Abstract
Human neural stem cells (NSCs) are particularly valuable for the study of neurogenesis process and have a therapeutic potential in treating neurodegenerative disorders. However, current progress in the use of human NSCs is limited due to the available NSC sources and the complicated isolation and culture techniques. In this study, we describe an efficient method to isolate and propagate human NSCs from the amniotic fluid with diagnosed neural tube defects (NTDs), specifically, anencephaly. These amniotic fluid-derived NSCs (AF-NSCs) formed neurospheres and underwent long-term expansion in vitro. In addition, these cells showed normal karyotypes and telomerase activity and expressed NSC-specific markers, including Nestin, Sox2, Musashi-1, and the ATP-binding cassette G2 (ABCG2). AF-NSCs displayed typical morphological patterns and expressed specific markers that were consistent with neurons, astrocytes, oligodendrocytes, and dopaminergic neurons after proper induction conditions. Furthermore, grafted AF-NSCs improved the physiological functions in a rat stroke model. The ability to isolate and bank human NSCs from this novel source provides a unique opportunity for translational studies of neurological disorders.
Introduction
Neural stem cells (NSCs) found in the central nervous system (CNS) have the capacity both to self-renew and to differentiate into each of the major cell types in the brain. Since these cells were first described in the mouse brain, NSCs have been the subject of intensive investigation because of their potential therapeutic use in treating neurodegenerative disorders [1,2]. Specifically, transplanting NSCs may induce cellular repair and functional recovery after CNS injury or disease [3–6]. Previous studies have supported this theory by demonstrating that NSCs grafted into the CNS not only form new neurons but also express protective and trophic factors that are released into the damaged area.
Previously identified sources of NSCs in the adult mammalian CNS include the subgranular zone of the hippocampus and the subventricular zone of the ventral forebrain [7]. Human NSCs are typically obtained from aborted fetuses, postmortem brains, or surgical specimens [7–9]. However, the variability in the donor age, storage, viability, and potential contamination of these samples makes it difficult to use them in therapeutic applications [10]. Other barriers include limited availability, technical difficulty in harvesting, and ethical concerns. Finally, the slow kinetics of human NSC growth in primary cultures severely limits the acquisition of sufficient numbers of quality cells for use in clinical applications. Recently, some immortalized neural stem/progenitor cell lines have been established [11,12], which possess a relatively higher capacity for proliferation than typical NSCs, while still retaining the ability to differentiate into different neural cell types. However, the use of oncogenic genes and viral infection in establishing these lines increased the high-risk concerns in medical-oriented applications. Other groups have established lines from pluripotent sources of stem cells such as embryonic stem (ES) cells or induced pluripotent stem cells [13–15]. While these methods do introduce a new source of NSCs, the possibility remains that undifferentiated cells will persist in these populations and will consequently form teratomas [16]. Therefore, the ability to use pluripotent stem cell-derived NSCs for therapeutic applications is limited by ethical issues, safety concerns, and poor efficiency.
Neural tube defects (NTDs) are the most common defects that occur when the neural tube develops abnormally, and these defects affect approximately 1 in 1,000 pregnancies [17]. The neural tube is formed during embryonic development and eventually gives rise to the entire CNS. When the neural tube does not close completely on either end, an NTD occurs. In humans, the most common NTDs are anencephaly and myelomeningocele. The former results from a failed closure of the rostral end of the neural tube and is characterized by a total or partial absence of the cranial vault and cerebral hemisphere, while the latter is a defective closure of the caudal neural tube and the vertebral column [18–20]. Anencephaly results in the incomplete formation of the brain and skull and is therefore lethal. Most individuals with myelomeningocele have a multiple system handicap and a limited lifespan. Either ultrasound technology or measurement of maternal serum alpha-fetal protein levels can be used to detect an NTD in utero [21]. Follow-up testing typically measures the levels of the alpha-fetal protein and acetylcholinesterase in the amniotic fluid (AF) to confirm that an NTD is present [22].
AF is known to contain multiple cell types that are derived from the developing fetus. Previous studies have demonstrated that multipotent stem cells can be isolated from this substance through amniocentesis. These AF-derived stem cells (AFSCs) express some pluripotent markers and can differentiate into cells of mesenchymal or neural lineages under inductive conditions [23–25]. Although AFSCs exhibit neural potentiality both in vivo and in vitro, they lack some typical properties of NSCs, such as proper growth, morphology, and neurosphere formation potential. In the present study, we established NSCs from human AF samples in pregnancies where an NTD had been diagnosed. We found that these unique NSCs could be isolated and expanded for long periods without losing their stem cell-specific properties. More importantly, these NSCs showed neuronal differentiation potential in vitro and beneficial functional recovery in a rat stroke model.
Materials and Methods
Sample collection
The AF samples used in this study were obtained from Cathay General Hospital in Taipei and Chang Gung Memorial Hospital in Tao-Yuan, Taiwan. Pregnant women aged 25–35 years underwent AF sampling for diagnostic purposes between 13 and 26 weeks gestation. Fetuses were diagnosed with either an anencephaly or a nonanencephaly NTD by ultrasound and maternal serum screening. In contrast, fetuses that showed negative NTD screening results and had normal karyotypes were defined as healthy donors. All procedures were approved by the Institutional Review Boards of Cathay General Hospital and Chang Gung Memorial Hospital, and all participants provided their written informed consent to participate in this study.
Cultivation of AF-NSCs
AF samples from healthy and NTD donors were centrifuged at 1,000 rpm for 5 min, and the cell pellets were resuspended in the NeuroCult™ NS-A proliferation medium (StemCell Technologies, Vancouver, BC, Canada) in T25 flasks at 37°C in a 5% CO2 humidified atmosphere. After 3–5 days, the suspended cells and debris were removed by changing the media. To maintain these cells, 1/5 volume of additional culture medium was added every 2–3 days. The initial mature neurospheres could be observed at 3–4 weeks after plating. These cells were designated as human AF-derived neural stem cells (AF-NSCs).
When the neurospheres grew to 50–100 μm in diameter, these cells were passaged. First, the cells were centrifuged at 800 rpm for 5 min and treated with TrypLE (Life Technologies, Gaithersburg, MD) at 37°C for 3 min. After an additional centrifugation step to remove the TrypLE solution, the cells were resuspended in the NeuroCult NS-A proliferation medium, and a single-cell suspension was obtained by pipetting carefully to avoid bubble formation. AF-NSCs were seeded at a density of 0.5–1×104/cm2 and maintained as described above. The neurospheres could also be cryopreserved for subsequent experiments.
To determine the optimal seeding density, the AF-NSCs were plated at various densities in T25 flasks and cultured as previously described. After 10 days, the neurospheres were collected and trypsinized. Cell counts were performed with a hemocytometer, and the doubling time per passage was calculated. Each of the described experiments was performed in triplicate.
For the cumulative cell number assay, AF-NSCs were seeded at 5,000 cells/cm2 in T25 flasks and cultured as described previously. The cells were passaged every 10–14 days, and cell counts were performed at each passage to calculate the fold increase in cells along with the total cell number.
Flow cytometry
AF-NSCs were trypsinized and resuspended as single cells in phosphate-buffered saline (PBS). For direct analysis, the cells were fixed with Cytofix™ (BD Biosciences, San Jose, CA), with or without permeabilization, and immunolabeled with the following anti-human antibodies: CD73-phycoerythrin (PE), CD105-fluorescein isothiocyanate (FITC), CD117-PE, HLA-I-PE, HLA-DR-PE, Nanog-PE, Oct-4-FITC, Sox2-PE, ABCG2-PE (all from BD Biosciences), SSEA-1-PE, SSEA-3-FITC, SSEA-4-PE, TRA-1-60-PE, TRA-1-81-PE, Nestin-FITC (all from R&D Systems, Minneapolis, MN), or CD133-PE (Merck Millipore, Billerica, MA). For indirect analysis, the cells were fixed, permeabilized with the Perm Buffer II (BD Biosciences), blocked, immunolabeled with Musashi-1 (R&D Systems), and stained with an Alexa Fluor 488 dye (Life technologies). All samples were processed using a FACSCanto II flow cytometer (BD Biosciences), and at least 30,000 events were captured per sample. The data acquisition and analysis were performed using FACSDiva 6.0 (BD Biosciences) and FCS Express V3.00 (De Novo Software, Thornhill, Canada).
Immunocytochemistry
The cells were fixed in 4% paraformaldehyde (Merck Millipore) and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich, St Louis, MO). After being blocked with 10% specific normal serum in PBS for 30 min, the cells were incubated with the following primary antibodies: Tuj-1 (Sigma-Aldrich), Nestin, Sox2, microtubule-associated protein 2 (MAP2), neural filament heavy chain (NFH), glial fibrillary acidic protein (GFAP), human neuron-specific nuclear protein (hNeuN), tyrosine hydroxylase (TH) (all from Merck Millipore), Musashi-1, O4, or aromatic L-amino acid decarboxylase (AADC) (all from R&D system) for 1 h. After two washes, the cells were then incubated with an Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibody (Life Technologies) for 1 h at room temperature. The resulting immunoreactive cells were visualized under a confocal microscope (TCS-SP5-X AOBS, Leica, Solms, Germany) or a fluorescence microscope (Axio Observer.Z1, Carl Zeiss, Oberkochen, Germany).
Telomerase activity assay
The telomerase activity was measured by the telomeric repeat amplification protocol (TRAP) using a commercially available TRAPeze RT kit (Merck Millipore). The amplified TRAP reaction products were separated on a 12.5% polyacrylamide gel and visualized as TRAP ladder patterns.
Quantitative polymerase chain reaction
Total RNA was extracted using the TRIzol Reagent (Invitrogen, Carlsbad, CA), and first-strand cDNA was synthesized according to the manufacturer's protocol using the M-MuLV Reverse Transcriptase (Thermo Scientific, San Jose, CA) and an oligo-dT primer. Quantitative polymerase chain reaction (qPCR) was performed with SYBR Green PCR Master Mix (Thermo Scientific) using an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). The relative expression level of β-actin was used as an internal control to normalize gene expression in each sample. Relative quantification of marker genes was performed according to the ΔΔCt method. The primer pairs used in this study are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
AF-NSC differentiation
The AF-NSC-derived neurospheres (passages #10-12) were trypsinized and seeded in culture dishes coated with 100 μg/mL poly-L-lysine (Sigma-Aldrich) and 10 μg/mL laminin (Sigma-Aldrich) in the presence of the NeuroCult™ NS-A proliferation medium at a density of 5×104 cells/cm2. Upon cell attachment to the bottom of the dishes, neural differentiation was induced by the addition of the NeuroCult™ NS-A differentiation medium (StemCell Technologies) according to the manufacturer's protocol. After induction, the differentiated cells were fixed in 4% paraformaldehyde for immunocytochemistry or collected for mRNA extraction and subsequent qPCR. To direct the differentiation of specific cell types, AF-NSCs were seeded on plates coated with 100 μg/mL poly-L-lysine at a density of 5×104 cells/cm2 in the presence of the NS-A proliferation medium. After attachment, the medium could be changed to the specific induction medium that would produce astrocytes, oligodendrocytes, and dopaminergic neurons according to previously published protocols [26]. AF-NSCs were cultured in the DMEM/F12 medium (Life Technologies) supplemented with 1×NEAA (Life Technologies), 2 mM l-glutamine (Life Technologies), 1×N2 (R&D Systems), and 1×B27 (Life Technologies) for 2 weeks to differentiate into astrocytes. For oligodendrocyte induction, AF-NSCs were cultured in DMEM/F12, 1×NEAA, 2 mM L-glutamine, 1×N2, 1×B27, 200 ng/mL Sonic hedgehog (Shh; R&D Systems), and retinoic acid (RA; Sigma-Aldrich) for 10 days, and then with 30 ng/mL NT3 (R&D Systems) and 10 ng/mL platelet-derived growth factor alpha (PDGF-α R&D Systems), but without Shh and RA, for an additional 2 weeks. Dopaminergic differentiation was initiated by inducing AF-NSCs for 10 days in the neurobasal medium (Life Technologies) containing 1×NEAA, 2 mM l-glutamine, B27, 200 ng/mL Shh, and 100 ng/mL FGF8 (R&D Systems). Shh and FGF8 were then withdrawn and replaced with 20 ng/mL brain-derived neurotrophic factor (BDNF; R&D Systems), 20 ng/mL glial cell-derived neurotrophic factor (GDNF; R&D Systems), 1 μM transforming growth factor beta-3 (TGF-β3; R&D Systems), 200 μM ascorbic acid (Sigma-Aldrich), and 1 mM cAMP (Sigma-Aldrich) for 3 weeks. After induction, the differentiated status could be confirmed by immunocytochemical staining and q-PCR.
Focal ischemia and AF-NSC transplantation
All Sprague-Dawley rats (8 weeks old, 250–300 g) were obtained from Lasco (Ilan, Taiwan) and housed in an animal facility at the National Chung Hsing University. All experimental procedures were devised with the welfare of the animals in mind and approved by the Institutional Animal Care and Use Committee of National Chung Hsing University. All rats were randomly assigned to one of three groups, including nonoperation healthy rats (control, n=7), ischemic rats given the medium alone (sham group, n=6), and ischemic rats given AF-NSCs (transplantation group, n=8). The ischemic rats were subjected to a 1.5-h-long suture occlusion of the middle cerebral artery (MCAO) in the right hemisphere [27]. On the first postoperative day, the medium alone (10 μL) or 1×106 AF-NSCs in the medium (passage #10-12, 10 μL) were injected at a rate of 1 μL/min into the damaged striatum at the location of AP: −0.4 R: 3.4 DV: 5. All AF-NSCs used in the animal experiments were from a single donor. The animals were sacrificed at 1 or 4 weeks after the operation and their brains were fixed with 4% paraformaldehyde by transcardial perfusion.
Behavioral assays
All rats performed similarly on both rotarod and grip strength assays after one week of training. All rats underwent behavioral testing before the MCAO procedure. For the rotarod test, all tested rats could remain on the cylinder as it was accelerated from 4 to 40 rpm within 300 s before MCAO. To test their grip strength, the rats grasped the pull bar with their forepaws, and their grip strength was quantified by an electronic sensor that was connected to the bar. After MCAO, the rotarod test was performed at 1, 2, 3, and 4 weeks, and the grip strength was tested at 1, 3, and 4 weeks. The results of three trials for each rat were recorded.
TTC staining and immunohistochemistry
MCAO rat brains were sectioned coronally into six slices, which were then immersed in 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 min at 37°C followed by formalin fixation. The infarcted areas were pale, while the normal brain tissue was stained red. The atrophied area was calculated by subtraction of the area of stoke hemisphere from the area of healthy hemisphere. The infarcted and atrophied areas were estimated with a computer image analysis system (Image-Pro Plus, Media Cybernetics, Carlsbad, CA), and the extent of tissue damage was calculated as a percentage of the total area of the contralateral healthy hemisphere.
For the immunohistochemical analysis, the brains were dehydrated in a sucrose gradient, embedded with OCT (Sakura Fine Technical, Tokyo, Japan), frozen at −70°C, and cryosectioned at a thickness of 40 μm. For immunostaining, the sections were rinsed in PBS containing 0.1% Tween-20 (PBST), permeabilized with 0.1% Triton X-100, and blocked with 10% specific normal serum in PBS for 30 min before overnight incubation with primary antibodies, including the human Nuclei (Merck Millipore) and Nestin (Merck Millipore). Next, the sections were washed twice with PBST and incubated with the appropriate rhodamine- or FITC-conjugated secondary antibody (Thermo Scientific) for 1 h at room temperature. Finally, a fluorescent microscope was used to visualize the distribution of labeled cells.
Statistical analysis
All results are presented as the mean±standard deviation (SD). Significant differences between the two mean values were compared using Student's t-test. One-way ANOVA with Scheffe's post hoc test was used to assess significant differences if more than two groups were compared. The results were considered statistically significant when P<0.05.
Results
Isolation of NSCs from AF
To isolate NSCs from AF, normal (n=7) and NTD-derived AF specimens (anencephaly n=6, nonanencephaly n=5) were collected by amniocentesis. When the cells were cultured, only a subset of the anencephaly samples produced some slightly attached neural-like cells and colonies during the first 3–5 days (Fig. 1A). After the unattached cells and cellular debris were removed carefully, these neural-like cells proliferated and rounded-up to form primary neurospheres that grew in suspension after 3 weeks (Fig. 1A). After passaging, the cells proliferated and reformed neurospheres in suspension with each passage, and the neurospheres possessed classic microspikes on their outer surfaces (Fig. 1B). These AF-NSCs could be expanded by continual passaging. Notably, AF-NSCs could only be established from AF samples taken from NTD patients that had been diagnosed with anencephaly (Table 1), and no neural-like cells or spheres developed from healthy or nonanencephaly NTD donor samples under these culture conditions. AF-NSC lines could be established from four of the six anencephaly samples (success rate, 67%), and all four lines had normal karyotypes (Supplementary Fig. S1).
FIG. 1.
Culture properties of amniotic fluid-derived neural stem cells (AF-NSCs). (A) After the initial seeding of the amniotic fluid cells, neural-like cells began to attach to the culture plate (left panel) and proliferated to form primary neurospheres (right panel). (B) AF-NSC-derived neurospheres contained a typical microspike structure over their outer surfaces (right, black arrows). Scale bar: 10 μm. (C) The doubling time of AF-NSCs was affected by the seeding density. *P<0.05; **P<0.01. (D) AF-NSC counts from long-term in vitro cultures. Two AF-NSC lines, 100 and 106, were cultured for 18 and 23 passages, respectively (black square: AF-NSC line #100; black circle: AF-NSC line #106), DIV, days in vitro.
Table 1.
Sources and Outcome of Amniotic Fluid Samples
| Patient Status | Total samples | # of AF-NSCs obtained | Success rate (%) |
|---|---|---|---|
| No defect | 7 | 0 | 0 |
| NTD | |||
| Anencephaly | 6 | 4 | 67 |
| Nonanencephaly | 5 | 0 | 0 |
AF-NSCs, amniotic fluid-derived neural stem cells; NTD, neural tube defect.
The rate of neurosphere growth was influenced by the density at which the AF-NSCs were seeded initially. The optimal seeding density was determined to be 5,000–10,000 cells/cm2 (Fig. 1C), with almost zero growth occurring at a density below 2,500 cells/cm2. The AF-NSCs doubled at a rate of 109.4±14.8 h when they were plated at a density under 10,000 cells/cm2. Although AF-NSCs showed large neurospheres in culture, the dividing rate slowed at a density of 20,000 cells/cm2.
To test the long-term expansion potential of the AF-NSCs, we grew two different lines (starting at passage 5) in vitro over the course of several months. Both lines maintained constant growth for at least 5 months (Fig. 1D), and one line could be propagated for over 8 months. These two cell lines could be expanded over 105–1010-fold.
Characterization of the AF-NSCs
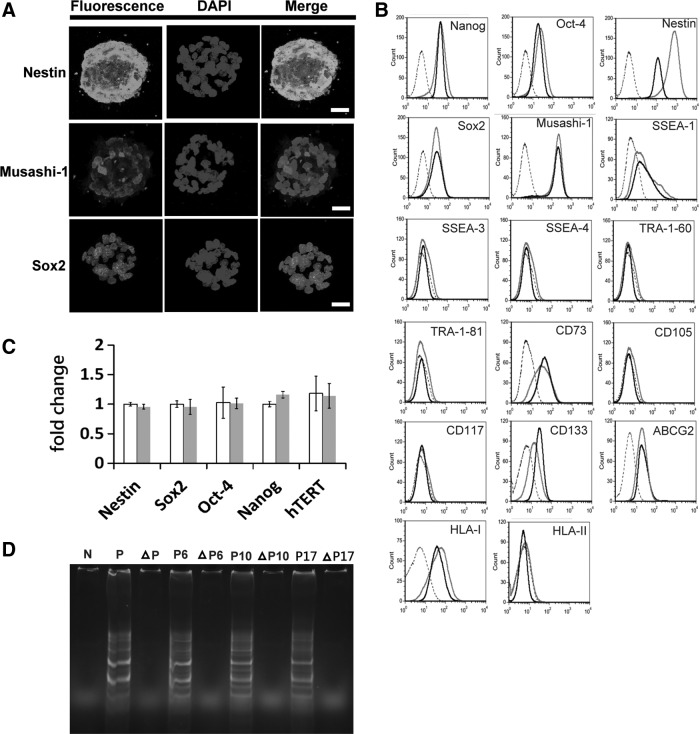
To characterize the AF-NSCs, immunocytochemistry and flow cytometry were used to detect the expression of NSC-specific markers. Confocal microscopy revealed that AF-NSC-derived neurospheres strongly expressed both Nestin and Musashi-1 within their cytoplasm. Expression of Sox2, a nuclear protein, could also be observed in the neurospheres (Fig. 2A).
FIG. 2.
Expression of NSC-specific markers and measurement of telomerase activity in AF-NSCs. (A) Confocal images of immunostained mature neurospheres (day 14) revealed the expression of specific NSC markers. Scale bar: 20 μm. (B) The expression of specific markers in AF-NSCs was analyzed by flow cytometry at early and late passage numbers. Dotted line: isotope control; gray line: early passages #10–12; black line: late passages #20–22. (C) qPCR was used to determine the mRNA levels of NSC-specific genes at early and late passages. White bar: early passage #10; gray bar: late passage #20. (D) Telomerase activity was measured at different times in culture (passages #6, 10, and 17). The triangle designates samples that were treated by heat inactivation. SSEA, stage-specific embryonic antigen; ABCG2, ATP-binding cassette subfamily G2; HLA, human leukocyte antigen; N, negative control; P, positive control.
Flow cytometry revealed that AF-NSCs express the NSC-specific markers Nestin, Sox2, Musashi-1, and ABCG2 (Fig. 2B). CD133 was initially expressed at a low level, but the intensity of the signal increased with subsequent passages. AF-NSCs expressed a set of transcription factors similar to that expressed in ES cells, including Nanog, Oct-4, and Sox2, as well as low levels of SSEA-1; however, they did not express SSEA-3, SSEA-4, TRA-1-60, and TRA-81. Furthermore, the pattern of human leukocyte antigen (HLA) expression in AF-NSCs was similar to that in most stromal cells, which express HLA class I but not HLA class II. Our results also showed that AF-NSCs did not express either CD105 or CD117 and only occasionally expressed CD73. Interestingly, the expression patterns of all detected markers were maintained in AF-NSCs throughout their time in vitro (Fig. 2B), and the percentages of Nestin+, Sox2+, and Nanog+ cells were not affected by freezing or passage (Supplementary Table S2).
We also employed qPCR to measure the expression levels of these markers throughout multiple passages and found that Nestin, Sox2, Oct-4, Nanog, and hTERT were all consistently expressed over the course of 20 passages (Fig. 2C), which confirmed our previous observations with flow cytometry. Finally, we assessed the telomerase activity of the AF-NSCs and determined that the activity levels were maintained despite increasing passage numbers in long-term cultures (Fig. 2D).
In vitro neural differentiation of AF-NSCs
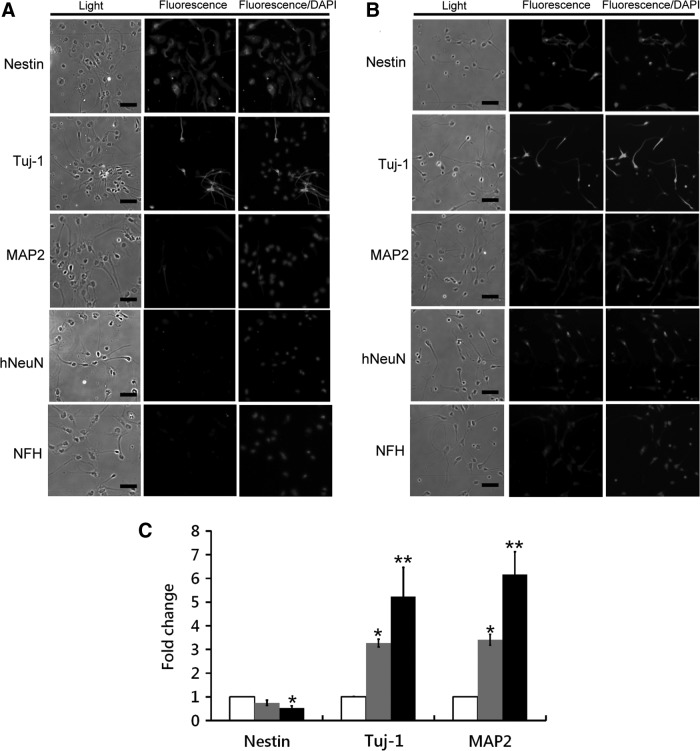
In the differentiation potential assay, the AF-NSCs began to undergo morphological changes, and only Nestin and Tuj-1 were expressed within cells at this stage after 2 days of induction (Fig. 3A). Additional neuron-specific markers, including Nestin, Tuj-1, MAP2, hNeuN, and NFH, could be detected after 7 days of induction (Fig. 3B), and the transcription levels of Tuj-1 and MAP2 were found to be upregulated by 5.2- and 6.2-fold, respectively (Fig. 3C). In contrast, the expression of Nestin, a NSC-specific gene, significantly decreased during this same period.
FIG. 3.
In vitro differentiation of AF-NSCs. The markers of early and mature neurons were detected by immunocytochemistry at 2 (A) and 7 (B) days postinduction. Scale bar: 20 μm. (C) qPCR was used to measure the expression levels of these neuronal markers. White bar: undifferentiated cells; gray bar: differentiated cells at day 2; black bar: differentiated cells at day 7. *P<0.05; **P<0.01. DAPI, 4′,6-diamidino-2-phenylindole.
Next, we induced the AF-NSCs into astrocytes, oligodendrocytes, and dopaminergic neurons by exposing them to defined differentiation media. Most (>80%) AF-NSCs could be induced to become GFAP-positive astrocytes (Fig. 4A) as determined by the dramatic 4,700-fold increase in GFAP expression after 2 weeks of induction (Fig. 4B). Oligodendrocytes were detected by immunostaining for the O4 antigen (Fig. 4A). Compared with undifferentiated AF-NSCs, the defined medium induced the expression of the oligodendrocyte-specific genes CNP, MBP, and O2 to increase by 2.15-, 4.97-, and 1.9-fold, respectively. Moreover, the presence of TH- and AADC-positive cells after 1 month suggested that AF-NSCs could give rise to dopaminergic neurons (Fig. 4A). This observation was verified by qPCR analysis, which determined that dopaminergic neuron-specific markers, including AADC, Pax2, Lmx-1b, and Nurr-1, were significantly upregulated after differentiation (Fig. 4B).
FIG. 4.
Directed differentiation of AF-NSCs into astrocytes, oligodendrocytes, and dopaminergic neurons. (A) Differentiated AF-NSCs were immunostained for specific markers to confirm the presence of astrocytes (GFAP), oligodendrocytes (O4), and dopaminergic neurons (TH and AADC). Scale bar: 20 μm. (B) qPCR was used to analyze gene expression of astrocyte-, oligodendrocyte (left)-, and dopaminergic neuron (right)-specific markers. White bar: before differentiation; black bar: after differentiation. *P<0.05; **P<0.01. GFAP, Glial fibrillary acidic protein; TH, tyrosine hydroxylase; AADC, aromatic L-amino acid decarboxylase; DAPI, 4′,6-diamidino-2-phenylindole.
AF-NSCs were transplanted into ischemia rats and induced functional recovery
To determine whether AF-NSCs could induce functional recovery from stroke in vivo, 1×106 cells were transplanted into the ischemic boundary zone of rat brains that had undergone MCAO. Sham-injected rats consistently showed impaired motor performance when compared to healthy controls as assessed by the rotarod and grip strength tests.
In the rotarod test, the AF-NSC-treated MCAO group experienced a 43%±18% reduction in time when compared to sham-injected MCAO rats, which suggests that the presence of AF-NSCs improved the motor deficits observed in ischemic rats. Notably, the AF-NSCs promoted a rapid recovery of motor function during the first week postinjection (85%±26%), and this improvement was maintained for 4 weeks postengraftment (98%±18%; Fig. 5A).
FIG. 5.
Engrafting AF-NSCs into MCAO ischemic rats has therapeutic effects. After the AF-NSCs were transplanted, ischemia rats underwent a rotarod test (A) and a grip strength test (B). Black bar: healthy control; white bar: sham group; gray bar: AF-NSC transplantation after MCAO. (C) Quantification of the hemispheric lesion area by TTC at 4 weeks after AF-NSC transplantation. (D) Top: one week after AF-NSC transplantation, immunohistochemistry revealed Nestin expression in the injection region. Bottom: higher magnification of this region revealed that grafted AF-NSCs coexpress Nestin and human nuclei (arrows) in the damaged area. Scale bar: 50 μm.
A similar improvement in function was observed with the grip strength test (Fig. 5B). MCAO significantly decreased the maximum grip strength of the rats. One week after receiving AF-NSC transplants, the maximum grip strength of these ischemic rats was similar to that of the sham-operated group. However, the rats that received AF-NSCs could recover their normal grip strength by 3–4 weeks post-transplantation, whereas the sham-operated rats remained weak.
To determine the extent of damage after the MCAO, the engrafted brains were stained with TTC at 4 weeks after the AF-NSC transplant. The size of the infarcted area significantly decreased after transplantation (sham: 22.17%±1.12%; AF-NSC transplant: 0.62%±0.15%), whereas the atrophied region remained the same (sham: 7.21%±0.48%; AF-NSC transplant: 5.26%±0.57%; Fig. 5C). One week after transplantation, we used immunohistochemistry to determine whether the AF-NSCs survived in the rat brains by double labeling cryosections with antibodies against Nestin and human nuclei antibodies. Our results showed that the Nestin and human nuclei double-positive cells were close to the injection area (Fig. 5D), which suggests that the engrafted AF-NSCs could survive and integrate into the host brain.
Discussion
Turner et al. have reported that NSCs could be isolated and cultured from rat AF with retinoic acid-treated NTDs; however, these cells were absent in healthy control rats [28]. These AF-derived cells exhibited typical neural progenitor morphology and robustly expressed the NSC-specific markers Nestin and Sox2. Previous studies have also reported that the number of NSCs that can be derived from the AF of rats with spina bifida is statistically significantly higher compared to that from the AF of those animals with exencephaly alone or with both spina bifida and exencephaly [29]. In this study, we isolated AF-NSCs from human AF collected from fetuses that had been diagnosed with anencephaly (67%), but not from normal fetuses or from those with a nonanencephaly NTD. However, the small number of donors may have limited our ability to isolate AF-NSCs in the setting of an NTD other than anencephaly.
NSCs can self-renew, differentiate into all CNS cell types, and are typically grown as neurospheres under serum-free conditions in vitro [30]. In our study, all four AF-NSC lines that we established could be maintained as neurospheres for >5 months and developed typical microspikes on their surface. Previous studies have suggested that neural precursor cells isolated from human embryos have decreased the telomerase activity, which declines to an undetectable level after 20 population doublings [31]. Conversely, our AF-NSCs could be expanded over several months and maintain their telomerase activity even at later passage numbers. However, the AF-NSCs did not form teratomas in vivo when injected into severe combined immunodeficiency mice. Typical human NSCs isolated from various CNS structures in 6–14.5-week-old human fetuses experience a long doubling time (5–10 days) in vitro [9]. Comparatively, our AF-NSCs divided every 4–5 days, which suggested that these cells were suitable for expansion and banking. Neurosphere culture appears sensitive to the culturing processes. Variations in cell density will alter the microenvironment of neurospheres, which in turn may affect the proliferation capacity of the neurospheres [32]. Similar to previous reports [33,34], the cell number and sphere size of the AF-NSCs in the present study increased initially with the seeding density. However, the difficulty of oxygen and nutrient acquisition and the heterogeneity of differentiated cells increase with sphere size [32,35]. A high density of AF-NSC culture (≥20,000 cells/cm2) may reduce cell division because of large neurosphere aggregation.
Nestin, Sox2, CD133, ABCG2, SSEA-1, and Musashi-1 are recognized as NSC-specific markers [36–38]. Our AF-NSCs maintained or increased their expression of these markers during long-term cultivation in vitro. AF has been identified as an ideal source to isolate fetal multipotent stem cells (AFSCs) [24,39]. AFSCs also express some markers with mesenchymal stem cells, such as CD73, CD105, and CD117, and some pluripotent stem cell markers, such as Oct-4, Nanog, and Sox2. However, in this study, AF-NSCs did not express either CD105 or CD117 and only slightly expressed CD73 on their surface. In addition, AF-NSCs showed differences in morphology, doubling time, and telomerase activity compared with AFSCs. Taken together, these results suggest that AF-NSCs are distinct from previously identified AFSCs.
Another hallmark of NSCs is their ability to differentiate into multiple CNS cell types. We have demonstrated that AF-NSCs have the potential to become neurons, astrocytes, oligodendrocytes, and dopaminergic neurons in vitro. During oligodendrocyte development, O4 is expressed in both oligodendrocyte precursors and mature oligodendrocytes, whereas CNP and MBP are only expressed in mature myelinating oligodendrocytes [40]. AF-NSCs could be induced into O4 immunoreactive cells successfully in vitro; however, these cells did not express either CNP or MBP at the protein level (data not shown), although significant increases in CNP and MBP mRNA could be detected by qPCR in multiple AF-NSC lines. This discrepancy may reflect a need for further stimulation in vitro to produce mature oligodendrocytes. Previous studies have demonstrated that brain-derived NSCs can provide functional improvement in the behavior of ischemic rats [41–43]. Similar to fetal NSCs, undifferentiated AF-NSCs grafted efficiently near the lesioned region in a rat stroke model. Furthermore, AF-NSCs can induce the recovery of the ischemia-induced reduction in grip strength and rotarod performance. One hypothesis is that NSC transplantation might induce tissue repair because these cells attenuate inflammation, increase the antiapoptotic activity, or reduce infarct size [41,44]. In this study, we observed a marked reduction of infarct size in ischemic rats after the injection of AF-NSCs. Taken together, these results suggest that the AF-NSCs not only have the potential to differentiate in vitro but also have a neuroprotective effect on ischemic rats.
Using high-resolution ultrasonography and amniocentesis to detect an NTD during pregnancy would allow human AF-NSCs to be collected efficiently. Human AF stem cells have been considered a model for investigating genes involved in human genetic diseases or oncogenesis [45,46]. Therefore, human AF-NSC banks could be established for developmental studies and preclinical testing purposes. NTDs in humans are known to be affected by complex genetic and environmental factors [18–20]. Fifty-one entities are associated with anencephaly alone in the Online Mendelian Inheritance in Man (OMIM) (www.ncbi.nlm.nih.gov/omim/?term=anencephaly). Bone morphogenetic proteins, which are part of the transforming growth factor-beta superfamily, are shown to be important in the formation and closure of the neutral tube during embryo development [20]. A recent study showed that mesenchymal cells derived from the AF of fetuses with diagnosed NTDs did not express sufficient type I collagen upon the treatment with TGF-beta 1 [47]. Therefore, one of the limitations of this study is that we did not exhaustively identify the mutations in potentially causative genes in these NTD-derived AF-NSC lines, although the AF-donating mothers in this study did not have diabetes or consume a folate antagonist or valproic acid. Apparently, NTD-derived AF-NSC lines are valuable for neuron development studies, but need full characterization before clinical use.
Conclusion
In conclusion, AF-NSCs derived from the AF of fetuses with diagnosed NTDs share common characteristics with other sources of human NSCs, including the ability to form neurospheres, the expression of stem cell-specific makers, the ability to undergo long-term proliferation, the potential to differentiate in vitro, and the potential to have therapeutic effects in a rat stroke model. Therefore, this novel source of human NSCs could have a significant effect on future neuron development studies.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Economic Affairs (104-EC-17-A-22-0525), Taiwan, and the Ministry of Science and Techonology (NSC 101-2311-B-080-001, MOST 103-2325-B-080-001), Taiwan.
Author Disclosure Statement
The authors report no potential conflicts of interest.
References
- 1.Okano H. (2002). Neural stem cells: progression of basic research and perspective for clinical application. Keio J Med 51:115–128 [DOI] [PubMed] [Google Scholar]
- 2.Reynolds BA. and Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 [DOI] [PubMed] [Google Scholar]
- 3.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP, et al. (1995). Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N Engl J Med 332:1118–1124 [DOI] [PubMed] [Google Scholar]
- 4.Hwang DH, Lee HJ, Park IH, Seok JI, Kim BG, Joo IS. and Kim SU. (2009). Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther 16:1234–1244 [DOI] [PubMed] [Google Scholar]
- 5.Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, et al. (2011). Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134:1777–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abematsu M, Tsujimura K, Yamano M, Saito M, Kohno K, Kohyama J, Namihira M, Komiya S. and Nakashima K. (2010). Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest 120:3255–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Perez O. (2012). Neural stem cells in the adult human brain. Biol Biomed Rep 2:59–69 [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK. and Klassen H. (2003). Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res 74:838–851 [DOI] [PubMed] [Google Scholar]
- 9.Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Panlilio M, Colombo A. and Galli R. (1999). Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol 156:71–83 [DOI] [PubMed] [Google Scholar]
- 10.Earl CD, Reum T, Xie JX, Sautter J, Kupsch A, Oertel WH. and Morgenstern R. (1996). Foetal nigral cell suspension grafts influence dopamine release in the non-grafted side in the 6-hydroxydopamine rat model of Parkinson's disease: in vivo voltammetric data. Exp Brain Res 109:179–184 [DOI] [PubMed] [Google Scholar]
- 11.Ryder EF, Snyder EY. and Cepko CL. (1990). Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol 21:356–375 [DOI] [PubMed] [Google Scholar]
- 12.De Filippis L, Ferrari D, Rota Nodari L, Amati B, Snyder E. and Vescovi AL. (2008). Immortalization of human neural stem cells with the c-myc mutant T58A. PLoS One 3:e3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SC, Wernig M, Duncan ID, Brustle O. and Thomson JA. (2001). In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19:1129–1133 [DOI] [PubMed] [Google Scholar]
- 14.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A. and Ben-Hur T. (2001). Neural progenitors from human embryonic stem cells. Nat Biotechnol 19:1134–1140 [DOI] [PubMed] [Google Scholar]
- 15.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M. and Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, et al. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27:743–745 [DOI] [PubMed] [Google Scholar]
- 17.Botto LD, Moore CA, Khoury MJ. and Erickson JD. (1999). Neural-tube defects. N Engl J Med 341:1509–1519 [DOI] [PubMed] [Google Scholar]
- 18.Copp AJ. and Greene ND. (2010). Genetics and development of neural tube defects. J Pathol 220:217–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Copp AJ, Stanier P. and Greene ND. (2013). Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol 12:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi Y. and Miura M. (2013). How to form and close the brain: insight into the mechanism of cranial neural tube closure in mammals. Cell Mol Life Sci 70:3171–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy D, Chitayat D, Winsor EJ, Silver M. and Toi A. (1998). Prenatally diagnosed neural tube defects: ultrasound, chromosome, and autopsy or postnatal findings in 212 cases. Am J Med Genet 77:317–321 [DOI] [PubMed] [Google Scholar]
- 22.Wald N, Cuckle H. and Nanchahal K. (1989). Amniotic fluid acetylcholinesterase measurement in the prenatal diagnosis of open neural tube defects. Second report of the Collaborative Acetylcholinesterase Study. Prenat Diagn 9:813–829 [DOI] [PubMed] [Google Scholar]
- 23.Tsai MS, Lee JL, Chang YJ. and Hwang SM. (2004). Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod 19:1450–1456 [DOI] [PubMed] [Google Scholar]
- 24.De Coppi P, Bartsch G, Jr., Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, et al. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106 [DOI] [PubMed] [Google Scholar]
- 25.Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G. and Hengstschlager M. (2004). Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 191:309–314 [DOI] [PubMed] [Google Scholar]
- 26.Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L. and Zeng X. (2010). Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28:1893–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longa EZ, Weinstein PR, Carlson S. and Cummins R. (1989). Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91 [DOI] [PubMed] [Google Scholar]
- 28.Turner CG, Klein JD, Wang J, Thakor D, Benedict D, Ahmed A, Teng YD. and Fauza DO. (2013). The amniotic fluid as a source of neural stem cells in the setting of experimental neural tube defects. Stem Cells Dev 22:548–553 [DOI] [PubMed] [Google Scholar]
- 29.Pennington EC, Gray FL, Ahmed A, Zurakowski D. and Fauza DO. (2013). Targeted quantitative amniotic cell profiling: a potential diagnostic tool in the prenatal management of neural tube defects. J Pediatr Surg 48:1205–1210 [DOI] [PubMed] [Google Scholar]
- 30.Reynolds BA. and Rietze RL. (2005). Neural stem cells and neurospheres—re-evaluating the relationship. Nat Methods 2:333–336 [DOI] [PubMed] [Google Scholar]
- 31.Ostenfeld T, Caldwell MA, Prowse KR, Linskens MH, Jauniaux E. and Svendsen CN. (2000). Human neural precursor cells express low levels of telomerase in vitro and show diminishing cell proliferation with extensive axonal outgrowth following transplantation. Exp Neurol 164:215–226 [DOI] [PubMed] [Google Scholar]
- 32.Jensen JB. and Parmar M. (2006). Strengths and limitations of the neurosphere culture system. Mol Neurobiol 34:153–161 [DOI] [PubMed] [Google Scholar]
- 33.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF. and van der Kooy D. (1999). Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208:166–188 [DOI] [PubMed] [Google Scholar]
- 34.Morshead CM. and van der Kooy D. (2004). Disguising adult neural stem cells. Curr Opin Neurobiol 14:125–131 [DOI] [PubMed] [Google Scholar]
- 35.Xiong F, Gao H, Zhen Y, Chen X, Lin W, Shen J, Yan Y, Wang X, Liu M. and Gao Y. (2011). Optimal time for passaging neurospheres based on primary neural stem cell cultures. Cytotechnology 63:621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Kong W, Falk A, Hu J, Zhou L, Pollard S. and Smith A. (2009). CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One 4:e5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauer HC, Tempfer H, Bernroider G. and Bauer H. (2006). Neuronal stem cells in adults. Exp Gerontol 41:111–116 [DOI] [PubMed] [Google Scholar]
- 38.Capela A. and Temple S. (2002). LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35:865–875 [DOI] [PubMed] [Google Scholar]
- 39.Tsai MS, Hwang SM, Tsai YL, Cheng FC, Lee JL. and Chang YJ. (2006). Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol Reprod 74:545–551 [DOI] [PubMed] [Google Scholar]
- 40.Zhang SC. (2001). Defining glial cells during CNS development. Nat Rev Neurosci 2:840–843 [DOI] [PubMed] [Google Scholar]
- 41.Zhang P, Li J, Liu Y, Chen X, Kang Q, Zhao J. and Li W. (2009). Human neural stem cell transplantation attenuates apoptosis and improves neurological functions after cerebral ischemia in rats. Acta Anaesthesiol Scand 53:1184–1191 [DOI] [PubMed] [Google Scholar]
- 42.Chen B, Gao XQ, Yang CX, Tan SK, Sun ZL, Yan NH, Pang YG, Yuan M, Chen GJ, et al. (2009). Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res 1284:1–11 [DOI] [PubMed] [Google Scholar]
- 43.Monni E, Cusulin C, Cavallaro M, Lindvall O. and Kokaia Z. (2014). Human fetal striatum-derived neural stem (ns) cells differentiate to mature neurons in vitro and in vivo. Curr Stem Cell Res Ther 9:338–346 [DOI] [PubMed] [Google Scholar]
- 44.Bliss T, Guzman R, Daadi M. and Steinberg GK. (2007). Cell transplantation therapy for stroke. Stroke 38:817–826 [DOI] [PubMed] [Google Scholar]
- 45.Rosner M. and Hengstschlager M. (2013). Amniotic fluid stem cells and fetal cell microchimerism. Trends Mol Med 19:271–272 [DOI] [PubMed] [Google Scholar]
- 46.Rosner M, Dolznig H, Schipany K, Mikula M, Brandau O. and Hengstschlager M. (2011). Human amniotic fluid stem cells as a model for functional studies of genes involved in human genetic diseases or oncogenesis. Oncotarget 2:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosper NA, Bank RA. and van den Berg PP. (2014). Human amniotic fluid-derived mesenchymal cells from fetuses with a neural tube defect do not deposit collagen type i protein after TGF-beta1 stimulation in vitro. Stem Cells Dev 23:555–562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.