Neoadjuvant treatment may result in better quality of life and functional status 1 year after diagnosis.
Abstract
Purpose:
Many patients do not receive guideline-recommended neoadjuvant chemoradiotherapy for resectable rectal cancer. Little is known regarding long-term quality of life (QOL) associated with various treatment approaches. Our objective was to determine patient characteristics and subsequent QOL associated with treatment approach.
Methods:
Our study was a geographically diverse population- and health system–based cohort study that included adults age 21 years or older with newly diagnosed stage II/III rectal cancer who were recruited from 2003 to 2005. Eligible patients were contacted 1 to 4 months after diagnosis and asked to participate in a telephone survey and to consent to medical record review, with separate follow-up QOL surveys conducted 1 and 7 years after diagnosis.
Results:
Two hundred thirty-nine patients with stage II/III rectal cancer were included in this analysis. Younger age (< 65 v ≥ 65 years: odds ratio, 2.49; 95% CI, 1.33 to 4.65) was significantly associated with increased odds of receiving neoadjuvant or adjuvant chemoradiotherapy. The adjuvant chemoradiotherapy group had significantly worse mean EuroQol-5D (range, 0 to 1) and Short Form-12 physical health component scores (standardized mean, 50) at 1-year follow-up than the neoadjuvant chemoradiotherapy group (0.75 v 0.85; P = .002; 37.2 v 43.3; P = .01, respectively) and the group that received only one or neither form of treatment (0.75 v 0.85; P = .02; 37.2 v 45.1; P = .008, respectively).
Conclusion:
Neoadjuvant treatment may result in better QOL and functional status 1 year after diagnosis. Further evaluation of patient and provider reasons for not pursuing neoadjuvant therapy is necessary to determine how and where to target process improvement and/or education efforts to ensure that patients have access to recommended treatment options.
Introduction
Since 1990, a multimodality approach of surgery and chemoradiotherapy has been the standard of care for stages II/III rectal cancer.1 Subsequently, neoadjuvant chemoradiotherapy was shown to improve local control and reduce toxicity in the German Rectal Cancer Study Group trial that was published in 20042; these results persisted after a median follow-up of 11 years.3 Although neoadjuvant chemoradiotherapy is the National Comprehensive Cancer Network (NCCN) guideline–recommended treatment approach,4 many patients do not receive neoadjuvant or even adjuvant chemoradiotherapy.
Analyses of SEER data demonstrate that the proportion of patients with stages II and III rectal cancer who do not receive any radiotherapy is slowly decreasing,5,6 but approximately one quarter of patients diagnosed in 2010 still did not receive radiotherapy.7 The reasons for non–guideline concordant care are unclear.5,6,8 Studies that were based on SEER data for those with stages II and III rectal cancer found that patients who did not receive radiotherapy were older compared with those who did receive it.5,6,9,10 Black patients are also less likely to receive radiotherapy compared with white patients.9–11 Unfortunately, SEER data do not allow for examination of patient preferences, comorbidities, adverse therapy effects, and other clinical factors that may elucidate possible explanations for nonreceipt of chemoradiotherapy.
Although neoadjuvant chemoradiotherapy has been demonstrated to decrease toxicity and local recurrence compared with adjuvant chemoradiotherapy, few studies have examined long-term differences in quality of life (QOL) between the two approaches.12,13 Neoadjuvant or adjuvant chemoradiotherapy can increase the risk for long-term bowel/anorectal, urinary, and sexual dysfunction.13–24 In this study, we address these gaps in our understanding by using data from CanCORS (the Cancer Care Outcomes Research and Surveillance Consortium). The two objectives of this study were to examine patient characteristics associated with the receipt and sequence of therapy, and to evaluate the impact of therapy receipt and sequence on long-term QOL and functional status among disease-free survivors.
Methods
Study Population and Design
CanCORS is a geographically diverse population- and health system–based cohort study that included 4,723 adults age 21 years or older with newly diagnosed, pathologically confirmed, invasive colorectal cancer who were recruited between 2003 and 2005. Patients were recruited from four geographically based cancer registries in Northern California, Los Angeles County, North Carolina, and Alabama, from five large health maintenance organizations (HMOs) that are part of the Cancer Research Network, and from five Veterans Affairs (VA) hospitals.
As described previously,25 eligible patients from these sites were contacted approximately 4 months after cancer diagnosis and asked to participate in a baseline telephone survey, with interview type (full, brief, surrogate interview for live patient, surrogate interview for deceased patient) dependent on patient status. Interviews included questions about sociodemographic characteristics, treatments, providers, goals/beliefs/preferences with regard to treatment options, symptoms, and quality of life.26
Medical records were abstracted from 3 months before diagnosis through at least 15 months after diagnosis. The CanCORS medical record abstraction (MRA) database contains information on tumor characteristics and the acute treatment phase, including provider types visited, staging procedures, and surgery, chemotherapy, and radiotherapy regimens.25–27 Medical record information was also used to assign American Joint Committee on Cancer collaborative stage28 and to determine Adult Comorbidity Evaluation–2729 comorbidity indicators.
CanCORS participants were also surveyed approximately 1 and 7 years after diagnosis. Functional status was measured at baseline and in both follow-up surveys using the Short Form-12 (SF-12).30,31 QOL was measured by the EuroQoL-5D (EQ-5D) scale.32–34 Defecation problems such as frequency of bowel movements, unintentional release of stools, difficulty or pain with moving bowels, and blood with stools were assessed via a Defecation Scale developed by CanCORS investigators.26 The lower the Defecation Scale score, the more problems reported by the participant (ie, higher scores correspond to better function).
Analyses included patients with stage II or III adenocarcinoma of the rectum with no previous history of cancer, except for nonmelanoma skin cancers. Stage was based on a hierarchy of best available evidence with Collaborative Stage (calculated American Joint Committee on Cancer stage based on medical record–abstracted tumor size, extension, lymph nodes, and metastases) at the top of the hierarchy. Pretreatment clinical stage was generally used for patients who received neoadjuvant therapy as opposed to pathologic staging at surgery. The algorithm for determining stage is described in detail elsewhere.35 Those with tumors at the rectosigmoid junction were excluded, given that administration of chemotherapy and radiotherapy is not included in guidelines for higher lesions. The study was approved by human subjects committees at all participating institutions.
Data Analysis
Patients were divided into two groups: first, those who received neoadjuvant chemoradiotherapy or adjuvant chemoradiotherapy; and second, those who received only chemotherapy or only radiotherapy (regardless of when administered) or neither. χ2 and Fisher's exact tests were used to compare the groups on key variables obtained from the CanCORS survey and MRA database. Multivariable logistic regression was used to examine patient characteristics associated with the two groups described; all variables listed in Table 1 and Appendix Table A1 (online only) were considered for inclusion in the models. Variables that were not significant predictors after adjustment for other covariates (P > .10) were removed in a backward selection process.
Table 1.
Patient Demographic and Clinical Characteristics
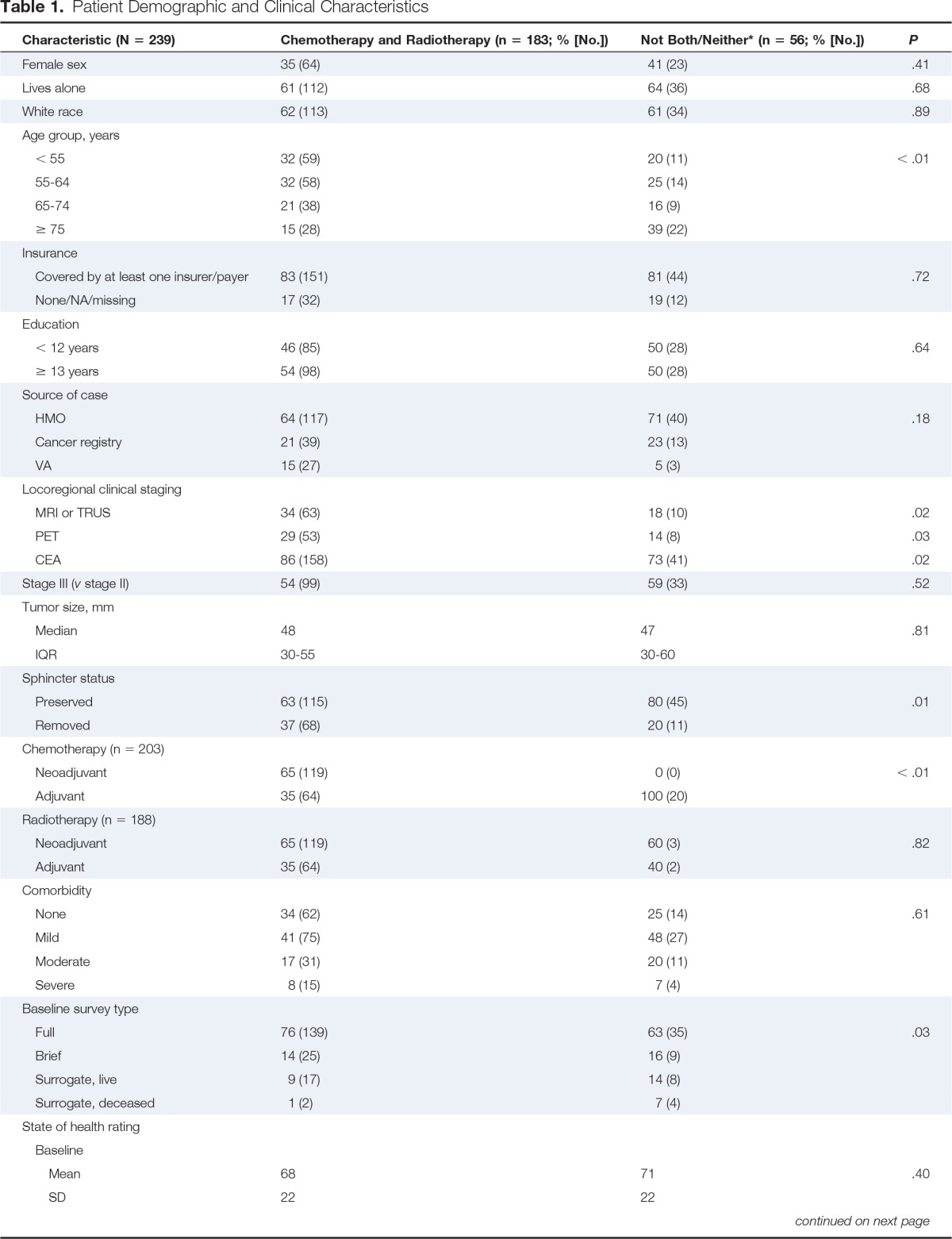
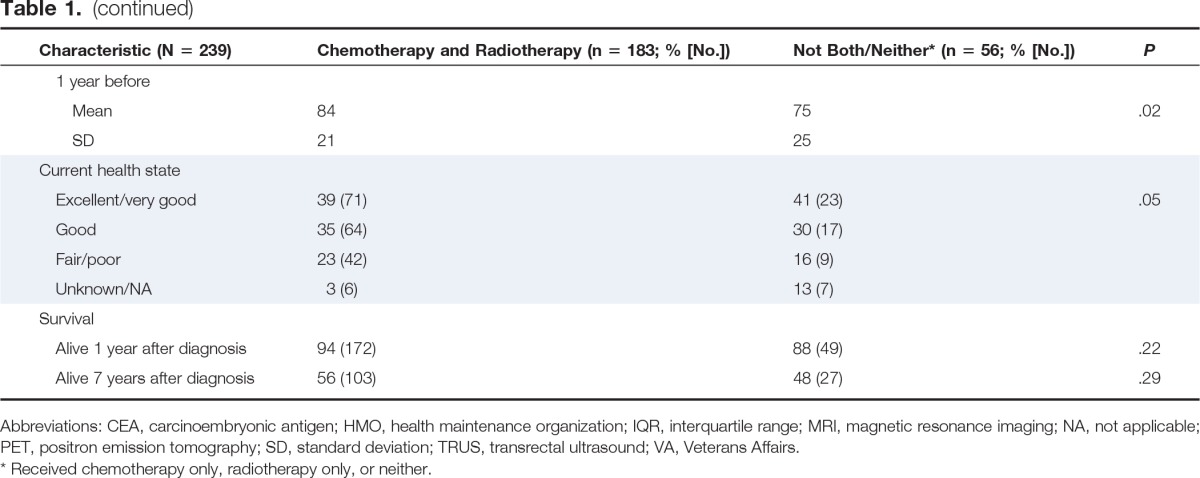
| Characteristic (N = 239) | Chemotherapy and Radiotherapy (n = 183; % [No.]) | Not Both/Neither* (n = 56; % [No.]) | P |
|---|---|---|---|
| Female sex | 35 (64) | 41 (23) | .41 |
| Lives alone | 61 (112) | 64 (36) | .68 |
| White race | 62 (113) | 61 (34) | .89 |
| Age group, years | |||
| < 55 | 32 (59) | 20 (11) | < .01 |
| 55-64 | 32 (58) | 25 (14) | |
| 65-74 | 21 (38) | 16 (9) | |
| ≥ 75 | 15 (28) | 39 (22) | |
| Insurance | |||
| Covered by at least one insurer/payer | 83 (151) | 81 (44) | .72 |
| None/NA/missing | 17 (32) | 19 (12) | |
| Education | |||
| < 12 years | 46 (85) | 50 (28) | .64 |
| ≥ 13 years | 54 (98) | 50 (28) | |
| Source of case | |||
| HMO | 64 (117) | 71 (40) | .18 |
| Cancer registry | 21 (39) | 23 (13) | |
| VA | 15 (27) | 5 (3) | |
| Locoregional clinical staging | |||
| MRI or TRUS | 34 (63) | 18 (10) | .02 |
| PET | 29 (53) | 14 (8) | .03 |
| CEA | 86 (158) | 73 (41) | .02 |
| Stage III (v stage II) | 54 (99) | 59 (33) | .52 |
| Tumor size, mm | |||
| Median | 48 | 47 | .81 |
| IQR | 30-55 | 30-60 | |
| Sphincter status | |||
| Preserved | 63 (115) | 80 (45) | .01 |
| Removed | 37 (68) | 20 (11) | |
| Chemotherapy (n = 203) | |||
| Neoadjuvant | 65 (119) | 0 (0) | < .01 |
| Adjuvant | 35 (64) | 100 (20) | |
| Radiotherapy (n = 188) | |||
| Neoadjuvant | 65 (119) | 60 (3) | .82 |
| Adjuvant | 35 (64) | 40 (2) | |
| Comorbidity | |||
| None | 34 (62) | 25 (14) | .61 |
| Mild | 41 (75) | 48 (27) | |
| Moderate | 17 (31) | 20 (11) | |
| Severe | 8 (15) | 7 (4) | |
| Baseline survey type | |||
| Full | 76 (139) | 63 (35) | .03 |
| Brief | 14 (25) | 16 (9) | |
| Surrogate, live | 9 (17) | 14 (8) | |
| Surrogate, deceased | 1 (2) | 7 (4) | |
| State of health rating | |||
| Baseline | |||
| Mean | 68 | 71 | .40 |
| SD | 22 | 22 | |
| 1 year before | |||
| Mean | 84 | 75 | .02 |
| SD | 21 | 25 | |
| Current health state | |||
| Excellent/very good | 39 (71) | 41 (23) | .05 |
| Good | 35 (64) | 30 (17) | |
| Fair/poor | 23 (42) | 16 (9) | |
| Unknown/NA | 3 (6) | 13 (7) | |
| Survival | |||
| Alive 1 year after diagnosis | 94 (172) | 88 (49) | .22 |
| Alive 7 years after diagnosis | 56 (103) | 48 (27) | .29 |
Abbreviations: CEA, carcinoembryonic antigen; HMO, health maintenance organization; IQR, interquartile range; MRI, magnetic resonance imaging; NA, not applicable; PET, positron emission tomography; SD, standard deviation; TRUS, transrectal ultrasound; VA, Veterans Affairs.
Received chemotherapy only, radiotherapy only, or neither.
For QOL analyses, the study population was limited to patients who were alive and disease-free at 7 years postdiagnosis. Group one was further stratified into patients who received neoadjuvant chemoradiotherapy (as a proxy measure for attempted guideline care) versus adjuvant chemoradiotherapy. Patients receiving both neoadjuvant chemoradiotherapy as well as adjuvant chemotherapy were placed in the neoadjuvant group. Multivariable linear regression was used to determine the relationship at baseline between the functional status/QOL scores mentioned previously and candidate predictor variables listed in Table 1 and Appendix Table A1. This set of candidate predictors, plus the baseline value of the outcome variable, was also used to model functional status/QOL measured in the 1- and 7-year follow-up surveys. Treatment was considered a key predictor and was not a candidate for removal in the backward selection procedure, as were baseline scores for the 1- and 7-year outcome measures. For all logistic regressions, patients were excluded if they were missing information for any variable included in that specific model. The minimum number of covariates in any of the models was one, and the maximum number was eight. All statistical analyses were performed in SAS software (version 9.3; SAS Institute, Cary, NC) on version 12 of the CanCORS surveys and MRA (version17 core data set).
Results
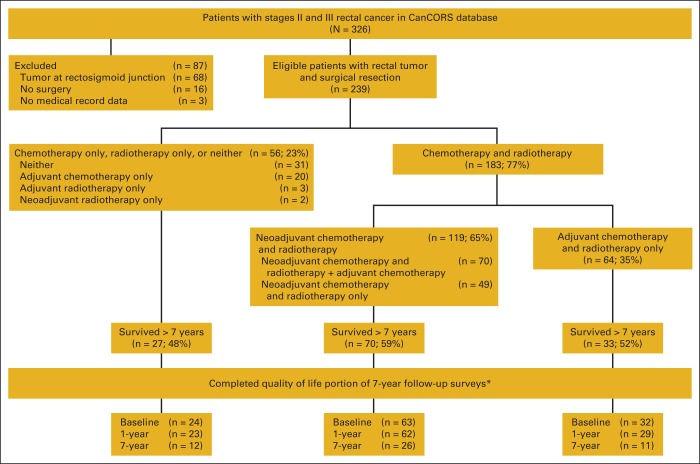
A total of 239 patients met the inclusion criteria for the objective 1 analysis. One hundred eighty-three patients (77%) received either neoadjuvant or adjuvant chemoradiotherapy, and 56 (23%) received only one treatment modality or neither (Figure 1). Among those who received chemoradiotherapy, 119 (65%) received neoadjuvant chemoradiotherapy and 64 (35%) received adjuvant chemoradiotherapy (Figure 1). Figure 1 shows the number who received each therapy and the sequence in which therapy was received; more detailed information on those receiving neoadjuvant versus adjuvant chemotherapy and radiotherapy is described in the article by Charlton et al.8
Figure 1.
Patient flow diagram for the Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) patients with stages II and III rectal cancer. * Reasons for missing quality-of-life (QOL) scores: baseline (BL): 10 patients had surrogates complete survey, and QOL scores were not available from these surveys; one patient did not answer the QOL questions. 1-year follow-up: 10 refused; four could not be contacted; two did not answer the QOL questions. 7-year follow-up: six patients had advanced disease so did not take the disease-free survivor survey; 33 refused; three incapable of responding; 39 could not be contacted.
Patient Characteristics Associated With Receipt of Chemoradiotherapy
The characteristics of the two study groups are presented in Table 1. Compared with the group that received only one or neither treatment modality, higher proportions of the group that received both (either neoadjuvantly or adjuvantly) were younger than age 75 years, had received recommended magnetic resonance imaging of the pelvis or transrectal ultrasound for locoregional staging, positron emission tomography and carcinoembryonic antigen testing during the staging/pretreatment period, and underwent sphincter-preserving surgery. Although both groups had similar self-reported health ratings at the time of diagnosis, those who received chemoradiotherapy had higher average ratings when asked about their general health status 1 year before diagnosis. A significantly lower proportion of the group that received chemoradiotherapy was deceased at the time of the baseline survey compared with the group that did not receive both (1% v 7%; P = .03; data not shown). However, the difference in the proportions alive 1 year after diagnosis was not significant (94% v 88%; P = .22).
A higher proportion of those who received only one treatment modality or neither indicated that they had made the decisions themselves (with little or no input from physicians) on whether or not to have chemotherapy and/or radiotherapy, whereas half of those who received chemoradiotherapy reported making the decision to receive these treatments together with their physician. A higher proportion of the group who received chemoradiotherapy indicated that chemotherapy would likely prolong their lives, help with their symptoms, and have adverse effects, and that radiotherapy would likely cure their cancer, prolong their lives, and help with their symptoms as compared with those who received only one or neither treatment modality (Table A1, online only).
Of those who did not receive chemotherapy, 44% reported that no physician ever talked to them about having chemotherapy. Similarly, 41% of those who did not receive radiotherapy said that no physician ever talked to them about having radiotherapy. On the basis of medical record abstraction, 20% of those who did not receive chemotherapy visited a medical oncologist, and 33% of those who did not receive radiotherapy visited a radiation oncologist (results not shown).
After considering all variables in Table 1 and Appendix Table A1, results of multivariable analyses indicated younger age (< 65 years v ≥ 65 years: odds ratio, 2.49; 95% CI, 1.33 to 4.65) was significantly associated with increased odds of receiving chemoradiotherapy. Preservation of the sphincter was associated with decreased odds of receiving chemoradiotherapy (odds ratio, 0.34; 95% CI, 0.16 to 0.73, results not shown), potentially as a result of surgeons being less likely to refer patients for neoadjuvant chemoradiotherapy when they had higher tumors and sphincter preservation was possible without the need to preoperatively reduce tumor bulk.
QOL Among Disease-Free Survivors
Of those who survived at least 7 years and completed the QOL and functional status questions at baseline or 1-year follow-up (n = 130), 49 (38%) completed the 7-year disease-free follow-up survey; six people who were contacted had advanced disease and therefore took a different survey. There were no significant differences in the demographic or clinical characteristics between those who did and did not complete the 7-year survey, although participants who completed the 7-year survey were marginally more likely to have had at least some college education (P = .06) and were enrolled onto the study via a cancer registry versus the VA or an HMO (P = .07). We also compared the characteristics of participants who survived at least 7 years with those who did not. Those who survived at least 7 years were significantly younger than those who did not. They also more frequently lived alone, and were enrolled onto the study through an HMO (results not shown).
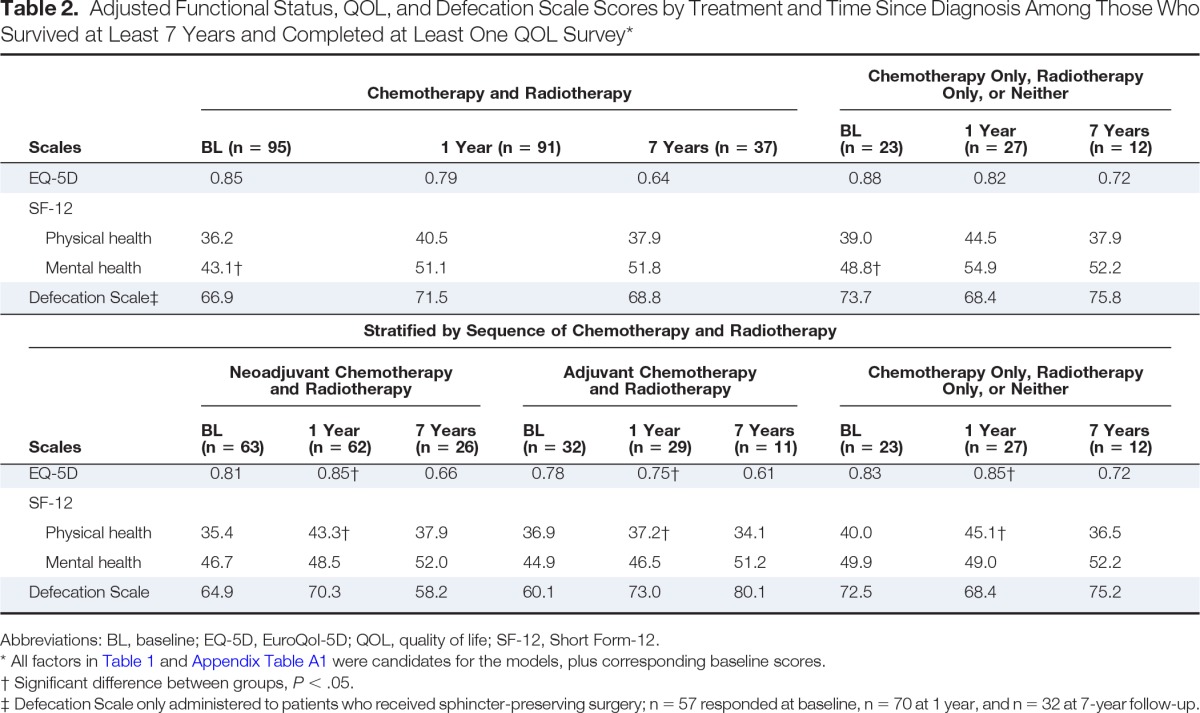
The adjusted EQ-5D, SF-12, and Defecation Scale scores by time since diagnosis (ie, baseline, 1 year, 7 years) for patients who received chemoradiotherapy compared with those who received only one or neither treatment modality are presented in Table 2. There were no significant differences in QOL or functional status at any time point except for the SF-12 mental health component score at baseline (ie, 1 to 4 months after diagnosis); those receiving chemoradiotherapy had a significantly lower score than those who received only one treatment modality or neither (43.1 v 48.8; P = .04). The adjuvant chemoradiotherapy group had significantly worse EQ-5D scores than the neoadjuvant chemoradiotherapy group and the group who received only one or neither treatment modality at 1-year follow-up (0.75, 0.85, 0.85, respectively; P = .002, .02), with a similar pattern seen for the SF-12 physical health component score (37.2, 43.3, 45.1, respectively; P = .01, .008). There were no significant differences between the three treatment groups in the SF-12 mental health component score or Defecation Scale at any time point.
Table 2.
Adjusted Functional Status, QOL, and Defecation Scale Scores by Treatment and Time Since Diagnosis Among Those Who Survived at Least 7 Years and Completed at Least One QOL Survey*
| Scales | Chemotherapy and Radiotherapy |
Chemotherapy Only, Radiotherapy Only, or Neither |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BL (n = 95) | 1 Year (n = 91) | 7 Years (n = 37) | BL (n = 23) | 1 Year (n = 27) | 7 Years (n = 12) | ||||
| EQ-5D | 0.85 | 0.79 | 0.64 | 0.88 | 0.82 | 0.72 | |||
| SF-12 | |||||||||
| Physical health | 36.2 | 40.5 | 37.9 | 39.0 | 44.5 | 37.9 | |||
| Mental health | 43.1† | 51.1 | 51.8 | 48.8† | 54.9 | 52.2 | |||
| Defecation Scale‡ | 66.9 | 71.5 | 68.8 | 73.7 | 68.4 | 75.8 | |||
| Stratified by Sequence of Chemotherapy and Radiotherapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scales | Neoadjuvant Chemotherapy and Radiotherapy |
Adjuvant Chemotherapy and Radiotherapy |
Chemotherapy Only, Radiotherapy Only, or Neither |
||||||
| BL (n = 63) | 1 Year (n = 62) | 7 Years (n = 26) | BL (n = 32) | 1 Year (n = 29) | 7 Years (n = 11) | BL (n = 23) | 1 Year (n = 27) | 7 Years (n = 12) | |
| EQ-5D | 0.81 | 0.85† | 0.66 | 0.78 | 0.75† | 0.61 | 0.83 | 0.85† | 0.72 |
| SF-12 | |||||||||
| Physical health | 35.4 | 43.3† | 37.9 | 36.9 | 37.2† | 34.1 | 40.0 | 45.1† | 36.5 |
| Mental health | 46.7 | 48.5 | 52.0 | 44.9 | 46.5 | 51.2 | 49.9 | 49.0 | 52.2 |
| Defecation Scale | 64.9 | 70.3 | 58.2 | 60.1 | 73.0 | 80.1 | 72.5 | 68.4 | 75.2 |
Abbreviations: BL, baseline; EQ-5D, EuroQol-5D; QOL, quality of life; SF-12, Short Form-12.
All factors in Table 1 and Appendix Table A1 were candidates for the models, plus corresponding baseline scores.
Significant difference between groups, P < .05.
Defecation Scale only administered to patients who received sphincter-preserving surgery; n = 57 responded at baseline, n = 70 at 1 year, and n = 32 at 7-year follow-up.
Discussion
These results are among the first to illustrate the effects of rectal cancer treatment approach across real-world settings among patients of all ages and states of health, and across several regions of the United States. Our analyses demonstrate that a substantial proportion (23%) of CanCORS patients with stage II/III rectal cancer did not receive chemoradiotherapy. In addition, we found that functional status and QOL were significantly lower 1 year after diagnosis among those who received adjuvant chemoradiotherapy compared with those who received neoadjuvant chemoradiotherapy and only one or neither treatment. Although decreased risk of recurrence, toxicity, and permanent colostomies has previously been demonstrated among patients receiving neoadjuvant chemoradiotherapy,2,3 the finding that the neoadjuvant therapy group had functional status and QOL scores similar to those of the group that received chemotherapy only, radiotherapy only, or neither may provide a more compelling rationale for adherence to the recommended sequence, given that the group receiving only one or neither therapy would be expected to experience the least long-term effects from treatment.
Our findings with respect to the proportion of patients receiving recommended therapy were consistent with previous evaluations of SEER data,5,6,9 which found that 30% to 40% of patients with stage II/III rectal cancer did not receive any radiotherapy, and that younger age was associated with receipt of radiotherapy. These studies included earlier years of data before wider uptake of NCCN guidelines for neoadjuvant chemoradiotherapy, which may explain why their estimates of radiotherapy nonreceipt are somewhat higher. Of the group that received only chemotherapy, only radiotherapy, or neither, 73% had no or mild comorbidities, 88% were still alive 1 year after diagnosis, and almost no patients reported that they expected to die in less than 5 years. Therefore, it does not seem that lack of therapy in most cases was simply a result of imminent death or patients being too ill to receive chemotherapy and/or radiotherapy. The association between nonsphincter-preserving surgery and receipt of chemoradiotherapy may suggest that some surgeons predominantly refer patients with lower-lying tumors for chemoradiotherapy in an attempt to save the sphincter, and do not consistently refer patients with higher tumors.
More than 40% of patients who did not receive chemoradiotherapy reported that no physician ever discussed the possibility of chemotherapy or radiotherapy with them, and medical record data confirmed that 70% to 80% did not visit an oncologist. It is possible that those who did not visit an oncologist refused to engage in a discussion about these therapies or did not recall that a different type of physician had spoken with them about it. However, given the high percentage of patients who did not have medical record data indicating a visit to an oncologist, it is also possible that the ideal multidisciplinary approach to treatment planning for patients with rectal cancer was not executed in the majority of these patients who did not receive chemotherapy and radiotherapy, and that no one fully described the treatment options and benefits to these individuals. Among the 27 (48%) members of the group who did not receive both chemotherapy and radiotherapy and survived at least 7 years, it is likely that they would not have gained benefit from additional neoadjuvant or adjuvant therapy. However, the 52% who did not survive that long may have benefitted. It is not currently possible to precisely predict at diagnosis who will and will not benefit from neoadjuvant chemoradiotherapy, which is why NCCN guidelines recommend it for nearly all patients with stage II/III disease.
It should also be noted that a significantly higher percentage of patients not receiving chemoradiotherapy reported that they had made the decision concerning whether or not to receive therapy, as opposed to making the decision together with their physician or their physician alone making the decision. It is possible that this is indicative of strong patient preference and deference to patient autonomy on the part of the physician, or alternatively, may indicate ineffective physician communication regarding the risk/benefit ratio for these treatments and/or a lack of data clearly indicating their benefit.
Although the results indicated that there was no difference in QOL at any time point between those who received chemoradiotherapy and those who did not, except for the SF-12 mental health component score at baseline, the stratified analysis by chemotherapy-radiotherapy-surgery sequence demonstrated that those receiving adjuvant chemoradiotherapy had lower EQ-5D and SF-12 physical health component scores 1 year after diagnosis after adjusting for baseline scores and other covariates. Statistically significant differences also fell within previously reported minimal clinically important difference ranges for the EQ-5D and SF-12.36–40 There were no other significant differences among groups at the follow-up time points. Two other long-term follow-up studies found that patients receiving neoadjuvant radiotherapy reported fewer bowel movements compared with patients receiving adjuvant radiotherapy,12,13 but did not find differences in overall functional status or QOL.13 It is possible that the main benefits of neoadjuvant chemoradiotherapy compared with adjuvant chemoradiotherapy occur in the short term, and that over time, the effects of any treatment for rectal cancer on QOL become more similar.
A potential limitation of this study is that self-reported information may have been collected before oncologist visits and treatment discussions with other providers. However, the fact that both medical record and self-report data could be used and compared for variables such as oncologist visits was a strength of this study. We also do not have information on whether patients were discussed in a Tumor Board or other multidisciplinary setting. Another potential limitation is the possibility of confounding by unmeasured characteristics that may have differed between treatment groups such as exact location of the tumor, hospital type/size, specialty of the surgeon, and other provider characteristics. Finally, small sample size, especially at the point of 7 years after diagnosis as a result of loss to follow-up or death, reduced statistical power for some subgroup analyses, and we were unable to stratify on the basis of specific chemotherapy drugs, chemotherapy and radiotherapy dosage, and exact timing of administration to determine complete compliance with guidelines.
In conclusion, the majority of patients with stage II and III rectal cancer received chemoradiotherapy, but a substantial proportion of patients did not. It is possible that patient preferences or proximal tumor location explain some of this variation, but on the basis of the finding that patients often reported not being told of these therapies, it is questionable whether or not many patients were given the opportunity to learn more about treatment options directly from oncologists. Among patients who did receive chemoradiotherapy, those who received guideline-recommended neoadjuvant therapy had the best QOL and physical health scores 1 year later. Further evaluation of patient and provider reasons for not pursuing recommended neoadjuvant chemoradiotherapy is necessary to determine how and where to target process improvements and education to ensure that patients have access to recommended treatments. More immediately, ensuring that patients have an opportunity to have a thorough, individualized discussion regarding chemoradiotherapy before surgery would also likely increase receipt of guideline-concordant care.
Acknowledgment
Supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (Grant No. U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana-Farber Cancer Institute/Cancer Research Network, Grant No. U01 CA093332; Harvard Medical School/Northern California Cancer Center, Grant No. U01 CA093324; RAND/University of California, Los Angeles, Grant No. U01 CA093348; University of Alabama at Birmingham, Grant No. U01 CA093329; University of Iowa, Grant No. U01 CA093339; University of North Carolina, Grant No. U01 CA093326) and by a Department of Veterans Affairs (VA) grant to the Durham VA Medical Center (Grant No. CRS 02-164). Also supported by Grant No. IRG-77-004-34 from the American Cancer Society, administered through the Holden Comprehensive Cancer Center at the University of Iowa, and by the University of Iowa Holden Comprehensive Cancer Center, which is funded in part by National Institutes of Health/NCI Grant No. P30 CA086862.
Presented in part as an abstract poster at the 2014 Gastrointestinal Cancers Symposium, cosponsored by the American Society of Clinical Oncology, American Gastroenterological Association Institute, American Society for Radiation Oncology, and Society of Surgical Oncology, San Francisco, CA, January 16-18, 2014.
Appendix
Table A1.
Patient Beliefs and Treatment Preferences
| Preference or Belief | Chemotherapy and Radiotherapy (n = 183; %, [No.]) | Not Both/Neither* (n = 56; %, [No.]) | P |
|---|---|---|---|
| Patient role in decision making about therapies†‡§‖ (4 missing responses; 40 not asked¶) | |||
| Decision to have chemotherapy | |||
| Patient made decision | 32 (52) | 52 (17) | .02 |
| Patient made decision together with physician | 50 (81) | 33 (11) | |
| Physicians made decision | 17 (27) | 15 (5) | |
| Do not know | 1 (2) | 0 (0) | |
| Decision to have radiotherapy | |||
| Patient made decision | 35 (58) | 47 (14) | < .01 |
| Patient made decision together with physician | 50 (83) | 30 (9) | |
| Physicians made decision | 12 (19) | 17 (5) | |
| Do not know | 3 (5) | 7 (2) | |
| Patient beliefs about therapies†‡§ (chemotherapy: 46 not asked¶; 4 missing; radiotherapy: 45 not asked¶; 3 missing ) | |||
| Chemotherapy (“very likely” or “somewhat likely”) | |||
| Would likely cure their cancer | 69 (111) | 56 (15) | .24 |
| Would likely prolong their life | 88 (142) | 59 (16) | < .01 |
| Would likely help with their symptoms | 77 (109) | 50 (12) | .02 |
| Would likely have side effects | 86 (140) | 67 (18) | .03 |
| Radiotherapy (“very likely” or “somewhat likely”) | |||
| Would likely cure their cancer | 61 (101) | 32 (8) | .03 |
| Would likely prolong their life | 80 (133) | 36 (9) | < .01 |
| Would likely help with their symptoms | 70 (105) | 41 (9) | .05 |
| Would likely have side effects | 71 (118) | 52 (13) | .06 |
| Role patient generally prefers to play in decision making and role family played†§ (5 missing) | |||
| Preferred role | |||
| Patient make decision on his/her own | 32 (49) | 39 (16) | .82 |
| Make the decision together with physician | 58 (89) | 51 (21) | |
| Physicians make the decision | 10 (15) | 10 (4) | |
| Role family played | |||
| Patient made decision | 45 (68) | 50 (19) | .81 |
| Make the decision together with family | 53 (81) | 47 (18) | |
| Family made decision | 2 (3) | 3 (1) | |
| Preferences regarding extension of life†§ (5 missing) | |||
| Extension of life v pain and discomfort | |||
| Prefers treatment that extends life as much as possible, even if it means having more pain/discomfort | 53 (82) | 32 (13) | .09 |
| Prefers treatment that focuses on relieving pain as much as possible, even if it means not living as long | 35 (54) | 51 (21) | |
| Refused to answer/do not know | 12 (19) | 17 (7) | |
| Extension of life v cost of treatment | |||
| Prefers treatment that extends life as much as possible, even if it means using up financial resources | 63 (97) | 49 (20) | .25 |
| Prefers treatment that costs less, even if means not living as long | 26 (41) | 32 (13) | |
| Refused to answer/do not know | 11 (17) | 19 (8) | |
| Expected time to live, fatalism beliefs, and concerns about treatment† | |||
| Expected time to live | |||
| Less than 5 years | 4 (6) | 6 (2) | .88 |
| At least 5 years | 62 (84) | 64 (21) | |
| In God's hands/do not know | 34 (46) | 30 (10) | |
| Fatalism beliefs (“strongly agree” or “agree”) | |||
| When bad things happen, we are not supposed to know why, we are just supposed to accept them | 40 (57) | 53 (18) | .34 |
| People die when it is their time, and nothing can change it | 62 (87) | 56 (19) | .30 |
| Everything that happens is a part of God's plan | 76 (107) | 71 (24) | .50 |
| If bad things happen, it is because they were meant to be | 44 (62) | 44 (15) | .74 |
| Treatment concerns (“very worried” or “somewhat worried”) about: | |||
| Adverse effects from treatment | 63 (87) | 53 (18) | .26 |
| Cost of treatment | 21 (29) | 26 (9) | .81 |
| Taking time away from family | 33 (46) | 41 (14) | .80 |
| Taking time away from work | 32 (44) | 44 (15) | .76 |
| Transportation to treatment | 13 (18) | 14 (5) | .45 |
Received chemotherapy only, radiotherapy only, or neither.
Full survey.
Brief survey.
Survey of surrogate (live patient).
Survey of surrogate (deceased patient).
Items not asked of those reporting that no one ever talked to them about chemotherapy/radiotherapy, or that they were told not to have it.
Authors' Disclosures of Potential Conflicts of Interest
Disclosures provided by the authors are available with this article at jop.ascopubs.org.
Author Contributions
Conception and design: All authors
Collection and assembly of data: Mary E. Charlton, Chi Lin, Jennifer A. Schlichting, Thorvardur R. Halfdanarson, Grelda Yazmin Juarez, Jane F. Pendergast, Elizabeth A. Chrischilles, Robert B. Wallace
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Predictors of Long-Term Quality of Life for Survivors of Stage II/III Rectal Cancer in the Cancer Care Outcomes Research and Surveillance Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Mary E. Charlton
No relationship to disclose
Karyn B. Stitzenberg
Stock or Other Ownership: Johnson & Johnson, Merck
Chi Lin
No relationship to disclose
Jennifer A. Schlichting
No relationship to disclose
Thorvardur R. Halfdanarson
Consulting or Advisory Role: Novartis, Tekmira
Research Funding: Jennerex, Boston Biomedical
Grelda Yazmin Juarez
No relationship to disclose
Jane F. Pendergast
No relationship to disclose
Elizabeth A. Chrischilles
No relationship to disclose
Robert B. Wallace
No relationship to disclose
References
- 1.[No authors listed] NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal cancer. Version. 2014:3. doi: 10.6004/jnccn.2009.0057. [DOI] [PubMed] [Google Scholar]
- 5.Lin C, Charlton ME, Meza JL, et al. Temporal and regional variations in the use of preoperative radiation therapy for rectal cancer. Am J Clin Oncol. 2010;33:443–447. doi: 10.1097/COC.0b013e3181b4b175. [DOI] [PubMed] [Google Scholar]
- 6.Mak RH, McCarthy EP, Das P, et al. Adoption of preoperative radiation therapy for rectal cancer from 2000 to 2006: A Surveillance, Epidemiology, and End Results Patterns-of-Care Study. Int J Radiat Oncol Biol Phys. 2011;80:978–984. doi: 10.1016/j.ijrobp.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Databases: Research Data (1973-2010) based on the November 2012 submission. National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch, released April 2013. http://seer.cancer.gov/data/seerstat/nov2012/
- 8.Charlton ME, Lin C, Jiang D, et al. Factors associated with use of preoperative chemoradiation therapy for rectal cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Am J Clin Oncol. 2013;36:572–579. doi: 10.1097/COC.0b013e318261082b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baxter NN, Rothenberger DA, Morris AM, et al. Adjuvant radiation for rectal cancer: Do we measure up to the standard of care? An epidemiologic analysis of trends over 25 years in the United States. Dis Colon Rectum. 2005;48:9–15. doi: 10.1007/s10350-004-0792-8. [DOI] [PubMed] [Google Scholar]
- 10.Schrag D, Gelfand SE, Bach PB, et al. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from Surveillance, Epidemiology, and End Results–Medicare. J Clin Oncol. 2001;19:3712–3718. doi: 10.1200/JCO.2001.19.17.3712. [DOI] [PubMed] [Google Scholar]
- 11.Morris AM, Billingsley KG, Baxter NN, et al. Racial disparities in rectal cancer treatment: A population-based analysis. Arch Surg. 2004;139:151–155. doi: 10.1001/archsurg.139.2.151. [DOI] [PubMed] [Google Scholar]
- 12.Nathanson DR, Espat NJ, Nash GM, et al. Evaluation of preoperative and postoperative radiotherapy on long-term functional results of straight coloanal anastomosis. Dis Colon Rectum. 2003;46:888–894. doi: 10.1007/s10350-004-6679-x. [DOI] [PubMed] [Google Scholar]
- 13.Hassan I, Larson DW, Cima RR, et al. Long-term functional and quality of life outcomes after coloanal anastomosis for distal rectal cancer. Dis Colon Rectum. 2006;49:1266–1274. doi: 10.1007/s10350-006-0640-0. [DOI] [PubMed] [Google Scholar]
- 14.Birgisson H, Påhlman L, Gunnarsson U, et al. Late gastrointestinal disorders after rectal cancer surgery with and without preoperative radiation therapy. Br J Surg. 2008;95:206–213. doi: 10.1002/bjs.5918. [DOI] [PubMed] [Google Scholar]
- 15.Dahlberg M, Glimelius B, Graf W, et al. Preoperative irradiation affects functional results after surgery for rectal cancer: Results from a randomized study. Dis Colon Rectum. 1998;41:543–549. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- 16.Kollmorgen CF, Meagher AP, Wolff BG, et al. The long-term effect of adjuvant postoperative chemoradiotherapy for rectal carcinoma on bowel function. Ann Surg. 1994;220:676–682. doi: 10.1097/00000658-199411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange MM, Maas CP, Marijnen CA, et al. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg. 2008;95:1020–1028. doi: 10.1002/bjs.6126. [DOI] [PubMed] [Google Scholar]
- 18.Lange MM, Marijnen CA, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer. 2009;45:1578–1588. doi: 10.1016/j.ejca.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Lundby L, Krogh K, Jensen VJ, et al. Long-term anorectal dysfunction after postoperative radiotherapy for rectal cancer. Dis Colon Rectum. 2005;48:1343–1349. doi: 10.1007/s10350-005-0049-1. [DOI] [PubMed] [Google Scholar]
- 20.Mannaerts GH, Schijven MP, Hendrikx A, et al. Urologic and sexual morbidity following multimodality treatment for locally advanced primary and locally recurrent rectal cancer. Eur J Surg Oncol. 2001;27:265–272. doi: 10.1053/ejso.2000.1099. [DOI] [PubMed] [Google Scholar]
- 21.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 22.Pollack J, Holm T, Cedermark B, et al. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br J Surg. 2006;93:1519–1525. doi: 10.1002/bjs.5525. [DOI] [PubMed] [Google Scholar]
- 23.Herman JM, Narang AK, Griffith KA, et al. The quality-of-life effects of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2013;85:e15–e19. doi: 10.1016/j.ijrobp.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiv M, Puyraveau M, Mineur L, et al. Long-term quality of life in patients with rectal cancer treated with preoperative (chemo)-radiotherapy within a randomized trial. Cancer Radiother. 2010;14:530–534. doi: 10.1016/j.canrad.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the Cancer Care Outcomes Research and Surveillance Consortium relative to the Surveillance, Epidemiology, and End Results program. Med Care. 2013;51:e9–e15. doi: 10.1097/MLR.0b013e318222a711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients' experience and outcomes: Development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14:837–848. doi: 10.1007/s00520-005-0902-8. [DOI] [PubMed] [Google Scholar]
- 27.Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Staging Manual. ed 6. Philadelphia, PA: Lippincott Raven; 2002. pp. 113–124. [Google Scholar]
- 29.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 30.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. ed 3. Lincoln, RI: QualityMetric; 1998. [Google Scholar]
- 32.Brooks R. EuroQol: The current state of play. Health Policy. 1996;37:53–72. doi: 10.1016/0168-8510(96)00822-6. [DOI] [PubMed] [Google Scholar]
- 33.EuroQol Group. EuroQol: A new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 34.Kind P, Brooks R, Rabin R, editors. EQ-5D Concepts and Methods. Dordrecht, the Netherlands: Springer; 2005. [Google Scholar]
- 35.Cancer Care Outcomes Research and Surveillance Consortium. Appendix 12: CanCORS Data Documentation, 5/12 update. https://www.cancors.org/public/servlets/open/home/home.cmd?itab=7.
- 36.Bennett SJ, Oldridge NB, Eckert GJ, et al. Comparison of quality of life measures in heart failure. Nurs Res. 2003;52:207–216. doi: 10.1097/00006199-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: A critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14:221–233. doi: 10.1586/14737167.2014.894462. [DOI] [PubMed] [Google Scholar]
- 38.Parker SL, Godil SS, Shau DN, et al. Assessment of the minimum clinically important difference in pain, disability, and quality of life after anterior cervical discectomy and fusion: Clinical article. J Neurosurg Spine. 2012;18:154–160. doi: 10.3171/2012.10.SPINE12312. [DOI] [PubMed] [Google Scholar]
- 39.Parker SL, Mendenhall SK, Shau D, et al. Determination of minimum clinically important difference in pain, disability, and quality of life after extension of fusion for adjacent-segment disease. J Neurosurg Spine. 2012;16:61–67. doi: 10.3171/2011.8.SPINE1194. [DOI] [PubMed] [Google Scholar]
- 40.Parker SL, Mendenhall SK, Shau DN, et al. Minimum clinically important difference in pain, disability, and quality of life after neural decompression and fusion for same-level recurrent lumbar stenosis: Understanding clinical versus statistical significance. J Neurosurg Spine. 2012;16:471–478. doi: 10.3171/2012.1.SPINE11842. [DOI] [PubMed] [Google Scholar]