Abstract
Introduction
Increasing social interaction could be a promising intervention for improving cognitive function. We examined the feasibility of a randomized controlled trial to assess whether conversation-based cognitive stimulation through personal computers, webcams, and a user-friendly interactive Internet interface had high adherence and a positive effect on cognitive function among older adults without dementia.
Methods
Daily 30-minute face-to-face communications were conducted during a 6-week trial period in the intervention group. The control group received only a weekly telephone interview. The cognitive status of normal subjects and those with mild cognitive impairment was operationally defined as a global clinical dementia rating of 0 and 0.5, respectively. Age, sex, education, mini mental state examination score, and clinical dementia rating score were balancing factors in randomization. The subjects were recruited using mass-mailing invitations. The pre- to postintervention differences in the cognitive test scores and loneliness scores were compared between the control and intervention groups using linear regression models.
Results
Eighty-three subjects participated (41 in the intervention group and 42 in the control group). Their mean ± standard deviation age was 80.5 ± 6.8 years. Adherence to the protocol was high. There was no dropout and mean percentage of days completed of the targeted trial days among the intervention group was 89% (range 77%–100%). Among the cognitively intact participants, the intervention group improved more than did the control group on a semantic fluency test (P = .003) at the post-trial assessment and a phonemic fluency test (P = .004) at the 18-week assessments. Among those with mild cognitive impairment, a trend (P = .04) toward improved psychomotor speed was observed in the intervention group.
Conclusion
Daily conversations by way of user-friendly Internet communication programs demonstrated high adherence. Among the cognitively intact, the intervention group showed greater improvement in tests of language-based executive functions. Increasing daily social contacts through communication technologies could offer cost-effective home-based prevention methods. Additional studies with a longer follow-up duration are required to examine whether the intervention slows cognitive declines and delays the onset of dementia.
Keywords: Social engagement, Conversational interaction, Internet, Communication technology, Oregon Center for Aging and Technology (ORCATECH), Randomized controlled clinical trial, Prevention study, Mild cognitive impairment
1. Introduction
Almost 2 decades ago, Rowe and Kahn [1] suggested the key elements of successful aging, including (1) a low probability of disease, (2) high levels of function, and (3) active engagement with life. The definition of “active engagement with life” varies across individuals and cultures. In epidemiologic studies, self-reported social engagement—one component of active engagement with life—has been extensively examined in relation to cognitive well-being. However, no set of standard activities was used across studies. Various activities were included, such as reading, playing games or musical instruments, going to classes, doing crossword puzzles, playing cards, going to the cinema or theater (often categorized as cognitive activities), visiting friends or relatives and attending organizations (as social activities), and dancing and walking (as physical activities). Furthermore, larger social networks (a structural aspect of social connectedness) were also found to be protective against dementia [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. It is as yet unknown which factors of social engagement or networking might reduce the risk of dementia. For example, playing games has often been categorized as an intellectual/cognitive stimulating activity; however, playing games with someone requires social interaction. Thus, the question is whether it is the social interaction or playing the game itself that is protective against cognitive decline. Randomized controlled trials (RCTs) with clearly specified elements and doses of social engagement are needed to clarify the mechanism of the protective function of social engagement and networks on cognitive function and, ultimately to translate this knowledge into actionable programs.
One integral component of being socially active is the ability to interact with others. Linguistic ability is known to be highly correlated with late-life changes in cognition in healthy older adults and those with dementia [13], [14], [15]. Furthermore, the results from psychological studies have suggested that the task of conversation is highly cognitively stimulating. Conversations require attention, working memory, the organization and control of thought (executive functions), and social cognition to understand others' intentions and feelings [16], [17], in addition to linguistic ability. To develop a prevention approach against cognitive decline that can be easily adapted to the oldest-old and those with mild cognitive impairment (MCI) or those with low motivation or apathy, we developed a clinical RCT, focusing on conversation. We examined whether face-to-face conversation—a core component of social interaction—can enhance cognitive functions by stimulating social cognition. To facilitate efficiency and quantification of outcomes, we used contemporary technologies, including personal computers (PCs), webcams, and the Internet, to deliver the conversational interventions. From the epidemiologic and psychological data discussed in the Introduction, we hypothesized that our trial intervention would lead to improved attention, executive function, verbal fluency, and memory (i.e., domains frequently impaired among patients with Alzheimer's disease. The objectives of our study were to assess the feasibility, adherence, and post-trial changes in cognitive functions and loneliness. In the report, we present the protocol and results of the RCT.
2. Methods
2.1. Subject recruitment
From November 2011 to August 2012, we distributed 2000 survey questionnaires targeting those living in retirement communities and senior centers located in the Portland, metropolitan area, within an approximately 1-hour commute from the Oregon Health & Science University (Portland, OR). Sixteen retirement communities and senior centers that covered a wide range of socioeconomic status (including low-income household retirement communities designated by the municipal government) and that had agreed to collaborate in research studies with our university were included. We conducted information sessions at each community and center to explain the upcoming trial. The survey was distributed at the conclusion of the information session and also by mail through the retirement communities and senior center administrative offices.
In the survey, we collected information, including demographic data, types and frequencies of social engagement, loneliness, and PC usage. After a brief introductory paragraph describing our trial, we asked individuals whether they would be interested in participating in the trial, and, if so, to provide their contact information. They were informed that they could decline to participate any time after learning about the study. The main information collected in the survey is listed in Table 1.
Table 1.
Information collected in survey questionnaire and baseline screening in-person interview
| Survey information |
| Demographic data Nature and frequencies of social, cognitive, and physical activities Self-rated health 3-Item loneliness measurement [18] Older Americans resources and services, activities of daily living, and instrumental activities of daily living [19] Brief questions on Internet and personal computer usage: (1) “Do you use a personal computer?” (yes/no). If yes, the subject was asked where (check all that apply: at home, at the library, at a senior center/community center, at a friend's or relative's house, other [write in]); how often (less than once a year, a few times a year, a few times a month, a few times a week, almost every day); and for what (check all that apply: send/receive e-mail, make documents, browse websites for information, shopping, Facebook or other social network sites, games, video chat (Skype, etc.), and other [write in]). Willingness (yes/no) to participate in the future clinical trial (after a brief explanation of the prevention study protocol) and provide contact information if willing to be contacted (described in detail in Dodge et al [20]). |
| Baseline interviews (subjects selected from those who had provided contact information in the survey) |
| Demographic information (confirming answers listed in the survey questionnaire) NEO Big-5 personality inventory [21] Geriatric depression scale, 15-item scale [22] Clinical dementia rating scale [23] Informant contact information to complete the clinical dementia rating scale List of current prescription and over the counter medications Neuropsychological assessment (mini mental state examination [24], category and letter fluency tests [25], Consortium to Establish a Registry for Alzheimer's Disease word list learning and recall [26], trail making tests A and B [27], Stroop test [25], wide range achievement test-revised [28], computer assessment of mild cognitive impairment computerized test [29], 3 subitems from Cogstate computerized test [30]) Feedback on computerized tests (fatigue, easiness to follow, preference for Computer Assessment of Mild Cognitive Impairment versus Cogstate, after these tests) |
2.2. Randomization
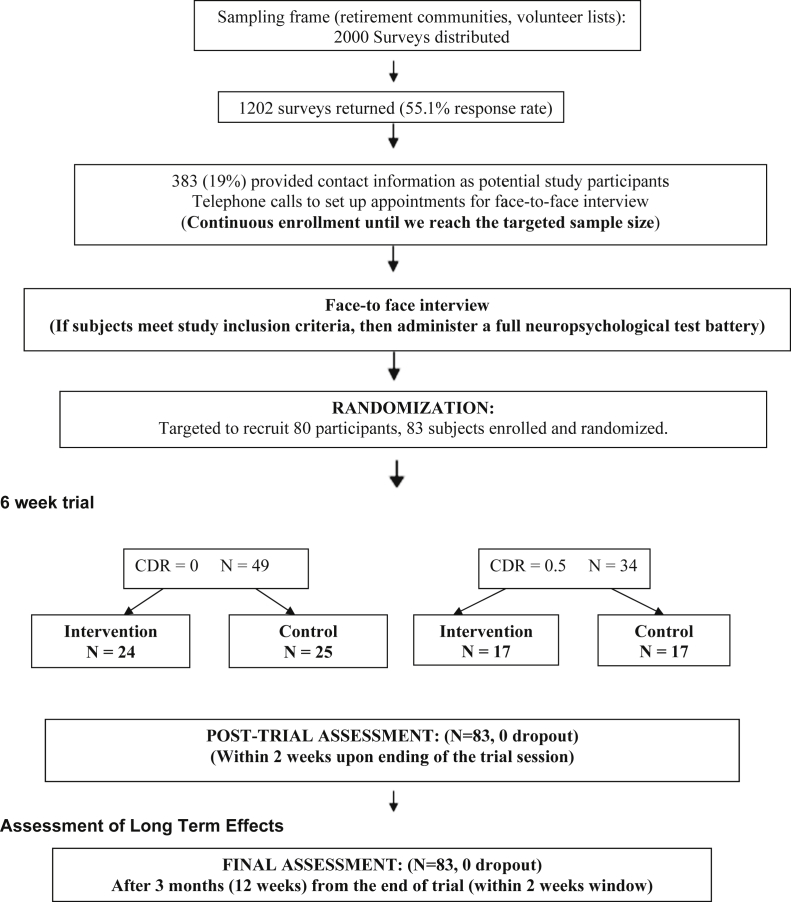
We invited those who had provided their contact information to participate in in-person screening interviews (Figure 1). The information collected at the interview is listed in Table 1, and the study inclusion and exclusion criteria are listed in Table 2. Trained research associates conducted the interviews. The subjects were randomly assigned to either the control or intervention group using the balancing factors of age (3 groups: aged 65–74, 75–84, and 85 years or older), sex, clinical dementia rating (CDR) scale score of 0 or 0.5 [31], mini mental state examination score (3 groups: less than 24, 24–26, 27 or more), and years of education (3 categories: less than 12, 12–15, and 16 or more). The cognitive status of normal subjects and those with MCI was operationally defined as a global CDR score of 0 and 0.5, respectively. A modified randomized minimization algorithm was used [32].
Fig. 1.
Study flow chart. CDR, clinical dementia rating.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria |
| Age ≥ 70 y CDR score of 0 or 0.5 Sufficient vision and hearing to engage in conversation using personal computer Sufficient English language skills to complete all testing General health status will not interfere with ability to complete longitudinal study (conditions likely to lead this problem were included in the exclusion criteria) |
| Exclusion criteria |
| A plan to start taking new classes, traveling requiring > 2 nights away, or participating in significant social events such as a family wedding or family reunion, during the scheduled prevention trial Diseases associated with dementia such as Alzheimer's disease, ischemic vascular dementia, normal pressure hydrocephalus, or Parkinson's disease Significant disease of central nervous system (e.g., brain tumor, seizure disorder, subdural hematoma, cranial arteritis) Current (within previous 2 years) alcohol or substance abuse Current major depression, schizophrenia, or other major psychiatric disorder Unstable or significantly symptomatic cardiovascular disease (e.g., coronary artery disease with frequent angina or congestive heart failure with shortness of breath at rest) Active systemic cancer within 5 years of study entry Illness requires >1 visit per month to a clinician Progressive vision loss (age-related macular degeneration already beginning to significantly degrade vision) Oxygen supplementation required for adequate function Medications Frequent use of high doses of analgesics Sedative medications, except for those used occasionally for sleep (use limited to no more than twice per week) Applicable to CDR of 0.5 group only: Subjects with unstable dosing of cholinesterase inhibitors (stable dosing for 2 months' duration required) |
Abbreviation: CDR, clinical dementia rating.
2.3. Duration and protocol of conversational trial
The intervention group engaged in face-to-face conversations with trained interviewers 5 days a week (Monday through Friday) for 6 weeks by way of a dedicated video-chat-enabled PC provided to each subject. Each conversational session was designed to last 30 to 35 minutes. The control group received weekly telephone calls to assess their social engagement activities during the previous week (i.e., no PC or Internet provided). If the participants in the control group were already using a PC before the trial, they were allowed to continue. After randomization, within 2 weeks before the start of the conversational intervention, we administered a comprehensive neuropsychological test battery. After the trial (within 2 weeks after trial completion) and at the endpoint (12 weeks after the post-trial assessment or 18 weeks from baseline), assessments were conducted to examine the post-trial effect and its durability.
2.4. CDR assessment
The CDR assessment was conducted by trained research nurses in a standardized manner, including information from informants or the collateral source [31].
2.5. Development of a user-friendly web-enabled conversational system
We created our own version of a chat system in which participants did not need to know how to use a computer, other than to touch the touch screen of a computer preconfigured to receive calls and automatically begin the conversational session. The study computer was enabled to record the trial sessions and store encrypted audio data automatically. Technical support personnel visited each participant's home and set up the equipment.
2.6. Development of the conversational protocol
Conversation requires synthesis of multiple cognitive functions. To present an understandable story or “rationality,” the speaker must organize their ideas and thoughts and pay attention to the other's response. Thus, attention, executive function, and abstract reasoning are simultaneously engaged. To take full advantage of this synthetic aspect of conversation, we placed an emphasis on spontaneous responses rather than structured answers (i.e., the participants had to organize their thoughts). We used unstructured conversations such as talking about the participants' “childhood memories,” “hobbies,” “siblings and parents,” and “movies/books.” A topic that engages one participant's attentiveness and interest might not do the same for others. Nevertheless, we attempted to create a degree of standardization by using a daily picture prompt to stimulate the conversation. For example, we presented Norman Rockwell paintings or pictures of famous events (e.g., the first moon landing) on screen as evocative pictures and asked the participant about what was happening in the picture. Next, we asked whether the subject could connect their experience with the story seen in the picture. We aimed to primarily engage the executive functions, attention, semantic memory, and abstract reasoning with this type of a semistructured session approach.
2.7. Standardization of interviewers
The interviewers practiced conversational sessions with our staff members and elderly volunteers to standardize their skills before the trial began. We also recorded each conversational session to monitor their interview quality. Permission for recording each trial session was included in the consent form. Additionally, we randomly selected three recorded conversational sessions per interviewer, one session each during the baseline, third, and sixth week and had them transcribed by a single professional transcriber. The proportion of words spoken by the interviewers was used as a tool to standardize the conversational sessions. The deviation observed in the number of spoken words contributed by the participant or interviewer during the recorded conversations served as a metric to improve the standardization of the individual interviewers' interview skills. The interviewers were unaware of the cognitive status of the participants.
2.8. Primary outcome: Cognitive function
We administered the following neuropsychological tests: (1) for immediate memory, the Consortium to Establish a Registry for Alzheimer's Disease word list learning [26]; (2) for delayed memory, the Consortium to Establish a Registry for Alzheimer's Disease word list delayed recall [26]; (3) for language, the composite of verbal fluency for letters (F, A, and S) [25]; (4) for psychomotor speed, the trail making A test [27]; (5) for executive function, the trail making B test [27] and verbal fluency for category animals [25]; (6) for selective attention/inhibition, the Stroop test [25]; and (7) for premorbid and general intelligence, the wide range achievement test-revised [28]. We also used the following items from computerized cognitive test batteries: two domains from the Cogstate [30] (for psychomotor speed, the detection test and for working memory, the one back and two back), and the full battery of the computer assessment of mild cognitive impairment [29].
2.9. Secondary outcome: Loneliness score
The changes in loneliness from before to after the trial were assessed using a 3-item loneliness scale developed by Hughes et al [18]. The measurement asks three questions: “How often do you feel” (1) that you lack companionship, (2) left out, and (3) isolated from others? (1, hardly ever [or never]; 2, some of the time; and 3, often). A higher score indicates greater levels of perceived loneliness.
2.10. Control variables
Symptoms of depression can mediate possible treatment effects, especially as they relate to socialization. Therefore, we controlled for symptoms of low mood measured using the geriatric depression scale, 15-item scale [22] in the primary analysis. As an exploratory analysis, we also examined personality measured using the NEO-5 factor personality scales [21] and controlled for them in the multivariate analyses, hypothesizing that personality could affect changes in the primary outcomes. Finally, we included the interaction effect of PC usage (yes/no; the questions are listed in Table 1) and the study group (intervention versus control group, with the latter group as a reference) to examine whether the trial efficacy differed by PC usage/experience. This was because our previous study found that those who provided contact information in the survey were significantly more likely to be PC users [20]. If PC users have higher or lower efficacy compared with non-PC users, this information would be useful for generalizing our study results to nonparticipants.
Our institutional review board approved the study protocol (protocol no. 5590), and all participants provided written informed consent. The project is listed in ClinicalTrials.gov (NCT01571427), and the final face-to-face interview with the participants was conducted on August 30, 2013.
2.11. Statistical analysis
The characteristics were compared between the intervention and control groups using Pearson chi-square tests for categorical variables and the t test or nonparametric Wilcoxon ranked sum test for continuous variables. Adherence was calculated as the proportion of days the subjects in the intervention group completed the experiment. The pre- to postintervention differences in the cognitive tests and loneliness scores were compared between the control and intervention groups using t tests (univariate analysis) and linear regression models (multivariate analysis). Statistical significance was set at P < .004, the Bonferroni multiple comparison adjusted P value. All analyses were performed using SAS, version 9.3, software (SAS Institute, Cary, NC).
3. Results
3.1. Participants
Of 2000 surveys distributed, 1102 were returned (55.1% response rate). Of these, 383 subjects (19.1%) provided contact information (Figure 1). The characteristics associated with those who provided contact information in the survey compared with those who returned the survey without providing the information (potential volunteer bias) have been previously summarized in detail [20]. In brief, those who provided contact information were more likely to be PC users and physically active and to have higher social isolation scores, with PC usage the most significant predictor after controlling for education and other confounders. A total of 83 subjects were enrolled and randomized (41 in the intervention group and 42 in the control group; Figure 1). The participant characteristics at baseline are listed in Table 3. The mean age was 80.5 years, and 76% were women. Per protocol, the age, sex, education, CDR score, and mini mental state examination score distributions were similar between the intervention and control groups. The other characteristics not used for randomization (i.e., marital status, wide range achievement test-revised scores, and PC usage) were also comparable between the two groups. We also compared the baseline characteristics between those with a CDR score of 0 and those with a CDR score of 0.5. The MCI group was somewhat older and more likely to be women. Although the CDR score was assessed independently, all conventional neuropsychological test scores, except for letter fluency, were lower among the CDR 0.5 group, with the most significant difference observed for category fluency and word list delayed recall (P < .0001) and the mini mental state examination (P < .0001), supporting the validity of our CDR assessment. The computer assessment of mild cognitive impairment overall scores were significantly lower among the MCI group, although the items in the Cogstate computerized tests showed no differences between the two groups.
Table 3.
Baseline characteristics of subjects
| Variable | Total (n = 83) | Intervention group (A) (n = 41) | Control group (B) (n = 42) | P value (difference between A and B) | CDR = 0 (C) (n = 49) | CDR = 0.5 (D) (n = 34) | P value (difference between C and D) |
|---|---|---|---|---|---|---|---|
| For randomization | |||||||
| Age (y) | 80.5 ± 6.8 | 80.9 ± 7.2 | 80.2 ± 6.6 | .65 | 78.9 ± 5.5 | 82.8 ± 7.9 | .02 |
| Female gender (%) | 75.9 | 78 | 73.8 | .65 | 71.4 | 82.4 | .25 |
| High school completed or greater education (%) | 96.4 | 97.6 | 95.2 | .57 | 100 | 91.1 | .03 |
| CDR 0.5 (%) | 41 | 41.5 | 40.5 | .93 | — | — | — |
| Mini-mental state examination score | 28.3 ± 1.8 | 28.2 ± 1.7 | 28.3 ± 1.8 | .87 | 28.9 ± 1.3 | 27.3 ± 1.9 | <.0001 |
| Other (not for randomization) | |||||||
| Marital status (% married) | 46.3 | 45.0 | 47.6 | .81 | 52.1 | 38.2 | .21 |
| WRAT-R | 72.0 ± 12.1 | 72.0 ± 12.9 | 72.0 ± 11.5 | .75 | 75.1 ± 10.5 | 67.6 ± 13.2 | .007 |
| Used PC (%) | 14.6 | 15.0 | 14.3 | .99 | 10.4 | 12.6 | .82 |
| Primary outcome variables | |||||||
| Category fluency | 19.9 ± 5.1 | 19.5 ± 5.3 | 20.4 ± 4.9 | .42 | 21.8 ± 4.6 | 17.3 ± 4.6 | <.0001 |
| Letter fluency | 37.4 ± 13 | 37 ± 13.2 | 37.7 ± 12.9 | .82 | 39.1 ± 11.9 | 34.9 ± 14.1 | .16 |
| Word list acquisition | 19 ± 4.5 | 19 ± 4.8 | 18.9 ± 4.2 | .94 | 20.2 ± 3.7 | 17.2 ± 4.9 | .004 |
| Word list delayed recall | 4.8 ± 2.3 | 4.8 ± 2.2 | 4.8 ± 2.4 | .96 | 5.6 ± 2.0 | 3.6 ± 2.2 | <.0001 |
| Trail making test A | 41.3 ± 15.8 | 44.6 ± 17 | 38.0 ± 14.0 | .06 | 36.4 ± 11.3 | 48.6 ± 18.8 | .002 |
| Trail making test B | 120.1 ± 62.3 | 123.1 ± 60.5 | 117.4 ± 64.5 | .68 | 102.9 ± 45.7 | 144.5 ± 74.1 | .005 |
| Stroop test | 29.3 ± 8.7 | 29.9 ± 10.5 | 28.8 ± 6.5 | .55 | 32.0 ± 7.9 | 25.5 ± 8.5 | .001 |
| Cogstate computerized tests | |||||||
| Detection test (log of speed of performance) | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | .45 | 2.6 ± 0.1 | 2.6 ± 0.1 | .72 |
| One back accuracy (working memory test) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.1 | .75 | 1.2 ± 0.1 | 1.1 ± 0.2 | .09 |
| Two back accuracy (working memory test) | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.2 | .78 | 1.1 ± 0.2 | 1.0 ± 0.2 | .14 |
| CAMCI test, total score∗ | −0.05 ± 0.68 | −0.12 ± 0.78 | 0.03 ± 0.56 | .32 | 0.19 ± 0.45 | −0.38 ± 0.80 | .0004 |
| Secondary outcome variable | |||||||
| Loneliness score (range 3–9) | 4.0 ± 1.6 | 4.3 ± 1.9 | 3.6 ± 1.0 | .05 | 3.7 ± 1.2 | 4.3 ± 1.9 | .09 |
| Control variable | |||||||
| GDS-15 | 1.7 ± 2.2 | 2.0 ± 2.3 | 1.5 ± 2.1 | .30 | 1.5 ± 1.9 | 2.0 ± 2.5 | .37 |
| Exploratory analysis (NEO 5-factor personality scale) | |||||||
| Extraversion | 3.5 ± 0.8 | 3.4 ± 0.8 | 3.6 ± 0.8 | .16 | 3.5 ± 0.9 | 3.4 ± 0.8 | .47 |
| Agreeable | 4.3 ± 0.6 | 4.2 ± 0.7 | 4.4 ± 0.5 | .05 | 4.3 ± 0.6 | 4.3 ± 0.6 | .97 |
| Conscientious | 3.9 ± 0.7 | 4.0 ± 0.8 | 3.8 ± 0.6 | .46 | 3.9 ± 0.8 | 3.9 ± 0.7 | .68 |
| Neuroticism | 2.3 ± 0.8 | 2.4 ± 0.9 | 2.2 ± 0.7 | .15 | 2.3 ± 0.8 | 2.3 ± 0.8 | .77 |
| Openness | 4.0 ± 0.6 | 4.0 ± 0.6 | 4.0 ± 0.6 | .65 | 4.0 ± 0.6 | 4.0 ± 0.7 | .85 |
Abbreviations: CAMCI, computer assessment of mild cognitive impairment; GDS-15, geriatric depression scale, 15-item scale; WRAT-R, wide range achievement test-revised.
NOTE. Data presented as mean ± standard deviation or percentages.
Z score compared with normative scores generated by the CAMCI, based on their normative distribution [8].
3.2. Adherence
All participants completed the pre- and post-trial neuropsychological tests. Among the intervention group, session adherence was 89% (range 77%–100%). All subjects (control and intervention groups) completed the 6- and 18-week final assessments.
3.3. Outcome measures
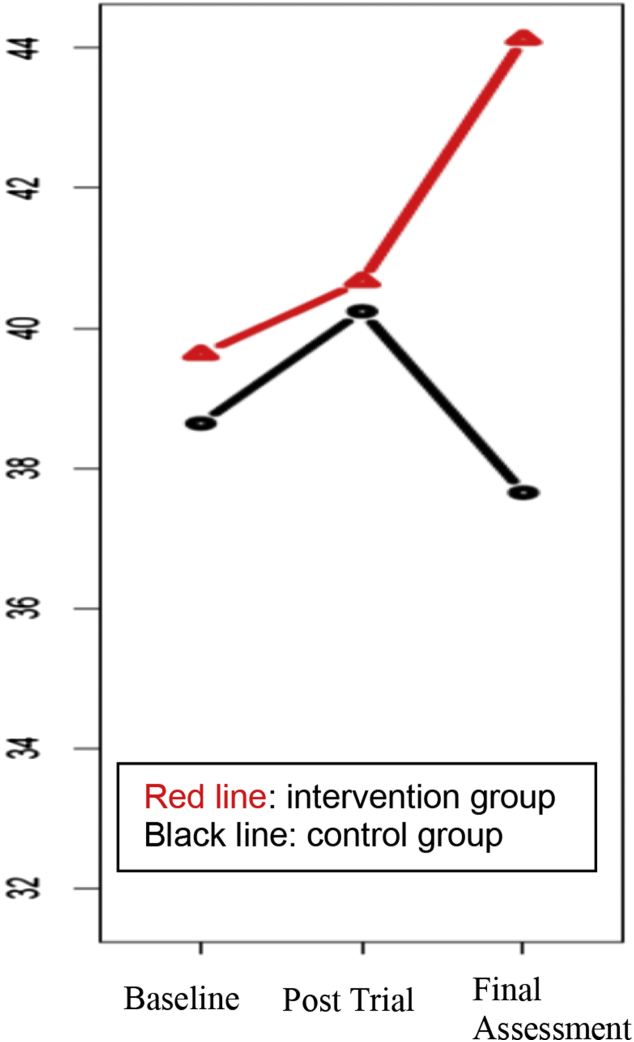
The results of the linear regression models in which the outcome was the differences in the test scores between the baseline and post-trial assessments (post-trial score minus the baseline score) with the study group as the independent variable and controlling for depressive symptoms (geriatric depression scale, 15-item scale), are listed in Table 4. The coefficients reported in Table 4 are “additional” changes obtained by the intervention group beyond those obtained by the control group. We found category fluency scores (semantic fluency scores) had improved more in the intervention group than in the control group (P = .02). The stratified analysis showed that this effect came mainly from the CDR 0 group (P = .003). Among the MCI group, the intervention group gained psychomotor speed, indicated by the Cogstate detection tests, compared with the control group (P = .04), although the differences were not significant using the multiple comparison-adjusted P value. At 12 weeks after the end of the trial, we examined the durability of the effects (data not shown). The category fluency scores no longer differed between the two groups, but the letter fluency scores showed greater improvement among those with a CDR of 0 in the intervention group (P = .004). Although both groups had similar levels of improvement/learning effects at the post-trial assessment, the letter fluency scores had improved further in the intervention group after the end of the trial sessions (Figure 2) but had declined in the control group. No difference was found between intervention and control groups in the pre- to post-trial changes in the loneliness score, the secondary outcome. As an exploratory analysis, we controlled for the personality scores, in addition to the geriatric depression scale scores, which did not influence the obtained results. Finally, we included the interaction of the study group (intervention versus control) and PC usage. No interaction effect was found, and it did not influence the obtained results.
Table 4.
Linear regression results with outcome being pre-post trial differences: overall and stratified by cognitive status (CDR 0 or 0.5)
| Outcome variable | Total (n = 83) |
CDR 0 (n = 49) |
CDR 0.5 (n = 34) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient for intervention group∗ | SE | P value | Coefficient for intervention group∗ | SE | P value | Coefficient for intervention group∗ | SE | P value | |
| Changes in neuropsychological tests | |||||||||
| Mini mental state examination | −0.41 | 0.35 | .25 | −0.49 | 0.42 | .25 | −0.11 | 0.62 | .85 |
| Category fluency | 2.20 | 0.92 | .02 | 4.00 | 1.28 | .003† | 0.52 | 1.14 | .65 |
| Letter fluency | 0.03 | 1.33 | .98 | −0.09 | 1.63 | .96 | 0.51 | 2.38 | .83 |
| Word list acquisition | −0.27 | 0.64 | .68 | −0.34 | 0.84 | .69 | −0.18 | 1.02 | .86 |
| Word list delayed recall | −0.04 | 0.43 | .92 | 0.06 | 0.61 | .92 | −0.05 | 0.62 | .94 |
| Trail making test A | −2.11 | 2.84 | .46 | −1.07 | 2.08 | .61 | −1.66 | 6.42 | .80 |
| Trail making test B | 3.25 | 8.88 | .72 | 2.26 | 11.10 | .84 | 12.39 | 14.40 | .40 |
| Stroop test | −0.81 | 0.91 | .38 | −1.12 | 1.31 | .39 | −0.68 | 1.26 | .59 |
| Cogstate computerized tests | |||||||||
| Detection test (log of speed of performance: psychomotor speed test) | −0.05 | 0.02 | .03 | −0.03 | 0.03 | .24 | −0.09 | 0.04 | .04 |
| One back accuracy (working memory test) | −0.02 | 0.05 | .65 | 0.02 | 0.05 | .64 | −0.08 | 0.10 | .42 |
| Two back accuracy (working memory test) | −0.03 | 0.04 | .45 | 0.004 | 0.05 | .93 | −0.07 | 0.08 | .40 |
| CAMCI total score‡ | −0.09 | 0.09 | .30 | 0.03 | 0.10 | .75 | −0.24 | 0.15 | .12 |
| Secondary outcome | |||||||||
| Loneliness score | −0.21 | 0.27 | .44 | −0.06 | 0.27 | .82 | −0.34 | 0.56 | .54 |
Abbreviations: CAMCI, computer assessment of mild cognitive impairment; CDR, clinical dementia rating; GDS-15, geriatric depression scale, 15-item scale; SE, standard error.
NOTE. All models were controlled for GDS-15 scores.
Coefficients reported are “additional” changes obtained by the intervention group beyond those obtained by the control group (changes among the control group used as the reference). For example, the data can be interpreted as follows: among those with intact cognition, the category fluency scores improved by 4 points among the intervention group beyond the changes observed among the control group.
Significant using the Bonferroni multiple comparison adjusted P value < .004.
Percentile compared with normative scores provided by CAMCI.
Fig. 2.
Letter fluency test results at baseline, post-trial, and final assessments among clinical dementia rating (CDR) 0 group. Among the CDR 0 group, the intervention group kept improving the test score after the trial, but the control group experienced a decline at the final assessment, leading to a significant difference between the two groups in the gain in scores from baseline to the final assessment (P = .004).
4. Discussion
We conducted a pilot behavioral clinical trial to improve cognitive functions among nondemented older elderly subjects by enhancing their social interaction through Internet-based conversation. We achieved high adherence to the protocol, and the intervention groups showed improvements in language-based executive functions within a short-duration trial period.
Cognitive stimulation through Internet-based face-to-face conversation has some ideal features as a prevention approach. First, unlike video game-invoked cognitive training, the subjects participate in naturalistic “human” interactions that might be more engaging and require less motivation on the part of older participants, thus allowing those with low motivation and/or apathy to participate and remain in the trial. Second, one might achieve more cost-effective execution of trials by allowing a few interviewers to interact with many participants daily using the Internet and also gain access to those who are home-bound or in remote locations. Third, conversations with interviewers through the Internet eliminate potential trial confounders such as indirect effects of tangible support that could affect overall and cognitive health (e.g., transportation service). Fourth, the trial differs in nature from the neuropsychological test itself. Therefore, the observed gains in the neuropsychological test scores at the post-trial assessment (beyond the learning effects observed among the control group) should reflect improvement in cognitive function that could not be attributed to “test-taking” or “limited trained skills.” Finally, the method provides the ability to record all interactions for off-line analysis with the participants' consent. Thus, acoustic speech characteristics, word selections, and sentence complexity associated with cognitive function, an area of growing research interest, could be analyzed for additional information [13], [33], [34], [35], [36], [37], [38].
We paid special attention to creating a user-friendly environment to achieve high adherence, including a large touch screen monitor that allowed eye-to-eye contact as experienced in in-person conversations to retain attention and pop-up pictures on the screen to evoke conversations without any effort by the participants. The published psychological data suggest that with age, adult cognition becomes more tightly linked to socioemotional systems, and emotional motives play an important role in driving engagement and enhancing cognitive outcomes in later adulthood [39], [40]. We believe that tailoring existing technologies to suit the current generation of the elderly, together with naturalistic human contact, is key to achieving high adherence when using contemporary communication technologies.
We found improvements in semantic fluency immediately after the trial sessions among the intervention group compared with the control group and at the 18-week assessment from baseline in phonemic fluency. In Alzheimer's disease, semantic fluency has been found to be disproportionately impaired, with phonemic fluency ability less impaired in some [41], [42], although not all studies agree [43]. It has been hypothesized that the disproportionate impairment in semantic fluency, compared with phonemic fluency, could occur because the former relies more on temporal lobe semantic stores, the area affected by Alzheimer's disease, and the latter on frontal lobe functions. It is noteworthy that the intervention group continued to show improvement in the phonemic fluency test. The stimulation obtained from the present trial might have led to sustained or an increased amount of social interaction, even after the termination of the trial sessions, although we do not have data to confirm this hypothesis. Future studies that examine the post-trial changes in functional and structural connectivity between medial temporal lobe and frontal lobe using functional magnetic resonance imaging and diffusion tensor imaging could be useful in identifying the underlying mechanisms of the finding.
The improvement in cognitive function we have described was limited to those with intact cognition, although we saw a trend toward improvement in psychomotor speed among the MCI group. The lack of improvement in cognitive functions among those with MCI likely was because our study was not powered to see changes among the MCI group (the sample size was predetermined for a combined analysis in this phase I study). Also, those with MCI are a heterogeneous group, and the efficacy is likely to vary depending on whether the subjects have only memory impairment or also have impairment in other domains (multidomain MCI). The CDR sum of box scores in our MCI group ranged from 0.5 to 3, suggesting variability in the types of MCI present. Future studies that allow for stratified analyses by MCI and its subtypes are warranted. Also, it will be important to identify the biomarker characteristics of those who improved and those who did not improve in cognitive functions, to examine the underlying mechanisms of those differences. This could aid in identifying who should be targeted for this type of behavioral trial and also in reducing the confounding effects and variability in outcomes.
We did not see any improvement in the loneliness scores (the secondary outcome). The scale we used asked only 3 questions (lack companionship, left out, and isolated from others), with each having 3 possible answers. It is possible that the variation in the scale is not great, and, therefore, it would be difficult to capture within-individual changes within a short period. Alternatively, loneliness is a subjective state, indicating a gap between desired levels of social interactions and the amount of available social network and support. Increasing the opportunity to converse or socialize might not be sufficient to modify the level of loneliness.
Recent magnetic resonance imaging studies found associations between the size and complexity of the real-world social networks and the density of the gray matter [44] and amygdala volume [45]. Modifiable effects of larger social networks on the symptomatic outcomes of Alzheimer's disease pathologic features have also been shown [6]. Nonhuman research has suggested that the social network size could actually contribute to changes in both brain structure and function, providing additional support for causal links [46]. We have previously outlined the possible mechanisms for social interaction's effects on cognitive function [47]. Despite the accumulating evidence, just a few RCTs have examined engagement-evoked cognitive changes targeted to older adults [48], [49]. We searched the clinicaltrial.gov website where active and completed trials in the United States and 187 countries are registered, using the following search words: cognition, dementia, social engagement, prevention, intervention. Only five studies were identified other than ours (as of August 2014). This is in contrast to the relatively large number of computerized cognitive training prevention studies targeting subjects with intact cognition [50]. Increasing social engagement through user-friendly devices using modern telecommunication technologies holds high promise as a translational, large-scale national prevention protocol for both cognitively intact and impaired individuals.
The limitations of our study included a selection bias. As shown in our previous study [20], those who volunteer to participate in such studies differ from the general population. For example, the high adherence we observed could have been in part because the participants were self-selected volunteers. The sample size for the present pilot study was determined for the normal/MCI combined analyses, not for analyses stratified by cognitive status. Also, our trial duration was only 6 weeks, and the retention effects were limited to 18 weeks from baseline. To confirm whether the rates of decline in cognitive functions are actually different between the intervention and control groups, we would need to monitor the participants for at least 6 months to 1 year to be able to observe and compare the natural history of cognitive declines with those in the intervention group. Finally, more effort is required to control for confounding effects such as the duration of daily conversation enacted outside the trial sessions. We intended to measure the amount of daily conversations; however, the currently available devices were limited in their battery life, and we were unable to include this confounder in the analyses.
The strengths of our study included that rigorous approaches were taken to standardize the interviewers, including intensive practice sessions before the trial initiation and assessment of recorded conversations, including examination of the proportion of words spoken by the interviewers and the participants during the trial sessions. Second, our study participants were relatively old (mean age 80 years). This age group is the fastest growing segment of the population in most developed countries and faces the greatest risk of developing cognitive impairment or dementia because of their risk factor of age alone. Developing prevention approaches with high adherence that could delay the onset of dementia for even a few years could have a large effect on the overall disease burden, especially among the oldest of the old group, and is urgently needed. To our knowledge, the present study is one of the first RCTs aimed at increasing social interactions among this age group.
5. Conclusion
Our social engagement intervention (daily conversations) using user-friendly Internet communication programs demonstrated high adherence. The intervention group showed significantly greater improvement in the neuropsychological test scores that evaluate both semantic and phonetic fluencies, despite the short duration of the trial period. Increasing daily social contacts through communication technologies could offer cost-effective execution of home-based prevention trials. Additional studies are needed that are powered to analyze the efficacy among those with MCI and have longer follow-up durations to examine the differences in the rate of decline in cognitive functions between the intervention and control groups. These studies should also examine the pre- and postintervention differences in biomarkers to identity the potential mechanism and should be able to assess the translational effects on everyday living.
Research in Context.
-
1.
Systematic review: We reviewed the available English-language data in PubMed to March 2014 using the search terms “social network,” “social engagement” or “social interaction,” and “randomized controlled clinical trials” to find studies to include in our discussion.
-
2.
Interpretation: Previous epidemiologic studies have demonstrated that a larger social network or more frequent social interactions might have protective effects on the development of Alzheimer's disease. We conducted a randomized, controlled clinical trial aimed at improving cognitive functions among nondemented older subjects (mean age 80 years) by enhancing their social interaction through Internet-based conversation. We achieved high adherence to the protocol, and the intervention groups showed improvements in language-based executive functions.
-
3.
Future directions: The cognitive stage at which this type of intervention is most effective should be identified. The study should be expanded to have a longer follow-up duration to examine the differences in the rate of decline in cognitive functions between the intervention and control groups. The pre- and postintervention differences in biomarker levels should be examined to identity the potential mechanisms. Finally, an effective, yet sustainable, dose of social interactions and methods to deliver the program to a larger community, scalable to a national level, should be identified.
References
- 1.Rowe J.W., Kahn R.L. Pantheon Books; New York: 1998. Successful Aging. [Google Scholar]
- 2.Fratiglioni L., Paillard-Borg S., Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 3.Amieva H., Stoykova R., Matharan F., Helmer C., Antonucci T.C., Dartigues J.F. What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosom Med. 2010;72:905–911. doi: 10.1097/PSY.0b013e3181f5e121. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L.L., Mendes de Leon C.F., Wilson R.S., Bienias J.L., Evans D.A. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 5.Beland F., Zunzunegui M.V., Alvarado B., Otero A., Del Ser T. Trajectories of cognitive decline and social relations. J Gerontol B Psychol Sci Soc Sci. 2005;60:P320–P330. doi: 10.1093/geronb/60.6.p320. [DOI] [PubMed] [Google Scholar]
- 6.Bennett D.A., Schneider J.A., Tang Y., Arnold S.E., Wilson R.S. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 7.Fratiglioni L., Wang H.X., Ericsson K., Maytan M., Winblad B. Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 8.Holtzman R.E., Rebok G.W., Saczynski J.S., Kouzis A.C., Wilcox Doyle K., Eaton W.W. Social network characteristics and cognition in middle-aged and older adults. J Gerontol B Psychol Sci Soc Sci. 2004;59:P278–P284. doi: 10.1093/geronb/59.6.p278. [DOI] [PubMed] [Google Scholar]
- 9.Zunzunegui M.V., Alvarado B.E., Del Ser T., Otero A. Social networks, social integration, and social engagement determine cognitive decline in community-dwelling Spanish older adults. J Gerontol B Psychol Sci Soc Sci. 2003;58:S93–S100. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarmeas N., Stern Y. Cognitive reserve: Implications for diagnosis and prevention of Alzheimer's disease. Curr Neurol Neurosci Rep. 2004;4:374–380. doi: 10.1007/s11910-004-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saczynski J.S., Pfeifer L.A., Masaki K., Korf E.S., Laurin D., White L. The effect of social engagement on incident dementia: The Honolulu-Asia Aging Study. Am J Epidemiol. 2006;163:433–440. doi: 10.1093/aje/kwj061. [DOI] [PubMed] [Google Scholar]
- 12.Hughes T.F., Flatt J.D., Fu B., Chang C.C., Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: The MYHAT study. Int Psychogeriatr. 2013;25:587–595. doi: 10.1017/S1041610212002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemper S., Marquis J., Thompson M. Longitudinal change in language production: Effects of aging and dementia on grammatical complexity and propositional content. Psychol Aging. 2001;16:600–614. doi: 10.1037//0882-7974.16.4.600. [DOI] [PubMed] [Google Scholar]
- 14.Kemper S. The role of working memory in language development over the life span. In: de Bot K., Makoni S., Schrauf R., editors. Language Development over the Life Span. Erlbaum; Mahwah, NJ: 2009. pp. 217–287. [Google Scholar]
- 15.Riley K.P., Snowdon D.A., Desrosiers M.F., Markesbery W.R. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Ybarra O., Burnstein E., Winkielman P., Keller M.C., Manis M., Chan E. Mental exercising through simple socializing: Social interaction promotes general cognitive functioning. Pers Soc Psychol Bull. 2008;34:248–259. doi: 10.1177/0146167207310454. [DOI] [PubMed] [Google Scholar]
- 17.Ybarra O., Winkielman P. On-line social interactions and executive functions. Front Hum Neurosci. 2012;6:75. doi: 10.3389/fnhum.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes M.E., Waite L.J., Hawkley L.C., Cacioppo J.T. A short scale for measuring loneliness in large surveys: Results from two population-based studies. Res Aging. 2004;26:655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillenbaum G.G., Smyer M.A. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 20.Dodge H.H., Katsumata Y., Zhu J., Mattek N., Bowman B.A., Gregor B.A. Characteristics associated with willingness to participate in a randomized controlled behavioral clinical trial using home-based personal computers and a webcam. Trials. 2014;15:508. doi: 10.1186/1745-6215-15-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa P.T., Robert R.M. The five-factor model of personality and its relevance to personality disorders. J Personal Disord. 1992;6:343–359. [Google Scholar]
- 22.Sheikh J.I., Yesavage J.A. Geriatric depression scale (GDS): Recent evidence and development of a shorter version. In: Brink T., editor. Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press, Inc.; New York: 1986. pp. 165–173. [Google Scholar]
- 23.Morris J.C., Ernesto C., Schafer K., Coats M., Leon S., Sano M. Clinical dementia rating training and reliability in multicenter studies: The Alzheimer's Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 24.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Lezak M.D., Howieson D.B., Bigler E.D., Tranel D. Oxford University Press; New York: 2012. Neuropsychological Assessment. [Google Scholar]
- 26.Morris J.C., Heyman A., Mohs R.C., Hughes J.P., van Belle G., Fillenbaum G. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Reitan R.M. Validity of the trail-making tests as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 28.Jastak S., Wilkinson G. Jastak Associates, Inc; Wilmington, DE: 1984. The Wide Range Achievement Test-Revised. [Google Scholar]
- 29.Saxton J., Morrow L., Eschman A., Archer G., Luther J., Zuccolotto A. Computer assessment of mild cognitive impairment. Postgrad Med. 2009;121:177–185. doi: 10.3810/pgm.2009.03.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darby D., Brodtmann A., Woodward M., Budge M., Maruff P. Using cognitive decline in novel trial designs for primary prevention and early disease-modifying therapy trials of Alzheimer's disease. Int Psychogeriatr. 2011;23:1376–1385. doi: 10.1017/S1041610211000354. [DOI] [PubMed] [Google Scholar]
- 31.Morris J.C. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 32.Schouten H.J. Adaptive biased urn randomization in small strata when blinding is impossible. Biometrics. 1995;51:1529–1535. [PubMed] [Google Scholar]
- 33.Mehl M.R., Vazire S., Ramirez-Esparza N., Slatcher R.B., Pennebaker J.W. Are women really more talkative than men? Science. 2007;317:82. doi: 10.1126/science.1139940. [DOI] [PubMed] [Google Scholar]
- 34.Stark A, Shafran I, Jeffrey K. Hello, who is calling? Can words reveal the social nature of conversations? Presented at the 2012 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies (NAACL HLT '12), 2012; Stroudsburg, PA, USA: 112–119. [PMC free article] [PubMed]
- 35.Thelwall M., Buckley K., Paltoglou G., Cai D., Kappas A. Sentiment strength detection in short informal text. J Am Soc Inf Sci Technol. 2010;61:2544–2558. [Google Scholar]
- 36.Ahmed S., Haigh A.M., de Jager C.A., Garrard P. Connected speech as a marker of disease progression in autopsy-proven Alzheimer's disease. Brain. 2013;136:3727–3737. doi: 10.1093/brain/awt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forbes-McKay K.E., Venneri A. Detecting subtle spontaneous language decline in early Alzheimer's disease with a picture description task. Neurol Sci. 2005;26:243–254. doi: 10.1007/s10072-005-0467-9. [DOI] [PubMed] [Google Scholar]
- 38.Satt A, Sorin A, Toledo-Ronen O, Barkan O, Kompatsiaris I, Kokonozi A, et al. Evaluation of speech-based protocol for detection of early-stage dementia. In: Bimbot F, Cerisara C, Fougeron C, Gravier G, Lamel L, Pellegrino F, et al (eds): INTERSPEECH. Presented at the 14th Annual Conference of the International Speech Communication Association, Lyon, France, August 25–29, 2013; ISCA, 2013:1692–1696.
- 39.Adams C., Smith M.C., Pasupathi M., Vitolo L. Social context effects on story recall in older and younger women: does the listener make a difference? J Gerontol B Psychol Sci Soc Sci. 2002;57:P28–P40. doi: 10.1093/geronb/57.1.p28. [DOI] [PubMed] [Google Scholar]
- 40.Isaacowitz D., Charles S., Carstensen L. Erlbaum; Mahwah, NJ: 2000. Emotion and Cognition. [Google Scholar]
- 41.Cerhan J.H., Ivnik R.J., Smith G.E., Tangalos E.C., Petersen R.C., Boeve B.F. Diagnostic utility of letter fluency, category fluency, and fluency difference scores in Alzheimer's disease. Clin Neuropsychol. 2002;16:35–42. doi: 10.1076/clin.16.1.35.8326. [DOI] [PubMed] [Google Scholar]
- 42.Henry J.D., Crawford J.R., Phillips L.H. Verbal fluency performance in dementia of the Alzheimer's type: A meta-analysis. Neuropsychologia. 2004;42:1212–1222. doi: 10.1016/j.neuropsychologia.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Brandt J., Manning K.J. Patterns of word-list generation in mild cognitive impairment and Alzheimer's disease. Clin Neuropsychol. 2009;23:870–879. doi: 10.1080/13854040802585063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanai R., Bahrami B., Roylance R., Rees G. Online social network size is reflected in human brain structure. Proc R Soc B. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bickart K.C., Wright C.I., Dautoff R.J., Dickerson B.C., Barrett L.F. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sallet J., Mars R.B., Noonan M.P., Andersson J.L., O'Reilly J.X., Jbabdi S. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 47.Dodge H.H., Ybarra O., Kaye J.A. Tools for advancing research into social networks and cognitive function in older adults. Int Psychogeriatr. 2014;26:533–539. doi: 10.1017/S1041610213001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lautenschlager N.T., Anstey K.J., Kurz A.F. Non-pharmacological strategies to delay cognitive decline. Maturitas. 2014;79:170–173. doi: 10.1016/j.maturitas.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 49.Schneider N., Yvon C. A review of multidomain interventions to support healthy cognitive ageing. J Nutr Health Aging. 2013;17:252–257. doi: 10.1007/s12603-012-0402-8. [DOI] [PubMed] [Google Scholar]
- 50.Lampit A., Hallock H., Valenzuela M. Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11:e1001756. doi: 10.1371/journal.pmed.1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]