Abstract
Objectives: The aim of this study was to investigate whether a fibrinogen biomatrix improves the transplantation effectiveness of induced pluripotent stem cells (iPSCs) in a model of myocardial infarction.
Background: Early retention, engraftment, and cell proliferation are important factors for successful cardiac stem cell therapy. Common transplantation techniques involve the direction injection of cells in aqueous media. However, this approach yields low retention and variable cell biodistribution, leading to reduced grafts that are unable to sufficiently regenerate damaged myocardium. Biologically compatible scaffolds that improve the retention of injected cells can improve cardiac stem cell therapy.
Methods: Murine iPSCs were transfected for luciferase reporter gene expression. First, in vitro experiments were performed comparing cell viability in fibrinogen and medium. Second, iPSCs were transplanted intramyocardially by direct injection into ischemic myocardium of immunodeficient mice, following permanent left coronary artery ligation. Cells were delivered in medium or fibrinogen. Follow-up included graft assessment by bioluminescence imaging, the evaluation of cardiac function by magnetic resonance imaging, and histology to evaluate graft size and determine the extent of myocardial scarring.
Results: In vitro experiments showed proliferation of iPSCs in fibrinogen from 6.4×103±8.0×102 after 24 h to 2.1×104±3.2×103 after 72 h. Early cardiac cell amount in control group animals was low (23.7%±0.7%) with massive cell accumulation in the right (46.3%±1.0%) and the left lung (30.0%±0.6%). When iPSCs were injected applying the fibrinogen biomatrix, intramyocardial cell amount was increased (66.3%±0.9%) with demonstrable graft proliferation over the experimental time course. Left ventricle-function was higher in the fibrinogen group (42.9%±2.8%), also showing a higher fraction of refilled infarcted-area (66.9%±2.7%).
Conclusions: The fibrinogen biomatrix improved cardiac iPSc retention, sustaining functional improvement and cellular refill of infarcted myocardium. Therefore, fibrinogen can be considered an ideal biological scaffold for intramyocardial stem cell transplantations.
Introduction
Coronary artery disease is a progressive pathology with high morbidity and mortality rates worldwide.1 Current projections estimate that by 2020, more than 7 million people will die due to cardiovascular disease.2 After myocardial infarction, the limited ability of self-regeneration often leads to irreversible heart failure.3 Despite great advances in medical treatment,4,5 terminal heart failure can only be treated by cardiac transplantation or with ventricular assist device (VAD) therapy.6,7 In the context of a shortage of donor organs and limited long-term experience with VAD-support, stem cell transplantation poses a promising alternative for the treatment of ischemic cardiomyopathy.8–10 A common aspect in many stem cell therapy studies is the injection of stem cells suspended in aqueous media.11–16 Although this technique results in viable grafts, it has been demonstrated that it entails massive early cell loss by different mechanisms, including venous drainage from the injection site to the right atrium leading to pulmonary graft accumulation.17 Several studies have shown that cardiac cell retention is lower than 10% immediately after injection.17–22 Thus, it could be argued that this loss of cells hinders the chances of success of cell therapy before the cell phenotype has the possibility to influence restoration. Consequently, several groups have been working on improving cellular retention by using special injection scaffolds or tissue-engineered constructs.23–32 Although fixing stem cell constructs to the surface of the infarcted myocardium can help to avoid cell discharge, it cannot regenerate the infarcted area. Therefore, direct intramyocardial injection is an important delivery technique since the infarction (or its border zone) can be targeted directly, generating high local tissue concentrations.22 In this context, considering massive early cell loss by this technique, studies focused on improving retention of injected cells are required. For this purpose, biocompatible scaffolds provide an excellent medium to transport and retain stem cells in the myocardium. Some biological scaffolds can contribute additionally to myocardial restoration, with positive effects for graft viability and paracrine properties.27 Among different types of materials, fibrinogen stands out due to its advanced clinical translation. As it is part of the intrinsic pathway of blood coagulation, it is widely used in its polymerized state as fibrin glue for hemostatic therapy in cardiac surgery. Previous studies have investigated the effect of fibrin glue as a scaffold for the intramyocardial injection of skeletal myoblasts in infarcted rat hearts.31,32 These studies revealed that the combination of fibrin and skeletal myoblasts resulted in a two-fold higher level of stem cell grafts within the infarction when compared to controls. Other groups applied fibrin as biogel to inject stem cell-derived cardiomyocyte sheets,33 cardiac progenitor cells,34 and adipose-derived mesenchymal stem cells.35 However, fibrin is difficult to apply as a polymer in small animal models, where needles with small diameters are used. Its rigid state also reduces nutrient exchange between host myocardium and transplanted stem cells, hampering graft viability and cell proliferation. Therefore, fibrinogen presents a promising alternative as it is viscous enough to retain cells, but liquid enough to guarantee graft nutrition once transplanted. Thus, the main hypothesis of our work was that using fibrinogen as biomatrix would improve retention and biodistribution of intramyocardial injected induced pluripotent stem cells (iPSCs) following myocardial infarction.
Materials and Methods
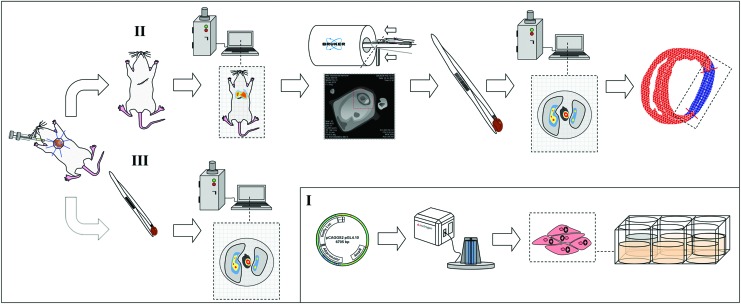
An overview of the experimental design is shown in Figure 1.
FIG. 1.
Experimental study design. (I) Murine induced pluripotent stem cells (iPSCs) were transfected for firefly Luciferase gene expression. (II, III) One million iPSCs were intramyocardially injected in fibrinogen following myocardial infarction. The first experimental group (III) was evaluated 10 min after injection to investigate early cell retention and biodistribution. Remaining animals (II) were kept in follow-up for 14 days to investigate iPSC viability over the experimental time course and to perform functional assessment by magnetic resonance imaging (MRI) and histology. Color images available online at www.liebertpub.com/tea
Optically bioluminescent miPS cells
Undifferentiated murine iPSCs were maintained in culture as previously described.36 Stable clones were generated by electroporative transfection (Neon™ Transfection System; Invitrogen) with a plasmid carrying a chicken-beta-actin promotor driving the expression of firefly luciferase reporter gene (fluc) and an ampicillin resistance (Fig. 1-I). The culture medium was composed of Dulbecco's modified Eagles medium (Invitrogen) supplemented with 15% fetal calf serum (Thermo Scientific), 0.2 mM l-glutamine (Invitrogen), 0.1 mM β-mercaptoethanol (Invitrogen), 0.1 mM nonessential amino acid stock (Invitrogen), and 0.1% human leukemia inhibitory factor conditioned medium. Genetically modified cells were selected by antibiotic resistance and the brightest clone was chosen for experiments.
In vitro evaluation of cell growth and viability
In vitro iPSC assays for cell viability (n=3) (live/dead cell staining kit II, Promokine; PromoCell) and proliferation (n=3) (WST-8 assay, Promokine; PromoCell) were performed on 3 consecutive days. To differentiate living from dead cells, 3×104 iPSCs were seeded on a 24-well plate. Well chambers were assigned to three groups: fibrinogen, fibrin, and medium. In each group, chambers were assigned for the respective time point at which measurements were performed: 24, 48, and 72 h. At each time point, cells were stained: green, living cells; red, dead cells. Following microscopy, representative areas of live and dead cells were measured using ImageJ (v. 1.48 for Mac OS X).
In vitro cell proliferation analysis was performed applying the WST-8 assay (Promokine; PromoCell). A 96-well plate was arranged for cell culture with three different groups: fibrin, fibrinogen and medium. Every group also included noncellular controls. In each well chamber 5×103 iPSCs were seeded and brought into culture. Separately, standard dilutions of iPSCs ranging from 2.5×103 to 1×104 cells were assembled at each time point for cell quantification. Quantification was performed using a microplate reader (Paradigm Detection Platform; Beckman Coulter) at 24, 48, and 72 h applying the WST-8 substrate.
Animal care
All surgical interventions and animal care were provided following the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, volume 25, no 28, revised 1996) and in accordance with institutional and federal regulations.
Injections
For each injection, 1×106 iPSCs were resuspended in 15 μL fibrinogen (Tissuecol Duo S; Baxter). Controls received 15 μL of medium. Injections were performed using a microsyringe with a 33G needle.
Murine myocardial infarction model and injections
For this study, a total of 38 immunodeficient SCID bg mice (16–21 g, 8–10 weeks; Charles River Laboratories) were used. Myocardial infarction was induced in all animals by permanent ligation of the left anterior descending coronary artery, as previously described.37 First, aliquots of 1×106 iPSCs were injected (n=20, 10 fibrinogen vs. 10 medium). After 10 min of beating heart action, bioluminescence imaging (BLI) was performed with closed thorax. Afterward, organs (heart and lungs) were explanted and again analyzed using BLI for cell quantification (Fig. 1-III). In the second group (n=18, 9 fibrinogen vs. 9 medium), 1×106 iPSCs were injected intramyocardially. Following chest closure, animals were kept in follow-up for 14 days. Follow-up examinations included BLI at different time points and magnetic resonance imaging (MRI). Animals were euthanized at day 14. Organs were explanted for graft quantification with BLI. Finally, histological assessment of explanted heart was performed to investigate graft size and infarct extension.
Bioluminescence imaging
The hearts and lungs of all animals were harvested and imaged with the IVIS Lumina II (Caliper Lifesciences) for BLI signal detection. In the 10-min groups, cell aliquots were resuspended in 5 μL of beetle luciferin (Promega) before injection for signal induction. In the follow-up-group, 100 μL of substrate was intraperitonealy injected and cells were quantified in explanted organs when peak bioluminescence signal was reached. Cell quantification of iPSC in explanted organs was calculated by linear correlation of the bioluminescence signal with the respective signals of in vitro standard dilutions containing known iPSC concentrations, as previously described.17
Magnetic resonance imaging
Small animal MRI was performed using a 7-Tesla scanner (Pharmascan 70/16 Bruker BioSpin). In the follow-up group, animals were anesthetized and scanned on day 14 to assess left ventricular ejection fraction (LV-EF). Cine FLASH-sequences (5–10 short axis slides) were captured and LV-EF was measured applying Simpson's method.
Histology
Hearts were processed following standard protocols for cryosectioning and histochemistry. Masson's trichrome staining was applied at 300 μm intervals to delineate cell allocation in line with infarct anatomy, as previously described.38 Infarct and graft extension were measured using ImageJ software (v. 1.48 for Mac OS X).
Statistics
GraphPad Prism 5.03 was used for statistical analysis. Data are expressed as the mean±standard error. For statistical analysis, we performed two-way ANOVA. Differences were considered significant at p<0.05. All reported p-values are two-sided.
Results
In vitro cell viability assays
Live cell staining
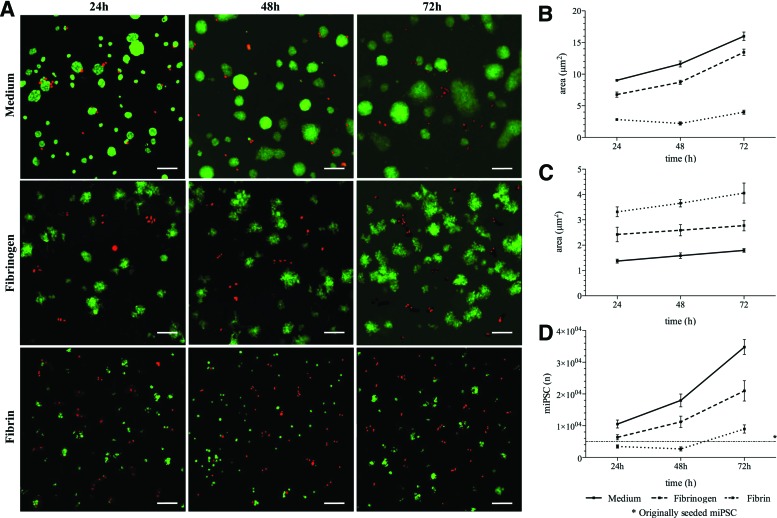
An overview of results is shown in Figure 2A and B. Cells cultivated in fibrin showed an area of 2.8±0.1 μm2 after 24 h, 2.2±0.3 μm2 after 48 h, and 4.0±0.3 μm2 after 72 h. The fibrinogen group showed an area of 6.8±0.4 μm2 at 24 h. After 48 and 72 h, the living cell area increased to 8.7±0.3 and 13.5±0.5 μm2, respectively. The medium group showed an area of 9.0±0.2 μm2 after 24 h. After 48 and 72 h, the living cell area was 11.6±0.5 and 16.0±0.7 μm2. Differences between both groups were not significant.
FIG. 2.
In vitro iPSC viability in fibrinogen. (A) iPSCs were kept in culture for 72 h in fibrinogen, fibrin, and medium. Living cells are marked in green, whereas red spots show dead cell colonies. The area of fibrinogen-cultured cells showed good cell survival (B) with small dead areas (C). (D) The cell proliferation assay also showed strong proliferation rates in the fibrinogen group. Differences between both groups were not considered significant. Color images available online at www.liebertpub.com/tea
Dead cell staining
An overview of results is shown in Figure 2A and C. Cells cultivated in fibrin showed an area of 3.3±0.2 μm2 after 24 h, 3.7±0.2 μm2 after 48 h and 4.1±0.4 μm2 after 72 h. The fibrinogen group showed an area of 2.5±0.3 μm2 at 24 h. After 48 and 72 h, the dead cell area was 2.6±0.2 and 2.8±0.2 μm2, respectively. The medium group showed an area of 1.4±0.1 μm2 after 24 h. After 48 and 72 h, dead cell staining showed areas of 1.6±0.1 and 1.8±0.1 μm2. Differences between both groups were not significant.
Cell proliferation assay
Results are graphically shown in Figure 2D. Cells cultivated in fibrin were calculated as 3.4×103±5.0×102 after 24 h, 2.7×103±6.3×102 after 48 h, and 8.9×103±1.3×103 after 72 h. The fibrinogen group showed 6.4×103±8.0×102 cells at 24 h. After 48 and 72 h, the cell numbers increased to 1.1×104±1.8×103 and 2.1×104±3.2×103, respectively. The medium group showed 1.0×104±1.2×103 cells after 24 h. After 48 and 72 h, cell amounts were calculated as 1.8×104±2.0×103 and 3.5×104±2.3×103. Differences between both groups were not significant.
Cardiac retention and biodistribution of iPSCs after 10 min
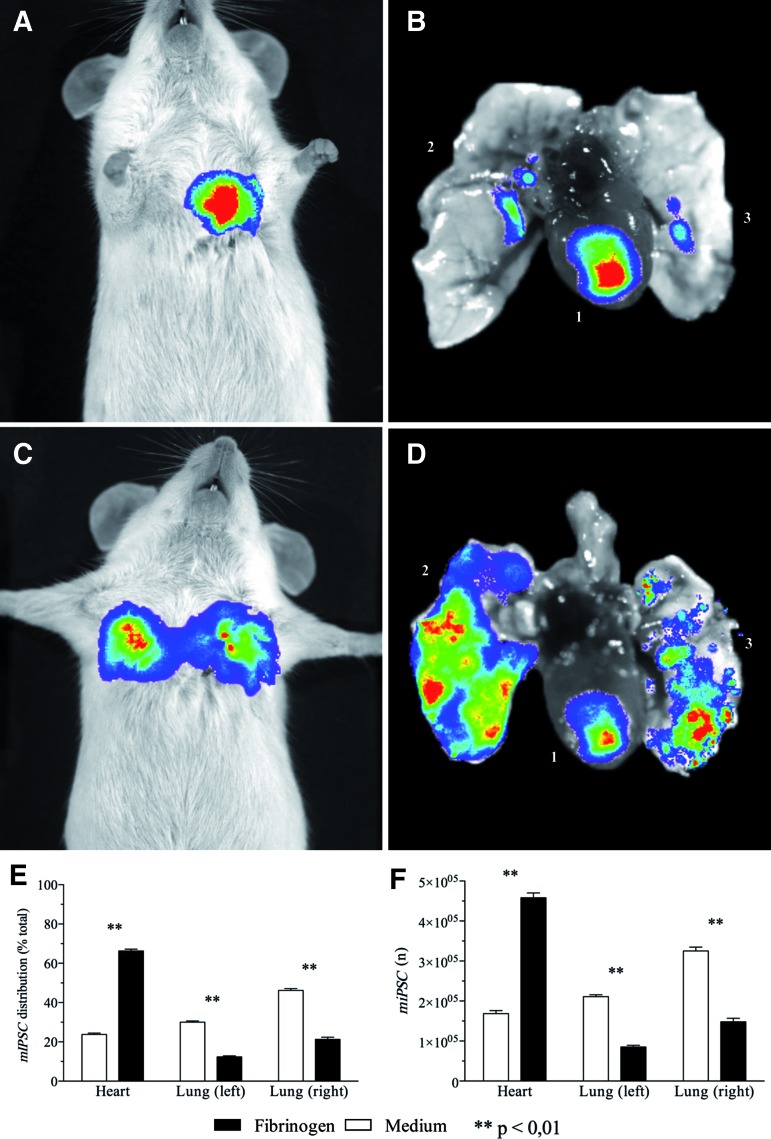
An overview of all results is shown in Figure 3. After 10 min of beating heart action, calculated cardiac retention in the medium control group was 1.7×105±7.8×103 cells. Analysis of the lungs showed cell distribution of 3.3×105±9.4×103 to the right lung and 2.1×105±4.6×103 cells to the left lung. The fibrinogen group had a cardiac retention of 4.6×105±1.2×104 cells. Analysis of the lungs showed cell distribution to the right lung (1.5×105±8.9×103 cells) and to the left lung (4.3×104±1.8×103 cells). Differences between means of each individual organ signal between both groups were significant (p<0.01).
FIG. 3.
Early biodistribution of injected iPSCs. (A) Fibrinogen-treated animals showed a rather focused thoracic bioluminescence imaging (BLI) signal, with most of injected iPSCs retained in the heart (B; 1, heart; 2, right lung; 3, left lung). (C) In the medium group, thoracic projection of BLI signal suggests lung biodistribution of injected iPSCs. (D) BLI assessment of organ explants unveiled massive iPSC accumulation in the right and the left lung. (E) Comparison between both groups showed that, in the fibrinogen group, more than 60% of total cells were retained in the heart, whereas in the medium group only 22% of all cells could be found in the heart. (F) Calculation of absolute iPSC amounts also showed significant higher number of intramyocardial iPSCs with lower lung concentrations of injected iPSCs in the fibrinogen group compared with medium controls. Color images available online at www.liebertpub.com/tea
Bioluminescence signal of iPSCs in time course (14 days follow-up)
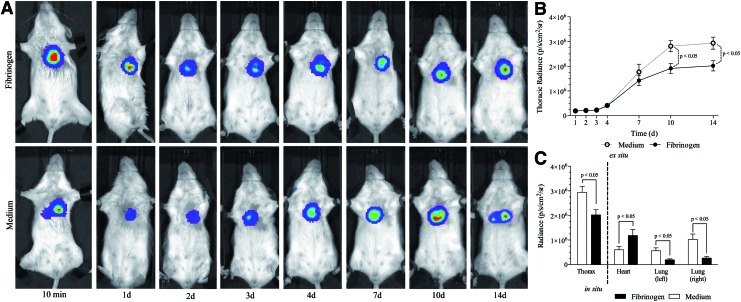
In this experimental setting, iPSCs were injected in fibrinogen or medium. After chest closure, thoracic BLI signals were assessed at different time points for a period of 14 days (Fig. 4A). An overview of the absolute radiance values of both groups is shown in Table 1. Figure 4A shows an exemplary trend of BLI signals in both groups during follow-up. A graphical plot of BLI signal development is shown in Figure 4B. After day 7, differences between group means became statistically significant (p<0.05).
FIG. 4.
Follow-up of iPSCs following intramyocardial transplantation. (A) Example of thoracic BLI signals over the time course (14 days) following intramyocardial transplantation with and without the fibrinogen biomatrix. (B) Comparison between both groups shows a first constant course of BLI signals according to the engraftment time required. After 72 h, total mean BLI signals began to rise consequently with significant higher mean radiances in the medium group. (C) Although overall BLI signals were higher in medium-treated animals, the analysis of organ explants showed significantly higher cardiac concentrations in the fibrinogen group and high pulmonary iPSC concentrations in the medium group. Color images available online at www.liebertpub.com/tea
Table 1.
Time Course of Mean Thoracic Bioluminescence Imaging Signals of fLuc Expressing Induced Pluripotent Stem Cells Following Intramyocardial Transplantation (Fibrinogen vs. Medium)
| 10 min | 1 day | 2 days | 3 days | 4 days | 7 days | 10 days | 14 days | |
|---|---|---|---|---|---|---|---|---|
| Fibrinogen,±SEM | 1.8×107±1.0×106 | 8.2×105±5.0×104 | 8.8×105±4.5×105 | 1.1×106±3.8×104 | 1.9×106±3.8×104 | 5.8×106±4.1×105 | 7.9×106±5.0×105 | 1.0×107±5.9×105 |
| Medium,±SEM | 1.8×107±2.9×106 | 6.1×106±4.8×104 | 6.9×105±5.9×104 | 8.6×105±5.4×104 | 1.8×106±1.7×105 | 9.3×106±3.1×105 | 1.4×107±5.8×105 | 1.8×107±6.6×105 |
| p | NS | NS | NS | NS | NS | <0.05 | <0.001 | <0.001 |
Radiance values are given in (p/s/cm2/sr).
SEM, standard error of the mean.
On day 14, organs were explanted directly after measuring the thoracic BLI signal of all animals (Fig. 4C). The total mean thoracic signal was higher in the medium group (p<0.01). Following organ explantation, the mean cardiac BLI signal in the medium group was 6.1×105±4.0×104 (p/s/cm2/sr), whereas in the fibrinogen group a mean radiance of 1.2×106±8.0×104 (p/s/cm2/sr) was assessed for the hearts (p<0.01). The analysis of the left lungs showed a mean radiance of 5.7×105±7.7×104 (p/s/cm2/sr) in the medium group and 1.9×105±1.3×104 (p/s/cm2/sr) in the fibrinogen group (p<0.01). Examination of the right lungs unveiled a mean BLI signal of 1.0×106±6.8×104 (p/s/cm2/sr) in the medium group and 2.7×105±4.8×104 (p/s/cm2/sr) in fibrinogen-treated animals (p<0.01).
Histological assessment of cardiac graft extension
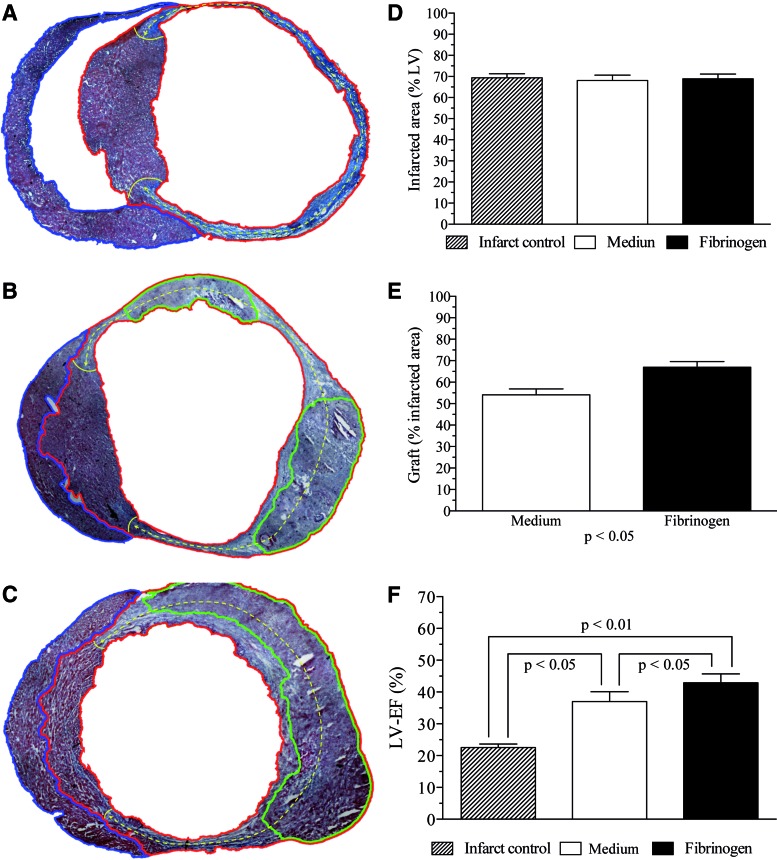
The mean infarct extension of the infarct control group (Fig. 5A) was 69.4%±2.0% of the whole LV circumference. In medium (Fig. 5B) and fibrinogen (Fig. 5C) groups, infarct extension was 68.1%±2.5% and 68.9%±2.3%, respectively. There was no statistically relevant difference regarding the infarct extensions between all groups (Fig. 5D). Furthermore, iPSC graft extension in the infarcted area was compared between medium and fibrinogen. Medium-treated animals had a mean graft extension of 54.2%±2.7%, whereas fibrinogen animals showed a graft extension of 67.0%±2.7% (p<0.05) (Fig. 5E).
FIG. 5.
iPSC graft extension and functional assessment of left ventricle (LV) function 14 days after intramyocardial transplantation of iPSCs in myocardial infarction. (A) Infarct extension (dashed yellow line) in the infarct-control group was quantified as percentage of the whole LV (red) circumference. Right ventricle is marked by the blue line. (B) Medium-treated animals showed several smaller grafts (marked in green) in the LV. (C) In the fibrinogen group grafts were bigger and confined to one part of the LV. (D) There was no difference regarding the extension of infarcted myocardium following experimental infarction by left anterior descending artery ligation. (E) Both cellular groups showed cardiac engraftment of iPSCs. However, graft size (% of the infarcted circumference of the LV) was superior in the fibrinogen group. (F) Both cellular groups showed improvement of the left ventricular ejection fraction (LV-EF), as measured by MRI on day 14. Functional improvement is related to ventricular geometry and correlates with the extent of refilled infarction. Color images available online at www.liebertpub.com/tea
Assessment of LV function by MRI
At day 14, before animals were sacrificed, LV-EF was measured by MRI (Fig. 5C). The infarct control group showed a mean LV-EF of 22.5%±1.1%. The mean LV-EF of the medium group was 37.0%±3.1%. Fibrinogen-treated animals showed a mean LV-EF of 42.9%±2.8%. Differences between groups were statistically significant (p<0.05).
Discussion
Cardiac stem cell treatment by intramyocardial injections is associated with poor and variable graft concentrations, especially when aqueous injection media are used for cell transfer. Previously, our group developed a BLI-based imaging method capable of monitoring cardiac injection efficacy, which unveiled substantial cardiac cell loss by venous drainage through the coronary sinus, culminating in an accumulation of iPSCs in the lungs.17 The aim of this study was to find an injectable biomaterial capable of enhancing cardiac stem cell retention, limiting biodistribution to the targeted cardiac area and enhancing graft survival. We chose iPSCs, a very promising regenerative cell source. In our experimental setting, cells were applied in an undifferentiated state. While for biodistribution analysis, potential teratoma formation is neglectable, further studies should involve iPSC-derived cardiomyocytes, which have more relevance regarding clinical translation. Moreover, generation and purification of iPSC-derived cardiomyocytes is already feasible and can be performed by antibiotic-resistance gene transfer and cell selection.39
First, we performed in vitro assays to investigate the effect of fibrinogen on iPSC viability and compared it to a fibrin control group. The cell survival, death, and proliferation assays showed that the less viscous fibrinogen was superior to fibrin in all tests. The considerable thicker fibrin possibly influenced negatively nutrition, cellular communication, and cell junctions.40 Fibrinogen is a plasma protein, which is converted by thrombin into fibrin during blood coagulation. It is a widely available autologous source, which does not provoke an immunogenic reaction and has positive effects on cell viability.41 Additionally, fibrinogen is already in use in the clinic, since it is commonly used in surgery as a component of fibrin glue for achieving hemostasis. First, we performed in vitro assays to investigate the effect of fibrinogen on iPSC viability and compared it to a medium control group. The in vitro assays showed that iPSCs could survive and proliferate in the fibrinogen matrix. However, in vitro results suggest that fibrinogen and fibrin take influence on proliferation and survival of seeded cells. Especially, more viscous fibrin showed lower cell viability. This phenomenon is probably explained by the matrix consistency that takes influence on nutrition, cellular communication, and cell junctions.40 However, by means of viscosity, fibrinogen can be adjusted according to its application. This is especially relevant for small animal models since viscosity limits the usage of microsyringes. When fibrinogen was applied as a biomatrix, intramyocardial retention of injected cells noticeably improved after 10 min beating heart action and in the follow-up group. Compared to the medium control group, cellular biodistribution to both lungs was also strongly reduced in fibrinogen-treated animals. In the follow-up experiments, overall thoracic BLI signals were compared between the medium and fibrinogen group. In the first 24 h, both groups showed a constant expression of the thoracic BLI signal, which coincides with the time needed for engraftment. After 72 h, BLI signal intensity began to rise in both groups, which is a clear sign of positive cell engraftment. Subsequently, total bioluminescence signals were higher in the medium group. However, analysis of explanted organs unveiled that all animals in the medium group showed a massive accumulation of proliferating iPSCs in the lungs. Thus, the overall amount of cells was higher in medium-treated animals. Nevertheless, fibrinogen-treated animals had considerably higher cardiac iPSC grafts with low lung accumulation of transplanted cells. This was also verified in the histological assessment, where the graft extension was also higher in fibrinogen-treated animals. Our study showed functional improvement in both cell-treated groups compared with the nontreated controls. The functional improvement was higher in the fibrinogen group and correlated with higher graft extensions. We did not see any evidence of new contractile myocardium. Moreover, when undifferentiated iPSCs are injected, teratoma formation is very likely.42 One possible explanation for functional improvement is by the change of LV geometry through cellular refill. By augmentation of left ventricular wall thickness in infarcted myocardium, left ventricular geometry is reconstructed. As shown in our study, fibrinogen-treated animals showed larger cell grafts leading to more refill of the infarcted area with significantly reduced infarct sizes.43,44
Conclusions
Our results show that fibrinogen is perfectly suited as a scaffold for cell-based cardiac repair. It improves retention and limits the biodistribution of iPSCs when injected in the early phase of myocardial infarction, when ischemia, inflammation, and other adverse physiological conditions are significant graft-limiting factors. Therefore, fibrinogen can be recommended as an injection vehicle for intramyocardial stem cell transplantations.
Acknowledgments
We would like to thank Nina McGuinness for review. This work was supported by the Integrated Research and Treatment Center Transplantation (IFB-Tx, Federal Ministry of Research, Germany), CORTISS Hannover GmbH the Cluster of Excellence REBIRTH (Deutsche Forschungsgemeinschaft).
Disclosure Statement
No competing financial interests exist.
References
- 1.Mathers C.D., Boerma T., and Ma Fat D. Global and regional causes of death. Br Med Bull 92, 7, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Mathers C.D., and Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3, e442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunwald E. Heart failure. JACC: Heart Fail 1, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J.V., Adamopoulos S., Anker S.D., Auricchio A., Böhm M., Dickstein K., Falk V., Filippatos G., Fonseca C., Gomez-Sanchez M.A., Jaarsma T., Køber L., Lip G.Y.H., Maggioni A.P., Parkhomenko A., Pieske B.M., Popescu B.A., Rønnevik P.K., Rutten F.H., Schwitter J., Seferovic P., Stepinska J., Trindade P.T., Voors A.A., Zannad F., Zeiher A.; ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33, 1787, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Nabel E.G., and Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med 366, 54, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Slaughter M.S., Rogers J.G., Milano C.A., Russell S.D., Conte J.V., Feldman D., Sun B., Tatooles A.J., Delgado R.M., Long J.W., Wozniak T.C., Ghumman W., Farrar D.J., Frazier O.H.; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 361, 2241, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Strueber M., O'Driscoll G., Jansz P., Khaghani A., Levy W.C., and Wieselthaler G.M. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol 57, 1375, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Laflamme M.A., and Murry C.E. Regenerating the heart. Nat Biotechnol 23, 845, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Murry C.E. Cell-based cardiac repair: reflections at the 10-year point. Circulation 112, 3174, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Passier R., Van Laake L.W., and Mummery C.L. Stem-cell-based therapy and lessons from the heart. Nature 453, 322, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Mauritz C., Martens A., Rojas S.V., Schnick T., Rathert C., Schecker N., Menke S., Glage S., Zweigerdt R., Haverich A., Martin U., and Kutschka I. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J 32, 2634, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Martens A., Gruh I., Dimitroulis D., Rojas S.V., Schmidt-Richter I., Rathert C., Khaladj N., Gawol A., Chikobava M.G., Martin U., Haverich A., and Kutschka I. Rhesus monkey cardiosphere-derived cells for myocardial restoration. Cytotherapy 13, 864, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Nelson T.J., Martinez-Fernandez A., Yamada S., Perez-Terzic C., Ikeda Y., and Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120, 408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Laake L.W., Passier R., Doevendans P.A., and Mummery C.L. Human embryonic stem cell-derived cardiomyocytes and cardiac repair in rodents. Circ Res 102, 1008, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Lee S.-T., White A.J., Matsushita S., Malliaras K., Steenbergen C., Zhang Y., Li T.-S., Terrovitis J., Yee K., Simsir S., Makkar R., and Marbán E. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J Am Coll Cardiol 57, 455, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Williams A.R., Hatzistergos K.E., Addicott B., McCall F., Carvalho D., Suncion V., Morales A.R., Da Silva J., Sussman M.A., Heldman A.W., and Hare J.M. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127, 213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens A., Rojas S.V., Baraki H., Rathert C., Schecker N., Zweigerdt R., Schwanke K., Rojas-Hernandez S., Martin U., Saito S., Schmitto J.D., Haverich A., and Kutschka I. Substantial early loss of induced pluripotent stem cells following transplantation in myocardial infarction. Artif Organs 38, 978, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Terrovitis J.V., Smith R.R., and Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res 106, 479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrovitis J., Lautamäki R., Bonios M., Fox J., Engles J.M., Yu J., Leppo M.K., Pomper M.G., Wahl R.L., Seidel J., Tsui B.M., Bengel F.M., Abraham M.R., and Marbán E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol 54, 1619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng C.J., Luo J., Chiu R.C.J., and Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg 132, 628, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hudson W., Collins M.C., deFreitas D., Sun Y.S., Muller-Borer B., and Kypson A.P. Beating and arrested intramyocardial injections are associated with significant mechanical loss: implications for cardiac cell transplantation. J Surg Res 142, 263, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Martens A., Rojas S.V., Baraki H., Rathert C., Schecker N., Hernandez S.R., Schwanke K., Zweigerdt R., Martin U., Saito S., Haverich A., and Kutschka I. Macroscopic fluorescence imaging: a novel technique to monitor retention and distribution of injected microspheres in an experimental model of ischemic heart failure. PLoS One 9, e101775, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekine H., Shimizu T., Hobo K., Sekiya S., Yang J., Yamato M., Kurosawa H., Kobayashi E., and Okano T. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118, S145, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Sekine H., Shimizu T., Dobashi I., Matsuura K., Hagiwara N., Takahashi M., Kobayashi E., Yamato M., and Okano T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng Part A 17, 2973, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann W.H. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 90, 223, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kutschka I. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation 114(1 Suppl), 1167, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kofidis T., Lebl D.R., Martinez E.C., Hoyt G., Tanaka M., and Robbins R.C. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation 112, I173, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Lisi A., Briganti E., Ledda M., Losi P., Grimaldi S., Marchese R., and Soldani G. A combined synthetic-fibrin scaffold supports growth and cardiomyogenic commitment of human placental derived stem cells. PLoS One 7, e34284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutschka I. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation 114, 1174, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Tudorache I., Kostin S., Meyer T., Teebken O., Bara C., Hilfiker A., Haverich A., and Cebotari S. Viable vascularized autologous patch for transmural myocardial reconstruction. Eur J Cardiothorac Surg 36, 306, 2009; discussion 311. [DOI] [PubMed] [Google Scholar]
- 31.Christman K., Vardanian A., Fang Q., Sievers R., Fok H., and Lee R. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44, 654, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Christman K.L., Fok H.H., Sievers R.E., Fang Q., and Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10, 403, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Guo J., Zhang P., Xiong Q., Wu S.C., Xia L., Roy S.S., Tolar J., O'Connell T.D., Kyba M., Liao K., and Zhang J. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ Heart Fail 8, 156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellamy V., Vanneaux V., Bel A., Nemetalla H., Boitard S.E., Farouz Y., Joanne P., Perier M.-C., Robidel E., Mandet C., Hagège A., Bruneval P., Larghero J., Agbulut O., and Menasche P. Long-term functional benefits of human embryonic stem cell-derived cardiac progenitors embedded into a fibrin scaffold. J Heart Lung Transplant 2014. DOI: 10.1016/j.healun.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 35.Sun C.-K., Zhen Y.-Y., Leu S., Tsai T.-H., Chang L.-T., Sheu J.-J., Chen Y.-L., Chua S., Chai H.-T., Lu H.-I., Chang H.-W., Lee F.-Y., and Yip H.-K. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int J Cardiol 173, 410, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Mauritz C., Schwanke K., Reppel M., Neef S., Katsirntaki K., Maier L.S., Nguemo F., Menke S., Haustein M., Hescheler J., Hasenfuss G., and Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 118, 507, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Van Laake L.W., Passier R., Monshouwer-Kloots J., Nederhoff M.G., Ward-Van Oostwaard D., Field L.J., Van Echteld C.J., Doevendans P.A., and Mummery C.L. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat Protoc 2, 2551, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Takagawa J., Zhang Y., Wong M.L., Sievers R.E., Kapasi N.K., Wang Y., Yeghiazarians Y., Lee R.J., Grossman W., and Springer M.L. Myocardial infarct size measurement in the mouse chronic infarction model: comparison of area- and length-based approaches. J Appl Physiol 102, 2104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kensah G., Roa Lara A., Dahlmann J., Zweigerdt R., Schwanke K., Hegermann J., Skvorc D., Gawol A., Azizian A., Wagner S., Maier L.S., Krause A., Dräger G., Ochs M., Haverich A., Gruh I., and Martin U. Murine and human pluripotent stem cell-derived cardiac bodies form contractile myocardial tissue in vitro. Eur Heart J 34, 1134, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Nicodemus G.D., and Bryant S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 14, 149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo H.-D., Wang H.-J., Tan Y.-Z., and Wu J.-H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A 17, 45, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez-Aranda I., Ramos-Mejia V., Bueno C., Munoz-Lopez M., Real P.J., Mácia A., Sanchez L., Ligero G., Garcia-Parez J.L., and Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 28, 1568, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai W., Wold L.E., Dow J.S., and Kloner R.A. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol 46, 714, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yu J., Christman K.L., Chin E., Sievers R.E., Saeed M., and Lee R.J. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg 137, 180, 2009 [DOI] [PubMed] [Google Scholar]