Abstract
The possibility of a gender-related difference in recovery after spinal cord injury (SCI) remains a controversial subject. Current empirical animal research lacks sizable test groups to definitively determine whether significant differences exist. Evaluating locomotor recovery variances between sexes following a precise, clinically relevant spinal cord contusion model can provide valuable insight into a possible gender-related advantage in outcome post-SCI. In the current study, we hypothesized that by employing larger sample sizes in a reproducible contusive SCI paradigm, subtle distinctions in locomotor recovery between sexes, if they exist, would be elucidated through a broad range of behavioral tests. During 13 weeks of functional assessment after a thoracic (T8) contusive SCI in rat, significant differences owing to gender existed for the Basso, Beattie, and Bresnahan score and CatWalk hindlimb swing, support four, and single stance analyses. Significant differences in locomotor performance were noticeable as early as 4 weeks post-SCI. Stereological tissue-volume analysis determined that females, more so than males, also exhibited greater volumes of preserved gray and white matter within the injured cord segment as well as more spared ventral white matter area at the center of the lesion. The stereological tissue analysis differences favoring females directly correlated with the female rats' greater functional improvement observed at endpoint.
Key words: : BBB, CatWalk, gender, GridWalk, spinal cord injury
Introduction
The significant functional loss associated with traumatic spinal cord injury (SCI) currently affects more than 250,000 chronically injured people in the United States—80.6% of whom are males.1 Current acute injury management in the clinic is inadequate without a clinically approved therapeutic agent proven to be effective in restoring locomotor function once function has been lost,2 thus the identification of therapeutic targets3 and the development of neuroprotective and reparative agents in experimental paradigms remain a primary focus in SCI treatment.4
Subsequent to the initial mechanical insult, a phase of secondary damage can persist for hours or even weeks post-SCI and extends tissue and functional loss—it is this phase of injury that is amenable to neuroprotective interventions. Secondary injury occurs in response to the release of cytotoxic metabolites, proteases, and oxidative species,5,6 ensuing mitochondrial dysfunction,7 immune cell activation,8 and the production of proinflammatory cytokines,9,10 as well as excitotoxic levels of neurotransmitters and calcium-induced neuronal cell death.11 Improved understanding of these secondary injury mechanisms has led to the identification of a number of promising neuroprotective therapeutics in experimental SCI paradigms that remain to be validated in the clinical setting.4
Numerous studies have reported that, across species, the sex hormones estrogen, progesterone, and testosterone can limit tissue damage and improve functional outcome post-SCI or following other types of nervous system injury.12–29 The beneficial effects of progesterone appear to occur through a reduction of inflammation and microglial cell activation, the prevention of cell swelling and apoptosis, as well as the enhancement of oligodendrocyte maturation from oligodendrocyte precursor cells, myelin synthesis, and ensuing remyelination repair.19 Thus, progesterone likely behaves as a glioactive factor by favoring remyelination and inhibiting reactive gliosis. Estrogen at supra- and normal physiological levels has also been shown to be neuroprotective post-SCI,14,16,30 though a lack of tissue preservation and functional improvement with estrogen supplementation post-SCI has been observed in one study.31 Mechanisms by which estrogen is thought to be beneficial postinjury include an alteration of mitochondrial Ca2+ loading and the maintenance of mitochondrial membrane potential under cellular stress, as well as its positive regulation of antiapoptotic protein expression and localization.32 Estrogen protects against glutamate excitotoxicity by increasing B-cell lymphoma 2 (bcl-2) expression and allowing neurons to sequester increased concentrations of cytosolic Ca2+,33 whereas 17beta-estradiol has been reported to confer neuroprotection in male rats after transient global ischemia by blocking cytochrome c translocation from the mitochondria to the cytosol during the early stages of neuronal death.28 As with the female sex hormones, the male sex hormone, testosterone, is also thought to have neuroprotective effects.26,34 Recent work has provided evidence that testosterone can protect motor neurons from death post-SCI25,35,36 and is neuroprotective in neurodegenerative disorders, such as Alzheimer's disease.37,38 Owing to the central role that both male and female sex hormones appear to play in mechanisms of cell death and neurodegeneration, it is not readily apparent whether there would be a gender-based advantage in functional recovery post-SCI. Bramlett and Dietrich demonstrated that female rats (both proestrous and nonproestrous) have significantly smaller cortical contusion sizes after traumatic brain injury, compared to males, and suggested that endogenous circulating hormones were involved.39 These results imply that, after SCI, another form of traumatic central nervous system injury, a similar gender-based effect may occur.
In humans, previous studies analyzing individuals with SCI have not been definitive as to whether gender differences in outcome exist. Reports have shown that there are no gender-related differences in the analysis of Functional Independence Measure motor scores, motor efficiencies, or daily changes between acute care admission or rehabilitation discharge,40 and that there is no correlation of gender with neurological outcome after acute traumatic cervical SCI.41 Conversely, other reports have shown that females have greater functional motor improvement than males with incomplete SCI,42 and that gender-related differences in nontraumatic SCI recovery exist in elderly patients, favoring women.43 Additionally, Sipski and colleagues concluded, in their study, that “women may have more natural neurologic recovery than men; however, for a given level and degree of neurologic injury, men tend to do better functionally than women at time of discharge from rehabilitation.”44 Finally, a recent report concluded that more research is needed to determine whether gender should be considered a predictor for neurological and functional recovery post-SCI owing to a lack of class I or II study data.45
Given the absence of conclusive evidence, it remains an important question as to whether there exists a gender-based difference in functional recovery post-SCI. Experimental SCI models, which enact a reproducible and clinically relevant injury, provide a paradigm that can properly address this question. To date, experimental studies employing both male and female cohorts have used smaller sample sizes and experienced conflicting results for whether gender differences in SCI locomotor recovery and neuroprotection exist.23,31,46–49 There have also been no published studies investigating whether body-weight differences could account for subtle differences in locomotor recovery post-SCI in animal models. Further, it has been shown that male rats tend to be less active than females.50 Different forms of activity, such as physical training51 or exercise,52 are known to enhance locomotor recovery post-SCI and alter injury pathology by reducing inflammation,52 enhancing the production of growth factors,52–55 and suppressing cell apoptosis.56
This study was part of a larger investigation examining functional recovery after varying doses of Schwann cells, a cellular therapeutic, to treat SCI. The differences between doses were expected to be subtle. Thus, the study was powered to detect these subtle differences. The findings of differences between males and females that received only a SCI with no cellular therapeutic was not the overall goal of the study, but was a discovery of interest made through careful observation, data collecting, and analysis. Thus, although not initially planned for, the present work provides significant evidence that female rats have improved locomotor recovery and more preservation of gray (GM) and white matter (WM) at the site of the injury post-SCI than males. The exact cause of this effect, whether it is the result of differences in sex hormones, physical activity, or some other unidentified gender related mechanism, is currently unknown and warrants further investigation.
Methods
Animals
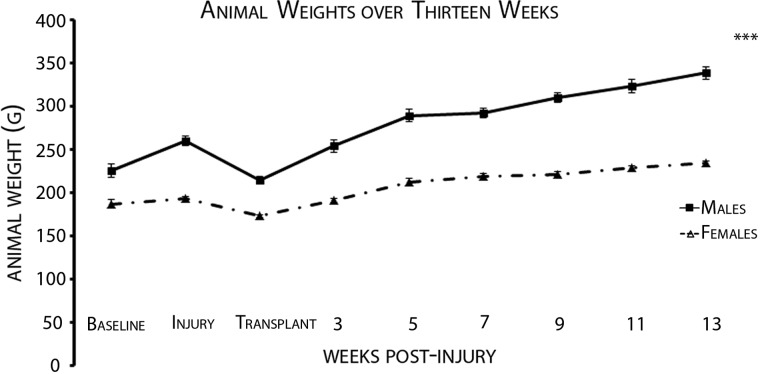
A total of 59 Fischer rats (male, n=26, and female, n=33; Harlan Laboratories, Inc., Frederick, MD) were included in the study. All animals were first acclimatized to handling and behavioral testing equipment and then were evaluated for baseline function. Subsequently, they received moderate (12.5-mm) contusion injuries followed by locomotor recovery assessment every week post-SCI using the Basso, Beattie, and Bresnahan (BBB) open-field locomotor test and every other week using CatWalk and GridWalk analysis until endpoint (13 weeks post-SCI). Rats were housed in pairs in the animal facility and maintained under conditions of constant temperature and humidity on a 12-h light/dark cycle. Mean age and weight for males and females at the time of injury was 14.22 weeks and 260.36 g for males and 18.02 weeks and 193.14 g for females. Temporal weight recordings of animals through the course of the study are shown in Figure 1.
FIG. 1.
Male rats are significantly heavier than female rats throughout the study. This study considers the weight of the animal to be a covariate with gender given that it may interfere with the locomotor measures. This figure shows the progression of the animal weights throughout the study. Error bars indicate standard error of the mean. A repeated-measures analysis of variance was done on these data and showed that the difference in weights throughout the study was significant. The three asterisks indicate p<0.001.
Preoperative preparation
Adult female and male Fischer rats were housed according to National Institutes of Health guidelines and the Guide for the Care and Use of Animals. All animal procedures were approved by the University of Miami Miller School of Medicine Institutional Animal Care and Use Committee (Miami, FL). Before surgical procedures, animals were weighed and anesthetized with a mixture of 2% isoflurane and 30% oxygen. An adequate level of anesthesia was determined by monitoring the corneal and hindlimb withdrawal reflexes. The backs were then shaved and aseptically prepared with chlorhexidine (Phoenix Pharmaceutical Inc., St. Joseph, MO). Lacrilube ophthalmic ointment (Allergan Pharmaceuticals, Irvine, CA) was applied to the eyes to prevent drying. Throughout surgery, rats were kept on a homeothermic blanket system (Harvard Apparatus Ltd., Kent, UK) to maintain body temperature at 37±0.5°C as assessed by a rectal probe.
Moderate thoracic spinal cord contusion injury
Rats were subjected to a moderate contusion injury using the MASCIS impactor.57 A laminectomy at thoracic vertebra T8 exposed the dorsal surface of the spinal cord underneath without disrupting the dura mater. Stabilization clamps were placed around the vertebrae at T7 and T9 to support the column during impact. The exposed spinal cord was moderately injured by dropping a 10.0-g rod from a height of 12.5 mm. The contusion impact height, velocity, and compression were monitored. Animals were excluded immediately when height or velocity errors exceeded 7% or if the compression distance was not within the range of 1.25–1.75 mm. A total of 6 animals (2 males and 4 females) were immediately excluded from the study because of poor impact parameters. An additional 2 male rats were also excluded from the study because of either a bladder rupture at 7 days post-SCI or pre-endpoint death at 14 days post-SCI. After injury, muscles were sutured in layers and the skin closed with metal wound clips.
Postoperative care
Rats were allowed to recover in a warmed cage with water and food easily accessible. Gentamicin (5 mg/kg, intramuscular; Abbott Laboratories, North Chicago, IL) was administered immediately postsurgery and then daily for 7 days. The analgesic, Buprenex (0.03 mg/kg, subcutaneous [s.c.]; Reckitt Benckiser, Richmond, VA), was delivered postsurgery and daily for 2 days. Lactated Ringers (5 cc, s.c.) was given twice a day for 7 days, or longer if needed. Bladders were manually expressed by gentle abdominopelvic compression twice-daily (Crede's method) until bladder function returned. There was no difference in bladder care between male and female rats. Signs of hematuria or bladder infection (either blood in urine or cloudy urine) were observed in 86.3% of males and 79.3% of females initially post-SCI. Using a Pearson's chi-square test, this difference was not statistically significant, and the condition resolved quickly over time. Animals had access to food and water ad libitum. Animals were caged in pairs with Alpha Dri® bedding (changed three times a week) and were provided water bottles with long curved sipper tubes.
Behavioral testing
BBB and BBB subscore
Hindlimb motor function was assessed using the open-field (BBB) scale,58 a test developed to assess deficits and recovery after thoracic contusion injuries. Hindlimb function was scored from 0 (no observable movements) to 21 (normal locomotion). Individual rats were placed in a metal elliptical enclosure, allowed to accommodate to the enclosure, and then observed for 4 min by two trained observers. This test was performed weekly for the 13-week duration of the experiment.
A BBB subscore analysis was performed as previously described.59 For the BBB subscore, only animals that achieved consistent weight support were analyzed.60 BBB subscores were assigned while monitoring performance during the BBB test. Subscores were assigned as follows: paw position: parallel paw rotation at initial contact (1 point/limb) and liftoff (1 point/limb); toe clearance: occasional clearance (<50% of the time, 1 point/limb), frequent clearance (51–95%, 2 points/limb), consistent clearance (>95%, 3 points/limb); tail position: down (0 point), alternating (1 point), up (2 points); and trunk stability (1 point). The cumulative scores for each hindlimb and tail position were summed to yield a single score (maximum of 13 points per rat). The BBB subscore was calculated from the weekly BBB test scoring forms for the 13-week duration of the experiment.
GridWalk test
For the GridWalk behavior test, deficits in descending fine motor control were examined by assessing the ability of the animals to navigate across a 1-m-long runway enclosed in a clear Plexiglass chamber with irregularly assigned gaps (0.5–5.0 cm) between round metal bars, as described previously.61 Pretraining procedures included acclimating the rats to the GridWalk by giving them treats, having low lights, talking to them in low tones, petting them, and having them walk across the grid for two runs for 6 min for 4 days. Rats were also trained to follow the scents of treats, Q-tips, and water bottles. Crossing the runway required that the animals accurately place their limbs on the bars. In baseline and postinjury testing, six crossings were analyzed. The number of footfall errors (where the hindlimb failed to grasp a bar and instead fell between them) was counted in each crossing, and a mean error rate was calculated and expressed as a percentage of the total hindlimb steps. This test was performed preinjury (baseline) and then every other week, starting at 3 weeks postinjury, for the 13-week duration of the experiment.
CatWalk gait analysis
Gait was analyzed during overground locomotion using the CatWalk device (Noldus Information Technology Inc., Leesburg, VA),62 where the walking patterns of all four limbs were filmed from underneath while the animal crossed an enclosed walkway. The walkway had a glass floor in which light was shone from one of the long edges. Paw-floor contact showed up brightly, whereas the rest of the floor appeared dark. A good run was determined when a rat took three or more consecutive steps without stopping while crossing the walkway, and the average of all the good runs per animal were then analyzed. Using the CatWalk software (v10.1), paw prints were labeled and 176 different parameters were analyzed for baseline and at endpoint. Thereafter, 10 parameters of interest were longitudinally analyzed for each time point: base of support; stride length (forelimbs); stride length (hindlimbs); speed; regularity index; support diagonal; support fours; couplings of diagonal paw pair; single stance; and hindlimb swing. Rats that were not able to take weight-supported hindpaw steps (BBB <10) and instead dragged their torso across the walkway were excluded from the analysis of these 10 parameters and are summarized in Table 1. In performing the CatWalk, these animals relied upon their forelimbs for forward propulsion, dragging the rest of their body when traversing the walkway. This locomotor behavior made it difficult to accurately count the number of hindlimb steps. Therefore, in order to include all the animals in comparative CatWalk analysis (dragging and nondragging), two parameters were additionally chosen for examination: the ratio of intensity of forepaw to hindpaw prints and speed of the animal when crossing. These parameters could be consistently measured in all animals. Post-SCI, there is increase in the use of forelimbs to move across the runway, which should be detected as an increase in the pawprint intensity of the forelimbs to the hindlimbs on the glass floor when transversing the runway. Additionally, for dragging animals, given that the force of the dragged hindlimbs is dissipated over a larger surface area, the intensity of the hindlimb prints should be less than those of rats able to take weight-supported steps.
Table 1.
Comparison of the Number of Male and Female Animals That Were Unable to Take Weight-Supported Plantar Steps Post-SCI
| Time point after SCI (weeks) | Females | Males |
|---|---|---|
| 3 | 4 | 7 |
| 5 | 4 | 6 |
| 7 | 3 | 5 |
| 9 | 3 | 5 |
| 11 | 4 | 5 |
| 13 | 4 | 6 |
There were 2 males and 1 female that were unable to bear their weight 3 weeks after injury (the first CatWalk time point after SCI), but regained that ability in later weeks. Additionally, 1 male and 1 female rat, who were taking weight-supported steps post-SCI, eventually lost this ability. At endpoint, 6 males and 4 females were unable to take consistent weight-supported plantar steps.
Histology
At 13 weeks postinjury, all rats received i.p. injections of terminal anesthesia (100 mg/kg of ketamine and 10 mg/kg of xylazine) and transcardially perfused with cold phosphate-buffered saline, then 4% paraformaldehyde (0.1 M, pH 7.4) as previously described.63 The T6–T10 thoracic spinal cord segments (2 cm), which contained the complete lesion, were dissected and embedded in paraffin. Paraffin-embedded cord segments were cut into 10-μm-thick sagittal sections and used to examine histological staining. These thinner sections allowed better study of damaged versus normal-appearing tissue after staining. Every 10th sagittal section of the paraffin-embedded T6–T10 segment (i.e., every 100-μm interval spanning the width of the spinal cord) was mounted onto slides, stained with haematoxylin, eosin, and Luxol fast blue, dehydrated, and cover-slipped in Pro-Texx mounting medium (Baxter Diagnostics, Deerfield, IL) for histological analysis.
Estimation of volumes
Stained sections were used to quantify volumes of normal-appearing GM and WM within the spinal cord piece using computer-assisted microscopy and MBF Biosciences Stereo Investigator software (version 10; MicroBrightField, Inc., Williston, VT). Contours were traced using the 4× objective, and the 20× objective was used to help determine borders between healthy and injured tissue. Algorithms for computations of Z-stack volumes of the spinal cord piece were performed according to Neuroleucida Explorer 5.64 (MicroBrightField) using the three-dimensional enclosed volume contour calculation. In each section, the perimeter (contour) of the total spinal cord section, the normal-appearing GM, and normal-appearing WM were separately traced on live images. Normal-appearing GM was distinguished from damaged tissue by the presence of healthy neurons and normal cellular density (without the presence of numerous nuclei, which is indicative of immune cell infiltration). Normal-appearing WM was defined as being nonfragmented, filamentous, darkly blue stained (not pale blue), and lacking immune cell infiltration.64 Each traced section was logged into the software using serial section manager, which tracked the positions of each section within the 20-mm-long Z-stack to enable volume calculations using the NeuroExplorer algorithms. In addition to volume analysis, we estimated the lesion length as the distance between the most rostral and the most caudal extent of the lesion in the mid-section as well as the cross-sectional area of the ventral white matter (VWM) at the injury epicenter. The VWM contains important axonal tracts involved in the locomotion of the rat, as demonstrated in studies targeting specific transection of WM regions of the rat thoracic spinal cord.65
Statistical analysis
An analysis of covariance (ANCOVA), excluding baseline data, analyzed using SAS software (Version 9.2; SAS Institute Inc., Cary, NC), was applied to determine whether there was an overall gender effect in all outcome measures, with gender as the between-subjects factor, time point as the within-subjects factor, and age and weight at time of injury and baseline score as covariates. An ANCOVA should be used when a covariate, or “a secondary variable that can affect the relationship between the dependent variable and other independent variables of primary interests,” is present.66 Additionally, it is more appropriate to use an ANCOVA with the baseline score as an addtional covariate than performing an ANOVA on the percentage change from baseline.67 In this experiment, an ANCOVA was used to account for the covariates of age, weight, and baseline score when determining the effect of gender on recovery. Age and weight are considered covariates as well because they can change the relationship between the independent variable of gender and the behavioral measures (BBB, GridWalk, and CatWalk). For post-hoc analysis, Bonferroni's correction for multiple tests was applied. The results were then converted to a percentage change by using mean baseline scores. Linear correlations, using GraphPad Prism software (v. 6.05; GraphPad Software, Inc., La Jolla, CA), were also performed on the stereological data obtained from the male and female rats with their respective BBB score at endpoint. p values obtained were corrected to account for differing sample sizes. Results and figures are presented as means±standard error of the mean. Statistical significance was determined when the adjusted p<0.05 was found and indicated by an asterisk, p<0.01 is assigned two asterisks, and p<0.001 is assigned thee asterisks.
Results
BBB and BBB subscore analysis
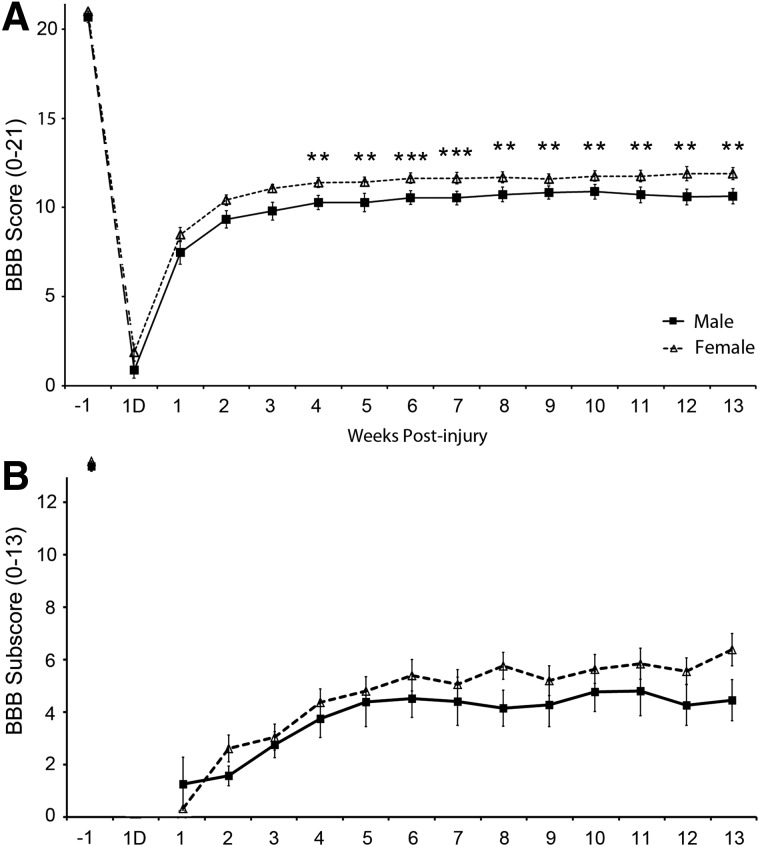
To investigate functional recovery in female rats versus male rats after SCI, gross locomotor performance in the open field was assessed weekly using the BBB score and subscore analysis (Fig. 2). By utilizing an ANCOVA with an adjusted Bonferroni's correction and baseline score, weight, and age as covariates, statistical comparison of the effects of gender on BBB scores showed a significant difference between genders starting at week 4 and remained significantly different at each time point thereafter. Values for each significant weekly time point were as follows: postinjury week 4 (F: 11.39±0.26 vs. M: 10.27±0.38; p=0.006); week 5 (F: 11.43±0.26 vs. M: 10.27±0.50; p=0.003); week 6 (F: 11.64±0.29 vs. M: 10.54±0.38; p=0.001); week 7 (F: 11.63±0.33 vs. M: 10.52±0.39; p=0.001); week 8 (F: 11.69±0.31 vs. M: 10.72±0.41; p=0 .008); week 9 (F: 11.60±0.29 vs. M: 10.34±0.61; p=0.018); week 10 (F: 11.76±0.28 vs. M: 10.88±0.41; p=0.014); week 11 (F: 11.75±0.32 vs. M: 10.70±0.45; p=0.006); week 12 (F: 11.90±0.41 vs. M: 10.59±0.44; p=0.004); and week 13 (F: 11.90±0.33 vs. M: 10.63±0.43; p=0.003). At endpoint, the proportion of females and males with a BBB score of at least 11 was 23 of 29 and 15 of 22, respectively.
FIG. 2.
Female rats show significantly higher locomotor performance in the open field post-SCI, compared to males. (A and B) Open-field locomotor performance was assessed weekly for 13 weeks post-SCI using the BBB scale (A) and BBB subscore analysis (B). Although transient increases in open-field locomotor performance on both the BBB scale and subscore with females was observed over males, at endpoint females exhibited significantly greater walking ability than males in the open field as evidenced by significantly higher values in both scores. An analysis of covariance with an adjusted Bonferroni's correction and baseline score, weight, and age as covariates was used to compare outcome means between males and females within a given week. Weeks that showed a significant gender difference are shown by an adjusted p value: *p<0.05; **p<0.01; ***p<0.001. At 1 day postinjury, all rats were excluded from BBB subscore analysis because none were taking weight-supported steps. BBB, Basso, Beattie, and Bresnahan.
BBB subscore analysis did not reveal an overall significant difference with gender between female and male rats by an ANCOVA analysis with age, weight, and baseline score as covariates (F=0.85; p=0.363). There were no significant differences with weight (F=0.18; p=0.674) or age (F=0.02; p=0.897) either. Additionally, following Bonferroni's correction, there were no significant differences in BBB subscore between genders in any individual weeks.
GridWalk testing
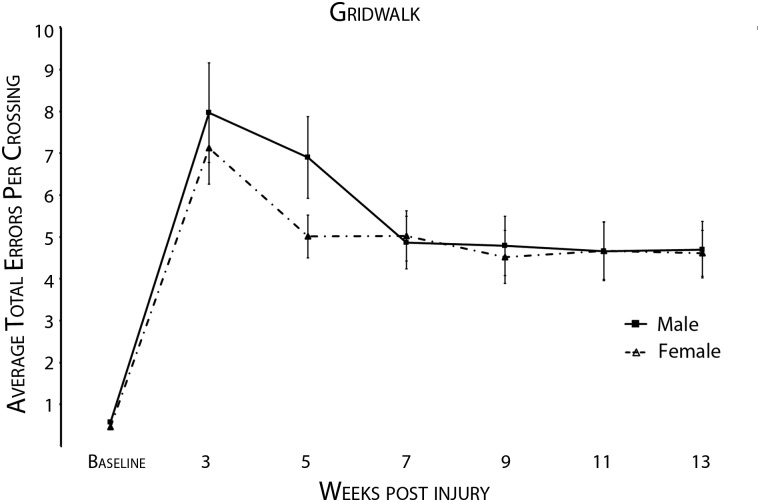
At baseline and at every other week starting at 3 weeks post-SCI, male and female rats were assessed for footfall hindlimb errors on the GridWalk test. Data were assessed by evaluating the total number of hindlimb errors per crossing (Fig. 3). Three male rats, but no female rats, were found dragging their torso across the runway, instead of taking weight-supported steps on the runs—they were excluded from analysis. Statistical comparison using an ANCOVA with an adjusted Bonferroni's correction and baseline score, weight, and age as covariates showed no significant difference of GridWalk hindlimb errors owing to gender (F=2.85; p=0.099), age (F=1.17; p=0.285), or weight (F=2.09; p=0.155). Additionally, there were no significant differences owing to gender in any individual week.
FIG. 3.
No gender improvement was observed on the GridWalk test after hindlimb total error analysis with animal exclusions. This graph shows the total number of hindlimb errors per crossing. GridWalk scores were recorded before injury and biweekly for 13 weeks post-SCI, beginning at 3 weeks postinjury. Three males who were not attempting to take any weight-supported hindlimb steps were excluded from this analysis. There were no weeks that showed a significant gender difference (adjusted p>0.05).
CatWalk gait analysis
At preinjury baseline and every other week starting at 3 weeks post-SCI, male and female rats were assessed for CatWalk gait analysis. Animals that were dragging their hindlimbs are listed in Table 1. Ten parameters of interest were studied longitudinally for the nondragging animals and then graphed as a percentage change to each respective baseline (Fig. 4). The number of animals analyzed for CatWalk each week postinjury of the 29 females and 22 males were: 3 weeks (21 females and 13 males); 5 weeks (20 females and 15 males); 7 weeks (25 females and 15 males); 9 weeks (26 females and 16 males); 11 weeks (25 females and 16 males); and 13 weeks (25 females and 15 males). For the CatWalk gait assessment, animals not able to take weight-supported steps are typically excluded (this same exclusion criteria applies to GridWalk and BBB subscore analysis). However, additional investigation that included all animals (dragging and nondragging) was conducted for two parameters: the animal's speed in crossing and the ratio of the intensity of forepaw to hindpaw prints (Fig. 5).
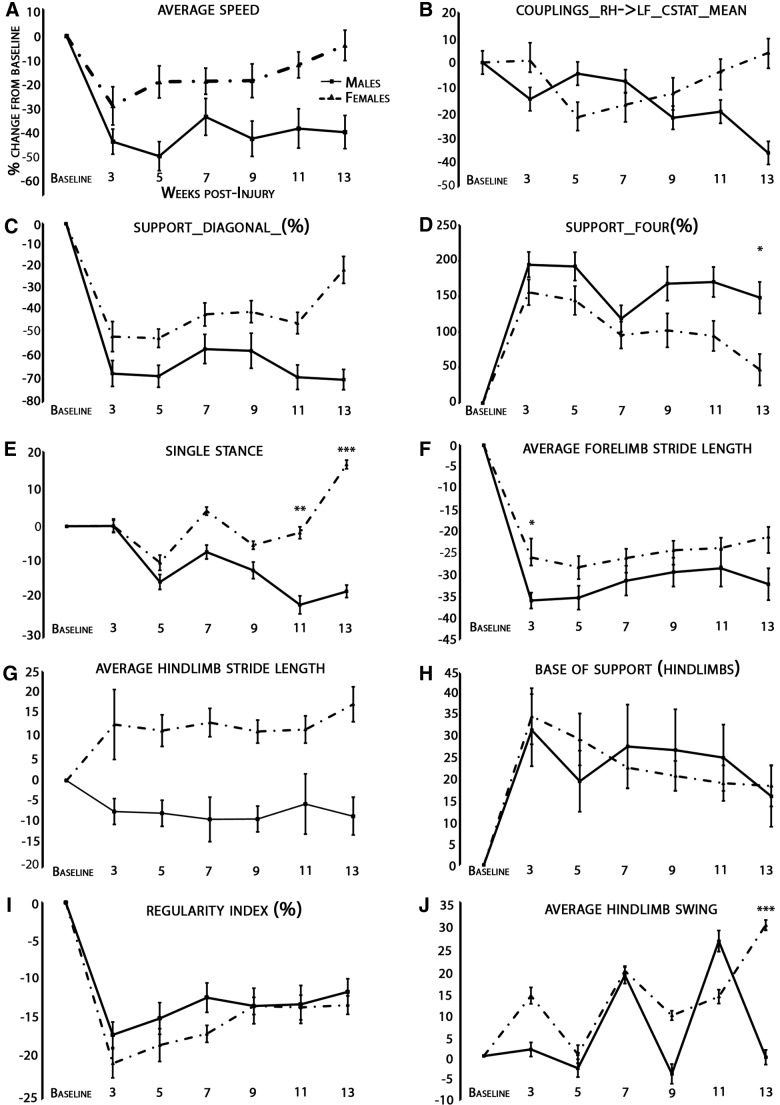
FIG. 4.
Female animals show a less injury-induced deficit than males in support four, hindlimb swing, and single stance at endpoint post-SCI. Temporal analysis of a variety of CatWalk parameters (as calculated according to a percent error of gender-specific baseline values), including speed (A), couplings of a diagonal paw pair (B), support diagonal % (C), support four % (D), single stance (E), forelimb stride length (F), hindlimb stride length (G), hindlimb base of support (H), regularity index % (I), and hindlimb swing (K), showed that females have significantly less injury-induced deficit in support four, hindlimb swing, and single stance at endpoint than males after analysis of covariance with Bonferroni's correction and age, weight, and baseline score as covariates. CatWalk analysis was performed before injury, 3 weeks postinjury, and biweekly for 13 weeks post-SCI. This analysis was performed after exclusion of rats that were dragging their hindlimbs. Weeks that showed a significant gender difference are shown by an adjusted p value: *p<0.05; **p<0.01; ***p<0.001.
FIG. 5.
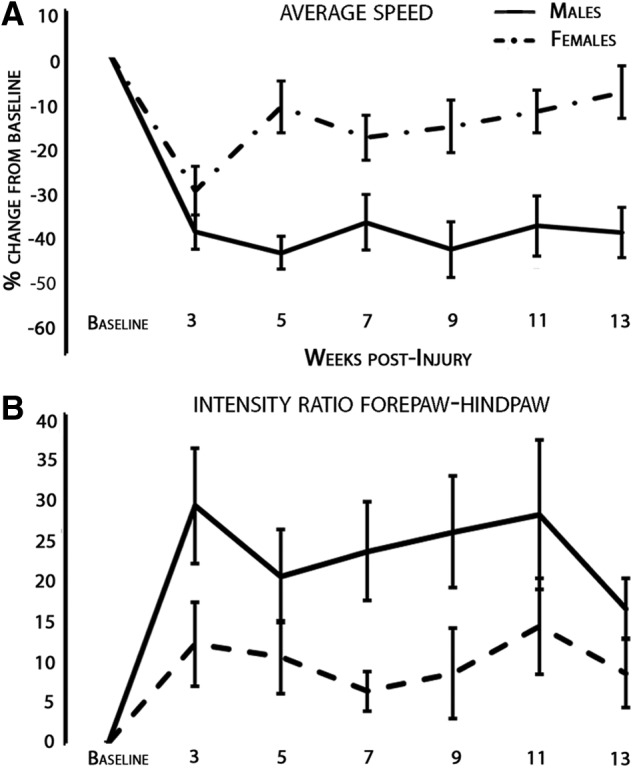
CatWalk analysis including rats that were dragging produced no significant difference owing to gender. CatWalk is unable to accurately count the hindlimb steps of animals that cannot take full weight-supported steps, and these animals are usually excluded from analysis. However, in an attempt to take these animals into consideration, the intensity ratio of forelimb to hindlimb steps and the speed of the animals were measured. The animals that could not take weight supported steps are listed in Table 1. The injury impacted both of these parameters as anticipated from baseline levels, but after analysis of covariance with Bonferroni's correction and age, weight, and baseline score as covariates there was no significant difference at any week for either parameter owing to gender (adjusted p>0.05).
Statistical comparison using an ANCOVA with Bonferroni's correction analyzing the difference of gender with age, weight, and baseline score as covariates over all the time points for only nondragging animals showed no significant difference between genders at any time point for average speed, support diagonal, forelimb stride length, couplings_RH->LF_Cstat_mean, hindlimb stride length, base of support, and the regularity index. Couplings_RH->LF_Cstat_Mean is a measure of the temporal relationship between placement of the right hind and left front paws using circular statistics (Cstat). The remaining parameters demonstrated the following significant differences. For hindlimb swing, there was a significant difference at weeks 9 (p=0.01) and 13 (p<0.001). For support four, there was a significant difference at week 13 (p=0.03). For single stance, there was a significant difference at weeks 11 (p=0.02) and 13 (p<0.001). These results are shown in Figure 4.
For the paramaters in which both dragging and nondragging animals were accounted for, there was no significant difference at any week for speed. When investigating the ratio of forepaw to hindpaw intensity, there was no significant difference owing to gender (p=0.35), nor was there any effect on this parameter owing to weight (p=0.525). These results are detailed in Figure 5.
Stereological assessment of injury length and tissue sparing
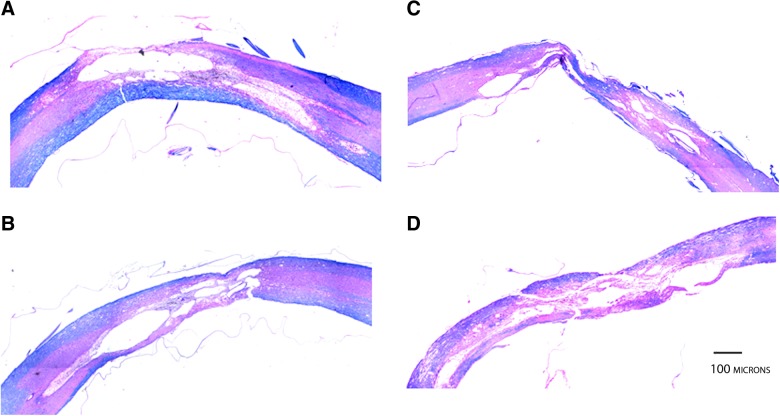
Contusion injuries in both male and female rats produced characteristic necrosis, gliosis, and cavitation at the injury epicenter. However, male SCI cords exhibited a decreased total tissue volume as a result of increased atrophy and/or cavitation expansion, as compared female SCI cords (Fig. 6).
FIG. 6.
Female rats have better tissue preservation at the injury epicenter. Representative haematoxylin, eosin, and Luxol fast blue staining of sagittal sections (10 um) from the contused spinal cords of female (A and B) and male (C and D) Fischer rats. Note the higher degree of gray and white matter preservation in females, in contrast to the significant tissue disruption and increased atrophy (C) and/or cavity expansion (D) observed in males. Color image is available online at www.liebertpub.com/neu
At 13 weeks postinjury, all animals were sacrificed and their cords were subjected to volumetric analysis (see Methods above). ANCOVA analysis for the effect of gender alone on total volume, preserved WM volume, preserved GM volume, and sparing of the VWM area at the lesion epicenter with age and weight as covariates resulted in gender having a significant effect on total volume (F=6.92; p=0.013), GM volume (F=11.21; p=0.002), VWM area sparing at the injury epicenter (F=5.33; p=0.025), and WM volume (F=5.77; p=.022), but not on the length of lesion extent (F=1.04; p=0.313), as noted by the data presented in Table 2 and images in Figure 6.
Table 2.
Comparison of Tissue Volume Analysis at Injury Epicenter and Length of Lesion Extent
| Females | Males | p value | |
|---|---|---|---|
| Total volume (mm3) | 21.8±1.2 | 16.8±2.0 | 0.013* |
| Preserved white matter (mm3) | 7.8±0.6 | 4.96±0.6 | 0.022* |
| Preserved gray matter (mm3) | 4.1±0.3 | 2.53±0.3 | 0.002** |
| Ventral white matter area (mm2) | 0.034±0.003 | 0.018±0.005 | 0.025* |
| Length of lesion (cm) | 0.95±0.005 | 1.22±0.006 | 0.313 |
Fischer rats were subjected to moderate spinal contusion by a weight drop (10.0 g) from a height of 12.5 mm, using the MASCIS impactor. After surviving 13 weeks postinjury and undergoing extensive behavioral analysis, rats were sacrificed and a length of 1.5-cm analyzed on spinal cord sections spanning the injury epicenter were histologically analyzed. Results are presented as means±standard error of the mean. An analysis of covariance, with age and weight as covariates, was performed on each measured factor to determine whether there was a significant difference owing to gender. A significant gender difference is shown by an adjusted p value: *p<0.05; **p<0.01.
Correlation assessment of injury length and tissue sparing with locomotor function
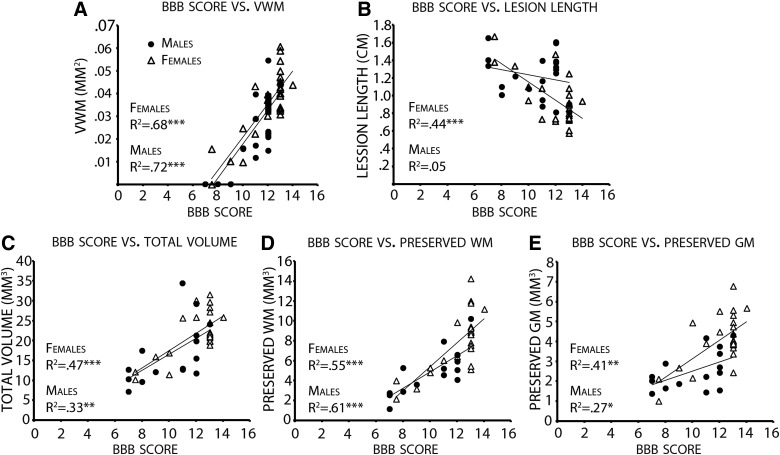
Using the stereological data obtained from the male and female rats combined, linear correlations with BBB score at endpoint were performed. The results of this analysis are reported in Figure 7. Strong and significant linear correlations existed between all histological parameters and BBB score at endpoint. The strongest correlation was with spared VWM area at the injury epicenter (R2=0.7), then preserved WM volume (R2=0.62), total volume (R2=0.47), and preserved GM volume (R2=0.47), and the weakest correlation was between BBB and lesion length (R2=0.3).
FIG. 7.
Linear correlation analysis shows that strong correlations exist between open-field locomotor performance and several stereological tissue analysis assessments. Linear correlation analysis was performed to determine whether correlations existed between tissue preservation and postinjury functional recovery (BBB score) at endpoint. Significant correlations for both males and female rats existed between spared VWM area (A), total volume (C), preserved WM volume (D), and preserved GM volume (E). For lesion length, there was a correlation for female rats, but not for male rats (B). The Pearson's product-moment correlation coefficient is included in each respective graph. VWM, ventral white matter; BBB, Basso, Beattie, and Bresnahan; WM, white matter; GM, gray matter.
Following correlation analysis of males and females individually, females presented results similar to the correlation analysis of male and female combined. For females, the strongest correlation was with spared VWM area at the injury epicenter (R2=0.68), then preserved WM volume (R2=0.55), total volume (R2=0.47), lesion length (R2=0.44), and then preserved GM volume (R2=0.41). For the male group, there was no correlation between BBB and lesion length (R2=0.05) and the strongest correlation was with spared VWM area at injury epicenter (R2=0.72). The correlations with GM (R2=0.27) and total volume (R2=0.33) were significant, but weak.
Discussion
Prior to this study, it was unclear whether a significant variance in functional recovery existed post-SCI between males and females. Through careful observation and detailed examination with an array of locomotor tests, our results show a significant difference in functional recovery and suggest distinctions in neuroprotection between male and female rats that have undergone thoracic contusive SCI. Significant differences in locomotor recovery, favoring females, were detected as early as 4 weeks post-SCI and remained significant at study endpoint, 13 weeks post-SCI. At endpoint, females also had more preserved WM and GM, a smaller length of the extent of the lesion, and more sparing of the VWM at the injury epicenter; these outcomes correlated strongly with locomotor function, as measured using the BBB scale.
Of the behavioral tests employed, the BBB scale and CatWalk hindlimb swing, support four, and single stance values showed a statistically significant difference in outcomes at endpoint when an overall gender effect was examined post-SCI. On the other hand, other CatWalk parameters, GridWalk, and BBB subscore showed no significant gender differences post-SCI. It is interesting that not all the tests were able to detect subtle, but significant, differences in locomotor performance, which signifies that either the former tests are more sensitive than the latter in detecting improved recovery post-SCI or that the type of improved locomotor recovery in females is owing to a preservation of neuronal circuits involved in motor strength and coordination, signifying that the protective effect is not tract specific and is more global in nature.
Further, in addition to performing CatWalk analysis on nondragging animals, we attempted to analyze CatWalk for all animals that were both dragging and not dragging. In the only previously published study comparing differences between male and female rats using CatWalk, Singh and colleagues excluded the animals that were dragging from their CatWalk analysis as well.49 To incorporate the dragging animals, we analyzed the speed of crossing and the ratio of intensity of forepaw to hindpaw prints for all animals. We postulated that postinjury, there would be an increase in the use of forelimbs to move across the runway, seen as an increase in intensity of the forepaws, compared to intensity of the hindlimbs, on the glass floor.
Both of the parameters used to detect differences in all animals (dragging and nondragging) significantly changed postinjury for both male and female rats from baseline levels. However, there were no significant differences in recovery owing to gender during any of the postinjury weeks. These analyses do highlight a limitation of the CatWalk analysis—the inability to include dragging with nondragging animals for analysis post-SCI. Nonetheless, perhaps by undertaking measures to increase the sensitivity of the CatWalk apparatus to detect intensity differences or by increasing the numbers of animals tested, significant differences would have become apparent, if they had existed.
Because there were more male rats that were unable to perform the CatWalk test than females owing to dragging (Table 1) and the performance of the male rats that were not dragging was worse than the nondragging females in all parameters tested (Fig. 4), it is our conclusion that the performance in CatWalk as well as the BBB scale (Fig. 2) show a gender-related effect in Fischer rats, favoring females over males, for an improved locomotor recovery after moderate thoracic contusive SCI. Additionally, because the histological correlations we performed also directly support this functional recovery (Fig. 7), there is a reasonable degree of certainty that this functional improvement is owing to a possible neuroprotective effect versus other plausible reasons for such a difference, such as a disparity in the overall activity of male and female rats or a behavioral effect unrelated to direct neuroprotection. However, activity is an important factor that future studies should take into account, which can be measured by placing subjects into an automated open-field test. This output could be used as another covariate in analysis, which could enhance the sensitivity of detecting significant differences.
Our review of the available literature on the effect of gender differences on neuroprotection and locomotor recovery post-SCI in animals did not find any analysis on the possible effects of age and weight disparities. In addition, the studies thus far included much smaller sample sizes and have produced conflicting results. Hauben and colleagues48 showed improved functional recovery of females over males in BBB score and the incline plane test post-SCI and histologically greater tissue preservation in Spague-Dawley rats after contusive SCI at T8 and in Balb/c mice after compressive SCI at T12. However, this behavioral analysis had only 4 animals per group and did not take differences in weight into consideration. Farooque and colleagues46 showed improved BBB score performance in C57BL/6 mice after compressive SCI at T10 in females over male mice post-SCI and more preserved spinal cord architecture with 9 and 10 mice per group. Swartz and colleagues31 found no improvement in BBB scoring between males and females, but found improved tissue sparing and length of injury favoring females with 6–8 animals per group. Ung and colleagues47 found no significant differences between males and females in locomotor recovery after T9/10 transection in mice with 11 mice per group. Fee and colleagues23 reported no behavioral or morphological differences between genders after moderate T10 contusion. In their study, different doses of progesterone were administered to both male and female Sprague-Dawley rats. However, their analysis of gender was pooled among all treated groups. There was no comparison of males and females, independent of treatment, that only received the vehicle in their study (dimethyl sulfoxide). Also, it is unclear how many animals were in the control groups, which could be 6 or less, and after examination of the graphed BBB performance of this group in their study, it appears that the female control groups fared better than the males. In the study by Singh and colleagues49 the effect of staircase training after contusive SCI in male and female Sprague-Dawley rats was investigated. Locomotor recovery was assessed using GridWalk, CatWalk, and BBB analysis. Their untreated groups that only received a SCI were 5 males and 6 females of weights 225–250 g. They state that there was no gender effect of any of these tests, so the male and female groups were pooled for analysis. To have the males and females to be the same weight, though, there would need to be a significant discrepancy in age, which was neither further discussed nor investigated in the article.
In this study, we found significant gender differences in functional outcome within the first 4 weeks post-SCI, suggestive of either an acute gender difference in secondary tissue damage or post-SCI plasticity. The only other study to investigate the effect of gender difference on locomotor recovery was by Singh and colleagues49 which utilized GridWalk, CatWalk, and BBB. Also, no previous studies reported the average ages and weights of male versus female rats, nor did they consider their role in the final analysis. Thus, the opposite conclusion that gender does not have an effect on locomotor recovery could likely have been inconsistent owing to not having a large enough sample size and disregarding the effects that age and weight could have had on their samples.
In light of the current study's finding that a gender-related difference in functional recovery exists post-SCI—upon analysis using various tests independent of body weight or age—it is now important to determine why such differences exist. Based upon the aforementioned acute post-SCI findings with estrogen in ovariectomized females31 and similar effects in TBI,39,68,69 we theorize that a likely possibility for these gender differences is the effect of female sex hormones, estrogen, and/or progesterone, which may have neuroprotective action14,16,27,30,70,71 at endogenous levels. The effects of these hormones on neurons may reduce neuronal cell death, axonal injury, and/or lead to a significant reduction of inflammation and a delay in neurodegeneration. Estrogen has also been shown to protect Schwann cells,72 the glial cells that support neuronal function in the peripheral nervous system (PNS). Normally found in the PNS, endogenous Schwann cells migrate in large numbers into the spinal cord postinjury.73 Surgical transplantation of Schwann cells into the site of the injury post-SCI has proven to be neuroprotective.63,74,75 Thus, enhancing the survival of endogenous, infiltrating Schwann cells and their ensuing ability to repair the injured spinal cord might, in part, explain the gender-related locomotor improvement in females observed in this study.
Therefore, given that females recover to a greater extent than males from traumatic SCI, it is possible that estrogen and/or progesterone may provide a neuroprotective advantage that is beneficial in recovery. Though the present study demonstrates that females recover from SCI more completely and rapidly than males, it neither supports nor refutes the possible neuroprotective action of testosterone shown in previous studies.25,35,36 However, from our study, one might conclude that the neuroprotective ability of estrogen and/or progesterone is greater than that of testosterone.
Differing levels of physical activity between male and female rats may also, in part, account for discrepancies in recovery between genders post-SCI. Although the present study does not regulate exercise regimen or physical activity among the different genders, it has been observed previously that females are more physically active than males.50 Forced physical activity by treadmill running improves functional recovery from SCI through increased protection from secondary tissue degeneration.52 Improved neuroprotection can be attributed to a decrease in inflammatory response and an increase in neurotrophic factors, such as insulin growth factor, glial-cell–derived neurotrophic factor, and brain-derived neurotrophic factor (BDNF), after exercise.52,54 The neurotrophic factor, BDNF, particularly, is important in both spontaneous and exercise-induced recovery.55 Also, benefits to neuronal plasticity, by up-regulation of gene and protein expression, is especially responsive to both voluntary and forced exercise.52–54,76 In further examining the associations between physical activity, BDNF, and locomotor recovery, studies have demonstrated that BDNF regulates molecules involved in synaptic plasticity, such as synapsin I and cyclic adenosine monophosphate–response-element binding protein, as well as neurotrophin 3 messenger RNA levels.54,55,76 Therefore, the improved recovery among females, compared to males, in our study could result from improved neuroprotection and wound-repair processes from enhanced levels of physical activity. To address increased activity as a possible cause for motor recovery favoring females, future studies accounting for discrepancies in physical activity and BDNF levels between genders are needed.
It is also possible that this gender-based phenomenon post-SCI is either not related to, or only partly explained by, the role of female sex hormones. As previously discussed, Swartz and colleagues31 did not find estrogen-dependent protection after contusive SCI, although a non-estrogen-dependent difference favoring female rats over males in the length of injury and the percentage of spared tissue at the site of injury was observed. Examination of the functional recovery patterns of ovariectomized females or male rats receiving estrogen and/or progesterone in the same SCI paradigm (with high animal numbers per group as used in the current study) would provide important data for concluding whether sex hormones play a role in the observed gender-related difference in functional recovery post-SCI.
For human SCI, the interpretation of results of gender-related differences in recovery has been inconclusive, with one study indicating there is no significant difference in recovery across genders,40 another claiming an improvement that was not significant,41 with other reports presenting a gender effect that favors females.42,43 Owing to the lack of available statistics to generalize this gender comparison, it is necessary to continue to study the roles of gender and gender-related factor(s) in human SCI recovery. It is challenging to compare and generalize gender effects on recovery in humans post-SCI because the complexities of the injury vary between individuals in severity, cause, and location. Additionally, the patient population at the time of SCI also has different known and unknown comorbidities and lifestyles, including medications, diet, alcohol, smoking, drug, and recreational habits. These differences may overshadow gender-related differences and make it difficult to determine whether a gender-related difference in outcome after SCI exists clinically. Being that these factors can all be controlled for in experimental animal models, such models are ideal for gathering data for gender-based effects on recovery owing to the ability to enact a reproducible injury in sufficient numbers for statistical comparison. Our study has provided some important supporting evidence that a female advantage in outcome post-SCI does exist. Future studies should look to identify the putative gender-related factor(s) involved in SCI recovery. Not only could these factor(s) be used for the purpose of enhancing recovery in males, but they could also further improve locomotor recovery in females.
In conclusion, the present work provides evidence that females have a gender-related advantage in functional recovery and, possibly even neuroprotection, after thoracic, contusive SCI in the adult rat. The effects, though subtle, are significant and persistent. Although previously published SCI studies with rodents have shown conflicting results, our study suggests that there are indeed locomotor recovery differences between genders. Unlike earlier studies, ours used much larger sample sizes and an array of behavioral tests as well as tissue analysis. This allowed us to detect more subtle and specific differences in locomotor performance than previously explored, and our study also accounted for other factors that could affect performance, including age and body weight, which previous studies did not investigate. Whether the gender-related advantage in females observed in this SCI model is owing to sex hormones or another sex-related cause or factor, such as activity, remains to be determined.
Acknowledgments
The authors thank Denise Koivisto, Eva Juarez, Alex Basagoitia, Ronald Zambrano, Miguel Martinez, and Ramon German for help with animal care; Paulo Diaz for surgical assistance; Carlos Concepcion, Layne Keathly, Isabel Lee, Jingwen Yang, and Vivien Chen for critical review and proofreading of the manuscript; Dr. Richard Morris for guidance and help with statistical analysis; Lindsay Connor for data analysis; and Leah Colucci and Hanna Mathers for data organization, quality assurance review of the CatWalk data, and proofreading of the manuscript. This research was supported by The Department of Defense Congressionally Directed Medical Research Programs SCIRP (Award No. W81XWH-10-1-0793), The Miami Project to Cure Paralysis, and The Buoniconti Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Devivo M. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 2.Rabchevsky A., Patel S., and Springer J. (2011). Pharmacological interventions for spinal cord injury: where do we stand? How might we step forward? Pharmacol. Ther. 132, 15–29 [DOI] [PubMed] [Google Scholar]
- 3.Kwon B., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L., Fehlings M., Tetzlaff W. (2011). A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samantaray S., Thakore N., Matzelle D., Varma A., Ray S., and Banik N. (2010). Neuroprotective drugs in traumatic CNS injury. Open Drug Discov. J. 2, 174–180 [Google Scholar]
- 5.Antunes F., Cadenas E. (2001). Cellular titration of apoptosis with steady state concentrations of H(2)O(2): submicromolar levels of H(2)O(2) induce apoptosis through Fenton chemistry independent of the cellular thiol state. Free Radic. Biol. Med. 30, 1008–1018 [DOI] [PubMed] [Google Scholar]
- 6.Pearse D., Chatzipanteli K., Marcillo A., Bunge M., and Dietrich W. (2003). Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J. Neuropathol. Exp. Neurol. 62, 1096–1107 [DOI] [PubMed] [Google Scholar]
- 7.Barut S., Canbolat A., Bilge T., Aydin Y., Cokneseli B., and Kaya U. (1993). Lipid peroxidation in experimental spinal cord injury: time-level relationship. Neurosurg. Rev. 16, 53–59 [DOI] [PubMed] [Google Scholar]
- 8.Carlson S., Parrish M., Springer J., Doty K., and Dossett L. (1998). Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 151, 77–88 [DOI] [PubMed] [Google Scholar]
- 9.Benowitz L., and Popovich P. (2011). Inflammation and axon regeneration. Curr. Opin. Neurol. 24, 577–583 [DOI] [PubMed] [Google Scholar]
- 10.Pearse D., and Jarnagin K. (2010). Abating progressive tissue injury and preserving function after CNS trauma: the role of inflammation modulatory therapies. Curr. Opin. Investig. Drugs 1, 1207–1210 [PubMed] [Google Scholar]
- 11.Park E., Velumian A., and Fehlings M. (2004). The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 21, 754–774 [DOI] [PubMed] [Google Scholar]
- 12.Stein D., and Hoffman S. (2003). Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. J. Pediatr. Rehabil. Med. 6, 13–22 [DOI] [PubMed] [Google Scholar]
- 13.Sribnick E., Wingrave J., Matzelle D., Ray S., and Banik N. (2003). Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann. N. Y. Acad. Sci. 993, 125–133 [DOI] [PubMed] [Google Scholar]
- 14.Sribnick E., Samantaray S., Das A., Smith J., Matzelle D., Ray S., and Banik N. (2010). Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 88, 1738–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samantaray S., Smith J., Das A., Matzelle D., Varma A., Ray S., and Banik N. (2011). Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem. Res. 36, 1809–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samantaray S., Matzelle D., Ray S., and Banik N. (2010). Physiological low dose of estrogen-protected neurons in experimental spinal cord injury. Ann. N. Y. Acad. Sci. 1199, 86–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrovska S., Dejanova B., and Jurisic V. (2012). Estrogens: mechanisms of neuroprotective effects. J. Physiol. Biochem. 68, 455–460 [DOI] [PubMed] [Google Scholar]
- 18.Thomas A., Nockels R., Pan H., Shaffrey C., and Chopp M. (1999). Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine 24, 2134–2138 [DOI] [PubMed] [Google Scholar]
- 19.Schumacher M., Guennoun R., Stein D., and De Nicola A. (2007). Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol. Ther. 116, 77–106 [DOI] [PubMed] [Google Scholar]
- 20.Labombarda F., Gonzalez S., Lima A., Roig P., Guennoun R., Schumacher M., and De Nicola A. (2009). Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia 57, 884–897 [DOI] [PubMed] [Google Scholar]
- 21.Labombarda F., Gonzalez S., Gonzalez D., Guennoun R., Schumacher M., and De Nicola A. (2002). Cellular basis for progesterone neuroprotection in the injured spinal cord. J. Neurotrauma 19, 343–355 [DOI] [PubMed] [Google Scholar]
- 22.Labombarda F., Gonzalez S., Lima A., Roig P., Guennoun R., Schumacher M., and De Nicola A. (2011). Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp. Neurol. 231, 135–146 [DOI] [PubMed] [Google Scholar]
- 23.Fee D., Swartz K., Joy K., Roberts K., Scheff N., and Scheff S. (2007). Effects of progesterone on experimental spinal cord injury. Brain Res. 1137, 146–152 [DOI] [PubMed] [Google Scholar]
- 24.Fargo K., Foster A., and Sengelaub D. (2009). Neuroprotective effect of testosterone treatment on motoneuron recruitment following the death of nearby motoneurons. Dev. Neurobiol. 69, 825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byers J., Huguenard A., Kuruppu D., Liu N., Xu X., and Sengelaub D. (2012). Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurobiol. 520, 2683–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bialek M., Zaremba P., Borowicz K., and Czuczwar S. (2004). Neuroprotective role of testosterone in the nervous system. Pol. J. Pharmacol. 56, 509–518 [PubMed] [Google Scholar]
- 27.Stein D., Wright D., and Kellermann A. (2008). Does progesterone have neuroprotective properties?. Ann. Emerg. Med. 51,164–172 [DOI] [PubMed] [Google Scholar]
- 28.Bagetta G., Chiappetta O., Amantea D., Iannone M., Rotiroti D., Costa A., Nappi G., and Corasaniti M. (2004). Estradiol reduces cytochrome c translocation and minimizes hippocampal damage caused by transient global ischemia in rat. Neurosci. Lett. 368, 87–91 [DOI] [PubMed] [Google Scholar]
- 29.Elkabes S., and Nicot A. (2014). Sex steroids and neuroprotection in spinal cord injury: a review of preclinical investigations. Exp. Neurol. 259, 28–37 [DOI] [PubMed] [Google Scholar]
- 30.Samantaray S., Sribnick E., Das A., Thakore N., Matzelle D., Yu S., Ray S., Wei L., and Banik N. (2010). Neuroprotective efficacy of estrogen in experimental spinal cord injury in rats. Ann. N. Y. Acad. Sci. 1199, 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartz K., Fee D., Joy K., Roberts K., Sun S., Scheff N., Wilson M., and Scheff S. (2007). Gender differences in spinal cord injury are not estrogen-dependent. J. Neurotrauma 24, 473–480 [DOI] [PubMed] [Google Scholar]
- 32.Singh M., Dykens J., and Simpkins J. (2006). Novel mechanisms for estrogen-induced neuroprotection. Exp. Biol. Med. (Maywood) 231, 514–521 [DOI] [PubMed] [Google Scholar]
- 33.Nilsen J., and Diaz Brinton R. (2003). Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc. Natl. Acad. Sci. U. S. A. 100, 2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammond J., Le Q., Goodyer C., Gelfand M., Trifiro M., and LeBlanc A. (2001). Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 77, 1319–1326 [DOI] [PubMed] [Google Scholar]
- 35.Tehranipour M., and Moghimi A. (2010). Neuroprotective effects of testosterone on regenerating spinal cord motoneurons in rats. J. Mot. Behav. 42, 151–155 [DOI] [PubMed] [Google Scholar]
- 36.Little C., Coons K., and Sengelaub D. (2009). Neuroprotective effects of testosterone on the morphology and function of somatic motoneurons following the death of neighboring motoneurons. J. Comp. Neurol. 512, 359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAllister C., Long J., Bowers A., Walker A., Cao P., Honda S., Harada N., Staufenbiel M., Shen Y., and Li R. (2010). Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J. Neurosci. 30, 7326–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morinaga A., Ono K., Takasaki J., Ikeda T., Hirohata M., and Yamada M. (2011). Effects of sex hormones on Alzheimer's disease-associated beta-amyloid oligomer formation in vitro. Exp. Neurol. 228, 298–302 [DOI] [PubMed] [Google Scholar]
- 39.Bramlett H., and Dietrich W. (2001). Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J. Neurotrauma 18, 891–900 [DOI] [PubMed] [Google Scholar]
- 40.Greenwald B., Seel R., Cifu D., and Shah A. (2001). Gender-related differences in acute rehabilitation lengths of stay, charges, and functional outcomes for a matched sample with spinal cord injury: a multicenter investigation. Arch. Phys. Med. Rehabil. 82, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 41.Furlan J., Krassioukov A., and Fehlings M. (2005). The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J. Neurotrauma 22, 368–381 [DOI] [PubMed] [Google Scholar]
- 42.Bracken M., Shepard M., Hellenbrand K., Collins W., Leo L., Freeman D., Wagner F., Flamm E., Eisenberg H., and Goodman J. (1985). Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J. Neurosurg. 63, 704–713 [DOI] [PubMed] [Google Scholar]
- 43.Kay E., Deutsch A., Chen D., Manheim L., and Rowles D. (2010). Effects of etiology on inpatient rehabilitation outcomes in 65- to 74-year-old patients with incomplete paraplegia from a nontraumatic spinal cord injury. PM R. 2, 504–513 [DOI] [PubMed] [Google Scholar]
- 44.Sipski M., Jackson A., Gomez-Marin O., Estores I., and Stein A. (2004). Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1826–1836 [DOI] [PubMed] [Google Scholar]
- 45.Al-Habib A., Attabib N., Ball J., Bajammal S., Casha S., and Hurlbert R. (2011). Clinical predictors of recovery after blunt spinal cord trauma: systematic review. J. Neurotrauma 28, 1431–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farooque M., Suo Z., Arnold P., Wulser M., Chou C., Vancura R., Fowler S., and Festoff B. (2006). Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord 44, 182–187 [DOI] [PubMed] [Google Scholar]
- 47.Ung R., Lapointe N., Tremblay C., Larouche A., and Guertin P. (2007). Spontaneous recovery of hindlimb movement in completely spinal cord transected mice: a comparison of assessment methods and conditions. Spinal Cord 45, 367–379 [DOI] [PubMed] [Google Scholar]
- 48.Hauben E., Mizrahi T., Agranov E., and Schwartz M. (2002). Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur. J. Neurosci. 16, 1731–1740 [DOI] [PubMed] [Google Scholar]
- 49.Singh A., Murray M., and Houle J. (2011). A training paradigm to enhance motor recovery in contused rats: effects of staircase training. Neurorehabil. Neural Repair 25, 24–34 [DOI] [PubMed] [Google Scholar]
- 50.Belviranli M., Atalik K., Okudan N., and Gokbel H. (2012). Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test. Neuroscience 227, 1–9 [DOI] [PubMed] [Google Scholar]
- 51.Battistuzzo C., Callister R.J., Callister R., and Galea M. (2012). A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J. Neurotrauma 29, 1600–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrade M., Mendonca L., and Chadi G. (2010). Treadmill running protects spinal cord contusion from secondary degeneration. Brain Res. 1346, 266–278 [DOI] [PubMed] [Google Scholar]
- 53.Tajiri N., Yasuhara T., Shingo T., Kondo A., Yuan W., Kadota T., Wang F., Baba T., Tayra J., Morimoto T., Jing M., Kikuchi Y., Kuramoto S., Agari T., Miyoshi Y., Fujino H., Obata F., Takeda I., Furuta T., and Date I. (2010). Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 1310, 200–207 [DOI] [PubMed] [Google Scholar]
- 54.Vaynman S., and Gomez-Pinilla F. (2005). License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil. Neural Repair 19, 283–295 [DOI] [PubMed] [Google Scholar]
- 55.Ying Z., Roy R., Zhong H., Zdunowski S., Edgerton V., and Gomez-Pinilla F. (2008). BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 155, 1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung S., Kim D., Yune T., Shin D., Baek S., and Kim C. (2014). Treadmill exercise reduces spinal cord injury-induced apoptosis by activating the PI3K/Akt pathway in rats. Exp. Ther. Med. 7, 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gruner J. (1992). A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 9, 123–128 [DOI] [PubMed] [Google Scholar]
- 58.Basso D., Beattie M., and Bresnahan J. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 [DOI] [PubMed] [Google Scholar]
- 59.Pearse D., Sanchez A., Pereira F., Andrade C., Puzis R., Pressman Y., Golden K., Kitay B., Blits B., Wood P., and Bunge M. (2007). Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: survival, migration, axon association, and functional recovery. Glia 55, 976–1000 [DOI] [PubMed] [Google Scholar]
- 60.Basso D., Fisher L., Anderson A., Jakeman L., McTigue D., and Popovich P. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 61.Metz G., Merkler D., Dietz V., Schwab M., and Fouad K. (2000). Efficient testing of motor function in spinal cord injured rats. Brain Res. 883, 165–177 [DOI] [PubMed] [Google Scholar]
- 62.Hamers F., Lankhorst A., Van Laar T., Veldhuis W., and Gispen W. (2001). Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J. Neurotrauma 18, 187–201 [DOI] [PubMed] [Google Scholar]
- 63.Takami T., Oudega M., Bates M., Wood P., Kleitman N., and Bunge M. (2002). Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 22, 6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearse D., Lo T., Jr., Cho K., Lynch M., Garg M., Marcillo A., Sanchez A., Cruz Y., and Dietrich W. (2005). Histopathological and behavioral characterization of a novel cervical spinal cord displacement contusion injury in the rat. J. Neurotrauma 22, 680–702 [DOI] [PubMed] [Google Scholar]
- 65.Schucht P., Raineteau O., Schwab M., and Fouad K. (2002). Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 176, 143–153 [DOI] [PubMed] [Google Scholar]
- 66.Everitt B. (2002). The Cambridge Dictionary of Statistics (2nd ed.). Cambridge University Press: Cambridge, UK [Google Scholar]
- 67.Vickers A. (2001). The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med. Res. Methodol. 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kasturi B., and Stein D. (2009). Progesterone decreases cortical and sub-cortical edema in young and aged ovariectomized rats with brain injury. Restor. Neurol. Neurosci. 27, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O'Connor C., Cernak I., Johnson F., and Vink R. (2007). Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp. Neurol. 205, 145–153 [DOI] [PubMed] [Google Scholar]
- 70.Gonzalez S., Labombarda F., Deniselle M., Mougel A., Guennoun R., Schumacher M., and De Nicola A. (2005). Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J. Steroid Biochem. Mol. Biol. 94, 143–149 [DOI] [PubMed] [Google Scholar]
- 71.Roof R., and Hall E. (2000). Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma 2000,17, 367–388 [DOI] [PubMed] [Google Scholar]
- 72.Siriphorn A., Chompoopong S., and Floyd C. (2010). 17β-estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J. Neurochem. 115, 864–872 [DOI] [PubMed] [Google Scholar]
- 73.Beattie M., Bresnahan J., Komon J., Tovar C., Van Meter M., Anderson D., Faden A., Hsu C., Noble L., Salzman S., and Young W. (1997). Endogenous repair after spinal cord contusion injuries in the rat. Exp. Neurol. 148, 453–463 [DOI] [PubMed] [Google Scholar]
- 74.Pearse D., Pereira F., Marcillo A., Bates M., Berrocal Y., Filbin M., and Bunge M. (2004). cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 10, 610–616 [DOI] [PubMed] [Google Scholar]
- 75.Schaal S., Kitay B., Cho K., Lo T., Jr., Barakat D., Marcillo A., Sanchez A., Andrade C., and Pearse D. (2007). Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant. 16, 207–228 [DOI] [PubMed] [Google Scholar]
- 76.Perreau V., Adlard P., Anderson A., and Cotman C. (2005). Exercise-induced gene expression changes in the rat spinal cord. Gene Expr. 12, 107–121 [DOI] [PMC free article] [PubMed] [Google Scholar]