Abstract
STUDY QUESTION
What are the direct effects of progesterone (P4) and estradiol (E2) on the development and function of primate follicles in vitro from the pre-antral to early antral stage?
SUMMARY ANSWER
In a steroid-depleted milieu, E2 improved follicle survival, growth, antrum formation and oocyte health, whereas P4 exerted minimal beneficial effects on follicle survival and reduced oocyte health.
WHAT IS KNOWN ALREADY
Effects of P4 and E2 on follicle development have been studied primarily in large antral and pre-ovulatory follicles. Chronic P4 exposure suppresses antral follicle growth, but acute P4 exposure promotes oocyte maturation in pre-ovulatory follicles. Effects of E2 can be stimulatory or inhibitory depending upon species, dose and duration of exposure.
STUDY DESIGN, SIZE, DURATION
Non-human primate model, randomized, control versus treatment. Macaque (n = 6) secondary follicles (n = 24 per animal per treatment group) were cultured for 5 weeks.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Adult rhesus macaque secondary follicles were encapsulated in 0.25% alginate and cultured individually in media containing follicle stimulating hormone plus (i) vehicle, (ii) a steroid-synthesis inhibitor, trilostane (TRL, 250 ng/ml), (iii) TRL + low E2 (100 pg/ml) or progestin (P, 10 ng/ml R5020) and (iv) TRL + high E2 (1 ng/ml E2) or P (100 ng/ml R5020). Follicles reaching the antral stage (≥750 µm) were treated with human chorionic gonadotrophin for 34 h. End-points included follicle survival, antrum formation, growth pattern, plus oocyte health and maturation status, as well as media concentrations of P4, E2 and anti-Müllerian hormone (AMH).
MAIN RESULTS AND THE ROLE OF CHANCE
In a steroid-depleted milieu, low dose, but not high dose, P improved (P < 0.05) follicle survival, but had no effect (P > 0.05) on antrum formation and AMH production. Low-dose P increased (P < 0.05) P4 production in fast-grow follicles, and both doses of P elevated (P < 0.05) E2 production in slow-grow follicles. Additionally, low-dose P increased (P < 0.05) the percentage of no-grow follicles, and high-dose P promoted oocyte degeneration. In contrast, E2, in a steroid-depleted milieu, improved (P < 0.05) follicle survival, growth, antrum formation and oocyte health. E2 had no effect on P4 or E2 production. Follicles exposed to E2 yielded mature oocytes capable of fertilization and early cleavage, at a rate similar to untreated control follicles.
LIMITATIONS, REASONS FOR CAUTION
This study is limited to in vitro effects of P and E2 during the interval from the secondary to small antral stage of macaque follicles.
WIDER IMPLICATIONS OF THE FINDINGS
This study provides novel information on the direct actions of P4 and E2 on primate pre-antral follicle development. Combined with our previous report on the actions of androgens, our findings suggest that androgens appear to be a survival factor but hinder antral follicle differentiation, E2 appears to be a survival and growth factor at the pre-antral and early antral stage, whereas P4 may not be essential during early folliculogenesis in primates.
STUDY FUNDING/COMPETING INTEREST(S)
NIH P50 HD071836 (NCTRI), NIH ORWH/NICHD 2K12HD043488 (BIRCWH), ONPRC 8P51OD011092. There are no conflicts of interest.
Keywords: ovary, follicle development, estrogen, progesterone
Introduction
The ultimate goal of folliculogenesis is to produce a mature oocyte. The process requires early follicular growth (proliferation of oocyte-surrounding granulosa and theca cells and the formation of fluid-filled antrum) and subsequent follicular differentiation that facilitates maturation of the oocyte and ovulation. Granulosa and theca cells are also steroid-secreting cells; the latter synthesize progesterone (P4) and androgens, which diffuse to granulosa cells and serve as precursors for estrogen (E) synthesis (Hillier et al., 1994). P4 and E have important endocrine functions and are crucial for ovulation; however, whether growing ovarian follicles are direct targets for P4 or E′s action are less clear, especially in the primates.
To understand the role of P4 and E signaling in the ovary, knockout (KO) mouse models lacking genomic progesterone receptor (PR), progesterone receptor membrane component 1 (PGRMC1) and estrogen receptors (ERs; two subtypes, α and β) were generated (for review, see Barnett et al., 2006; Peluso and Pru, 2014). Defects during early follicle growth were observed in βERKO mice that were subfertile with decreased numbers of large antral follicles (Krege et al., 1998), as well as in PGRMC1 KO mice that exhibited a dysregulation in pre-antral to antral follicle development (Peluso and Pru, 2014). In contrast, deficiencies in ovulation were observed in PRKO and αERKO mice (Lydon et al., 1996; Couse and Korach, 1999). While transgenic mouse models provide insight into P4 and E′s roles in ovarian function, there are known species-specific differences in PR and ER expression, signaling and action in the ovary between rodents and primates. Nuclear PR is temporarily expressed in granulosa cells of pre-ovulatory follicles following the gonadotrophin surge in rodents, monkeys, pigs and human (Iwai et al., 1990; Natraj and Richards, 1993; Teilmann et al., 2006; Durlej et al., 2010). However, while PR is also observed in the theca of pre-antral and small antral follicles in rhesus monkeys (Hild-Petito et al., 1988) and women (Suzuki et al., 1994), it is absent in all stages of mouse follicles (Shao et al., 2003). In addition, recent discovery of the membrane-bound PGRMC1, which locates in granulosa and theca cells of pre-antral and antral follicles, postulates a greater involvement of P4’s action during follicle development (for review, see Peluso, 2013). It is generally believed that P4 suppresses follicle growth, as demonstrated by a correlation between circulating P4 levels and delayed antral follicle development in rats, rabbit and monkeys (Buffler and Roser, 1974; diZerega and Hodgen, 1982; Setty and Mills, 1987). However, local actions of P4 on growing follicles remain to be established.
ER expression in the ovary is more conserved among different species: ERβ is expressed in granulosa cells, while ERα is expressed in theca cells of growing follicles in rodents (Tetsuka et al., 1998; Sar and Welsch, 1999), cows(Rosenfeld et al., 1999), pigs (Slomczynska and Wozniak, 2001) and primates (Pelletier and El-Alfy, 2000; Saunders et al., 2000). However, E′s ovarian action is more complex and can be stimulatory or inhibitory depending on the species, dosage and treatment duration (for review, see Hutz, 1989). Diethylstilbestrol, a synthetic estrogen, stimulates granulosa cell proliferation and follicle growth in vivo (Goldenberg et al., 1972; Reiter et al., 1972), and 17β estradiol (E2) promotes DNA synthesis in cultured granulosa cells (Bendell and Dorrington, 1991) of rats. On the contrary, exogenous diethylstilbestrol did not alter follicle growth and E2 exposure disrupted ovulation and caused follicle atresia in monkeys (Clark et al., 1981; Dierschke et al., 1985). Additionally, a synergistic effect of E and follicle stimulating hormone (FSH) in granulosa cell proliferation and function is well established in rodents (Richards, 1980). Whether such synergism exits in primates is unclear (for review, see Hutz, 1989; Zelinski-Wooten and Stouffer, 1996; Chaffin and VandeVoort, 2013). Together, studies to date have not tackled direct actions of P4 and E on primate follicles during early folliculogenesis.
Our group developed a 3-dimensional (D) follicle culture system that allows the manipulation of individual follicles from rhesus macaques, and the study of their growth and function (Xu et al., 2010). Using this system, we observed that macaque secondary follicles can grow, form an antrum, produce steroid hormones (including P4 and E2) and growth factors, and in some instances yield healthy oocytes capable of maturation, fertilization and early cleavage. Previously, using an ‘ablation-replacement’ approach, we reported that trilostane (TRL), a steroid-synthesis inhibitor, diminished survival, growth and maturation of macaque follicles in vitro (Rodrigues et al., 2015). Replacement of androgens, testosterone (T) or dihydrotestosterone (DHT) demonstrated direct actions of these steroids on the pre-antral and antral-stage follicles in primates (Rodrigues et al., 2015). In the current study, two doses of R5020, a non-metabolizable progestin (P) analog, and E2, were used to study P4 and E′s direct effects, respectively, on early follicular growth, function, as well as oocyte competence in a steroid-depleted milieu in the rhesus macaque. The doses of E2 and P were chosen based on circulating levels (low dose, Young et al., 2003) as well as levels produced by the follicles (high dose, Xu et al., 2010) of P4 and E2.
Materials and Methods
Ethical approval
The studies were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by ONPRC's Institutional Animal Care and Use Committee.
Animals and ovary collection
The general care and housing of rhesus macaque monkeys (Macaca mulatta) was provided by the Division of Comparative Medicine, Oregon National Primate Research Center (ONPRC). Animals were pair-caged in a temperature-controlled (22°C) light-regulated (12L:12D) room and fed monkey chow twice a day and water ad libitum.
Ovaries were collected at necropsy from six adults, female rhesus monkeys (9.5 ± 2.1 years old) terminated for reasons unrelated to reproductive health. All animals exhibited regular menstrual cycles prior to necropsy. Ovaries were placed in 3-(N-morpholino) propane sulfonic acid-buffered holding media (CooperSurgical, Inc., Trumbull, CT, USA) and immediately transported to the laboratory at 37°C. All chemicals used in the study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
Follicle isolation, encapsulation and culture
Two or three outermost layers (0.5-mm thick) of the ovarian cortex were collected using a Stadie-Riggs tissue slicer (Thomas Scientific, Swedesboro, NJ, USA). Cortical slices were cut into 1 × 1 mm2 fragments using a McIlwain tissue chopper (Ted Pella, Inc., Redding, CA, USA). Follicle isolation, encapsulation and culture were performed as described previously (Xu et al., 2010). Briefly, healthy secondary follicles (125–250 µm) were isolated mechanically in holding media using 31-gauge needles. Each follicle was then placed individually in ∼5 µl of 0.25% (w/v) sterile sodium alginate (FMC BioPolymers, Philadelphia, PA, USA), and the droplet cross linked in 50 mM CaCl2 solution. Encapsulated follicles were cultured individually in 300 μl of alpha minimum essential medium (Invitrogen, Carlsbad, CA, USA) supplemented with 0.3% (v/v) human Serum Protein Substitute (CooperSurgical), 0.5 mg/ml bovine fetuin, 5 μg/ml transferrin, 0.5 μg/ml insulin and 5 ng/ml sodium selenite. Follicles were cultured at 37°C under 5% O2 and 6% CO2 with recombinant human FSH (NV Organon, Oss, Netherlands) at 3 ng/ml (100 mIU) for the first 21 days to promote pre-antral follicle survival; then the FSH concentration was decreased to 0.3 ng/ml for the remainder of the culture period to support antral follicle and oocyte growth (Sanchez et al., 2010; Xu et al., 2011, 2013b). Every other day, half of the culture media was exchanged with fresh media (prepared weekly) and stored at −20°C for subsequent hormone measurements. Recombinant human chorionic gonadotrophin (hCG; 100 ng/ml; Ovidrel, Serono, Geneva, Switzerland) was added to the culture medium to initiate meiotic oocyte maturation in a subset of follicles that reached the antral stage and 750 µm in diameter (Peluffo et al., 2010).
Experiment 1: progesterone replacement
The 3β-hydroxysteroid dehydrogenase (3β-HSD) inhibitor, TRL (Vetoryl, Dechra Veterinary Products, Overland Park, KS, USA) was used to produce a steroid-hormone depleted milieu. At 250 ng/ml, TRL effectively blocks P4 production by primate granulosa cells (Duffy et al., 1996) as well as secondary follicles (Rodrigues et al., 2015) in vitro. To evaluate the direct action of P4 during folliculogenesis, two doses of R5020 (in 100% ethanol), a non-aromatizable P, thus eliminating the conversion of P4 to E or androgen, were added in addition to TRL (in 100% ethanol), plus a vehicle control (CONT) group. Rhesus monkey (n = 3) secondary follicles (n = 24 per monkey per treatment group) were randomly divided into four treatment groups: (i) CONT: control media plus vehicle (100% ethanol), (ii) TRL (250 ng/ml, Rodrigues et al., 2015), (iii) TRL + low R5020 concentration (10 ng/ml, TRL + LP, Young et al., 2003) and (iv) TRL + high R5020 concentration (100 ng/ml, TRL + HP; Xu et al., 2010).
Experiment 2: estrogen replacement
Similarly, to evaluate the direct action of E, two doses of E2 (in 100% ethanol) were added to the culture media containing TRL, plus vehicle control and TRL alone. Rhesus monkey (n = 3) secondary follicles (n = 24 per monkey per treatment group) were randomly divided into four treatment groups: (i) CONT: control media plus vehicle (100% ethanol), (ii) TRL (250 ng/ml), (iii) TRL + low E2 concentration (100 pg/ml; TRL + LE; Young et al., 2003) and (iv) TRL + high E2 concentration (1 ng/ml; TRL + HE; Xu et al., 2010).
Follicle survival, growth, antrum formation and classification of follicular growth
All follicles were cultured for 5 weeks. Follicles that underwent hCG treatment were maintained for up to 1 additional week of culture. Weekly images were taken using an Olympus DP11 digital camera attached to an Olympus CK40 microscope (Olympus Imaging America, Inc., Center Valley, PA, USA), and survival as well as antrum formation were assessed (Xu et al., 2010). Follicles were considered to be degenerating if the oocyte became dark or no longer surrounded by a layer of granulosa cells, the granulosa cells were dark or lysed, or the diameter of the follicle decreased. For surviving follicles, two diameters (the longest diameter of the follicle plus a second measurement perpendicular to the first) were measured using the ImageJ 1.44 p software (National Institutes of Health, Bethesda, MD, USA) and the average was recorded as the follicle diameter. Based on prior studies (Xu et al., 2010), surviving follicles were stratified into three categories according to their size at Week 5: (i) fast-grow: diameters ≥500 µm, (ii) slow-grow: diameters between 250 and 500 µm and (iii) no-grow: diameters <250 µm.
Anti-Müllerian hormone and ovarian steroid measurements
Peak concentrations (Xu et al., 2010) of anti-Müllerian hormone (AMH) (Week 3), P4 (Week 5) and E2 (Week 5) in media of fast-grow and slow-grow follicles were measured by the ONPRC Endocrine Technology Support Core. Hormones produced by no-grow follicles are minimal (Xu et al., 2010) and not measured. AMH levels were analyzed by enzyme-linked immunosorbent assay using a DSL-10-14400 kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA) based on the manufacturers' instructions. An Immulite 2000 chemiluminescence based automatic clinical platform (Siemens Healthcare Diagnostics, Deerfield, IL, USA) was used for steroid assays. All assays were validated previously for macaque follicle culture media (Xu et al., 2010).
Oocyte retrieval, maturation and fertilization
The cumulus–oocyte complex was harvested 34 h after hCG treatment, oocytes stripped of granulosa and cumulus cells (hyaluronidase, 2 mg/ml; Peluffo et al., 2010) and their meiotic maturation status determined. Conventional in vitro fertilization (IVF) was performed using oocytes reaching the metaphase I (MI) or II (MII) stage of meiosis, and fresh sperm (Assisted Reproductive Technologies Core, ONPRC) as previously described (Lanzendorf et al., 1990). Fourteen to sixteen hour post-IVF, oocytes were stripped of attached sperm and fertilization was confirmed by the presence of two polar bodies and two pronuclei. Fertilized oocytes were transferred to Global embryo culture media supplemented with Life Global Protein Supplement (Life Global; Guilford, CT, USA) and embryo images and development was recorded every other day (Xu et al., 2013a).
Oocyte immunofluorescence
Healthy oocytes harvested from hCG-treated follicles that remained at the germinal vesicle (GV) stage were fixed in 4% paraformaldehyde at 37°C, immunolabeled for actin (Alexa488-phalloidin; Invitrogen), tubulin (anti-alpha-tubulin; Sigma) and DNA (Hoechst 33342; Invitrogen) as described previously (Peluffo et al., 2012). Fluorescent labeled oocytes were analyzed with confocal microscopy (Leica SP5 AOBS spectral confocal system, Buffalo Grove, IL, USA; ONPRC Imaging Support Core).
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was determined using one-way analysis of variance (ANOVA) and Student–Newman–Keuls post hoc analysis (SigmaPlot 11.0; Systat Software, Inc., San Jose, CA, USA) for data comparison among different treatment groups. One-way ANOVA with repeated measurements was performed for comparing data among different time points within the same group. Difference was considered significant when P is ≤ 0.05.
Results
Follicle survival and growth
Five-week survival rate
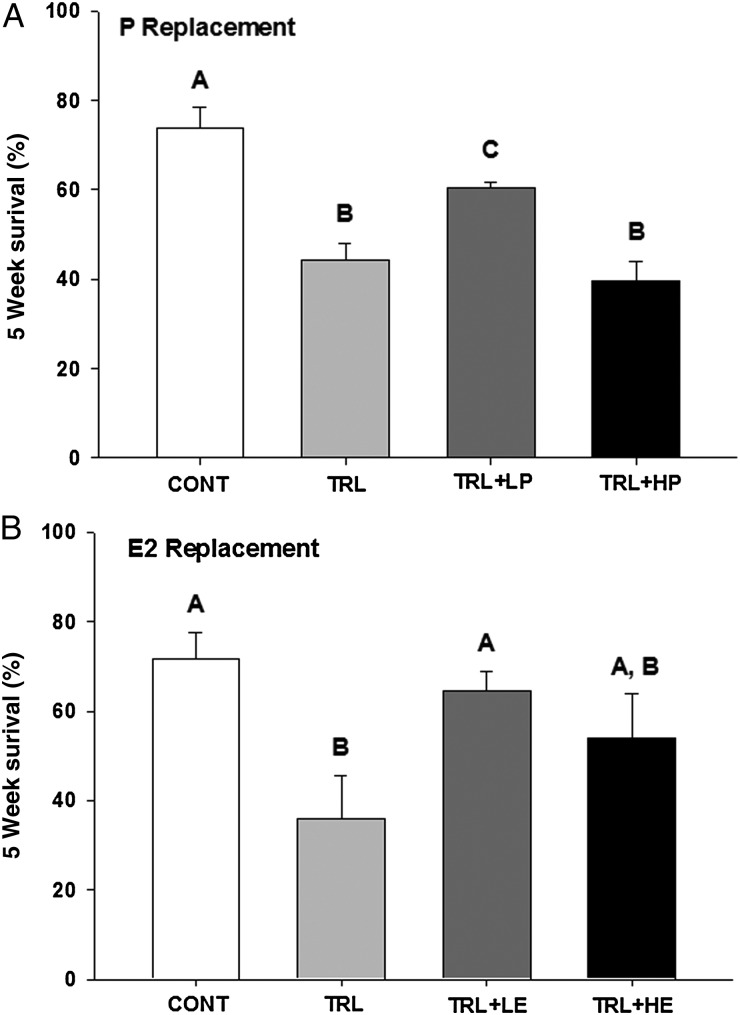
An average of 74 ± 5 and 72 ± 6% of control follicles remained healthy after 5 weeks in culture in the P (Fig. 1A) and E2 (Fig. 1B) replacement experiments, respectively. Survival rate decreased (P < 0.05) in TRL, compared with vehicle control (CONT) in both experiments. Low-dose R5020 (LP, 10 ng/ml) increased (P < 0.05) survival rate compared with TRL alone (Fig. 1A), but remained lower (P < 0.05) than the control level. Survival rates in TRL + high P (HP; 100 ng/ml R5020) were not different from TRL alone. In the E2 replacement experiment, survival was restored to the CONT level by the addition of low-dose E2 (LE, 100 pg/ml) in TRL, but not as well by the higher concentration (HE, 1 ng/ml) in TRL.
Figure 1.
Follicle (n = 72 follicles/treatment group) survival rate after 5 weeks (Wks) of culture in progestin (P; R5020, A) and estradiol (E2, B) replacement experiments. CONT, control; TRL, trilostane; LP, low-dose P; HP, high-dose P; LE, low-dose E2 and HE, high-dose E2. Data are presented as mean ± SEM. Different letters represent significant differences between treatment groups in each experiment.
Growth characteristics
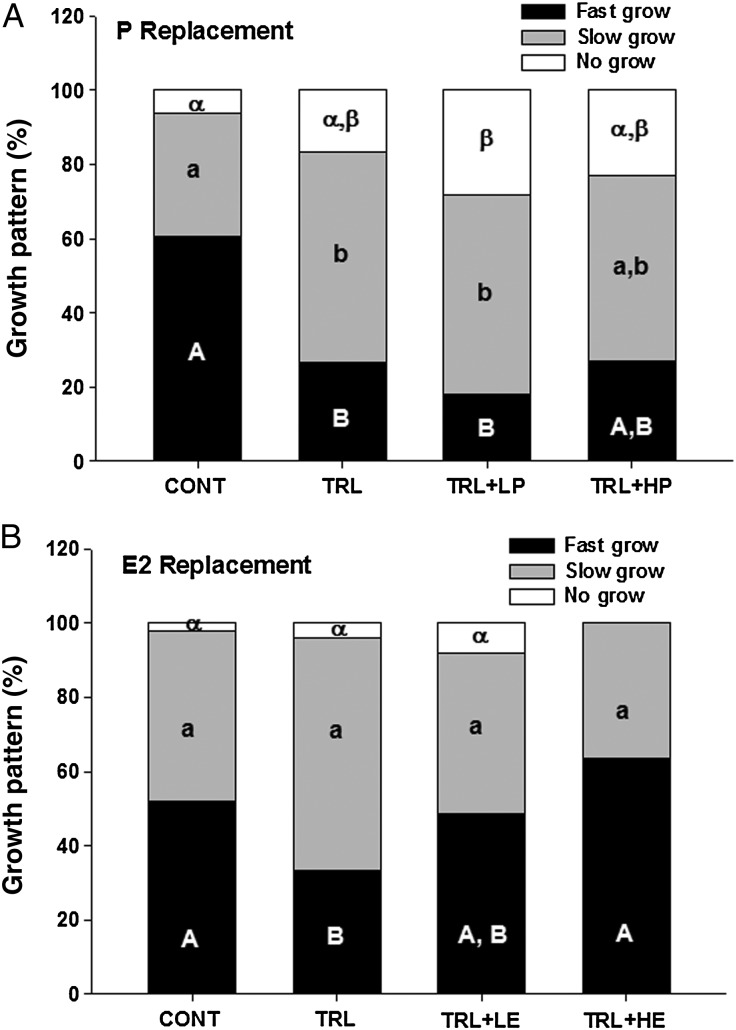
At Week 5, surviving follicles were stratified into three groups (fast-grow, slow-grow and no-grow). In the P replacement experiment, the control group was comprised of 60 ± 10% fast-grow, 33 ± 5% slow-grow and 6 ± 4% no-grow follicles (Fig. 2A). The percentage of fast-grow follicles decreased (P < 0.05) and the percentage of slow-grow increased during TRL treatment. Neither dose of R5020 was able to restore the proportion of fast-grow and slow-grow follicles in the presence of TRL. In fact, low-dose R5020 increased the percentage of no-grow follicles in TRL + LP, compared with CONT. In the E2 replacement experiment, the control group was comprised of 52 ± 12% fast-grow, 46 ± 10% slow-grow and 2 ± 2% no-grow follicles (Fig. 2B). The percentage of fast-grow follicles again decreased (P < 0.05) during TRL treatment. This effect was partially prevented by the low-dose E2 with TRL and completely reversed by the high-dose E2 with TRL, to the control level. No significant changes were found in the proportion of slow-grow and no-grow populations; however, no-grow follicles were absent in TRL + HE.
Figure 2.
Growth characteristics of surviving follicles in progestin (P, A) and estradiol (E2, B) replacement experiments. CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2. Different capital, lower case and Greek letters represent statistical differences in fast-grow, slow-grow and no-grow follicle populations, respectively, between treatment groups in each experiment.
Antrum formation rate
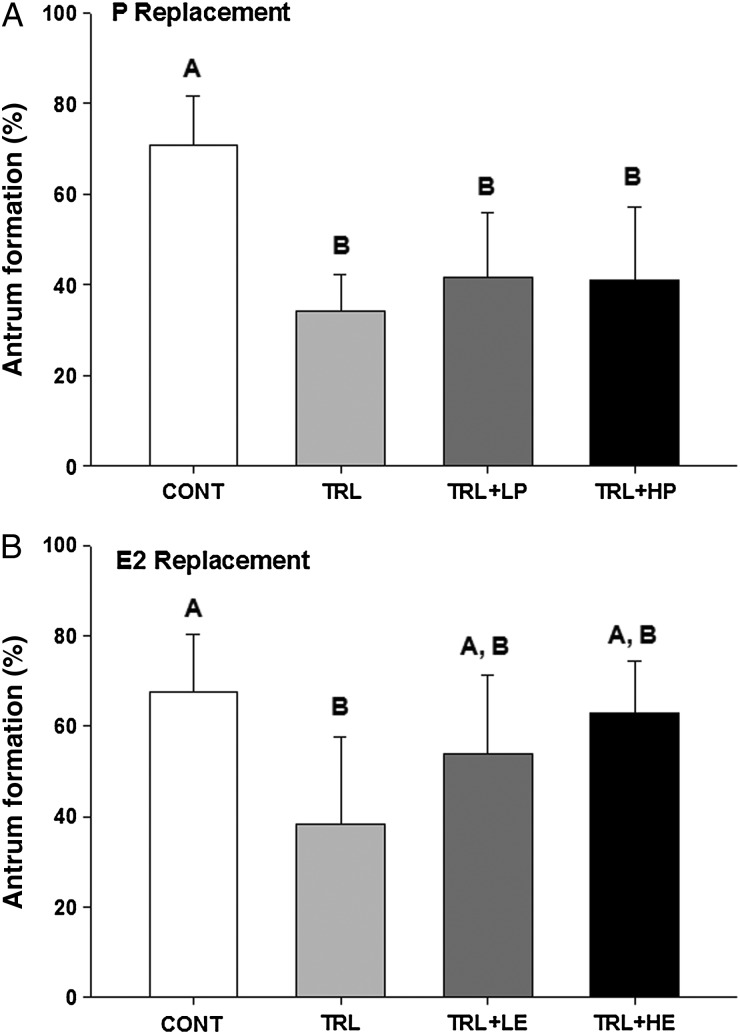
An average of 71 ± 11 and 68 ± 3% of the surviving control follicles formed an antrum in P (Fig. 3A) and E2 (Fig. 3B) replacement experiments, respectively. TRL decreased (P < 0.05) antrum formation rates, compared with the controls, in both experiments. Neither low- or high-dose R5020 increased antrum formation, compared with TRL alone (Fig. 3A). In contrast, E2 addition to TRL seemed to restore the antrum formation rate to the CONT level (not different from control or TRL, Fig. 3B).
Figure 3.
Antrum formation rate of surviving follicles in progestin (P, A) and estradiol (E2, B) replacement experiments. CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2. Data are presented as mean ± SEM. Different letters represent significant differences between treatment groups in each experiment.
AMH, P and E concentrations
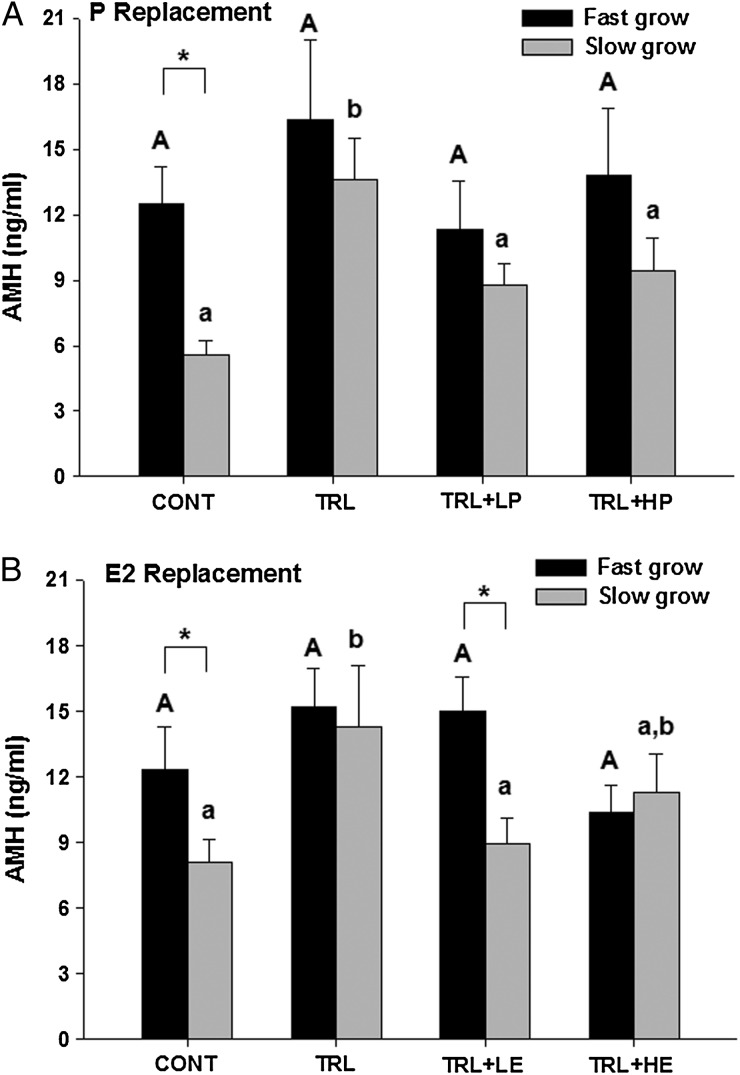
AMH levels in control fast-grow and slow-grow follicles at Week 3 of culture averaged 12 ± 2 and 6 ± 1 ng/ml, respectively, in the P replacement experiment (Fig. 4A), and 12 ± 2 and 8 ± 1 ng/ml, respectively, in the E2 replacement experiment (Fig. 4B). As expected (Xu et al., 2013a), fast-grow follicles produced higher (P < 0.05) levels of AMH compared with slow-grow follicles in control conditions in both experiments. AMH concentrations remained similar among different treatment groups in fast-grow follicles in both experiments. However, in slow-grow follicles, AMH levels increased (P < 0.05) during TRL exposure but returned to that of controls with the addition of R5020 in TRL + LP and TRL + HP groups, or E2 in TRL + LE and TRL + HE groups.
Figure 4.
Media anti-Müllerian Hormone (AMH) concentrations (ng/ml) in fast-grow and slow-grow follicles in progestin (P, A) and estradiol (E2, B) replacement experiments at Week 3 of culture. CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2. Data are presented as mean ± SEM. Different capital and lower case letters represent differences in fast-grow and slow-grow follicle populations, respectively, between treatment groups in each experiment. Asterisk represents differences between fast-grow and slow-grow follicles within the same treatment group.
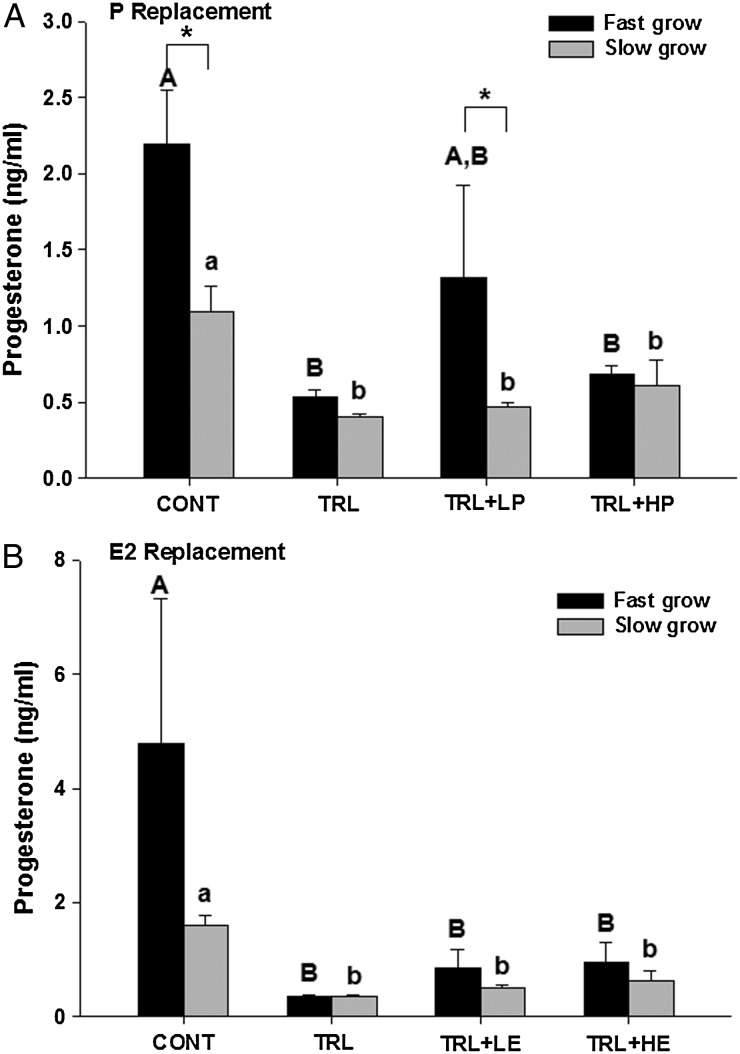
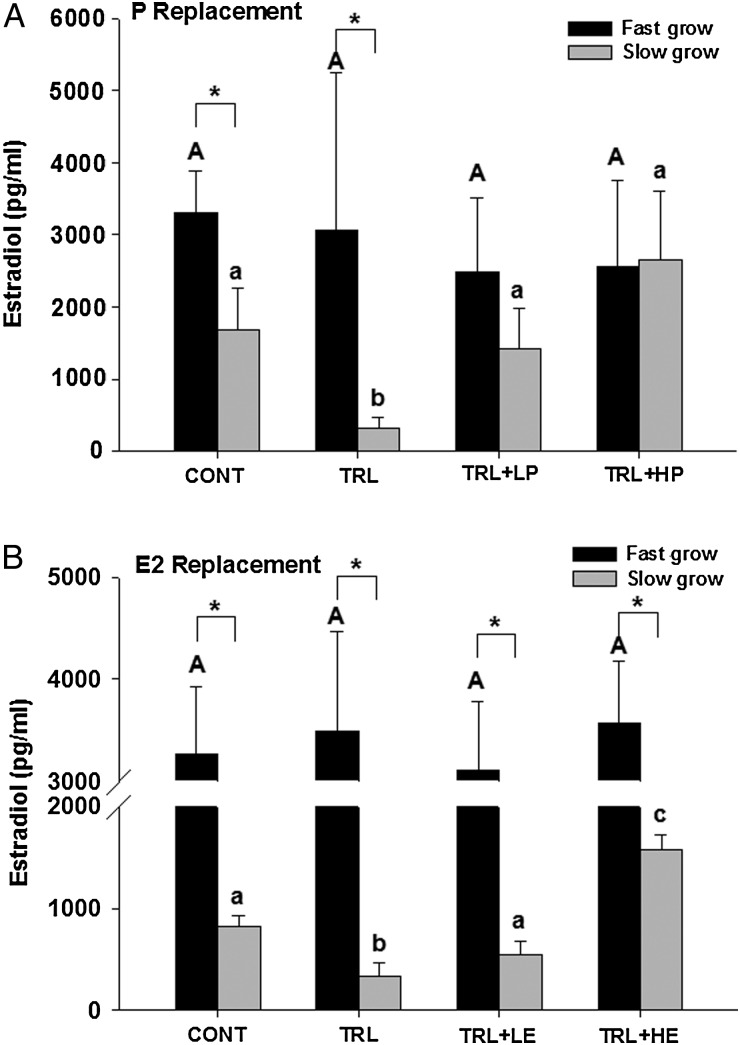
As expected (Xu et al., 2013a), fast-grow follicles produced higher (P < 0.05) levels of P4 compared with slow-grow follicles in control conditions during the P (Fig. 5A) replacement experiments. Additionally, TRL exposure markedly decreased (P < 0.05) P4 levels in both fast-grow and slow-grow populations in both P (Fig. 5A) and E2 (Fig. 5B) replacement experiments. However, low-dose P seemed to increase P4 in fast-grow follicles to levels not significantly different from controls, even though R5020 does not cross react with P4 in the assay utilized and is not reflected in the P4 levels (Fig. 5A). High-dose P did not alter P4 levels compared with TRL in slow-grow or fast-grow follicles. Neither dose of E2 altered P4 concentrations, compared with the suppressed (P < 0.05) P4 levels in TRL alone for either fast- or slow-grow follicles (Fig. 5B).
Figure 5.
Media progesterone concentrations (ng/ml) in fast-grow and slow-grow follicles in progestin (P, A) and estradiol (E2, B) replacement experiments at Week 5 of culture. CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2. Data are presented as mean ± SEM. Different capital and lower case letters represent differences in fast-grow and slow-grow follicle populations, respectively, between treatment groups in each experiment. Asterisk represents differences between fast-grow and slow-grow follicles within the same treatment group.
As expected (Xu et al., 2013a), fast-grow follicles produced higher (P < 0.05) levels of E2 compared with slow-grow follicles in control conditions of both the P (Fig. 6A) and E2 (Fig. 6B) replacement experiments. For fast-grow follicles, E2 levels remained similar among all treatment groups in both experiments. For slow-grow follicles, TRL exposure decreased (P < 0.05) E2 levels, compared with CONT in both experiments. This reduction was reversed to control levels by the addition of either dose of R5020 (Fig. 6A), as well as low-dose E2 (Fig. 6B). The addition of high-dose E2 elevated (P < 0.05) E2 levels above that of controls.
Figure 6.
Media estradiol (E2) concentrations (pg/ml) in fast-grow and slow-grow follicles in progestin (P, A) and E2 (B) replacement experiments at Week 5 of culture. CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2. Data are presented as mean ± SEM. Different capital and lower case letters represent differences in fast-grow and slow-grow follicle populations, respectively, between treatment groups in each experiment. Asterisk represents differences between fast-grow and slow-grow follicles within the same treatment group.
Follicle, oocyte and embryo development
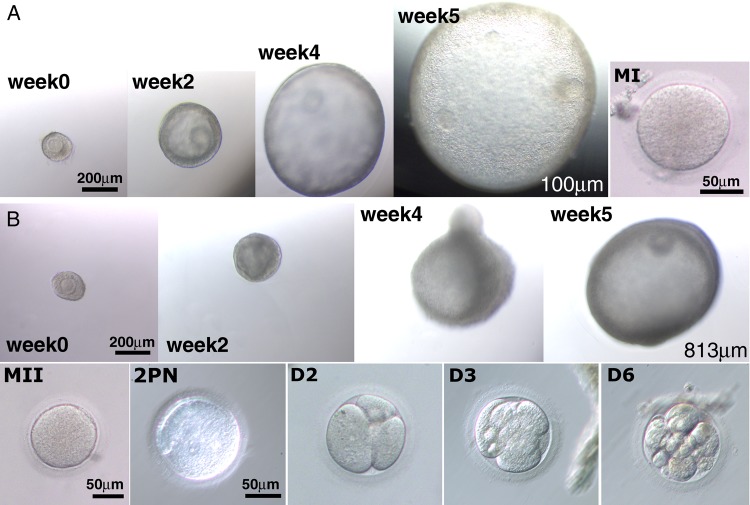
During 5 weeks of culture, fast-grow follicles exhibited increased diameters and formed an antrum in both P (Fig. 7A) and E2 (Fig. 7B) replacement experiments. The number of follicles harvested and oocytes retrieved after hCG treatment, as well as oocyte meiotic status and diameter, is summarized in Table I. TRL treatment increased (P < 0.05) the percentage of degenerated oocytes, compared with controls in both experiments; therefore, fewer healthy oocytes were available for analysis. Neither dose of R5020 was able to prevent oocyte degeneration. In fact, high-dose P increased (P < 0.05) the percentage of degenerated oocytes compared with controls. One oocyte showed progression to the MI stage in control follicles in the P replacement study, but failed to show evidence of fertilization after IVF (Fig. 7A). In contrast, low- or high-dose E2, reduced oocyte degeneration to control levels (Table I). Oocyte maturation to the MI and MII stage (n = 1–2 per treatment group) was observed in controls, and follicles treated with low- and high-dose E2 with TRL. Mature oocytes (MII) displayed a diameter of ≥112 µm and were capable of fertilization and early cleavage (Fig. 7B).
Figure 7.
Follicle, oocyte and embryo development in progestin (P, A) and estradiol (E2, B) replacement experiments. Represented photomicrographs from both experiments showing secondary follicles that grew and formed an atrum (∼week [Wk] 3) with diameters that can reach 1.2 mm (not shown) during 5 weeks of culture. Thirty-four hours after human chorionic gonadotrophin treatment, some oocytes matured to the metaphase I (MI) or MII stage and when subjected to in vitro fertilization, a few exhibited two pronuclei (2PN) formation and early cleavage (3–4 cell on Day [D] 2, 6–8 cell on D3 and 10–16 cell on D4).
Table I.
Health, meiotic maturation status and diameter of oocytes retrieved from antral follicles at Week 5 (34 h after the addition of hCG) in progestin (P, R5020) and estradiol (E2) replacement experiments.
| Number (n) of |
Oocyte diameter (µm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Follicles harvested | Oocytes retrieved | Degenerated oocytes (%) | Healthy oocytes |
GV | MI | MII | |||
| GV | MI | MII | |||||||
| P replacement | |||||||||
| Control | 22 | 22 | 9 (41 ± 10)a | 12 | 1 | 0 | 102 ± 1 | 108 | – |
| TRL | 8 | 8 | 5 (58 ± 8)b | 3 | 0 | 0 | 101 ± 4 | – | – |
| TRL + LP | 12 | 11 | 6 (47 ± 7)a,b | 5 | 0 | 0 | 99 ± 1 | – | – |
| TRL + HP | 5 | 5 | 4 (83 ± 17)b | 1 | 0 | 0 | 101 | – | – |
| E2 replacement | |||||||||
| Control | 13 | 12 | 5 (38 ± 7)A | 6 | 0 | 1 | 102 ± 2 | – | 115 |
| TRL | 9 | 9 | 7 (87 ± 13)B | 2 | 0 | 0 | 96 ± 1 | – | – |
| TRL + LE | 14 | 13 | 4 (28 ± 15)A | 7 | 1 | 1 | 100 ± 1 | 103 | 112 |
| TRL + HE | 9 | 9 | 3 (25 ± 14)A | 5 | 1 | 0 | 104 ± 3 | 107 | – |
CONT, control, TRL, trilostane, LP, low-dose P, HP, high-dose P, LE, low-dose E2 and HE, high-dose E2.
Different lower and capital case letters represent differences in P and E2 replacement experiments, respectively, between treatment groups.
GV chromatin and TZPs in GV oocytes
Many oocytes retrieved from hCG-exposed antral follicles did not reinitiate meiosis, but appeared to be healthy. Immunoflourescent labeling revealed that these oocytes exhibited normal chromatin configuration at the GV stage with extensive transzonal projections (TZPs; Fig. 8).
Figure 8.
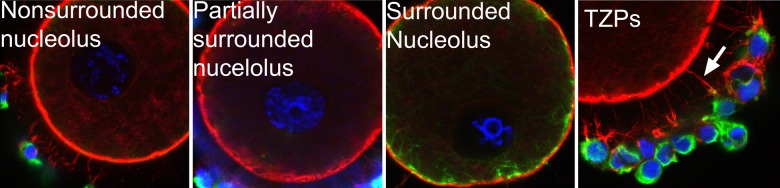
Immunofluorescent labeling for actin (red), tubulin (green) and DNA (blue) in germinal vesicle (GV)-intact oocytes from in vitro grown follicles. Healthy GV oocytes showed normal chromatin configuration including non-surrounded nucleolus (diffused), partially surrounded nucleolus and surrounded nucleolus. Extensive development of the transzonal projections (TZPs) was also evident in cumulus–oocyte complexes.
Discussion
As previously reported, the steroid synthesis inhibitor, TRL decreased survival, growth and function including antrum formation and hormone production in macaque secondary follicles in culture (Rodrigues et al., 2015), suggesting important roles of steroid hormones in primate folliculogenesis. In addition, we recently reported that androgen (T and/or DHT) replacement, in a steroid-depleted milieu, promoted pre-antral follicle survival and growth, but suppressed follicular activities such as AMH and E2 production (Rodrigues et al., 2015). The current study examined the direct effects of E (E2 replacement) or P4 (R5020 replacement) on early primate folliculogenesis in a steroid-depleted milieu, using a 3D culture system.
Our results suggest that P4 acts directly on pre-antral follicles and at a lower, but not higher, concentration, promotes follicle survival. However, low-dose R5020 only partially restored follicle survival, suggesting other steroid hormones, such as androgen (Rodrigues et al., 2015) or E, are required for normal follicular health. The role of P4 as an anti-apoptotic factor was observed in granulosa cells of large antral folliclesin rodents (Peluso and Pappalardo, 1998; Peluso et al., 2006), monkeys (Chaffin and Stouffer, 2000; Puttabyatappa et al., 2013) and women (Svensson et al., 2001). In addition, it is well established that P4 exhibits two modes of action on ovarian follicles: anti-apoptotic (Peluso, 1997) and growth inhibitory (Peluso and Pappalardo, 1998). Indeed, concentrations of norgestomet, another synthetic P, correlate negatively with the rate of ovarian follicle growth (Sanchez et al., 1995). Therefore, it is possible that the high dose, unlike the low dose, of R5020 exerted inhibitory effect on follicle survival in the current study. In contrast, both doses of E2, in the presence of TRL, improved and restored follicle survival rate to the control level. This is consistent with previous reports demonstrating that E inhibits granulosa cell apoptosis (Billig et al., 1993) and diminishes follicle atresia (Harman et al., 1975) in rats. Thus, E2 and to a lesser extent, Pact as pro-survival factors in primate follicles in culture.
Our results showed that the addition of P did not improve follicle growth. This might be expected as P4 is generally considered a suppressor of follicle growth (for review, see Peluso, 2006). On the contrary, the addition of E2 promoted follicle growth, and high-dose E2 completely eliminated no-grow follicles. This is consistent with studies showing that E increases the number and size of rodent and bovine follicles in vivo and in vitro (for review, see Hutz, 1989; Rosenfeld et al., 2001). Thus, E2, but not P, promotes early follicular growth in primate follicles in culture.
FSH stimulates antrum-like reorganization of granulosa cells in rats, which is augmented by E2 (Gore-Langton and Daniel, 1990). In the current study, TRL disrupted antrum formation in cultured follicles, suggesting that even in the presence of FSH, steroid hormones are important for antrum formation. Our results demonstrated that P is not crucial during antrum development. This is not surprising as P4 suppresses antrum follicle development as mentioned above (Peluso and Pappalardo, 1998). On the contrary, E2 restored antrum formation rates to the control level, implying a role of E in antrum formation in primate follicles. Effects of E on antrum formation were not reported previously, although mice lacking ERβ exhibit lack of progression from small to large antral follicles (Emmen et al., 2005). Whether this is a direct effect of E2 or a combined effect with FSH is unclear. Thus, E2, but not P, promotes antrum formation in primate follicles in vitro.
AMH is produced by granulosa cells and its level correlates with follicle size and growth pattern in pre-antral follicles, but decreases in large antral follicles in monkeys and women (Weenen et al., 2004; Thomas et al., 2007; Xu et al., 2010). Previously, we suggested that high AMH production in the pre-antral stage predicts those follicles destined to be fast-grow follicles and yield MII oocytes (Xu et al., 2013a). In the current study, there is no treatment effect on AMH levels in fast-grow follicles. It is possible that once stratified based on their size, follicles produce maximal levels of AMH in the presence of FSH regardless of their treatment. We speculate that a modestly increased AMH after TRL treatment in slow-grow follicles is due to TRL's inhibitory effect on granulosa cell differentiation and antrum development; therefore, AMH levels remained high in TRL-treated follicles which have delayed or diminished antrum development. Thus, neither E2 nor P affected AMH production by primate follicles in culture.
TRL, a competitive inhibitor of 3β-HSD (Schane et al., 1979), decreased, but did not totally eliminate, the conversion of pregnenolone to P4 by slow- and fast-grow follicles in vitro. Levels of P4 and E2 correlate with antral follicle development and are markers for antral follicle differentiation (Xu et al., 2010). In the current study, although it is unclear how, low dose R5020 stimulated P4 production. A similar observation occurred in our androgen replacement study where low dose, but not high dose, T increased P4 levels (Rodrigues et al., 2015). As mentioned above, follicle survival also exhibited such dose-dependent action of P4. In contrast, neither dose of E2 altered P4 levels, presumably due to already diminished P4 levels following TRL exposure. Others showed that exogenous E reduced P4 levels produced by pre-ovulatory follicles in monkeys (Hutz et al., 1986; Hutz et al., 1989) and women (Veldhuis et al., 1983). However, local actions of E on steroidogenesis of primate pre-antral and early antral follicles have not been previously examined. Notably, despite low P4 levels in both E2 replacement groups, follicles showed high survival and growth, implying again that P is not critical for early folliculogenesis in primates.
E2 production by fast-grow follicles was not altered by TRL or the addition of P or E2 treatment. As mentioned above, TRL did not eliminate P4 production which can serve as a substrate for E2 synthesis (spillover). This may coincide with increased FSH sensitivity and/or elevated aromatase activity in fast-grow follicles. In contrast, TRL inhibited E2 production in slow-grow follicles, possibly due to limited substrate availability for E2 production. Both doses of R5020 increased E2, even though R5020 is non-aromatizable and cannot serve as a substrate for E2 synthesis. The reason for this is unclear since in vitro exposure to R5020 has no effect on E2 production and inhibits FSH-stimulated E2 production by granulosa cells from rats (Schreiber et al., 1980; Fortune and Vincent, 1983). The addition of E2 increased E2 levels in a substrate-dependent manner. This is most likely due to levels of the exogenous E2 which is detectable in the E2 assay. While it is difficult to distinguish effect of E2 on follicular E2 production in the current study, others showed that E2 treatment reduced E2 levels in follicular fluid of pre-ovulatory follicles in monkeys (Hutz et al., 1986). Thus, P, in a dose-dependent manner, and E2, in a substrate-dependent manner, elevated E2 production by small antral follicles in culture.
In the current study, macaque secondary follicles (∼200 µm) grew to a maximum diameter of 1278 µm after 5 weeks in culture. However, few oocytes matured to the MII stage following hCG treatment. This is comparable with recent studies where ∼10% of small antral follicles treated with hCG yielded MI or MII oocytes (Xu et al., 2013a; Rodrigues et al., 2015). Nevertheless, fertilization and early embryonic cleavage was achieved in oocytes reaching the MI and MII stages. Those oocytes that did not respond to hCG appear healthy at the GV-stage with an extensive TZP network. TZPs are essential for communication between the granulosa/cumulus cells and oocyte, as well as for follicular development and maturation (Plancha et al., 2005). In addition, chromatin condensation or remodeling from non-surrounded to surrounded nucleolus around the nucleolus is associated with global transcriptional silencing and completion of oocyte growth in pre-ovulatory oocytes just prior to resumption of meiosis (Mattson and Albertini, 1990; Wiekowski et al., 1997; De La Fuente et al., 2004). Therefore, while the majority of oocytes from in vitro grown follicles failed to resume meiosis, the healthy GV oocytes showed progression towards oocyte competency.
The addition of R5020 was associated with an increased percentage of degenerating oocytes compared with controls and did not yield any mature oocytes. Previous studies showed that R5020, administered during the periovulatory interval, prevented TRL-induced follicle atresia and promoted oocyte maturation during the periovulatory interval in monkeys (Borman et al., 2004). In the current study, TRL and R5020 were added to pre-antral follicles and we speculate that R5020 was unable to support normal follicle development which subsequently led to reduced oocyte health. On the contrary, the current study suggests that local administration of E2, especially at the circulating level, improved oocyte health and yielded MII oocytes. This finding differs from previous studies showing that in vivo E2 exposure induced atresia of the dominant follicle in monkeys (Hutz et al., 1986) as well as in vitro E2 exposure delayed or inhibited oocyte meiotic maturation during secondary follicle culture in mice (Tarumi et al., 2014). However, in vivo E2 treatment led to a reduced local E2 production by the dominant follicle and E2 concentration used in the latter experiment is 1000 times over the physiological level. Whether E2 acts directly on the oocyte or on the somatic cells to support oocyte health and development is yet to be determined.
We can now compare the effects of P and E2 with those recently reported for T and DHT (Rodrigues et al., 2015), on various parameters of macaque follicles cultured in a 3D alginate matrix in a steroid-depleted milieu. The marked disruption in the growth and maturation of macaque pre-antral follicles in a steroid-depleted (but FSH replete) milieu is likely due to the loss of androgen (Rodrigues et al., 2015), E2, and to a lesser extend P actions on the follicle. Of the three steroids, androgen (DHT), followed by E2, were survival factors. In addition, E2 appeared to be a growth factor, promoting the rate of growth and formation of antral-stage follicles. Moreover, androgen (T, DHT) appeared to inhibit follicular differentiation or key activities, such as AMH and E2 production, with little effect of P or E2 on these functions. These results are consistent with the concept that E2 is an important factor promoting early folliculogenesis in primates, whereas P may not be essential. Although androgen is a survival factor for early growing follicles, its primary action switches to suppression of follicular development by the late pre-antral to early antral stage. Further studies are warranted to examine the effects of steroid combinations as a function of the stage of follicular development, as well as the receptor signaling pathways and cellular processes involved in steroid-regulated survival, growth and differentiation of primate follicles. This information could be valuable for understanding the perturbations in folliculogenesis associated with diseases such as polycystic ovarian syndrome, as well as improving the in vitro culture system for generating mature fertilizable oocytes from follicles of infertility patients.
Authors’ roles
A.Y.T., J.X. and R.L.S. were involved in the study conception, design, interpretation and analysis as well as manuscript preparation. A.Y.T and J.X. were involved in follicle isolation and culture. A.Y.T. was responsible for data acquisition and analysis. All authors have approved the final version and submission of this manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) P50 HD071836 (NCTRI), NIH ORWH/NICHD 2K12HD043488 (BIRCWH), ONPRC 8P51OD011092.
Conflict of interest
None declared.
Acknowledgements
We thank Dr. Cecily Bishop and Maralee Lawson for technical assistance. We are also grateful to the ONPRC Endocrine Technology Support Core (Dr. Francis Pau, Director), Imaging Support Core (Dr. Anda Cornea, Director), Assisted Reproductive Technologies Core Laboratory (Dr. Jon Hennebold, Director) and Division of Comparative Medicine.
References
- Barnett KR, Schilling C, Greenfeld CR, Tomic D, Flaws JA. Ovarian follicle development and transgenic mouse models. Hum Reprod Update 2006;5:537–555. [DOI] [PubMed] [Google Scholar]
- Bendell JJ, Dorrington J. Estradiol-17 beta stimulates DNA synthesis in rat granulosa cells: action mediated by transforming growth factor-beta. Endocrinology 1991;5:2663–2665. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Hsueh AJ. Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 1993;5:2204–2212. [DOI] [PubMed] [Google Scholar]
- Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod 2004;1:366–373. [DOI] [PubMed] [Google Scholar]
- Buffler G, Roser S. New data concerning the role played by progesterone in the control of follicular growth in the rat. Acta Endocrinol (Copenh) 1974;3:569–578. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL. Role of gonadotrophins and progesterone in the regulation of morphological remodelling and atresia in the monkey peri-ovulatory follicle. Hum Reprod 2000;12:2489–2495. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, VandeVoort CA. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp Biol Med (Maywood) 2013;5:539–548. [DOI] [PubMed] [Google Scholar]
- Clark JR, Dierschke DJ, Wolf RC. Hormonal regulation of ovarian folliculogenesis in rhesus monkeys: III. Atresia of the preovulatory follicle induced by exogenous steroids and subsequent follicular development. Biol Reprod 1981;2:332–341. [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Reproductive phenotypes in the estrogen receptor-alpha knockout mouse. Ann Endocrinol (Paris) 1999;2:143–148. [PubMed] [Google Scholar]
- De La Fuente R, Viveiros MM, Burns KH, Adashi EY, Matzuk MM, Eppig JJ. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol 2004;2:447–458. [DOI] [PubMed] [Google Scholar]
- Dierschke DJ, Hutz RJ, Wolf RC. Induced follicular atresia in rhesus monkeys: strength-duration relationships of the estrogen stimulus. Endocrinology 1985;4:1397–1403. [DOI] [PubMed] [Google Scholar]
- diZerega GS, Hodgen GD. The interovarian progesterone gradient: a spatial and temporal regulator of folliculogenesis in the primate ovarian cycle. J Clin Endocrinol Metab 1982;3:495–499. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Molskness TA, Stouffer RL. Progesterone receptor messenger ribonucleic acid and protein in luteinized granulosa cells of rhesus monkeys are regulated in vitro by gonadotropins and steroids. Biol Reprod 1996;4:888–895. [DOI] [PubMed] [Google Scholar]
- Durlej M, Tabarowski Z, Slomczynska M. Immunohistochemical study on differential distribution of progesterone receptor A and progesterone receptor B within the porcine ovary. Anim Reprod Sci 2010;1–2:167–173. [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology 2005;6:2817–2826. [DOI] [PubMed] [Google Scholar]
- Fortune JE, Vincent SE. Progesterone inhibits the induction of aromatase activity in rat granulosa cells in vitro. Biol Reprod 1983;5:1078–1089. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Vaitukaitis JL, Ross GT. Estrogen and follicle stimulation hormone interactions on follicle growth in rats. Endocrinology 1972;6:1492–1498. [DOI] [PubMed] [Google Scholar]
- Gore-Langton RE, Daniel SA. Follicle-stimulating hormone and estradiol regulate antrum-like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod 1990;1:65–72. [DOI] [PubMed] [Google Scholar]
- Harman SM, Louvet JP, Ross GT. Interaction of estrogen and gonadotrophins on follicular atresia. Endocrinology 1975;5:1145–1152. [DOI] [PubMed] [Google Scholar]
- Hild-Petito S, Stouffer RL, Brenner RM. Immunocytochemical localization of estradiol and progesterone receptors in the monkey ovary throughout the menstrual cycle. Endocrinology 1988;6:2896–2905. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol 1994;1–2:51–54. [DOI] [PubMed] [Google Scholar]
- Hutz RJ. Disparate effects of estrogens on in vitro steroidogenesis by mammalian and avian granulosa cells. Biol Reprod 1989;4:709–713. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Dierschke DJ, Wolf RC. Markers of atresia in ovarian follicular components from rhesus monkeys treated with estradiol-17 beta. Biol Reprod 1986;1:65–70. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Morgan PM, Krueger GS, Durning M, Dierschke DJ. Direct effect of estradiol-17β on progesterone accumulation by ovarian granulosa cells from rhesus monkeys. Am J Primatol 1989;17:87–92. [DOI] [PubMed] [Google Scholar]
- Iwai T, Nanbu Y, Iwai M, Taii S, Fujii S, Mori T. Immunohistochemical localization of oestrogen receptors and progesterone receptors in the human ovary throughout the menstrual cycle. Virchows Arch A Pathol Anat Histopathol 1990;5:369–375. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 1998;26:15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzendorf SE, Zelinski-Wooten MB, Stouffer RL, Wolf DP. Maturity at collection and the developmental potential of rhesus monkey oocytes. Biol Reprod 1990;4:703–711. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol 1996;56:67–77. [DOI] [PubMed] [Google Scholar]
- Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev 1990;4:374–383. [DOI] [PubMed] [Google Scholar]
- Natraj U, Richards JS. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 1993;2:761–769. [DOI] [PubMed] [Google Scholar]
- Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab 2000;12:4835–4840. [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod 2010;4:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod 2012;8:2430–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ. Placing progesterone in the apoptotic pathway. Trends Endocrinol Metab 1997;7:267–271. [DOI] [PubMed] [Google Scholar]
- Peluso JJ. Multiplicity of progesterone's actions and receptors in the mammalian ovary. Biol Reprod 2006;1:2–8. [DOI] [PubMed] [Google Scholar]
- Peluso JJ. Progesterone receptor membrane component 1 and its role in ovarian follicle growth. Front Neurosci 2013;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A. Progesterone mediates its anti-mitogenic and anti-apoptotic actions in rat granulosa cells through a progesterone-binding protein with gamma aminobutyric acidA receptor-like features. Biol Reprod 1998;5:1131–1137. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction 2014;5:R169–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology 2006;6:3133–3140. [DOI] [PubMed] [Google Scholar]
- Plancha CE, Sanfins A, Rodrigues P, Albertini D. Cell polarity during folliculogenesis and oogenesis. Reprod Biomed Online 2005;4:478–484. [DOI] [PubMed] [Google Scholar]
- Puttabyatappa M, Brogan RS, Vandevoort CA, Chaffin CL. EGF-like ligands mediate progesterone's anti-apoptotic action on macaque granulosa cells. Biol Reprod 2013;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter EO, Goldenberg RL, Vaitukaitis JL, Ross GT. A role for endogenous estrogen in normal ovarian development in the neonatal rat. Endocrinology 1972;6:1537–1539. [DOI] [PubMed] [Google Scholar]
- Richards JS. Maturation of ovarian follicles: actions and interactions of pituitary and ovarian hormones on follicular cell differentiation. Physiol Rev 1980;1:51–89. [DOI] [PubMed] [Google Scholar]
- Rodrigues JK, Navarro PA, Zelinski MB, Stouffer RL, Xu J. Direct actions of androgens on the survival, growth and secretion of steroids and anti-Mullerian hormone by individual macaque follicles during three-dimensional culture. Hum Reprod 2015;3:664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Yuan X, Manikkam M, Calder MD, Garverick HA, Lubahn DB. Cloning, sequencing, and localization of bovine estrogen receptor-beta within the ovarian follicle. Biol Reprod 1999;3:691–697. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CS, Wagner JS, Roberts RM, Lubahn DB. Intraovarian actions of oestrogen. Reproduction 2001;2:215–226. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Wehrman ME, Kojima FN, Cupp AS, Bergfeld EG, Peters KE, Mariscal V, Kittok RJ, Kinder JE. Dosage of the synthetic progestin, norgestomet, influences luteinizing hormone pulse frequency and endogenous secretion of 17 beta-estradiol in heifers. Biol Reprod 1995;2:464–469. [DOI] [PubMed] [Google Scholar]
- Sanchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod 2010;4:514–524. [DOI] [PubMed] [Google Scholar]
- Sar M, Welsch F. Differential expression of estrogen receptor-beta and estrogen receptor-alpha in the rat ovary. Endocrinology 1999;2:963–971. [DOI] [PubMed] [Google Scholar]
- Saunders PT, Millar MR, Williams K, Macpherson S, Harkiss D, Anderson RA, Orr B, Groome NP, Scobie G, Fraser HM. Differential expression of estrogen receptor-alpha and -beta and androgen receptor in the ovaries of marmosets and humans. Biol Reprod 2000;4:1098–1105. [DOI] [PubMed] [Google Scholar]
- Schane HP, Potts GO, Creange JE. Inhibition of ovarian, placental, and adrenal steroidogenesis in the rhesus monkey by trilostane. Fertil Steril 1979;4:464–467. [DOI] [PubMed] [Google Scholar]
- Schreiber JR, Nakamura K, Erickson GF. Progestins inhibit FSH-stimulated steroidogenesis in cultured rat granulosa cells. Mol Cell Endocrinol 1980;2:165–173. [DOI] [PubMed] [Google Scholar]
- Setty SL, Mills TM. The effects of progesterone on follicular growth in the rabbit ovary. Biol Reprod 1987;5:1247–1252. [DOI] [PubMed] [Google Scholar]
- Shao R, Markstrom E, Friberg PA, Johansson M, Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod 2003;3:914–921. [DOI] [PubMed] [Google Scholar]
- Slomczynska M, Wozniak J. Differential distribution of estrogen receptor-beta and estrogen receptor-alpha in the porcine ovary. Exp Clin Endocrinol Diabetes 2001;4:238–244. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Kimura N, Tamura M, Fukaya T, Yajima A, Nagura H. Immunohistochemical distribution of progesterone, androgen and oestrogen receptors in the human ovary during the menstrual cycle: relationship to expression of steroidogenic enzymes. Hum Reprod 1994;9:1589–1595. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markstrom E, Shao R, Andersson M, Billig H. Progesterone receptor antagonists Org 31710 and RU 486 increase apoptosis in human periovulatory granulosa cells. Fertil Steril 2001;6:1225–1231. [DOI] [PubMed] [Google Scholar]
- Tarumi W, Itoh MT, Suzuki N. Effects of 5alpha-dihydrotestosterone and 17beta-estradiol on the mouse ovarian follicle development and oocyte maturation. PLoS One 2014;6:e99423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol 2006;3:525–535. [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Milne M, Hillier SG. Expression of oestrogen receptor isoforms in relation to enzymes of oestrogen synthesis in rat ovary. Mol Cell Endocrinol 1998;1–2:29–35. [DOI] [PubMed] [Google Scholar]
- Thomas FH, Telfer EE, Fraser HM. Expression of anti-Mullerian hormone protein during early follicular development in the primate ovary in vivo is influenced by suppression of gonadotropin secretion and inhibition of vascular endothelial growth factor. Endocrinology 2007;5:2273–2281. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Klase PA, Sandow BA, Kolp LA. Progesterone secretion by highly differentiated human granulosa cells isolated from preovulatory Graafian follicles induced by exogenous gonadotropins and human chorionic gonadotropin. J Clin Endocrinol Metab 1983;1:87–93. [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 2004;2:77–83. [DOI] [PubMed] [Google Scholar]
- Wiekowski M, Miranda M, Nothias JY, DePamphilis ML. Changes in histone synthesis and modification at the beginning of mouse development correlate with the establishment of chromatin mediated repression of transcription. J Cell Sci 1997;110:1147–1158. [DOI] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski-Wooten MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young and older adult, rhesus monkeys during encapsulated three-dimensional (3D) culture: effects of gonadotropins and insulin. Reproduction 2010;140:685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, Stouffer RL. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod 2011;5:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod 2013a;8:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, Zelinski MB, Stouffer RL. Primate follicular development and oocyte maturation in vitro. Adv Exp Med Biol 2013b;761:43–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod 2003;11:2257–2263. [DOI] [PubMed] [Google Scholar]
- Zelinski-Wooten MB, Stouffer RL. Steroid receptors and action in the primate follicle. Trends Endocrinol Metab 1996;5:177–183. [DOI] [PubMed] [Google Scholar]