [Google Scholar]
- 12.Peng S., Wuu J., Mufson E.J., Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 13.Narisawa-Saito M., Wakabayashi K., Tsuji S., Takahashi H., Nawa H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport. 1996;7:2925–2928. doi: 10.1097/00001756-199611250-00024. [DOI] [PubMed] [Google Scholar]
- 14.Li G., Peskind E.R., Millard S.P., Chi P., Sokal I., Yu C.E. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laske C., Stransky E., Leyhe T., Eschweiler G.W., Wittorf A., Richartz E. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- 16.Laske C., Stransky E., Leyhe T., Eschweiler G.W., Wittorf A., Richartz E. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Laske C., Stellos K., Hoffmann N., Stransky E., Straten G., Eschweiler G.W. Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- 18.Yu H., Zhang Z., Shi Y., Bai F., Xie C., Qian Y. Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry. 2008;69:1104–1111. doi: 10.4088/jcp.v69n0710. [DOI] [PubMed] [Google Scholar]
- 19.Forlenza O.V., Diniz B.S., Teixeira A.L., Ojopi E.B., Talib L.L., Mendonça V.A. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 20.Erickson K.I., Prakash R.S., Voss M.W., Chaddock L., Heo S., McLaren M. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30:5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunstad J., Benitez A., Smith J., Glickman E., Spitznagel M.B., Alexander T. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J Geriatr Psychiatry Neurol. 2008;21:166–170. doi: 10.1177/0891988708316860. [DOI] [PubMed] [Google Scholar]
- 22.Rohe M., Synowitz M., Glass R., Paul S.M., Nykjaer A., Willnow T.E. Brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J Neurosci. 2009;29:15472–15478. doi: 10.1523/JNEUROSCI.3960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arancibia S., Silhol M., Moulière F., Meffre J., Höllinger I., Maurice T. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol Dis. 2008;31:316–326. doi: 10.1016/j.nbd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Nagahara A.H., Merrill D.A., Coppola G., Tsukada S., Schroeder B.E., Shaked G.M. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler D. Harcourt Brace Jovanovich; 1987. WMS-R: Wechsler Memory Scale-revised: manual.http://books.google.com/books?id=Q2RIPwAACAAJ Available at: Accessed April 30, 2015. [Google Scholar]
- 31.Jack C.R., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 33.Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 34.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr;18:192–205. [PubMed]
- 35.Mazziotta J., Toga A., Evans A., Fox P., Lancaster J., Zilles K. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shattuck D.W., Sandor-Leahy S.R., Schaper K.A., Rottenberg D.A., Leahy R.M. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 37.Jagust W.J., Bandy D., Chen K., Foster N.L., Landau S.M., Mathis C.A. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soares H.D., Potter W.Z., Pickering E., Kuhn M., Immermann F.W., Shera D.M. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69:1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lommatzsch M., Zingler D., Schuhbaeck K., Schloetcke K., Zingler C., Schuff-Werner P. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Egan M.F., Kojima M., Callicott J.H., Goldberg T.E., Kolachana B.S., Bertolino A. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 41.Apostolova L.G., Steiner C.A., Akopyan G.G., Dutton R.A., Hayashi K.M., Toga A. Three-dimensional gray matter atrophy mapping in mild cognitive impairment and mild Alzheimer disease. Arch Neurol. 2007;64:1489–1495. doi: 10.1001/archneur.64.10.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arai A., Kaneta T., Okamura N., Tashiro M., Iwata R., Takanami K. Pitfalls of Voxel-Based Amyloid PET Analyses for Diagnosis of Alzheimer ' s Disease :Artifacts due to Non-Specific Uptake in the White Matter and the Skull. Tohoku J Exp Med. 2014;234:175–181. doi: 10.1620/tjem.234.175. [DOI] [PubMed] [Google Scholar]
- 43.Seibyl J, Sabri O, Barthel H, Barret O, Marek K, Reininger C. Modeling the influence of white matter contamination on detectability of brain amyloid changes in longitudinal studies of Alzheimer’s progression: segmentation analyses using the PET β-amyloid tracer 18F NAV4694.
- 44.Cohen A.D., Goodheart A., Tamburo E., Minhas D.S., Aizenstein H., Weissfeld L. Binding of Pittsburgh Compound B to both normal and abnormal white matter in elderly cognitively normal control. Alzheimers Dement. 2013;10:P16. [Google Scholar]
- 45.Lowe V, Senjem M, Przybelski S, Weigand S, Knopman D, Boeve B, et al. Increased PiB accumulation occurs in the white matter of PiB positive subjects. Available at: http://www.worldeventsforum.com/hai/HAI2015_Book.pdf. Accessed April 30, 2015.
- 46.Pan W., Banks W.A., Fasold M.B., Bluth J., Kastin A.J. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]