Abstract
The replacement of abnormal hematopoietic stem cells (HSCs) with normal transplanted HSCs can correct a wide range of hematologic disorders. Here, we provide evidence that transplantation of more differentiated progenitor cells can be used to more rapidly correct lymphoid deficiencies in unconditioned immunocompromised mice. Transplantation of flk2+ multipotent progenitors led to robust B and T cell reconstitution that was maintained for at least 16 weeks. Antigenic challenge at 16 weeks post-transplantation revealed that reconstituted lymphocytes maintained a functional repertoire. In contrast to the persistent lymphocytic engraftment, myeloid chimerism was lost by 12 weeks post-transplantation consistent with the fact that flk2+ progenitors are non-self-renewing. Thus, while more differentiated progenitors are capable of rescuing lymphoid deficiencies, transplantation of HSCs must be used for the correction of non-lymphoid disorders, and, we propose, very long-term immune reconstitution. Based on recent evidence, we discuss novel strategies to achieve the replacement of abnormal HSCs without the use of cytotoxic conditioning regimens.
Accessing the stem cell niche
HSCs possess the remarkable ability to self-renew and yet maintain full differentiation potential for the lifetime of an organism. These two properties allow for the proper maintenance of hematopoietic homeostasis, but genetic abnormalities within HSCs can lead to profound negative consequences such as immunodeficiency, anemia, or leukemia. Because the replacement of functionally compromised HSCs with normal HSCs can correct some of these diseases, it is of critical importance to understand how best to achieve such an exchange while minimizing risk to the patient.
The hypothesis that HSCs require a fixed tissue microenvironment within the bone marrow to function properly was first proposed by Schofield over 30 years ago1. The idea that such a "niche" exists was based on the observations that the bone marrow, but not the spleen, could sustain hematopoiesis through serial transplantations. In support of the niche hypothesis, later studies showed that irradiation was required in order to facilitate sustained donor bone marrow engraftment, presumably to clear endogenous HSC from their niches2, 3.
These and other studies also suggested that HSCs have the ability to home efficiently to these empty niches upon intravenous transplantation. Very early work provided some evidence that some HSCs might be present in the blood4, 5, but until recently the etiologic purpose of the inherent ability of HSCs to home to their specialized microenvironments through intravascular circulation was not clear. To determine whether HSCs circulate and re-home to their niches under physiologic conditions, Wright et al. turned to a parabiosis model in which cross circulation between congenically distinguishable mice was rapidly established6. After separation, these mice maintained long-term blood chimerism, suggesting that functional HSC cross-engraftment had occurred during the period of parabiosis and in the absence of any radiaton or chemotherapy to open niches. Indeed, HSC chimerism was detectable directly within the bone marrow of both partners. Additionally, approximately 100 HSCs were detectable in the blood of unmanipulated mice at any given point. Because the intravascular residence time of HSCs is no more than 5 minutes, it was estimated that ~30,000 HSCs flux through the blood per day. Thus, the ability to exit and relocate the appropriate niche seems to be a normal part of the homeostatic behavior of HSC; it is almost certainly linked to the ability of HSC to home to the correct microenvironments and support long-term blood cell reconstitution in the clinical context of bone marrow transplantation. The biological importance of homeostatic HSC circulation is still poorly understood, but the constant exchange of HSC between and within bone marrow compartments presumably contributes to the maintenance of proper hematopoietic balance, and may represent a strategy for rapidly responding to acute or focal hematopoietic stress or injury. If indeed such an exchange occurs, a small number of HSC niches must be free for engraftment by these circulating HSCs at any given point. However, because sustained donor HSC engraftment is rarely seen in the absence of irradiation or other cytotoxic conditioning2, 3, the prevailing dogma has been that under normal conditions HSC niches are occupied and must be cleared prior to transplantation.
In order to resolve these apparently contradictory observations, we recently performed experiments in which histocompatible HSCs were transplanted into both unconditioned wild type and immunodeficient animals7. These data showed that approximately 0.5% of HSC niches are open and available for productive stem cell engraftment in the absence of any myeloablative or suppressive conditioning, and that HSC engraftment of these rare niches permitted complete and sustained rescue of the immunodeficiencies exhibited in SCID mice through the temporal and considerable expansion of donor B cell and T cell progenitors. Our experiments further revealed that access to these HSC niches was restricted through immunosurveillance by a CD4+ T cell-dependent mechanism sufficient for the rejection of HSC with even single antigenic differences. Consistent with this, CD4+ T cell immunodepletion allowed for functional HSC engraftment even in the presence of minor histocompatibility differences. These experiments provide proof of principle of a general mechanism by which transplanted stem cells can functionally engraft recipients to rescue a severe genetic deficiency without the use of highly cytotoxic preparative regimens.
Rapid Lymphopoiesis by Non-Self-Renewing Progenitors
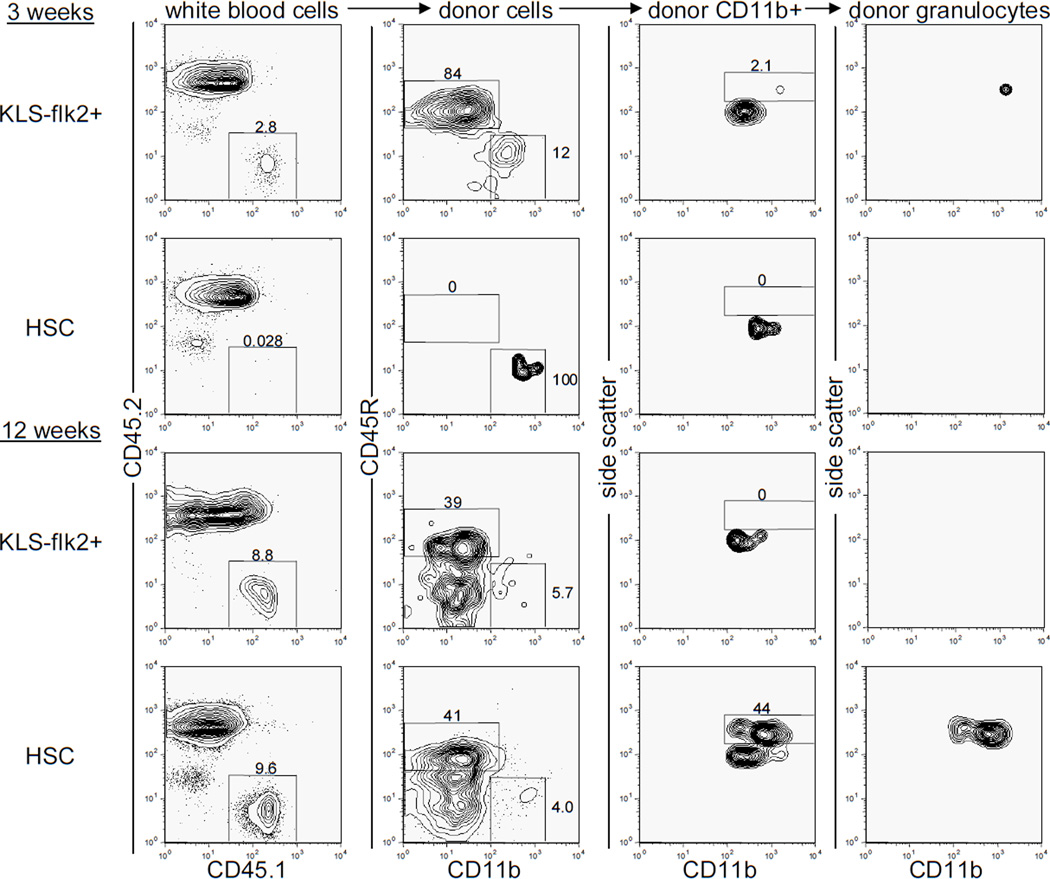
Because many lymphocytes can persist for very long periods of time in the absence of input from the bone marrow8, 9, we hypothesized that even non-self-renewing multipotent progenitor cells might be able to reconstitute the lymphoid compartment of immunocompromised mice. Because such progenitors can reconstitute hosts more rapidly than HSCs, transplantation of these cells could potentially generate functional immunity in recipients more rapidly than transplanted HSCs. For instance, studies using common lymphoid progenitors have shown that a rapid burst of lymphopoiesis can protect mice against lethal doses of cytomegalovirus after irradiation and HSC transplantation10. To test the ability of non-self-renewing progenitors to generate immunity in unconditioned immunocompromised recipients, we transplanted 180 c-kit+ lineage− Sca-1+ flk2+ (KLS flk2+) progenitor cells or 500 c-kit+ lineage− Sca-1+ CD34− flk2− HSCs into unconditioned recombinase activating gene 2 and interleukin-2 receptor common gamma chain-deficient (RAG2−/−γc−/−) mice11. These KLS flk2+ cells retain myeloid and lymphocytic differentiation potential, but lack the ability to self-renew for the lifetime of the organism12, 13. Indeed, at 12 weeks post transplantation donor lymphocytes persisted but donor granulocytes, which accurately reflect the presence of engrafted donor HSCs 6, 7, were not observed in any of the recipients transplanted with KLS flk2+ cells (Figure 1). Importantly, transplantation of KLS flk2+ cells led to the generation of B cells in all recipients by 3 weeks post-transplant. In contrast, no B cells were detected at this timepoint upon transplantation of HSCs (Figure 1). Thus, the non-self-renewing KLS flk2+ cells generate B cells approximately 1–2 weeks earlier than do HSCs7. The findings that these progenitors are more abundant than HSCs (D. Bryder, D.J.R., and I.L.W., submitted) and can more rapidly generate functional lymphocytes than HSCs emphasize the potential clinical use of KLS flk2+ cells for the transient treatment of inherited or acquired immunodeficiency.
Figure 1. Rapid B cell and transient granulocyte reconstitution after transplantation of non-self-renewing multipotent progenitor cells in unconditioned immunodeficient animals.
Unconditioned CD45.2+ RAG2−/− γc−/− animals were transplanted with 180 CD45.1+ c-kit+ lineage− Sca-1+ CD34+ flk2+ (KLS flk2+) or 500 c-kit+ lineage− Sca-1+ CD34− flk2− (HSC) cells and peripheral blood was analyzed for donor contribution at 3 and 12 weeks post-transplantation. Representative plots for KLS flk2+ cell-transplanted animals (n=5) and HSC-transplanted animals (n=14) are shown. Donor B lymphocytes were identified as CD45.1+ CD45.2− CD45R+ CD11b− cells. Donor granulocytes were identified as CD45.1+ CD45.2− CD45R− CD11b+ side scatterhigh cells. The subset of cells upon which each plot is gated is listed above every column.
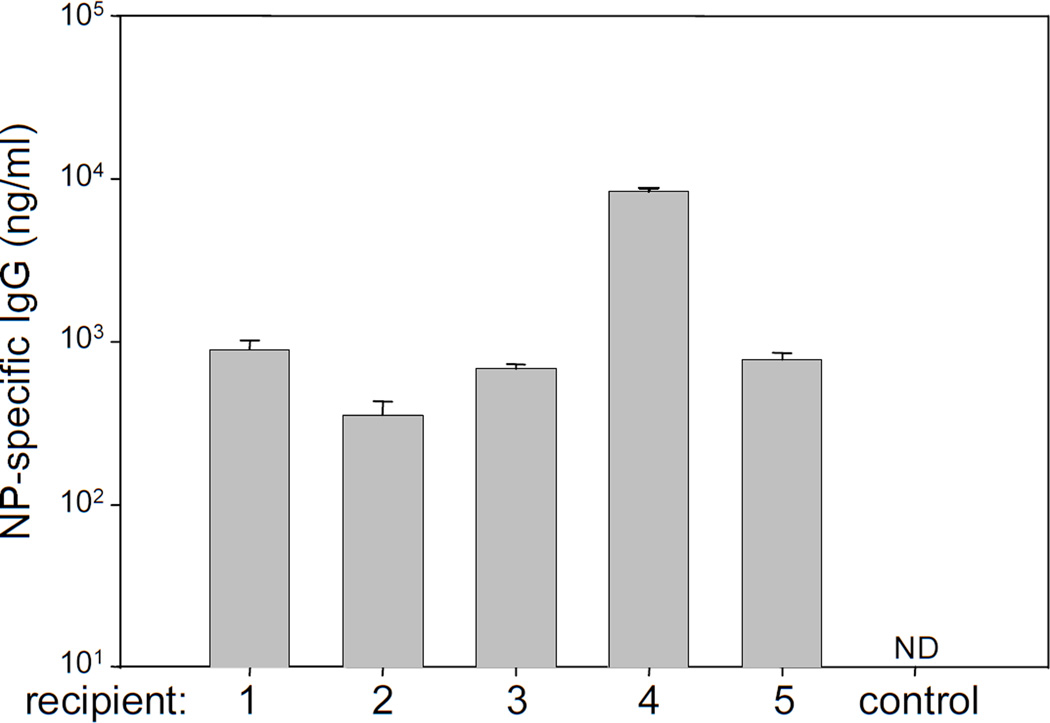
Previous studies have shown that as mature lymphocytes homeostatically proliferate in the secondary lymphoid tissues of lymphocyte-deficient hosts, the antigen-specific repertoire can decrease through the oligoclonal expansion of B and T cells with particular antigen receptor rearrangements14. To determine if functional repertoires were maintained in RAG2−/− γc−/− mice transplanted with the non-self-renewing KLS flk2+ cells described above, we immunized recipients with the T cell-dependent antigen alum-precipitated 4-Hydroxy-3-nitrophenylacetyl conjugated to chicken gamma globulin (NP-CGG)15, 16 at 16 weeks post-transplantation. NP-specific antibody responses were seen in all mice, demonstrating that a functional antigen-specific repertoire had been both generated and maintained (Figure 2). Taken together, these data demonstrate that transplantation of non-self-renewing KLS flk2+ cells can be used to rapidly rescue lymphoid deficiencies in unconditioned immunocompromised mice. However, because animals engrafted with KLS flk2+ cells did not maintain donor granulocyte chimerism, the data suggest that HSCs rather than their more differentiated progeny must be used for the treatment of non-lymphoid defects. Further, because naive lymphocytes have prolonged but not indefinite lifespans17, the immunity generated by transplantation of KLS flk2+ cells is unlikely to be permanent. While the memory lymphocytes generated during the period of functional immunity might persist because of their intrinsic ability to survive18, 19 and/or self-renew20–22, the naive lymphocytes would eventually be expected to be lost and with them the ability to respond to new antigens. Because of these caveats, it is important to achieve HSC engraftment alongside progenitor cell engraftment for both long-lasting and rapid correction of hematopoietic deficiencies.
Figure 2. Functional donor lymphocytes persist after transplantation of non-self-renewing progenitor cells.
Unconditioned RAG2−/−γc−/− mice were transplanted with 180 KLS FLK2+ cells from wild type mice and immunized with 100µg of the T-dependent antigen NP-CGG 14 weeks after transplantation. NP-specific antibody levels were measured 1 week after immunization in 5 mice that had been immunized and 1 wild type untransplanted mouse that had not been immunized. Serum from each mouse was tested in triplicate and the standard deviations for each sample are shown. ND=not detectable.
Strategies to Maximize HSC Engraftment
Maximizing the efficiency of functional HSC engraftment upon transplantation has long been a priority for the treatment of a number of diseases. The two major barriers to efficient HSC engraftment are thought to be immune rejection of the graft and the lack of access of donor HSCs to appropriate niches. Both of these barriers can be lowered in part through the treatment of the recipient with cytotoxic conditioning drugs prior to transplantation. However, the use of these drugs themselves carries considerable risk for the patient, particularly for the treatment of non-malignancies. Well-documented side effects of commonly used cytotoxic treatments such as total body irradiation, busulfan, and cyclophosphamide include prolonged loss of platelets, infertility, and secondary malignancies23. Thus, methods to achieve HSC engraftment without such toxic conditioning regimens should be of great interest to the medical community.
In previous work, we demonstrated that approximately 0.5% of HSC niches are available for engraftment at any given point in unconditioned animals provided the transplanted HSCs can evade host immune surveillance7. Since engraftment of wild type HSCs into these rare niches leads to a dramatic expansion of lymphoid progenitors in unconditioned immunodeficient animals, immunocompetence can be restored by astoundingly few numbers of transplanted HSCs. However, unless donor HSCs have a significant competitive advantage, these levels of engraftment in unconditioned hosts are likely too low to treat mature myeloid or erythroid defects since these cell types turn over very rapidly and thus require constant regeneration24. For example, studies using a mouse model of sickle cell anemia showed that a donor HSC chimerism of ~25% was required to reverse some of the detrimental effects associated with the disease25. The transplantation of very high doses of bone marrow into unconditioned hosts leads to chimerism, and it was suggested this results from displacement of resident stem cells from their niches26. However, the data presented in our study suggest that transplanted HSCs in excess of temporary open niches will not displace filled niches, but might enter niches for non self-renewing progenitors. In our view a closed niche at one moment has a finite probability of opening as a resident HSC moves out into the peripheral blood. Because this implies that chimerism could be increased by repetitive transplantation, the saturation of HSC niches should not be permanent and HSC transit into and out of niches should be an ongoing dynamic process. Consistent with this, we have found that repetitive transplantation increases HSC chimerism over that which can be achieved by a single transplantation of HSCs7. Since the number of empty HSC niches is similar to the number of HSCs estimated to exist in the blood at any given point6, it is tempting to speculate that each circulating HSC represents a cell that has left vacant its bone marrow niche. If true, it is likely that the rate of HSC niche emptying and filling is quite high. Thus, donor HSC engraftment can potentially be increased to very high levels through the repetitive transplantation of small numbers of highly purified HSCs. Alternatively, it might be possible to achieve high levels of chimerism through the slow continuous infusion of purified donor HSCs. It is even conceivable that agents to deplete only HSC’s will be developed, or to mobilize high fractions of HSC out of niches. In these settings, high levels of donor HSC engraftment would be efficiently achieved without the risks associated with cytotoxic conditioning or graft versus host disease, which does not occur after transplantation of highly purified HSCs27.
Avoiding Rejection
Our earlier work also showed that antibody-mediated depletion of CD4 T cells allowed HSC engraftment when only minor histocompatibility differences existed between the host and donor, but that this engraftment was lost at ~8 weeks post-transplant as host CD4 T cells were regenerated7. We have shown that injection of neonates with HY incompatible hematolymphoid cells can induce and maintain transplant tolerance with less than 1% chimerism28, 29, while more recently, Taniguchi and colleagues have shown that a blood chimerism of >10% can generate permanent tolerance to allografts in adults30. It may be possible to achieve this level of chimerism through the repetitive transplantation of MHC-matched HSCs into CD4 and/or fully T cell depleted hosts prior to the regeneration of CD4 T cells and thus maintain permanent tolerance to the graft even in fully immunocompetent individuals. Importantly, several recent clinical trials using humanized antibodies directed against CD231 and CD432 have shown some efficacy in the depletion of host T cells and in some cases, the generation of tolerance to allografts33.
Concluding Remarks
Through a better understanding of the dynamic interactions of HSCs with their bone marrow niches, it now appears possible to manipulate this game of musical chairs for clinical benefit. The ability to purify HSCs coupled with new methods to transiently suppress the immune system might allow for high levels of HSC engraftment with little or no risk to the patient. Such advances might transform the way bone marrow transplants are performed and could greatly benefit individuals suffering from hematopoietic disorders.
Materials and Methods
Animals
All animal procedures were approved by the International Animal Care and Use Committee. The C57Bl/Ka-Thy1.1 CD45.1 strain was derived and maintained in our laboratory. The RAG2−/− γc−/− mice have been described previously 11 and were bred onto the C57Bl/Ka-Thy1.2 CD45.2 background. Peripheral blood was sampled from the tail vein and HSC transplants were performed by injection into the retroorbital sinus of isoflurane-anesthetized mice. Donor mice were 10–12 weeks old and recipient mice ranged from 4–6 weeks of age.
Antibodies
The following monoclonal antibodies were purified and conjugated using hybridomas maintained in our laboratory: 2C11 (anti-CD3), GK1.5 (anti-CD4), 53–7.3 (anti-CD5), 53–6.7 (anti-CD8), 6B2 (anti-B220), 8C5 (anti-Gr-1), M1/70 (anti-Mac-1), TER119 (anti-Ter119), A20.1.7 (anti-CD45.1), AL1-4A2 (anti-CD45.2), 2B8 (anti-c-kit), and E13-161-7 (anti-Sca-1). Antibodies were conjugated to biotin, phycoerythrin (PE), allophycocyanin (APC), or Alexa 488 (Molecular Probes, Eugene, OR), according to manufacturer's instructions. Antibodies against CD3, CD4, CD8, B220, Mac-1, Ter119, and Gr-1 conjugated to PE-Cy5, anti-ckit and anti-Mac-1 conjugated to PE-Cy7, anti-Sca-1 conjugated to PE-Cy5.5, anti-B220 conjugated to APC-Cy7, streptavidin conjugated to APC, and anti-flk2 (A2F10) conjugated to PE were purchased from eBiosciences (San Diego, CA). Anti-CD34 (RAM34) conjugated to biotin was purchased from BD Pharmingen (San Diego, CA).
Fluorescence Activated Cell Sorting and Analysis
All cells were sorted and analyses performed on a BD FACS-Aria (Becton Dickinson, Mountain View, CA). Peripheral blood was obtained from the tail vein, red blood cells were sedimented with 2% dextran, and the remaining red blood cells were lysed with an ammonium chloride solution. The remaining white blood cells were stained with anti-CD45.2-Alexa 488, anti-CD45.1-PE, anti-Ter119-PE-Cy5, anti-Mac-1-PE-Cy7, anti-CD3-APC, and anti-B220-APC-Cy7. For HSC and progenitor isolation, bone marrow was first enriched using anti-c-kit beads and immunomagnetic selection on an AutoMACS machine (Miltenyi Biotec, Bergisch Gladbach, Germany). Enriched cells were stained with anti-flk2-PE, anti-lineage (CD3, CD4, CD8, B220, Ter119, Mac-1, Gr-1)-PE-Cy5, anti-Sca-1-PE-Cy5.5, anti-c-kit-PE-Cy7 and anti-CD34-biotin followed by streptavidin-APC. C-kit+ lineage− Sca-1+ CD34− Flk2− (HSCs) or c-kit+ lineage− Sca-1+ CD34+ flk2+ (KLS flk2+) cells were double sorted prior to transplantation.
Immunizations
Mice were immunized intraperitoneally with 100µg NP-CGG (Biosearch Technologies, Novato, CA) precipitated in 10% aluminum potassium sulfate (Sigma-Aldrich, St. Louis, MO). NP-specific enzyme-linked immunosorbent assays were performed with serum obtained 7 days after immunization on high-protein binding 96-well plates coated with 5µg NP-BSA/ well (Biosearch Technologies). Wells were developed with anti-mouse IgG-horseradish peroxidase (SouthernBiotech, Birmingham, AL) followed by 1 mg/ml ABTS reagent (Sigma-Aldrich) and the reactions were stopped by the addition of 0.1% sodium azide (Sigma-Aldrich). Absorbance was read at a wavelength of 405 nm. Concentrations of NP-specific antibody were calculated through comparisons with standard dilution curves of mouse IgG (Sigma-Aldrich).
Acknowledgements
We thank L. Jerabek for laboratory management and C. Richter for antibody production. This work was supported by NIH grants 5R01HL058770 and 2R01AI047457 (I.L. Weissman). D. Bhattacharya is supported by a fellowship from the Cancer Research Institute, D. Bryder was supported by a scholarship from the Swedish Medical Research Council (STINT), and D.J. Rossi is supported by a fellowship from the Damon Runyon Cancer Foundation. Affiliations that might be perceived to have biased this work are as follows: (1) I.L.W. was a member of the scientific advisory board of Amgen and owns significant Amgen stock; and (2) I.L.W. cofounded and consulted for Systemix; is a cofounder and director of Stem Cells, Inc.; and recently cofounded Cellerant, Inc.
Footnotes
All other authors do not have a conflict of interest.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Tarbell NJ, Amato DA, Down JD, Mauch P, Hellman S. Fractionation and dose rate effects in mice: a model for bone marrow transplantation in man. Int J Radiat Oncol Biol Phys. 1987;13:1065–1069. doi: 10.1016/0360-3016(87)90046-0. [DOI] [PubMed] [Google Scholar]
- 3.Tomita Y, Sachs DH, Sykes M. Myelosuppressive conditioning is required to achieve engraftment of pluripotent stem cells contained in moderate doses of syngeneic bone marrow. Blood. 1994;83:939–948. [PubMed] [Google Scholar]
- 4.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. [PubMed] [Google Scholar]
- 5.McCredie KB, Hersh EM, Freireich EJ. Cells capable of colony formation in the peripheral blood of man. Science. 1971;171:293–294. doi: 10.1126/science.171.3968.293. [DOI] [PubMed] [Google Scholar]
- 6.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc Natl Acad Sci U S A. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron Y, Sprent J. Prolonged survival in vivo of unprimed B cells responsive to a T-independent antigen. J Exp Med. 1985;161:1581–1586. doi: 10.1084/jem.161.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arber C, BitMansour A, Sparer TE, Higgins JP, Mocarski ES, Weissman IL, Shizuru JA, Brown JM. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood. 2003;102:421–428. doi: 10.1182/blood-2002-12-3834. [DOI] [PubMed] [Google Scholar]
- 11.Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 12.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolfsson J, Borge OJ, Bryder D, Theilgaard-Monch K, Astrand- Grundstrom I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 14.La Gruta NL, Driel IR, Gleeson PA. Peripheral T cell expansion in lymphopenic mice results in a restricted T cell repertoire. Eur J Immunol. 2000;30:3380–3386. doi: 10.1002/1521-4141(2000012)30:12<3380::AID-IMMU3380>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Tesch H, Smith FI, Muller-Hermes WJ, Rajewsky K. Heterogeneous and monoclonal helper T cells induce similar anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibody populations in the primary adoptive response. I. Isotype distribution. Eur J Immunol. 1984;14:188–194. doi: 10.1002/eji.1830140215. [DOI] [PubMed] [Google Scholar]
- 16.Smith FI, Tesch H, Rajewsky K. Heterogeneous and monoclonal helper T cells induce similar anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibody populations in the primary adoptive response. II. Lambda light chain dominance and idiotope expression. Eur J Immunol. 1984;14:195–200. doi: 10.1002/eji.1830140216. [DOI] [PubMed] [Google Scholar]
- 17.Rocha B, Penit C, Baron C, Vasseur F, Dautigny N, Freitas AA. Accumulation of bromodeoxyuridine-labeled cells in central and peripheral lymphoid organs: minimal estimates of production and turnover rates of mature lymphocytes. Eur J Immunol. 1990;20:1697–1708. doi: 10.1002/eji.1830200812. [DOI] [PubMed] [Google Scholar]
- 18.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 19.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 20.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 21.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001;293:248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 22.Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferry C, Socie G. Busulfan-cyclophosphamide versus total body irradiation-cyclophosphamide as preparative regimen before allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia: what have we learned? Exp Hematol. 2003;31:1182–1186. doi: 10.1016/j.exphem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202:1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iannone R, Luznik L, Engstrom LW, Tennessee SL, Askin FB, Casella JF, Kickler TS, Goodman SN, Hawkins AL, Griffin CA, Noffsinger L, Fuchs EJ. Effects of mixed hematopoietic chimerism in a mouse model of bone marrow transplantation for sickle cell anemia. Blood. 2001;97:3960–3965. doi: 10.1182/blood.v97.12.3960. [DOI] [PubMed] [Google Scholar]
- 26.Rao SS, Peters SO, Crittenden RB, Stewart FM, Ramshaw HS, Quesenberry PJ. Stem cell transplantation in the normal nonmyeloablated host: relationship between cell dose, schedule, and engraftment. Exp Hematol. 1997;25:114–121. [PubMed] [Google Scholar]
- 27.Shizuru JA, Jerabek L, Edwards CT, Weissman IL. Transplantation of purified hematopoietic stem cells: requirements for overcoming the barriers of allogeneic engraftment. Biol Blood Marrow Transplant. 1996;2:3–14. [PubMed] [Google Scholar]
- 28.Weissman IL. Studies on the mechanism of split tolerance in mice. Transplantation. 1966;4:565–571. doi: 10.1097/00007890-196609000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Weissman IL. Transfer of tolerance. Transplantation. 1973;15:265–269. doi: 10.1097/00007890-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi H, Abe M, Shirai T, Fukao K, Nakauchi H. Reconstitution ratio is critical for alloreactive T cell deletion and skin graft survival in mixed bone marrow chimeras. J Immunol. 1995;155:5631–5636. [PubMed] [Google Scholar]
- 31.Przepiorka D, Phillips GL, Ratanatharathorn V, Cottler-Fox M, Sehn LH, Antin JH, LeBherz D, Awwad M, Hope J, McClain JB. A phase II study of BTI-322, a monoclonal anti-CD2 antibody, for treatment of steroid-resistant acute graft-versus-host disease. Blood. 1998;92:4066–4071. [PubMed] [Google Scholar]
- 32.Rep MH, van Oosten BW, Roos MT, Ader HJ, Polman CH, van Lier RA. Treatment with depleting CD4 monoclonal antibody results in a preferential loss of circulating naive T cells but does not affect IFN-gamma secreting TH1 cells in humans. J Clin Invest. 1997;99:2225–2231. doi: 10.1172/JCI119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer TR, McAfee SL, Dey BR, Colby C, Hope J, Grossberg H, Preffer F, Shaffer J, Alexander SI, Sachs DH, Sykes M. Nonmyeloablative haploidentical stem-cell transplantation using anti-CD2 monoclonal antibody (MEDI-507)-based conditioning for refractory hematologic malignancies. Transplantation. 2003;75:1748–1751. doi: 10.1097/01.TP.0000064211.23536.AD. [DOI] [PubMed] [Google Scholar]