Abstract
Several epidemiological studies have found a positive association between diabetic retinopathy (DR) and subclinical hypothyroidism (SCH), but the findings are varied or even contradictory. In the work, we performed a meta-analysis to ascertain the relationship between DR and SCH. We searched relevant studies on the relationship between DR and SCH. All English reports were used Medline, EMbase, Web of Science, Google scholar, and all Chinese ones used CBMDisc (Chinese Biochemical Literature on Disc) and CNKI (China National Knowledge Infrastructure) database. Meta-analysis was performed using RevMan 5.1 software. We obtained eight observational studies. Random-effects meta-analysis indicated a significant association between DR and SCH (odds ratio = 2.13, 95% confidence interval = 1.41 – 3.23, p < 0.001). Based on currently evidence, SCH is probably a significant risk factor for DR.
Type 2 diabetes mellitus (T2DM) is becoming increasingly prevalent worldwide. Diabetic retinopathy (DR) is the most common ocular complication of diabetes, and is the leading cause of visual impairment and blindness in working-aged people1. DR is common in diabetic patients but is asymptomatic until a significant visual impairment occurs. Late diagnosis of DR results in the socio-economic burden of illness associated with diabetes2. According to the World Health Organization (WHO), blindness due to DR accounts for 4.8% of the total thirty-seven million cases of blindness around the world in 20063. Therefore, it is important to investigate the risk factors that promote or predict the onset and development of DR.
Subclinical hypothyroidism (SCH) is a common endocrine disorder and characterized as elevated serum thyroid-stimulating hormone (TSH) levels in the presence of serum free thyroxine (FT4) and triiodothyronine (T3) levels within the reference range4. In general population screening surveys, the prevalence of SCH has been reported to range from 4% to 10%5, and the risk factors of SCH are baseline TSH level6, iodine-sufficient7,8,9, old age, female sex, and the presence of thyroid autoantibodies10,11,12,13,14.
The association between T2DM and SCH is well known, with the reported prevalence of SCH in diabetes varying between 2.2% to 17%15,16. Recently, several researches investigate the relationship between SCH and DR, but the results were inconsistent. Kim Bo-Yeon et al.17 considered that SCH was associated with DR, but Chen et al.18 took the opposite opinion.
Consequently, we performed this meta-analysis of eligible observational studies in order to more precisely investigate the relationship between SCH and DR.
Results
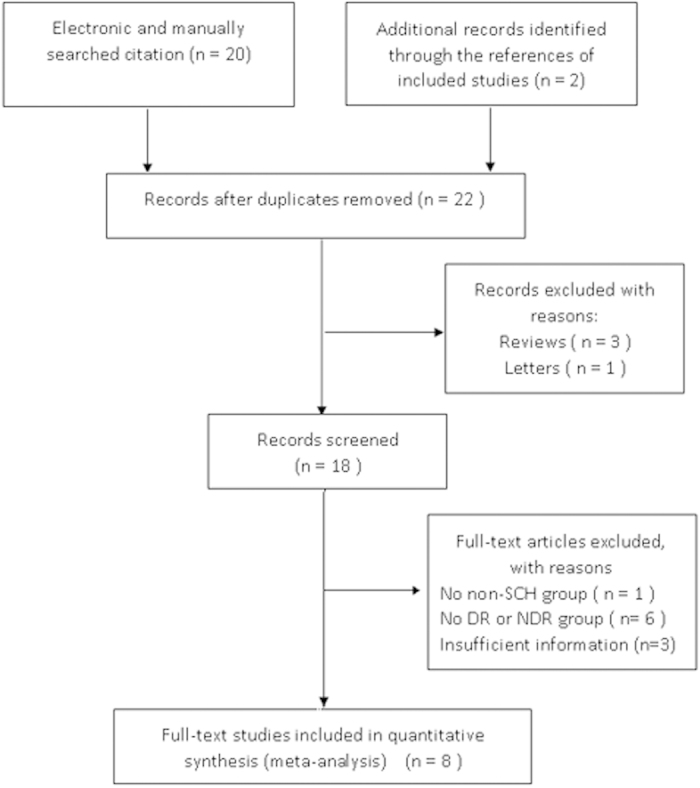
We screened 22 articles published from 2010 to 2014. Of these, 14 studies were excluded for the following reasons: reviews, letter and not provide sufficient information for estimating the relationship between DR and SCH. Thus, 8 studies were included in this meta-analysis. Figure 1 was the selection process and reasons for excluding studies.
Figure 1. Flow chart demonstrating those studies that were processed for inclusion in the meta-analysis.
The major characteristics of the eight studies are shown in Table 1. The included studies were published in Chinese and English, and preformed in Chinese mainland, Taiwan and Korea. All of them were hospital-based case-control studies and SCH were all defined as TSH level >4.0 uIU/mL in the presence of normal serum free thyroxine level (0.7–2.0 ng/dL).
Table 1. Characteristics of included studies in the meta-analysis.
| First Author | Region | Study design | Publication year | Relationship between DR and SCH | Sample size | Age(years) | SCH sample | DR sample |
|---|---|---|---|---|---|---|---|---|
| Kim Bo-Yeon7 | Korea | Case control | 2011 | YES | 489 | 61.7 ± 9.8 | 61 | 207 |
| Chen HS8 | Taiwan | Case control | 2007 | NO | 588 | 67.2 ± 10.8 | 41 | 158 |
| Yang Jinkui11 | China | Case control | 2010 | YES | 327 | 61.0 ± 13.7 | 127 | 158 |
| Chen Jihai12 | China | Case control | 2012 | YES | 400 | 75.53 ± 6.53 | 45 | 29 |
| Guo Dan13 | China | Case control | 2012 | YES | 162 | 55.84 ± 13.03 | 40 | 30 |
| Zheng Yongqiang14 | China | Case control | 2012 | NO | 138 | 60.2 ± 11.8 | 28 | 39 |
| Yang Guangran53 | China | Case control | 2010 | YES | 371 | 59.94 ± 10.61 | 83 | 187 |
| Tang Jiandong54 | China | Case control | 2012 | YES | 1156 | 64.6 ± 10.2 | 164 | 610 |
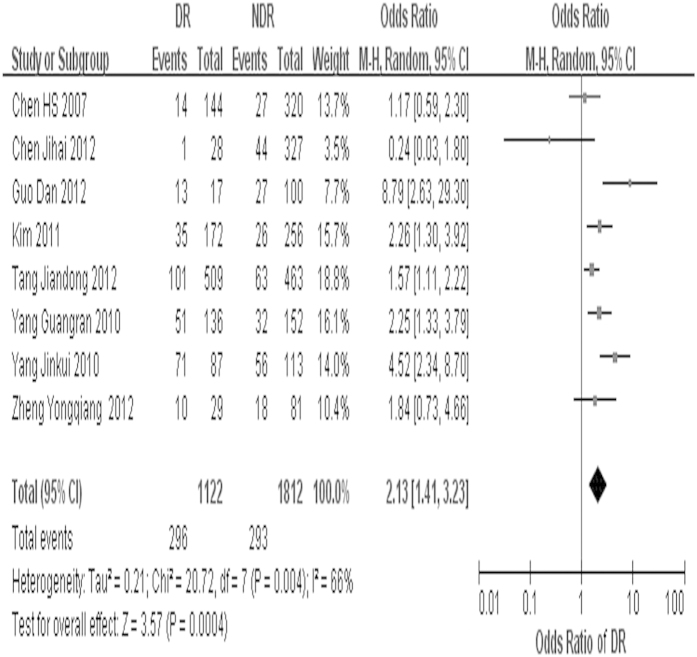
The overall OR estimates for each study were pooled to give a total estimated of risk (Fig. 2). Obviously heterogeneity was observed (p = 0.004, I2 = 66%), so the result based on the random-effects model showed that exposure to SCH can increase the DR risk 2.13 times (OR = 2.13, 95%CI = 1.41 – 3.23, p < 0.001).
Figure 2. Forest plot of SCH and risk of DR, studies are pooled with random-effects model.
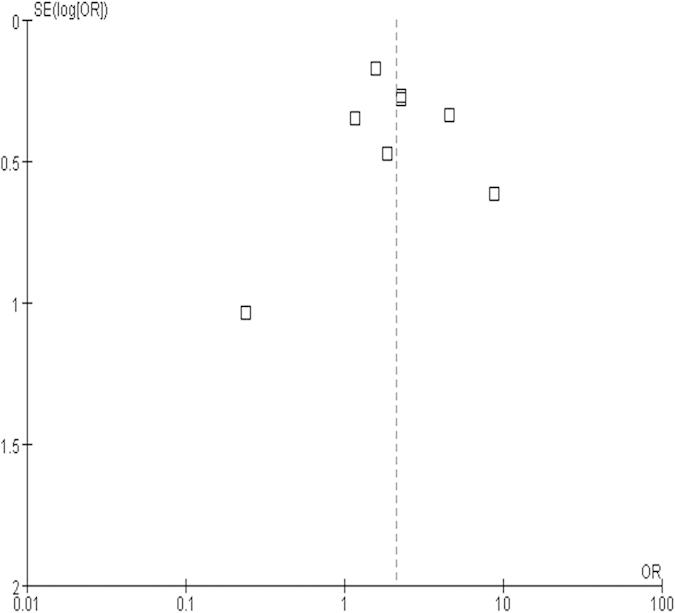
Figure 3 was the funnel plot of the research. The result shows that the figure was not asymmetrical, which demonstrated that there was no significant publication bias.
Figure 3. Funnel plot based on 8 case-control studies.
Discussion
It is well-known that, DR is one of the most common microvascular complications and the leading cause of blindness worldwide. Recently, as the number of people with T2DM increased, the prevalence of DR is rapidly increasing19,20. The results of a meta-analysis from the population-based studies around the world showed that the overall age-standardized prevalence of any DR was 34.6%21. Because the symptoms of the early stage in DR are not apparent, patients often miss the best opportunity for treatment when diagnosed, which is leading to a high rate of blinding. According to the World Health Organization (WHO), blindness due to DR accounts for 4.8% of the total thirty-seven million cases of blindness around the world22. Therefore, it is important to investigate the risk factors for DR. In the past researches, the well-known risk factors for the development of DR include duration of diabetes, poor glycemic control, elevated blood pressure, and dyslipidemia, of which the latter three are potentially amenable to therapeutic intervention23,24.
In recent years, several investigators discussed the relationship between DR and SCH, but the results were contradictory. Accordingly, this study was aimed to estimate the pooled size of SCH in DR risk by a meta-analysis.
In this research, the pooled effect estimated from eight included papers demonstrated a 2.13 fold (95%CI = 1.41 – 3.23, p < 0.001) increased risk of DR in SCH patients compared with non-SCH individuals. But substantial heterogeneity was observed among the studies. To find out the sources of heterogeneity, we exclude each one study at a time. Heterogeneity was obviously decreased when excluding the following three studies respectively, which indicating that the three studies were the source of heterogeneity. They are studies by Yang Jinkui et al.25, Chen Jihai et al.26 and Guo Dan et al.27. Sample size of study by Guo Dan et al. was only 157, that may be the reason for its heterogeneity. Chen Jihai et al. only selected over 65 years old people as study objects. So there may be selection bias in that study. Similarly, Yang Jinkui et al. randomly selected 127 SCH patients and 200 non-SCH people from 1170 T2DM patients, but the method of selecting was unclear.
Above all, this is the first meta-analysis investigating the association between DR and SCH, and it demonstrated that DM patient suffering from SCH could increase the risk of DR. But it differed from those of Chen et al.18 and Zheng Yongqiang et al.28. Reasons for this discrepancy may be related to differences in characteristics of the participants.
Several mechanisms may be involved in the association between DR and SCH.
First of all, insulin resistance. Several studies have found that fasting hyperinsulinemia or insulin resistance (IR) was associated with SCH29,30. Previous studies indicated that IR was associated with the presence of DR in T2DM31,32, and the main mechanism was defective fibrinolysis or impaired vasodilation associated with IR33,34. It may be correlated with the reduction of vasodilation ability and fibrosis caused by IR, which led to destruction of retinal vessel and secondary revascularization35,36.
Secondly, serum C-reactive protein (CRP). Researches by Christ-Crain et al.37 and Kvetny et al.38 indicated that the level of CRP in patients with SCH was obviously higher than that in non-SCH people. The study of Van Hecke et al.39 shown that there was significant relationship between CRP and DR. As is well known, DR is a Chronic inflammatory disease, which is correlated with the inflammation-mediated injury of vascular endothelial cell. Moreover, CRP is recognized one of the primary and most sensitive acute phase protein in human nonspecific inflammation reaction40.
The third, the level of serum homocysteine (Hcy) in SCH patients was much higher than non-SCH people. Hcy is a reactive amino acid about vascular injuries. Looker et al.41 demonstrated that elevated levels of Hcy was the risk factor for DR. The reason may be that Hcy could enhance the lipid peroxidation42, which leads to increased levels of oxidized low density lipoprotein(OX-LDL), accelerating the progresses of vascular disease43. Besides, some scholars have found that the mRNA expression of VEGF was significantly enhanced with the concentration enrichment of Hcy44. Moreover, it is generally recognized that higher increase of VEGF was significantly associated with DR. Consequently, Hcy may promote the occurrence and development of DR by approaches mentioned above.
The forth, oxidative stress. The activity of paraoxonase 1(PON1) and superoxide dismutase (SOD) in the plasma of SCH patients is significantly lower than that of normal control. That is to say, antioxidative capacity of SCH has dropped significantly. However, there were some literatures have shown that oxidative stress was an important risk factor for promoting the occurrence and development of DR45.
The last, correlation between DR and dyslipidemia has been reported46, and atherogenic disturbances in lipid metabolism have been observed in patients with SCH47,48. Thus, dyslipidemia in SCH may be one of the reasons for the association between DR and SCH.
This study demonstrated that SCH could increase the risk of DR, but whether SCH could be a biomarker for DR is also unclear. Therefore, animal experiments should be performed to investigate the correlated mechanism between DR and SCH. The results can give implications for clinical practice and support the link between the two diseases.
All the included studies were performed in Asian countries. Therefore, we suggest the western countries to conduct relevant studies to verify this prediction.
We acknowledge that the current findings are cross-sectional in nature and the availability of prospective data would further improve confidence in these associations.
Although this is the first meta-analysis investigating the association between DR and SCH, which is very important for prevention of DR, there were some limitations. First, all the literature searches were hospital-based studies, absence of population-based studies. Second, as we cannot have access to unpublished results, publication bias cannot be excluded. Third, the literature search was limited to be published in English or Chinese. The last point, the number of studies for our meta-analysis is not large and the sample sizes in some studies were slightly small. Compared with the studies having a large sample size, studies with a small sample size may overestimate the true association. A large sample study with either finding may better reflect a true association because of its sufficient statistical power.
In conclusion, the meta-analysis of all published epidemiological studies on DR and SCR revealed that SCH was associated with DR in diabetes and exposure to SCH can increase the DR risk 2.13 times.
Methods
Search Strategy
Our research objects are all human species. We searched all English publications using Medline, Embase, Web of Science, Google scholar, and searched all Chinese publications manually and on-line using CNKI (China National Knowledge Infrastructure) and CBMDisc (Chinese Biochemical Literature on Disc) database. The search keywords were: (1) “diabetic retinopathy” OR “diabetic microangiopathy”; (2) “subclinical hypothyroidism”; (3) “association” OR “relationship”. A total of 22 reports published in the period from 1991 to 2013 were identified.
Inclusion and Exclusion Criteria
Reports were considered eligible for inclusion if the following criteria were met: (1) full-text could obtain; (2) clear diagnostic criteria for SCH and DR were reported; (3) the adjusted or unadjusted hazard ratios (HRs), relative risks (RRs), or odds ratios (Ors) could obtain, associated 95% confidence intervals (Cis) or the numbers of events that can calculate them were reported. If more than one study covered the same population, only the study of highest quality was included.
Reports were excluded if the following criteria were met: (1) the study without specific sample origins; (2) the study with a sample size less than 50; (3) the data in the study was obviously paradoxical or not present clearly enough. A total of 22 relevant studies were screened. After systematic review, only 8 of these were included in the meta-analysis. The progress for study inclusion is shown in Fig. 1.
Data Extraction
There were two researchers, Jingyang Wu and Lei Liu, independently reviewing all the potentially relevant papers through assessing the eligibility of each article and abstracting data with standardized data-abstraction forms. Disagreements were resolved through discussion. The characteristics of these studies included in this meta-analysis on the association of DR and SCH are shown in Table 1.
Data Analysis
Ors and relevant 95% Cis were computed using Review Manager (RevMan, version 5.1, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Ors were used to measure association across the studies. Heterogeneity evaluation of all the studies were used the Chi-square based Q test and I2 test49. Heterogeneity was assessed with low, moderate, and high I2 values of 25%, 50%, and 75%, respectively with the I2 statistic50. If moderate or high level heterogeneity existing, a random-effects meta-analysis was performed, unless using fixed-effects models. Publication bias was assessed by visually inspecting a funnel plot. A p value less than 0.05 was considered statistically significant51,52.
Additional Information
How to cite this article: Wu, J. et al. Relationship between Diabetic Retinopathy and Subclinical Hypothyroidism: a meta-analysis. Sci. Rep. 5, 12212; doi: 10.1038/srep12212 (2015).
Footnotes
Author Contributions J.Y.W. and L.L. have contributed to the design of the study, analysis and interpretation of data, and prepared all figures and tables. J.Y.W., L.L., W.P.T. and L.C. drafted a part of manuscript. S.Y., J.G. and L.M.L. took part in analyzing data, and drafting a part of manuscript. All authors reviewed the manuscript.
Author Contributions This study was supported by National Natural Science Foundation of China (81300783); Important Platform of Science and Technology for the University in Liaoning Province (16010).
References
- Yamada M. et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol 17, 50–57 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 304, 649–656 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., He M. & Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol 60, 428–431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J. R. et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American association of Clinical endocrinologists and the American Thyroid association. Endocr Pract 18, 988–1028 (2012). [DOI] [PubMed] [Google Scholar]
- McDermott M. T. & Ridgway E. C. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 86, 4585–4590 (2001). [DOI] [PubMed] [Google Scholar]
- Imaizumi M. et al. Risk for progression to overt hypothyroidism in an elderly Japanese population with subclinical hypothyroidism. Thyroid 21, 1177–1182 (2011). [DOI] [PubMed] [Google Scholar]
- Laurberg P. et al. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocrinol Metab 83, 765–769 (1998). [DOI] [PubMed] [Google Scholar]
- Teng W. et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 354, 2783–2793 (2006). [DOI] [PubMed] [Google Scholar]
- Konno N., Makita H., Yuri K., Iizuka N. & Kawasaki K. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab 78, 393–397 (1994). [DOI] [PubMed] [Google Scholar]
- Hollowell J. G. et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87, 489–499 (2002). [DOI] [PubMed] [Google Scholar]
- Bagchi N., Brown T. R. & Parish R. F. Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med 150, 785–787 (1990). [PubMed] [Google Scholar]
- Canaris G. J., Manowitz N. R., Mayor G. & Ridgway E. C. The Colorado thyroid disease prevalence study. Arch Intern Med 160, 526–534 (2000). [DOI] [PubMed] [Google Scholar]
- Tunbridge W. M. et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 7, 481–493 (1977). [DOI] [PubMed] [Google Scholar]
- Parle J. V., Franklyn J. A., Cross K. W., Jones S. C. & Sheppard M. C. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocrinol (Oxf) 34, 77–83 (1991). [DOI] [PubMed] [Google Scholar]
- Perros P., McCrimmon R. J., Shaw G. & Frier B. M. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med 12, 622–627 (1995). [DOI] [PubMed] [Google Scholar]
- Uzunlulu M., Yorulmaz E. & Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J 54, 71–76 (2007). [DOI] [PubMed] [Google Scholar]
- Kim B. Y. et al. Association between subclinical hypothyroidism and severe diabetic retinopathy in Korean patients with type 2 diabetes. Endocr J 58, 1065–1070 (2011). [DOI] [PubMed] [Google Scholar]
- Chen H. S. et al. Subclinical hypothyroidism is a risk factor for nephropathy and cardiovascular diseases in Type 2 diabetic patients. Diabet Med 24, 1336–1344 (2007). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Prevalence of diabetic retinopathy in mainland china: a meta-analysis. PloS One 7, e45264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek M. et al. Medical management of diabetic retinopathy: an overview. Arch Iran Med 15, 635–640 (2012). [PubMed] [Google Scholar]
- Yau J. W. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., He M. & Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol 60, 428–431 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton I. M. et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 44, 156–163 (2001). [DOI] [PubMed] [Google Scholar]
- Barnett A. H. et al. Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia 49, 2234–2246 (2006). [DOI] [PubMed] [Google Scholar]
- Yang J. K., Liu W., Shi J. & Li Y. B. An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care 33, 1018–1020 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. H. et al. Influence of subclinical hypothyroidism for type 2 diabetic retinopathy. Pract Geriatr 26, 497–500 (2012). [Google Scholar]
- Guo D. The correlation of subclinical hypothyroidism and microvascular complications in type 2 diabetic patients. Available at: http://d.wanfangdata.com.cn/Thesis_Y2115203.aspx. (Accessed: 28th November 2012)
- Zheng Y. Q., Xue L., Bie M. J., Li X. J. & Chen D. Analyzation of lipids and hs-CRP level for type 2 diabetic patients with subclinical hypothyroidism. Modern Preventive Medicine 39, 4485–4487 (2012). [Google Scholar]
- Maratou E. et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol 160, 785–790 (2009). [DOI] [PubMed] [Google Scholar]
- Tuzcu A., Bahceci M., Gokalp D., Tuzun Y. & Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive C-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J 52, 89–94 (2005). [DOI] [PubMed] [Google Scholar]
- Parvanova A. et al. Insulin resistance and proliferative retinopathy: a cross-sectional, case-control study in 115 patients with type 2 diabetes. J Clin Endocrinol Metab 89, 4371–4376 (2004). [DOI] [PubMed] [Google Scholar]
- Park C. Y. et al. Prevalence of and risk factors for diabetic retinopathy in Koreans with type II diabetes: baseline characteristics of Seoul Metropolitan City-Diabetes Prevention Program (SMC-DPP) participants. Br J Ophthalmol 96, 151–155 (2012). [DOI] [PubMed] [Google Scholar]
- Yudkin J. S. Abnormalities of coagulation and fibrinolysis in insulin resistance. Evidence for a common antecedent? Diabetes Care 22, C25–30 (1999). [PubMed] [Google Scholar]
- Kurioka S., Koshimura K., Murakami Y., Nishiki M. & Kato Y. Reverse correlation between urine nitric oxide metabolites and insulin resistance in patients with type 2 diabetes mellitus. Endocr J 47, 77–81 (2000). [DOI] [PubMed] [Google Scholar]
- Yudkin J. S. Abnormalities of coagulmion and fibrinolysis in insulin resistance. Evidence for a common antecedent? Diabetes Care 22(Suppl 3), C25–30 (1999). [PubMed] [Google Scholar]
- Kurioka S. et al. Reverse correlation between urine nitric oxide metabolites and insulin resistance in patients with type 2 diabetes mellitus. Endocr J 47, 77–81 (2000). [DOI] [PubMed] [Google Scholar]
- ChristCrain M. et al. Elevated C-reactive protein and homocysteinevalues: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis 166, 379–386 (2003). [DOI] [PubMed] [Google Scholar]
- Kvetny J., Heldgaard P. E. & Bladbjerg E. M. Subclinical hypothyroidism is associated with a low-grate inflammation, increased triglyceride levels and predicts cardiovascular disease in males below 50 years. Clin Endocrinol(Oxf) 61, 232–238 (2004). [DOI] [PubMed] [Google Scholar]
- VanHecke M. V. et al. Inflammation and endothelial dysfunction are associated with retinopathy: The Hoom Study. Diabetologia 48, 1300–1306 (2005). [DOI] [PubMed] [Google Scholar]
- Volanakis J. E. Human C reactive protein: expression,structure and function. Mol Immunl 38, 189–197 (2001). [DOI] [PubMed] [Google Scholar]
- Looker H. C. et al. Homocysteine as a risk factor for nephropathy and retinopathy in Type 2 diabetes. Diabetologia 46, 766–772 (2003). [DOI] [PubMed] [Google Scholar]
- Poddar R. et al. Homoeysteine induces expression and secretion of monogyte chemoattractant protein-l and inter leukin-8 in human aortic endothelial ceils: implications for vascular disease. Circulmion 103, 2717–2723 (2001). [DOI] [PubMed] [Google Scholar]
- Blom H. J. et al. Lipid peroxidation and susceptibility of low-density lipoprotein to in vitro oxidation in hyperhomocysteinaemia. Eur J Clin Invest 25, 149–154 (1995). [DOI] [PubMed] [Google Scholar]
- Maeda M. et al. Homocysteine induces vascular endothelial growth factor expression in differentiated THP-1macrophages. Biochim Biophys Acta 1623, 41–46 (2003). [DOI] [PubMed] [Google Scholar]
- Baskol G. et al. The role of advanced oxidation protein products and total thiols in diabetic retinopathy. Eur J Ophthalmol 18, 792–820 (2008). [DOI] [PubMed] [Google Scholar]
- Popescu T. & Mota M. Dyslipidemia and hypertension in patients with type 2 diabetes and retinopathy. Rom J Interm Med 47, 235–241 (2009). [PubMed] [Google Scholar]
- Althaus B. U. et al. LDL/HDL-changes in subclinical hypothyroidism: possible risk factors for coronary heart disease. Clin Endocrinol (Oxf) 28, 157–163 (1988). [DOI] [PubMed] [Google Scholar]
- Staub J. J. et al. Spectrum of subclinical and overt hypothyroidism: effect on thyrotropin, prolactin, and thyroid reserve, and metabolic impact on peripheral target tissues. Am J Med 92, 631–642 (1992). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Mantel J. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]
- Der Simonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Yang G. R., Yang J. K., Zhang L., An Y. H. & Lu J. K. Association between subclinical hypothyroidism and proliferative diabetic retinopathy in type 2 diabetic patients: a case-control study. Tohoku J Exp Med 222, 303–310 (2010). [DOI] [PubMed] [Google Scholar]
- Tang J. D. et al. Analyzation of clinical index for type 2 diabetic patients with subclinical hypothyroidism. Clin J Prev Contr Chron Dis 20, 591–593 (2012). [Google Scholar]