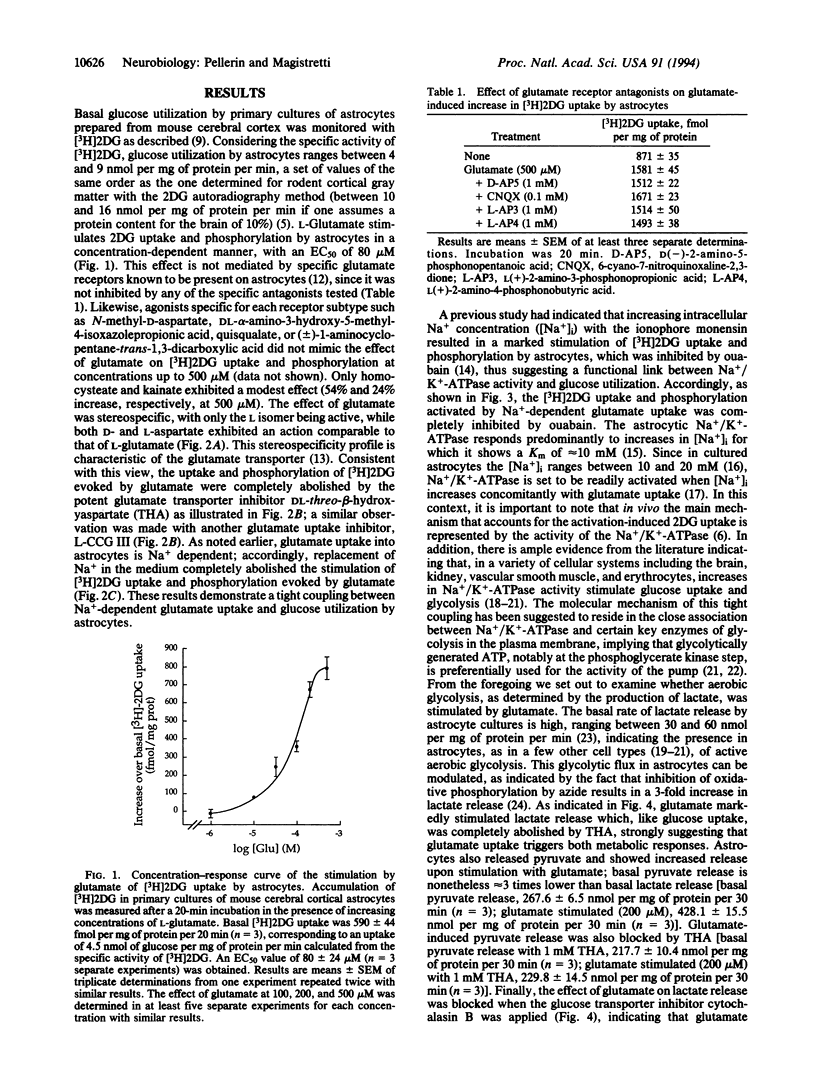
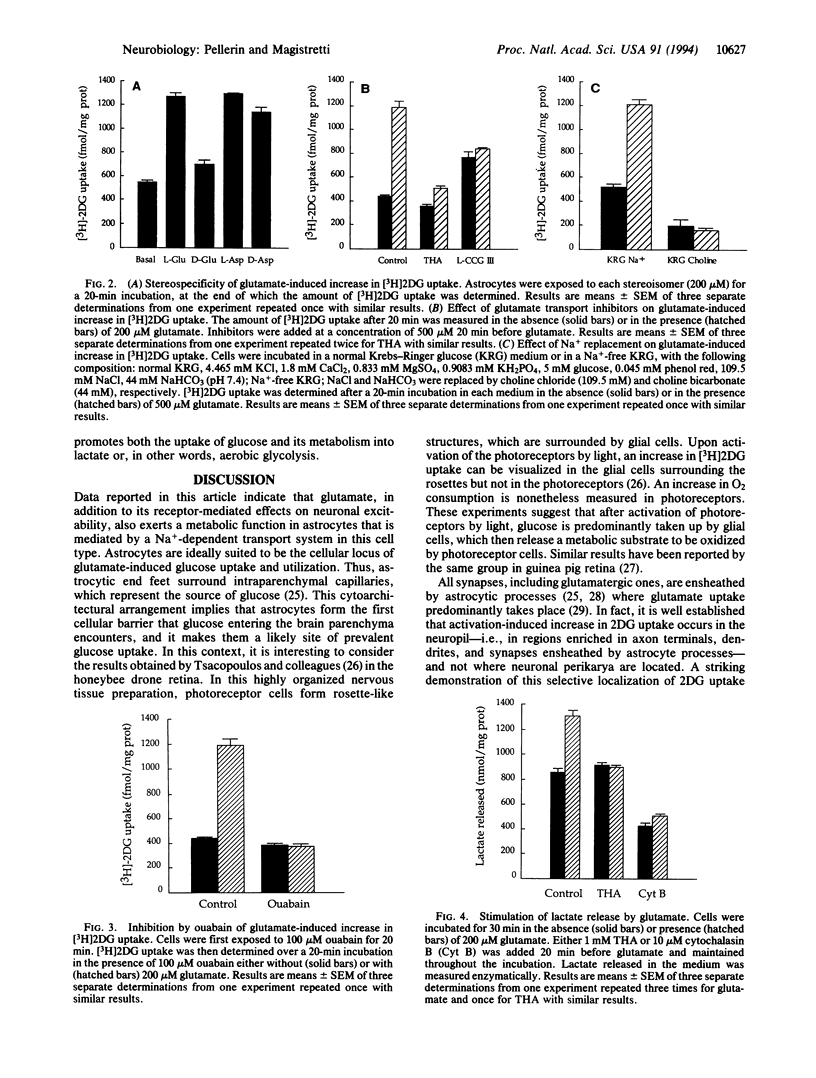
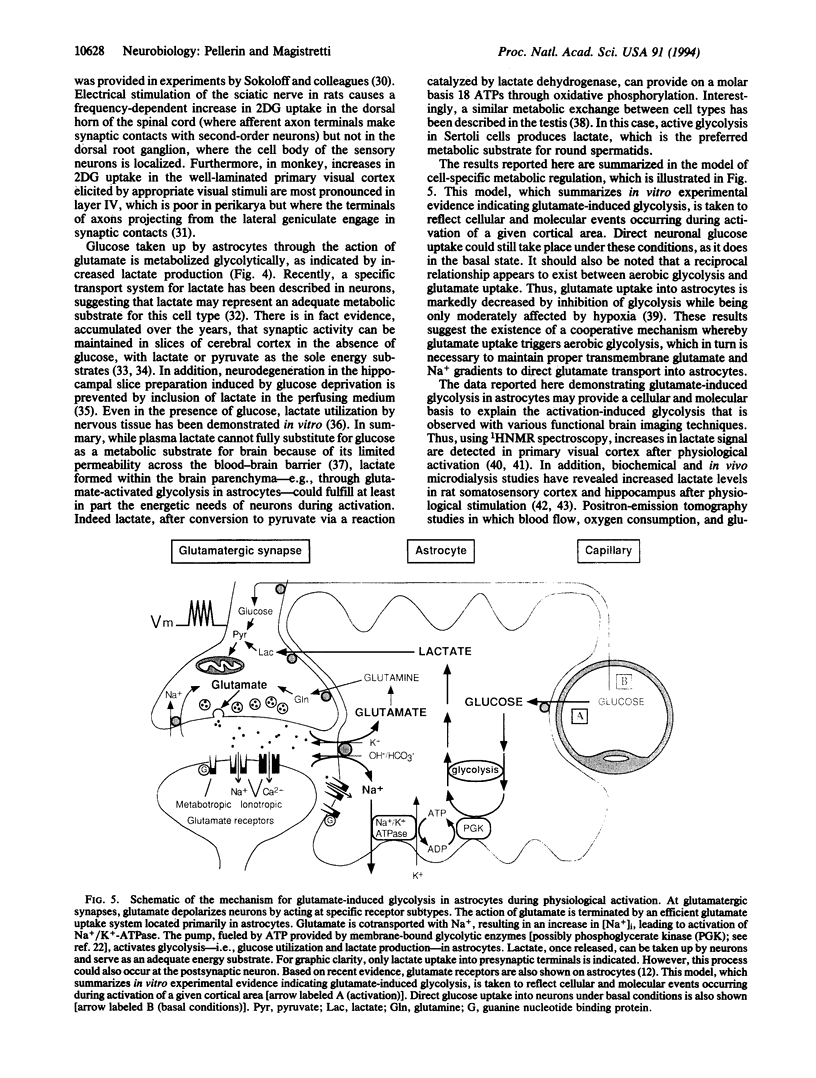
Abstract
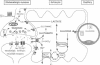
Glutamate, released at a majority of excitatory synapses in the central nervous system, depolarizes neurons by acting at specific receptors. Its action is terminated by removal from the synaptic cleft mostly via Na(+)-dependent uptake systems located on both neurons and astrocytes. Here we report that glutamate, in addition to its receptor-mediated actions on neuronal excitability, stimulates glycolysis--i.e., glucose utilization and lactate production--in astrocytes. This metabolic action is mediated by activation of a Na(+)-dependent uptake system and not by interaction with receptors. The mechanism involves the Na+/K(+)-ATPase, which is activated by an increase in the intracellular concentration of Na+ cotransported with glutamate by the electrogenic uptake system. Thus, when glutamate is released from active synapses and taken up by astrocytes, the newly identified signaling pathway described here would provide a simple and direct mechanism to tightly couple neuronal activity to glucose utilization. In addition, glutamate-stimulated glycolysis is consistent with data obtained from functional brain imaging studies indicating local nonoxidative glucose utilization during physiological activation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvier M., Szatkowski M., Amato A., Attwell D. The glial cell glutamate uptake carrier countertransports pH-changing anions. Nature. 1992 Dec 3;360(6403):471–474. doi: 10.1038/360471a0. [DOI] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Derouiche A., Frotscher M. Astroglial processes around identified glutamatergic synapses contain glutamine synthetase: evidence for transmitter degradation. Brain Res. 1991 Jun 28;552(2):346–350. doi: 10.1016/0006-8993(91)90103-3. [DOI] [PubMed] [Google Scholar]
- Dringen R., Gebhardt R., Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993 Oct 1;623(2):208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Dringen R., Wiesinger H., Hamprecht B. Uptake of L-lactate by cultured rat brain neurons. Neurosci Lett. 1993 Nov 26;163(1):5–7. doi: 10.1016/0304-3940(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Fellows L. K., Boutelle M. G., Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem. 1993 Apr;60(4):1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Benz A. M., Zorumski C. F., Olney J. W. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. Neuroreport. 1994 Jan 31;5(5):617–620. doi: 10.1097/00001756-199401000-00021. [DOI] [PubMed] [Google Scholar]
- Kadekaro M., Crane A. M., Sokoloff L. Differential effects of electrical stimulation of sciatic nerve on metabolic activity in spinal cord and dorsal root ganglion in the rat. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6010–6013. doi: 10.1073/pnas.82.17.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I. Glutamate transporters from brain. A novel neurotransmitter transporter family. FEBS Lett. 1993 Jun 28;325(1-2):95–99. doi: 10.1016/0014-5793(93)81421-u. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Des Rosiers M. H., Sakurada O., Shinohara M., Reivich M., Jehle J. W., Sokoloff L. Metabolic mapping of the primary visual system of the monkey by means of the autoradiographic [14C]deoxyglucose technique. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4230–4234. doi: 10.1073/pnas.73.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H. K., Biddelcome S., Narumi S., Bourke R. S. ATPase and carbonic anhydrase activities of bulk-isolated neuron, glia and synaptosome fractions from rat brain. Brain Res. 1978 Feb 10;141(2):305–323. doi: 10.1016/0006-8993(78)90200-7. [DOI] [PubMed] [Google Scholar]
- Kwong K. K., Belliveau J. W., Chesler D. A., Goldberg I. E., Weisskoff R. M., Poncelet B. P., Kennedy D. N., Hoppel B. E., Cohen M. S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrabee M. G. Lactate uptake and release in the presence of glucose by sympathetic ganglia of chicken embryos and by neuronal and nonneuronal cultures prepared from these ganglia. J Neurochem. 1983 May;40(5):1237–1250. doi: 10.1111/j.1471-4159.1983.tb13562.x. [DOI] [PubMed] [Google Scholar]
- Lipton P., Robacker K. Glycolysis and brain function: [K+]o stimulation of protein synthesis and K+ uptake require glycolysis. Fed Proc. 1983 Sep;42(12):2875–2880. [PubMed] [Google Scholar]
- Lynch R. M., Balaban R. S. Coupling of aerobic glycolysis and Na+-K+-ATPase in renal cell line MDCK. Am J Physiol. 1987 Aug;253(2 Pt 1):C269–C276. doi: 10.1152/ajpcell.1987.253.2.C269. [DOI] [PubMed] [Google Scholar]
- Mata M., Fink D. J., Gainer H., Smith C. B., Davidsen L., Savaki H., Schwartz W. J., Sokoloff L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980 Jan;34(1):213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- McLennan H. The autoradiographic localization of L-[3h]glutamate in rat brain tissue. Brain Res. 1976 Oct 8;115(1):139–144. doi: 10.1016/0006-8993(76)90828-3. [DOI] [PubMed] [Google Scholar]
- Mercer R. W., Dunham P. B. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J Gen Physiol. 1981 Nov;78(5):547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita M., Hall P. F. Metabolism of round spermatids from rats: lactate as the preferred substrate. Biol Reprod. 1982 Apr;26(3):445–455. doi: 10.1095/biolreprod26.3.445. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977 Jan;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- Parker J. C., Hoffman J. F. The role of membrane phosphoglycerate kinase in the control of glycolytic rate by active cation transport in human red blood cells. J Gen Physiol. 1967 Mar;50(4):893–916. doi: 10.1085/jgp.50.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R. J., Bauer M., Pease W. Vascular smooth muscle: aerobic glycolysis linked to sodium and potassium transport processes. Science. 1979 Dec 21;206(4425):1414–1416. doi: 10.1126/science.505014. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate C., Tsacopoulos M. Glial (Müller) cells take up and phosphorylate [3H]2-deoxy-D-glucose in mammalian retina. Neurosci Lett. 1991 Jan 28;122(2):241–244. doi: 10.1016/0304-3940(91)90868-t. [DOI] [PubMed] [Google Scholar]
- Prichard J., Rothman D., Novotny E., Petroff O., Kuwabara T., Avison M., Howseman A., Hanstock C., Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. C., Rush B. F. An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin Chem. 1966 May;12(5):299–307. [PubMed] [Google Scholar]
- Rosenberg P. A., Amin S., Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J Neurosci. 1992 Jan;12(1):56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappey-Marinier D., Calabrese G., Fein G., Hugg J. W., Biggins C., Weiner M. W. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992 Jul;12(4):584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- Schurr A., West C. A., Rigor B. M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988 Jun 3;240(4857):1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sorg O., Magistretti P. J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991 Nov 1;563(1-2):227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- Swanson R. A. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci Lett. 1992 Dec 7;147(2):143–146. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M., Evêquoz-Mercier V., Perrottet P., Buchner E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8727–8731. doi: 10.1073/pnas.85.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M., Linn F., Hossmann K. A. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1988 Aug;8(4):486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Walz W., Mukerji S. Lactate release from cultured astrocytes and neurons: a comparison. Glia. 1988;1(6):366–370. doi: 10.1002/glia.440010603. [DOI] [PubMed] [Google Scholar]
- Yarowsky P., Boyne A. F., Wierwille R., Brookes N. Effect of monensin on deoxyglucose uptake in cultured astrocytes: energy metabolism is coupled to sodium entry. J Neurosci. 1986 Mar;6(3):859–866. doi: 10.1523/JNEUROSCI.06-03-00859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Martin J. L., Stella N., Magistretti P. J. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4042–4046. doi: 10.1073/pnas.90.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]