Although insurers varied in terms of public statements regarding coverage intentions, bevacizumab use declined similarly among all payers, suggesting that provider decision making, rather than payer-specific coverage policies, drove reductions.
Abstract
Purpose:
In February 2008, the US Food and Drug Administration (FDA) granted accelerated approval for bevacizumab for metastatic breast cancer. After public hearings in July 2010, and June 2011, the FDA revoked this approved indication in November 2011, on the basis of additional evidence regarding its risk/benefit profile. The Centers for Medicare and Medicaid Services, local Medicare contractors, and commercial payers varied in their stated intentions to cover bevacizumab after FDA's regulatory actions. We examined payer-specific trends in bevacizumab use after the FDA's regulatory actions.
Methods:
We used outpatient medical claims compiled by IMS Health to evaluate trends in bevacizumab use for breast cancer for Medicare-insured and commercially insured patients (N = 102,906) using segmented regression. Given that Medicare coverage policies may vary across regional contractors, we estimated trends in bevacizumab use across 10 local coverage areas. In a sensitivity analysis, we estimated trends in bevacizumab use for breast cancer compared with trends in use for lung cancer using difference-in-differences models.
Results:
Among chemotherapy infusions for breast cancer, bevacizumab use decreased from 31% in July 2010, to 4% in September 2012. Use decreased by 11% among commercially insured and 13% among Medicare-insured patients after July 2010 (interaction P = .68) and continued to decline by 9% per month (interaction P = .61). We observed no contractor-level variation in bevacizumab use among Medicare beneficiaries. During the same period, bevacizumab use for lung cancer was stable.
Conclusion:
Although insurers varied in public statements regarding coverage intentions, bevacizumab use declined similarly among all payers, suggesting that provider decision making, rather than payer-specific coverage policies, drove reductions.
Introduction
Bevacizumab is an infused anticancer therapy that was first approved by the US Food and Drug Administration (FDA) in 2004 for use in metastatic colorectal cancer.1 Since its initial approval, bevacizumab has also received approval for use alone or in combination for treating glioblastoma, non–small-cell lung cancer, and renal cell cancer.2–4 In February 2008, the FDA granted accelerated approval to expand use of bevacizumab to include first-line therapy of metastatic, human epidermal growth factor receptor 2–negative breast cancer.5,6 The accelerated approval was given on the basis of initial promising results that were related to progression-free survival in ECOG (Eastern Cooperative Oncology Group) trial E2100, when bevacizumab was added to docetaxel.7
As a condition of the approval, the FDA required that the manufacturer provide additional data to confirm the clinical benefits of bevacizumab with respect to progression-free and overall survival. Evidence from these follow-up studies failed to demonstrate anticipated overall survival benefits of bevacizumab for breast cancer beyond the benefits provided by chemotherapy alone; the evidence also highlighted increased risks of severe adverse effects.5,6,8–10 In July 2010, in light of the new data, the FDA's Oncologic Drugs Advisory Committee (ODAC) voted 12 to one to remove bevacizumab's labeled indication for treating metastatic breast cancer. This meeting was followed by a hearing in June 2011, in which the ODAC voted unanimously (six to zero) to withdraw the approval of the breast cancer indication. In November 2011, the FDA formally withdrew bevacizumab's breast cancer indication.11–13
Major payers, including Medicare and commercial health plans, varied in their public responses regarding their intended coverage policies after the FDA's removal of bevacizumab's breast cancer indication. For example, one large private insurer, Blue Shield of California, announced their intention to stop paying for bevacizumab for breast cancer after the June 2011 hearing,14 but after outcry from consumer groups, they quickly softened their public stance, noting that bevacizumab would be covered for patients on a case-by-case basis.15
At the same time, the Centers for Medicare and Medicaid Services (CMS) announced their intention to continue to cover bevacizumab for breast cancer, regardless of the FDA's decision to withdraw approval.12,16 Medicare, although a federal program, allows coverage for some services to differ across Medicare claims processing contractors, potentially creating variation in product use even within the Medicare program.17–22 In the case of bevacizumab, there was noted variation in stated coverage policies between local Medicare claims processing contractors after the indication withdrawal.23
Previous research has documented that there were significant decreases in bevacizumab use over time, with large declines coinciding with the timing of each advisory committee meeting or regulatory action beginning in June 2010.24 It is unknown whether or to what extent public statements regarding intended coverage translated into differences in the use of bevacizumab for breast cancer by payer or local coverage area. We examined Medicare- and commercial insurer–specific trends in bevacizumab use to explore the role of coverage policies in reducing bevacizumab use for breast cancer after the July 2010 ODAC meeting and the FDA's subsequent indication withdrawal.
Methods
Data Source
We used IMS Health LifeLink private practice provider medical claims database from January 2007 to September 2012 for all analyses. This administrative data source compiles outpatient medical claims submitted by private practitioners for reimbursement by insurers using the standardized billing form CMS-1500.25 IMS Health receives approximately one billion claims per year from physicians working in the United States. These data are available in near real-time; approximately 95% of claims are received within 21 days of electronic submission for payment by the provider.
Sample Selection
We included claims for in-office–administered chemotherapy that included International Classification of Diseases ninth edition (ICD-9) codes for breast cancer (174.x) between January 2007 and September 2012. Because bevacizumab was widely used for the treatment of macular degeneration and diabetic retinopathy during this time period, we included only chemotherapy claims for which breast cancer was the primary diagnosis code (N = 125,765). We excluded individuals living outside of the contiguous United States (n = 42). We also excluded individuals whose primary payer was not Medicare or a commercial health plan because these two insurance categories covered 82% of the individuals meeting our study criteria and were the focus of public statements regarding coverage determinations, which was the primary focus of this study (n = 22,817; n = 8,460 in Medicaid and n = 14,357 covered by “other” insurance). This resulted in 102,906 individuals with breast cancer diagnoses for our analysis.
We separated individuals into two payer categories—Medicare or commercial insurance—to determine whether there was a differential impact of the FDA's indication withdrawal by payer. Additionally, because Medicare coverage policies may vary by local coverage areas, we divided states into coverage area regions as described in the Local Coverage Areas section to ensure that we did not mask regional changes in use, should they have existed.
Local Coverage Areas
We defined Medicare local coverage areas at the state level. We used the CMS Web site to identify contractors by state.26 We searched for notices of contract awards during the study period and abstracted the Medicare Administrative Contractor (MAC) jurisdictions affected, the date of planned implementation, and the previous MAC that managed claims for each affected state. Award implementation dates were used to assign local coverage area by year, with the contractor that serviced the state for the majority of the year assigned as the MAC. We assumed that the date of implementation was that announced in the award notice, unless documentary evidence indicated otherwise.
Analysis
We estimated changes in monthly bevacizumab use as a proportion of all injectable anticancer therapy use between January 2007 and September 2012. To estimate changes in bevacizumab use during the study period, we used an interrupted time series design.27 We considered the first ODAC meeting at the FDA to be the month of the intervention (July 2010) because other evidence suggests that use began declining steeply at this time.24 We also included a study month indicator to account for the base trend in bevacizumab use before the ODAC meeting (1 to 69 for time between January 2007 and September 2012) and a postintervention month indicator to estimate the postintervention change in trend (months 44 to 69). We tested interactions between payer and time to determine if there were significant differences in responses by payer. These models were estimated using generalized estimating equations with a log link and binominal distribution to generate risk and risk ratios directly and to adjust SEs for repeated measures.
Because Medicare coverage policies may vary by local coverage area,20 we also estimated changes in bevacizumab use for breast cancer over time by local coverage area to determine whether there were regional contractor–level differences in responsiveness to the labeled indication withdrawal. Similar to the previous models, we used generalized estimating equations with a log link and binomial distribution. We included the local coverage area variable as a class-level indicator and tested the independent and interactive effects of the local coverage area variable with each of the time-related variables. Significant interactions between local coverage areas and time would suggest that there was variation in bevacizumab use across areas within Medicare.
Sensitivity Analyses
Because we were concerned that changes in the use of bevacizumab over time, independent of regulatory actions and potential coverage changes, could influence our findings, we also used a difference-in-differences model to evaluate trends in use for breast cancer in comparison with trends in use for lung cancer (n = 78,671 patients with primary ICD-9 diagnosis code 162.x). We selected lung cancer because bevacizumab received FDA approval for the treatment of metastatic, nonsquamous non–small-cell lung cancer in 2006,28 and the lung cancer indication did not change during the study period. Therefore, we expected that payer decisions with respect to limiting coverage for bevacizumab would be targeted toward use for breast cancer and would have little effect on cancer in other sites, such as lung cancer.
Results
Among the 102,906 individuals with breast cancer who used chemotherapy and were included in our sample, 70.9% were commercially insured and 29.1% were Medicare insured; the mean age was 58.3 years (standard deviation,12.6; Appendix Table A1, online only). As shown in Figure 1, bevacizumab as a proportion of all chemotherapy use for breast cancer declined from a peak of 38% in July 2009 and 31% in July 2010 to only 4% by September 2012, whereas bevacizumab use for lung cancer declined only slightly during this period, from 47% to 40% (data not shown). As indicated in Figure 1, there were no differences in the baseline use of bevacizumab for breast cancer by payer; Medicare and commercial enrollees received bevacizumab for approximately 26% of their infused anticancer treatments during 2007 through mid-2010, before the advisory period.
Figure 1.
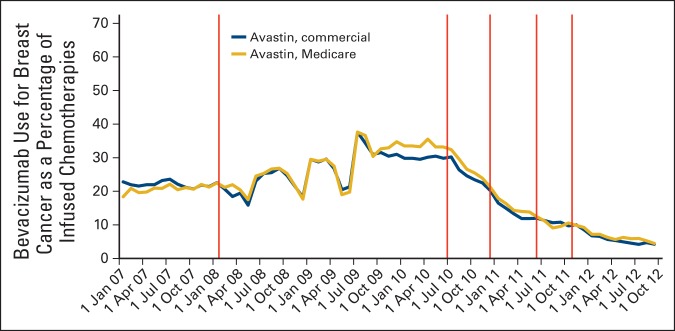
Trends in bevacizumab use for breast cancer as a proportion of infused chemotherapies by payer. Regulatory actions identified by vertical lines: February 2008, US Food and Drug Administration (FDA) approval for metastatic breast cancer. July 2010, FDA Oncologic Drugs Advisory Committee (ODAC) meeting on evidence supporting breast cancer indication withdrawal. December 2010, FDA announces intent to withdraw bevacizumab's breast cancer indication. June 2011, Second ODAC meeting. November 2011, FDA indicates withdrawal.
In the interrupted time series analysis stratified by payer (Table 1), bevacizumab use declined among commercially insured individuals by 11% after the July 2010 advisory committee meeting (risk ratio [RR], 0.89; 95% CI, 0.84 to 0.95) and by an additional 9% per month subsequently (monthly decline from August 2010 through September 2012; RR, 0.91; 95% CI, 0.91 to 0.92). Similarly, among Medicare beneficiaries, there was a 13% decrease in use after July 2010 (RR, 0.87; 95% CI, 0.80 to 0.96) and an additional 9% monthly decline during the following months (RR, 0.91; 95% CI, 0.90 to 0.92).
Table 1.
Interrupted Time Series Results Assessing Change in Bevacizumab Use for Breast Cancer, Combined and Stratified by Payers
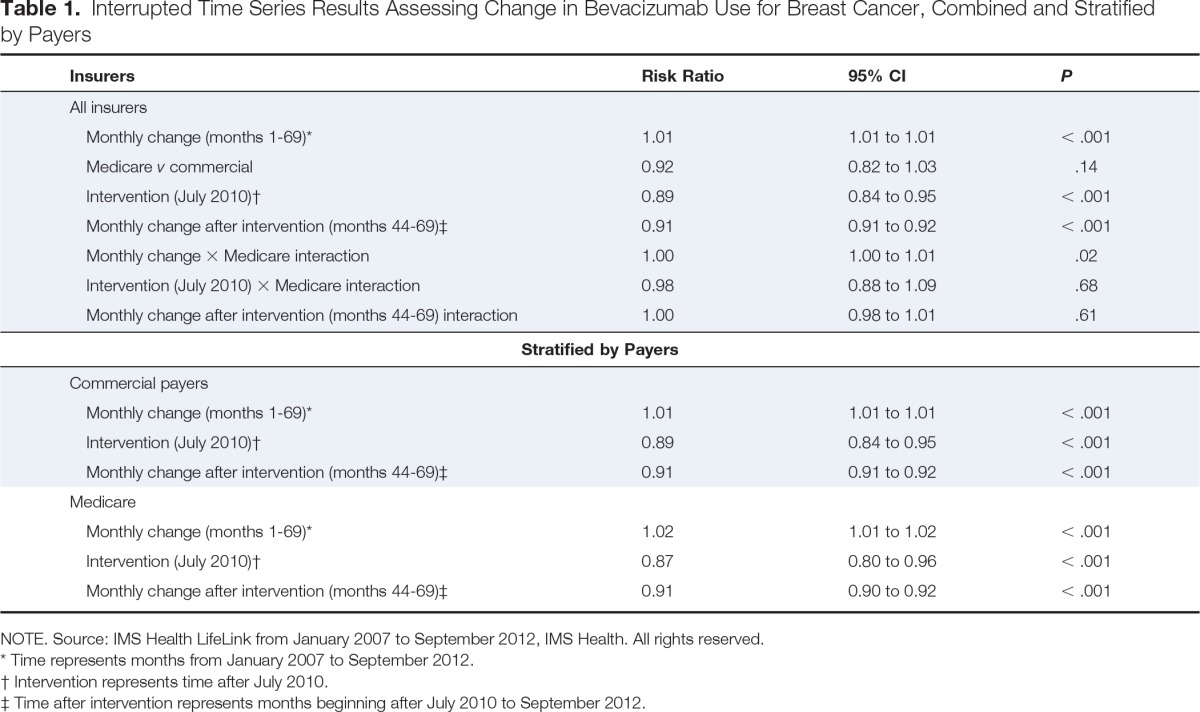
| Insurers | Risk Ratio | 95% CI | P |
|---|---|---|---|
| All insurers | |||
| Monthly change (months 1-69)* | 1.01 | 1.01 to 1.01 | < .001 |
| Medicare v commercial | 0.92 | 0.82 to 1.03 | .14 |
| Intervention (July 2010)† | 0.89 | 0.84 to 0.95 | < .001 |
| Monthly change after intervention (months 44-69)‡ | 0.91 | 0.91 to 0.92 | < .001 |
| Monthly change × Medicare interaction | 1.00 | 1.00 to 1.01 | .02 |
| Intervention (July 2010) × Medicare interaction | 0.98 | 0.88 to 1.09 | .68 |
| Monthly change after intervention (months 44-69) interaction | 1.00 | 0.98 to 1.01 | .61 |
| Stratified by Payers | |||
|---|---|---|---|
| Commercial payers | |||
| Monthly change (months 1-69)* | 1.01 | 1.01 to 1.01 | < .001 |
| Intervention (July 2010)† | 0.89 | 0.84 to 0.95 | < .001 |
| Monthly change after intervention (months 44-69)‡ | 0.91 | 0.91 to 0.92 | < .001 |
| Medicare | |||
| Monthly change (months 1-69)* | 1.02 | 1.01 to 1.02 | < .001 |
| Intervention (July 2010)† | 0.87 | 0.80 to 0.96 | < .001 |
| Monthly change after intervention (months 44-69)‡ | 0.91 | 0.90 to 0.92 | < .001 |
NOTE. Source: IMS Health LifeLink from January 2007 to September 2012, IMS Health. All rights reserved.
Time represents months from January 2007 to September 2012.
Intervention represents time after July 2010.
Time after intervention represents months beginning after July 2010 to September 2012.
Finally, when considering the impact of Medicare local coverage area on the use of bevacizumab during the study period, we observed no evidence of variation in the responses to the regulatory actions or potential coverage changes (P > .05 for all interactions; data not shown).
In a sensitivity analysis comparing time trends in the use of bevacizumab for individuals with breast cancer versus those with lung cancer, there was only a slight decline in bevacizumab use among patients with lung cancer. In particular, we observed no change in the use of bevacizumab after the ODAC meeting in July 2010 (RR, 1.01; 95% CI, 0.97 to 1.04) and only a small decrease in monthly use after that time (RR, 0.99; 95% CI, 0.99 to 0.99; Table 2). Estimates from the difference-in-differences model showed that bevacizumab use among patients treated for breast cancer decreased by an additional 12% during the intervention period and by an additional 8% per month in the postintervention period above that observed for patients treated for lung cancer.
Table 2.
Difference-in-Differences Results Assessing Change in Bevacizumab Use for Breast Cancer, Compared With Change in Use for Lung Cancer
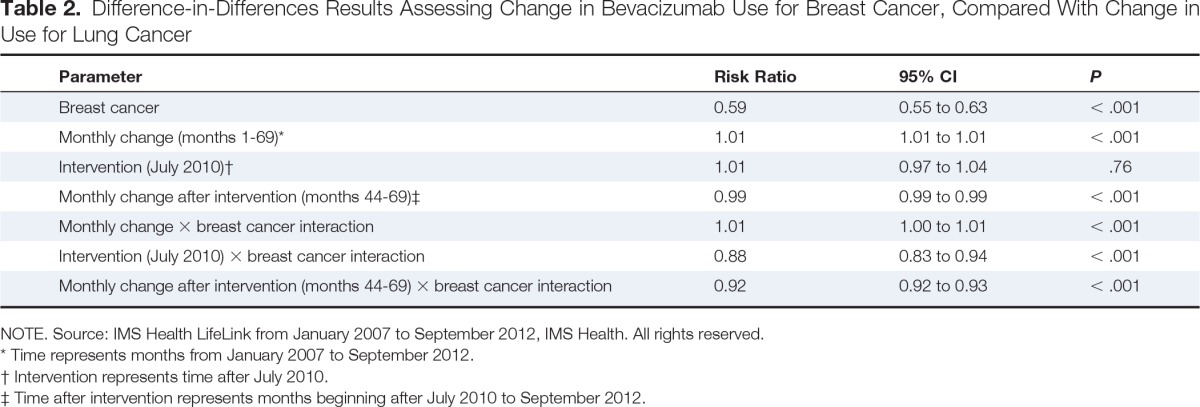
| Parameter | Risk Ratio | 95% CI | P |
|---|---|---|---|
| Breast cancer | 0.59 | 0.55 to 0.63 | < .001 |
| Monthly change (months 1-69)* | 1.01 | 1.01 to 1.01 | < .001 |
| Intervention (July 2010)† | 1.01 | 0.97 to 1.04 | .76 |
| Monthly change after intervention (months 44-69)‡ | 0.99 | 0.99 to 0.99 | < .001 |
| Monthly change × breast cancer interaction | 1.01 | 1.00 to 1.01 | < .001 |
| Intervention (July 2010) × breast cancer interaction | 0.88 | 0.83 to 0.94 | < .001 |
| Monthly change after intervention (months 44-69) × breast cancer interaction | 0.92 | 0.92 to 0.93 | < .001 |
NOTE. Source: IMS Health LifeLink from January 2007 to September 2012, IMS Health. All rights reserved.
Time represents months from January 2007 to September 2012.
Intervention represents time after July 2010.
Time after intervention represents months beginning after July 2010 to September 2012.
Discussion
Although payers varied in their public statements regarding their intentions to cover bevacizumab for breast cancer,14,16 bevacizumab use decreased rapidly among patients with both commercial insurance and Medicare after the FDA's first ODAC meeting in July 2010 through late 2012. Importantly, these declines were not seen among individuals with diagnoses for lung cancer, suggesting that physicians were likely responding to the negative risk-benefit profile and potential for coverage changes for breast cancer–specific use. Furthermore, although Medicare contractors varied in their stated intent to cover bevacizumab,23 we did not observe such variation. Previous studies exploring the role of local coverage–area group policies have seen little difference in physician behavior and use of services, suggesting that the lack of variation by contractors could have been anticipated in this case.22,29,30
One possible explanation for the lack of observed variation is that coverage policies were not the primary drivers of bevacizumab use. Providers may have reduced bevacizumab use because of their concerns regarding the safety and effectiveness of this agent for their patients. Given the timing of declines in use, physicians seemed to respond quite rapidly to efficacy and safety data that were presented at advisory committee meetings before the FDA's formal withdrawal of bevacizumab's indication. In fact, several other studies have shown that physicians respond rapidly to new information related to safety and effectiveness of therapies used among patients with cancer.24,31,32 This responsiveness to regulatory actions may be unique to cancer-related therapies, given the rapid advances in treatment and the preeminence of clinical guidelines in this medical area. Alternatively, physician's rapid responses could also be a result of their anticipation of changes in bevacizumab coverage. Private or community-based practices may have been more financially vulnerable if payers changed their coverage policy for bevacizumab, making it more difficult for physicians to recoup the costs of this high-priced therapy.
Second, changes in pharmaceutical product use during periods after regulatory actions can be driven by many factors, aside from insurer coverage policies.33 These factors include changes in pharmaceutical promotion by manufacturers, dissemination of drug risk information through the media and lay press, peer-reviewed publications, physician-patient communication, and modification of treatment guidelines, among others. Additionally, the data on which regulatory decisions are based may also be used by clinicians and payers to shift practice patterns even before formal regulatory actions are made. Although there were no formal changes in the National Comprehensive Care Network Guidelines regarding the use of bevacizumab among patients with breast cancer during our study time frame, it is possible that less formal communications were occurring among oncologists that shaped prescribing practices, among other factors.
A third potential explanation for a lack in observed variation across payers is that commercial payers varied in their use and implementation of coverage restrictions. For example, in a previous study, Conti et al24 reported that two large commercial payers indicated that they did not change coverage policies for bevacizumab as a result of the FDA's regulatory actions. In addition, among plans that intended to change coverage, coverage restrictions may not have been fully implemented because of public outcry and the negative publicity that surrounded initial efforts to restrict access to bevacizumab for breast cancer. Blue Shield of California and the Medicare local contractor, Palmetto GBA, who announced plans to restrict coverage, both quickly reversed course after the negative publicity that surrounded these decisions.13,15,23 Importantly, declines in bevacizumab use for breast cancer were observed before the announced coverage policy changes, suggesting that these events would likely have little impact on use.
There are many reasons why insurers, Medicare, and particularly the regional contractors might have difficulty implementing a change in coverage policies in this setting.34 First, bevacizumab is a product that is approved for treating multiple cancers and, as such, was not being removed from the marketplace with the regulatory actions. Instead, the labeled indication specific to breast cancer was removed. Given that physicians may use prescription drug products for off-label indications, it would be difficult for insurers to completely eliminate bevacizumab's use for breast cancer if the clinical community still believed that it was a safe and effective product for their patients, although, admittedly, physicians would risk having such claims denied by Medicare or other payers. Second, within Medicare there are two mechanisms for affecting coverage policy: local coverage determinations and national determinations. The case of bevacizumab illustrates how the two policies may conflict. Although in January 2011, Palmetto GBA issued a local coverage determination that limited coverage for bevacizumab, CMS later issued a national coverage determination, in November 2011, that declared continued coverage of bevacizumab. Presumably, the national determination would supersede the local coverage determination, causing Palmetto GBA to change its policies; however, whether such a reversal would affect changes in behavior would presume that local coverage determinations were effectively implemented. Foote and Town34 document several factors that may make it difficult for regional contractors to ensure that their own local coverage determinations are followed, including inability to apply local coverage determinations at the point of payment because of the lack of data that are necessary to evaluate the claim and a lack of incentives and infrastructure to enforce coverage policies.
There are several important limitations to note. We did not have any information on individual patient and provider characteristics, including tumor stage, grade, hormone receptor status, or line of therapy, which limited our ability to model whether other patient, provider-level, or disease characteristics drove bevacizumab discontinuation. Although our data do not allow for evaluation of receipt of treatment by stage, a previous study on the topic reports that the majority of bevacizumab use was for metastatic indications and that changes in use were not significantly different by tumor stage.24 We also lacked data on patient and physician decision making or beliefs about bevacizumab's safety and effectiveness and the setting in which care was received. It is possible that smaller or privately owned practices are more financially vulnerable and thus react more quickly to anticipated coverage changes. In a previous study we found earlier declines in bevacizumab use among private practices, which may support this anticipatory behavior.24 We also only observed outpatient medical claims for bevacizumab, which could underestimate use overall if bevacizumab were reimbursed through inpatient or pharmacy claims or used in clinical trials. Although nearly all breast cancer chemotherapy is delivered in outpatient settings,35 providers might have increased their use of bevacizumab in the inpatient setting or in clinical trials to minimize financial risks to their practices. In addition, our time series only included the period from 2007 through 2012, so changes in bevacizumab use before 2007 are not evident in our data. Next, using trends in bevacizumab use for lung cancer as a negative control may be inappropriate if there were other factors that influenced bevacizumab use for lung cancer during the study period. We observed only small changes in the use of bevacizumab for lung cancer during this time period. We compared breast cancer use with lung cancer use as a sensitivity analysis, rather than a primary analysis, given the potential concerns regarding differences in the use of bevacizumab across cancer sites. Finally, we lacked data on actual insurance coverage policies that were in place, coverage generosity, and other market-level factors that may have influenced product use outside of the regulatory communications.
In conclusion, bevacizumab use declined rapidly and consistently among women with breast cancer who were commercially insured and Medicare insured (across areas with potentially varying coverage policies) after concerns were stated regarding the efficacy and safety of the product for treating breast cancer. Given the timing and consistency of the declines that were observed across payers, it is likely that physician decision making (either related to perceptions of a lack of drug benefit or coverage concerns), rather than payer-specific policies, drove reductions in bevacizumab use for breast cancer.
Acknowledgment
Supported by the Agency for Healthcare Research and Quality (Grant No. RO1 HS0189960). S.B.D. is supported by the National Institutes of Health Building Interdisciplinary Research Careers in Women's Health K12 Program and the North Carolina Translational and Clinical Sciences Institute (Grant No. UL1TR001111). N.L.K. is supported by K24CA181510 from the National Cancer Institute (NCI). R.M.C. received funding from Grant No. K07 CA138906 from the NCI to the University of Chicago. A.W. is supported by the Royster Society of Fellows at Graduate School of the University of North Carolina, Chapel Hill. The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health information services: IMS Health LifeLink, from January 2007 to September 2012, IMS Health. All rights reserved. Such statements, findings, conclusions, views, and opinions are not necessarily those of IMS Health or any of its affiliated or subsidiary entities.
Appendix
Table A1.
Baseline Characteristics of Chemotherapy Users by Cancer Type

| Characteristic | Breast Cancer (n = 102,906) | Lung Cancer (n = 78,671) |
|---|---|---|
| Age, years | ||
| Mean | 58.3 | 67.3 |
| SD | 12.6 | 9.8 |
| Payer type, % | ||
| Commercial | 70.9 | 45.7 |
| Medicare | 29.1 | 54.3 |
| Medicare local coverage area, % | ||
| CGS Administrators, LLC | 5.7 | 6.7 |
| Cahaba Government Benefit Administrators | 6.7 | 6.8 |
| California | 10.1 | 6.8 |
| First Coast Service Options | 8.6 | 11.3 |
| National Government Services | 10.9 | 11.0 |
| NHIC | 1.5 | 1.8 |
| Noridian Healthcare Solutions | 6.9 | 6.4 |
| Novita Solutions | 29.0 | 27.5 |
| Palmetto GBA | 10.3 | 10.3 |
| Wisconsin Physicians Service Insurance Corporation | 10.3 | 11.4 |
NOTE. Source: IMS Health LifeLink from January 2007 to September 2012, IMS Health. All rights reserved.
Abbreviation: SD, standard deviation.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: Stacie B. Dusetzina, Rena M. Conti, Haiden A. Huskamp, Nancy L. Keating
Financial support: G. Caleb Alexander
Collection and assembly of data: Stacie B. Dusetzina, Shellie Ellis
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
How Do Payers Respond to Regulatory Actions? The Case of Bevacizumab
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Stacie B. Dusetzina
No relationship to disclose
Shellie Ellis
No relationship to disclose
Rachel A. Freedman
No relationship to disclose
Rena M. Conti
No relationship to disclose
Aaron N. Winn
No relationship to disclose
James D. Chambers
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: Baxter International, Astellas Pharma
Research Funding: Pfizer (Inst), Genentech (Inst), Bayer (Inst)
G. Caleb Alexander
Consulting or Advisory Role: IMS Health
Expert Testimony: US Senate
Other Relationship: FDA Peripheral and Central Nervous System Advisory Committee
Haiden A. Huskamp
Travel, Accommodations, Expenses: IMS Health
Nancy L. Keating
No relationship to disclose
References
- 1.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: Bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 2.Summers J, Cohen MH, Keegan P, et al. FDA drug approval summary: Bevacizumab plus interferon for advanced renal cell carcinoma. Oncologist. 2010;15:104–111. doi: 10.1634/theoncologist.2009-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: Bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: Bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14:1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 5.O'Shaughnessy J, Miles D, Gray RJ, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28(suppl):115s. abstr 1005. [Google Scholar]
- 6.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 7.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 8.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 9.D'Agostino RB., Sr Changing end points in breast-cancer drug approval: The Avastin story. N Engl J Med. 2011;365:e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365:e3. doi: 10.1056/NEJMp1107201. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration. FDA begins process to remove breast cancer indication from Avastin label: Drug not shown to be safe and effective in breast cancer patients. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm237172.htm.
- 12.Neergaard L. FDA revokes approval of Avastin for breast cancer. Associated Press. 2011 Nov 8; [Google Scholar]
- 13.Pollack A. Another setback for Avastin as a breast cancer treatment. The New York Times. 2011. Jan 7, http://query.nytimes.com/gst/fullpage.html?res=940CE6DC113BF934A35752C0A9679D8B63.
- 14.Pollack A. Blue Shield of California won't cover breast cancer drug. The New York Times. 2011 Oct 3;:B3. [Google Scholar]
- 15.Shivinsky S. Blue Shield of California statement regarding changes to coverage policy for Avastin. https://www.blueshieldca.com/bsca/about-blue-shield/newsroom/avastin-coverage-policy-changes.sp.
- 16.Pollack A. Medicare Will Pay for Avastin in Treating Breast Cancer. The New York Times. 2011. Jun 30, http://prescriptions.blogs.nytimes.com/2011/06/30/medicare-will-pay-for-avastin-in-treating-breast-cancer/
- 17.Centers for Medicare & Medicaid Services. Local Coverage Determination (LCD): PEGFILGRASTIM (Neulasta®) (L28946) www.cms.gov/medicare-coverage-database/details/lcd-details.aspx?LCDId=28946&ContrId=368&ver=14&ContrVer=1&CoverageSelection=Both&ArticleType=All&PolicyType=Final&s=All&KeyWord=pegfilgrastim&KeyWordLookUp=Title&KeyWordSearchType=And&CptHcpcsCode=J2505&bc=gAAAABAAAAAAAA%3d%3d&.
- 18.Noridian Healthcare Solutions. Pegfilgrastim (Neulasta) J2505 Revised. Medicare B News Issue 283. 2013. Jan 23, https://www.noridianmedicare.com/shared/partb/bulletins/2013/283_jan/Pegfilgrastim_-_Neulasta_-_J2505_-_Revised.htm.
- 19.Levinson DR. Department of Health and Human Services, Office of the Inspector General; 2014. Local coverage determinations create inconsistency in Medicare coverage. http://oig.hhs.gov/oei/reports/oei-01-11-00500.pdf. [Google Scholar]
- 20.Foote SB, Wholey D, Rockwood T, et al. Resolving the tug-of-war between Medicare's national and local coverage. Health Aff (Millwood) 2004;23:108–123. doi: 10.1377/hlthaff.23.4.108. [DOI] [PubMed] [Google Scholar]
- 21.Foote SB. Focus on locus: Evolution of Medicare's local coverage policy. Health Aff (Millwood) 2003;22:137–146. doi: 10.1377/hlthaff.22.4.137. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson M, O'Malley AJ, Earle CC, et al. Does reimbursement influence chemotherapy treatment for cancer patients? Health Aff (Millwood) 2006;25:437–443. doi: 10.1377/hlthaff.25.2.437. [DOI] [PubMed] [Google Scholar]
- 23.Pollack A. Medicare coverage for breast cancer drug ends in some states. The New York Times. 2011. Jan 6, http://prescriptions.blogs.nytimes.com/2011/01/06/medicare-coverage-for-breast-cancer-drug-ends-in-some-states/
- 24.Conti RM, Dusetzina SB, Herbert AC, et al. The impact of emerging safety and effectiveness evidence on the use of physician-administered drugs: The case of bevacizumab for breast cancer. Med Care. 2013;51:622–627. doi: 10.1097/MLR.0b013e318290216f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hess GP, Hill J. Final report on the results from the OMOP Methods Experiment. http://omop.org/sites/default/files/SDI%20Final%20Report%20OMOP%20Research%20Feb%202011.pdf.
- 26.Center for Medicaid and Medicare Services. LCDs by state index results, 2014. www.cms.gov/medicare-coverage-database/indexes/lcd-state-index.aspx?s=All&DocType=Active&bc=AggAAAAAAAAAAA%3d%3d&#ResultsAnchor.
- 27.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 28.Gentzler RD, Patel JD. Optimal first-line and maintenance treatments for advanced-stage nonsquamous non-small cell lung cancer. J Natl Compr Canc Netw. 2014;12:889–897. doi: 10.6004/jnccn.2014.0083. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians' responses to a reimbursement change. N Engl J Med. 2011;365:2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foote SB, Virnig BA, Town RJ, et al. The impact of Medicare coverage policies on health care utilization. Health Serv Res. 2008;43:1285–1301. doi: 10.1111/j.1475-6773.2008.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dusetzina SB, Alexander GC, Freedman RA, et al. Trends in co-prescribing of antidepressants and tamoxifen among women with breast cancer, 2004-2010. Breast Cancer Res Treat. 2013;137:285–296. doi: 10.1007/s10549-012-2330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dusetzina SB, Higashi AS, Dorsey ER, et al. Impact of FDA drug risk communications on health care utilization and health behaviors: A systematic review. Med Care. 2012;50:466–478. doi: 10.1097/MLR.0b013e318245a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foote SB, Town RJ. Implementing evidence-based medicine through Medicare coverage decisions. Health Aff (Millwood) 2007;26:1634–1642. doi: 10.1377/hlthaff.26.6.1634. [DOI] [PubMed] [Google Scholar]
- 35.Halpern MT, Yabroff KR. Prevalence of outpatient cancer treatment in the United States: Estimates from the Medical Panel Expenditures Survey (MEPS) Cancer Invest. 2008;26:647–651. doi: 10.1080/07357900801905519. [DOI] [PubMed] [Google Scholar]


