Incorporation of US Preventive Services Task Force genetic counseling recommendations as part of primary care is feasible, and warrants further investigation as a strategy for addressing disparities in breast cancer mortality.
Abstract
Purpose:
The US Preventive Services Task Force recommends identifying candidates for breast cancer (BC) chemoprevention and referring them for genetic counseling as part of routine care. Little is known about the feasibility of implementing these recommendations or how low-income women of color might respond to individualized risk assessment (IRA) performed by primary care providers (PCPs).
Methods:
Women recruited from a federally qualified health center were given the option to discuss BC risk status with their PCP. Comprehensive IRA was performed using a software tool designed for the primary care environment combining three assessment instruments and providing risk-adapted recommendations for screening, prevention, and genetic referral. Logistic regression models assessed factors associated with wanting to learn and discuss BC risk with PCP.
Results:
Of 237 participants, only 12.7% (n = 30) did not want to discuss IRA results with their PCP. Factors associated with lower odds of wanting to learn results included having private insurance and reporting ever having had a mammogram. Factors associated with higher odds of wanting to learn results included older age (50 to 69 years) and increased BC worry. For all women wishing to learn results, IRA was successfully completed and delivered to the PCP immediately before the encounter for incorporation into the well-visit evaluation.
Conclusion:
Incorporation of US Preventive Services Task Force recommendations as part of routine primary care is feasible. Interest in IRA seems high among underserved women. This approach warrants further investigation as a strategy for addressing disparities in BC mortality.
Introduction
The US Preventive Services Task Force (USPSTF) recently recommended that primary care providers (PCPs) engage in shared decision making about medications to reduce the risk of breast cancer (BC) with women at increased risk.1 The USPSTF now also recommends that PCPs screen women with family members with breast, ovarian, fallopian tube, or primary peritoneal cancer to identify candidates for genetic counseling.2 Implementation of these and other national guidelines3–5 will require widespread application of comprehensive, quantitative BC risk assessment in primary care (PC). However, information about the feasibility and effect of individualized risk assessment (IRA) in PC settings is limited.1 Available evidence suggests this is not standard practice for US PCPs.6,7
Efforts to incorporate USPSTF recommendations into standard practice face several challenges. For example, different risk assessment models are required for identifying candidates for chemoprevention1 versus genetic counseling2 versus magnetic resonance imaging (MRI) screening as an adjunct to mammography.4 Limited research indicates that many PCPs lack sufficient time and feel unequipped to provide IRA and counseling.8,9 The optimal strategies and sites for delivering comprehensive, population-based IRA are unknown.
Women's decisions regarding genetic testing for BC risk have been extensively researched,10–12 but few studies have looked at women's decisions to undergo IRA for BC. One study that examined IRA as a standard component of PC was conducted in a private, not-for-profit health care system and was limited in the scope of the assessment, did not explore patients' attitudes, and did not report racial/ethnic or sociodemographic characteristics.13 Another study used intensive patient navigation efforts to direct women to a community-based breast health center for IRA.14 The USPSTF highlights the need for additional research, including the need for trials of different approaches to risk screening and strategies to improve access to genetic counseling and BRCA testing for high-risk individuals.2 Little is known about the effects on women who are identified as high risk in a PC environment and what happens to them after that designation.
The USPSTF also emphasizes the need for research involving diverse study populations.2 Identifying minority women at increased BC risk for targeted interventions to enhance screening may be one of the most cost-effective strategies for eliminating the BC mortality disparity that exists among underserved women of color,15 which is a national public health priority.16 Population-based risk assessment that would allow implementation of a tailored approach to cancer control with risk-specific interventions is a promising yet untested strategy for reducing BC mortality among underserved women. IRA may also support targeted interventions to improve adherence to standard screening measures among the subgroup of nonadherent women who would benefit the most from screening (ie, those at increased risk). Understanding one's BC risk may promote lifestyle modifications (eg, weight control) that have a salutary effect on both breast health and many other health outcomes. Knowledge of risk can inform other health-related decisions (eg, whether to use hormone-replacement therapy).17 Including IRA as part of routine PC of underserved women could be highly beneficial and is a strategy that should be explored.
Here we report on a pilot study to investigate the feasibility of a health system–wide policy to implement USPSTF mandates for routine BC risk assessment as part of standard PC among an ethnically diverse population of underserved women in federally qualified health centers (FQHCs). Data presented were collected as part of an exploratory study of the potential implications of administering IRA in PC clinics. We report results from baseline surveys on women's decisions to learn IRA results and discuss them with their PCP, including predictors of wanting to know results or not (including demographic characteristics and psychosocial measures).
Methods
Participants
Recruitment occurred from September 2012 to April 2013 at two FQHC clinic sites in Chicago. Female patients age 25 to 69 years18–20 with no previous history of BC and at least one intact breast presenting for a scheduled annual well-visit appointment with their PCP were invited to participate in a study on women's views of BC. Of 448 women identified as eligible for participation, 261 were approached, 246 agreed to participate, and 243 enrolled (Figure 1).
Figure 1.
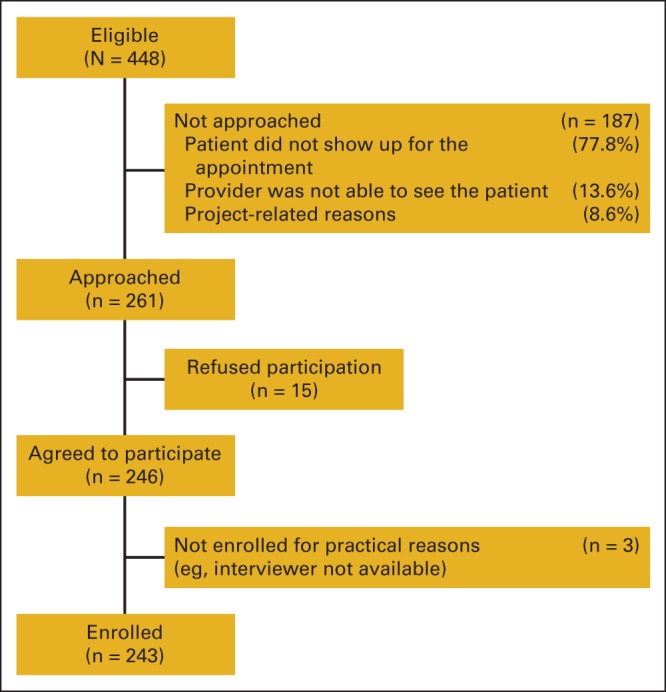
Eligibility and recruitment.
Research staff administered a baseline survey and collected risk factor data from all participants immediately before their visit with the PCP. For those wishing to learn their IRA results, these were calculated immediately before seeing the PCP. The protocol was approved by institutional review board of the University of Illinois at Chicago.
Measures
Individualized BC risk assessment.
The BC Risk Screening (BRS) tool created for this study includes the modified version of the Gail model,21 available as the National Cancer Institute Breast Cancer Risk Assessment Tool (which also includes the CARE [Contraceptive and Reproductive Experiences] model developed for African American women22) and the Claus model.23 We chose models recommended by standard guidelines for use in identifying candidates for specific screening and risk-reduction interventions. The US Food and Drug Administration (FDA) indication for tamoxifen and raloxifene as BC chemoprevention agents is based on a Gail model assessment, and national guidelines endorse the Gail model for identifying chemoprevention candidates.24 The American College of Surgeons (ACS) endorses the Claus model as one of three models that can be used to identify candidates for screening breast MRI.4 Of these, the Claus model is the only one that could be readily incorporated into the BRS tool. However, the Gail and Claus models do not account for several features that indicate increased risk for hereditary BC and may fail to identify women at risk for hereditary breast or ovarian cancer and non-BRCA hereditary syndromes.17 Therefore, the BRS tool also includes the pedigree assessment tool (PAT),25,26 a component for evaluating family history to identify women at risk for BRCA-related and non-BRCA hereditary BCs who require referral to genetic counseling. The PAT, developed by one of the authors (K.F.H.) for screening women in PC,25,26 is one of four instruments recommended by the USPSTF for identifying women who should be referred for genetic counseling. None of these instruments has been shown to be superior to the others.2
The BRS tool is a Web-based software application that combines data entry for all three instruments and calculates results for each model in a single step. It also provides guideline-based recommendations for screening, prevention, and genetic counseling referral based on assessment results in one document. The tool was administered by research staff members (with no prior medical training) who received 4 hours of tool-specific training. The tool provides risk-adapted recommendations for screening, including initiation of more frequent clinical breast examinations and early initiation of mammography per National Comprehensive Cancer Network guidelines.27 It identifies candidates for breast MRI as an adjunct to mammography based on ACS guidelines.4 It also provides recommendations for cancer risk-reduction (lifestyle) modifications for reducing BC risk for women in all risk categories based on ACS recommendations, chemoprevention for women at increased risk meeting the FDA indication,28 and referral for genetic counseling based on results of the PAT and current NCCN guidelines.27,29 The tool assigns a patient to one of three risk categories based on a composite risk assessment derived from the Gail, Claus, and PAT models: general population risk (5-year risk < 1.7% and lifetime risk ≤ 12%), moderately increased risk (5-year risk ≥ 1.7% or lifetime risk 13% to 20%), or high risk (lifetime risk ≥ 20% or PAT score ≥ 8). Only women with possible hereditary BC syndromes according to the Claus model and PAT were assigned to the high-risk category.2,4 A Claus estimate of ≥ 20% lifetime risk was chosen, because that represents the cut point in the ACS guidelines for adding breast MRI as an adjunct to mammography for women with hereditary risk.4 A PAT score of ≥ 8 was identified in validation studies as the optimal threshold for referral for genetic counseling.25 The moderate-risk group represented women who might be candidates for chemoprevention or enhanced screening with more frequent clinical breast examinations or earlier initiation of mammography but who did not meet criteria for MRI screening or referral for genetic counseling (ie, are not at high risk for hereditary cancer syndrome). Because the PAT was designed specifically to identify women at risk for a hereditary syndrome, it was not used to assign women to the moderate-risk category. For our analyses here, we grouped women at moderately increased risk and high risk into one group (ie, increased risk).
Baseline survey.
Participants were surveyed at baseline (before meeting with their PCP and learning their risk level) to obtain information on demographic characteristics and measures hypothesized to correlate with the interest in learning BC risk assessment results.30–35 Research staff administered surveys using an electronic data capture system while patients waited to see their PCP.36
Decision to learn risk assessment results.
After all data were collected, participants were given the option to learn their individual risk level from their PCP. For participants who wanted to know their IRA results, research staff calculated risk estimates and delivered results to the PCP to be discussed at that visit. Hardcopy printouts of the assessment and recommendations were scanned into the electronic health record. If a participant declined to learn the assessment results, the risk calculation was performed at a later date after data were deidentified; the PCP did not receive the assessment, and it was not included in the medical record.
Data Analysis
Bivariate relationships between demographic and psychosocial variables and whether participants wanted to learn and discuss the IRA were compared. We used the χ2 test for categorical variables and the Fisher's exact test for categorical variables with small cell sizes. The Wilcoxon rank sum test was used for continuous variables. The dependent measure used in multivariable analysis was agreeing to learn and discuss risk results with the PCP. A logistic regression model was used to estimate the factors that influenced agreeing to learn and discuss risk results with the PCP. Clinic and risk status were excluded from the regression model because of the presence of cells with no subjects. All analyses were conducted using STATA software (version 13.0; STATA, College Station, TX).
Results
Sample
Table 1 lists participants' demographic characteristics. Of 237 participants, 30 (12.7%) decided not to learn the results of their IRA. All 30 women (100%) who refused to learn their IRA results were later identified as being at average risk for BC. Of the 207 women who desired to know their risk status, 191 (92.3%) were identified as being at average risk, and 16 (7.7%) were identified as being at increased risk.
Table 1.
Characteristics by Participant Decision to Learn and Discuss IRA Results and Recommendations With PCP
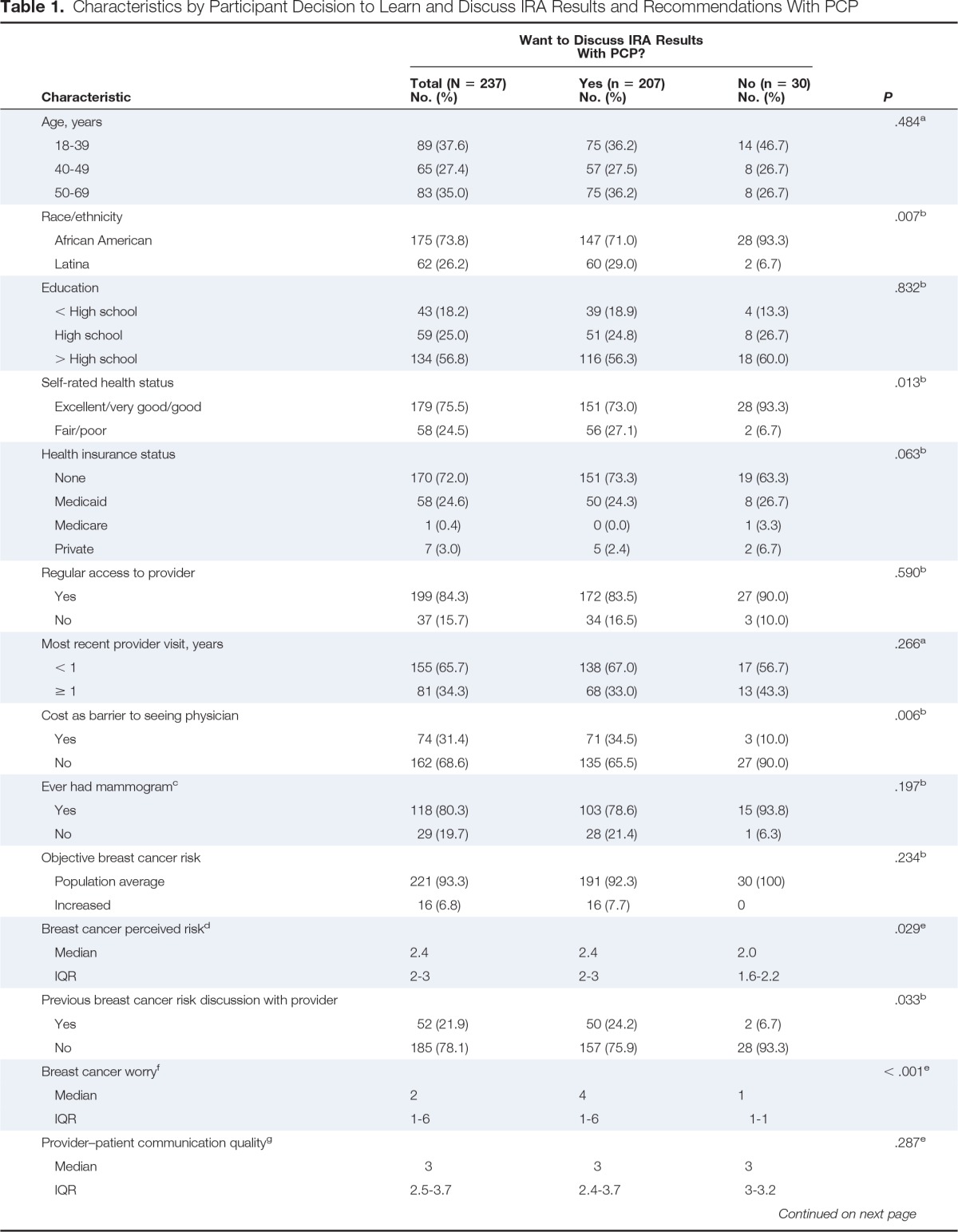
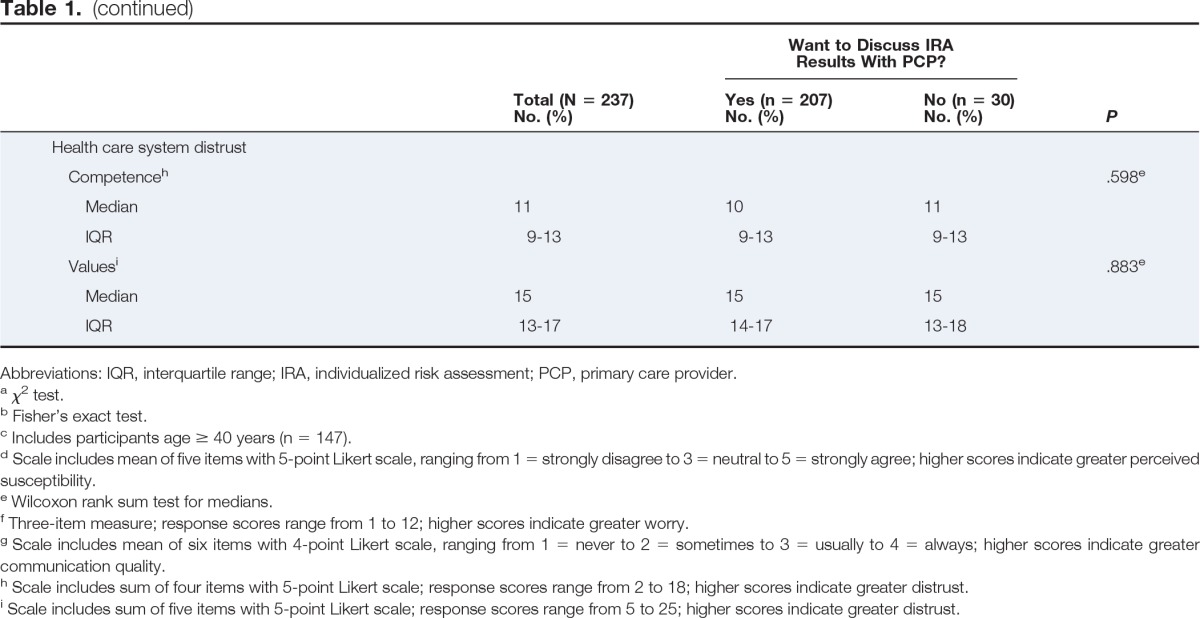
| Characteristic | Want to Discuss IRA Results With PCP? |
P | ||
|---|---|---|---|---|
| Total (N = 237) No. (%) | Yes (n = 207) No. (%) | No (n = 30) No. (%) | ||
| Age, years | .484a | |||
| 18-39 | 89 (37.6) | 75 (36.2) | 14 (46.7) | |
| 40-49 | 65 (27.4) | 57 (27.5) | 8 (26.7) | |
| 50-69 | 83 (35.0) | 75 (36.2) | 8 (26.7) | |
| Race/ethnicity | .007b | |||
| African American | 175 (73.8) | 147 (71.0) | 28 (93.3) | |
| Latina | 62 (26.2) | 60 (29.0) | 2 (6.7) | |
| Education | .832b | |||
| < High school | 43 (18.2) | 39 (18.9) | 4 (13.3) | |
| High school | 59 (25.0) | 51 (24.8) | 8 (26.7) | |
| > High school | 134 (56.8) | 116 (56.3) | 18 (60.0) | |
| Self-rated health status | .013b | |||
| Excellent/very good/good | 179 (75.5) | 151 (73.0) | 28 (93.3) | |
| Fair/poor | 58 (24.5) | 56 (27.1) | 2 (6.7) | |
| Health insurance status | .063b | |||
| None | 170 (72.0) | 151 (73.3) | 19 (63.3) | |
| Medicaid | 58 (24.6) | 50 (24.3) | 8 (26.7) | |
| Medicare | 1 (0.4) | 0 (0.0) | 1 (3.3) | |
| Private | 7 (3.0) | 5 (2.4) | 2 (6.7) | |
| Regular access to provider | .590b | |||
| Yes | 199 (84.3) | 172 (83.5) | 27 (90.0) | |
| No | 37 (15.7) | 34 (16.5) | 3 (10.0) | |
| Most recent provider visit, years | .266a | |||
| < 1 | 155 (65.7) | 138 (67.0) | 17 (56.7) | |
| ≥ 1 | 81 (34.3) | 68 (33.0) | 13 (43.3) | |
| Cost as barrier to seeing physician | .006b | |||
| Yes | 74 (31.4) | 71 (34.5) | 3 (10.0) | |
| No | 162 (68.6) | 135 (65.5) | 27 (90.0) | |
| Ever had mammogramc | .197b | |||
| Yes | 118 (80.3) | 103 (78.6) | 15 (93.8) | |
| No | 29 (19.7) | 28 (21.4) | 1 (6.3) | |
| Objective breast cancer risk | .234b | |||
| Population average | 221 (93.3) | 191 (92.3) | 30 (100) | |
| Increased | 16 (6.8) | 16 (7.7) | 0 | |
| Breast cancer perceived riskd | .029e | |||
| Median | 2.4 | 2.4 | 2.0 | |
| IQR | 2-3 | 2-3 | 1.6-2.2 | |
| Previous breast cancer risk discussion with provider | .033b | |||
| Yes | 52 (21.9) | 50 (24.2) | 2 (6.7) | |
| No | 185 (78.1) | 157 (75.9) | 28 (93.3) | |
| Breast cancer worryf | < .001e | |||
| Median | 2 | 4 | 1 | |
| IQR | 1-6 | 1-6 | 1-1 | |
| Provider–patient communication qualityg | .287e | |||
| Median | 3 | 3 | 3 | |
| IQR | 2.5-3.7 | 2.4-3.7 | 3-3.2 | |
| Health care system distrust | ||||
| Competenceh | .598e | |||
| Median | 11 | 10 | 11 | |
| IQR | 9-13 | 9-13 | 9-13 | |
| Valuesi | .883e | |||
| Median | 15 | 15 | 15 | |
| IQR | 13-17 | 14-17 | 13-18 | |
Abbreviations: IQR, interquartile range; IRA, individualized risk assessment; PCP, primary care provider.
χ2 test.
Fisher's exact test.
Includes participants age ≥ 40 years (n = 147).
Scale includes mean of five items with 5-point Likert scale, ranging from 1 = strongly disagree to 3 = neutral to 5 = strongly agree; higher scores indicate greater perceived susceptibility.
Wilcoxon rank sum test for medians.
Three-item measure; response scores range from 1 to 12; higher scores indicate greater worry.
Scale includes mean of six items with 4-point Likert scale, ranging from 1 = never to 2 = sometimes to 3 = usually to 4 = always; higher scores indicate greater communication quality.
Scale includes sum of four items with 5-point Likert scale; response scores range from 2 to 18; higher scores indicate greater distrust.
Scale includes sum of five items with 5-point Likert scale; response scores range from 5 to 25; higher scores indicate greater distrust.
Bivariate analysis (Table 1) revealed that a greater percentage of participants who did not want to discuss risk with their PCP were African American (P = .007), reported excellent/very good or good health status (P = .01), did not view cost as a barrier to seeing a physician (P = .006), had lower perceived BC risk (P = .02), had not had a previous discussion with their provider about BC risk (P = .03), and reported lower levels of worry (P ≤ .001).
Predictors of Decision to Learn Results of BC Risk Assessment
Table 2 summarizes the logistic regression analyses. Compared with women age < 40 years, those in the age categories of 40 to 49 and 50 to 69 years were more likely to want to learn and discuss risk results with their PCP (odds ratio [OR], 5.4; 95% CI, 1.09 to 26.67 and OR, 7.99; 95% CI, 1.47 to 43.44, respectively). Compared with those having no insurance, women with private insurance were significantly less likely to want to learn and discuss IRA results (OR, 0.07; 95% CI, 0.01 to 0.76). Women who had ever had a mammogram were less likely to want to learn and discuss risk results (OR, 0.23; 95% CI, 0.05 to 0.95). As worry about BC increased, women were more likely to want to learn and discuss IRA results (OR, 1.52; 95% CI, 1.12 to 2.06).
Table 2.
Characteristics Associated With Agreeing to Discuss IRA Results and Recommendations for Screening and Prevention With PCP
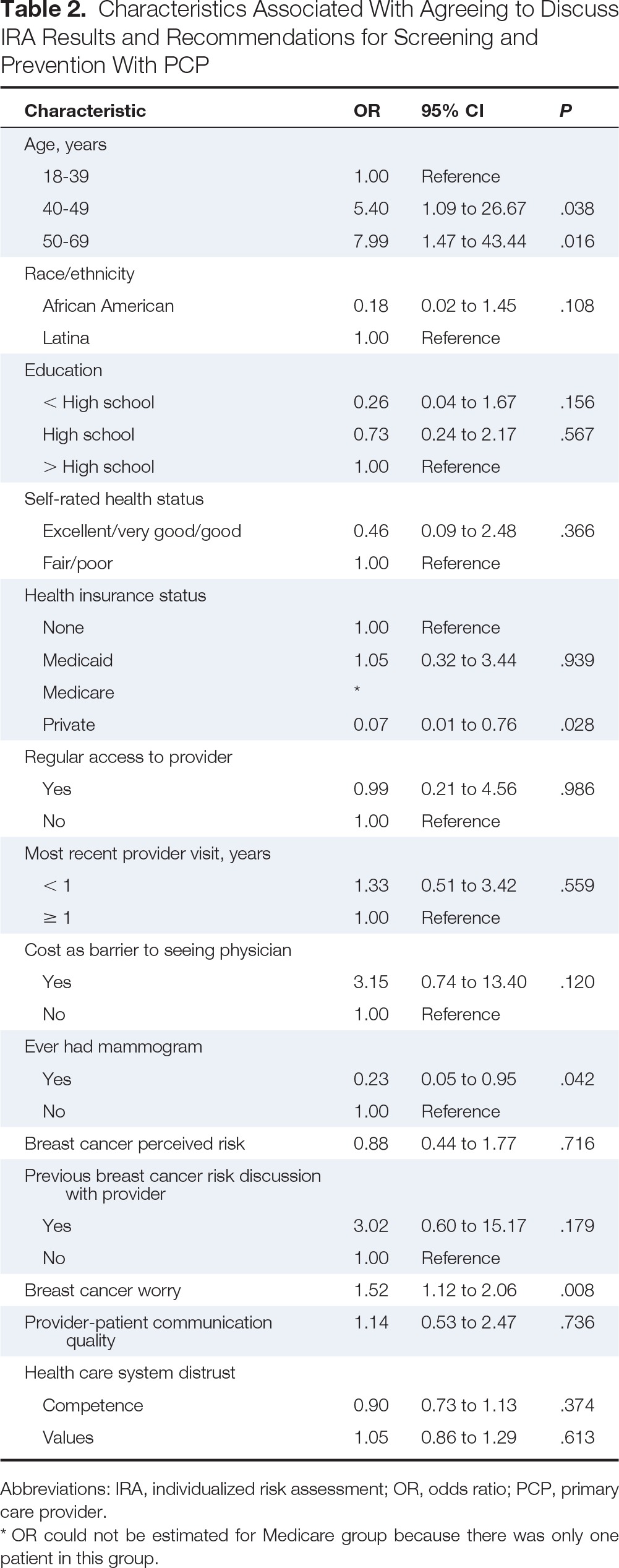
| Characteristic | OR | 95% CI | P |
|---|---|---|---|
| Age, years | |||
| 18-39 | 1.00 | Reference | |
| 40-49 | 5.40 | 1.09 to 26.67 | .038 |
| 50-69 | 7.99 | 1.47 to 43.44 | .016 |
| Race/ethnicity | |||
| African American | 0.18 | 0.02 to 1.45 | .108 |
| Latina | 1.00 | Reference | |
| Education | |||
| < High school | 0.26 | 0.04 to 1.67 | .156 |
| High school | 0.73 | 0.24 to 2.17 | .567 |
| > High school | 1.00 | Reference | |
| Self-rated health status | |||
| Excellent/very good/good | 0.46 | 0.09 to 2.48 | .366 |
| Fair/poor | 1.00 | Reference | |
| Health insurance status | |||
| None | 1.00 | Reference | |
| Medicaid | 1.05 | 0.32 to 3.44 | .939 |
| Medicare | * | ||
| Private | 0.07 | 0.01 to 0.76 | .028 |
| Regular access to provider | |||
| Yes | 0.99 | 0.21 to 4.56 | .986 |
| No | 1.00 | Reference | |
| Most recent provider visit, years | |||
| < 1 | 1.33 | 0.51 to 3.42 | .559 |
| ≥ 1 | 1.00 | Reference | |
| Cost as barrier to seeing physician | |||
| Yes | 3.15 | 0.74 to 13.40 | .120 |
| No | 1.00 | Reference | |
| Ever had mammogram | |||
| Yes | 0.23 | 0.05 to 0.95 | .042 |
| No | 1.00 | Reference | |
| Breast cancer perceived risk | 0.88 | 0.44 to 1.77 | .716 |
| Previous breast cancer risk discussion with provider | |||
| Yes | 3.02 | 0.60 to 15.17 | .179 |
| No | 1.00 | Reference | |
| Breast cancer worry | 1.52 | 1.12 to 2.06 | .008 |
| Provider-patient communication quality | 1.14 | 0.53 to 2.47 | .736 |
| Health care system distrust | |||
| Competence | 0.90 | 0.73 to 1.13 | .374 |
| Values | 1.05 | 0.86 to 1.29 | .613 |
Abbreviations: IRA, individualized risk assessment; OR, odds ratio; PCP, primary care provider.
OR could not be estimated for Medicare group because there was only one patient in this group.
Discussion
This study demonstrates that the use of a tool providing a rapid, comprehensive BC risk assessment and tailored recommendations based on national guidelines successfully overcomes two principal barriers to performance of IRA in the PC environment: lack of time and insufficient provider knowledge.2,8,9 This strategy identifies women who are appropriate for consideration of chemoprevention and enhanced screening, as well as those who should be referred for genetic counseling for possible hereditary BC risk.
It is noteworthy that although the study protocol stipulated that patients be brought to the examination room when clinic staff were ready to room the patient, regardless of whether they had completed all research activities, IRA was completed for all 207 women who desired to know IRA results. The assessments were performed by research associates without prior medical training and only 4 hours of training on using the BRS tool. Thus, we have demonstrated that it is feasible to implement USPSTF mandates for BC IRA as part of standard well-care visits in urban FQHCs without disrupting normal clinic flow or provider schedules, using nonlicensed clinic staff with minimal training.
This approach also seems to be acceptable to the large majority of African American and Latina women seen at an urban FQHC. This study makes a unique contribution to the literature on the use of BC risk assessment in PC, which thus far has been implemented mostly in specialty care clinics and therefore limited primarily to insured white women.13 Better understanding the decisions of low-income women of color across risk levels can ensure that IRA is implemented in an ethical and respectful manner that minimizes potential psychosocial harm and enhances potential health benefits.37
Most women in our sample chose to learn their IRA results. However, although the proportion of women who chose not to learn their IRA results (12.7%) was relatively small, it may be clinically significant. More research is needed to explore the reasons why some women may not want to undergo IRA and the potential implications of these reasons for systems-level policies.
The decision to opt in or out of learning risk assessment results was not associated with perceived or actual risk. Level of worry, age, insurance status, and ever having had a mammogram were associated with this decision. Women wanting to know their risk assessment results worried more about BC. Other studies have found similar results; compared with those with at least a moderate amount of worry, women with low BC worry are less likely to report recent mammography screening.38 Older age (> 40 years) was associated with wanting to learn risk results. It may be that older women are more willing to learn about their risk, because they are age eligible for mammography screening and may therefore have more information about BC available to them. Furthermore, women with private insurance were less likely to want to learn results. Insured women may have felt that they could discuss information about BC with their provider at any time, whereas women without health insurance may have felt the need to take advantage of all information and tests offered, because they may not return for care soon. Finally, women who refused risk results were more likely to have had a previous mammogram. If their previous mammogram results were normal, women may have felt that they did not have to worry about BC or discuss it with their PCP.
The primary limitation of this study is that we were unable to gather more information regarding reasons for refusal from the 30 women who did not want to know their IRA results (only one agreed to a follow-up interview). It is uncertain whether they were making a clear, informed choice not to know this information or if their refusal was merely related to concerns about time commitment, burden of continued research participation, or a lack of understanding of risk assessment. Another limitation is that our study population may not be representative of all low-income women of color seeking PC at urban FQHCs. Our sample of potentially eligible women had high no-show rates, and we recruited only from the pool of eligible women who presented for a well-care visit during our recruitment period. Participants also reported rates of mammography similar to those reported in national surveys, indicating a certain level of participation in preventive health care. Furthermore, because we only recruited women scheduled for an annual well-care visit, these patients were more likely to be concerned about their health. Also, our population was generally healthy compared with the entire clinic population (75% of our participants rated their health status as excellent, very good, or good). Thus, we do not have any information about the acceptability of IRA to women who are not regularly accessing care or who are dealing with chronic disease or acute illness. Further research is needed to understand the acceptability of BC risk assessment to women dealing with significant health issues or not regularly engaged with the health care system, who may have different attitudes toward preventive care and health care use.
We did not study adherence to screening and prevention recommendations. The ultimate public health impact of widespread risk assessment and implementation of recent guidelines advocating enhanced interventions for women at increased BC risk1,2 will largely depend on patient adherence to recommended interventions. This will require that some now-healthy patients access screening services more frequently and accept preventive medical and/or surgical interventions previously used to treat disease. More research is needed to address this issue and identify potential barriers to adherence. Although the risk of psychological harm (eg, stress, worry) or unnecessary testing or interventions resulting from overinflated risk is primarily theoretic,39 one study did find IRA to have potentially adverse effects on mammography rates in poorly educated women.40 More longitudinal research is needed on the impact of IRA on health and health care behavior.
Our ongoing work will address questions related to adherence as well as the potential emotional and psychological sequelae for women who learn that they are at high risk for developing BC in a PC context without immediate access to genetic counselors or other specialized cancer risk professionals. Research is needed to determine how best to educate PCPs on risk assessment guidelines, enhance their comfort in talking about BC risk, and improve their communication skills. Research is also needed to determine how best to provide adequate patient education and what care delivery model integrates genetic counselors and other cancer risk specialists into the assessment, counseling, and patient management processes in the most cost-effective manner. It will be important to demonstrate that implementation of USPSTF guidelines has the desired impact on population health.
Acknowledgment
Supported by Awards No. 2P50CA106743(Center)-07S2 (bioethics supplement) and -07S1 (diversity supplement) from the National Cancer Institute and award UL1RR029879, National Center for Advancing Translational Sciences. Development of the Breast Cancer Risk Screening tool was supported by OSF Saint Anthony Foundation, Rockford, IL.
Presented in part at the Fifth Annual American Association for Cancer Research Conference on the Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, San Diego, CA, October 28, 2012; and as a poster at the 2013 Centers for Population Health and Health Disparities Annual Meeting, Boston, MA, May 1, 2013.
We thank our collaborators at Chicago Family Health Centers, Alicia Carrillo, Veena Korah, Loraine Moreno, and Maria Rojas, and all of our participants. The source code for the National Cancer Institute Breast Cancer Risk Assessment Tool was generously provided by Mitchell Gail and David Pee.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Collection and assembly of data: Emily E. Anderson, Silvia Tejeda
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Breast Cancer Risk Assessment Among Low-Income Women of Color in Primary Care: A Pilot Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Emily E. Anderson
No relationship to disclose
Silvia Tejeda
No relationship to disclose
Kimberly Childers
No relationship to disclose
Melinda R. Stolley
No relationship to disclose
Richard B. Warnecke
No relationship to disclose
Kent F. Hoskins
No relationship to disclose
References
- 1.Moyer VA. Medications for risk reduction of primary breast cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:698–708. doi: 10.7326/0003-4819-159-10-201311190-00717. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:271–281. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 3.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 5.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 6.Burke W, Culver J, Pinsky L, et al. Genetic assessment of breast cancer risk in primary care practice. Am J Med Genet A. 2009;149A:349–356. doi: 10.1002/ajmg.a.32643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272–279. doi: 10.3122/jabfm.2009.03.080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katapodi MC, Lee KA, Facione NC, et al. Predictors of perceived breast cancer risk and the relation between perceived risk and breast cancer screening: A meta-analytic review. Prev Med. 2004;38:388–402. doi: 10.1016/j.ypmed.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Katapodi MC, Dodd MJ, Lee KA, et al. Underestimation of breast cancer risk: Influence on screening behavior. Oncol Nurs Forum. 2009;36:306–314. doi: 10.1188/09.ONF.306-314. [DOI] [PubMed] [Google Scholar]
- 10.Glenn B, Chawla N, Bastani R. Barriers to genetic testing for breast cancer risk among ethnic minority women: An exploratory study. Ethn Dis. 2012;22:267–273. [PubMed] [Google Scholar]
- 11.Hughes C, Gomez-Caminero A, Benkendorf J, et al. Ethnic differences in knowledge and attitudes about BRCA1 testing in women at increased risk. Patient Educ Couns. 1997;32:51–62. doi: 10.1016/s0738-3991(97)00064-5. [DOI] [PubMed] [Google Scholar]
- 12.Thompson HS, Valdimarsdottir HB, Jandorf L, et al. Perceived disadvantages and concerns about abuses of genetic testing for cancer risk: Differences across African American, Latina and Caucasian women. Patient Educ Couns. 2003;51:217–227. doi: 10.1016/s0738-3991(02)00219-7. [DOI] [PubMed] [Google Scholar]
- 13.Owens WL, Gallagher TJ, Kincheloe MJ, et al. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7:85–88. doi: 10.1200/JOP.2010.000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mays D, Sharff M, DeMarco T, et al. Outcomes of a systems-level intervention offering breast cancer risk assessments to low-income underserved women. Fam Cancer. 2012;11:493–502. doi: 10.1007/s10689-012-9541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandelblatt JS, Schechter CB, Yabroff KR, et al. Benefits and costs of interventions to improve breast cancer outcomes in African American women. J Clin Oncol. 2004;22:2554–2566. doi: 10.1200/JCO.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. ed 2. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 17.Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med. 2000;342:564–571. doi: 10.1056/NEJM200002243420807. [DOI] [PubMed] [Google Scholar]
- 18.Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: Breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 19.Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727–737. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 22.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 23.Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643–651. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 25.Hoskins KF, Zwaagstra A, Ranz M. Validation of a tool for identifying women at high risk for hereditary breast cancer in population-based screening. Cancer. 2006;107:1769–1776. doi: 10.1002/cncr.22202. [DOI] [PubMed] [Google Scholar]
- 26.Teller P, Hoskins KF, Zwaagstra A, et al. Validation of the pedigree assessment tool (PAT) in families with BRCA1 and BRCA2 mutations. Ann Surg Oncol. 2010;17:240–246. doi: 10.1245/s10434-009-0697-9. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network. NCCN guidelines: Breast cancer screening and diagnosis, 2013. www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp.
- 28.Waters E, McNeel T, Stevens W, et al. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134:875–880. doi: 10.1007/s10549-012-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. NCCN guidelines: Breast cancer risk reduction, 2013. www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp.
- 30.Buchanan AH, Skinner CS, Rawl SM, et al. Patients' interest in discussing cancer risk and risk management with primary care physicians. Patient Educ Couns. 2005;57:77–87. doi: 10.1016/j.pec.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Behavioral risk factor surveillance system survey questionnaire. www.cdc.gov/brfss/questionnaires/index.htm.
- 32.Champion VL, Monahan PO, Springston JK, et al. Measuring mammography and breast cancer beliefs in African American women. J Health Psychol. 2008;13:827–837. doi: 10.1177/1359105308093867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman C, Trock B, Rimer BK, et al. Psychological side effects of breast cancer screening. Health Psychol. 1991;10:259–267. doi: 10.1037//0278-6133.10.4.259. [DOI] [PubMed] [Google Scholar]
- 34.Shea J, Micco E, Dean L, et al. Development of a revised health care system distrust scale. J Gen Intern Med. 2008;23:727–732. doi: 10.1007/s11606-008-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Underhill ML, Kiviniemi MT. The association of perceived provider-patient communication and relationship quality with colorectal cancer screening. Health Educ Behav. 2012;39:555–563. doi: 10.1177/1090198111421800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris P, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartle-Haring S, Toviessi P, Katafiasz H. Predicting the use of individualized risk assessment for breast cancer. Womens Health Issues. 2008;18:100–109. doi: 10.1016/j.whi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen MR, Smith R, Meischke H, et al. Breast cancer worry and mammography use by women with and without a family history in a population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12:314–320. [PubMed] [Google Scholar]
- 39.Anderson E, Hoskins K. Individual breast cancer risk assessment in underserved populations: Integrating empirical bioethics and health disparities research. J Health Care Poor Underserved. 2012;23(suppl):34–46. doi: 10.1353/hpu.2012.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz MD, Rimer BK, Daly M, et al. A randomized trial of breast cancer risk counseling: The impact on self-reported mammography use. Am J Public Health. 1999;89:924–926. doi: 10.2105/ajph.89.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]


