Abstract
STUDY QUESTION
Do women who give birth after assisted reproductive technology (ART) have an increased risk of cancer compared with women who give birth without ART?
SUMMARY ANSWER
Without correction, the results indicate an increase in overall cancer risk, as well as a 50% increase in risk of CNS cancer for women giving birth after ART, however the results were not significant after correcting for multiple analyses.
WHAT IS KNOWN ALREADY
Studies regarding the effects of hormonal treatments involved with ART on subsequent cancer risk have provided inconsistent results, and it has also been suggested that infertility itself could be a contributory factor.
STUDY DESIGN, SIZE, DURATION
A population-based cohort consisting of all women registered in the Medical Birth Registry of Norway as having given birth between 1 January 1984 and 31 December 2010 was assembled (n = 812 986). Cancers were identified by linkage to the Cancer Registry of Norway. Study subjects were followed from start of first pregnancy during the observational period until the first cancer, death, emigration, or 31 December 2010.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Of the total study population (n = 806 248), 16 525 gave birth to a child following ART. Cox regression analysis computed hazard ratios (HR) and 95% confidence intervals (CI) comparing cancer risk between ART women and non-ART women; for overall cancer, and for cervical, ovarian, uterine, central nervous system (CNS), colorectal and thyroid cancers, and for malignant melanoma.
MAIN RESULTS AND THE ROLE OF CHANCE
A total of 22 282 cohort members were diagnosed with cancer, of which 338 were ART women and 21 944 non-ART women. The results showed an elevated risk in one out of seven sites for ART women. The HR for cancer of the CNS was 1.50 (95% CI 1.03– 2.18), and among those specifically subjected to IVF (without ICSI) the HR was 1.83 (95% CI 1.22–2.73). Analysis of risk of overall cancer gave an HR of 1.16 (95% CI 1.04–1.29). Among those who had delivered only one child by the end of follow-up, the HR for ovarian cancer was 2.00 (95% CI 1.08–3.65), and for those nulliparous at entry the HR was 1.80 (95% CI 1.04–3.11). However, all findings became non-significant after correcting for multiple analyses.
LIMITATIONS, REASONS FOR CAUTION
The results of elevated risk of overall cancer and CNS cancer lost significance when adjusting for multiple analyses, implying an important limitation of the study. The follow-up time was relatively short, especially for ART women. In addition, as the cohort was relatively young, there were few incident cancers, especially for some rarer cancer forms, such as uterine cancer. Risk assessments according to different causes of infertility could not be done.
WIDER IMPLICATIONS OF THE FINDINGS
In light of the findings in the present study, further studies should be made on risk of CNS and ovarian cancer, and continued monitoring of all those treated with ART is encouraged. Our findings may only be generalizable to women who give birth after ART, and the risk for women who remain nulliparous after ART remains to be assessed.
STUDY FUNDING/COMPETING INTEREST
The study was funded by the Norwegian National Advisory Unit on Women's Health. All authors claim no competing interests.
Keywords: brain tumor, neoplasm of the central nervous system, cancer, IVF, assisted reproductive technology
Introduction
Increasing infertility rates (Slama et al., 2012; Louis et al., 2013) and use of assisted reproductive technology (ART) (Wright et al., 2004; Sunderam et al., 2013) have raised concern regarding potential effects on subsequent cancer risk. The role of endogenous or exogenous sex hormones in cancer development is well recognized for breast and gynecological cancers (Key and Pike, 1988a,b; Moodley et al., 2003). However, it has also been suggested that these hormones are associated with cancers at other sites, such as central nervous system (CNS) (Wigertz et al., 2008), and thyroid cancers (Rossing et al., 2000), as well as colorectal cancer (CRC) (Althuis et al., 2005; Nichols et al., 2005) and cutaneous malignant melanoma (CMM) (Koomen et al., 2009).
Although some studies have indicated that women treated with fertility hormones are at an increased risk of ovarian tumors (Whittemore et al., 1992; Rossing et al., 1994; Lerner-Geva et al., 2003; van Leeuwen et al., 2011) or breast cancer (Jensen et al., 2007; Orgeas et al., 2009; Silva Idos et al., 2009; Brinton et al., 2014), a recent meta-analysis concluded that controlled ovarian hyperstimulation for IVF resulted in no increased risks of ovarian, uterine or cervical cancers, once confounding effects were taken into account (Siristatidis et al., 2013). Studies have also focused on possible associations of ART with tumors of the CNS (Kallen et al., 2011; Yli-Kuha et al., 2012), CRC (Althuis et al., 2005; Kallen et al., 2011; Yli-Kuha et al., 2012), thyroid (Althuis et al., 2005; Hannibal et al., 2008b; Pazaitou-Panayiotou et al., 2014) and CMM (Rossing et al., 1994; Althuis et al., 2005; Hannibal et al., 2008a; Stewart et al., 2013), with discrepant results.
Many of the aforementioned studies on cancer in women treated with fertility hormones do not distinguish between ART and other types of medically assisted reproduction when defining exposure, and only some have specifically focused on the effects of ART alone. Additionally, many studies have had short follow-up times, few cancer cases in comparison groups, and incomplete information on exposure and cancer diagnoses. Since pregnancy and childbirth exert a protective effect on certain cancers (Risch et al., 1994; Althuis et al., 2004; Horn et al., 2014), the outcome of fertility treatment can significantly affect risk differences between treated and untreated women.
In a population-based cohort study, we assessed cancer risk among women in Norway who had given birth over a 27-year period (1984–2010), which allowed for adjustment for the known alterations of risk following childbirth. The same cohort has been used in a previous study where we found a significant increase in breast cancer risk among women who gave birth following ART (Reigstad et al., 2015). In the present study, the objective was to compare the risk of cancer among women who gave birth following ART to that of women who had given birth without the use of ART. Overall cancer risk and site-specific risks of cancers of the cervix, ovary, uterus, CNS, and thyroid and of CRC and CMM were examined.
Materials and Methods
The study population
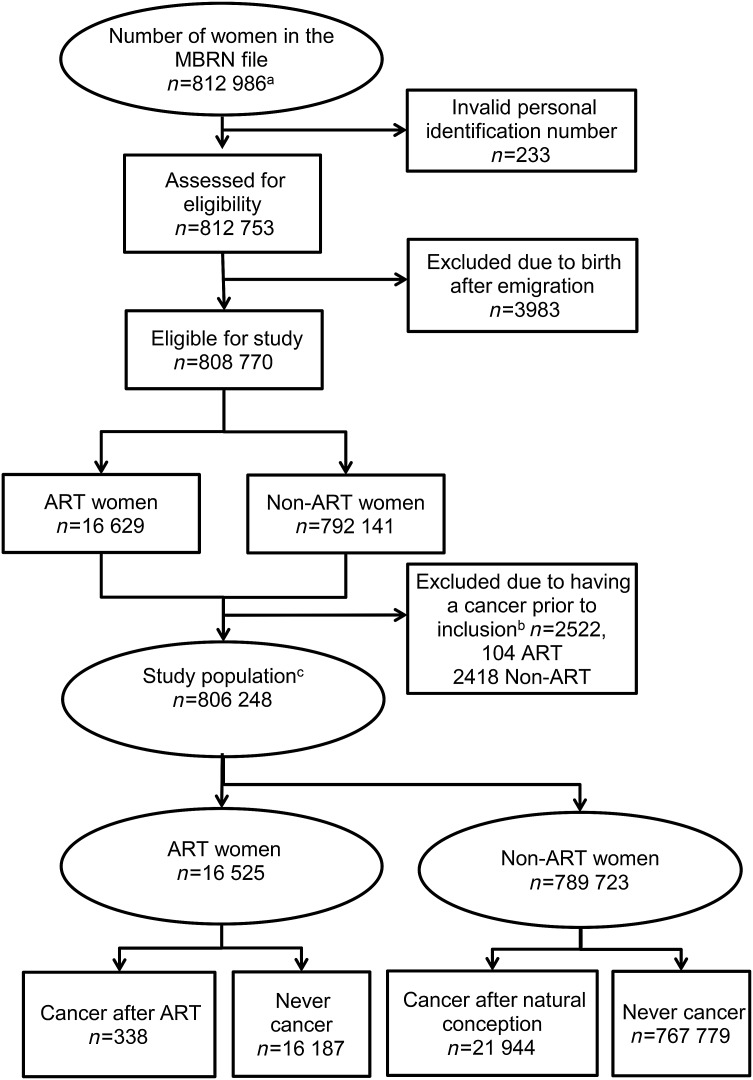
Women registered in the Medical Birth Registry of Norway (MBRN) as having given birth in Norway between 1 January 1984 and 31 December 2010 constituted the study cohort (n = 812 986). The study cohort has been described in detail previously in an article addressing effects on breast cancer risk (Reigstad et al., 2015). After removal of subjects who delivered after emigrating from Norway, and those with a cancer prior to the start of follow-up, 806 248 study subjects were included in the analyses (Fig. 1).
Figure 1.
Number of women constituting the study cohort. aWomen registered in the MBRN as having given birth between 1 January 1984 and 31 December 2010. bWomen with a cancer at any site were excluded from the study population in analysis of overall cancer. cThe number of subjects in the study population in the analysis of overall cancer. MBRN: Medical Birth Registry of Norway; ART: assisted reproductive technology; ART women: those with at least one ART delivery as registered in the MBRN; Non-ART women: those without a registered ART delivery in the MBRN.
All deliveries (from Week 22) in Norway have been recorded in the MBRN since its establishment in 1967. The reporting of data on ART pregnancies started in 1984 (the year the first baby was born after IVF in Norway). For each child born, the following data were extracted from the MBRN: date of birth of mother and child, parity, present region of residence, exposure to ART, the specific method of ART. The MBRN categorizes method of ART into four groups: (i) conventional IVF, (ii) IVF with ICSI, (iii) a combination of the two or treatment with a different ART procedure (such as frozen embryo replacement, gamete donation or ART abroad; the MBRN does not allow distinction between these latter treatment types), (iv) unknown/unspecified.
Ascertainment of exposure
Women who had at least one pregnancy initiated by ART were classified as ART women, and women who had no registered ART pregnancies were classified as non-ART. In this study we did not have information about the type of medication given during each ART procedure, only that each delivery was a result of one of the four categories described above. Categories three and four were treated as one category ‘others’, due to low numbers.
Identification of cancer cases
All women with at least one diagnosis of invasive cancer during the period 1 January 1953 through 31 December 2010 were identified through linkage of the MBRN data to the Cancer Registry of Norway (CRN) using each woman's unique personal identification number. The latest update of the cancer data was 31 December 2010, at the time of merging of the two files.
The information on cancer diagnoses was categorized according to the International Classification of Diseases version 10 (ICD-10), and information on cancer diagnoses (C00-96) was extracted from the CRN.
For women who were diagnosed with more than one cancer, only the first case was counted in the analysis of overall cancer risk. In the site-specific analyses, the first case of the cancer of interest was counted. In analyses of risk of overall cancer, all women with any cancer before the start of follow-up were excluded from the analyses. In the site-specific analyses, only those with a cancer at the index site prior to the start of follow-up were excluded from the analyses. Analyses were made separately for cervical (invasive cancers only) (C53), ovarian (excluding borderline tumors) (C56), uterine (C54–55), CNS (C70–72), colorectal (C18–20) and thyroid (C73) cancers, as well as CMM (C43). For CNS cancer separate analyses were undertaken for glioma and meningioma, obtained by ICD-O-3 morphology (see Supplementary Data).
Follow-up
All study subjects started follow-up at the starting date of their first pregnancy registered in the MBRN between 1 January 1984 and 31 December 2010. This date was obtained by subtracting the gestational length from the date of birth of each child born with the aid of ART during the observational period. Where the gestational length was missing, start of follow-up was obtained by subtracting the mean length of a pregnancy (282 days), from the birth date of the first child. All women were followed until the date of their first cancer diagnosis of interest, the date of death or emigration, or to 31 December 2010, whichever occurred first. Some study subjects had a non-ART pregnancy prior to the first ART pregnancy. These women first contributed person years to the non-ART group and, after ART exposure, contributed person years to the ART group. To model this, ART exposure was treated as a time-varying covariate, and these study subjects switched group, from the non-ART to the ART group upon exposure (the start of their first ART pregnancy).
Statistical analyses
Descriptive statistics are presented as median/interquartile range and frequency/percentage where appropriate. Demographic data at baseline are presented for the whole cohort, as well as for ART women and non-ART women separately (Table I).
Table I.
Demographic data of the study cohort* at baseline, by method of conception, as registered in the Medical Birth Registry of Norway.
| Characteristics of study population | ART women** |
Non-ART women |
Total cohort* | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Number of study subjects | 16 525 | 789 723 | 806 248 | |||
| Numbers of study subjects with cancer | 338 | 21 944 | 22 282 | |||
| Age at start follow-up, years (median, IQR) | 31.2 (28.0–34.3) | 26.3 (22.9–29.9) | 26.4 (22.9–30.0) | |||
| Age at diagnosis, years (median, IQR) | 41.7 (37.3–46.0) | 43.5 (37.1–49.4) | 43.5 (37.1–49.3) | |||
| Age at end, years (median, IQR) | 40.5 (36.0–45.5) | 42.0 (35.0–49.0) | 42.0 (35.1–48.9) | |||
| Follow-up time, person years (median, IQR) | 7.3 (3.7–12.3) | 16.0 (8.0–22.9) | 15.9 (7.8–22.8) | |||
| Total follow-up time, person years | 139 776 | 12 162 146 | 12 301 922 | |||
| Age at start follow-up (years) | ||||||
| Less than 25 | 1896 | 11% | 315 820 | 40% | 317 716 | 39% |
| 25–29 | 4674 | 28% | 281 274 | 36% | 285 948 | 35% |
| 30–34 | 6629 | 40% | 140 511 | 18% | 147 140 | 18% |
| 35–39 | 3111 | 19% | 44 865 | 6% | 47 976 | 6% |
| 40–44 | 209 | 1% | 6989 | 1% | 7198 | 1% |
| 45 and older | 6 | 0% | 264 | 0% | 270 | 0% |
| Total | 16 525 | 100% | 789 723 | 100% | 806 248 | 100% |
| Parity at entry | ||||||
| Nulliparous | 15 589 | 94% | 628 959 | 80% | 644 548 | 80% |
| One child | 807 | 5% | 94 943 | 12% | 95 750 | 12% |
| More than one child | 129 | 1% | 65 821 | 8% | 65 950 | 8% |
| Total | 16 525 | 100% | 789 723 | 100% | 806 248 | 100% |
| Parity at exit | ||||||
| One | 5572 | 34% | 172 773 | 22% | 178 354 | 22% |
| Two | 7208 | 44% | 338 957 | 43% | 346 165 | 43% |
| Three | 2956 | 18% | 200 475 | 25% | 203 431 | 25% |
| Four or more | 789 | 5% | 77 518 | 10% | 78 307 | 10% |
| Total | 16 525 | 100% | 789 723 | 100% | 806 257 | 100% |
| Method of ART | ||||||
| IVF | 10 057 | 61% | – | – | – | – |
| ICSI | 4933 | 30% | – | – | – | – |
| Other | 1535 | 9% | – | – | – | – |
| Total | 16 525 | 100% | ||||
IQR, inter quartile range.
*This constitutes the cohort used in analysis of risk of total cancer, i.e. excluding all those with a cancer at any site prior to inclusion (n =2522).
**3233 ART women also contributed person years to the non-ART group, due to one or more non-ART deliveries before the ART delivery.
A Cox proportional hazards model was used to compare risk between ART and non-ART women, first for overall cancer and subsequently for the seven cancer sites. To assess the risk of cancer after exposure to ART, the data were split at the time of exposure to ART for exposed study subjects. The assumption of proportional hazards was tested using Schoenfeld residuals (Schoenfeld, 1982). To adjust for the age difference between ART women and non-ART women, we used the age of study subjects as the timescale (Commenges et al., 1998). Confounder adjustment was made for age at start of follow-up, calendar period, region of residence in Norway and parity as a time-varying covariate, as previously described (Reigstad et al., 2015).
Stratified analyses were performed by age at follow-up, parity at inclusion, parity at end of follow-up, method of ART and time from inclusion to end of follow-up, as previously described (Reigstad et al., 2015).
Sensitivity analyses were performed after excluding those with cancers diagnosed within the first year after inclusion to remove the possibility that a pre-existing cancer was diagnosed after inclusion. Analyses by specific cancer sites were also conducted by excluding all study subjects with cancers at any site (as well as the index cancer) prior to inclusion.
To adjust for multiple testing, a Benjamini-Hochberg correction was made for all analyses at the final stage of analysis, where the P-value was set at 0.01 (Benjamini et al., 2001).
All analyses were conducted using the software package STATA version 13.0 (StataCorp. Stata Statistical Software: Release 13. College Station, TX, USA).
Ethical approval
The study was approved by the Committee for Medical and Health Research Ethics, South Eastern Health region of Norway.
Results
Of the total study population (n = 806 248), 16 525 gave birth to a child following ART, and 789 723 gave birth without ART. A total of 338 ART women and 21 944 non-ART women were registered with a cancer diagnosis during the study period, 1 January 1984 and 31 December 2010 (Fig. 1).
The total follow-up time was 12 301 922 person years; 139 776 for ART women, and 12 162 146 for non-ART women. Median follow-up time for ART women was 7.3 years and for non-ART women 16.0 years (Table I). Non-ART women were younger at start of follow-up and older at the end of follow-up. Of those with at least one cancer diagnosis, the age at diagnosis of overall cancer was slightly lower for ART women compared with non-ART women. Of those not diagnosed with cancer, 765 889 women were followed until the end of the observational period and of these 3663 died (11 ART women) and 14 414 were censored due to emigration (101 ART women) (data not shown). Among the ART women, the majority (44%) started follow-up during the last decade of the study period (2003–2010), whereas the majority (46%) of non-ART women started follow-up during the first decade of the study period (1983–1992) (data not shown).
The risk of overall cancer for ART women compared with non-ART women is shown in Table II. After adjustment for age at start of follow-up, parity, region of residence and calendar year at follow-up, the HR for overall cancer in ART women compared with non-ART women was 1.16 (95% CI 1.04–1.29). Sensitivity analyses excluding those with cancer within the first year of inclusion did not alter this estimate (HR 1.16, 95% CI 1.04–1.30). Significantly elevated risks were seen for ART women compared with non-ART women among those nulliparous at start of follow-up (1.17, 95% CI 1.04–1.32) and in ART women compared with non-ART women among those who were exposed to IVF alone (1.22, 95% CI 1.07–1.38) (Table II).
Table II.
Hazard ratios (HR) with 95% confidence intervals (CI) of overall cancer for ART women compared with non-ART women. All women with a cancer at any site prior to inclusion were omitted from analyses.
| ART women |
Non-ART women |
HR | Lower CI | Upper CI | |||
|---|---|---|---|---|---|---|---|
| N | p.yrs | N | p.yrs | ||||
| Crude risk | 338 | 139 776 | 21 944 | 12 162 146 | *1.23 | 1.11 | 1.37 |
| Adjusted riska | 338 | 139 776 | 21 944 | 12 162 146 | *1.16 | 1.04 | 1.29 |
| Excl. cancer during the first yearb | 329 | 139 770 | 21 610 | 12 161 963 | *1.16 | 1.04 | 1.30 |
| Age at follow-up, years | |||||||
| 30–40 | 133 | 86 928 | 7868 | 8 273 366 | *1.25 | 1.05 | 1.49 |
| 40–50 | 173 | 46 773 | 9075 | 3 074 518 | *1.21 | 1.04 | 1.42 |
| ≥50 | 32 | 6074 | 5001 | 814 262 | 0.84 | 0.59 | 1.20 |
| Total | 338 | 139 775 | 21 944 | 12 162 146 | |||
| Parity at entry | |||||||
| P0 | 302 | 128 250 | 13 024 | 8 827 019 | *1.17 | 1.04 | 1.32 |
| P1+ | 36 | 11 527 | 8920 | 3 335 126 | 1.19 | 0.85 | 1.65 |
| Total | 338 | 139 777 | 21 944 | 12 162 145 | |||
| Parity at exit | |||||||
| One | 163 | 78 480 | 4133 | 3 673 723 | 1.07 | 0.91 | 1.26 |
| Two | 138 | 49 014 | 9233 | 4 842 584 | *1.23 | 1.04 | 1.46 |
| Three or more | 37 | 12 282 | 8578 | 3 645 839 | 1.37 | 0.99 | 1.90 |
| Total | 338 | 139 776 | 21 944 | 12 162 146 | |||
| Calendar year at follow-up | |||||||
| 1983–1992 | 6 | 3778 | 1561 | 1 972 460 | 1.51 | 0.68 | 3.39 |
| 1993–2002 | 71 | 40 547 | 6723 | 4 777 423 | 1.06 | 0.83 | 1.34 |
| 2003–2010 | 261 | 95 451 | 13 660 | 5 412 262 | *1.18 | 1.05 | 1.34 |
| Total | 338 | 139 776 | 21 944 | 12 162 145 | |||
| Method of ART | |||||||
| IVF | 257 | 96 017 | 21 944 | 12 162 146 | *1.22 | 1.07 | 1.38 |
| ICSI | 45 | 28 632 | 21 944 | 12 162 146 | 0.98 | 0.73 | 1.32 |
| Other | 36 | 15 127 | 21 944 | 12 162 146 | 1.03 | 0.75 | 1.43 |
| Total | 338 | 139 776 | |||||
| Duration of follow-up, years | |||||||
| <1 year | 11 | 13 240 | 349 | 791 255 | 0.84 | 0.59 | 1.20 |
| 1–5 | 63 | 44 411 | 2221 | 2 903 417 | 1.14 | 0.86 | 1.44 |
| >5–10 | 102 | 39 949 | 3821 | 3 033 330 | *1.23 | 1.01 | 1.51 |
| >10 | 162 | 42 176 | 15 553 | 5 434 144 | 1.14 | 0.97 | 1.33 |
| Total | 338 | 139 776 | 21 944 | 12 162 146 | |||
aHazard ratios are adjusted for attained age, age at start of follow-up, parity, region of residence and calendar period.
bThose with cancer diagnosed within the first year of follow-up were omitted from this analysis.
Number of cancers in each group (n), person years (p.yrs).
*Hazard rates which were statistically significant between ART and non-ART women, lost statistical significance once corrections were made for multiple analyses, using the Benjamini-Hochberg method.
The adjusted HR for cervical cancer was 0.86 (95% CI 0.57–1.29) in ART women compared with non-ART women (Table III), whilst for ovarian cancer the HR was 1.56 (95% CI 0.94–2.60). Among those who had delivered only one child by the end of follow-up, the HR for ovarian cancer was 2.00 (95% CI 1.08–3.65), and for those nulliparous at entry the HR was 1.80 (95% CI 1.04–3.11) (Table III) in ART women compared with non-ART women.
Table III.
Risk of cervical and ovarian cancer for ART women compared with non-ART women, stratified analyses.
| Cervical cancer (C53) |
Ovarian cancer (C56) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ART | Non-ART | HR | Lower bound | Upper bound | ART | Non-ART | HR | Lower bound | Upper bound | |
| n | n | n | n | |||||||
| Number of cancers during the observational period | 24 | 2156 | 16 | 800 | ||||||
| Crude risk | 24 | 2156 | 0.83 | 0.55 | 1.24 | 16 | 800 | 1.67 | 1.02 | 2.74 |
| Adjusted riska | 24 | 2156 | 0.86 | 0.57 | 1.29 | 16 | 800 | 1.56 | 0.94 | 2.60 |
| Excl. cancer during the first yearb | 23 | 2102 | 0.87 | 0.58 | 1.32 | 14 | 781 | 1.43 | 0.83 | 2.45 |
| Parity at entry | ||||||||||
| P0 | 19 | 1459 | 0.78 | 0.50 | 1.24 | 14 | 402 | *1.80 | 1.04 | 3.11 |
| P1+ | 5 | 697 | 2.03 | 0.84 | 4.93 | 2 | 398 | 1.60 | 0.40 | 6.45 |
| Total | 24 | 2156 | 16 | 800 | ||||||
| Parity at exit | ||||||||||
| One | 15 | 498 | 0.99 | 0.59 | 1.67 | 12 | 172 | *2.00 | 1.08 | 3.65 |
| Two | 9 | 910 | 0.85 | 0.44 | 1.64 | 3 | 305 | 0.95 | 0.30 | 2.98 |
| Three or more | 0 | 748 | NA | – | – | 1 | 323 | 1.22 | 0.17 | 8.71 |
| Total | 24 | 2156 | 16 | 800 | ||||||
| Method of ART | ||||||||||
| IVF | 17 | 2156 | 0.88 | 0.55 | 1.43 | 11 | 800 | 1.45 | 0.79 | 2.65 |
| ICSI | 4 | 2156 | 0.70 | 0.26 | 1.88 | 2 | 800 | 1.51 | 0.37 | 6.11 |
| Other | 3 | 2156 | 1.04 | 0.33 | 3.22 | 3 | 800 | 2.33 | 0.75 | 7.28 |
| Total | 24 | 16 | ||||||||
| Duration of follow-up, years | ||||||||||
| <1 year | 2 | 56 | 1.08 | 0.25 | 4.54 | 2 | 19 | *5.59 | 1.12 | 28.0 |
| 1–5 | 11 | 317 | 1.70 | 0.91 | 3.15 | 3 | 73 | 2.01 | 0.60 | 6.67 |
| >5–10 | 9 | 608 | 1.10 | 0.57 | 2.15 | 6 | 133 | 1.98 | 0.85 | 4.64 |
| >10 | 2 | 1175 | 0.20 | 0.05 | 0.81 | 5 | 575 | 0.96 | 0.39 | 2.33 |
| Total | 24 | 2156 | 16 | 800 | ||||||
aHazard ratios are adjusted for attained age, age at start of follow-up, parity, region of residence and calendar period.
bThose with an index cancer diagnosed within the first year of follow-up were omitted from this analysis.
*Hazard rates which were statistically significant between ART and non-ART women, lost statistical significance once corrections were made for multiple analyses, using the Benjamini-Hochberg method.
The crude HR of uterine cancers in ART women compared with non-ART women was 0.73 (95% CI 0.30–1.77), and adjusted HR was 0.69 (95% CI 0.28–1.68) (data not shown). Removing those with uterine cancer within the first year gave an HR of 0.69 (95% CI 0.28–1.68) in ART women compared with non-ART women (data not shown). These estimates were based on only five cases in the ART group, and stratified analyses are therefore precluded.
The HR for cancer of the CNS was 1.50 (95% CI 1.03–2.18) comparing women treated with ART to non-ART women. Restricting analyses to those who were parous at inclusion gave an HR of 2.78 (95% CI 1.14–6.76), and restricting to those who were treated specifically with IVF gave an HR of 1.83 (95% CI 1.22–2.73) (Table IV). Removing study subjects with a CNS cancer diagnosis within the first year after ART, did not alter the risk of CNS cancer (HR 1.57, 95% CI 1.08–2.28) (Table IV).
Table IV.
Risk of cancer of the central nervous system and cutaneous malignant melanoma for ART women compared with non-ART women, stratified analyses.
| Cancer of the central nervous system (C70-73) |
Cutaneous malignant melanoma (C43) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ART | Non-ART | HR | Lower bound | Upper bound | ART | Non-ART | HR | Lower bound | Upper bound | |
| n | n | n | n | |||||||
| Number of cancers during the observational period | 29 | 1489 | 41 | 2509 | ||||||
| Crude risk | 29 | 1489 | *1.55 | 1.07 | 2.24 | 41 | 2509 | 1.28 | 0.94 | 1.74 |
| Adjusted riska | 29 | 1489 | *1.50 | 1.03 | 2.18 | 41 | 2509 | 1.24 | 0.91 | 1.70 |
| Excl. cancer during the first yearb | 29 | 1461 | *1.57 | 1.08 | 2.28 | 39 | 2419 | 1.25 | 0.91 | 1.73 |
| Parity at entry | ||||||||||
| P0 | 24 | 945 | 1.32 | 0.87 | 1.99 | 38 | 1745 | 1.25 | 0.90 | 1.74 |
| P1+ | 5 | 544 | *2.78 | 1.14 | 6.76 | 3 | 762 | 1.20 | 0.38 | 3.75 |
| Total | 29 | 1489 | 41 | 2507 | ||||||
| Parity at exit | ||||||||||
| One | 13 | 278 | 1.30 | 0.73 | 2.30 | 20 | 588 | 1.12 | 0.71 | 1.78 |
| Two | 13 | 634 | 1.66 | 0.95 | 2.90 | 15 | 1052 | 1.20 | 0.72 | 2.01 |
| Three | 3 | 577 | 1.58 | 0.51 | 4.92 | 6 | 869 | 2.09 | 0.93 | 4.67 |
| Total | 29 | 1489 | 41 | 2509 | ||||||
| Method of ART | ||||||||||
| IVF | 25 | 1487 | *1.83 | 1.22 | 2.73 | 29 | 2509 | 1.25 | 0.86 | 1.81 |
| ICSI | 4 | 1487 | 1.16 | 0.43 | 3.12 | 8 | 2509 | 1.30 | 0.64 | 2.60 |
| Other | 0 | 1487 | NA | 4 | 2509 | 1.08 | 0.40 | 2.87 | ||
| Total | 29 | 4461 | 41 | |||||||
| Duration of follow-up, years | ||||||||||
| <1 year | 0 | 29 | NA | – | – | 2 | 96 | 0.94 | 0.22 | 3.90 |
| 1–5 | 8 | 175 | 1.83 | 0.88 | 3.81 | 9 | 383 | 1.00 | 0.51 | 1.96 |
| >5–10 | 8 | 284 | 1.48 | 0.72 | 3.04 | 15 | 524 | 1.54 | 0.91 | 2.61 |
| >10 | 13 | 1001 | 1.54 | 0.88 | 2.67 | 15 | 1506 | 1.21 | 0.72 | 2.02 |
| Total | 29 | 1489 | 41 | 2509 | ||||||
aHazard ratios are adjusted for attained age, age at start of follow-up, parity, region of residence and calendar period.
bThose with an index cancer diagnosed within the first year of follow-up were omitted from this analysis.
*Hazard rates which were statistically significant between ART and non-ART women, lost statistical significance once corrections were made for multiple analyses, using the Benjamini-Hochberg method.
There were 12 cases of meningioma in the ART group and 585 in the non-ART group; similarly there were 12 cases of glioma in the ART group and 498 in the non-ART group. Risk of meningioma in ART women versus non-ART women was 1.49 (95% CI 0.83–2.68) and risk of glioma was 2.21 (95% CI 1.23–3.98) (data not shown). For those exposed to IVF the HRs were 1.88 (95% CI 1.02–3.45) and 2.87 (95% CI 1.56–5.29) for meningioma and glioma, respectively (data not shown).
The HR for CMM in ART women was 1.24 (95% CI 0.91–1.70) (Table IV), and risk of colorectal cancer was 1.31 (95% CI 0.85–2.01) (Table V). There was no difference in risk of thyroid cancer between ART women and non-ART women, HR 1.15 (95% CI 0.66–2.00) (Table V).
Table V.
Risk of colorectal and thyroid cancer for ART women compared with non-ART women, stratified analyses.
| Colorectal cancer (C18-20) |
Thyroid cancer (C73) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ART | Non-ART | HR | Lower bound | Upper bound | ART | Non-ART | HR | Lower bound | Upper bound | |
| n | n | n | n | |||||||
| Number of cancers during the observational period | 25 | 1364 | 13 | 919 | ||||||
| Crude risk | 22 | 1364 | 1.36 | 0.89 | 2.07 | 13 | 919 | 1.14 | 0.66 | 1.97 |
| Adjusted riska | 22 | 1364 | 1.31 | 0.85 | 2.01 | 13 | 919 | 1.15 | 0.66 | 2.00 |
| Excl. cancer during the first yearb | 22 | 1354 | 1.34 | 0.87 | 2.06 | 13 | 894 | 1.18 | 0.68 | 2.06 |
| Parity at entry | ||||||||||
| P0 | 20 | 659 | 1.43 | 0.91 | 2.25 | 12 | 640 | 1.07 | 0.60 | 1.91 |
| P1+ | 2 | 705 | 0.90 | 0.22 | 3.60 | 1 | 279 | 0.97 | 0.13 | 6.93 |
| Total | 22 | 1364 | 13 | 919 | ||||||
| Parity at exit | ||||||||||
| One | 9 | 183 | 1.13 | 0.57 | 2.23 | 3 | 201 | 0.50 | 0.15 | 1.55 |
| Two | 10 | 572 | 1.41 | 0.75 | 2.65 | 7 | 403 | 1.59 | 0.75 | 3.39 |
| Three or more | 3 | 609 | 1.81 | 0.58 | 5.64 | 3 | 315 | 2.60 | 0.83 | 8.15 |
| Total | 22 | 1364 | 13 | 919 | ||||||
| Method of ART | ||||||||||
| IVF | 16 | 1364 | 1.27 | 0.77 | 2.10 | 9 | 919 | 1.15 | 0.59 | 2.23 |
| ICSI | 2 | 1364 | 0.95 | 0.24 | 3.80 | 2 | 919 | 0.87 | 0.22 | 3.51 |
| Other | 4 | 1364 | 1.86 | 0.69 | 4.98 | 2 | 919 | 1.59 | 0.40 | 6.40 |
| Total | 22 | 13 | ||||||||
| Duration of follow-up, years | ||||||||||
| <1 year | 0 | 10 | NA | – | – | 0 | 26 | NA | – | – |
| 1–5 | 6 | 81 | 2.27 | 0.96 | 5.41 | 3 | 186 | 0.82 | 0.26 | 2.61 |
| >5–10 | 5 | 158 | 1.13 | 0.46 | 2.82 | 3 | 238 | 0.81 | 0.26 | 2.58 |
| >10 | 11 | 1115 | 1.16 | 0.64 | 2.12 | 7 | 469 | 1.84 | 0.87 | 3.93 |
| Total | 22 | 1364 | 13 | 919 | ||||||
aHazard ratios are adjusted for attained age, age at start of follow-up, parity, region of residence and calendar period.
bThose with an index cancer diagnosed within the first year of follow-up were omitted from this analysis.
The main HR estimates for all cancer sites are also demonstrated graphically (Fig. 2). Removing all those with a previous cancer at any site did not alter the main HR estimates in the site-specific analyses (data not shown). Removing breast and CNS cancers from the main estimate gave an HR of 1.10 (95% CI 0.94–1.27), for ART women compared with non-ART women (data not shown).
Figure 2.
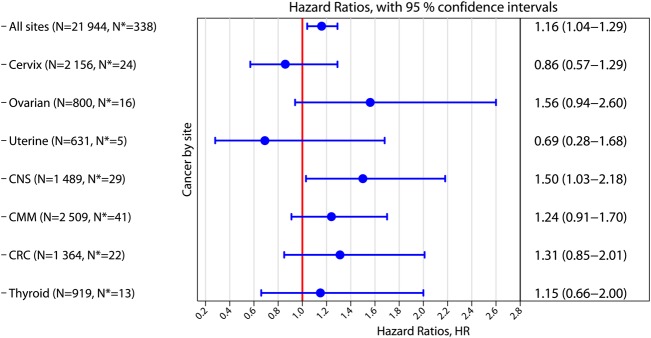
Hazard ratios for cancer by site for ART women compared with non-ART women. The right column displays numerically hazard ratios (HR) and 95% confidence intervals (CI). Adjustments have been made for attained age, age at start of follow-up, parity, region of residence and calendar period. N denotes the number of cancers occurring after the start of follow-up in the non-ART group. N* denotes the number of cancers in the ART group. CMM: cutaneous malignant melanoma; CNS: cancer of the central nervous system; CRC, colorectal cancer. Hazard rates which were statistically significant between ART and non-ART women, lost statistical significance after adjustments for multiple analyses using the Benjamini-Hochberg method.
Once corrections for multiple analyses were made, all HRs that initially were significant lost statistical significance (data not shown).
Discussion
This study used a population-based cohort design to compare the risk of cancer in women who gave birth following ART to that in women who gave birth without ART. Without correction, the results indicate an increase in overall cancer risk, as well as a 50% increase in risk of CNS cancer for women giving birth after ART, however the results were not significant once correction for multiple analyses was made.
In a previous paper we have demonstrated a 20% increase in risk of breast cancer for ART women (Reigstad et al., 2015) using the same population-based cohort. In the present study we found no excess risk of overall cancer when excluding breast and CNS cancer cases, implying that the elevated overall cancer risk is due to the increased risk of these two specific cancers.
CNS cancer
Before correction for multiple analyses, we found an elevated risk of CNS cancer in ART women, and in analyses by histologic subtype found the risk highest for gliomas. A Swedish population-based study of parous women found a significant increase in CNS cancer risk in women treated with IVF before they underwent treatment, compared with the general population, but the risk increase was not significant after IVF (Kallen et al., 2011). Our finding is in contrast to a Finnish study that did not find an increased risk of CNS cancer for women who were registered in a prescription database as having purchased fertility drugs for ART, but the analysis was based on only 16 cases of CNS cancer (Yli-Kuha et al., 2012). Neither of the aforementioned studies subdivided their tumors according to histology.
In analyses restricted to women exposed to conventional IVF treatment (without ICSI), there was elevated risk of CNS cancer. It is possible that it is not the hormone treatment itself, but some other association between women selected for IVF and risk of CNS cancer. Caution should be taken in interpreting this result; however, as almost all the cases were in the IVF group, and as mentioned under limitations below, the indications for treating couples with ICSI have widened in recent years.
Apart from ionizing radiation and some rare hereditary conditions, the risk factors for the development of CNS cancers are largely unknown (Wrensch et al., 2002). Findings of an increased risk of CNS tumors related to use of menopausal hormone therapy (Benson et al., 2010; Andersen et al., 2013) have prompted an interest in the role of other exogenous hormones. In line with this, a recent study from Denmark found an association between glioma and the use of oral contraceptives, with a nearly 2-fold increase in risk (Andersen et al., 2015). Estrogen has been shown to exert a protective effect on glial cells in vitro (Shy et al., 2000), and it could be that infertile women, or some subgroups thereof, harbor an altered steroid hormone profile, in some way associated with elevated risk of CNS tumors. A group of researchers from the USA found a 50% lower risk of glioma in women who were younger at first childbirth (below 20 years), and suggested that long-term alterations in steroid hormones may play a role (Hatch et al., 2005). ART women are known to be older than non-ART women at their first birth, also in line with findings in the present study.
Gynaecological cancers
Several authors have suggested decreased risks of cervical cancer in infertile women or women treated with fertility drugs. Both a Finnish study (Yli-Kuha et al., 2012), and a study in Israel (Brinton et al., 2013) found reductions in the risk of cervical cancer among women who had received IVF, and in the latter study there was a significant reduction in the risk of in situ cervical cancers. Decreased risks of cervical cancer were observed in women assessed for infertility in the UK, in two different studies (Doyle et al., 2002; Silva Idos et al., 2009), as well as in a meta-analysis including many of the above studies, which found reduced cervical cancer risk in women treated with IVF (Siristatidis et al., 2013). These findings might be due to observation bias, as women seeking fertility treatment possibly have gynecological examinations with cervical cytology more frequently, which is known to reduce the risk of development of cervical cancers through a screening effect (Scarinci et al., 2010). A British study did indeed find that women assessed for infertility were ‘healthier’, i.e. had a lower mortality rate, than the general population (Silva Idos et al., 2009). We found a tendency of lower, although not significant, risk of invasive cervical cancer in ART women compared with non-ART women.
No significantly elevated risk of ovarian cancer was observed among ART women in our study. This is in accordance with a recent meta-analysis regarding ovarian cancer, which concluded no increased risk after fertility treatment (Siristatidis et al., 2013). Others have suggested elevated risks following fertility treatment, associated with the use of clomiphene citrate and/or other types of fertility medications (Rossing et al., 1994; Calderon-Margalit et al., 2009; van Leeuwen et al., 2011), as well as progesterone, which in a Danish study from 2015 was associated with an elevated risk of borderline ovarian tumors (Bjornholt et al., 2015). Parity is an important protective factor in the development of ovarian cancer (Adami et al., 1994) and thus one might conclude that in our study of namely parous women our findings of no increased risk of ovarian cancer reflect the protective effect of childbirth. In stratified analyses, we observed elevated risk of ovarian cancer in women who remained uniparous throughout the study, as well as in women who were nulliparous when receiving ART treatment. Although this risk estimate lost statistical significance after correction for multiple analyses, it is noteworthy that several other authors have suggested that those with resistant infertility may represent a subgroup of infertile women that harbors a higher risk (Ness et al., 2002; Rossing et al., 2004; Trabert et al., 2013), which could explain our finding.
Risk of uterine cancer was not elevated for ART women. There were, however, only five cases of uterine cancer in the ART group, so this prompts further observation as the population of ART women ages. The Siristatidis meta-analysis identified an increased risk of endometrial cancers, based on five studies and a total of 18 cancer cases in the IVF group, but when adjusting for the effect of infertility, no elevated risk was found (based on only 2 studies and 11 cancer cases) (Siristatidis et al., 2013).
Other cancers
We also had the opportunity to evaluate the risk of several other cancers. The risk of CRC was not elevated among ART women, which was in accordance with study results from Finland (Yli-Kuha et al., 2012) and Sweden (Kallen et al., 2011). However, Althuis and colleagues reported on use of clomiphene citrate, and found that infertile women had an increased risk of colon cancer compared with the general population, and also found increased risk following more than six cycles of treatment with clomiphene citrate (Althuis et al., 2005).
Our results for thyroid cancer were in line with a pooled risk analysis which, based on 13 studies, observed no definite association between use of exogenous hormones and thyroid cancer (La Vecchia et al., 1999). On the other hand, Hannibal and colleagues in 2008 found that CC and possibly progesterone increased the risk of thyroid cancer in parous women, but found no association with gonadotrophins, hCG or GnRH analogues (Hannibal et al., 2008b).
In our study, no elevated risk of CMM was observed following ART, in agreement with previous studies on fertility treatment (Hannibal et al., 2008a), and treatment with clomiphene citrate (Rossing et al., 1995). Althuis however, reported increased risk after treatment with clomiphene citrate, but not gonadotrophins (Althuis et al., 2005). In a recent paper Stewart and colleagues reported no overall risk increase from a study of 21 604 women treated with IVF or ICSI (Stewart et al., 2013). However, they did note that those who ended up giving birth had higher risks of CMM associated with ART compared with those who remained nulliparous, a difference we could not analyze in our data set consisting of parous women only. Results were also reassuring in a Dutch study from 2015, which found no overall increased risk of CMM in the period 1989 through 2009, although in subgroup analyses the risk of CMM was found to be increased among women who were older at first child birth (Spaan et al., 2015).
Correction for multiple analyses
The results of elevated risk of overall cancer and CNS cancer lost significance when adjusting for multiple analyses, demonstrating the risk of a type I error and implying an important limitation of the study. However, with the amount of data available to us, it was important in the planning phase to address the most common cancer forms for women in this cohort, and to do so in the most comprehensive manner. The present findings, including the slightly elevated overall cancer risk and the magnitude of the main estimate of CNS cancer, should still be alerting to clinicians and researchers in the field of ART and cancer. Additionally, although subgroup analyses of ovarian cancer lost statistical significance when correcting for multiple analyses, it must be pointed out that several other researchers in the field find slight elevations of ovarian cancer risk. Our results should prompt other researchers to looking at risks in infertile women, specifically for CNS cancer but also for ovarian cancer. With longer follow-up time, reassessment of the current cohort will be essential.
Strengths
The registry-based design of this cohort study allowed for unbiased collection of exposure data, regardless of the outcome. By focusing on women who had given birth we were able to address the effects of primary infertility and by adjusting for the number of births could account for effects of secondary infertility (Brinton et al., 2004). The completeness of the CRN enabled accurate ascertainment of cancer, with negligible losses to follow-up (Larsen et al., 2009). The time-dependent analysis using the Cox proportional hazards model provided the opportunity to take into account changes in cancer incidence observed since the early 1980s, as well as increases in the use of ART over the last three decades. To our knowledge, no other study has made separate analyses by IVF with and without ICSI. This is important because when a male factor of infertility exists, a couple is always selected for ICSI (Practice Committees of the American Society for Reproductive Medicine, 2012). Thus, in the ICSI group there may be a higher proportion of couples suffering from male infertility. This distinction may be used as a marker of the type of infertility the couple suffers from, although with some limitations. As mentioned, it is known that the indication for ICSI has become much wider in recent years, for example in cases of unexplained infertility and when few eggs are collected at oocyte retrieval (Andersen et al., 2008), and therefore selection to ICSI may be a somewhat inaccurate measure of female/male infertility.
Limitations
Despite the many strengths of the study, it also has the following limitations. Notably, the follow-up time was still relatively short, especially for women who received ART. As the cohort was relatively young, there were few incident cancers, especially for some rarer tumors, such as uterine cancer, which could have led to type II errors. Information on potential confounders, such as BMI, socioeconomic level and earlier use of oral contraceptives and menopausal hormone therapy, was also unavailable. As mentioned previously, there are indications that ART women may have better health. This healthy cohort effect may cause selection bias, especially for cervical cancer, giving rise to the slightly reduced risk of cervical cancer observed for ART women. Risk assessments according to different causes of infertility could not be done, and indeed different risk profiles have been shown within different infertility categories (Venn et al., 1999). Some research has pointed out that women exposed to many ART cycles may be at increased risk. In this study, we were unable to determine how many treatment cycles a woman was exposed to in order to become pregnant with a child. We did not have data on which specific types of hormone treatments ART women received, and consequently could not assess whether certain medications were associated with elevated cancer risk, as has been described by other investigators. Non-ART women may have been exposed to ART later during their follow-up period, but without a following pregnancy, thus misclassified as unexposed. However, one would expect these to be very few, as only 20% of women receiving ART are parous, and approximately 40% of those exposed to ART do not conceive (R. Storeng, personal communication).
To conclude, our population-based study indicates a possible elevation of risk of CNS cancer as well as a slightly increased risk of overall cancer for women who give birth following ART, although the risk estimates were not statistically significant after adjusting for multiple analyses. Results were largely reassuring for all other cancers, although the number of cancer cases was low for some sites, e.g. uterine cancer. Additionally, stratified analyses suggested that subgroups of ART women may have increased risk of ovarian cancer. The follow-up time is indeed still short for the ART group (median 7.3 years) and the population is still young (median 42 years at end of follow-up). Thus, the increasing use of ART should encourage patients and health care personnel to remain vigilant regarding cancer risk in these women, as they age into a period of life where cancer is more common.
Hopefully, this cohort can be updated and reanalyzed later, to allow for longer follow-up time, and continued monitoring of cancer risk in ART women, especially for CNS and ovarian cancer. Additional large cohort studies on infertile women and women exposed to ART need to be performed to confirm a possible association between infertility or ART and CNS cancer. Furthermore, cancer risk among women who remain childless after ART treatment in Norway remains to be assessed.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org.
Authors' roles
R.S. had the initial idea for the study. R.S., I.K.L., T.E.R. and M.M.R. contributed to study design. M.M.R., I.K.L. and T.Å.M. performed the data linkages and analyses. All authors have contributed to interpretation of results, reviewed, critically revised and approved the final manuscript.
Funding
This work was funded by the Norwegian National Advisory unit on Women's Health, Oslo University Hospital, Oslo, Norway. Funding to pay the Open Access publication charges for this article was provided by The Norwegian National Advisory Unit on Women's Health, Oslo University Hospital.
Conflict of interest
All authors declare no conflicts of interest and have signed the ICMJE statement.
Supplementary Material
Acknowledgements
The authors are grateful to the Medical Birth Registry of Norway and the Cancer Registry of Norway for supplying the data. The interpretation and reporting of these data is the sole responsibility of the authors, and no endorsement by the Medical Birth Registry of Norway is intended nor should be inferred.
References
- Adami HO, Hsieh CC, Lambe M, Trichopoulos D, Leon D, Persson I, Ekbom A, Janson PO. Parity, age at first childbirth, and risk of ovarian cancer. Lancet 1994;344:1250–1254. [DOI] [PubMed] [Google Scholar]
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004;13:1558–1568. [PubMed] [Google Scholar]
- Althuis MD, Scoccia B, Lamb EJ, Moghissi KS, Westhoff CL, Mabie JE, Brinton LA. Melanoma, thyroid, cervical, and colon cancer risk after use of fertility drugs. Am J Obstet Gynecol 2005;193:668–674. [DOI] [PubMed] [Google Scholar]
- Andersen AN, Carlsen E, Loft A. Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update 2008;14:593–604. [DOI] [PubMed] [Google Scholar]
- Andersen L, Friis S, Hallas J, Ravn P, Gaist D. Hormone replacement therapy and risk of glioma: a nationwide nested case-control study. Cancer Epidemiol 2013;37:876–880. [DOI] [PubMed] [Google Scholar]
- Andersen L, Friis S, Hallas J, Ravn P, Kristensen BW, Gaist D. Hormonal contraceptive use and risk of glioma among younger women a nationwide case-control study. Br J Clin Pharmacol 2015;79:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- Benson VS, Pirie K, Green J, Bull D, Casabonne D, Reeves GK, Beral V, Million Women Study C. Hormone replacement therapy and incidence of central nervous system tumours in the Million Women Study. Int J Cancer 2010;127:1692–1698. [DOI] [PubMed] [Google Scholar]
- Bjornholt SM, Kjaer SK, Nielsen TS, Jensen A. Risk for borderline ovarian tumours after exposure to fertility drugs: results of a population-based cohort study. Hum Reprod 2015;30:222–231. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Lamb EJ, Moghissi KS, Scoccia B, Althuis MD, Mabie JE, Westhoff CL. Ovarian cancer risk after the use of ovulation-stimulating drugs. Obstet Gynecol 2004;103:1194–1203. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Trabert B, Shalev V, Lunenfeld E, Sella T, Chodick G. In vitro fertilization and risk of breast and gynecologic cancers: a retrospective cohort study within the Israeli Maccabi Healthcare Services. Fertil Steril 2013;99:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton LA, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, Ruggieri D, Trabert B, Lamb EJ. Long-term relationship of ovulation-stimulating drugs to breast cancer risk. Cancer Epidemiol Biomarkers Prev 2014;23:584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Margalit R, Friedlander Y, Yanetz R, Kleinhaus K, Perrin MC, Manor O, Harlap S, Paltiel O. Cancer risk after exposure to treatments for ovulation induction. Am J Epidemiol 2009;169:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenges D, Letenneur L, Joly P, Alioum A, Dartigues JF. Modelling age-specific risk: application to dementia. Stat Med 1998;17:1973–1988. [DOI] [PubMed] [Google Scholar]
- Doyle P, Maconochie N, Beral V, Swerdlow AJ, Tan SL. Cancer incidence following treatment for infertility at a clinic in the UK. Hum Reprod 2002;17:2209–2213. [DOI] [PubMed] [Google Scholar]
- Hannibal CG, Jensen A, Sharif H, Kjaer SK. Malignant melanoma risk after exposure to fertility drugs: results from a large Danish cohort study. Cancer Causes Control 2008a;19:759–765. [DOI] [PubMed] [Google Scholar]
- Hannibal CG, Jensen A, Sharif H, Kjaer SK. Risk of thyroid cancer after exposure to fertility drugs: results from a large Danish cohort study. Hum Reprod 2008b;23:451–456. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Linet MS, Zhang J, Fine HA, Shapiro WR, Selker RG, Black PM, Inskip PD. Reproductive and hormonal factors and risk of brain tumors in adult females. Int J Cancer 2005;114:797–805. [DOI] [PubMed] [Google Scholar]
- Horn J, Opdahl S, Engstrom MJ, Romundstad PR, Tretli S, Haugen OA, Bofin AM, Vatten LJ, Asvold BO. Reproductive history and the risk of molecular breast cancer subtypes in a prospective study of Norwegian women. Cancer Causes Control 2014;25:881–889. [DOI] [PubMed] [Google Scholar]
- Jensen A, Sharif H, Svare EI, Frederiksen K, Kjaer SK. Risk of breast cancer after exposure to fertility drugs: results from a large Danish cohort study. Cancer Epidemiol Biomarkers Prev 2007;16:1400–1407. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnstrom O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Malignancies among women who gave birth after in vitro fertilization. Hum Reprod 2011;26:253–258. [DOI] [PubMed] [Google Scholar]
- Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988a;57:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key TJ, Pike MC. The role of oestrogens and progestogens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol 1988b;24:29–43. [DOI] [PubMed] [Google Scholar]
- Koomen ER, Joosse A, Herings RM, Casparie MK, Guchelaar HJ, Nijsten T. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case-control study. Ann Oncol 2009;20:358–364. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Ron E, Franceschi S, Dal Maso L, Mark SD, Chatenoud L, Braga C, Preston-Martin S, McTiernan A, Kolonel L et al. A pooled analysis of case-control studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control 1999;10:157–166. [DOI] [PubMed] [Google Scholar]
- Larsen I, Smastuen M, Johannesen T, Langmark F, Parkin D, Bray F, Moller B. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–1231. [DOI] [PubMed] [Google Scholar]
- Lerner-Geva L, Geva E, Lessing JB, Chetrit A, Modan B, Amit A. The possible association between in vitro fertilization treatments and cancer development. Int J Gynecol Cancer 2003;13:23–27. [DOI] [PubMed] [Google Scholar]
- Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the United States from a male perspective: evidence from a nationally representative sample. Andrology 2013;1:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodley M, Moodley J, Chetty R, Herrington CS. The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: a review. Int J Gynecol Cancer 2003;13:103–110. [DOI] [PubMed] [Google Scholar]
- Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, Purdie DM, Risch HA, Vergona R, Wu AH. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol 2002;155:217–224. [DOI] [PubMed] [Google Scholar]
- Nichols HB, Trentham-Dietz A, Hampton JM, Newcomb PA. Oral contraceptive use, reproductive factors, and colorectal cancer risk: findings from Wisconsin. Cancer Epidemiol Biomarkers Prev 2005;14:1212–1218. [DOI] [PubMed] [Google Scholar]
- Orgeas CC, Sanner K, Hall P, Conner P, Holte J, Nilsson SJ, Sundfeldt K, Persson I, Chia KS, Wedren S et al. Breast cancer incidence after hormonal infertility treatment in Sweden: a cohort study. Am J Obstet Gynecol 2009;200:72 e71–77. [DOI] [PubMed] [Google Scholar]
- Pazaitou-Panayiotou K, Toulis KA, Mandanas S, Tarlatzis BC. Thyroid cancer after in vitro fertilization: a retrospective, non-consecutive case-series analysis. Gynecol Endocrinol 2014;30:569–572. [DOI] [PubMed] [Google Scholar]
- Practice Committees of the American Society for Reproductive Medicine. Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: a committee opinion. Fertil Steril 2012;98:1395–1399. [DOI] [PubMed] [Google Scholar]
- Reigstad MM, Larsen IK, Myklebust TÅ, Robsahm TE, Oldereid NB, Omland AK, Vangen S, Brinton LA, Storeng R. Risk of breast cancer following fertility treatment-A registry based cohort study of parous women in Norway. Int J Cancer 2015;136:1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol 1994;140:585–597. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Ovarian tumors in a cohort of infertile women. N Engl J Med 1994;331:771–776. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Daling JR, Weiss NS, Moore DE, Self SG. Risk of cutaneous melanoma in a cohort of infertile women. Melanoma Res 1995;5:123–127. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Voigt LF, Wicklund KG, Daling JR. Reproductive factors and risk of papillary thyroid cancer in women. Am J Epidemiol 2000;151:765–772. [DOI] [PubMed] [Google Scholar]
- Rossing MA, Tang MT, Flagg EW, Weiss LK, Wicklund KG. A case-control study of ovarian cancer in relation to infertility and the use of ovulation-inducing drugs. Am J Epidemiol 2004;160:1070–1078. [DOI] [PubMed] [Google Scholar]
- Scarinci IC, Garcia FA, Kobetz E, Partridge EE, Brandt HM, Bell MC, Dignan M, Ma GX, Daye JL, Castle PE. Cervical cancer prevention: new tools and old barriers. Cancer 2010;116:2531–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika 1982;69:239–241. [Google Scholar]
- Shy H, Malaiyandi L, Timiras PS. Protective action of 17beta-estradiol and tamoxifen on glutamate toxicity in glial cells. Int J Dev Neurosci 2000;18:289–297. [DOI] [PubMed] [Google Scholar]
- Silva Idos S, Wark PA, McCormack VA, Mayer D, Overton C, Little V, Nieto J, Hardiman P, Davies M, MacLean AB. Ovulation-stimulation drugs and cancer risks: a long-term follow-up of a British cohort. Br J Cancer 2009;100:1824–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, Psaltopoulou T, Skalkidou A, Petridou ET. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer—a systematic review and meta-analysis. Hum Reprod Update 2013;19:105–123. [DOI] [PubMed] [Google Scholar]
- Slama R, Hansen OK, Ducot B, Bohet A, Sorensen D, Giorgis Allemand L, Eijkemans MJ, Rosetta L, Thalabard JC, Keiding N et al. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod 2012;27:1489–1498. [DOI] [PubMed] [Google Scholar]
- Spaan M, van den Belt-Dusebout AW, Schaapveld M, Mooij TM, Burger CW, van Leeuwen FE, OMEGA-project group. Melanoma risk after ovarian stimulation for in vitro fertilization. Hum Reprod 2015;30:1216–1228. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Holman CD, Finn JC, Preen DB, Hart R. Association between in-vitro fertilization, birth and melanoma. Melanoma Res 2013;23:489–495. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Crawford S, Anderson JE, Folger SG, Jamieson DJ, Barfield WD, Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. Assisted reproductive technology surveillance—United States, 2010. MMWR Surveill Summ 2013;62:1–24. [PubMed] [Google Scholar]
- Trabert B, Lamb EJ, Scoccia B, Moghissi KS, Westhoff CL, Niwa S, Brinton LA. Ovulation-inducing drugs and ovarian cancer risk: results from an extended follow-up of a large United States infertility cohort. Fertil Steril 2013;100:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen FE, Klip H, Mooij TM, van de Swaluw AM, Lambalk CB, Kortman M, Laven JS, Jansen CA, Helmerhorst FM, Cohlen BJ et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod 2011;26:3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn A, Watson L, Bruinsma F, Giles G, Healy D. Risk of cancer after use of fertility drugs with in-vitro fertilisation. Lancet 1999;354:1586–1590. [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies. IV. The pathogenesis of epithelial ovarian cancer. Collaborative Ovarian Cancer Group. Am J Epidemiol 1992;136:1212–1220. [DOI] [PubMed] [Google Scholar]
- Wigertz A, Lonn S, Hall P, Auvinen A, Christensen HC, Johansen C, Klaeboe L, Salminen T, Schoemaker MJ, Swerdlow AJ et al. Reproductive factors and risk of meningioma and glioma. Cancer Epidemiol Biomarkers Prev 2008;17:2663–2670. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol 2002;4:278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright VC, Schieve LA, Reynolds MA, Jeng G, Kissin D. Assisted reproductive technology surveillance—United States, 2001. MMWR Surveill Summ 2004;53:1–20. [PubMed] [Google Scholar]
- Yli-Kuha AN, Gissler M, Klemetti R, Luoto R, Hemminki E. Cancer morbidity in a cohort of 9175 Finnish women treated for infertility. Hum Reprod 2012;27:1149–1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.