Abstract
Mesenchymal stem cell (MSC) differentiation is dramatically reduced after long-term in vitro culture, which limits their application. MSCs derived from induced pluripotent stem cells (iPSCs-MSCs) represent a novel source of MSCs. In this study, we investigated the therapeutic effect of iPSC-MSCs on diabetic mice. Streptozocin-induced diabetic mice transplanted with 400 islets alone or with 1×106 iPSC-MSCs were examined following rapamycin injection (0.1 mg/kg/day, i.p., from days 0 to 9) after transplantation. Our results showed that iPSC-MSCs combined with rapamycin significantly prolonged islet allograft survival in the diabetic mice; 50% of recipients exhibited long-term survival (>100 days). Histopathological analysis revealed that iPSC-MSCs combined with rapamycin preserved the graft effectively, inhibited inflammatory cell infiltration, and resulted in substantial release of insulin. Flow cytometry results showed that the proportion of CD4+ and CD8+ T cells was significantly reduced, and the number of T regulatory cells increased in the spleen and lymph nodes in the iPSC-MSCs combined with the rapamycin group compared with the rapamycin-alone group. Production of the Th1 proinflammatory cytokines interleukin-2 (IL-2) and interferon-γ was reduced, and secretion of the anti-inflammatory cytokines IL-10 and transforming growth factor-β was enhanced compared with the rapamycin group, as determined using enzyme-linked immunosorbent assays. Transwell separation significantly weakened the immunosuppressive effects of iPSC-MSCs on the proliferation of Con A-treated splenic T cells, which indicated that the combined treatment exerted immunosuppressive effects through cell–cell contact and regulation of cytokine production. Taken together, these findings highlight the potential application of iPSC-MSCs in islet transplantation.
Introduction
Islet transplantation is a promising therapy for diabetes. However, it does not have an ideal postoperative survival time because of immune rejection and islet toxicity of immunosuppressive agents [1,2]. The immunosuppressive effect and low immunogenicity of mesenchymal stem cells (MSCs) make them ideal candidates for immunosuppressive strategies [3,4]. Adult MSCs have been used widely in the allogeneic heart [5–11], liver [12], islet [13–17], kidney [18,19], and composite tissue transplants [20,21]. Bone marrow mesenchymal stem cells (BM-MSCs) alone prolong heart allograft survival [8]. However, some studies showed that MSCs alone had no significant effect on graft survival in a completely allogeneic heart transplant model. In contrast, combining MSCs with mycophenolate mofetil led to prolonged allograft survival [10], and MSCs plus rapamycin (Rapa) induced immune tolerance of heart allografts [9]. Furthermore, MSCs combined with cyclosporine A (CsA) induced tolerance of islet allografts in immune-deficient mice [14]. In a kidney allograft model, MSCs led to long-term graft acceptance in rodents [19] and had immunosuppressive effects in renal transplant recipients [22–24], which suggested that MSCs may reduce immunosuppressant dosage [25,26]. Collectively, these studies suggested that under certain conditions, MSCs could prolong allograft survival in combination with clinical immunosuppressants.
MSCs showed various degrees of efficacy in preclinical animal studies [27]; however, their limited accessibility is a major factor inhibiting their use in routine clinical treatment. Current methods to obtain MSCs from patients are invasive and labor intensive. Furthermore, MSCs have a limited capacity to expand in culture. Successive passages slow the proliferation rate, and MSCs progressively lose their multipotency and lack immunosuppressive activity. In addition, aging and age-related disorders significantly impair the survival and differentiation potential of BM-MSCs, thus limiting their therapeutic efficacy [28–32]. Therefore, it is important to identify alternative sources of MSCs before they can be used as a mainstream treatment for organ transplantation. A breakthrough in the generation of human-induced pluripotent stem cells (iPSCs) from adult somatic cells offered the possibility of generating a high yield of MSCs [33–35].
Several laboratories have found that iPSC-derived MSCs have the same in vitro and in vivo characteristics as MSCs derived from adult sources. Previous studies indicate that iPSC-MSCs grown on a calcium phosphate scaffold enhanced osteogenic differentiation and promoted bone regeneration [36–38]. iPSC-MSCs could form mature mineralized structures that were histologically similar to mature bone, facilitating periodontal regeneration [39,40]. Transplanting iPSC-MSCs attenuated severe hindlimb ischemia and improved the hepatic function in mouse models [33,41,42]. These results suggested that iPSC-MSCs have high potential for tissue-engineering applications. In addition to their tissue repair ability, iPSC-MSCs also exhibit immunomodulatory properties [43–45]. For example, iPSC-MSCs displayed long-lasting immunosuppressive properties toward natural killer cells by interfering in their activation, thus protecting target cells [44]. Human iPSC-MSCs exerted immunomodulatory effects on T-cell subsets in the peripheral blood from allergic rhinitis patients by modulating T-cell phenotypes toward Th2 suppression and inducing T regulatory cell (Treg) expansion [45]. iPSC-MSCs also prevented allergic airway inflammation in mice [43]. Therefore, iPSC-MSCs may be a novel source of tolerance induction, although their immunosuppressive activity in organ transplantation remains to be explored. The aim of this study was to assess the efficacy of iPSC-MSCs in combination with Rapa in islet transplantation immunosuppressive therapy in streptozocin (STZ)-induced diabetic mice.
Materials and Methods
Animals
Female BALB/c mice (8–12 weeks old) and C57BL/6 were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and used as graft donors and recipients, respectively. The care and handling of the animals were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Animal Studies Committee of Xiamen University, China.
Drugs
Rapamycin was purchased from LC Laboratories (LC Labs, Woburn, MA) and dissolved in PBS at 0.02 mg/mL for injection. The recipient mice received Rapa at doses of 0.1 mg/kg/day i.p. from days 0 to 9 after transplantation.
Phenotypic analysis of iPSC-MSCs
iPSC-MSCs were kindly provided by Prof. Qi-Zhou Lian of the University of Hong Kong. The iPSC lines were prepared from iPSCs, which were reprogrammed from human fibroblast cells, and differentiated into MSCs according to a previously described protocol [33]. Briefly, MSCs were purified by sorting for CD105+/CD24− cells and maintained in a medium containing 90% knockout Dulbecco's Modified Eagle's Medium (Gibco, Invitrogen Corporation, Carlsbad, CA) supplemented with 10% serum replacement medium (Gibco) and basic fibroblast growth factor (10 ng/mL; Gibco). The morphology of iPSC-MSCs was very similar to BM-MSCs and they have the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts [33]. Millipore's FlowCellect™ Human Mesenchymal Stem Cell Characterization Kit was used for the phenotypic analysis of iPSC-MSCs. iPSC-MSCs (passage 5–10) were resuspended in an assay buffer and then incubated with an antibody working cocktail solution for 30 min on ice in the dark. The antibody working cocktail solution contained anti-CD105/PE-, anti-CD90/FITC-, anti-CD73/APC-, and anti-CD14/CD34/CD45/PerPC-conjugated antibodies. Each fluorescence analysis included the appropriate FITC-, PE-, or cytochrome-conjugated isotype Ab controls. Cells were separated using flow cytometry (FACS Calibur; Becton-Dickinson, San Diego, CA) and not sorted. The use of iPSC-MSCs in this study was approved by the Ethics Committee of Xiamen University, China.
Chemical induction of diabetes
Diabetes was induced in female C57BL/6 mice by intraperitoneal injection of streptozocin (180–220 mg/kg; Sigma-Aldrich, St. Louis, MO) [46]. Blood glucose was measured using a FreeStyle glucose meter (Abbott, Alameda, CA), and diabetes onset was defined as two consecutive daily blood glucose measurements above 16.7 mmol/L.
Islet isolation, purification, and transplantation
BALB/c islets were isolated using the digestion method [47,48] with collagenase P (1 mg/mL; Roche, Basel, Switzerland). The pancreas was perfused through bile duct cannulation with 3 mL of 1 mg/mL collagenase P per mouse and then excised. Briefly, the pancreas was digested at 37°C–38°C for 20 min and then shaken vigorously in cold Hank's Balanced Salt Solution (HBSS) containing 10% fetal bovine serum (FBS; Shanghai ExCell Biology, Shanghai, China). The digested pancreatic tissues were filtered through a 200-μm mesh, washed thrice [49], and then purified using human mononuclear cells and granulocyte separation media Histopaque-10771 and Histopaque-11191 (Sigma-Aldrich). The islets were sorted manually under the microscope.
Four hundred BALB/c islets were used for a single transplantation. Islets were transplanted under the kidney capsule of diabetic C57BL/6 mice. Blood glucose levels were monitored in the recipient mice. Islet transplants were considered functional when two consecutive blood glucose measurements were <8 mmol/L, and graft rejection was defined as a blood glucose level of >11.1 mmol/L on 2 consecutive days. Body weight was recorded (daily) until complete graft rejection occurred.
Recipient therapy and experimental groups
Four hundred islets isolated from BALB/c mice with or without 1×106 iPSC-MSCs were transplanted into diabetic mice. The control group received islets alone. The Rapa group was treated with 0.1 mg/kg/day Rapa alone. The iPSC-MSCs+Rapa group received islets with iPSC-MSCs and was treated with 0.1 mg/kg/day Rapa. Rapa treatments were conducted from days 0 to 9.
Mixed lymphocyte reaction
Nylon wool columns (Wako, Osaka, Japan) were used to isolate T cells from the spleen of the recipient mice, which were used as responder cells. Spleen cells obtained from the BALB/c mice were used as stimulator cells. The responder cells (5×105 cells) were cultured in 96-well plates in the presence of stimulator cells (5×104 cells), pretreated with mitomycin C (40 μg/mL; Amresco, Solon, OH) in 200 μL RPMI 1640 supplemented with 10% FBS and 1% penicillin and streptomycin, and incubated at 37°C in a 5% CO2 humidified atmosphere for 72 h. Cell proliferation was measured using a bromodeoxyuridine (BrdU) cell proliferation assay kit (Roche Applied Science, Mannheim, Germany). The magnitude of the absorbance is proportional to the quantity of BrdU incorporated into cells, which is a direct indication of the cell proliferation rate. The optical density values were measured in an enzyme-linked immunosorbent assay (ELISA) reader (Model 680; BIO-RAD, Hercules, CA) at 450 nm (the reference wavelength was 690 nm). Measurements were performed in triplicate.
Transwell experiments
For the Transwell experiments, 24-well Transwell plates with a 4-μm-pore membrane (Costar, Corning, NY) were used to separate T cells from the iPSC-MSCs. T cells were isolated from the spleens of C57BL/6 mice using nylon wool columns (Wako). iPSC-MSCs were plated into the lower chamber at 5×104 cells/well, and 5 μg/mL Con A (Sigma-Aldrich)-stimulated T cells (5×105 cells/well) were cultured in the upper chamber of the Transwell insert. Cell culture media were supplemented with 2 ng/mL Rapa. After 3 days of coculture, T cells were harvested and placed in a 96-well plate at a concentration of 1×105 cells/well (n=6). Cell proliferation was measured using the BrdU cell proliferation assay kit (Roche Applied Science), as described above. Measurements were performed in triplicate.
Flow cytometry analysis
Recipient splenic lymphocytes and T cells (isolated using nylon wool columns) were prepared in 100 μL PBS per 1×106 cells. The splenic lymphocyte cells were incubated with PE-Cy5-anti-CD4 (GK1.5), FITC-anti-CD8 (53-6.7), and their isotype controls (purchased from BioLegend, San Diego, CA) at 4°C for 30 min. T cells from lymph nodes were incubated with FITC anti-CD4 (RM4-5) and PE anti-Foxp3 (FJK-16s) (purchased from eBioscience, San Diego, CA) at 4°C for 30 min. Conjugated isotype antibodies were used as negative controls. The stained cells were detected on a FACScan flow cytometer (Partec Co., Munster, Germany), and the data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
The secretions of interleukin-2 (IL-2), IL-10, and interferon-γ (IFN-γ) into the recipient sera were detected using a Cytometric Bead Array™ (CBA; BD Biosciences, San Jose, CA), according to the manufacturer's instructions. The stained samples were detected on a BD FACS Aria Cell Sorter (BD Biosciences), and the data were analyzed using FlowJo software and FCAP Array software (BD Biosciences).
Histopathological analysis
Kidney islet grafts were removed from recipient mice at day 12 post-transplantation, fixed in 4% paraformaldehyde fixative (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China), and embedded in paraffin. Grafts were cut into 5-μm sections, stained with hematoxylin and eosin (H&E), and examined by a transplant pathologist who was blinded to treatment modality. Representative specimens (n=36) from all treatment modalities were ranked from 1 to 36 (from least to most) for overall rejection/inflammation, with the median in each group presented [47].
Immunohistochemistry
At day 12 after islet graft, the islet grafts were removed for pathological examination, fixed in zinc fixative (Biolegend), and embedded in paraffin. The paraffin tissues were cut into 5-μm sections, deparaffinized in xylene, hydrated through graded ethanol series, and immersed in absolute methanol, which contained 0.3% hydrogen peroxide, for 10 min to block the endogenous peroxidase activity. Sections were incubated with nonimmune goat serum for 20 min to prevent nonspecific binding and then with the primary Insulin Rabbit mAb (1:100; Cell Signaling Technology, Boston, MA) diluted in PBS for 1 h. The sections were incubated with Polymer Helper for 15 min, with poly-HRP anti-rabbit IgG for 30 min, and with the peroxidase substrate diaminobenzidine for 1 min. Slides were counterstained with hematoxylin. The slides were examined under a microscope and evaluated in a blinded manner.
Quantitative real-time reverse transcription PCR analysis
Kidney islet grafts were removed from recipient mice at day 12 post-transplantation, and the mRNA was extracted using TRIzol (Life Technologies, Carlsbad, CA). Reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR) were performed using commercially available reagents (Toyobo, Osaka, Japan). The StepOne Real-Time PCR System (ABI, Foster City, CA) was used to detect IL-2, IFN-γ, IL-10, transforming growth factor (TGF-β), and Foxp3. β-Actin served as the control. Calculation of the relative expression was performed using the 2−ΔΔCT method. The following primer sequences were used for qRT-PCR:
β-actin: forward 5′-CATCCGTAAAGACCTCTATGCC AAC-3′
and reverse 5′-ATGGAGCCACCGATCCACA-3′;
IFN-γ: forward 5′-CGGCACAGTCATTGAAAGCCTA-3′
and reverse 5′-GTTGCTGATGGCCTGATTGTC-3′;
IL-2: forward 5′-GGAGCAGCTGTTGATGGACCTAC-3′
and reverse 5′-AATCCAGAACATGCCGCAGAG-3′;
IL-10: forward 5′-GACCAGCTGGACAACATACTGC TAA-3′
and reverse 5′-GATAAGGCTTGGCAACCCAAGTAA-3′;
TGF-β: forward 5′-GACCAGCTGGACAACATACTGC TAA-3′
and reverse 5′-GATAAGGCTTGGCAACCCAAGTAA-3′;
Foxp-3: forward 5′-CAGCTCTGCTGGCGAAAGTG-3′
and reverse 5′-TCGTCTGAAGGCAGAGTCAGGA-3′.
Enzyme-linked immunosorbent assay
Supernatants from the mixed lymphocyte reaction (MLR) after a 72-h incubation and the sera of recipient mice were collected and frozen at −20°C. ELISAs were performed using commercially available kits (NeoBioscience Technology Co., Ltd., Beijing, China) to detect the secretion levels of IL-2, IFN-γ, IL-10, and TGF-β. The process was conducted according to the manufacturer's instruction. Each reaction was carried out in triplicate.
Statistical analyses
The median survival times of the four groups were calculated and compared using the Kaplan–Meier method. Data from MLR, FACS, ELISA, and CBA experiments were analyzed by one-way analysis of variance (ANOVA) and expressed as the mean±standard deviation. A Bonferroni correction was calculated and applied because multiple comparisons were made during the analysis. A P value<0.05 was considered statistically significant; P<0.01 and P<0.001 indicated highly significant differences. All analyses were performed using the GraphPad Prism® (GraphPad Software, Inc., San Diego, CA) software.
Results
Flow cytometry analysis of iPSC-MSC surface antigens
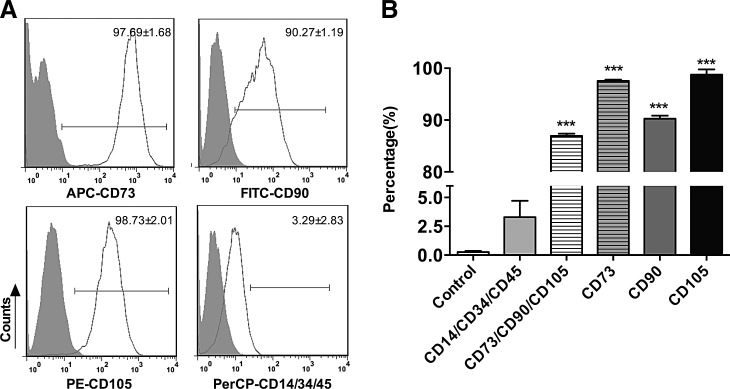
iPSC-MSCs exhibited a spindle-shaped morphology and their identity was confirmed by flow cytometry. The results showed that the percentage of CD73, CD90, and CD105 triple-positive cells and the percentage of CD14, CD34, and CD45 triple-negative cells were 86.94±0.87 and 3.29±2.83, respectively, where the latter showed no significant difference compared with the isotype control (Fig. 1). The results showed that iPSC-MSCs expressed the cell surface marker characteristic of MSCs (CD73, CD90, and CD105) and were negative for markers typically absent on MSCs (CD14, CD34, and CD45). These results suggest that iPS-MSCs display morphological characteristics of adult MSCs.
FIG. 1.
Cell surface antigens for iPSC-MSCs analyzed by flow cytometry. (A) iPSC-MSCs were stained with the indicated mAbs (open white plots) or Ig isotype controls (shaded gray plots) and analyzed by FACS (one representative FACS experiment is shown). The numbers represent the percentage of cells staining positive for the indicated marker and are shown as mean±SD (n=4, n represents the number of independent experiments). (B) Statistical analysis of cell surface antigens. The data are representative of four FACS experiments from the same cell line, but different passages. The percentage of CD14, CD34, and CD45 triple-negative cells showed no significant difference compared with the isotype control, while other cell surface antigens had significant differences compared with their corresponding isotype controls. iPSC-MSCs, induced pluripotent stem cells–mesenchymal stem cells.
Effect of iPSC-MSCs on islet allograft survival
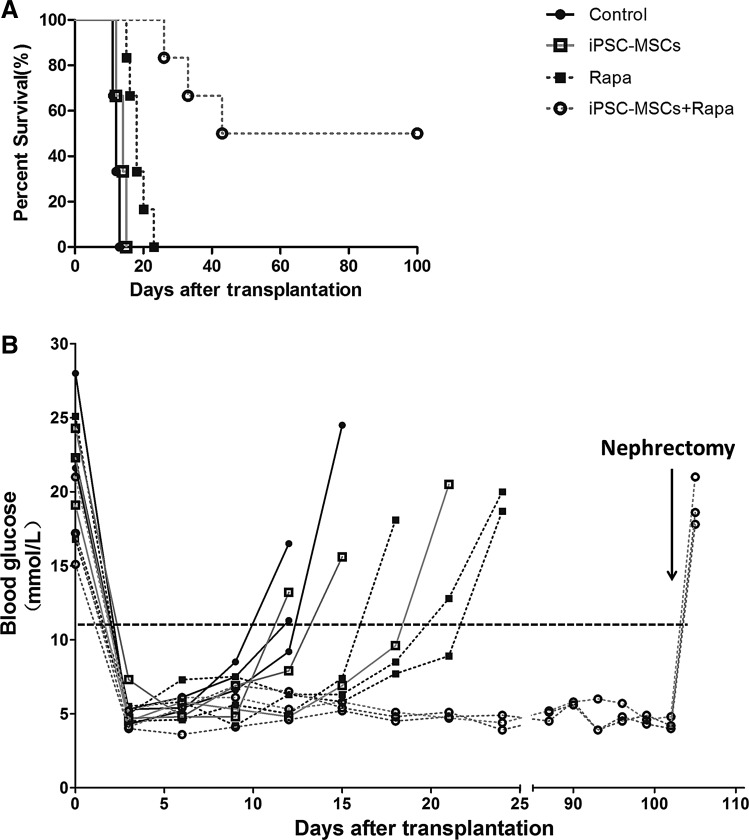
We investigated the effect of iPSC-MSCs on islet allograft survival in STZ-induced diabetic mice by cotransplantation into recipient mice by kidney subcapsular injection (Fig. 2A). The survival times of the iPSC-MSCs and Rapa groups were 14 and 18 days, respectively, both of which were significantly prolonged compared with 12 days in the controls (P<0.05, iPSC-MSC group; P<0.001, Rapa group). iPSC-MSCs combined with Rapa prolonged survival time, compared with Rapa alone (P<0.001), and induced immune tolerance in 50% of the recipients. Blood glucose values (measured every 3 days) remained normal in Rapa-treated iPSC-MSCs, until the mice were nephrectomized at day 102 post-transplantation (Fig. 2B).
FIG. 2.
Survival of islet allografts treated with iPSC-MSCs and Rapa. Islet graft survival and blood glucose levels in C57BL/6 mice with different treatments are shown, respectively (A, n=6) and (B, n=3). Diabetes was induced in C57BL/6 mice 4 days before transplantation. Four hundred islets isolated from BALB/c mice, with or without 1×106 iPSC-MSCs, were transplanted into recipient mice. The control group received islets alone, the iPSC-MSC group received islets with iPSC-MSCs, the Rapa group received islets following a 0.1 mg/kg/day Rapa treatment from days 0 to 9, and the iPSC-MSCs+Rapa group received islets with iPSC-MSCs and 0.1 mg/kg/day Rapa from days 0 to 9. Graft survival was calculated by the Kaplan–Meier method and compared using a log-rank test. Allograft nephrectomies were performed on mice after 100 days to ensure normal glucose levels had been maintained by the islet allograft.
Effect of iPSC-MSCs on the inflammatory response of STZ-induced diabetic mice following islet transplantation
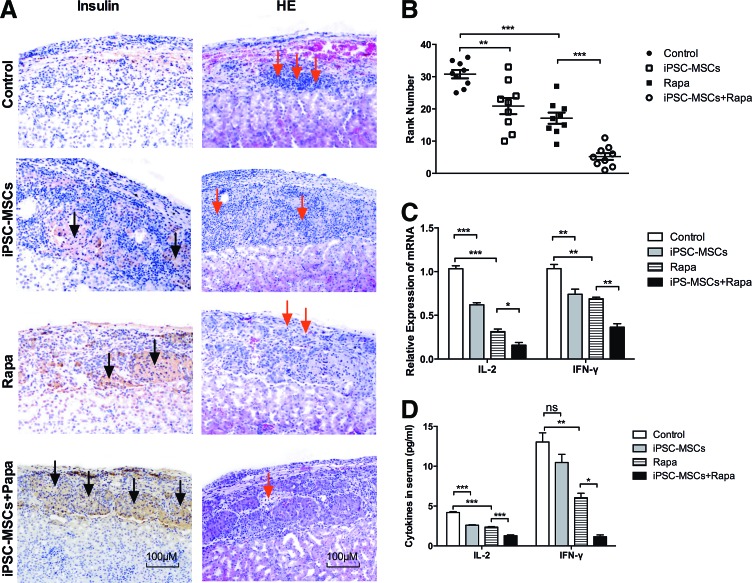
To investigate the effect of iPSC-MSCs on grafts in diabetic mice, grafts were dissociated from recipient mice at day 12 post-transplantation and processed for histological analysis (Fig. 3A). Grafts from control mice exhibited islet damage and little insulin secretion. Grafts from iPSC-MSCs or Rapa-treated mice showed less islet damage, more insulin secretion, and fewer infiltrating inflammatory cells. iPSC-MSCs+Rapa treatment preserved the graft most effectively and resulted in a substantial release of insulin. The overall ranking of rejection/inflammation is shown in Fig. 3B. The rankings given for the combined treatment group were significantly lower in terms of rejection/inflammation than those for the Rapa group (P<0.01). We next examined the expressions of inflammatory cytokines in graft and sera using qRT-PCR, ELISA, and CBA flow cytometry (Fig. 3C, D). IL-2 and IFN-γ in the iPSC-MSCs+Rapa treatment group were downregulated at both the mRNA and protein levels compared with the Rapa group. These results demonstrated that iPSC-MSCs and Rapa have synergistic effects on the expressions of inflammatory cytokines.
FIG. 3.
Preservation of islet graft and infiltration of inflammatory cells at 12 days post-transplantation. (A) Immunohistochemical staining for insulin (magnification×100) and H&E (magnification×100) in islet grafts, with dark arrows indicating islets and red arrows indicating inflammatory cells. (B) Representative specimens (n=36) from all treatment modalities were ranked from 1 to 36 (from least to most) for overall rejection/inflammation, and the median in each group is shown. (C) Effects of iPSC-derived MSCs and rapamycin on the relative mRNA expression of inflammatory cytokines in the graft. (D) Effects of iPSC-derived MSCs and rapamycin on the expression of inflammatory cytokines in serum. Each group was tested in triplicate and the data are representative of three independent experiments (n=3, *P<0.05; **P<0.01; ***P<0.001; ns, no significant difference). H&E, hematoxylin and eosin. Color images available online at www.liebertpub.com/scd
Effect of iPSC-MSCs on CD4+ and CD8+ ratios in STZ-induced diabetic mice
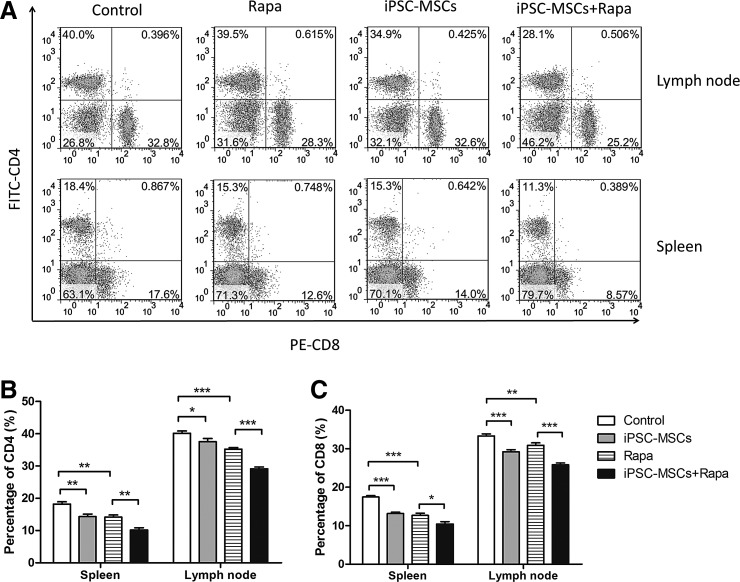
Flow cytometry was used to investigate the effect of iPSC-MSCs+Rapa treatment on CD4+ and CD8+ T lymphocytes at day 12 post-transplantation. Although iPSC-MSCs or Rapa alone effectively decreased the number of CD4+ and CD8+ T lymphocytes, iPSC-MSCs combined with Rapa showed the best inhibitory effect on the proliferation of CD4+ and CD8+ T lymphocytes, whether in the spleen or in lymph nodes (Fig. 4B).
FIG. 4.
Proportion of CD4+ and CD8+ T lymphocytes in the spleen and lymph nodes at 12 days post-transplantation. (A) Proportion of CD4+ and CD8+ T lymphocytes from one separate experiment. Statistical analyses of the proportion of CD4+ and CD8+ T lymphocytes are shown in (B) and (C), respectively. Data are representative of three separate experiments (n=3, *P<0.05; **P<0.01; ***P<0.001; ns, no significant difference).
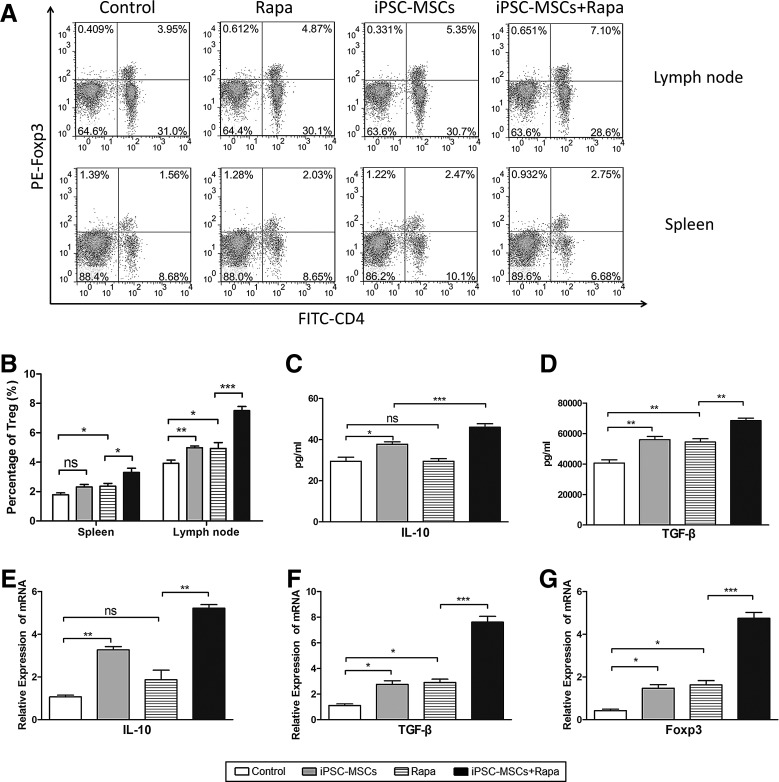
Protective effect of iPSC-MSCs+Rapa on islet allografts through Tregs induction
The proportion of splenic and lymph node Tregs was examined by flow cytometry (Fig. 5A). Although the results showed that both iPSC-MSCs and Rapa induce Tregs compared with the controls, iPSC-MSCs+Rapa treatment induced a larger numbers of Tregs compared with the Rapa group (Fig. 5B). We next examined IL-10, TGF-β, and Foxp3 expression in the islet grafts and sera of the recipient mice. iPSC-MSCs+Rapa treatment increased serum IL-10 and TGF-β concentrations (Fig. 5C, D). Furthermore, IL-10, TGF-β, and Foxp3 mRNA levels in the graft also increased compared with the Rapa group (Fig. 5E–G).
FIG. 5.
Protective effect of iPSC-MSCs+Rapa on islet allografts through T regulatory cell induction. (A) T regulatory cell proportions from one separate experiment in the spleen and lymph nodes; (B) statistical analysis of T regulatory cell proportions of three separate experiments. Effects of iPSC-MSCs+Rapa on IL-10 and TGF-β expression in sera are shown in (C) and (D); effects of iPSC-MSCs+Rapa on the relative expression of IL-10, TGF-β, and Foxp3 mRNA in grafts are shown in (E), (F), and (G). Data are presented as mean±SD of three independent experiments (n=3, *P<0.05; **P<0.01; ***P<0.001; ns, no significant difference). IL, interleukin; TGF, transforming growth factor.
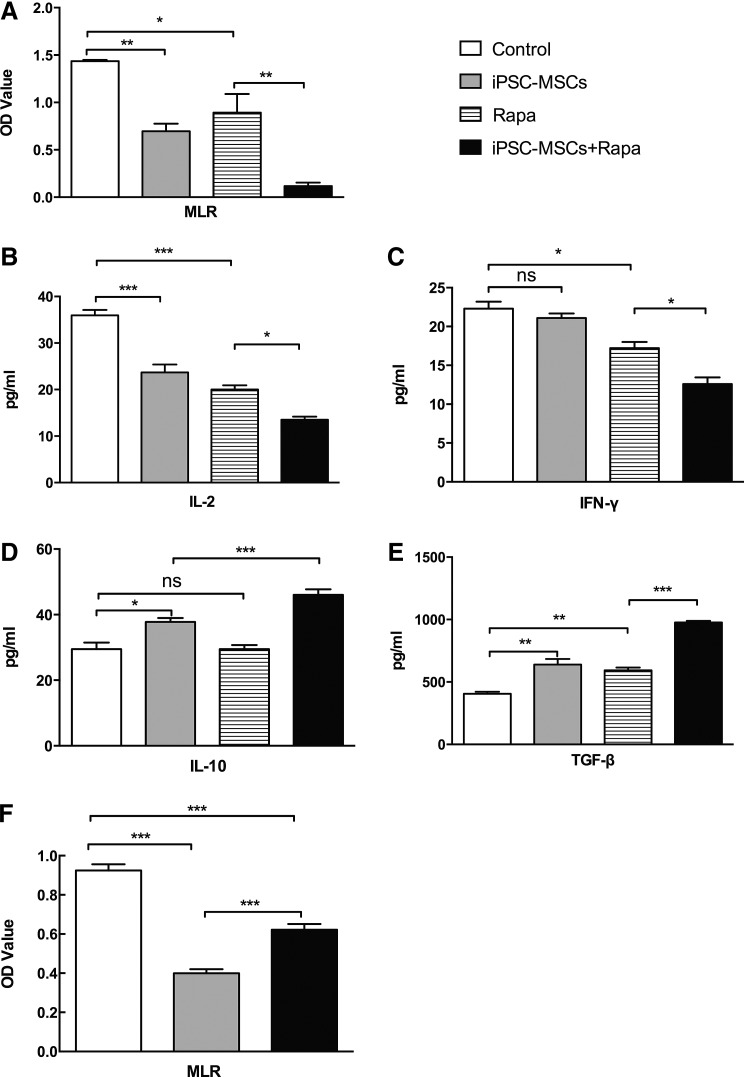
Immunosuppressive mechanisms of iPSC-MSCs+Rapa on T-lymphocyte proliferation and Treg production
T lymphocytes from the four groups were cocultured with mitomycin C-treated splenic lymphocytes from BALB/c mice in a 96-well plate for 3 days. The MLR test results indicated that recipient splenic T cells from combination-treated mice showed a reduced proliferative response when stimulated with mitomycin C-treated donor splenocytes compared with the Rapa group (P<0.01, Fig. 6A). In addition, IL-2, IFN-γ, IL-10, and TGF-β supernatant levels were determined using ELISA. iPSC-MSCs+Rapa treatment downregulated IL-2 and IFN-γ expression and upregulated IL-10 and TGF-β levels compared with the Rapa group (P<0.05, Fig. 6B–D). These results suggested that iPSC-MSCs+Rapa treatment suppressed Th1 function and increased Treg production.
FIG. 6.
Immunosuppressive effect of iPSC-MSCs+Rapa on T-lymphocyte proliferation. (A) T lymphocytes from four groups (named on the right of Fig. 6A) were cocultured with mitomycin C-treated splenic lymphocytes from BALB/c mice in 96-well plates with mixed lymphocyte reactions for 3 days; OD values represent T-cell proliferation. IL-2, IFN-γ, IL-10, and TGF-β supernatant levels were determined using ELISA and are shown in (B–E), respectively. (F) T lymphocytes from normal C57BL/6 mice were stimulated with Con A (5 μg/mL) for 3 days in the presence of iPSC-MSCs with transwell separation from MSCs. Data are presented as mean±SD of three independent experiments (n=3, *P<0.05; **P<0.01; ***P<0.001; ns, no significant difference). ELISA, enzyme-linked immunosorbent assay; IFN, interferon; OD, optical density.
To evaluate the molecular mechanisms underlying these immunomodulatory properties, we further examined the possible role of cell contact in the modulation of T-cell proliferation by iPSC-MSCs+Rapa using Transwell experiments. As shown in Fig. 6F, iPSC-MSCs+Rapa significantly decreased the number of Con A-treated splenic T cells from normal mice. Moreover, Transwell separation significantly weakened the immunomodulatory effects of iPSC-MSCs+Rapa on Con A-treated splenic T cells. These findings suggested that cell contact could, at least partially, interfere with the immunomodulatory effects on lymphocyte proliferation under iPSC-MSCs+Rapa treatment.
Discussion
MSCs have previously demonstrated their capacity to facilitate regeneration and regulate immune responses in a range of animal models; however, major factors related to life span and tumorigenicity limit their widespread use in a clinical setting [28–32]. Recent reports have described MSC-like cells derived from iPSCs [33–35] with a greater proliferative capacity, lower immunogenicity, and greater immunoregulatory function compared with primary MSC cultures [33,43,45]. In addition, these iPSC-MSCs did not exhibit the tumorigenic properties associated with iPSCs [50,51], implying that iPSC-MSCs may be a safer MSC source. This study investigated the effects of iPSC-MSCs on islet allografts without using BM-MSC treatment as a control. Rapa is used frequently in islet transplantation; however, recent research showed evidence of Rapa toxicity in islet transplantation in clinical studies. For example, Rapa exerts dual effects on the islet endothelium by inhibiting angiogenesis and downregulating receptors that are involved in lymphocyte adhesion and activation [52]. Furthermore, Rapa also inhibits the revascularization of isolated pancreatic islets [53] and has significant detrimental effects on peripheral insulin resistance and β-cell function and survival [54]. Rapa at ≥0.5 mg/kg had detrimental effects on islet engraftment, while lowering the concentration to 0.1 mg/kg did not affect engraftment when tested for preventing rejection in the full mismatch allogeneic transplant BALB/c to the C57BL/6 model [55]. This result suggested that the detrimental effects of Rapa were dose dependent. Therefore, 0.1 mg/kg Rapa was chosen for use in our study. However, we showed that Rapa alone was inefficient in preventing rejection, which was consistent with published studies [55]. Thus, a combination of 0.1 mg/kg Rapa with iPSC-MSCs was used to suppress immune rejection of islet allografts in this study.
Our results showed that iPSC-MSCs had the morphological characteristics of adult MSCs. Previous published experimental studies suggested that MSCs can, under certain conditions, induce allograft tolerance together with immunomodulatory drugs [9,10,14,19]. In our study, iPSC-MSCs+Rapa treatment effectively prolonged islet allograft survival time and even induced immune tolerance in 50% of the recipients, which was consistent with results using adult MSCs with 2 mg/kg/day Rapa treatment in heart allografts [9]. Our results demonstrated that the combined treatment had synergistic effects.
Adult MSCs isolated from various sources (adipose tissue and Wharton's jelly) have been reported to equally suppress proliferation of CD4+ and CD8+ T-cell subsets in a dose-dependent manner [56]. Furthermore, adult MSCs inhibit Th1 and IFN-γ secretion in vitro [57,58]. Using a rat model of STZ-induced diabetes, adult MSCs significantly improved glycemic control and reduced inflammatory cell infiltration in either allogeneic or syngeneic pancreatic islet transplantation [59]. In this study, iPSC-MSCs+Rapa treatment effectively decreased the proportion of splenic and lymph node CD4+ and CD8+ T lymphocytes in vivo and strongly inhibited T-cell proliferation. We also found that IL-2 and IFN-γ expression was downregulated. These results suggested that iPSC-MSCs+Rapa treatment reduces Th1 inflammatory cytokines and may suppress the Th1 response.
Several studies have shown that adult MSCs alone, or combined with immunosuppressive drugs, induce allograft immune tolerance through Treg induction in vivo [8,9,17]. Berman et al. first reported that infusions of donor or third-party MSCs reversed rejection episodes and prolonged islet function, associated with increased numbers of Tregs in peripheral blood [60]. We hypothesized that iPSC-MSCs act like adult MSCs to induce Tregs. TGF-β is the perpetrator of immune suppression through regulatory T cells [61]. Although the in vitro dependency of Treg suppression on TGF-β is compelling, immune suppression mediated by Treg in vivo clearly requires TGF-β, because administration of an antibody against TGF-β blocked protection from colitis [62]. Moreover, in a type 1 diabetes model, CD8+ T cells bearing a dominant-negative TβRII transgene were incapable of responding to Treg suppression, resulting in diabetes progression [63]. Thus, we examined TGF-β expression in the islet grafts and sera of the recipient mice. The results showed that iPSC-MSCs+Rapa treatment increased sera TGF-β concentrations and graft TGF-β and Foxp3 mRNA levels, compared with Rapa treatment; this result supported our hypothesis.
Although a number of studies have revealed the immunosuppressive effects of MSCs, the mechanisms that modulate this process have not been fully explained. Generally, contact-dependent mechanisms and soluble factors, including 2,3-dioxygenase, prostaglandin-E2, nitric oxide, TGF-β, and hepatocyte growth factor, are thought to collaborate to induce MSC-mediated immunosuppressive effects [64,65]. Moreover, IL-10 has been reported to be involved in MSC-mediated immune regulation. Studies have demonstrated that addition of MSCs to MLRs increases IL-10 expression, while adding a neutralizing IL-10 antibody to MLRs results in recovery of the MLR response in long-term surviving splenocytes. This suggested that IL-10 mediates MSC suppressive capacity in autologous MSC+CsA-treated rats [17]. In this study, we used ELISA and qRT-PCR to measure IL-10 expression levels. Our results showed that iPSC-MSCs alone, or in the combination with Rapa, increased IL-10 secretion in sera and IL-10 mRNA levels in the graft, whereas Rapa alone did not. Further research is required to determine whether IL-10 mediates the immunosuppressive effect of iPSC-MSCs+Rapa on T cells. In addition, Transwell separation significantly weakened the immunosuppressive effects of iPSC-MSCs on the proliferation of Con A-treated splenic T cells, which indicated that the combined treatment exerts its immunosuppressive effects through cell–cell contact and the regulation of cytokine production.
It is worth mentioning that MSCs from the umbilical cord matrix, adipose tissue, and BM exhibit different capability to suppress peripheral blood B, natural killer, and T cells [66]. MSCs derived from the umbilical cord Wharton's Jelly displayed the most prominent immunosuppressive effects on phytohemagglutinin-induced T-cell proliferation, compared with MSCs derived from BM, adipose tissue, and the placenta [67]. Equine MSCs from solid tissue-derived sources, including the adipose tissue and umbilical cord tissue, inhibited T-cell proliferation by inducing lymphocyte apoptosis, while MSCs from BM and cord blood induced lymphocyte cell cycle arrest [68]. These studies showed that MSCs from different tissue sources possess different immunomodulatory effects and modulate immune cell function through overlapping and unique mechanisms [66–68]. Thus, there may be differences in the immunosuppressive effects and mechanisms between iPSC-MSCs and MSCs from other sources, which require further study.
In conclusion, we have demonstrated that iPSC-MSCs combined with low-dose Rapa reduced the production of Th1 proinflammatory cytokines and significantly prolonged islet graft survival compared with iPSC-MSCs or Rapa alone. Furthermore, iPSC-MSCs alone, or combined with low-dose Rapa, induced IL-10 production in vivo and in vitro, which might have resulted from the immunomodulatory effects of iPSC-MSCs. In addition, iPSC-MSCs combined with low-dose Rapa significantly induced anti-inflammatory cytokines and Treg proliferation. The synergistic immunomodulatory effects of iPSC-MSCs and low-dose Rapa in islet transplantation suggest a promising strategy for preventing transplant rejection. This research also provides a preliminary experimental basis for applying MSCs not only in clinical islets but also in other solid organ transplants. However, there are still several questions that remain to be answered. Most importantly, the origin of the cell sources and the long-term effects of iPSC-MSCs need to be investigated to validate their safety and effectiveness in vivo. Finally, a more detailed understanding of iPSC-MSC functions should be determined in a transplant model.
Acknowledgments
This work was supported by grants from the Major State Scientific Research Program of China (no. 2012CBA01303), the National Natural Science Foundation of China (nos. 81302546, 31271038), the Xiamen Science and Technology Key Program Grant (no. 3502Z20100006), and partly from the Hong Kong Research Grant Council Collaborative Research Fund (HKU3/CRF/11R).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ruggenenti P, Remuzzi A. and Remuzzi G. (2008). Decision time for pancreatic islet-cell transplantation. Lancet 371:883–884 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM. and Rajotte RV. (2000). Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343:230–238 [DOI] [PubMed] [Google Scholar]
- 3.Casiraghi F, Perico N. and Remuzzi G. (2013). Mesenchymal stromal cells to promote solid organ transplantation tolerance. Curr Opin Organ Transplant 18:51–58 [DOI] [PubMed] [Google Scholar]
- 4.English K. and Wood KJ. (2013). Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med 3:a015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, Liu JC, Zhang JZ. and Wu TJ. (2006). Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc 38:3046–3051 [DOI] [PubMed] [Google Scholar]
- 6.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I. and Cuturi MC. (2007). A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 110:3691–3694 [DOI] [PubMed] [Google Scholar]
- 7.Popp FC, Eggenhofer E, Renner P, Slowik P, Lang SA, Kaspar H, Geissler EK, Piso P, Schlitt HJ. and Dahlke MH. (2008). Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol 20:55–60 [DOI] [PubMed] [Google Scholar]
- 8.Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, et al. (2008). Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol 181:3933–3946 [DOI] [PubMed] [Google Scholar]
- 9.Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B. and Wang H. (2009). Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 9:1760–1772 [DOI] [PubMed] [Google Scholar]
- 10.Eggenhofer E, Renner P, Soeder Y, Popp FC, Hoogduijn MJ, Geissler EK, Schlitt HJ. and Dahlke MH. (2011). Features of synergism between mesenchymal stem cells and immunosuppressive drugs in a murine heart transplantation model. Transpl Immunol 25:141–147 [DOI] [PubMed] [Google Scholar]
- 11.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK. and Dahlke MH. (2006). Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation 81:1589–1595 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang A, Ye Z, Xie H. and Zheng S. (2009). Bone marrow-derived mesenchymal stem cells inhibit acute rejection of rat liver allografts in association with regulatory T-cell expansion. Transplant Proc 41:4352–4356 [DOI] [PubMed] [Google Scholar]
- 13.Xu DM, Yu XF, Zhang D, Zhang MX, Zhou JF, Tan PH. and Ding YC. (2012). Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice. Diabetologia 55:1091–1102 [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ. and Wood KJ. (2009). Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solari MG, Srinivasan S, Boumaza I, Unadkat J, Harb G, Garcia-Ocana A. and Feili-Hariri M. (2009). Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun 32:116–124 [DOI] [PubMed] [Google Scholar]
- 16.Li FR, Wang XG, Deng CY, Qi H, Ren LL. and Zhou HX. (2010). Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin Exp Immunol 161:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim YH, Wee YM, Choi MY, Lim DG, Kim SC. and Han DJ. (2011). Interleukin (IL)-10 induced by CD11b(+) cells and IL-10-activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allografts. Mol Med 17:697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge W, Jiang J, Arp J, Liu W, Garcia B. and Wang H. (2010). Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 90:1312–1320 [DOI] [PubMed] [Google Scholar]
- 19.Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, et al. (2012). Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant 12:2373–2383 [DOI] [PubMed] [Google Scholar]
- 20.Kuo YR, Chen CC, Shih HS, Goto S, Huang CW, Wang CT, Chen CL. and Wei FC. (2011). Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model. Plast Reconstr Surg 127:569–579 [DOI] [PubMed] [Google Scholar]
- 21.Kuo YR, Chen CC, Goto S, Huang YT, Wang CT, Tsai CC. and Chen CL. (2012). Immunomodulatory effects of bone marrow-derived mesenchymal stem cells in a swine hemi-facial allotransplantation model. PLoS One 7:e35459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, et al. (2011). Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol 6:412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinders ME, de Fijter JW, Roelofs H, Bajema IM, de Vries DK, Schaapherder AF, Claas FH, van Miert PP, Roelen DL, et al. (2013). Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl Med 2:107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riella LV. and Chandraker A. (2012). Stem cell therapy in kidney transplantation. JAMA 308:130; author reply 130–131 [DOI] [PubMed] [Google Scholar]
- 25.Vanikar AV. and Trivedi HL. (2012). Stem cell transplantation in living donor renal transplantation for minimization of immunosuppression. Transplantation 94:845–850 [DOI] [PubMed] [Google Scholar]
- 26.Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, Li X, Chen Z, Ma J, et al. (2013). Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: a clinical pilot study. Transplantation 95:161–168 [DOI] [PubMed] [Google Scholar]
- 27.Vaegler M, Maerz JK, Amend B, da Silva LA, Mannheim JG, Fuchs K, Will S, Sievert KD, Stenzl A, Hart ML. and Aicher WK. (2014). Labelling and tracking of human mesenchymal stromal cells in preclinical studies and large animal models of degenerative diseases. Curr Stem Cell Res Ther 9:444–450 [DOI] [PubMed] [Google Scholar]
- 28.Briquet A, Dubois S, Bekaert S, Dolhet M, Beguin Y. and Gothot A. (2010). Prolonged ex vivo culture of human bone marrow mesenchymal stem cells influences their supportive activity toward NOD/SCID-repopulating cells and committed progenitor cells of B lymphoid and myeloid lineages. Haematologica 95:47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, et al. (2006). Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells 24:1095–1103 [DOI] [PubMed] [Google Scholar]
- 30.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V. and Ho AD. (2008). Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 3:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, et al. (2009). Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One 4:e5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG. and Cao Y. (2008). Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, et al. (2010). Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 121:1113–1123 [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA. and Kuhn LT. (2012). One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One 7:e33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ. and Fisk NM. (2012). Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med 1:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang M, Chen W, Liu J, Weir MD, Cheng L. and Xu HH. (2014). Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A 20:1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Chen W, Zhao Z. and Xu HH. (2013). Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials 34:7862–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.TheinHan W, Liu J, Tang M, Chen W, Cheng L. and Xu HH. (2013). Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res 4:371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hynes K, Menicanin D, Mrozik K, Gronthos S. and Bartold PM. (2014). Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev 23:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S. and Bartold PM. (2013). Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res 92:833–839 [DOI] [PubMed] [Google Scholar]
- 41.Moslem M, Valojerdi MR, Pournasr B, Muhammadnejad A. and Baharvand H. (2013). Therapeutic potential of human induced pluripotent stem cell-derived mesenchymal stem cells in mice with lethal fulminant hepatic failure. Cell Transplant 22:1785–1799 [DOI] [PubMed] [Google Scholar]
- 42.Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K. and Taniguchi H. (2014). Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc 9:396–409 [DOI] [PubMed] [Google Scholar]
- 43.Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J. and Fu QL. (2012). Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 30:2692–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A. and Bennaceur-Griscelli A. (2011). Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood 118:3254–3262 [DOI] [PubMed] [Google Scholar]
- 45.Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, Sun YQ, Wen W, Tse HF, Lian Q. and Xu G. (2012). Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 67:1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng PP, Xia JJ, Wang HL, Chen JB, Wang FY, Zhang Y, Huang X, Zhang QJ. and Qi ZQ. (2011). Islet transplantation reverses the effects of maternal diabetes on mouse oocytes. Reproduction 141:417–424 [DOI] [PubMed] [Google Scholar]
- 47.Xia J, Chen J, Shao W, Lan T, Wang Y, Xie B, Thorlacius H, Tian F, Huang R. and Qi Z. (2010). Suppressing memory T cell activation induces islet allograft tolerance in alloantigen-primed mice. Transpl Int 23:1154–1163 [DOI] [PubMed] [Google Scholar]
- 48.Peng Y, Chen J, Shao W, Wang F, Dai H, Cheng P, Xia J, Wang F, Huang R, Zhu Q. and Qi Z. (2011). Xenoreactive CD4+ memory T cells resist inhibition by anti-CD44 mAb and reject islet grafts via a Th2-dependent pathway. Xenotransplantation 18:252–261 [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Xia J, Chen J, Peng Y, Cheng P, Ekberg H, Wang X. and Qi Z. (2010). Combination of antibodies inhibits accelerated rejection mediated by memory T cells in xenoantigen-primed mice. Xenotransplantation 17:460–468 [DOI] [PubMed] [Google Scholar]
- 50.Villa-Diaz LG, Brown SE, Liu Y, Ross AM, Lahann J, Parent JM. and Krebsbach PH. (2012). Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells 30:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei H, Tan G, Manasi , Qiu S, Kong G, Yong P, Koh C, Ooi TH, Lim SY, Wong P, Gan SU. and Shim W. (2012). One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res 9:87–100 [DOI] [PubMed] [Google Scholar]
- 52.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, et al. (2006). Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant 6:2601–2611 [DOI] [PubMed] [Google Scholar]
- 53.Nishimura R, Nishioka S, Fujisawa I, Shiku H, Shimada M, Sekiguchi S, Fujimori K, Ushiyama A, Matsue T, et al. (2013). Tacrolimus inhibits the revascularization of isolated pancreatic islets. PLoS One 8:e56799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barlow AD, Nicholson ML. and Herbert TP. (2013). Evidence for rapamycin toxicity in pancreatic beta-cells and a review of the underlying molecular mechanisms. Diabetes 62:2674–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marzorati S, Melzi R, Citro A, Cantarelli E, Mercalli A, Scavini M. and Piemonti L. (2014). Engraftment versus immunosuppression: cost-benefit analysis of immunosuppression after intrahepatic murine islet transplantation. Transplantation 97:1019–1026 [DOI] [PubMed] [Google Scholar]
- 56.Najar M, Raicevic G, Boufker HI, Fayyad Kazan H, De Bruyn C, Meuleman N, Bron D, Toungouz M. and Lagneaux L. (2010). Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton's Jelly and bone marrow sources. Cell Immunol 264:171–179 [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal S. and Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A. and Shi S. (2009). Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 27:1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longoni B, Szilagyi E, Quaranta P, Paoli GT, Tripodi S, Urbani S, Mazzanti B, Rossi B, Fanci R, et al. (2010). Mesenchymal stem cells prevent acute rejection and prolong graft function in pancreatic islet transplantation. Diabetes Technol Ther 12:435–446 [DOI] [PubMed] [Google Scholar]
- 60.Berman DM, Willman MA, Han D, Kleiner G, Kenyon NM, Cabrera O, Karl JA, Wiseman RW, O'Connor DH, Bartholomew AM. and Kenyon NS. (2010). Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates. Diabetes 59:2558–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahl SM, Swisher J, McCartney-Francis N. and Chen W. (2004). TGF-beta: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J Leukoc Biol 76:15–24 [DOI] [PubMed] [Google Scholar]
- 62.Read S, Malmstrom V. and Powrie F. (2000). Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green EA, Gorelik L, McGregor CM, Tran EH. and Flavell RA. (2003). CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci U S A 100:10878–10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdi R, Fiorina P, Adra CN, Atkinson M. and Sayegh MH. (2008). Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57:1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N. and Yarmush ML. (2010). Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant 19:667–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Graos M, et al. (2013). Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther 4:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Bai J, Ji X, Li R, Xuan Y. and Wang Y. (2014). Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med 34:695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrade Holt DD, Wood JA, Granick JL, Walker NJ, Clark KC. and Borjesson DL. (2014). Equine mesenchymal stem cells inhibit T cell proliferation through different mechanisms depending on tissue source. Stem Cells Dev 23:1258–1265 [DOI] [PubMed] [Google Scholar]