Dear Editor,
Neurodegeneration with brain iron accumulation (NBIA) constitutes a group of genetic disorders characterized by iron deposition in the brain, and in particular the basal ganglia. These disorders are genetically heterogeneous, and their clinical features include cognitive impairment, psychiatric abnormality, and movement disorders such as parkinsonism and dystonia.1,2 Brain magnetic resonance imaging (MRI) is used to investigate iron accumulation in the basal ganglia.
In addition to the several known NBIA genes, beta-propeller protein-associated neurodegeneration (BPAN) was recently identified with heterozygous mutation in the WD repeat-containing protein 45 (WDR45) on the X-chromosome.3,4 While BPAN shares clinical and laboratory features with other NBIA disorders with respect to its X-linked inheritance pattern, it differs from all other previously identified genes for NBIA disorders, which are autosomal recessive or dominant traits. In this report we describe the case of a 43-year-old female with developmental retardation, chorea, and parkinsonism who was diagnosed with BPAN following genetic analysis of WDR45.
A 38-year-old female visited the neurologic department of our hospital due to sensations of slowness in her right upper and lower extremities of 20 years duration. The patient was born at full term via a normal delivery and without perinatal complications. There was no familial or medical history to account for her condition. She had a masked face with chorea on the forehead. Her speech was monotone, and there was no tremor movement during either resting posture or action states. There was marked joint rigidity of her wrists and knees. The patient stood upright without a stooped posture, and walked slowly without bilateral arm swing; however, there was no festination or propulsion. Laboratory testing included a complete blood count, blood chemistry, thyroid function test, parathyroid function test, HIV antibody, antistreptolysin O antibody, ferritin, serum copper level, ceruloplasmin, and peripheral blood smear test; all test results were within normal ranges.
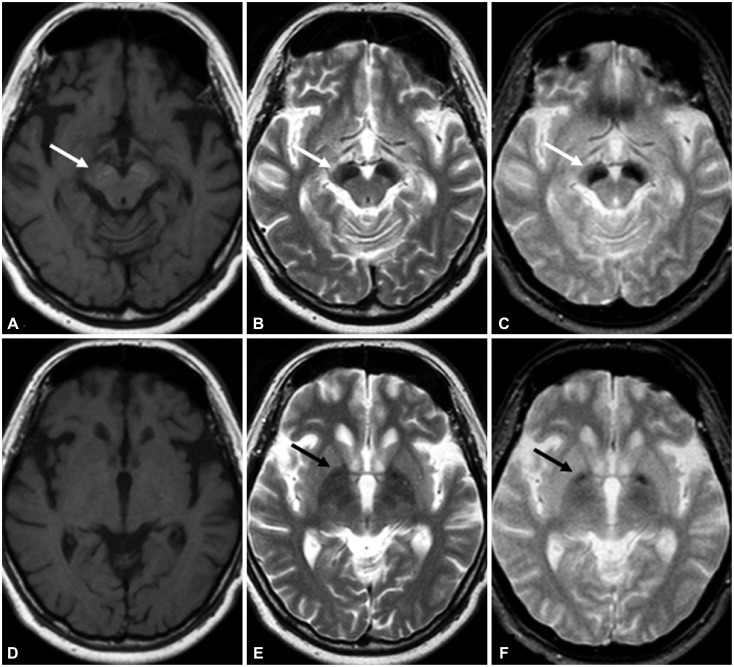
Conventional MRI using a 1.5-T magnetic resonance scanner (Gyroscan, Philips, Best, The Netherlands) revealed bilateral linear lesions in the substantia nigra surrounded by a hypertense 'halo' on the T1-weighted image. The corresponding lesions also appeared hypointense without linear signal abnormality on the T2-weighted and gradient-echo images. Bilateral hypointense lesions in the globus pallidus were evident in the T2-weighted and gradient-echo images, while there was no abnormal signal change on the T1-weighted image (Fig. 1).
Fig. 1. Magnetic resonance imaging reveals bilateral hypointense lesions in the substantial nigra surrounded by a hyperintense 'halo' on the T1-weighted image (A). The corresponding lesions appear hypointense on the T2-weighted (B) and gradient-echo (C) images (white arrows). While there is no abnormal signal change on the T1-weighted image (D), bilateral hypointense lesions in the globus pallidus are evident in the T2-weighted (E) and gradient-echo (F) images.
The patient's WDR45 status was analyzed. All exons and exon-intron boundaries of WDR45 were amplified by the polymerase chain reaction and Sanger sequenced directly. Primer design and amplification were performed as reported previously.4 Genomic DNA and complementary DNA sequencing revealed a novel mutation, c.345-1G>A, which resulted in deletion of exon 7 and the introduction of a premature stop codon in exon 8, r.345_439del. WDR45 mutation on the X-chromosome is associated with defective autophagy, which plays an important role in the cellular processing of abnormal protein degradation.5
In summary, this is the first published case of WDR45-related BPAN in Korea. The patient had phenotypically clinical features of BPAN with the development of movement disorders. In addition to clinical manifestations, a distinct pattern of iron deposition in the globus pallidus and substantia nigra revealed by MRI, could be a useful clue for distinguishing different forms of NBIA.
Acknowledgements
This study was supported by 2011 Research Grant from Kangwon National University.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Kruer MC, Boddaert N, Schneider SA, Houlden H, Bhatia KP, Gregory A, et al. Neuroimaging features of neurodegeneration with brain iron accumulation. AJNR Am J Neuroradiol. 2012;33:407–414. doi: 10.3174/ajnr.A2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhoeven WM, Egger JI, Koolen DA, Yntema H, Olgiati S, Breedveld GJ, et al. Beta-propeller protein-associated neurodegeneration (BPAN), a rare form of NBIA: novel mutations and neuropsychiatric phenotype in three adult patients. Parkinsonism Relat Disord. 2014;20:332–336. doi: 10.1016/j.parkreldis.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Haack TB, Hogarth P, Kruer MC, Gregory A, Wieland T, Schwarzmayr T, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet. 2012;91:1144–11 49. doi: 10.1016/j.ajhg.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitsu H, Nishimura T, Muramatsu K, Kodera H, Kumada S, Sugai K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet. 2013;45:445–449. 449e1. doi: 10.1038/ng.2562. [DOI] [PubMed] [Google Scholar]
- 5.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]