ABSTRACT
The presumptive transcriptional regulator YjjQ has been identified as being virulence associated in avian pathogenic Escherichia coli (APEC). In this work, we characterize YjjQ as transcriptional repressor of the flhDC operon, encoding the master regulator of flagellar synthesis, and of additional loci. The latter include gfc (capsule 4 synthesis), ompC (outer membrane porin C), yfiRNB (regulated c-di-GMP synthesis), and loci of poorly defined function (ybhL and ymiA-yciX). We identify the YjjQ DNA-binding sites at the flhDC and gfc promoters and characterize a DNA-binding sequence motif present at all promoters found to be repressed by YjjQ. At the flhDC promoter, the YjjQ DNA-binding site overlaps the RcsA-RcsB DNA-binding site. RcsA-RcsB likewise represses the flhDC promoter, but the repression by YjjQ and that by RcsA-RcsB are independent of each other. These data suggest that YjjQ is an additional regulator involved in the complex control of flhDC at the level of transcription initiation. Furthermore, we show that YjjQ represses motility of the E. coli K-12 laboratory strain and of uropathogenic E. coli (UPEC) strains CFT073 and 536. Regulation of flhDC, yfiRNB, and additional loci by YjjQ may be features relevant for pathogenicity.
IMPORTANCE Escherichia coli is a commensal and pathogenic bacterium causing intra- and extraintestinal infections in humans and farm animals. The pathogenicity of E. coli strains is determined by their particular genome content, which includes essential and associated virulence factors that control the cellular physiology in the host environment. However, the gene pools of commensal and pathogenic E. coli are not clearly differentiated, and the function of virulence-associated loci needs to be characterized. In this study, we characterize the function of yjjQ, encoding a transcription regulator that was identified as being virulence associated in avian pathogenic E. coli (APEC). We characterize YjjQ as transcriptional repressor of flagellar motility and of additional loci related to pathogenicity.
INTRODUCTION
Escherichia coli is a tremendously well-characterized genetic model organism and an invaluable tool in genetic engineering. However, E. coli is also an important pathogen causing a variety of intra- and extraintestinal infections in vertebrates, including humans and farm animals. The specific pathotype of individual E. coli strains is presumptively determined by their particular genome, which is composed of core and variable gene loci, including horizontally acquired genes (1, 2). The variable gene pool includes essential virulence factors and loci that apparently affect and regulate the cellular physiology and metabolism in the host environment (1). For example, the B2 lineage of the four phylogenetic lineages of E. coli A, B1, B2, and D (3, 4) includes a high proportion of extraintestinal and avian pathogenic strains (1, 4). However, currently the gene pools of commensal and various pathogenic E. coli strains are not clearly differentiated, and it is necessary to further characterize specific virulence traits (1, 5).
One of the many loci that have been identified as being virulence associated is yjjQ, encoding a putative transcription regulator (6, 7). This locus was found in a signature-tagged transposon mutagenesis screen of avian pathogenic E. coli (APEC) strain IMT5155 (O2:H5:K1) using a chicken infection model (7). Analyses of the Tn5 transposon-tagged APEC IMT5155 yjjQ mutant indicated a downregulation of iron metabolism-related genes (6). Furthermore, a Tn5 transposon-tagged yjjQ mutant was identified in a screen for mutants of uropathogenic E. coli (UPEC) strain CFT073 that are motile when simultaneously expressing type I fimbria. However, this phenotype was not confirmed with an isogenic yjjQ deletion mutant (8).
The yjjQ gene is the first gene of the yjjQ-bglJ operon. The second gene, bglJ, also encodes a transcriptional regulator that has a pleiotropic function (9–11). Transcription of the yjjQ-bglJ operon is repressed by the global repressor H-NS (heat-stable nucleoid structuring protein), and it is activated by the pleiotropic transcriptional regulator LeuO (12). The physiological conditions that cause induction of the yjjQ-bglJ operon are unknown, which resembles the current lack of knowledge about H-NS-repressed and LeuO-activated loci (13–17). However, regulation of the yjjQ-bglJ operon by H-NS and LeuO indicates that these genes are related to the control of stress responses and/or virulence-associated traits (15, 18, 19).
In this work, we addressed the function of the YjjQ protein. YjjQ is a dimeric presumptive transcriptional regulator carrying an FixJ/NarL-type C-terminal helix-turn-helix DNA-binding domain (11). We identify gene loci regulated by YjjQ and address the putative regulation of their promoters. We characterize a YjjQ DNA-binding motif and specific DNA-binding sites by mutagenesis. Furthermore, we show that YjjQ specifically represses the promoter of the flhDC operon encoding the master regulator FlhD4C2 of flagellar synthesis (20), among other loci. Correspondingly, ectopically expressed YjjQ inhibits motility of the E. coli laboratory strain K-12 as well as that of human extraintestinal pathogenic E. coli strains, such as UPEC strains CFT073 and 536.
MATERIALS AND METHODS
Strains, plasmids, and oligonucleotides.
The strains, plasmids, and oligonucleotides used in this study are listed in Tables S1, S2, and S3 in the supplemental material. Bacteria were grown in LB medium (10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter). Antibiotics were added to final concentrations of 25 μg/ml kanamycin, 15 μg/ml chloramphenicol, 50 μg/ml spectinomycin, and 50 μg/ml ampicillin where indicated. Plasmid cloning followed standard techniques (21). For mutagenesis of the flhDC promoter fragment, the PCR was performed with an unbalanced nucleotide ratio, as described previously (22).
Motility and biofilm assay.
For motility assays, the centers of LB soft agar plates (0.25% [wt/vol] Bacto agar supplemented with 25 μg/ml kanamycin, and 200 μM isopropyl-β-d-thiogalactopyranoside [IPTG] where indicated) were spotted with 3 μl of an overnight culture. Swimming radii were determined after incubation for 5 h at 37°C for K-12 strain BW30270 and after 12 h at 28°C for the UPEC strains. Biofilm assays were performed as described previously (57). Briefly, overnight cultures were diluted 100-fold in LB kanamycin medium supplemented with 1 mM IPTG where indicated. Of these dilutions, 100 μl was grown for 48 h at 28°C in quadruplets in 96-well microtiter plates with lids (flat bottom, polystyrene, non-tissue culture treated). The bacterial suspension was removed by pipetting, and the wells were washed once with 150 μl H2O. Then 150 μl 0.1% (wt/vol) crystal violet was added to each well, and the plates were incubated for 10 min at room temperature. The dye was removed by pipetting, the wells were washed twice with 200 μl H2O, and the plates were air dried. To solubilize the dye, 200 μl of 30% acetic acid was added to each well, and the plates were incubated at room temperature for a few minutes.
Mapping of transcription start sites by 5′-RACE.
Mapping of the transcription start sites by 5′ rapid amplification of cDNA ends (5′-RACE) was performed as described previously (10, 23). Briefly, RNA was isolated from strain T23 [Δ(yjjP-yjjQ-bglJ)] grown in LB to an optical density at 600 nm (OD600) of 0.5 using the RNAprotect and RNeasy minikit system with on-column DNase digestion (Qiagen, Germany). To distinguish primary 5′ ends of transcripts and 5′ ends generated by processing, 6 μg of the RNA was treated with tobacco acid pyrophosphatase (TAP) (Epicentre Biotechnologies). Then an RNA adapter (RNA oligonucleotide T268) was ligated to both 6 μg of untreated RNA and to the TAP-treated RNA preparations. Following phenol-chloroform-isoamyl alcohol (25:24:1) extraction and ethanol precipitation, the RNA samples were used for first-strand cDNA synthesis with random hexameric oligonucleotides as primers and the SuperScript III First Strand synthesis kit according to the instructions of the manufacturer (Invitrogen, Germany). The cDNA was used for PCRs with adapter-specific primer T265 and gene-specific primers (see Table S3 in the supplemental material). TAP-specific PCR fragments obtained for each locus were digested with EcoRI and XbaI, and the fragments were cloned into pUC12. At least 3 clones were sequenced for mapping of the primary transcription start sites.
Expression analyses.
For expression analyses of lacZ reporter fusions, β-galactosidase assays were performed as described previously (10, 24). Briefly, bacterial cultures were inoculated from fresh overnight cultures to an OD600 of 0.05 and grown to the exponential-growth-phase OD600 of 0.5 in LB medium. The medium of the overnight and exponential cultures was supplemented with antibiotics and 1 mM IPTG where indicated. The average units are based on at least three biological replicates, and in each assay, at least 4 technical replicates were measured.
To determine putative target genes of YjjQ, the Δ(yjjP-yjjQ-bglJ) strain S3922 was transformed with yjjQ-containing plasmid pKERV17 or the empty vector control pKES169. The transformants were grown in LB medium supplemented with kanamycin to an OD600 of 0.3, and then IPTG was added for induction, and the cultures were grown for additional 30 min to an OD600 of approximately 0.5. RNA was isolated using the RNAprotect and RNeasy minikit system with on-column DNase digestion (Qiagen, Germany). Hybridization to Affymetrix GeneChip E. coli Genome 2.0 microarrays was carried out according to the manufacturer's instructions. Microarrays were scanned using an Affymetrix GeneChip Scanner 3000 7G. Data were processed using the Affymetrix apt-probeset-summarize software (version 1.10) and RMA algorithm. Samples were normalized using the standard normalization probes present on the Affymetrix GeneChip, and differential expression values were calculated as fold changes. Data for all loci for which a 10-fold or higher repression in at least one of the strains was detected are shown in Table 1.
TABLE 1.
Putative YjjQ-regulated loci in E. coli K-12 and UPEC CFT073 identified by microarray analysis
| Locus | Fold repressiona |
Function | |
|---|---|---|---|
| K-12 | CFT073 | ||
| adiY | 39.6 | 29.3 | Arginine-dependent acid resistance |
| cbpA | 25.3 | 8.0 | DNA-binding cochaperone system |
| csrB | 11.3 | 5.2 | srRNA |
| flhDC | Mutantb | 9.8/6.0 | Master regulator of flagellar synthesis |
| gfcA | 53.1c | Group 4 capsule synthesis | |
| mdtJ | 31.1 | 13.7 | Spermidine exporter |
| nanC | 11.5 | 1.1 | Sialic acid metabolism |
| ompC | 36.6 | 17.2 | Porin |
| panD | 11.4 | 10.4 | l-Aspartate-α-decarboxylase |
| ucpA | 22.0 | 8.7 | Furan resistance |
| uspF | 6.4 | 10.6 | Universal stress protein |
| yfiR | 23.4 | 7.8 | Periplamic regulator of c-di-GMP synthase YfiN |
| yhjR | 15.5 | 12.9 | Cellulose synthesis |
| bcsE | 9.0 | 7.9 | Cellulose synthesis |
| c1618 | 21.4 | Unknown | |
| ybhL | 26.8 | 19.3 | Inner membrane transporter |
| ymiA/yciX | 23.3/28.6 | 10.4/10.1 | Unknown |
Listed are the fold repression values caused by expression of yjjQ carried by plasmid pKERV17 in transformants of E. coli K-12 strain S3922 and UPEC strain CFT073. Shown are the repression values for the first gene of each operon. For flhDC and ymiA/yciX, the folds of repression for both genes of the operon are given.
E. coli K-12 strain S3922 [BW30270 Δ(yjjP-yjjQ-bglJ)::KD3] is nonmotile, and the flhDC promoter region is rearranged.
The gfc operon is not expressed in E. coli K-12 due to an IS1 insertion element. However, the microarray probe maps between the promoter and the IS1 element.
RESULTS
Regulation of presumptive target promoters by YjjQ.
To identify target genes of the putative transcriptional regulator YjjQ, we analyzed the change of the expression pattern caused by plasmidic expression of yjjQ using a microarray approach. In this assay, high-level expression of yjjQ that was provided by high-copy-number plasmid pKERV17 caused downregulation of approximately 20 loci, both in the E. coli K-12 yjjQ deletion strain S3922, and in the UPEC strain CFT073 (Table 1). The UPEC strain CFT073 was chosen because UPEC and APEC strains are closely related (25, 26) and a representative microarray was available for CFT073 at that time but not for APEC strain IMT5155, in which yjjQ was identified as being virulence associated. To validate whether the presumptive target loci identified by this microarray analysis are indeed regulated by YjjQ at the level of transcription initiation, we chose 16 of the loci for construction of promoter lacZ fusions (see Fig. S1 in the supplemental material). These promoter-lacZ reporter fusions were integrated into the attB site of the E. coli K-12 yjjQ and lacZ deletion strain T23 [ΔlacZ Δ(yjjP-yjjQ-bglJ)] (Fig. 1; see Fig. S1). Regulation of these lacZ reporter fusions by YjjQ was tested using the low-copy-number yjjQ-expressing plasmid pKEKD31 and an empty vector control (Fig. 1; see Fig. S1). YjjQ was provided plasmidically, as expression of the native yjjQ-bglJ operon is repressed by H-NS during exponential growth under laboratory conditions (12). The expression analyses of the promoter-lacZ reporter fusions revealed that 6 of the 16 putative target promoters are repressed by YjjQ (Fig. 1), while the activities of the remaining promoters were unaffected under the conditions used (see Fig. S1). Possibly high expression levels of yjjQ in the microarray yielded that many false positives. Repression by YjjQ using the promoter-lacZ reporters was detected for the promoters of flhDC, ompC, gfc, ybhL, yfiRNB, and ymiA-yciX (Fig. 1). For comparison, these six YjjQ-repressed promoter-lacZ fusions were also tested in the yjjQ+ wild-type background S4197 (ΔlacZ) (Fig. 1), with similar results as in the yjjQ deletion strain (Fig. 1), which demonstrated that the presence of wild-type yjjQ at its native, H-NS-repressed locus has no effect. Of the six YjjQ repressed loci, flhDC encodes the master regulator FlhD4C2 of flagellar synthesis (27). This locus is repressed most by YjjQ (10-fold). Please note that in the initial microarray analysis, flhDC was identified as the putative target in UPEC strain CFT073 but not in the K-12 strain S3922. The latter strain, S3922, turned out to be nonmotile and to carry a mutation at the flhDC locus, which may explain this discrepancy. Other targets of YjjQ are ompC, encoding the outer membrane porin OmpC (28), and the gfc locus, encoding enzymes for group 4 capsule synthesis (29). The gfc locus is not expressed in E. coli K-12 due to an IS1 insertion between the promoter and the coding region (29). However, in the gfc promoter-lacZ reporter, this IS1 element was excluded. The ybhL gene product is an inner membrane protein and putative transporter (28). The yfiRNB locus encodes the c-di-GMP cyclase YfiN, the periplasmic regulatory protein YfiR, and the lipoprotein YfiB. Mutation of yfiR affects swarming and swimming motility, as well as early biofilm formation (28, 30). Furthermore, the Yfi system promotes CsgD-independent cellulose production under reducing conditions (31). Likewise, in the UPEC strain CFT073, yfiR mutants are affected in synthesis of curli and cellulose, and they are attenuated in mouse bladder and kidney infection (32). The YjjQ target locus ymiA-yciX codes for the small putative membrane protein YmiA (42 amino acids [aa]) (33) and a small putative protein YciX (55 aa) of unknown function (28).
FIG 1.
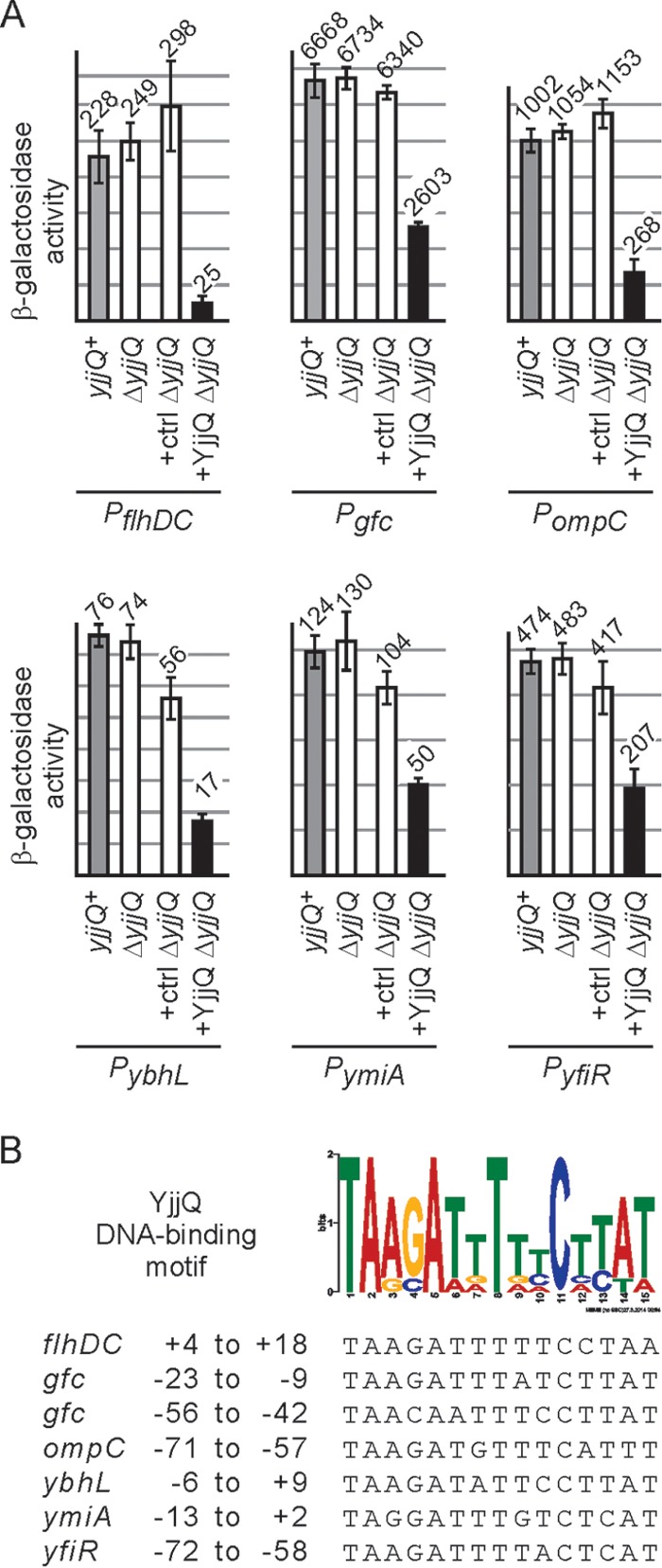
Regulation of target gene promoters by YjjQ and identification of a YjjQ DNA-binding motif. (A) The expression level of chromosomal promoter-lacZ reporter fusions was determined in the yjjQ+ strain background S4197 as well as in the yjjP-yjjQ-bglJ deletion strain T23 (ΔyjjQ). Further transformants of T23 (ΔyjjQ)-derived reporter strains with vector control plasmid pKESK22 (ctrl) and the yjjQ-containing plasmid pKEKD31 (+ YjjQ) were analyzed. Bacteria were grown to the mid-exponential phase (OD600 of 0.5) in LB medium, which was supplemented with kanamycin and 1 mM IPTG in the case of transformants. The β-galactosidase activities of at least three biological replicates were determined. The following strains were used: T2047 and T1266 (PflhDC), T2051 and T1541 (Pgfc), T2046 and T1268 (PompC), T2049 and T1533 (PybhL), T2050 and T1535 (PymiA), and T2048 and T1351 (PyfiR). (B) Analysis of 150-bp DNA sequence encompassing the target promoters using MEME Suite (34) yielded a putative YjjQ DNA-binding motif. The individual sequences matching this motif in the target promoter fragments and their relative positions to the transcription start site are indicated at the bottom. At the gfc promoter, two sites were identified.
Mapping of YjjQ-binding sites.
YjjQ-mediated repression of the target gene promoters presumably involves DNA binding by YjjQ. To map the YjjQ DNA-binding sites, the DNA sequences encompassing the YjjQ-regulated target promoters from positions −100 to +50 relative to their transcription start sites were searched for a common motif using the program MEME Suite (34). The position of the transcription start sites were taken from the literature for the flhDC and ompC promoters (for references, see reference 28), while they were mapped by 5′-RACE for the ybhL, ymiA-yciX, yfiRNB, and gfc promoters (see Fig. S2 in the supplemental material). The transcription start sites of the non-YjjQ-regulated promoters of the bcsE, yhjR, and panD genes were also mapped by 5′-RACE (see Fig. S1C). Indeed, the MEME Suite search of the YjjQ target promoter sequences yielded a motif present in all six fragments (Fig. 1; see Fig. S2). This 15-bp motif is palindromic, as it is typical for a DNA-binding site of a homodimeric transcriptional regulator and is of similar size to other DNA-binding motifs characterized for transcription regulators of the FixJ/NarL family (35, 36). The YjjQ DNA-binding motif overlaps with the core promoter or transcription initiation region in the cases of flhDC, gfc, ybhL, and ymiA (Fig. 2; see Fig. S2). At the gfc promoter, the motif sequence was identified twice, with one of them mapping between the −35 and −10 promoter sequences (see Fig. S2). At the ompC promoter, the motif maps upstream of the core promoter (centered at position −64 relative to the transcription start site of the main P1 promoter) and overlaps the binding sites of the activator OmpR (see Fig. S2). Likewise, at yfiR the motif maps upstream of the promoter, with the center of the motif mapping at position −65 relative to the transcription start site (see Fig. S2).
FIG 2.
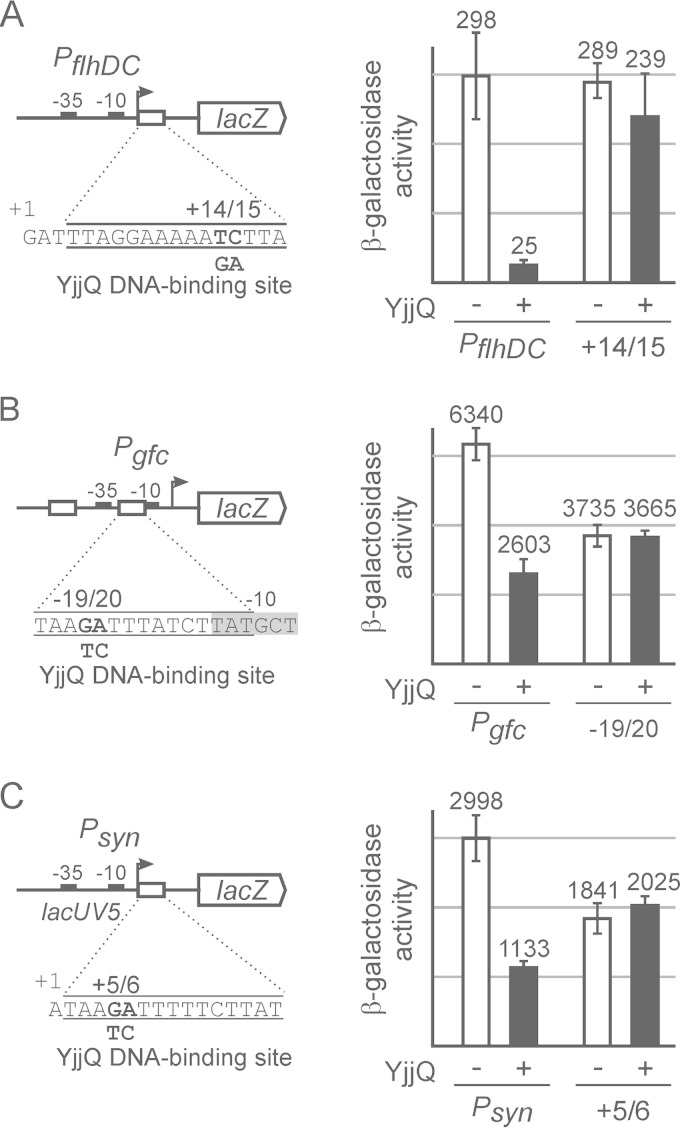
Mutation of the putative YjjQ DNA-binding site abrogates repression by YjjQ. Promoter-lacZ fusions and their mutant derivatives carrying mutations in the putative YjjQ DNA-binding site were integrated in strain T23. The expression level was determined for transformants of these reporter strains with control plasmid pKESK22 (−) and yjjQ-containing plasmid pKEKD31 in the presence of YjjQ (+). Schematically shown are the main features of the promoter-lacZ fusions, such as the transcription start site (+1), the −10 and the −35 boxes, as well as the sequences of the putative YjjQ DNA-binding sites and the mutations that were introduced. Tested were the flhDC promoter (PflhDC) (strain T1266) and its mutant derivative (+14/15) carrying a 2-nucleotide exchange at positions +14 and +15 relative to the transcription start (strain T1661) (A), the gfc promoter (Pgfc) (strain 1541) and its mutant derivative (−19/20 [strain T1664]) (B), and a synthetic promoter consisting of the lacUV5 core promoter and the YjjQ DNA-binding motif fused immediately downstream of the transcription initiation site (strain T1675), as well as its mutant derivative (+5/6 [strain T1667]) (C). Transformants of these reporter strains with the control plasmid pKESK22 (− YjjQ) and with plasmid pKEKD31 (+ YjjQ) were grown to an OD600 of 0.5 in LB medium supplemented with kanamycin and IPTG. Given is the average of enzyme activities determined from at least 3 independent biological replicates.
To experimentally validate whether the motif represents the YjjQ DNA-binding site, we chose the flhDC and the gfc promoters and mutated the motif sequence in the respective promoter-lacZ reporter fusions, as shown in Fig. 2. Expression analyses of these mutants in the absence and presence of YjjQ demonstrated that the mutation of the YjjQ DNA-binding motif abrogates repression of these promoters by YjjQ (Fig. 2). In the case of the flhDC promoter, the mutation of the YjjQ DNA-binding motif rendered the promoter YjjQ independent and affected the activity of the promoter only marginally (Fig. 2A). In the case of the gfc promoter, the mutation of the YjjQ DNA-binding motif likewise abrogated repression by YjjQ (Fig. 2B). However, the mutation also caused an approximately 2-fold reduction of the promoter activity (Fig. 2B). This reduction of activity is likely to be based on the proximity of the mutation to the −10 sequence. In a second approach to validate the YjjQ DNA-binding motif, we fused this motif to the lacUV5 core promoter (PlacUV5) and analyzed whether this confers repression by YjjQ (Fig. 2C). This synthetic PlacUV5 YjjQ motif construct was repressed 3-fold by YjjQ (Fig. 2C). As control, fusion of a mutant YjjQ DNA-binding motif to the lacUV5 core promoter did not confer repression by YjjQ (Fig. 2C). Again the mutation, which maps next to the transcription initiation site of this synthetic promoter, affected the promoter and reduced its activity approximately 1.5-fold (Fig. 2C). Taken together, the data from the mutational analyses suggest that the motif represents the YjjQ DNA-binding site.
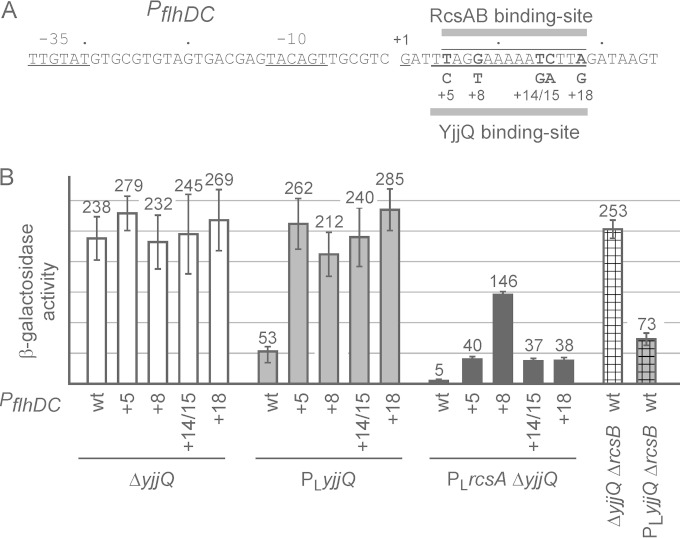
As a further unbiased approach to identify the YjjQ DNA-binding site, we performed a mutagenesis of the flhDC promoter fragment and screened for promoter mutants that are YjjQ independent (Fig. 3). For this mutagenesis screen, the flhDC promoter fragment was amplified by PCR using error-prone conditions and cloned into the lacZ reporter plasmid pKES268. The clones were screened for a changed phenotype in strain T1124 (PLyjjQ ΔlacZ), which carries a yjjQ allele that is constitutively expressed under the control of the phage lambda PL promoter. Colonies were screened on tetrazolium chloride lactose indicator plates, and clones with a phenotype different from that of the wild type were picked. This screen yielded 4 independent mutants mapping to 3 different nucleotides within the YjjQ DNA-binding site (Fig. 3). In addition, several clones with mutations mapping within the core promoter were isolated (see Fig. S3 in the supplemental material). The latter turned out to be promoter up- and promoter down-mutations (see Fig. S3). Intriguingly, all 3 mutations mapping within the YjjQ DNA-binding motif abrogated repression of the flhDC promoter by YjjQ but caused no change of the promoter activity, as shown by expression analyses (Fig. 3). For these expression analyses, the flhDC promoter and its mutants were integrated into the chromosome of strain T1124 carrying the PLyjjQ allele (conferring constitutive yjjQ expression) and strain T23 carrying a yjjQ deletion (Fig. 3). The data support the conclusion that the motif represents the YjjQ DNA-binding site.
FIG 3.
Characterization of YjjQ-independent mutants of the flhDC promoter and their regulation by RcsB and RcsA-RcsB. (A) Sequence of the flhDC promoter and YjjQ independent mutants mapping within the YjjQ DNA-binding site. The mutations at +5, +8, and +18 were identified in a mutagenesis screen, while mutations at +14 and +15 (+14/15) were generated by site-directed mutagenesis. The YjjQ DNA-binding site overlaps with the RcsAB box that was characterized as the DNA-binding site of RcsA-RcsB (37). (B) The wild-type and mutant flhDC promoter-lacZ reporter fusions were integrated chromosomally, and their expression was tested in the yjjQ deletion strain T23 (ΔyjjQ) and in strain T1124 carrying the yjjQ PLyjjQ allele that is constitutively expressed under the control of the phage lambda PL promoter. Furthermore, for constitutive expression of rcsA under the control of the lambda PL promoter, allele PLrcsA was transduced, resulting in PLrcsA ΔyjjQ strain backgrounds (see Table S1 in the supplemental material). In addition, ΔrcsB deletion mutants that carry a yjjQ deletion (ΔyjjQ) (strain T1674) or constitutive yjjQ allele, PLyjjQ (strain T1771), were used. The reporter strains were grown to the mid-exponential phase (OD600 of 0.5) in LB medium, and average values from at least three biological replicates are shown.
Control of flhDC by YjjQ and RcsAB.
The putative YjjQ DNA-binding site at the flhDC promoter overlaps the DNA-binding site for RcsA-RcsB and for RcsB that was identified previously (37) (Fig. 3). Therefore, repression of flhDC by YjjQ might be indirect and depend on RcsB or RcsA-RcsB. To address this, we analyzed repression of the flhDC promoter by YjjQ in an rcsB deletion background. In this strain background, repression can be mediated neither by RcsB nor by the heteromeric RcsA-RcsB. The expression data show that YjjQ represses the flhDC promoter-lacZ fusion similarly in the ΔrcsB background as in the isogenic rcsB+ strain (Fig. 3). This suggests that YjjQ-mediated repression is RcsB as well as RcsA-RcsB independent. Notably, the expression levels are moderately higher in the ΔrcsB mutant than in the wild type, which may be attributable to a weak repression of the flhDC promoter by the response regulator RcsB, even without induction of the Rcs signaling cascade. This is in accordance with previous data, where it was shown that overexpression of RcsB causes repression of flhDC (37). Furthermore, we analyzed RcsA-RcsB-mediated repression of the flhDC promoter-lacZ fusion and its YjjQ DNA-binding motif mutants. To analyze regulation of flhDC by RcsA-RcsB independently of regulation of rcsA, we replaced the rcsA promoter region by the phage lambda PL promoter conferring constitutive rcsA expression (allele PLrcsA). The rcsA promoter itself is H-NS repressed; its expression is positively autoregulated by RcsA-RcsB and requires induction of the Rcs signaling pathway (38). Constitutive expression of rcsA or stabilization of the RcsA protein in protease Lon mutants is sufficient for activation of RcsA-RcsB target genes in the absence of Rcs signal induction (37, 38). As expected, constitutive expression of rcsA (allele PLrcsA) caused strong repression of the flhDC promoter-lacZ fusion (compare 228 units and 5 units corresponding to a 45-fold repression in Fig. 3). The mutations mapping in the overlapping YjjQ and RcsA-RcsB DNA-binding sites all reduced repression by RcsA-RcsB (Fig. 3). Interestingly, the G-to-T exchange at position +8 relative to the transcription start had the strongest effect and almost completely abrogated repression of the flhDC promoter by RcsA-RcsB, while the other mutations reduced repression by RcsA-RcsB from 45-fold to 7-fold. This +8G residue maps to the most conserved bases, GGA, of the RcsA-RcsB DNA-binding site (37). Taken together, the data show that YjjQ and RcsA-RcsB repress the flhDC promoter independently, that the YjjQ and RcsA-RcsB DNA-binding sites overlap, and that individual nucleotides are of different levels of relevance for repression by YjjQ and RcsA-RcsB.
Repression of motility by YjjQ and effects on biofilm formation.
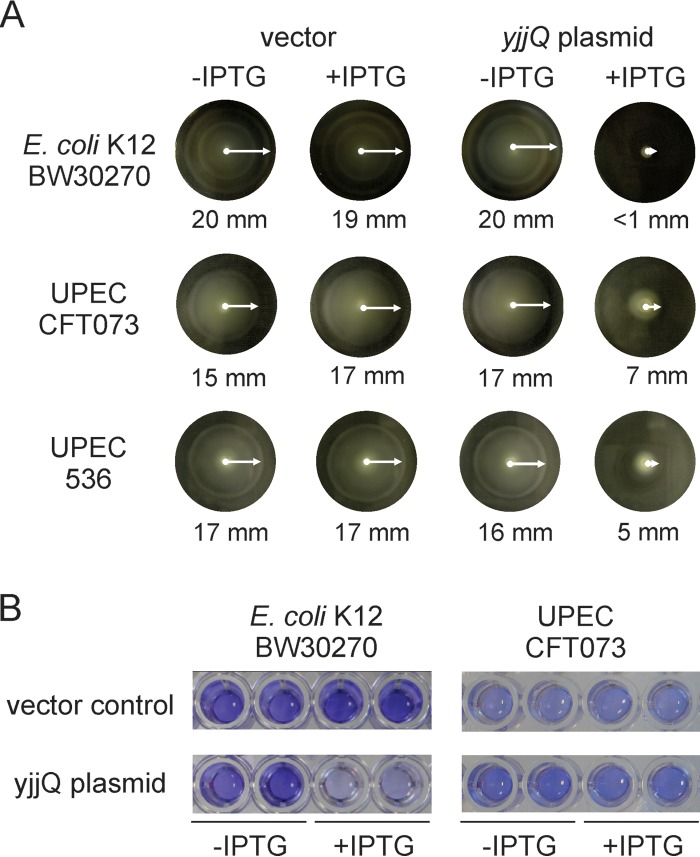
The promoter of the flhDC locus encoding the master regulator of flagellar synthesis is repressed by YjjQ, suggesting that YjjQ also represses motility of E. coli. To test this, the motile E. coli K-12 strain BW30270 was transformed with plasmid pKEKD31 carrying yjjQ under the control of the IPTG-inducible tac promoter as well as with the empty vector control. Motility assays using soft agar plates (0.25% agar) revealed a high motility of strain BW30270, which is presumably based on the insertion of an IS5 element upstream of the flhDC promoter (39). However, the motility was completely inhibited upon induction of yjjQ expression (Fig. 4A). Similar motility assays with UPEC strains 536 and CFT037 demonstrated that induction of YjjQ inhibits the motility of these strains as well (Fig. 4A). As regulation of flagellar synthesis may affect the motility-to-biofilm transition (40), we analyzed the effect of YjjQ on biofilm formation by the laboratory strain K-12 and UPEC strain CFT073 (Fig. 4B). Transformants of E. coli strains BW30270 (K-12) and CFT073 (UPEC) were grown in 96-well polystyrene microtiter plates for 48 h at 28°C, and biofilm formation was estimated by crystal violet staining (Fig. 4B). No staining was observed for UPEC strain 536 (data not shown), and therefore this strain was not used. In this assay, YjjQ inhibited biofilm formation by K-12 strain BW30270 but had no effect on UPEC strain CFT073 (Fig. 4B).
FIG 4.
Control of motility and biofilm formation by YjjQ. (A) Soft agar plates (0.25% agar) were used to test the motility of transformants of K-12 strain BW30270 and UPEC strains CFT073 and 536 with the empty vector control and yjjQ-expressing plasmids pKEKD31 (for K-12) and pKEKD30 (for UPEC strains). The plates were incubated for 5 to 6 h at 37°C for strains K-12 and 536 and for 12 h at 28°C in the case of CFT073. The swimming radii are indicated. IPTG was added to a 200 μM final concentration where indicated. (B) Biofilm formation of transformants of K-12 strain BW30270 and UPEC strain CFT073 with the vector control and the yjjQ plasmids pKEKD31 and pKEKD30, respectively, was analyzed by crystal violet staining of bacteria adhering to polystyrol microtiter plates. Bacteria were grown for 48 h at 28°C before staining. IPTG was added to a 1 mM final concentration where indicated.
DISCUSSION
Previously, the presumptive transcriptional regulator YjjQ of E. coli has been implicated in virulence of the avian pathogenic E. coli strain IMT5155 (6), and yjjQ expression has been shown to be regulated by H-NS and LeuO (12). Here we have shown that YjjQ represses transcription of several loci by binding to a palindromic DNA-binding motif. These loci include the flhDC operon, encoding the master regulator of flagellar synthesis FlhD4C2, gfc, encoding enzymes of group 4 capsule (O-antigen) synthesis, ompC, encoding the outer membrane porin C, and yfiRNB, coding for a c-di-GMP cyclase and regulatory proteins, as well as two additional loci of poorly defined function. Furthermore, we show that YjjQ represses motility of the laboratory E. coli K-12 strain and of UPEC strains and that YjjQ inhibits biofilm formation by K-12 but not by UPEC strain CFT073. These data suggest that YjjQ represents a transcriptional repressor in E. coli, which may contribute to the switch between the motile and adhesive lifestyles.
Transcriptional repression of the flhDC promoter by YjjQ adds an additional component to the complex regulation of this operon. In addition to the repression by YjjQ shown here, transcription initiation at the flhDC promoter is known to be controlled by OmpR, CRP, RcsA-RcsB and RcsB, HfdR, DksA/ppGpp, LrhA, and MatA (= EcpR) (37, 39, 41–46). Furthermore, several small regulatory RNAs (sRNAs) control expression of the flhDC operon at a posttranscriptional level (47). Tight control of flhDC expression, dependent on multiple inputs, is attributed both to the high energy consumption of flagellar synthesis as well as to the requirement to adapt a motile or sessile lifestyle, depending on the environment (48, 49). At the flhDC promoter, the YjjQ DNA-binding site and the RcsA-RcsB DNA-binding site overlap almost completely, but transcriptional regulation by YjjQ and that by RcsA-RcsB seem independent of each other. This may indicate that RcsA-RcsB and YjjQ compete for binding. However, another possibility is that repression by RcsA-RcsB or by YjjQ provides a means to control flhDC in response to different signals. RcsA-RcsB-mediated repression of flhDC is coupled to the Rcs signaling system that is sensing perturbation in the outer membrane protein assembly and peptidoglycan layer and is induced by antimicrobial peptides (50–53), while signals that induce yjjQ remain to be defined.
YjjQ also represses the gfc and yfiRNB operons, ompC, and two further loci (ybhL and ymiA-yciX). The gfc-encoded group 4 capsule is synthesized by various E. coli strains, as shown for enteropathogenic E. coli (EPEC) O127:H6, enterohemorrhagic E. coli (EHEC) O157:H7, and APEC O78:H9 (29, 54, 55). Furthermore, strains with mutation of the gfc locus in APEC O78:H9 strain X7122 are attenuated (54), while the group 4 capsule masks surface structures of EHEC O157:H7 and affects intestine colonization (55). The gfc locus is absent in UPEC strains (29). However, the yfiRNB operon, encoding a regulated c-di-GMP synthesis system, has been implicated in UPEC virulence, for which a yfiR mutant was shown to be attenuated in a murine urinary tract and kidney infection model (32). Thus, YjjQ represses a set of loci that are associated with several virulence traits in various pathogenic E. coli strains. A common feature of these loci is that they concern surface components (porin and capsule 4) and loci implicated in the control of the motile versus sessile lifestyle (flhDC and yfiRNB). However, a target gene relevant for iron homeostasis was not identified, although previous work suggested that YjjQ may control iron homeostasis in APEC (6). This discrepancy may be caused by a particular trait of the specific APEC IMT5155 strain or its derivative, the primary transposon-tagged yjjQ mutant that was analyzed previously. Such a trait might confer indirect control of iron metabolism by YjjQ.
How is YjjQ-mediated repression of flhDC and the other loci controlled? Expression of the yjjQ gene, which is encoded in the yjjQ-bglJ operon, is repressed by the global repressor H-NS and activated by the pleiotropic transcriptional regulator LeuO, as shown for the laboratory E. coli K-12 strain (12). LeuO, a regulator that is conserved in Enterobacteriaceae, is of pleiotropic function which includes virulence control in Salmonella enterica, and it is considered an antagonist of the global repressor H-NS (13, 15, 56). Present knowledge indicates that induction of the yjjQ gene determines whether repression by YjjQ occurs. It is not known whether YjjQ protein activity is also controlled. Furthermore, it needs to be worked out (i) how YjjQ integrates into regulatory networks controlling loci such as flhDC, gfc, yfiRNB, and ompC and (ii) how YjjQ may contribute to the control and modulation of virulence associated surface structures in various E. coli strains.
Supplementary Material
ACKNOWLEDGMENTS
We thank Thomas Stratmann and rotation students, who contributed to strain and plasmid constructions, and Lisa Rabes for discussions and critical reading of the manuscript. Microarray analysis was performed by the Cologne Center of Genomics (CCG).
This work was funded by the Deutsche Forschungsgemeinschaft through grants SCHN 371/10-1, SCHN 371/10-2, and WI 1436/5-3.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00263-15.
REFERENCES
- 1.Alteri CJ, Mobley HLT. 2012. Escherichia coli physiology and metabolism dictates adaptation to diverse host microenvironments. Curr Opin Microbiol 15:3–9. doi: 10.1016/j.mib.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Touchon M, Hoede C, Tenaillon O, Barbe V, Baeriswyl S, Bidet P, Bingen E, Bonacorsi S, Bouchier C, Bouvet O, Calteau A, Chiapello H, Clermont O, Cruveiller S, Danchin A, Diard M, Dossat C, Karoui ME, Frapy E, Garry L, Ghigo JM, Gilles AM, Johnson J, Le Bouguénec C, Lescat M, Mangenot S, Martinez-Jéhanne V, Matic I, Nassif X, Oztas S, Petit MA, Pichon C, Rouy Z, Ruf CS, Schneider D, Tourret J, Vacherie B, Vallenet D, Médigue C, Rocha EPC, Denamur E. 2009. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet 5:e1000344. doi: 10.1371/journal.pgen.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 4.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MCJ, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köhler C-D, Dobrindt U. 2011. What defines extraintestinal pathogenic Escherichia coli? Int J Med Microbiol 301:642–647. doi: 10.1016/j.ijmm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Ewers C, Laturnus C, Diehl I, Dai J, Antao E-M, Schnetz K, Wieler LH. 2008. Characterization of a yjjQ mutant of avian pathogenic E. coli (APEC). Microbiology 154:1082–1093. doi: 10.1099/mic.0.2007/015784-0. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Laturnus C, Ewers C, Wieler LH. 2005. Identification of genes required for avian Escherichia coli septicemia by signature-tagged mutagenesis. Infect Immun 73:2818–2827. doi: 10.1128/IAI.73.5.2818-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simms AN, Mobley HLT. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J Bacteriol 190:3747–3756. doi: 10.1128/JB.01870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salscheider SL, Jahn A, Schnetz K. 2014. Transcriptional regulation by BglJ-RcsB, a pleiotropic heteromeric activator in Escherichia coli. Nucleic Acids Res 42:2999–3008. doi: 10.1093/nar/gkt1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratmann T, Pul Ü, Wurm R, Wagner R, Schnetz K. 2012. RcsB-BglJ activates the Escherichia coli leuO gene, encoding an H-NS antagonist and pleiotropic regulator of virulence determinants. Mol Microbiol 83:1109–1123. doi: 10.1111/j.1365-2958.2012.07993.x. [DOI] [PubMed] [Google Scholar]
- 11.Venkatesh GR, Kembou Koungni FC, Paukner A, Stratmann T, Blissenbach B, Schnetz K. 2010. BglJ-RcsB heterodimers relieve repression of the Escherichia coli bgl operon by H-NS. J Bacteriol 192:6456–6464. doi: 10.1128/JB.00807-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratmann T, Madhusudan S, Schnetz K. 2008. Regulation of the yjjQ-bglJ operon, encoding LuxR-type transcription factors, and the divergent yjjP gene by H-NS and LeuO. J Bacteriol 190:926–935. doi: 10.1128/JB.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon SC, Espinosa E, Hokamp K, Ussery DW, Casadesús J, Dorman CJ. 2012. LeuO is a global regulator of gene expression in Salmonella enterica serovar Typhimurium. Mol Microbiol 85:1072–1089. doi: 10.1111/j.1365-2958.2012.08162.x. [DOI] [PubMed] [Google Scholar]
- 14.Espinosa E, Casadesús J. 2014. Regulation of Salmonella enterica pathogenicity island 1 (SPI-1) by the LysR-type regulator LeuO. Mol Microbiol 91:1057–1069. doi: 10.1111/mmi.12500. [DOI] [PubMed] [Google Scholar]
- 15.Hernández-Lucas I, Calva E. 2012. The coming of age of the LeuO regulator. Mol Microbiol 85:1026–1028. doi: 10.1111/j.1365-2958.2012.08175.x. [DOI] [PubMed] [Google Scholar]
- 16.Medina-Aparicio L, Rebollar-Flores JE, Gallego-Hernandez AL, Vazquez A, Olvera L, Gutierrez-Rios RM, Calva E, Hernandez-Lucas I. 2011. The CRISPR/Cas immune system is an operon regulated by LeuO, H-NS, and leucine-responsive regulatory protein in Salmonella enterica serovar Typhi. J Bacteriol 193:2396–2407. doi: 10.1128/JB.01480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westra ER, Pul U, Heidrich N, Jore MM, Lundgren M, Stratmann T, Wurm R, Raine A, Mescher M, Van Heereveld L, Mastop M, Wagner EG, Schnetz K, Van Der Oost J, Wagner R, Brouns SJ. 2010. H-NS-mediated repression of CRISPR-based immunity in Escherichia coli K-12 can be relieved by the transcription activator LeuO. Mol Microbiol 77:1380–1393. doi: 10.1111/j.1365-2958.2010.07315.x. [DOI] [PubMed] [Google Scholar]
- 18.Dillon SC, Dorman CJ. 2010. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol 8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 19.Navarre WW, McClelland M, Libby SJ, Fang FC. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev 21:1456–1471. doi: 10.1101/gad.1543107. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Fleming RT, Westbrook EM, Matsumura P, McKay DB. 2006. Structure of the Escherichia coli FlhDC complex, a prokaryotic heteromeric regulator of transcription. J Mol Biol 355:798–808. doi: 10.1016/j.jmb.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 2005. Current protocols in molecular biology. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 22.Zhou YH, Zhang XP, Ebright RH. 1991. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res 19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner EGH, Vogel J. 2005. Approaches to identify novel non-messenger RNAs in bacteria and to investigate their biological functions: functional analysis of identified non-mRNAs, p 632–642. In Hartmann RK, Bindereif A, Scḧon A, Westhof E (ed), Handbook of RNA biochemistry. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 24.Miller JH. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 25.Johnson TJ, Kariyawasam S, Wannemuehler Y, Mangiamele P, Johnson SJ, Doetkott C, Skyberg JA, Lynne AM, Johnson JR, Nolan LK. 2007. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J Bacteriol 189:3228–3236. doi: 10.1128/JB.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellata M. 2013. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis 10:916–932. doi: 10.1089/fpd.2013.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soutourina OA, Bertin PN. 2003. Regulation cascade of flagellar expression in Gram-negative bacteria. FEMS Microbiol Rev 27:505–523. doi: 10.1016/S0168-6445(03)00064-0. [DOI] [PubMed] [Google Scholar]
- 28.Keseler IM, Mackie A, Peralta-Gil M, Santos-Zavaleta A, Gama-Castro S, Bonavides-Martínez C, Fulcher C, Huerta AM, Kothari A, Krummenacker M, Latendresse M, Muñiz-Rascado L, Ong Q, Paley S, Schröder I, Shearer AG, Subhraveti P, Travers M, Weerasinghe D, Weiss V, Collado-Vides J, Gunsalus RP, Paulsen I, Karp PD. 2013. EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res 41:D605–D612. doi: 10.1093/nar/gks1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peleg A, Shifrin Y, Ilan O, Nadler-Yona C, Nov S, Koby S, Baruch K, Altuvia S, Elgrably-Weiss M, Abe CM, Knutton S, Saper MA, Rosenshine I. 2005. Identification of an Escherichia coli operon required for formation of the O-antigen capsule. J Bacteriol 187:5259–5266. doi: 10.1128/JB.187.15.5259-5266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girgis HS, Liu Y, Ryu WS, Tavazoie S. 2007. A comprehensive genetic characterization of bacterial motility. PLoS Genet 3:e154. doi: 10.1371/journal.pgen.0030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hufnagel DA, DePas WH, Chapman MR. 2014. The disulfide bonding system suppresses CsgD-independent cellulose production in Escherichia coli. J Bacteriol 196:3690–3699. doi: 10.1128/JB.02019-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raterman EL, Shapiro DD, Stevens DJ, Schwartz KJ, Welch RA. 2013. Genetic analysis of the role of yfiR in the ability of Escherichia coli CFT073 to control cellular cyclic dimeric GMP levels and to persist in the urinary tract. Infect Immun 81:3089–3098. doi: 10.1128/IAI.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemm MR, Paul BJ, Schneider TD, Storz G, Rudd KE. 2008. Small membrane proteins found by comparative genomics and ribosome binding site models. Mol Microbiol 70:1487–1501. doi: 10.1111/j.1365-2958.2008.06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Bassam MM, Bibb MJ, Bush MJ, Chandra G, Buttner MJ. 2014. Response regulator heterodimer formation controls a key stage in Streptomyces development. PLoS Genet 10:e1004554. doi: 10.1371/journal.pgen.1004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wehland M, Bernhard F. 2000. The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J Biol Chem 275:7013–7020. [DOI] [PubMed] [Google Scholar]
- 37.Francez-Charlot A, Laugel B, Van Gemert A, Dubarry N, Wiorowski F, Castanie-Cornet MP, Gutierrez C, Cam K. 2003. RcsCDB His-Asp phosphorelay system negatively regulates the flhDC operon in Escherichia coli. Mol Microbiol 49:823–832. [DOI] [PubMed] [Google Scholar]
- 38.Majdalani N, Gottesman S. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol 59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- 39.Lee C, Park C. 2013. Mutations upregulating the flhDC operon of Escherichia coli K-12. J Microbiol 51:140–144. doi: 10.1007/s12275-013-2212-z. [DOI] [PubMed] [Google Scholar]
- 40.Guttenplan SB, Kearns DB. 2013. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev 37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko M, Park C. 2000. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J Bacteriol 182:4670–4672. doi: 10.1128/JB.182.16.4670-4672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehnen D, Blumer C, Polen T, Wackwitz B, Wendisch VF, Unden G. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol 45:521–532. doi: 10.1046/j.1365-2958.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 43.Lehti TA, Bauchart P, Dobrindt U, Korhonen TK, Westerlund-Wikström B. 2012. The fimbriae activator MatA switches off motility in Escherichia coli by repression of the flagellar master operon flhDC. Microbiology 158:1444–1455. doi: 10.1099/mic.0.056499-0. [DOI] [PubMed] [Google Scholar]
- 44.Lemke JJ, Durfee T, Gourse RL. 2009. DksA and ppGpp directly regulate transcription of the Escherichia coli flagellar cascade. Mol Microbiol 74:1368–1379. doi: 10.1111/j.1365-2958.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin S, Park C. 1995. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol 177:4696–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. 1999. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol 181:7500–7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Lay N, Gottesman S. 2012. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Mol Microbiol 86:524–538. doi: 10.1111/j.1365-2958.2012.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6:455–465. doi: 10.1038/nrmicro1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mika F, Hengge R. 2013. Small regulatory RNAs in the control of motility and biofilm formation in E. coli and Salmonella. Int J Mol Sci 14:4560–4579. doi: 10.3390/ijms14034560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho S-H, Szewczyk J, Pesavento C, Zietek M, Banzhaf M, Roszczenko P, Asmar A, Laloux G, Hov A-K, Leverrier P, Van der Henst C, Vertommen D, Typas A, Collet J-F. 2014. Detecting envelope stress by monitoring β-barrel assembly. Cell 159:1652–1664. doi: 10.1016/j.cell.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 51.Clarke DJ. 2010. The Rcs phosphorelay: more than just a two-component pathway. Future Microbiol 5:1173–1184. doi: 10.2217/fmb.10.83. [DOI] [PubMed] [Google Scholar]
- 52.Farris C, Sanowar S, Bader MW, Pfuetzner R, Miller SI. 2010. Antimicrobial peptides activate the Rcs regulon through the outer membrane lipoprotein RcsF. J Bacteriol 192:4894–4903. doi: 10.1128/JB.00505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laubacher ME, Ades SE. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J Bacteriol 190:2065–2074. doi: 10.1128/JB.01740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziva F, Hauser H, Connor TR, van Diemen PM, Prescott G, Langridge GC, Eckert S, Chaudhuri RR, Ewers C, Mellata M, Mukhopadhyay S, Curtiss R, Dougan G, Wieler LH, Thomson NR, Pickard DJ, Stevens MP. 2013. Sequencing and functional annotation of avian pathogenic Escherichia coli serogroup O78 strains reveal the evolution of E. coli lineages pathogenic for poultry via distinct mechanisms. Infect Immun 81:838–849. doi: 10.1128/IAI.00585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shifrin Y, Peleg A, Ilan O, Nadler C, Kobi S, Baruch K, Yerushalmi G, Berdichevsky T, Altuvia S, Elgrably-Weiss M, Abe C, Knutton S, Sasakawa C, Ritchie JM, Waldor MK, Rosenshine I. 2008. Transient shielding of intimin and the type III secretion system of enterohemorrhagic and enteropathogenic Escherichia coli by a group 4 capsule. J Bacteriol 190:5063–5074. doi: 10.1128/JB.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoebel DM, Free A, Dorman CJ. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 57.Merritt JH, Kadouri DE, O'Toole GA. 2005. Growing and analyzing static biofilms. Curr Protoc Microbiol Chapter 1:Unit 1B.1. doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.