Abstract
Objectives
To test, in a prostate-cancer population-based database, the validity of the finding that in a single-institution series, palliative transurethral resection of prostate (TURP) is associated with an increased risk of progression.
Patients and methods
Using the Surveillance and End Epidemiology Registry, we identified men who had a TURP subsequent to their diagnosis of prostate cancer, from 1998 or 1999. The outcome of interest was disease progression, as defined by the initiation of androgen-deprivation therapy or procedures indicating progressive urinary obstruction. Multivariable logistic regression analysis was used to assess the adjusted odds of signal events related to disease progression adjusting for the concurrent effect of the covariates.
Results
There were 29 361 men with prostate cancer and 2742 (9.3%) had a TURP after the diagnosis. These men had a mean age of 75 years and were unlikely to undergo definitive primary treatment. Men receiving TURP were more likely to undergo orchidectomy than men who did not have a TURP (odds ratio 1.64; 95% confidence interval 1.03–2.60) even after adjusting for differences in cancer-directed treatment, tumour stage and grade, prostate-specific antigen level, race, and age at diagnosis. These men were also more likely to have malignant urinary obstruction (ureteric and bladder outlet) than were men who did not have TURP.
Conclusion
The requirement for TURP is an adverse prognostic marker even when this is adjusted for classical tumour characteristics. Although the exact reasons for this finding are unclear, consideration should be given to adjuvant treatment in patients undergoing TURP.
Keywords: prostatic neoplasms, transurethral resection of prostate, prognosis, SEER program
Introduction
In patients with metastatic prostate cancer, a palliative TURP can be safe, with improvement in urinary symptoms [1]. However, the use of TURP in patients with clinically localized disease is controversial, primarily because of evidence that this procedure is related to progression and dissemination of prostate cancer [2–5]. By contrast, other studies showed no significant effect on survival [6–9]. Some authors proposed that patients with stage T1–2 disease are not at risk of disease progression from TURP, and that this procedure negatively affects only patients with more advanced tumours [10,11].
There are plausible reasons why TURP could be directly responsible for worsening the prognosis of patients with prostate cancer by leading to a higher incidence of disseminated cancer. For example, TURP can facilitate intravascular dissemination of neoplastic cells due to the simultaneous transection of the cancer as well as venous and lymphatic channels in a setting of fluid under pressure. The RT-PCR technique has been used to search for cells expressing PSA or prostate-specific membrane antigen in extraprostatic sites, including the peripheral blood, lymph nodes, and bone marrow [12–14]. In this regard, several authors have reported the perioperative dissemination of prostate cancer cells after TURP by a RT-PCR assay for PSA mRNA [15]. However, recent understanding in the molecular biology of cancer metastasis indicates that the process of cancer dissemination is complex and is probably not simply explained by forced mechanical spread of tumour cells [16].
As these concerns are based on small, retrospective studies, the potential exists for undetected bias to mislead clinicians. We intended that by analysing a much larger cohort, we could determine first if TURP is indeed associated with disease progression, and second, what if any factors are associated with the progression In the present study we aimed to answer these important questions by using Surveillance, Epidemiology and End Results (SEER)-Medicare programme data to evaluate the association of TURP on the prognosis of patients with clinically localized prostate cancer, when this is adjusted for other concomitant patient and tumour variables.
Patients and methods
We used the SEER-Medicare database, which combines information from the National Cancer Institute’s SEER programme with Medicare claims and enrolment records [17]. The study population includes all men in the SEER registry diagnosed with incident prostate cancer in either 1998 or 1999, who were aged ≥66 years at diagnosis and eligible for Medicare at the time of their diagnosis. Information drawn from the SEER data was used to determine patient age at diagnosis, race, tumour stage, tumour grade, and whether an elevated PSA level was reported at diagnosis. Tumour stage is reported by SEER using the American Joint Committee on Cancer criteria, with stage 1 defined as impalpable tumours, stage 2 as tumours that are palpable but confined to the prostate with no clinical evidence of metastatic disease, stage 3 as tumours that are intracapsular extending into the prostatic apex, and stage 4 as tumours with extension beyond the prostate. Stage 1 and 2 by convention are considered localized prostate cancer, while stage 3 and 4 are no longer organ-confined. Histological grades are defined by SEER as grade 1 for well differentiated cells, grade 2 for moderately differentiated cells, grade 3 for poorly differentiated cells, and grade 4 as undifferentiated. Histological grade 1 corresponds to Gleason scores of 2–4, grade 2 to 5–7, and grade 3 to 8–10 [17].
Physician part B claims records were searched to identify patients in the study population with the Current Procedural Terminology (CPT) code 52601, (TURP) at any time from the date of diagnosis to December 2003, or to the date of death for those men who died before December 2003. Dates of death were obtained from the linked Medicare programme enrolment data. The number of months between the date of prostate cancer diagnosis and the date of TURP was calculated for each patient, and whether TURP occurred before receipt of cancer-directed therapies (CDTs).
We defined progression as the need for androgen-deprivation therapy (ADT), or urinary obstruction requiring a procedure. Bilateral orchidectomy and treatment with LHRH agonists occurring ≥6 months after prostate cancer diagnosis both represented the need for ADT, and we defined bladder obstruction as surgical intervention in the form of suprapubic tube, ureteric stent insertion or nephrostomy tube placement. Medicare records were queried for CPT codes relating to these endpoints during the follow-up [18,19]. Occurrences of orchidectomy and initiation of LHRH-agonist therapy were empirically limited to events occurring ≥6 months after diagnosis, to avoid including cases where these therapies were likely to represent treatment after the incident diagnosis rather than treatment for disease recurrence. Death occurring any time after diagnosis was also assessed. The interval between the date of prostate cancer diagnosis and the date of each event was calculated for each patient. Events that occurred before receipt of TURP were excluded as outcomes.
Multivariable logistic regression analysis was used to assess the adjusted odds of each event, except death, that occurred among men who had ad TURP within 3 months of diagnosis compared to men with no TURP during this period. Multivariable Cox proportional hazards regression was used to assess the adjusted hazard of death associated with receipt of TURP within 3 months, using the same covariates included in the logistic regression models. The relationship was assessed only for men receiving TURP within 3 months of diagnosis because TURP occurring after this period could itself be an indicator of disease progression. The multivariable odds of each of the six disease recurrence-related events occurring for men with TURP within 3 months of diagnosis were adjusted in each case for the concurrent effect of differences among patients in the types of CDT received, tumour stage and grade, PSA level, race, and age at diagnosis. Identical adjustments were included in the Cox proportional hazards model to assess the relative hazard of death associated with TURP within 3 months of diagnosis.
The overall statistical performance of each regression model was assessed using the C statistic, which measures the model’s ability to discriminate between patients with and without the event predicted by the model [20,21]. The C statistic is equivalent to the area under the receiver operating characteristic curve; it has a maximum value of 1.0 when all of the model’s predicted risks for the outcome among patients who have the outcome are greater than the model-predicted risks for the outcome among patients who did not have the outcome. The C statistic = 0.5 when the model’s discrimination between patients who did or did not experience the outcome is equal to random chance.
Results
The study population included 29 361 men with incident prostate cancer who fulfilled the above criteria. Table 1 shows the number and relative frequency of each characteristic measured for the total study population and for the subpopulation of men with TURP within 3 months of diagnosis. An elevated PSA level at diagnosis was recorded for 62% of the total, moderately differentiated tumour for 63%, and localized disease for 45% of the men in the total population. Among men with TURP within 3 months, 36% had an elevated PSA level at diagnosis, 51% had moderately differentiated tumour, and 54% had localized disease. Although only 15% of the total population had extraprostatic disease by DRE, stage information was missing for 39%. African-American men represented 11% of the total population and of men with TURP within 3 months. The mean age at diagnosis was 75 years in the total study population and 78 years for men with TURP within 3 months.
Table 1.
The characteristics of the study population
| n (%) variable* | All | TURP at < 3 months |
|---|---|---|
| Total | 29361 (100) | 2143 (100) |
| TURP | 2742 (9.34) | 2143 (100) |
| TURP before CDT | 248 (0.84) | 220 (10.27) |
| TURP after CDT | 171 (0.58) | 36 (1.68) |
| TURP with no CDT | 2323 (7.91) | 1887 (88.05) |
| Patients with CDT by type | ||
| RP | 3641 (12.40) | 80 (3.73) |
| EBRT | 1964 (6.69) | 132 (6.16) |
| Brachytherapy | 3135 (10.68) | 65 (3.03) |
| Cancer recurrence-related outcomes | ||
| Orchidectomy | ||
| ≥6 months after diagnosis | 188 (0.64) | 23 (1.07) |
| < 6 months after diagnosis | 480 (1.63) | 128 (5.97) |
| JJ stent any time after diagnosis or TURP | 229 (0.78) | 31 (1.45) |
| Suprapubic cystostomy after diagnosis or TURP | 218 (0.74) | 31 (1.45) |
| PCN any time after diagnosis or TURP | 129 (0.44) | 25 (1.17) |
| LHRH initiated | ||
| ≥6 months after diagnosis | 1793 (6.11) | 161 (7.51) |
| < 6 months after diagnosis | 7357 (25.06) | 520 (24.27) |
| Tumour grade | ||
| well differentiated | 2040 (6.95) | 436 (20.35) |
| moderately differentiated | 18393 (62.64) | 1091 (50.91) |
| poorly differentiated | 6329 (21.56) | 524 (24.45) |
| undifferentiated | 126 (0.43) | 18 (0.84) |
| unknown | 2471 (8.42) | 73 (3.41) |
| Tumour stage | ||
| clinically unapparent impalpable | 10448 (35.58) | 1101 (51.38) |
| clinically apparent palpable | 3033 (10.33) | 73 (3.41) |
| but confined to prostate | ||
| intracapsular extending into | 1849 (6.30) | 39 (1.82) |
| prostatic apex | ||
| extension beyond prostate | 2490 (8.48) | 212 (9.89) |
| unknown | 11541 (39.31) | 718 (33.50) |
| PSA listed as elevated at diagnosis | 18194 (61.97) | 782 (36.49) |
| in SEER data | ||
| African-American | 3168 (10.79) | 236 (11.01) |
| Mean age at diagnosis, years | 75.08 | 77.83 |
| Mean available follow-up, years | 3.50 | 3.26 |
| Died during available follow-up | 8114 (27.64) | 923 (43.07) |
Unless stated otherwise.
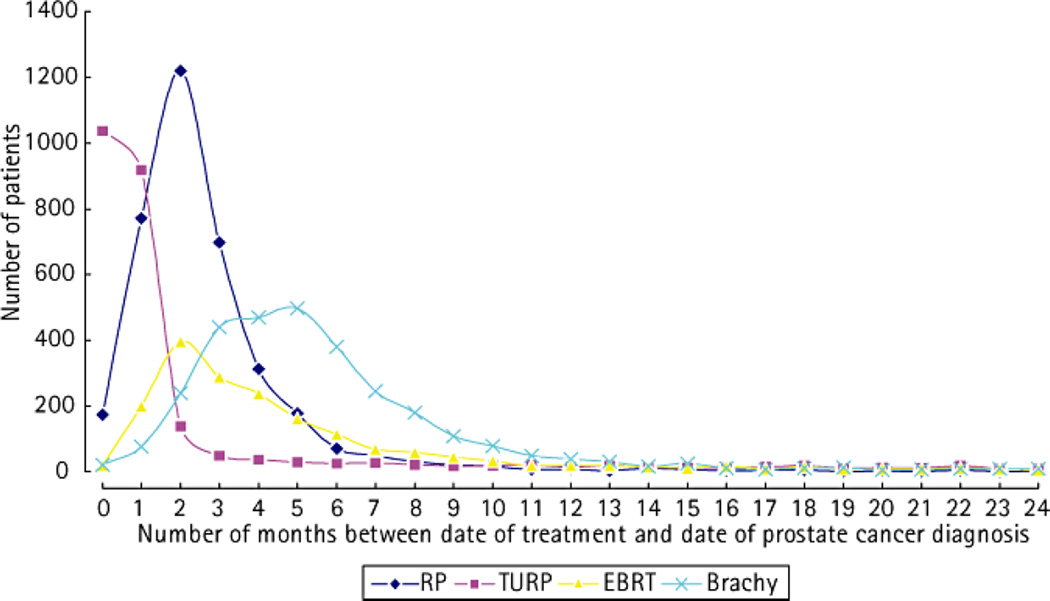
TURP was carried out in 2742 men (9.3%) at some point between the date of their prostate cancer diagnosis and the end of the available follow-up period. Of these, 419 were outside of the 3-month period used to exclude cases indicative of early disease progression. A mean of 3.4 years of follow-up after TURP was available. Importantly, most patients receiving TURP within 3 months of diagnosis did not receive definitive CDT. Radical prostatectomy (RP) was performed in 3.7%, external beam radiotherapy (EBRT) in 6%, and brachytherapy in 3% of men with TURP within 3 months of diagnosis. Figure 1 shows the frequency distributions of the time elapsed between the date of prostate cancer diagnosis and dates of CDT in the total population. Notably, brachytherapy often occurred at 4–5 months after the date of diagnosis, while other types CDT were commonly applied within 2–3 months of diagnosis. Only 10% of the total population received treatment at ≥;9 months after their diagnosis with RP, EBRT or brachytherapy. Figure 1 also shows the frequency of the time elapsed between the date of prostate cancer diagnosis and dates of TURP. TURP was performed within 3 months of prostate cancer diagnosis for 78% of the men who received TURP.
Fig. 1. The relationship of palliative transurethral resection of the prostate with disease progression in patients with prostate cancer.
The frequency distribution of the various potentially curative procedures and for TURP, showing the number of patients in the study population who had RP, TURP, EBRT, or brachytherapy (Brachy) by the time (months) elapsed between the date of their prostate cancer diagnosis and the date of the procedure.
Table 1 also lists the frequency of five cancer progression-related outcomes and deaths from any cause among patients in the total population and the subpopulation with TURP within 3 months of diagnosis. There were higher frequencies for each outcome among men with TURP. Table 2 shows the adjusted odds of ADT, receipt of a JJ stent, percutaneous nephrostomy (PCN), and suprapubic cystostomy associated with TURP within 3 months. Men receiving TURP were significantly more likely to have an orchidectomy beginning ≥6 months after TURP (odds ratio 1.64, 95% CI 1.03–2.60) than men who did not have TURP, after adjusting for differences in CDT (RP, EBRT, brachytherapy), tumour stage and grade, PSA level (elevated or not at diagnosis), African-American race, and age at diagnosis. The difference in the odds of LHRH therapy initiation at ≥6 months after diagnosis (odds ratio 1.09, 95% CI 0.92–1.30) for men receiving TURP was not statistically significant. Men receiving TURP were significantly more likely to receive a JJ stent (odds ratio 1.76, 95% CI 1.17–2.64), PCN (2.46, 1.53–3.95), and suprapubic cystostomy (1.90, 1.26–2.87), than to men who did not have TURP. Table 3 presents the adjusted all-cause mortality hazard associated with TURP within 3 months. Men receiving TURP had a significantly higher mortality risk over the period of available follow-up (hazard ratio 1.26, 95% CI 1.17–1.35), after simultaneous adjustments for differences in types of CDT, tumour grade, stage, elevated PSA level, race, and age at diagnosis. The C statistic obtained for the multivariable regression models was 0.584–0.75.
Table 2.
Adjusted odds of various CDTs at ≥ 6 months after diagnosis
| Odds ratio (95% CI) |
|||||
|---|---|---|---|---|---|
| Variable | Orchidectomy | LHRH | JJ stent | PCN | Cystostomy |
| TURP within 3 months of diagnosis | 1.64 (1.03–2.60) | 1.09 (0.92–1.30) | 1.76 (1.17–2.64) | 2.46 (1.53–3.95) | 1.90 (1.26–2.87) |
| RP | 1.39 (0.87–2.23) | 1.05 (0.88–1.24) | 1.66 (1.11–2.50) | 1.09 (0.57–2.09) | 2.57 (1.66–3.97) |
| EBRT | 1.72 (1.05–2.80) | 1.48 (1.24–1.75) | 1.88 (1.25–2.84) | 1.50 (0.81–2.77) | 1.21 (0.74–1.96) |
| Brachytherapy | 0.67 (0.37–1.22) | 1.11 (0.95–1.30) | 1.31 (0.86–1.98) | 1.47 (0.82–2.62) | 2.95 (2.05–4.25) |
| Grade | |||||
| well differentiated (reference) | |||||
| moderately differentiated | 2.08 (0.88–4.91) | 0.96 (0.79–1.18) | 0.83 (0.47–1.48) | 0.54 (0.26–1.10) | 1.24 (0.64–2.40) |
| poorly differentiated | 3.57 (1.50–8.5) | 1.08 (0.87–1.34) | 1.29 (0.71–2.34) | 1.79 (0.89–3.60) | 1.87 (0.95–3.70) |
| undifferentiated | 6.69 (1.61–27.8) | 1.13 (0.58–2.23) | 2.13 (0.59–7.67) | 1.21 (0.15–9.71) | 6.87 (2.26–20.86) |
| unknown | 1.25 (0.45–3.47) | 0.66 (0.51–0.85) | 0.89 (0.44–1.81) | 1.39 (0.64–2.99) | 1.30 (0.60–2.83) |
| Stage | |||||
| clinically unapparent, impalpable (reference) | |||||
| clinically apparent, palpable, | 1.57 (0.97–2.52) | 0.85 (0.71–1.03) | 1.17 (0.75–1.84) | 1.11 (0.54–2.28) | 0.68 (0.40–1.16) |
| but confined to prostate intracapsular,) | 0.81 (0.39–1.68) | 1.17 (0.94–1.45) | 0.42 (0.19–0.94) | 1.31 (0.58–2.98 | 0.52 (0.25–1.07) |
| extending to prostatic apex extension beyond prostate | 1.67 (1.04–2.70) | 1.09 (0.90–1.32) | 1.75 (1.14–2.67) | 1.66 (0.93–2.96) | 0.80 (0.48–1.33) |
| unknown | 1.17 (0.80–1.72) | 1.04 (0.92–1.18) | 0.83 (0.59–1.19) | 1.13 (0.69–1.85) | 0.84 (0.60–1.18) |
| PSA listed as elevated at diagnosis | 1.16 (0.85–1.59) | 0.85 (0.77–0.94) | 0.65 (0.50–0.86) | 0.45 (0.31–0.65) | 0.85 (0.64–1.12) |
| African-American | 1.16 (0.74–1.82) | 1.10 (0.95–1.28) | 0.64 (0.38–1.07) | 2.11 (1.36–3.27) | 1.49 (1.01–2.20) |
| Age at diagnosis | 1.05 (1.02–1.08) | 1.03 (1.02–1.04) | 1.00 (0.98–1.03) | 1.02 (0.99–1.05) | 1.06 (1.04–1.09) |
| Multivariable regression | 0.665 | 0.636 | 0.680 | ||
| model, C statistic | |||||
Table 3.
Adjusted hazard of death
| Variable | Hazard ratio (95% CI) |
|---|---|
| TURP within 3 months of diagnosis | 1.26 (1.17–1.35) |
| RP | 0.40 (0.36–0.45) |
| EBRT | 1.10 (1.00–1.21) |
| Brachytherapy | 0.62 (0.56–0.68) |
| Grade | |
| well differentiated (reference) | |
| moderately differentiated | 1.10 (0.98–1.21) |
| poorly differentiated | 1.62 (1.46–1.80) |
| undifferentiated | 2.38 (1.84–3.07) |
| unknown | 3.04 (2.74–3.38) |
| Stage | |
| clinically unapparent, impalpable (reference) | |
| clinically apparent, palpable, but confined to prostate | 1.01 (0.93–1.11) |
| intracapsular, extending into prostatic apex | 1.08 (0.95–1.21) |
| extension beyond prostate | 2.85 (2.65–3.05) |
| unknown | 1.15 (1.08–1.22) |
| PSA listed as elevated at diagnosis in SEER data | 0.77 (0.73–0.80) |
| African-American | 1.27 (1.19–1.36) |
| Age at diagnosis | 1.09 (1.08–1.10} |
| Multivariable regression model, C statistic | 0.756 |
Discussion
We intended that by analysing a much larger cohort we could determine first if TURP is indeed associated with disease progression and second, what if any factors are associated with the progression. Unfortunately, even by using the SEER-Medicare population-based database, we were still limited by the nature of claims data and a TURP population that was not homogenous. Our study does support the concern that TURP could be associated with disease progression, but we were unable to determine the strength of that association or identify factors associated with progression.
The mean age of the patients was 75 years, which is clearly older than most men currently presenting with prostate cancer. The widespread use of PSA testing was only just becoming common during the period for incident prostate cancer used here. Thus, it is not surprising that the mean age was higher and men were less likely to undergo definitive treatment. With the publication of the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, and the European Randomized Study of Screening for Prostate Cancer, the potential exists for less prostate cancer screening. If that occurs, we could again be treating patients diagnosed with palpable, locally advanced disease.
In addition to the theory that the fluid pressure used during TURP facilitates dissemination of neoplastic cells, epidemiological studies suggested that TURP might somehow promote vascular spread. Comparison of the outcomes of patients diagnosed by TURP with those diagnosed by needle biopsy suggest that the former approach had a higher incidence of distant metastasis, increased recurrence rate and lower survival, especially when TURP was carried out in T3 and T4 (Stage C) or moderately to poorly differentiated tumours [2–5]. By contrast, Fowler et al. [6] reported that TURP had no adverse effect on survival, while Kuban et al. [7] reported that only tumour grade and stage were reliable predictors of the potential to develop metastatic prostate cancer. They described that TURP, in itself, has no significant influence on the progression of disease, survival and bone metastasis [7,22]. They also stressed that local control correlated strongly with survival and incidence of metastasis. However, locally advanced disease, either at diagnosis or resulting for the lack of local control, can be accompanied by symptoms of BOO requiring TURP. Therefore, the use of TURP would probably be associated with larger tumours and thus more aggressive disease, and this information might not be captured fully by other tumour characteristics, such as stage or grade. Landmann et al. [23] examined this issue and reported an adverse effect of TURP on disease-free survival only in T3-T4 tumours of intermediate and poor differentiation.
Our study was limited by our inability to assess the incidence of distant metastasis with any reliability. We lack the clinical information to determine why a clinical course was chosen. For example, we do not have results of bone scans, CT or PSA doubling time. Although ADT might be initiated for metastatic disease, claims data limit our ability to determine if the instigating reason for the ADT was local progression or metastasis. The initiation of ADT is often subject to the personal philosophy of both the physician and patient. However, endpoints related to BOO are a more reliable method of determining local progression and less subject to personal philosophy. By using the large SEER-Medicare database, our analysis has the advantage of being able to adjust for many factors that might have confounded previous studies. In addition, because it is national, it is more representative of the overall effect of TURP in the general population of patients with prostate cancer. By adjusting for elevated PSA level, grade, stage and treatment, we are able to address the sole effect of TURP on outcomes. The national database also allows us to assess for relatively rare outcomes and in fact, the outcomes of interest occurred in a small fraction of the overall cohort. While these outcomes are statistically significant, we acknowledge that the clinical importance of the progression endpoints chosen is subject to interpretation. Certainly the association between TURP and increased all-cause mortality is clinically important. However, we have not proved causation, but just an association. It is still conceivable that we inadequately controlled for grade, stage or comorbidities and that the men with TURP within 3 months had more aggressive tumours.
Our data suggest that men receiving TURP were significantly more likely to receive a JJ stent (odds ratio 1.76), suprapubic cystostomy (1.90) and PCN (2.46) than men who did not receive TURP. These are all signs of local progression of disease. Indeed, local tumour control, TURP resected weight and obstructive uropathy are prognostic factors in prostate cancer. Obstructive uropathy (BOO, ureteric obstruction) results in significantly reduced survival in men with prostate cancer and can be rapidly progressive, especially in poorly differentiated tumour [24,25]. These are the same patients that might require TURP. In the report of Duncan et al. [26], patients with locally advanced disease who had a TURP also had a statistically significant higher probability of having a poorly differentiated cancer. However, although our analysis was able to adjust for factors such as grade and stage which are associated with more advanced tumour and higher incidence of local progression, the use of TURP was associated with local progression. Our work corroborates the smaller retrospective studies raising the possibility that the TURP procedure itself might induce biological changes that result in a more advanced tumour. Indeed, the all-cause mortality was higher in those undergoing TURP and it could be inferred that these men had more advanced tumour. The men treated by TURP could simply have more severe comorbid conditions and their underlying cancer might have had no effect on their longevity. Thus, caution must be used when inferring that the TURP ‘caused’ the local progression or increase in mortality.
Our study has several limitations. The results obtained in this analysis reflect only events observed for men aged ≥66 years at diagnosis, who were eligible for Medicare and living in localities that report cases to the SEER registry. Our research is pertinent only to the older patient diagnosed with more advanced disease who might require a TURP, not to a young man diagnosed by a change in PSA velocity who is undergoing definitive therapy. The limitations of this observational research include the potential for bias in the identification of disease recurrence-related events and the potential for errors in the measurement of patient covariates included in the multivariable models. Although most patients had ≥3 years of follow-up available, it is possible that the frequency of events occurring beyond the follow-up period could be substantially different across the groups being compared. The PSA level at diagnosis was measured as ‘elevated’, and the effects of this covariate might be under-adjusted compared to what could be obtained from the model if the specific PSA value and/or PSA slope were available. Further, our adjustments for the effects of differences in tumour grade are limited by the manner in which this information is reported in SEER.
Tumour grades reported in SEER are defined by the biopsy specimen for patients without surgery, and by pathology results for patients receiving surgery. Comparison of outcomes for patients with TURP or RP to outcomes for patients without surgery is potentially confounded by this bias in reported tumour grade. These results are also limited by the potential for unadjusted confounders that could meaningfully change the observed results if they were included in the models. This is suggested by the C statistics obtained for the five multivariable regression models, which were 0.584–0.75. At the lower end, these levels of discrimination imply that unmeasured confounders might exist that could change the observed results. None of the models perfectly discriminate (i.e. have a C statistic = 1.0) according to the risk of local progression. Comparison of the model C statistics indicates that unmeasured confounders have greater potential to change the results obtained for the models predicting the occurrence of LHRH therapy and JJ stents, and lower potential to change the observed results for model predicting occurrences of PCN. In essence, the association of TURP with an increased risk of local progression might, despite adjustments for elevated PSA level, stage and grade, be due to an unrecognized factor of ‘disease bulk’ that is not captured by the data.
A prospective single-institution study with individual patient data would better address the issue of tumour bulk. For example, if diagnostic testing before TURP, e.g. PSA level, PSA doubling time, degree of lymphadenopathy or Gleason score, were available, this would be a better surrogate for disease burden. If the patients treated by TURP still had disease progression at greater rates than matched controls, this design would better support causation.
In conclusion, using a large national cohort of men, our work corroborates that of several smaller retrospective studies suggesting that TURP carried out within the first few months after needle biopsy-based diagnosis of the prostate cancer is associated with a risk of local tumour progression and greater all-cause mortality. Although the exact reasons for these findings are unclear, consideration should be given to adjuvant treatment in patients undergoing this procedure.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumour registries in the creation of the SEER-Medicare database. This research has been reviewed and determined to be exempt human subject research by the Human Investigations Committee of the University of Virginia.
Funding: NIH CA104106 award to DT
Abbreviations
- SEER
Surveillance, Epidemiology and End Results
- RP
radical prostatectomy
- EBRT
external beam radiotherapy
- PCN
percutaneous nephrostomy
- ADT
androgen-deprivation therapy
- CDT
cancer-directed therapy
- CPT
Current Procedural Terminology
Footnotes
Patient Informed Consent: The data used in this study were reviewed and determined to be exempt for human subjects research by the Human Investigations Committee of the University of Virginia HIC#11621
References
- 1.Crain DS, Amling CL, Kane CJ. Palliative transurethral prostate resection for bladder outlet obstruction in patients with locally advanced prostate cancer. J Urol. 2004;171:668–671. doi: 10.1097/01.ju.0000104845.24632.92. [DOI] [PubMed] [Google Scholar]
- 2.Pilepich MV, Asbell SO, Krall JM, et al. Correlation of radiotherapeutic parameters and treatment related morbidity - analysis of RTOG Study 77–06. Int J Radiat Oncol Biol Phys. 1987;13:1007–1012. doi: 10.1016/0360-3016(87)90038-1. [DOI] [PubMed] [Google Scholar]
- 3.McGowan DG. The adverse influence of prior transurethral resection on prognosis in carcinoma of prostate treated by radiation therapy. Int J Radiat Oncol Biol Phys. 1980;6:1121–1126. doi: 10.1016/0360-3016(80)90163-7. [DOI] [PubMed] [Google Scholar]
- 4.Forman JD, Order SE, Zinreich ES, Lee DJ, Wharam MD, Mellits ED. The correlation of pretreatment transurethral resection of prostatic cancer with tumor dissemination and disease-free survival. A univariate and multivariate analysis. Cancer. 1986;58:1770–1778. doi: 10.1002/1097-0142(19861015)58:8<1770::aid-cncr2820580832>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Hanks GE, Leibel S, Kramer S. The dissemination of cancer by transurethral resection of locally advanced prostate cancer. J Urol. 1983;129:309–311. doi: 10.1016/s0022-5347(17)52069-8. [DOI] [PubMed] [Google Scholar]
- 6.Fowler JE, Jr, Fisher HA, Kaiser DL, Whitmore WF., Jr Relationship of pretreatment transurethral resection of the prostate to survival without distant metastases in patients treated with 125I–implantation for localized prostatic cancer. Cancer. 1984;53:1857–1863. doi: 10.1002/1097-0142(19840501)53:9<1857::aid-cncr2820530911>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Kuban DA, el-Mahdi AM, Schellhammer PF, Babb TJ. The effect of transurethral prostatic resection on the incidence of osseous prostatic metastasis. Cancer. 1985;56:961–964. doi: 10.1002/1097-0142(19850815)56:4<961::aid-cncr2820560443>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Pansadoro V, Sternberg CN, DePaula F, Florio A, Giannarelli D, Arcangeli G. Transurethral resection of the prostate and metastatic prostate cancer. Cancer. 1991;68:1895–1898. doi: 10.1002/1097-0142(19911101)68:9<1895::aid-cncr2820680908>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Anscher MS, Prosnitz LR. Transurethral resection of prostate prior to definitive irradiation for prostate cancer. Lack of correlation with treatment outcome. Urology. 1991;38:206–211. doi: 10.1016/s0090-4295(91)80345-8. [DOI] [PubMed] [Google Scholar]
- 10.Perez CA, Garcia D, Simpson JR, Zivnuska F, Lockett MA. Factors influencing outcome of definitive radiotherapy for localized carcinoma of the prostate. Radiother Oncol. 1989;16:1–21. doi: 10.1016/0167-8140(89)90066-2. [DOI] [PubMed] [Google Scholar]
- 11.Sandler HM, Hanks GE. Analysis of the possibility that transurethral resection promotes metastasis in prostate cancer. Cancer. 1988;62:2622–2627. doi: 10.1002/1097-0142(19881215)62:12<2622::aid-cncr2820621229>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Ignatoff JM, Oefelein MG, Watkin W, Chmiel JS, Kaul KL. Prostate specific antigen reverse transcriptase-polymerase chain reaction assay in preoperative staging of prostate cancer. J Urol. 1997;158:1870–1874. doi: 10.1016/s0022-5347(01)64150-8. [DOI] [PubMed] [Google Scholar]
- 13.Moreno JG, Croce CM, Fischer R, et al. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992;52:6110–6112. [PubMed] [Google Scholar]
- 14.Ellis WJ, Vessella RL, Corey E, et al. The value of a reverse transcriptase polymerase chain reaction assay in preoperative staging and followup of patients with prostate cancer. J Urol. 1998;159:1134–1138. [PubMed] [Google Scholar]
- 15.Heung YM, Walsh K, Sriprasad S, Mulvin D, Sherwood RA. The detection of prostate cells by the reverse transcription-polymerase chain reaction in the circulation of patients undergoing transurethral resection of the prostate. BJU Int. 2000;85:65–69. doi: 10.1046/j.1464-410x.2000.00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Rinker-Schaeffer CW, O’Keefe JP, Welch DR, Theodorescu D. Metastasis suppressor proteins. discovery, molecular mechanisms, and clinical application. Clin Cancer Res. 2006;12:3882–3889. doi: 10.1158/1078-0432.CCR-06-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Colombel M, Mallame W, Abbou CC. Influence of urological complications on the prognosis of prostate cancer. Eur Urol. 1997;31(Suppl. 3):21–24. doi: 10.1159/000474556. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Jr, Soloway MS, Young MJ. Complications of advanced prostate cancer. Urology. 1999;54:8–14. doi: 10.1016/s0090-4295(99)00448-3. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982 Apr;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 22.Kuban DA, el-Mahdi AM, Schellhammer PF. The effect of TURP on prognosis in prostatic carcinoma. Int J Radiat Oncol Biol Phys. 1987;13:1653–1659. doi: 10.1016/0360-3016(87)90161-1. [DOI] [PubMed] [Google Scholar]
- 23.Landmann C, Hunig R, Rutishauser G. Adverse effect of transurethral resection on disease-free survival in locally advanced prostatic cancer treated with irradiation. Int J Radiat Oncol Biol Phys. 1993;26:217–221. doi: 10.1016/0360-3016(93)90200-f. [DOI] [PubMed] [Google Scholar]
- 24.Cheng L, Bergstralh EJ, Scherer BG, et al. Predictors of cancer progression in T1a prostate adenocarcinoma. Cancer. 1999;85:1300–1304. doi: 10.1002/(sici)1097-0142(19990315)85:6<1300::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Oefelein MG. Prognostic significance of obstructive uropathy in advanced prostate cancer. Urology. 2004;63:1117–1121. doi: 10.1016/j.urology.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Duncan W, Catton CN, Warde P, et al. The influence of transurethral resection of prostate on prognosis of patients with adenocarcinoma of the prostate treated by radical radiotherapy. Radiother Oncol. 1994;31:41–50. doi: 10.1016/0167-8140(94)90412-x. [DOI] [PubMed] [Google Scholar]