Abstract
Midbrain dopamine (DA) neurons are essential for maintaining multiple brain functions. These neurons have also been implicated in relation with diverse neurological disorders. However, how these neurons are developed from neuronal stem cells (NSCs) remains largely unknown. In this study, we provide both in vivo and in vitro evidence that the thyroid hormone, an important physiological factor for brain development, promotes DA neuron differentiation from embryonic ventral midbrain (VM) NSCs. We find that thyroid hormone deficiency during development reduces the midbrain DA neuron number, downregulates the expression of tyrosine hydroxylase (TH) and the dopamine transporter (DAT), and impairs the DA neuron-dependent motor behavior. In addition, thyroid hormone treatment during VM NSC differentiation in vitro increases the production of DA neurons and upregulates the expression of TH and DAT. We also found that the thyroid hormone enhances the expression of Otx2, an important determinant of DA neurogenesis, during DA neuron differentiation. Our in vitro gene silencing experiments indicate that Otx2 is required for thyroid hormone-dependent DA neuron differentiation from embryonic VM NSCs. Finally, we revealed both in vivo and in vitro that the thyroid hormone receptor alpha 1 is expressed in embryonic VM NSCs. Furthermore, it participates in the effects of thyroid hormone-induced Otx2 upregulation and DA neuron differentiation. These data demonstrate the role and molecular mechanisms of how the thyroid hormone regulates DA neuron differentiation from embryonic VM NSCs, particularly providing new mechanisms and a potential strategy for generating dopamine neurons from NSCs.
Introduction
Midbrain dopaminergic neurons represent an important neuronal subtype and provide the major source of dopamine (DA) in the mammalian brain. These neurons have critical functional roles for maintaining normal brain functions [1]. They have also been implicated in many neurological and psychiatric disorders, including Parkinson's disease, depression, and schizophrenia [2,3]. DA neurons arise from embryonic neuronal stem cells (NSCs) in the ventral midbrain (VM) and are located in the substantia nigra pars compacta (SNc), the ventral tegmental area (VTA), and the retrorubral area [3].
Because of the physiological and clinical involvement of this neuronal subtype in common neurological disorders and normal brain functions, NSC differentiation toward DA neurons is of key interest. Revealing the extrinsic signals and intrinsic molecular mechanisms that control the generation of DA neurons during development is likely to facilitate the engineering of DA neurons from stem cells. These data may be critical for the development of stem cell-based therapies. However, much is still unknown regarding the extrinsic signals and intrinsic molecules involved in DA neuron differentiation from NSCs during brain development.
Thyroid hormones (the prohormone thyroxine T4 and its active metabolite 3,5,3′-triiodothyronine T3) are well-known essential factors for brain development [4]. Undiagnosed hypothyroidism in pregnant women may adversely affect their fetuses, resulting in severe mental retardation, spastic diplegia, and extrapyramidal rigidity [5,6]. During brain development, thyroid hormones are essential for embryonic neurogenesis [7,8]. However, the mechanisms, by which the thyroid hormone regulates the differentiation of NSCs into specific subtypes of neurons in different brain regions, remain largely unknown. The investigation of thyroid hormone actions and mechanisms underlying NSC biology may uncover new roles for the classic hormone.
In previous studies, we showed that the thyroid hormone promotes the differentiation of embryonic mouse cortex NSCs into neurons [9]. The effects of the thyroid hormone on NSCs in the adult hippocampus and the subventricular zone have also been well described [10,11]. However, the role of the thyroid hormone on NSC biology in the midbrain remains unclear.
A number of studies indirectly suggest that thyroid hormones influence DA system development. T3 was reported to affect the morphogenesis of DA neurons from the fetal mouse mesencephalon [12]. Neonatal hypothyroidism markedly decreases the concentration of DA and the activity of its rate-limiting enzyme, tyrosine hydroxylase (TH) [13]. In a genetic thyroid-stimulating hormone receptor mutant mouse model, a reduction in the number of midbrain DA neurons has been observed [14]. However, whether the thyroid hormone regulates DA neuron generation by affecting NSC differentiation directly is unknown. Examining this question will increase our knowledge of DA system development and reveal new mechanisms generating DA neurons from NSCs.
The development of DA neurons depends on a number of intrinsic transcription factors and several identified extrinsic signals. The identified intrinsic transcription factors, which are called DA neuron determinants, control a cascade of transcriptional machinery that regulates the cell fate and differentiation of DA neurons. These factors include Otx2, Nurr1, Pitx3, En1, En2, Foxa1, Foxa2, Ngn2, Lmx1α, Lmx1β, Msx1, and Msx2, which enable stem and progenitor cells to establish proper cell identity [15–17]. Extrinsic signals, such as Shh and Fgf8, specify DA progenitor identity; these factors are important for the patterning and expansion of DA neurons [3,18].
Previous studies suggested that there are interactions between several of these intrinsic transcription factors and thyroid hormones [19–21]. Whether the thyroid hormone is an efficient extrinsic signal that governs DA neuron generation through these DA neuron determinants during brain development is an unanswered question.
In this study, we identify a critical role of the thyroid hormone and its receptor alpha 1 in DA neuron differentiation from embryonic VM NSCs. In addition, we demonstrate that Otx2 signaling is essential for the effects of thyroid, hormone-dependent DA neuron differentiation. Our studies reveal a critical role of the thyroid hormone in DA neuron development and identify new mechanisms for generating DA neurons from NSCs.
Materials and Methods
Animal treatments
Timed pregnant BALB/c mice were used in the experiments. Hypothyroidism was induced by propyl-thio-uracil (PTU; Sigma-Aldrich) treatment, as previously reported [22,23]. Briefly, PTU was added daily to the drinking water (500 mg/L PTU) throughout the pregnancy. In addition, an intraperitoneal injection of PTU (10 mg/kg) was given at embryonic day 0 (E0). For thyroid hormone treatment, a physiological combination of T4 and T3 was added to the drinking water (0.2 μg T4/mL and 0.03 μg T3/mL; Sigma-Aldrich) based on previous reports [24].
The serum-free T3 (FT3) and FT4 levels were measured by a radioimmunoassay in the pregnant mice and their offspring to monitor the effects of PTU and thyroid hormone treatment, as previously described [9]. All the experiments conformed to the guidelines on the handling and training of laboratory animals of both the National Institutes of Health and the Third Military Medical University.
Behavioral testing
Open field test
The open field test was conducted with a TM-Vision system, as previously described [25]. Briefly, it contains a 50×50×30-cm black box, a computer controlled camera, and a surrounding curtain. In each trail, the mouse was placed in the center of the apparatus at the beginning of the test. The time spent moving and the total distance moved were measured during the 5-min trial.
Pole climbing test
This test was carried out as previously described [26]. Briefly, the pole test consisted of a 50-cm-high and 0.5-cm-diameter wooden pole. The pole was wrapped in gauze to prevent slipping, and the base was positioned on the floor. The mice were placed on the top of the wooden pole and allowed to climb down without interference. The time that the animal required to climb down the pole was measured. Each animal performed three successive trials with a 5-min intertrial interval. The average of the three trials was used for statistical analyses.
Wire suspension test
The wire suspension test was carried out as described previously [27]. Mice were hung from a horizontal wire by the forepaws and observed for 30 s. The mouse was scored according to the following scheme: 0, fell off; 1, hung onto the bar with two forepaws; 2, in addition to 1, attempted to climb onto the wire; 3, hung onto the wire with two forepaws and one or both hind paws; 4, hung onto the wire with all four paws with tail wrapped around the wire; 5, escaped to one of the supports.
Rotarod test
Briefly, the mice were first trained on a rotating rod at 10 revolutions per minute (rpm) for 2 days. On the third day, the mice were tested on the rotarod at 16 rpm for 300 s. The latency (s) to fall from the rod was measured. For each animal, the latency to fall was recorded for three trials, and the average was used for statistical analysis.
Cell culture, differentiation, and T3 treatment
NSCs were cultured in vitro from the VM of mouse embryos at E13.5, as described previously [28–31], with some modifications. Briefly, dissected brain tissues were mechanically triturated and plated at a final concentration of 2.5×105 cell/mL in 6- or 24-well plates. Embryonic VM NSCs were grown in a serum-free complete medium composed of a 1:1 (v/v) mixture of Dulbecco's Modified Eagle's Medium and F12 medium (Gibco) supplemented with the basic fibroblast growth factor (bFGF, 20 ng/mL; Sigma-Aldrich), EGF (20 ng/mL; Sigma-Aldrich), and N2 (1×) supplements (Gibco) under the floating condition. The medium was changed every other day.
For differentiation, VM NSCs were dissociated and adhered to 24-well plates. Cell differentiation was induced by withdrawing the bFGF and EGF from the medium, and T3 was added to the culture medium every 3 days to a final concentration of 0.3 nM.
Cell proliferation assay
The effects of T3 on the proliferation of embryonic neural stem cells (eNSCs) were studied by a Colorimetric Cell-Counting Kit-8 (CCK-8; Dojindo) assay and 5-bromo-2-deoxyuridine (BrdU) incorporation, as previously described [32].
High-performance liquid chromatography analysis of DA and its metabolites
The midbrain and striatum tissue levels of DA and its metabolite DOPAC were measured by high-performance liquid chromatography (HPLC) using an electrochemical detector (L-3500 A; Merck) following established methods [33]. In brief, midbrain tissues were rapidly dissected, weighed, homogenized, and centrifuged. The final supernatant was stored at −70°C until the HPLC assay. The HPLC data were obtained as an average of three measurements for each determination. The results are expressed in terms of pg of DA and DOPAC per g of wet tissue. For detecting the DA and DOPAC levels in cultured cells, cell lysates were used instead of tissue lysates. The results were expressed in terms of pg DA and DOPAC per mg of protein.
RNA isolation and real-time polymerase chain reaction analysis
Total RNA was extracted from mouse midbrain tissues or cultured cells using the TRIzol reagent (Takara). Reverse transcription–polymerase chain reaction (RT-PCR) was used to identify the expression of thyroid hormone receptors (TRs) using the following primers: TRα fwd 5′-ttcagcgagtttaccaagatca-3′ and rev 5′-gtcatccaggttaaaggcagag-3′ and TRβ fwd 5′-aaaagcaaggactctgacttgg-3′ and rev 5′-ggatggagacttttctgaatgg-3′. The gene-specific primers used for real-time PCR were β-actin fwd 5′-agattactgctctggctcctagc-3′ and rev 5′-actcatcgtactcctgcttgct-3′, TH fwd 5′-cctttgacccagacacagcag-3′ and rev 5′-tggatacgagaggcatagttcc-3′, and the dopamine transporter (DAT) fwd 5′-cctggttctacggtgtccag-3′ and rev 5′-gctgaccacgaccacataca-3′.
Real-time PCR was performed on a CFX96™ real-time system using SYBR® Master Mix (Life Technologies). Fold change in gene expression was calculated using the 2−ΔΔCT method [34]. All gene expression values were normalized to endogenous β-actin. Subsequently, relative expression levels of the target genes were calculated using the control as a reference.
Western blot analysis
Western blot analysis was performed and quantified with an Odyssey system (LI-COR), as previously described [32]. The primary antibodies used for western blot analysis were the rabbit anti-mouse TH antibody (1:1,000; Abcam), rabbit or goat anti-mouse DAT antibody (1:1,000; Santa Cruz), rabbit anti-mouse Otx2 antibody (1:1,000; Santa Cruz), rabbit anti-mouse Ngn2 antibody (1:1,000; Santa Cruz), rabbit anti-mouse Nurr1 antibody (1:1,000; Santa Cruz), and mouse anti-mouse β-actin antibody (1:5,000; Sigma-Aldrich). The secondary antibodies used were Odyssey-specific IRDye® 680 or 800 donkey anti-rabbit, donkey anti-goat, and donkey anti-mouse antibodies (1:5,000; LI-COR).
Immunohistochemistry and quantification of TH+ cells in vivo
Histology and immunohistochemistry were performed as described previously [35,36]. Briefly, heads or dissected brains (E13.5–P0) of mice were immersion fixed overnight with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at 4°C. Postnatal day 14 (P14) mice were transcardially perfused with 0.9% saline followed by 4% PFA. Mouse brains were sectioned at a thickness of 30 μm. Sections were blocked in PBS containing 5% normal serum and 0.25% Triton X-100 before incubation with antibodies. Sections were incubated overnight at 4°C with the appropriate primary antibody and then extensively washed in PBS and incubated for 1 h at room temperature with a secondary antibody.
The rabbit anti-mouse TH primary antibody (1:100; Abcam) was used to identify DA neurons. The mouse anti-mouse Nestin antibody (1:100; Millipore) was used to identify NSCs. For Nestin and TRα1 double staining, the rabbit anti-mouse TRα1 (1:100; Thermo) was used. Appropriate Alexa Fluor 488–597- or 647-labeled secondary antibodies were used for visualization. Cell nuclei were visualized by staining with Hoechst33342 (5 μg/mL; Sigma-Aldrich). All images were collected with a Leica TCS SP5 confocal microscope.
StereoLogic analysis was performed under blinded conditions according to previously reported methods [35,36]. The total number of TH+ neurons in the midbrain was calculated bilaterally for each animal using an optical fractionator, an unbiased cell counting method. To minimize bias (ie, systematic error) in stereologic estimates, appropriate protocols were adopted as summarized by Schmitz and Hof [37].
Briefly, every fifth consecutive section (30 μm) through the entire midbrain from the anterior to posterior was collected for quantification. Six animals were counted for each condition. For each section, the entire VTA and SNc were identified as the region of interest. Each section was viewed with a 4×objective and outlined. The numbers of TH+ cells were counted with a 60×oil objective using a 50×50-μm counting frame in a systematic manner. The average total numbers of TH+ cells were estimated using a previously established equation: N=total cells counted×t/h×1/asf×1/ssf [14]. In this equation, t/h is the thickness of the section divided by the height of the counting frame, asf is the area of the counting frame divided by the area of the programmed stage movements in the x- and y-axis, and ssf is the sampling fraction of the tissue sections.
Immunocytochemistry and cell counts
For immunocytochemistry, cells were allowed to adhere to poly-l-lysine-coated round glass coverslips (12 mm in diameter) and differentiated for an appropriate number of days, as indicated. The cells were then fixed in 4% PFA and stained as previously described [9]. The primary antibodies and secondary antibodies used were the same as those described in the Immunohistochemistry and Quantification of TH+ Cells In Vivo section. In addition, the following primary antibodies were used for double staining: rabbit anti-mouse Otx2 (1:100; Santa Cruz), rabbit anti-mouse beta-III tubulin (Tuj1, 1:100; GeneTex), and rabbit anti-mouse DAT (1:100; Santa Cruz). The mouse anti-mouse TH (1:100; GeneTex) antibody was used for TH double staining with TRα1 and Otx2.
For cell counts, Hoechst33342 (Sigma-Aldrich) staining was used for identification, and TH+Tuj1+, Tuj1+, and Hoechst+ cells were counted. The cells were counted in six different fields of each coverslip using a 20×objective. Each condition was analyzed in duplicate for every experiment, and three to four independent experiments were performed for every condition. Quantification was performed by an independent observer in a blinded manner. The TH+ neurons were expressed as a percentage of total cells determined by Hoechst33342 staining and also as a percentage of the total number of Tuj1+ neurons.
Cell transfection and RNA interference
SiRNAs against Otx2 were purchased from Sigma-Aldrich (EMU014781). TRα1-specific siRNAs were purchased from GenePharma, as reported previously [9]. The siRNAs were transfected into NSCs using Lipofectamine 2000 (Invitrogen). An oligonucleotide that was not homologous to any known gene was used as a negative control. The effect of siRNA silencing was identified by detecting the mRNA and protein expression of specific genes.
Statistics
All data were collected from at least four independent duplicate experiments unless otherwise stated. Data are expressed as the mean±standard error of the mean. The results of different groups were compared by one-way ANOVA followed by Fisher's post hoc test using the SPSS13.0 software package. When only two sets of data were compared, Student's t-test was used. A P-value <0.05 was considered statistically significant.
Results
Thyroid hormone deficiency impairs midbrain DA neuron generation in vivo
To investigate the effects of thyroid hormone on DA neuron development in vivo, a model of hypothyroidism in pregnant mice was established with PTU treatment, as previously reported [22,23]. Serum FT3 and FT4 levels were monitored to detect the effects of PTU treatment. The expression of TH and DAT, critical components of DA synthesis and transport, was used as markers to identify and analyze the differentiation of DA neurons. DA and DOPAC levels in brain tissues of the offspring were assessed to analyze the function of the DA neurons.
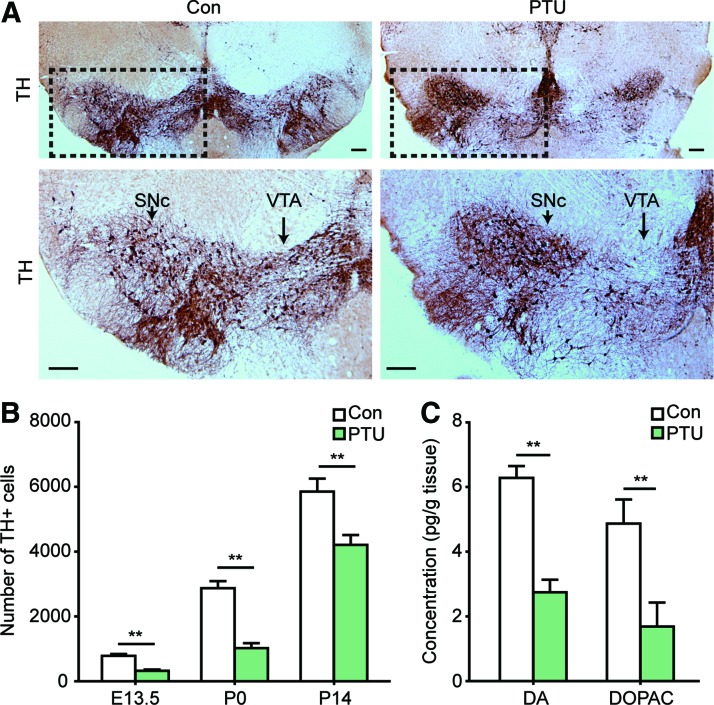
The results of serum FT3 and FT4 levels suggested a successful induction of thyroid hormone deficiency during the embryonic phase (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd). Subsequently, the number of midbrain DA neurons was calculated by immunostaining with the TH antibody. We found that PTU treatment robustly decreased the number of midbrain TH+ cells at E13.5, P0, and P14 (Fig. 1A, B and Supplementary Fig. S1A, B). In addition, both DA and DOPAC levels in the midbrain tissues were reduced by PTU treatment in the P0 offspring (Fig. 1C).
FIG. 1.
Hypothyroidism during pregnancy decreases the number of midbrain dopamine (DA) neurons in the offspring. (A, B) Pregnant mice were treated with propyl-thio-uracil (PTU), and the midbrain DA neurons in the offspring were stained with tyrosine hydroxylase (TH) and quantified as described in the Materials and Methods section. (A) Representative images of TH staining in the midbrain of the P0 offspring are shown. Row 2 is the amplification of the dotted rectangles. Arrow shows the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) of the midbrain. Scale bar: 200 μm. E13.5 and P14 representative images are shown in Supplementary Fig. S1. (B) The statistical results of TH+ cell counts in the midbrain of the offspring. n=6, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. (C) DA and DOPAC content in the brain tissues of the P0 offspring. Data are presented as pg of DA or DOPAC per g of wet tissue. n=12, Student's t-test, **P<0.01. For all experiments, data are presented as the mean±standard error of mean (SEM). Color images available online at www.liebertpub.com/scd
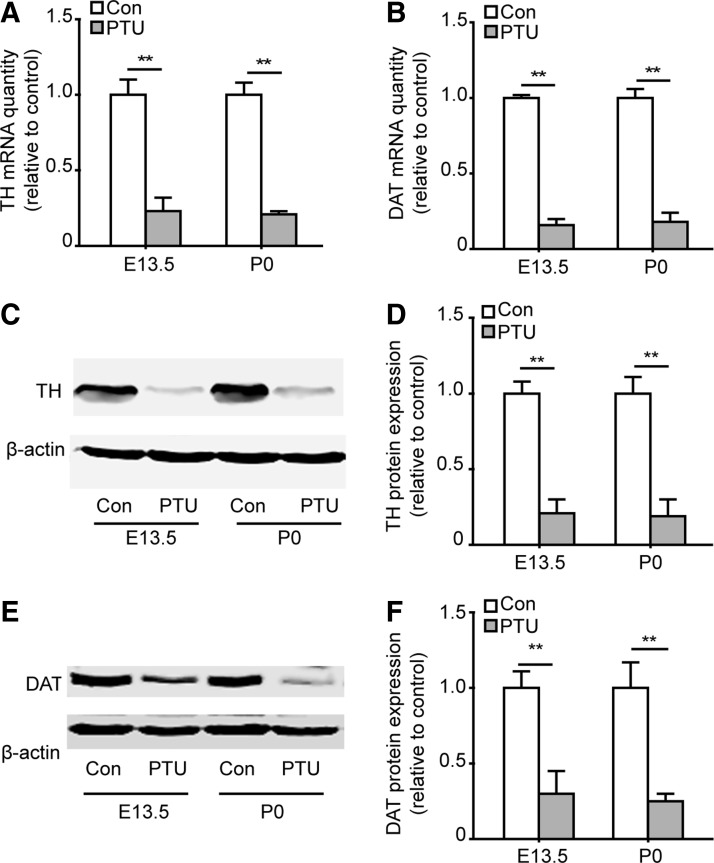
We also found that the mRNA and protein expression of TH and DAT in the PTU-treated offspring remained significantly lower than the controls, as detected at E13.5 and P0 (Fig. 2A–F). Together, these results suggest that thyroid hormone deficiency during the development impairs DA neuron differentiation.
FIG. 2.
Hypothyroidism during pregnancy decreases the expression of TH and dopamine transporter (DAT) in the midbrain. Pregnant mice were treated with PTU, and the midbrain mRNA and protein expression of TH and DAT was detected in the offspring at E13.5 and P0. The graphs show the fold change of mRNA and protein levels with respect to the control. (A) TH mRNA levels in each condition. (B) DAT mRNA expression detected at E13.5 and P0. (C) Representative images of TH western blots. (D) Statistical results of the fold change in TH protein expression. (E, F): DAT protein expression detected at E13.5 and P0. Data are presented as the mean±SEM, n=6, one-way ANOVA followed by Fisher's post hoc test, **P<0.01.
Thyroid hormone deficiency during pregnancy impairs offspring DA neuron-dependent motor behavior
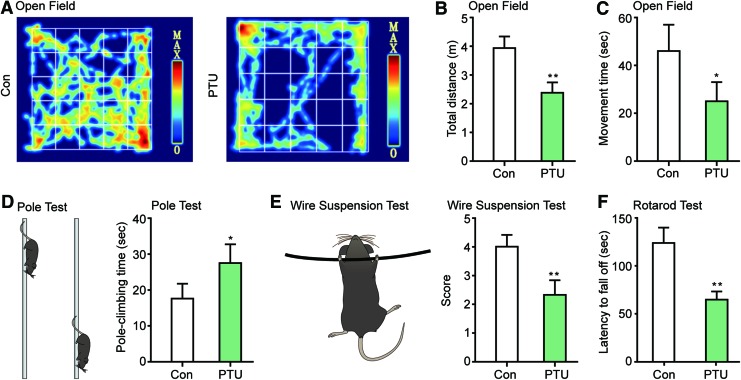
To further confirm the effects of thyroid hormone deficiency on DA system development, behavioral tests were carried out in the offspring of PTU-treated and control mice at 1 and 2 months after birth to detect the DA neuron-dependent motor ability. In the 1-month-old mice, a decrease in the center-area tracks, total distance, and locomotion time was detected in the offspring of PTU-treated mice in the open field test (Fig. 3A–C). Compared with the control, PTU-treated mice required a much longer time to climb down in the pole test (Fig. 3D) and received lower scores on the wire suspension test (Fig. 3E). Furthermore, the offspring of PTU-treated mice exhibited much shorter fall latencies in the rotarod test than the control offspring (Fig. 3F).
FIG. 3.
Hypothyroidism during pregnancy impairs the DA neuron-dependent motor function in the offspring. Pregnant mice were treated with PTU, and the motor functions of their offspring were tested with the open field test, pole climbing test, wire suspension test, and rotarod test 1 month after birth. (A) Movement traces of the mice in the open field test. (B) The total distance travelled during a 5-min period in the open field test. (C) The movement time in the open field test. (D) The time to descend the pole in the pole climbing test. (E) The score assigned in the wire suspension test. (F) The latency to fall off the rod in the rotarod test. For all experiments, data are got from 12 animals and are presented as the mean±SEM. Student's t-test, *P<0.05, **P<0.01. Color images available online at www.liebertpub.com/scd
At 2 months of age, although some of the parameters in the behavioral tests recovered, these offspring still moved a shorter total distance in the open field test and spent a longer time on the pole test compared to control mice (Supplementary Fig. S2A–D). These results demonstrated an impairment of DA-dependent motor abilities in the offspring of hypothyroid females and further confirm that thyroid hormone deficiency during the embryonic phase impairs DA system development and functions.
Thyroid hormone induces DA neuron differentiated from embryonic VM NSCs in vitro
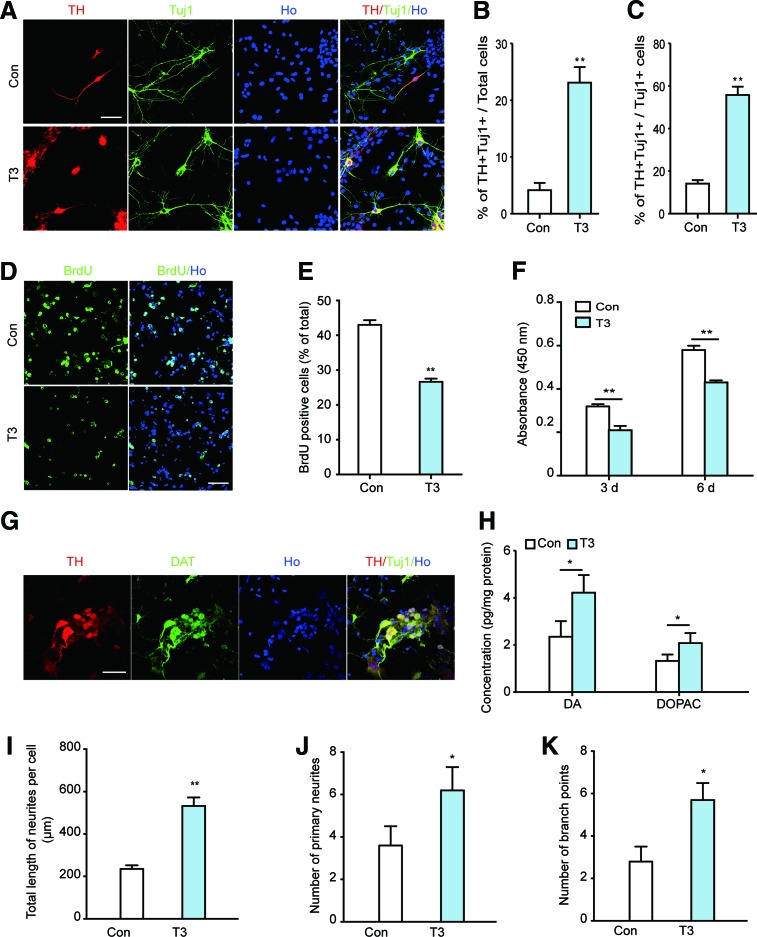
Embryonic NSCs in the VM are the original source of midbrain DA neurons. To study the role of the thyroid hormone in DA neuron development, we cultured NSCs from the embryonic mouse VM. The identification of NSC characteristics confirmed that the cultured cells were NSCs (Supplementary Fig. S3A). Next, the cells were induced to differentiate with 0.3 nM T3, a physiological level of the thyroid hormone [38–40]. The percentage of differentiated DA neurons was calculated by double-staining for TH and the neuronal-specific marker Tuj1 after T3 treatment for 6 days.
We found a significant increase in the percentage of TH and Tuj1 double-positive (TH+Tuj1+) cells in the T3-treated group compared with the control (Fig. 4A, B). This effect was due to an increase in DA neurons as opposed to an increase in the general neuron number because the percentage of TH+Tuj1+ relative to the total neuron population also increased after T3 treatment (Fig. 4C). This effect was further confirmed by the results that T3-induced DA neuron differentiated from cultured neurospheres (Supplementary Fig. S3B, C). In addition, this effect is independent of increased cell number, as demonstrated by the results that T3 slightly inhibited VM NSC proliferation (Fig. 4D–F). Furthermore, all the T3-induced TH+ cells coexpressed DAT, another unique marker of DA neurons (Fig. 4G).
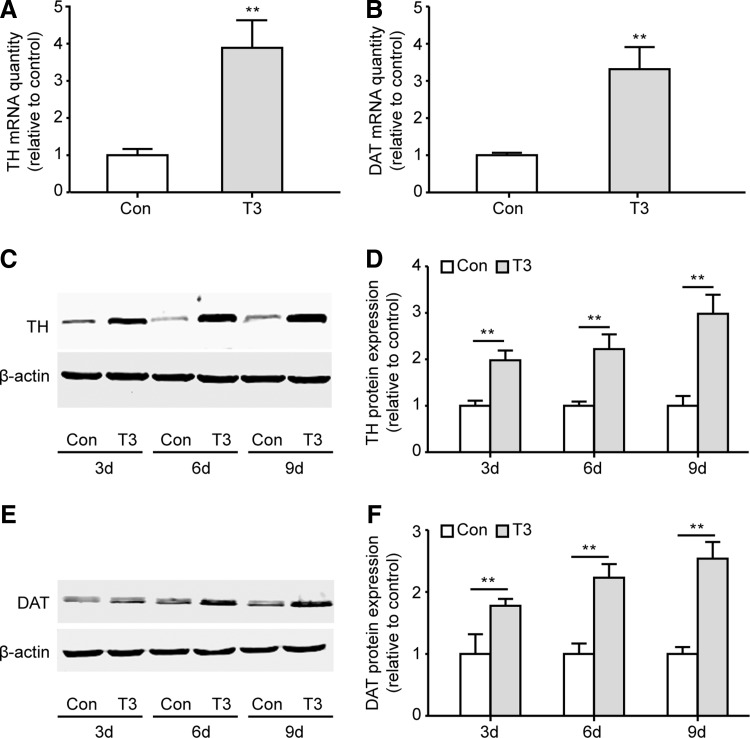
FIG. 4.
T3 treatment promotes DA neuron differentiation from ventral midbrain neuronal stem cells (VM NSCs) in vitro. (A–C) VM NSCs were treated with 0.3 nM T3 and differentiated for 6 days in vitro. The differentiated DA neurons were then identified by immunocytochemistry using antibodies against TH and Tuj1. The images in (A) are representative from four independent experiments (scale bar: 50 μm). (B, C) Represent the statistical results (Ho, Hoechst33342). n=4, Student's t-test, **P<0.01. (D, E) VM NSCs were treated with 0.3 nM T3 for 6 days. 5-bromo-2-deoxyuridine (BrdU) was added to the culture medium during the last 24 h. Images in (D) are representative images of BrdU and Hoechst33342 staining cells (Scale bar: 50 μm). (E) Statistical results of the percentage of BrdU+ cells in each group. BrdU+ cells were quantified in four random fields in each culture well, and 12 wells from four independent experiments were quantified for each condition. Student's t-test, **P<0.01. (F) VM NSCs were treated with 0.3 nM T3 for indicated times. Cell proliferation was assessed by the Cell-Counting Kit-8 (CCK-8) assay. n=4, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. (G) The differentiated DA neurons were further identified by TH and DAT double staining. Scale bar: 50 μm. (H) DA and DOPAC content in the differentiated cells was detected after T3 treatment for 9 days. n=6, Student's t-test, *P<0.05. (I–K) Neurite outgrowth in T3-induced TH+ cells and control TH+ cells. n=32 from four independent experiments; *P<0.05, **P<0.01 compared with the control, Student's t-test. For all experiments, data are presented as the mean±SEM. Color images available online at www.liebertpub.com/scd
To further evaluate DA neuron differentiation, the levels of DA and its metabolite DOPAC in the differentiated cells were measured. Both were markedly increased in T3-treated cells, as detected in cell lysates 9 days after differentiation (Fig. 4H). Furthermore, T3 promoted neurite outgrowth in differentiated TH+ neurons, as measured by increased neurite length, number of primary neurites, and branch points (Fig. 4I–K). In addition, TH and DAT mRNA expression was markedly upregulated after T3 treatment for 3 days (Fig. 5A, B). The protein levels of TH and DAT were notably increased at days 3, 6, and 9 after T3-induced differentiation (Fig. 5C–F). These results suggested that T3 robustly induced embryonic VM NSC differentiation into functional DA neurons in vitro.
FIG. 5.
T3 treatment upregulates TH and DAT expression from VM NSC-differentiated cells in vitro. (A) NSCs were differentiated with T3 for 3 days, and the mRNA expression of TH was detected by real-time polymerase chain reaction. (B) DAT mRNA expression after T3 treatment for 3 days. (A, B) n=4, Student's t-test, **P<0.01. (C, D) The protein expression of TH was detected by western blot analysis after T3 treatment for the indicated number of days. The images in (C) are representative of four independent experiments, and (D) indicates the fold changes in protein levels compared to the control. (E, F) DAT protein expression after T3 treatment for the indicated number of days. (D, F) n=4, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. For all experiments, data are presented as the mean±SEM.
Otx2 signaling is required for thyroid hormone-mediated DA neuron differentiation
To elucidate the molecular mechanism of T3-induced DA neuron differentiation, the mRNA expression of previously reported DA neuron development-related transcription factors was measured after T3 treatment for 2 days. We measured the mRNA levels of Otx2, Nurr1, Pitx3, En1, En2, Foxa1, Foxa2, Ngn2, Lmx1α, Lmx1β, Msx1, and Msx2. Only Otx2, Ngn2, and Nurr1 were found to be significantly upregulated (Table 1).
Table 1.
Fold Change in the Expression Level of Dopamine Neuron Determinants After T3 Treatment
| Symbol | Gene name | Primer | Fold change |
|---|---|---|---|
| Nurr1 | Nuclear receptor subfamily 4 | fwd 5′-atcagagggtgggcagagaag-3′ | 1.88±0.25a |
| rev 5′-ctgggttggacctgtatgctaag-3′ | |||
| Pitx3 | Paired-like homeodomain transcription factor 3 | fwd 5′-accctccgcttccagaacat-3′ | 1.11±0.26 |
| rev 5′-cttcttcttcagagagccgtcct-3′ | |||
| En1 | Engrailed 1 | fwd 5′-gactcacagcaacccctagtgtg-3′ | 0.85±0.12 |
| rev 5′-cgcttgtcttccttctcgttct-3′ | |||
| En2 | Engrailed 2 | fwd 5′-cagtgggagtgtgtcctgaa-3′ | 0.91±0.22 |
| rev 5′-gattccaactcgctctgcca-3′ | |||
| Otx2 | Orthodenticle homolog 2 | fwd 5′-ggacgacatttactagggcacag-3′ | 2.98±0.59b |
| rev 5′-cggcacttagctcttcgattct-3′ | |||
| Foxa1 | Forkhead box A1 | fwd 5′-actctccttatggcgctaccttg-3′ | 0.96±0.10 |
| rev 5′-ggaagtatttagcacgggtctgg-3′ | |||
| Foxa2 | Forkhead box A2 | fwd 5′-agaagatggctttcaggccc-3′ | 1.03±0.07 |
| rev 5′-aggtgagactgctcccttga-3′ | |||
| Ngn2 | Neurogenin 2 | fwd 5′-gtcatcctccaactccacgtc-3′ | 1.97±0.28a |
| rev 5′-aggcgcataacgatgcttctc-3′ | |||
| Lmx1α | LIM homeobox transcription factor 1 alpha | fwd 5′-gcagcatcagcaaggttgtc-3′ | 1.08±0.34 |
| rev 5′-gcaaccacctcatccacact-3′ | |||
| Lmx1β | LIM homeobox transcription factor 1 beta | fwd 5′-gtgcaagggtgactatgagaagg-3′ | 1.17±0.26 |
| rev 5′-catctccactgcctttactctgg-3′ | |||
| Msx1 | Homeobox, msh-like 1 | fwd 5′-actcggtgtcaaagtggaggact-3′ | 0.89±0.09 |
| rev 5′-gctgaagggcaggagtgaag-3′ | |||
| Msx2 | Homeobox, msh-like 2 | fwd 5′-gaaactccatgtcaggtccc-3′ | 0.96±0.11 |
| rev 5′-tgtaccgtgtgaggactgatg-3′ |
Data are obtained from four independent experiments detected by real-time polymerase chain reaction analysis and are presented as the mean±standard error of mean.
P<0.05 compared with the control, Student's t-test.
P<0.01 compared with the control, Student's t-test.
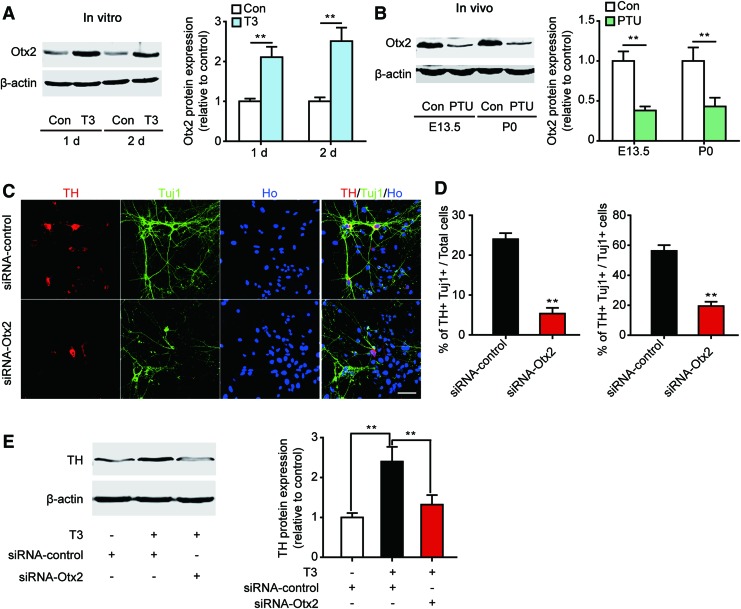
It was previously reported that the expression of Ngn2 and Nurr1 is dependent on Otx2 [41,42], indicating that Otx2 may play a central role in T3-induced DA neuron differentiation. To confirm this, we detected Otx2 expression in T3-induced DA neurons. We found that almost all of the T3-induced TH+ cells also expressed Otx2 (Supplementary Fig. S4). In addition, the protein expression of Otx2 was increased robustly after T3 treatment for 1 and 2 days (Fig. 6A). Furthermore, a significant reduction in both the mRNA and protein expression of Otx2 was found in the midbrain of the PTU-treated offspring at E13.5 and P0 (Fig. 6B and Supplementary Fig. S5).
FIG. 6.
T3 induces DA neuron differentiation through Otx2 signaling. (A) VM NSCs were treated with T3 for 1 or 2 days, and the protein level of Otx2 was detected. n=4, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. (B) Pregnant mice were treated with PTU, and the protein level of Otx2 in the midbrain of the offspring was detected at E13.5 and P0. n=4, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. (C, D) VM NSCs were transfected with control-siRNA or Otx2-siRNA, and T3 was administered for 6 days to induce differentiation. (C) Representative images (scale bar: 50 μm) of TH+ and Tuj1+ staining. (D) Statistical results of the percentage of TH+Tuj1+ cells. n=4, Student's t-test, **P<0.01. (E) NSCs were treated as described in (C), and the TH protein level was then detected. n=4, one-way ANOVA followed by Fisher's post hoc test, **P<0.01. For all experiments, data are presented as the mean±SEM. Color images available online at www.liebertpub.com/scd
We then silenced Otx2 expression in cultured NSCs and induced NSC differentiation with T3 for 6 days. The mRNA and protein level of Otx2 was decreased robustly after Otx2-siRNA transfection for 2 and 4 days, indicating a high efficiency of RNA silence (Supplementary Fig. S6). The percentage of differentiated TH+Tuj1+ cells and the TH protein levels were markedly decreased in Otx2-silenced cells compared with the controls (Fig. 6C–E). These results confirmed the critical role of Otx2 in T3-mediated DA neuron differentiation from embryonic VM NSCs.
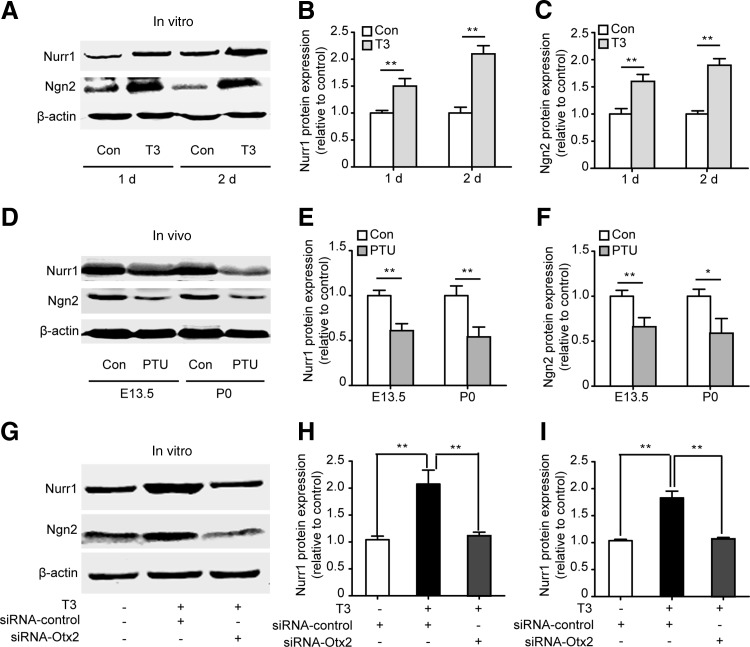
Ngn2 and Nurr1 also play a key role in DA neuron development, as previously reported [43,44]. To investigate the role of Ngn2 and Nurr1 in T3-induced DA neuron differentiation and their relationships with Otx2, we detected the protein expression of Ngn2 and Nurr1 during this process. In accordance with Otx2, Ngn2, and Nurr1, protein expression was also increased after T3 treatment for 1 and 2 days (Fig. 7A–C). In addition, in the midbrain of the PTU-treated offspring, a significant reduction in both the mRNA and protein expression of Ngn2 and Nurr1 was found at E13.5 and P0 (Fig. 7D–F and Supplementary Fig. S4). These results indicated that Ngn2 and Nurr1 are also involved in T3-induced DA neuron differentiation.
FIG. 7.
Otx2 is required for T3-induced Ngn2 and Nurr1 activation. (A–C) VM NSCs were treated with T3 for 1 or 2 days. The protein level of Ngn2 and Nurr1 was detected. Images in (A) are representative western blot bands from four independent experiments. (B, C) Indicate the fold changes in protein levels compared to the control. (D–F) Pregnant mice were treated with PTU. The protein level of Ngn2 and Nurr1 was detected at E13.5 and P0. Images in (D) are representative bands from four independent experiments. (E, F) The statistical results of the protein expression of Ngn2 and Nurr1. (G–I) NSCs were transfected with control-siRNA or Otx2-siRNA. T3 was administered to induce differentiation. Graphs contain the representative protein bands and statistical results of Ngn2 and Nurr1 from four independent experiments. For all experiments, data are presented as the mean±SEM. n=4, one-way ANOVA followed by Fisher's post hoc test, *P<0.05, **P<0.01.
To detect the relationship among Otx2, Ngn2, and Nurr1, we silenced Otx2 expression. We found that the effects of T3 on Ngn2 and Nurr1 protein expression were largely reversed (Fig. 7G–I) after Otx2 silencing. The result confirmed that the promotive effect of T3 on the expression of Ngn2 and Nurr1 was dependent on Otx2. Taken together, these results reveal that DA determinant Otx2 is required for T3-induced DA neuron differentiation from embryonic VM NSCs.
Thyroid hormone receptor alpha 1 participates in T3-induced Otx2 upregulation and DA neuron differentiation
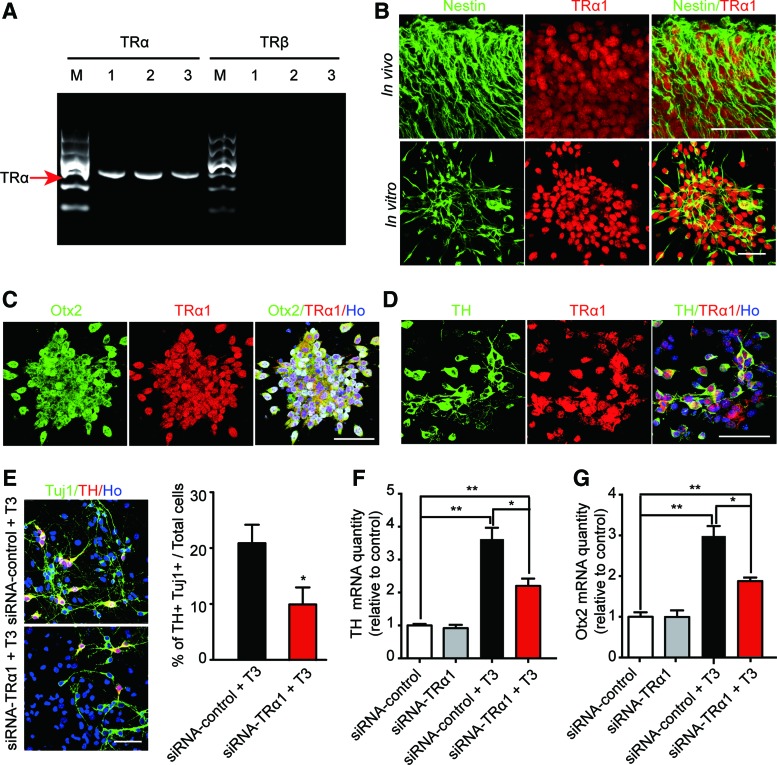
To detect whether TR is involved in the effects of T3 on Otx2 upregulation and DA neuron differentiation, the expression of TR was first characterized. There are two types of TRs in mammals, TRα and TRβ, encoded by two different genes. We found that TRα mRNA is expressed in cultured embryonic VM NSCs and VM NSC-differentiated cells in vitro. We also observed that TRα mRNA is expressed in E13.5 mouse midbrain tissues. However, TRβ mRNA was not found both in vivo and in vitro (Fig. 8A and Supplementary Fig. S7A). Alternative splicing of TRα mRNA gives rise to TRα1 and TRα2, but only TRα1 can bind T3 and is considered to be a true TR. We detected the protein expression of TRα1 in embryonic VM NSCs and found that TRα1 is coexpressed with Nestin, both in cultured VM NSCs and in midbrain tissues (Fig. 8B).
FIG. 8.
Thyroid hormone receptor alpha 1 (TRα1) participates in T3 regulation of Otx2 expression and DA neuron differentiation in VM NSCs. (A) TRα mRNA is expressed in VM NSCs and the embryonic midbrain, while TRβ is not. M, marker. Lane 1, fresh VM NSC population. Lane 2, VM NSCs differentiated with T3 for 3 days in vitro. Lane 3, E13.5 midbrain tissues. (B) TRα1 protein is expressed in Nestin+ cells in the E13.5 midbrain and in cultured VM NSCs. Scale bar: 50 μm. (C) TRα1 is coexpressed with Otx2 in VM NSCs. Scale bar: 50 μm. (D) TRα1 is expressed in T3-induced VM NSC-differentiated TH+ cells. Scale bar: 50 μm. (E) VM NSCs were treated with TRα1-siRNA for 3 days to silence TRa1 expression. The T3-induced DA neuron differentiation was then detected. Data from four independent experiments. Scale bar: 50 μm. Student's t-test, *P<0.05. (F, G) TRα1 silencing inhibited the effects of T3 on Otx2 and TH expression. n=4, one-way ANOVA followed by Fisher's post hoc test, *P<0.05, **P<0.01. For all experiments, data are presented as the mean±SEM. Color images available online at www.liebertpub.com/scd
Furthermore, we found that TRα1 is coexpressed with Otx2 in cultured VM NSCs (Fig. 8C). We also found that the T3-induced TH+ cells are immunoreactive to TRα1 (Fig. 8D). To further detect the role of TRα1 in mediating the effects of T3 on VM NSCs, we eliminated the TRα1 protein expression using TRα1-specific siRNAs (Supplementary Fig. S7B). We found that TRα1 silencing significantly reduced the effects of T3 on DA differentiation (Fig. 8E). In addition, the effect of T3 on the mRNA expression of TH and Otx2 expression was also decreased robustly after TRα1 silencing (Fig. 8F, G). These results suggested that TRα1 participates in the effects of T3-induced Otx2 upregulation and DA neuron differentiation.
Discussion
The thyroid hormone is critical for brain development. However, the role and mechanisms of the thyroid hormone in DA neuron development are still not well established. In this study, we found that the thyroid hormone plays a vital role in DA neuron differentiated from embryonic VM NSCs and that Otx2 signaling is essential for thyroid hormone-dependent DA neuron differentiation. The thyroid hormone increases Otx2 expression during DA neuron differentiation and upregulates Nurr1 and Ngn2 expression through Otx2. These effects together promote DA neuron differentiated from embryonic VM NSCs (Fig. 9). To our knowledge, this is the first report on the role and mechanism of the thyroid hormone in DA neuron differentiation from embryonic VM NSCs.
FIG. 9.
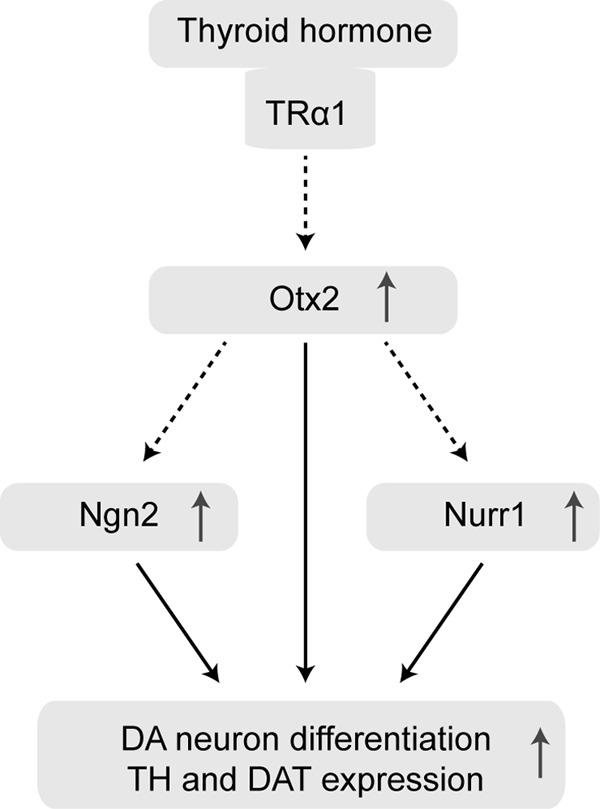
Model for the mechanism of thyroid hormone-induced DA neuron differentiation. During embryonic VM NSC differentiation, thyroid hormone-TRα1 upregulates Otx2 expression and enhances the expression of Ngn2 and Nurr1 through Otx2. The upregulation of these three DA determinants finally leads to DA neuron differentiation and enhanced the expression of TH and DAT.
The thyroid hormone has diverse actions on brain development, exhibiting region-specific effects, and acting on specific cellular targets [45]. Previous studies have identified the role and mechanisms of the thyroid hormone in the development of the cerebellum and cortex [7,46]. However, much is still unknown regarding the effects of the thyroid hormone in midbrain. Some investigations indicate that the thyroid hormone influences TH activity and SNc DA neuron number [13,14]. Our results provide comprehensive evidence that the thyroid hormone is critical for DA neuron differentiation from embryonic VM NSCs.
In this study, we used PTU to generate an animal model of hypothyroidism during pregnancy. This model induces thyroid hormone deficiency only during the embryonic phase, a critical period of NSC fate determination, as measured by FT3 and FT4 levels. We found a decrease in the DA neuron-specific proteins, TH and DAT, in the offspring of PTU-treated mice. In addition, we observed a loss of DA neurons and a decrease in DA and DOPAC levels in the midbrain.
We also demonstrated an impairment of the DA neuron-related motor ability in the offspring of PTU-treated mice. Some of the motor abilities recovered at 2 months of age, likely due to undetected compensatory mechanisms after thyroid hormone levels returned to normal in the offspring. Nevertheless, the impairment of neurobehavior indicates injury of the DA system. Together, the above findings reveal that the thyroid hormone is essential for the development of the DA neurons.
Loss of midbrain DA neurons was also observed in a genetic generated hypothyroidism rat model [14]. However, little focus has been placed on the influence of the thyroid hormone on the generation of DA neurons from VM NSCs during the embryonic phase. It is reported that hypothyroidism during brain development decreases the volume of the brain. The volume of neocortex and hippocampus is also decreased in thyroid hormone-deficient mice [7,47,48]. These results suggested that the thyroid hormone has generally positive effects on brain development.
We conclude that the thyroid hormone benefits DA neuron development by promoting embryonic VM NSC differentiation into DA neurons. This conclusion is based on our in vitro findings: first, increased TH+ neuron was observed when the thyroid hormone was applied to undifferentiated VM NSCs; second, the percentage of TH+ neurons relative to the total differentiated neuron population was increased after thyroid hormone treatment, suggesting that the effect was not due to an increase in general neuronal differentiation; third, induction of DA and DOPAC was found in differentiated cells. These findings strongly indicate that the thyroid hormone leads to the differentiation of DA neurons from VM NSCs.
Previous studies on the effects of the thyroid hormone were mostly conducted on postmitotic neurons after cell differentiation [4,49]. The effect of T3 on the morphogenesis of DA neurons has been studied on a mouse mesencephalic neuron model. T3 was found to increase the perikarya, without affecting the neurite density of mesencephalic DA neurons [12]. Parts of these results are in contrast with our research. The differences might be due to the higher plasticity of embryonic VM NSCs than mesencephalic neurons, indicating that the thyroid hormone plays an important role during the early stage of DA system development.
The effects of the thyroid hormone on embryonic NSCs from the E16 hippocampus were also investigated previously [50]. It was found that T3 promotes the differentiation of glial cells, including oligodendrocytes and astrocytes, but markedly decreased the neuronal population. The differences might be due to the fact that NSCs derived from the late stage of the embryonic phase are much more likely to differentiate into glial cells, as demonstrated before [51–53].
In addition, NSCs derived from different brain regions may have different characteristics [54,55]. As demonstrated by our group and others, the thyroid hormone is known to be a critical factor in promoting the neural differentiation of NSCs, both in the early stage of embryonic cortex and in the adult hippocampus [7,9,11]. These results suggested that the thyroid hormone probably acts in a different manner during different developmental stages.
In addition, the effect of the thyroid hormone in different brain regions might be inequable during brain development. Thus, revealing the effects of the thyroid hormone during embryonic VM NSC development and DA neuron differentiation is critical for understanding the role of the thyroid hormone on brain development. Our findings are important for understanding the role of the thyroid hormone on DA system development and the mechanisms underlying DA neuron differentiation from embryonic VM NSCs.
The development of DA neurons depends on a number of intrinsic transcription factors and several identified extrinsic signals. Our results suggest that the thyroid hormone is an efficient extrinsic signal that governs DA neuron differentiation. The thyroid hormone is necessary for embryonic DA neurogenesis in vivo and can act efficiently to generate DA neurons from embryonic VM NSCs in vitro. How the extrinsic signals and intrinsic transcription factors interact with each other is important not only for understanding the regulatory network of DA development but also for optimal stem cell engineering for cell replacement therapy in neurological diseases.
In our experiments, we explored the relationship between the thyroid hormone and transcription factors related to DA neuron development. We found that Otx2, which is activated by T3, plays a critical role in T3-induced DA neuron differentiation from embryonic VM NSCs. DA determinants, Ngn2 and Nurr1, were also activated by T3 treatment, which are Otx2 dependent, as shown by our results and demonstrated previously [41,42].
The transcript factor Otx2 is critical for the proliferation and differentiation of embryonic VM NSCs [41]. It also plays a crucial role in DA neuron subtype specification, regionalization, and differentiation [56–58]. The orphan nuclear receptor, Nurr1, and the proneural gene, Ngn2, are highly expressed in the developing VM, and mice lacking these two genes exhibit a specific loss of midbrain DA neurons [59–61]. Otx2, Nng2, and Nurr1 are all essential transcripts for the induction of DA neurons. Our results confirmed the critical role of these three DA determinants, Otx2, Nurr1, and Ngn2, in DA neuron generation.
In addition, we revealed that the thyroid hormone promotes DA neuron differentiation by upregulating the activity of Otx2 signaling. Our data provide evidence for the role of the thyroid hormone in the regulation of the intrinsic transcription factors that determine midbrain DA neuron differentiation. It is reported that Otx2 defines subpopulations of DA neurons already at the neural progenitor cell stage [62]. Otx2 and Ngn2 are both transcripts that play functions in the early stage of DA progenitor proliferation and differentiation, while Nurr1 is a late determinant of DA neurons [41,63]. Thus, we speculate that the thyroid hormone affects multiple stages of DA neuron development. Further investigation is required to elucidate the mechanistic details.
Previous studies revealed that TRs appear in the mammalian fetal brain early before embryonic neurogenesis and are critical for thyroid hormone functions in the brain [4,64]. The appearance of TRα1 in DA neurons has been reported before [65]. We found that TRα1 is expressed in embryonic VM NSCs, both in vivo and in vitro. We also found that TRα1 is expressed in T3-induced TH+ cells. However, the functions of TRα1 during DA neuron development are unclear. In this study, we revealed that TRα1 participates in the effects of T3-induced DA neuron differentiation, a novel physiological relevance to TR in DA system development.
Coexpression of TRβ and Otx2 is observed in retina progenitors [66]. However, there is no evidence to suggest that TRα1 or TRβ could direct regulate Otx2 transcription. In addition, we did not detect the binding site for TRα1 in the Otx2 gene. Thus, from our experiments, we could not get a conclusion that the thyroid hormone directly regulates Otx2 expression through TRα1. In addition, Nurr1 is a member of the steroid/TR family, suggesting that interactions between Nurr1 and the thyroid hormone exist. Thus, further studies are still needed to explore the interactions among the thyroid hormone, TRs, and DA neuron determinants. Both the genomic and nongenomic effects of the thyroid hormone on DA development need to be further identified.
Understanding the molecular and physiological mechanisms underlying midbrain DA neuron differentiation in cultured NSCs in vitro and in endogenous NSCs in vivo is critical for the development of therapies for DA neuron-related disorders. Consequently, our studies reveal the critical role of thyroid hormone-Otx2 signaling in DA neuron differentiation from embryonic VM NSCs. These results increase our understanding of the molecular mechanisms of thyroid hormone regulation during midbrain DA neuron development and probably provide a new strategy for generating DA neurons from NSCs.
Supplementary Material
Acknowledgments
The authors thank Jia Lou, Wei Sun, and Min Li for excellent technical assistance. This work was supported by the National Natural Science Foundation of China (grant no. 31370832) and the National Program on Key Basic Research Project (2011CB50370).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bromberg-Martin ES, Matsumoto M. and Hikosaka O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smidt MP. and Burbach JP. (2007). How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci 8:21–32 [DOI] [PubMed] [Google Scholar]
- 4.Horn S. and Heuer H. (2010). Thyroid hormone action during brain development: more questions than answers. Mol Cell Endocrinol 315:19–26 [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O'Heir CE, Mitchell ML, Hermos RJ, et al. (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:549–555 [DOI] [PubMed] [Google Scholar]
- 6.Bath SC, Steer CD, Golding J, Emmett P. and Rayman MP. (2013). Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 382:331–337 [DOI] [PubMed] [Google Scholar]
- 7.Mohan V, Sinha RA, Pathak A, Rastogi L, Kumar P, Pal A. and Godbole MM. (2012). Maternal thyroid hormone deficiency affects the fetal neocorticogenesis by reducing the proliferating pool, rate of neurogenesis and indirect neurogenesis. Exp Neurol 237:477–488 [DOI] [PubMed] [Google Scholar]
- 8.Stergiopoulos A. and Politis PK. (2013). The role of nuclear receptors in controlling the fine balance between proliferation and differentiation of neural stem cells. Arch Biochem Biophys 534:27–37 [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Zhou Z, Zhong M, Zhang Y, Li M, Zhang L, Qu M, Yang J, Wang Y. and Yu Z. (2012). Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRalpha1. Stem Cells Dev 21:2667–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordstrom K, Vennstrom B. and Vaidya VA. (2010). Unliganded thyroid hormone receptor alpha1 impairs adult hippocampal neurogenesis. FASEB J 24:4793–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Juarez A, Remaud S, Hassani Z, Jolivet P, Pierre Simons J, Sontag T, Yoshikawa K, Price J, Morvan-Dubois G. and Demeneix BA. (2012). Thyroid hormone signaling acts as a neurogenic switch by repressing Sox2 in the adult neural stem cell niche. Cell Stem Cell 10:531–543 [DOI] [PubMed] [Google Scholar]
- 12.Puymirat J, Faivre-Bauman A, Barret A, Loudes C. and Tixier-Vidal A. (1985). Does triiodothyronine influence the morphogenesis of fetal mouse mesencephalic dopaminergic neurons cultured in chemically defined medium? Brain Res 355:315–317 [DOI] [PubMed] [Google Scholar]
- 13.Chaube R. and Joy KP. (2003). Thyroid hormone modulation of brain in vivo tyrosine hydroxylase activity and kinetics in the female catfish Heteropneustes fossilis. J Endocrinol 179:205–215 [DOI] [PubMed] [Google Scholar]
- 14.Kincaid AE. (2001). Spontaneous circling behavior and dopamine neuron loss in a genetically hypothyroid mouse. Neuroscience 105:891–898 [DOI] [PubMed] [Google Scholar]
- 15.Hegarty SV, Sullivan AM. and O'Keeffe GW. (2013). Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol 379:123–138 [DOI] [PubMed] [Google Scholar]
- 16.Ang SL. (2006). Transcriptional control of midbrain dopaminergic neuron development. Development 133:3499–3506 [DOI] [PubMed] [Google Scholar]
- 17.Ferri AL, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA. and Ang SL. (2007). Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134:2761–2769 [DOI] [PubMed] [Google Scholar]
- 18.Swistowska AM, da Cruz AB, Han Y, Swistowski A, Liu Y, Shin S, Zhan M, Rao MS. and Zeng X. (2010). Stage-specific role for shh in dopaminergic differentiation of human embryonic stem cells induced by stromal cells. Stem Cells Dev 19:71–82 [DOI] [PubMed] [Google Scholar]
- 19.Emerson MM, Surzenko N, Goetz JJ, Trimarchi J. and Cepko CL. (2013). Otx2 and Onecut1 promote the fates of cone photoreceptors and horizontal cells and repress rod photoreceptors. Dev Cell 26:59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanamoto N, Tagami T, Ueda-Sakane Y, Sone M, Miura M, Yasoda A, Tamura N, Arai H. and Nakao K. (2012). Forkhead box A1 (FOXA1) and A2 (FOXA2) oppositely regulate human type 1 iodothyronine deiodinase gene in liver. Endocrinology 153:492–500 [DOI] [PubMed] [Google Scholar]
- 21.Navarro D, Alvarado M, Morte B, Berbel D, Sesma J, Pacheco P, Morreale de Escobar G, Bernal J. and Berbel P. (2014). Late maternal hypothyroidism alters the expression of camk4 in neocortical subplate neurons: a comparison with nurr1 labeling. Cereb Cortex 24:2694–2706 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez M, Pirondi S, Manservigi M, Giardino L. and Calza L. (2004). Thyroid hormone participates in the regulation of neural stem cells and oligodendrocyte precursor cells in the central nervous system of adult rat. Eur J Neurosci 20:2059–2070 [DOI] [PubMed] [Google Scholar]
- 23.Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA. and Vaidya VA. (2005). Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci 29:414–426 [DOI] [PubMed] [Google Scholar]
- 24.Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernandez-Lamo I, Garcia-Verdugo JM, Bernal J. and Guadano-Ferraz A. (2006). Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry 11:361–371 [DOI] [PubMed] [Google Scholar]
- 25.He MD, Xu SC, Zhang X, Wang Y, Xiong JC, Lu YH, Zhang L, Yu ZP. and Zhou Z. (2013). Disturbance of aerobic metabolism accompanies neurobehavioral changes induced by nickel in mice. Neurotoxicology 38:9–16 [DOI] [PubMed] [Google Scholar]
- 26.Luchtman DW, Meng Q. and Song C. (2012). Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson's disease. Behav Brain Res 226:386–396 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi E, Niimi K. and Itakura C. (2009). Motor coordination impairment in aged heterozygous rolling Nagoya, Cav2.1 mutant mice. Brain Res 1279:50–57 [DOI] [PubMed] [Google Scholar]
- 28.Campbell K, Olsson M. and Bjorklund A. (1995). Regional incorporation and site-specific differentiation of striatal precursors transplanted to the embryonic forebrain ventricle. Neuron 15:1259–1273 [DOI] [PubMed] [Google Scholar]
- 29.Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C, Kokaia M, Lindvall O. and Arenas E. (2008). Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest 118:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soldati C, Cacci E, Biagioni S, Carucci N, Lupo G, Perrone-Capano C, Saggio I. and Augusti-Tocco G. (2012). Restriction of neural precursor ability to respond to Nurr1 by early regional specification. PLoS One 7:e51798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaing ZZ. and Roberts JL. (2009). Embryonic mescencephalon derived neurospheres contain progenitors as well as differentiated neurons and glia. Restor Neurol Neurosci 27:611–620 [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Zhou Z, Zhong M, Li M, Yang X, Zhang Y, Wang Y, Wei A, Qu M, et al. (2011). Excess thyroid hormone inhibits embryonic neural stem/progenitor cells proliferation and maintenance through STAT3 signalling pathway. Neurotox Res 20:15–25 [DOI] [PubMed] [Google Scholar]
- 33.Del Pino J, Martinez MA, Castellano V, Ramos E, Martinez-Larranaga MR. and Anadon A. (2013). Effects of exposure to amitraz on noradrenaline, serotonin and dopamine levels in brain regions of 30 and 60 days old male rats. Toxicology 308:88–95 [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ. and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 35.Andersson E, Jensen JB, Parmar M, Guillemot F. and Bjorklund A. (2006). Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development 133:507–516 [DOI] [PubMed] [Google Scholar]
- 36.Tang M, Miyamoto Y. and Huang EJ. (2009). Multiple roles of beta-catenin in controlling the neurogenic niche for midbrain dopamine neurons. Development 136:2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz C. and Hof PR. (2005). Design-based stereology in neuroscience. Neuroscience 130:813–831 [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi S, Aoki N, Kitajima T, Iikubo E, Katagiri S, Matsushima T. and Homma KJ. (2012). Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat Commun 3:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bastian TW, Prohaska JR, Georgieff MK. and Anderson GW. (2010). Perinatal iron and copper deficiencies alter neonatal rat circulating and brain thyroid hormone concentrations. Endocrinology 151:4055–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B. and Morreale de Escobar G. (2002). Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab 87:1768–1777 [DOI] [PubMed] [Google Scholar]
- 41.Omodei D, Acampora D, Mancuso P, Prakash N, Di Giovannantonio LG, Wurst W. and Simeone A. (2008). Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135:3459–3470 [DOI] [PubMed] [Google Scholar]
- 42.Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y, Park S, Li Y, Bolshakov VY, Lamonerie T. and Kim KS. (2011). ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci U S A 108:9703–9708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, Arenas E. and Ang SL. (2006). Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133:495–505 [DOI] [PubMed] [Google Scholar]
- 44.Saucedo-Cardenas O, Quintana-Hau JD, Le WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP. and Conneely OM. (1998). Nurr1 is essential for the induction of the dopaminergic phenotype and the survival of ventral mesencephalic late dopaminergic precursor neurons. Proc Natl Acad Sci U S A 95:4013–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oppenheimer JH. and Schwartz HL. (1997). Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18:462–475 [DOI] [PubMed] [Google Scholar]
- 46.Koibuchi N. (2013). The role of thyroid hormone on functional organization in the cerebellum. Cerebellum 12:304–306 [DOI] [PubMed] [Google Scholar]
- 47.Powell MH, Nguyen HV, Gilbert M, Parekh M, Colon-Perez LM, Mareci TH. and Montie E. (2012). Magnetic resonance imaging and volumetric analysis: novel tools to study the effects of thyroid hormone disruption on white matter development. Neurotoxicology 33:1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behnam-Rassoli M, Herbert LC, Howard V, Pharoah PO. and Stanisstreet M. (1991). Effect of propylthiouracil treatment during prenatal and early postnatal development on the neocortex of rat pups. Neuroendocrinology 53:321–327 [DOI] [PubMed] [Google Scholar]
- 49.Dezonne RS, Stipursky J, Araujo AP, Nones J, Pavao MS, Porcionatto M. and Gomes FC. (2013). Thyroid hormone treated astrocytes induce maturation of cerebral cortical neurons through modulation of proteoglycan levels. Front Cell Neurosci 7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM. and McKay RD. (1996). Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev 10:3129–3140 [DOI] [PubMed] [Google Scholar]
- 51.Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA. and Temple S. (2000). Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28:69–80 [DOI] [PubMed] [Google Scholar]
- 52.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD. and Greenberg ME. (1997). Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278:477–483 [DOI] [PubMed] [Google Scholar]
- 53.Burrows RC, Wancio D, Levitt P. and Lillien L. (1997). Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron 19:251–267 [DOI] [PubMed] [Google Scholar]
- 54.Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C. and Muller U. (2012). Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 337:746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thor S. (2013). Neuroscience: stem cells in multiple time zones. Nature 498:441–443 [DOI] [PubMed] [Google Scholar]
- 56.Puelles E, Annino A, Tuorto F, Usiello A, Acampora D, Czerny T, Brodski C, Ang SL, Wurst W. and Simeone A. (2004). Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development 131:2037–2048 [DOI] [PubMed] [Google Scholar]
- 57.Simeone A. (2005). Genetic control of dopaminergic neuron differentiation. Trends Neurosci 28:62–65; discussion 65–66 [DOI] [PubMed] [Google Scholar]
- 58.Vernay B, Koch M, Vaccarino F, Briscoe J, Simeone A, Kageyama R. and Ang SL. (2005). Otx2 regulates subtype specification and neurogenesis in the midbrain. J Neurosci 25:4856–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park CH, Kang JS, Yoon EH, Shim JW, Suh-Kim H. and Lee SH. (2008). Proneural bHLH neurogenin 2 differentially regulates Nurr1-induced dopamine neuron differentiation in rat and mouse neural precursor cells in vitro. FEBS Lett 582:537–542 [DOI] [PubMed] [Google Scholar]
- 60.Thompson LH, Andersson E, Jensen JB, Barraud P, Guillemot F, Parmar M. and Bjorklund A. (2006). Neurogenin2 identifies a transplantable dopamine neuron precursor in the developing ventral mesencephalon. Exp Neurol 198:183–198 [DOI] [PubMed] [Google Scholar]
- 61.Zetterstrom RH, Solomin L, Jansson L, Hoffer BJ, Olson L. and Perlmann T. (1997). Dopamine neuron agenesis in Nurr1-deficient mice. Science 276:248–250 [DOI] [PubMed] [Google Scholar]
- 62.Panman L, Papathanou M, Laguna A, Oosterveen T, Volakakis N, Acampora D, Kurtsdotter I, Yoshitake T, Kehr J, et al. (2014). Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep 8:1018–1025 [DOI] [PubMed] [Google Scholar]
- 63.D'Ascenzo M, Piacentini R, Casalbore P, Budoni M, Pallini R, Azzena GB. and Grassi C. (2006). Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci 23:935–944 [DOI] [PubMed] [Google Scholar]
- 64.Schroeder AC. and Privalsky ML. (2014). Thyroid Hormones, T3 and T4, in the Brain. Front Endocrinol (Lausanne) 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jansen HT, Lubbers LS, Macchia E, DeGroot LJ. and Lehman MN. (1997). Thyroid hormone receptor (alpha) distribution in hamster and sheep brain: colocalization in gonadotropin-releasing hormone and other identified neurons. Endocrinology 138:5039–5047 [DOI] [PubMed] [Google Scholar]
- 66.Trimarchi JM, Harpavat S, Billings NA. and Cepko CL. (2008). Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev Biol 8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.