Abstract
This article is one of a series, summarising views expressed at the Orthopaedic Research Society New Frontiers in Tendon Research Conference. This particular article reviews the three workshops held under the “Functional Extracellular Matrix” stream. The workshops focused on the roles of the tendon extracellular matrix, such as performing the mechanical functions of tendon, creating the local cell environment and providing cellular cues. Tendon is a complex network of matrix and cells, and its biological functions are influenced by widely-varying extrinsic and intrinsic factors such as age, nutrition, exercise levels and biomechanics. Consequently, tendon adapts dynamically during development, ageing and injury. The workshop discussions identified research directions associated with understanding cell-matrix interactions to be of prime importance for developing novel strategies to target tendon healing or repair.
Keywords: tenocyte, cell, tendinopathy, collagen, proteoglycans, hierarchy, structure, mechanics
Overview
Tendons connect muscle to bone, carrying some of the highest forces experienced by any vertebrate tissue, as they facilitate movement and provide skeletal stability1. The ratio of matrix-to-cells in tendon is subsequently amongst the highest of any vertebrate tissues; thus understanding matrix composition and organisation, and how cells interact with matrix, is key to understanding the function, homeostasis and repair of tendons. While significant advances in our understanding of tendon function have been made, major unanswered questions remain, such as how cells establish the tendon matrix and how the matrix organisation explains the mechanical properties of tendon. This knowledge is a prerequisite for the development of novel strategies to improve tendon repair in the treatment of tendinopathies.
Tendon composition
The extracellular matrix (ECM) of tendon is composed predominantly of collagen, which accounts for ~ 60–85% of the dry weight of the tissue2. Roughly 95% of the collagen is type I, with small levels of collagen types III, V, XI, XII and XIV3; 4. The collagen forms fibre-like structures at a number of different hierarchical levels, each aligned close to the long axis of the tissue (the loading direction), conferring excellent uniaxial mechanical strength to the tendon (figure 1). Collagen fibrils are the principle tensile element in tendon, and can be millimetres in length5 and range in diameter from a few nanometers to over 300 nm6. Evidence from electron microscopy (e.g. see7; 8) suggests that collagen fibrils assemble at the plasma membrane of embryonic tenocytes9; 10; 11, with the force required for transport coming from non-muscle myosin II12. However, further research is needed to fully establish the molecular and mechanical mechanisms. Collagen fibrils are grouped into fibres, fascicles and finally the whole tendon.
Figure 1.
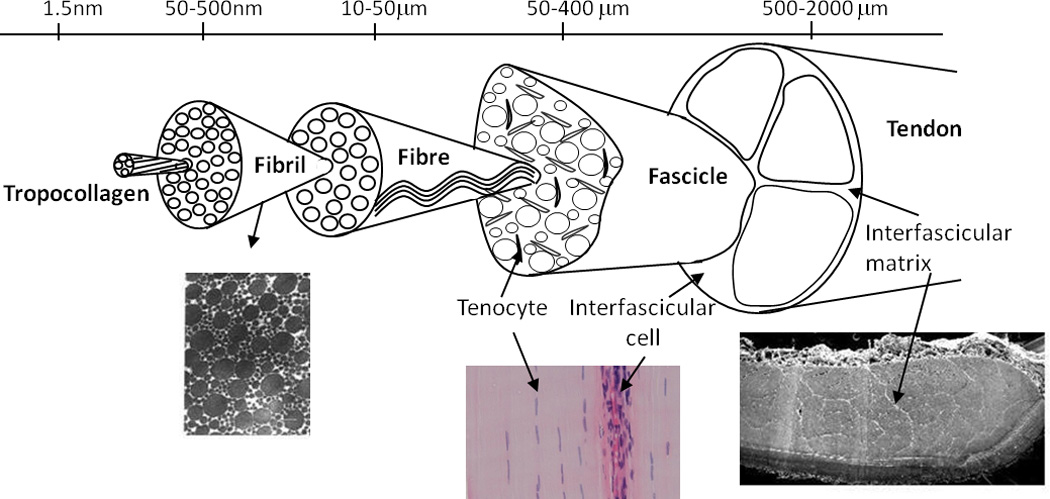
Schematic depicting the hierarchical structure of tendon, with inset images: Transverse sections show fibril and fascicle packing. The longitudinal histological section (H&E) shows the tendon cell populations.
Interspersed between the collagen units throughout the tendon hierarchy is a variety of other non-collagenous matrix components4. Many of these are found across a range of other connective tissues; however details of the amounts, organisation and hierarchical locations of these non-collagenous matrix components are generally less well defined. Within tendon, it is unclear how these matrix components give tendon its unique properties, both mechanical and biological. However, in recent years, some progress has been made to understand their nature and function. The non-collagenous proteins can be grouped into proteoglycans, glycoproteins and glycoconjugates. Proteoglycans are generally divided into 1) large aggregating PGs such as versican and aggrecan and 2) members of the small leucine-rich proteoglycan (SLRP) family.
SLRPs are the abundant proteoglycans in tendon, with decorin accounting for roughly 80% of the total proteoglycan content of the tissue13. The SLRP family is composed of 17 members that are sub-divided into classes I-V based on their protein and DNA sequence homology14. Decorin (named because of its ability to decorate collagen fibrils15) is one of the most widely studied class I SLRPs in tendon, alongside biglycan16; 17 (named because it contains two chains of attached glycosaminoglycans (GAGS)). The class II SLRPs fibromodulin18 and lumican13; 19; 20 are also present in tendon and, like decorin and biglycan, appear to have unique, but overlapping functions in fine-tuning collagen fibril assembly and subsequent tendon integrity19; 20; 21; 22; 23; 24.
The large aggregating proteoglycans such as versican and aggrecan are particularly prominent in the pericellular regions25, but also in compressive regions of tendon, for example where tendons wrap around joints26. Their role, increasing water content in these regions, provides resistance to compression27. The glycoproteins found in tendon include molecules such as lubricin28, tenascin-C, collagen oligomeric matrix component29 (COMP) and tenomodulin30. Elastic fibres, composed of elastin, fibrillins 1 and 2, as well as other elastic fibre-associated molecules are also present31; 32. In addition, microfibrillar structures containing fibrillins, not co-distributed with elastin, are localized throughout the tendon ECM32. Little is known about the function of these components. However, recent data has indicated that both lubricin and elastin are localised to the matrix between fascicles (the interfascicular matrix) where both may play a role in facilitating fascicle sliding and recoil4; 28; 32; 33. Lubricin is also found in the sheath around tendons, where it also may aid in sliding34; 35. The fibrillins have structural and instructive roles. The structural roles are dependent on the temporal and hierarchical assembly of elastic fibres and microfibrils. In contrast, the instructive roles are dependent on the ability of fibrillins to sequester transforming growth factor beta (TGFbeta) and bone morphogenetic protein (BMP) in the extracellular matrix36.
The tendon cell population is heterogenous and poorly defined, with no clear markers available to identify the cells. At least two distinct cell populations are evident within tendon; the highly elongated specialised fibroblasts within fascicles (tenocytes)37, that synthesise a collagen-rich extracellular matrix, and a population of more rounded cells in the interfascicular matrix, which appear more metabolically active38. A phenotypically different, active cell population also has been identified within the peritendon sheath39. Further analysis of these cells is necessary, to establish how they first establish, and subsequently maintain the tendon ECM. In addition, the lack of specific markers identifying cell type and functional state in tendons remains a major impediment to progress.
Tendon ECM Function
Like all connective tissues, tendon has a complex network of cells and surrounding ECM, performing a number of different functional roles. Appropriate composition and organisation of the ECM enables a tendon to perform its mechanical function of force transfer, but also ensures the biological function of tendon, maintaining the microenvironment to ensure cell and matrix health. Mechanical function and biological function are intrinsically linked and neither can be considered in isolation. However, different disciplines tend to use the word "function" generically, and multidisciplinary discussions may benefit from a clear articulation of "biological function" or "mechanical function" when reviewing the role of the matrix.
Mechanical Function
Tendons are predominantly loaded along their long axis in tension, enabling muscles to move the skeleton to position the body. While all tendons perform this positional role, some tendons have an additional role, stretching when loaded to store energy, which they can later return to the system to improve the efficiency of locomotion40. Such tendons are called energy storing tendons. Positional tendons are generally subjected to small strains, in the region of 2–3% in vivo, whilst energy storing tendons can experience strains in excess of 10% during use41; 42. Unsurprisingly, this more demanding mechanical environment leaves energy storing tendons more prone to injuries, termed tendinopathies.
An in-vitro analysis of tendon mechanical properties has identified differences in both the quasi-static and time-dependent mechanical properties of energy storing and positional tendons as befits their functional roles. Energy storing tendons are less stiff and more extensible than positional tendons42; 43, and also behave more elastically, showing less hysteresis and time dependent behaviour44; 45. An overview of differences in energy storing and positional tendon mechanical parameters is provided in figure 2.
Figure 2.
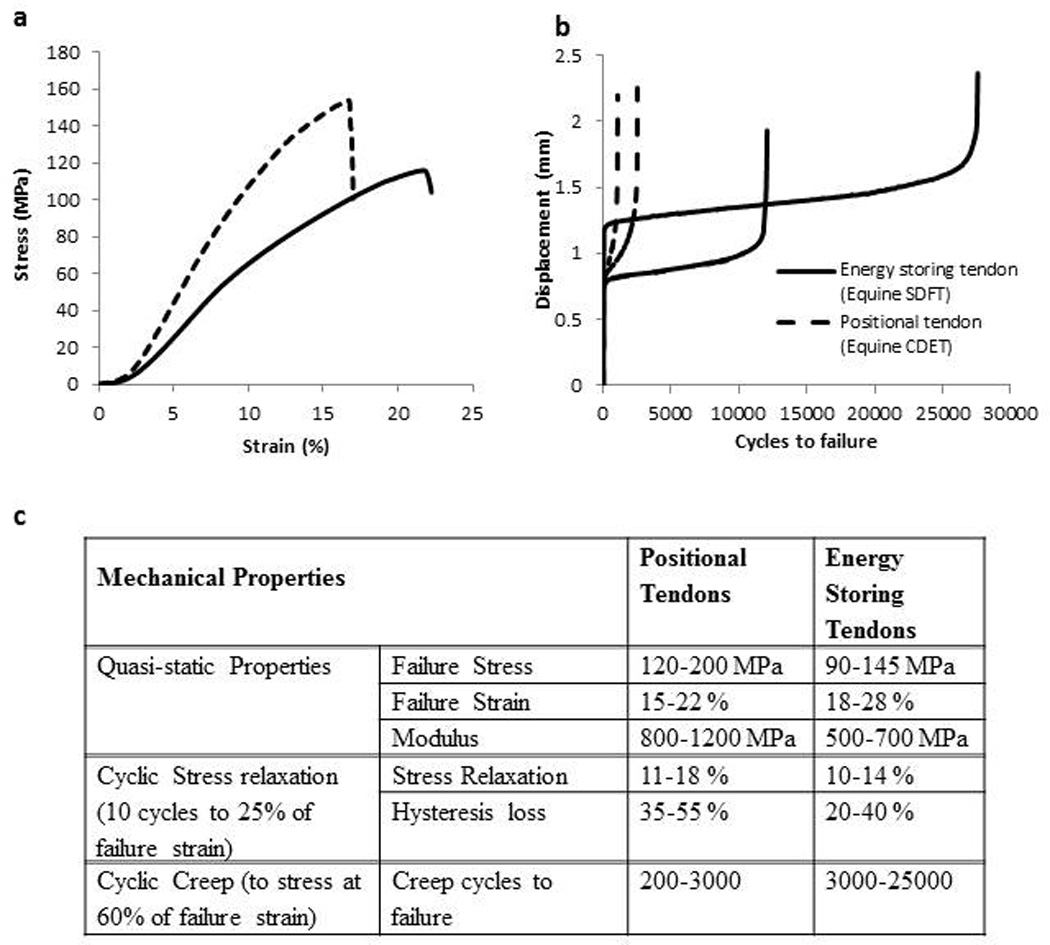
Quasi-static and time dependent mechanical properties differ between energy storing and positional tendons. Typical data in parts a and b highlight differences in (a) the quasi-static properties and (b) the time dependent properties of the equine superficial digital flexor tendon (SDFT - energy storing tendon - solid line) and the common digital extensor tendon (CDET - positional tendon - dotted line). The table (c) provides a more comprehensive overview of data from a series of studies comparing paired equine SDFT & CDET tendons. Note the less stiff, more extensible and more fatigue resistant energy storing tendon properties42; 44; 45; 46.
It is notable that much of the data comparing energy storing and positional tendons has focused on a comparison between the superficial digital flexor tendon (SDFT) and common digital extensor tendon (CDET) of the equine hoof. The SDFT provides a good model for the human Achilles tendon, prone to aetiologically similar tendinopathies47, but is also a particularly highly loaded energy storing tendon40, providing an excellent structure-function model to study the structural mechanisms enabling the tendon to perform its mechanical function.
Compositional differences between energy storing and positional tendons have been reported. For example, the energy storing SDFT has higher levels of GAG than the positional CDET indicating a greater proteoglycan content41; 43. It also has a greater abundance of COMP29, and collagen crosslinking also differs, with the mature crosslink hydroxylysinonorleucine (HHL) found only in the positional CDET48. More recent studies have attempted to localise the compositional differences between tendon types, with the aim of establishing how specific compositional or structural variations contribute to different tendon mechanical or biological behaviours. From a mechanics perspective, a tendon can be considered as a composite material, comprised of fibrous collagen units surrounded by non-collagenous matrix, at multiple hierarchical levels. The transfer of strain through this complex multilevel structure is subsequently highly inhomogenous, involving not just direct loading and extension of the collagen units, but also shearing or sliding between different levels of the collagen hierarchy, modulated by the non-collagenous matrix. Small local variations in the composition of the non-collagenous matrix at a single hierarchical level within the tendon can subsequently have a significant impact on tendon mechanical behaviour. These variations are, as yet, poorly understood, but data indicate that in positional tendons, proteoglycans located between fibrils enable fibril sliding49; 50; 51 and result in a more viscoelastic tendon behaviour, whilst energy storing tendons rely on lubricin and elastin between fascicles to enable a more elastic, recoverable fascicle sliding4; 42; 52. Energy storing tendon fascicles also appear to be helically arranged, contributing to a spring-like, elastic behaviour44; 45. Further work is necessary to understand how tendon structure is optimised to provide appropriate mechanical function across tendon types, and identify the specific matrix components / organisations providing that mechanical behaviour.
Biological Function
It is well known that cell-matrix interactions are dynamic, and connective tissues will not only turnover in homeostatic conditions to maintain health, but also will remodel in response to different stimuli. There is some evidence that these processes can be adaptive, and whilst the speed of adaptation is slow, tendon can thicken and strengthen in response to use53; 54. However, both overuse and underuse have been reported to initiate a more rapid catabolic cell response and tendon degeneration38; 55; 56; 57.
Investigating cell-matrix interactions is complex, as the relationships are not just dynamic, but are also cyclically linked, directly influencing one another. Tendon cells govern the production and organisation of the tendon matrix in response to mechanical and chemical cues58. However, the structure and composition of the matrix is directly responsible for controlling the cues reaching the cells59, generating complex networks and feedback loops linking cell and matrix fate within the tendon. To provide some examples, the mechanical load placed on a tendon when walking will lead to inhomogeneous strains throughout the tendon matrix, and it is these local strains that are perceived at a cellular level60. Matrix turnover is modulated in response to these local strains, adapting the matrix, so the same external load stimulus may be perceived very differently at the cell level over time. A similar dynamic relationship may occur with biological stimuli. The tendon ECM may act to manipulate local availability of the growth factor at a cell level, adjusting cell metabolism and subsequently influencing local matrix conditions and future availability of the growth factor61. The influence of individual matrix components in modulating growth factors may have multiple outcomes, including the regulation of stem cell maintenance, propagation and overall fate62. A major limitation in deepening our understanding in this area is the paucity of ways to track and monitor tendon progenitors63. In this regard, a systematic exploration of tendon cell character is needed. With new tendon markers in hand, the fate and function of tendon cells during development, aging and in disease and, in particular, the role of the ECM in these processes could be seriously addressed.
Dynamic and cyclic cell-matrix interactions are poorly understood in isolation, yet we have even less understanding of how they interact. Research building our understanding of dynamic cell-matrix interactions is critical to provide a cornerstone towards understanding functional extracellular matrix. To further complicate the issue, not only are cell-matrix interactions dynamic processes, but they must be considered in a temporal manner. The circadian cycle affects matrix homeostasis, whilst periods of mechanical stimulus associated with loading a tendon and rest create variable temporal stimulus patterns. The circadian clock regulates gene expression in anticipation of an expected environmental change64. Therefore it is no surprise that bone65, muscle66, cartilage67 and tendon68 are prominent peripheral clock tissues in which the expression of specific sets of genes are regulated in readiness for diurnal mechanical activity. In tendon, BMP signalling and the suppression of calcification is under strict circadian control68. Hormonal changes can influence tissues69, whilst nutritional and lifestyle factors can influence cell-matrix interactions and matrix metabolism over time-spans in the range of months or even years70.
Furthermore, recent data indicates that turnover rates differ between matrix components48, and whilst collagenous components have an exceptionally long half-life (in the hundreds of years71) and show little change during a life time, the non-collagenous components of the matrix are able to turn over and adapt to new stimuli much faster48, perhaps another indicator of their importance within tendon.
Tendinopathy
Tendinopathy is the term given to a broad spectrum of clinical tendon disorders, but is most commonly concerned with chronic painful tendon conditions, in which matrix degradation, neovascularisation and swelling are evident72; 73. The initiation and development of tendinopathy is a temporal and multifactorial process, likely initiated by overuse. However, it remains unclear if the initial driving factor towards tendinopathy is matrix disruption generated by mechanical overuse or a cellular response to altered loading conditions. It is likely that both factors are implicated, with local mechanical matrix damage initiating a biological cascade of events culminating in matrix deterioration74; 75. However, multiple extrinsic and intrinsic factors will influence the dynamic processes of matrix metabolism in health as well as propensity to injury, and will influence cell-matrix interactions in response to overuse.
Interrogating the aeitiology of tendinopathy presents a series of methodological difficulties. Any analysis of human tendinopathic tissue occurs in late stage disease, when matrix changes are chronic. These data have been highly beneficial in understanding chronic, degenerative tendon conditions76; 77, but with no controlled initiation or development of the tendinopathy and only late stage data for analysis, it is difficult to interrogate early tendinopathy. Animal models provide a mechanism for investigating the acute stages of tendinopathy, and more fully characterising temporal aspects of its aetiology78. However, the high degree of heterogeneity in structure, function and metabolism of tendons, both across species and functionally distinct tendons can make the resulting data difficult to interpret.
Questions associated with tendon matrix healthy function and the aetiology of tendinopathy are general complex and multifactorial. In order to begin unravelling these, it is necessary to select a model system in which to undertake highly controlled studies, to investigate a subset of the biological and mechanical parameters involved in these behaviours. Such studies are undoubtedly contributing to our understanding of tendon and the aetiology of tendinopathy, advancing our knowledge base. For example, recent data has begun to quantify the development of matrix disruption with tendon overuse, correlating this to reduced tendon mechanics79. Early overuse damage has been identified within the non-collagenous regions of tendon, and overuse shown to initiate an immediate inflammatory response in tendon38, alongside an upregulation of matrix degradation and cell apoptosis80. However, whilst these data provide important insights into tendinopathy, we still lack a functionally ideal model to enhance further studies. While a single perfect model is probably not attainable, further model development and characterization is a high priority. During this process, we must not forget the full spectrum of complex interactions between cell and matrix and the need for multidisciplinary approaches to interrogate tendon mechanobiology.
Future Directions
There remain multiple unanswered questions associated with ECM function in health and disease, and a number of key avenues for further exploration. We need to establish the molecular and mechanical mechanisms associated with how the ECM develops, and carry out a systemic exploration of the different tendon cell phenotypes, to establish their roles in development, health and disease. To this end, future studies characterizing markers for cell type and functional state will be critical. In addition, the development of animal and cell based models will be necessary to elevate these studies to the next level. We also need a more detailed understanding of both the interactions between matrix components and the interactions between cells and matrix, across the multiple hierarchical levels of the tendon ECM, to establish their biological and mechanical implications. This will facilitate efforts to establish how ECM structure provides mechanical function and also regulates dynamic cell-matrix interactions.
Characterising these behaviours in health provides a basis from which to investigate the aetiology of tendinopathy, and the processes governing ECM degeneration in disease. As part of these efforts, further development and characterisation of tendinopathy models is high priority81.
To continue progress and increased understanding of tendon biology and pathobiology, it is important to embrace multidisciplinary approaches and work in collaborative teams to identify the contributions of cells, matrix, and mechanical forces to achieve tendon biological and mechanical function. The ability to communicate more effectively across disciplines is critical and requires researchers and clinicians to move out of their traditional research focuses and embrace new approaches. We have an opportunity to support this learning as we train early career researchers; exposure to multiple laboratories, techniques and scientific viewpoints during training will help support effective cross-discipline work.
Acknowledgements
The authors would like to thank the Orthopaedic Research Society and the organisers of the New Frontiers in Tendon Research Conference for sponsoring the meeting.
The research in HRCS’s laboratory was supported by grants from the BBSRC, Arthritis Research UK and Orthopaedic Research UK.
The research in KEK’s laboratory was supported by grants from The Wellcome Trust.
The research in MFY’s laboratory was supported by grants from the DIR, NIDCR, part of the IRP, NIH and DHHS.
The research in DEB’s laboratory was supported by grants from the National Institutes of Health, NIAMS AR044745 and AR065995.
Footnotes
Author Contributions: The article summarising the views expressed during the functional extracellular matrix breakout sessions. All authors; Screen, Birk, Kadler, Ramirez and Young were all involved in managing the breakout sessions, and producing this manuscript based on those discussions. All authors have read and approved the final submitted manuscript.
References
- 1.Ker RF. Mechanics of tendon, from an engineering perspective. Int J Fatigue. 2007;29:1001–1009. [Google Scholar]
- 2.Kastelic J, Galeski A, Baer E. Multicomposite Structure of Tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 3.Riley GP, Harrall RL, Constant CR, et al. Tendon Degeneration and Chronic Shoulder Pain - Changes in the Collagen Composition of the Human Rotator Cuff Tendons in Rotator Cuff Tendinitis. Ann Rheum Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorpe CT, Birch HL, Clegg PD, et al. The role of the non-collagenous matrix in tendon function. Int J Exp Pathol. 2013;94:248–259. doi: 10.1111/iep.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig AS, Birtles MJ, Conway JF, et al. An Estimate of the Mean Length of Collagen Fibrils in Rat Tail-Tendon as a Function of Age. Connect Tissue Res. 1989;19:51–62. doi: 10.3109/03008208909016814. [DOI] [PubMed] [Google Scholar]
- 6.Parry DAD, Craig AS. Quantitative Electron-Microscope Observations of Collagen Fibrils in Rat-Tail Tendon. Biopolymers. 1977;16:1015–1031. doi: 10.1002/bip.1977.360160506. [DOI] [PubMed] [Google Scholar]
- 7.Birk DE, Zycband EI, Woodruff S, et al. Collagen fibrillogenesis in situ: Fibril segments become long fibrils as the developing tendon matures. Dev Dynam. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Starborg T, Kalson NS, Lu YH, et al. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat Protoc. 2013;8:1433–1448. doi: 10.1038/nprot.2013.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trelstad RL, Hayashi K. Tendon Collagen Fibrillogenesis - Intracellular Subassemblies and Cell-Surface Changes Associated with Fibril Growth. Dev Biol. 1979;71:228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- 10.Birk DE, Trelstad RL. Extracellular Compartments in Tendon Morphogenesis - Collagen Fibril, Bundle, and Macroaggregate Formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canty EG, Lu YH, Meadows RS, et al. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalson NS, Starborg T, Lu Y, et al. Non-muscle myosin II powered transport of newly-formed collagen fibrils at the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1314348110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix biology : journal of the International Society for Matrix Biology. 2004;23:127–140. doi: 10.1016/j.matbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Nikitovic D, Aggelidakis J, Young MF, et al. The Biology of Small Leucine-rich Proteoglycans in Bone Pathophysiology. J Biol Chem. 2012;287:33926–33933. doi: 10.1074/jbc.R112.379602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott JE. Elasticity in extracellular matrix 'shape modules' of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol-London. 2003;553:335–343. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. The tendon injury response is influenced by decorin and biglycan. Ann Biomed Eng. 2014;42:619–630. doi: 10.1007/s10439-013-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson PS, Lin TW, Reynolds PR, et al. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng. 2004;126:252–257. doi: 10.1115/1.1695570. [DOI] [PubMed] [Google Scholar]
- 18.Svensson L, Aszodi A, Reinholt FP, et al. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 19.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 20.Ezura Y, Chakravarti S, Oldberg A, et al. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–787. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birk DE, Nurminskaya MV, Zycband EI. Collagen Fibrillogenesis in-Situ - Fibril Segments Undergo Postdepositional Modifications Resulting in Linear and Lateral Growth during Matrix Development. Dev Dynam. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- 23.Kilts T, Ameye L, Syed-Picard F, et al. Potential roles for the small leucine-rich proteoglycans biglycan and fibromodulin in ectopic ossification of tendon induced by exercise and in modulating rotarod performance. Scand J Med Sci Sports. 2009;19:536–546. doi: 10.1111/j.1600-0838.2009.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry-Us. 1996;35:8795–8799. doi: 10.1021/bi960773t. [DOI] [PubMed] [Google Scholar]
- 25.Wang VM, Bell RM, Thakore R, et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. J Orthop Res. 2012;30:620–626. doi: 10.1002/jor.21558. [DOI] [PubMed] [Google Scholar]
- 26.Vogel KG. What happens when tendons bend and twist? Proteoglycans. J Musculoskelet Neuronal Interact. 2004;4:202–203. [PubMed] [Google Scholar]
- 27.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments - an adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funakoshi T, Schmid T, Hsu HP, et al. Lubricin distribution in the goat infraspinatus tendon: A basis for interfascicular lubrication. J Bone Joint Surg Am. 2008;90A:803–814. doi: 10.2106/JBJS.G.00627. [DOI] [PubMed] [Google Scholar]
- 29.Smith RK, Gerard M, Dowling B, et al. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: a proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet J Suppl. 2002:241–244. doi: 10.1111/j.2042-3306.2002.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 30.Alberton P, Dex S, Popov C, et al. Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev. 2014 doi: 10.1089/scd.2014.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosline J, Lillie M, Carrington E, et al. Elastic proteins: biological roles and mechanical properties. Philos Trans R Soc Lond B Biol Sci. 2002;357:121–132. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritty TM, Ditsios K, Starcher BC. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat Rec. 2002;268:430–440. doi: 10.1002/ar.10175. [DOI] [PubMed] [Google Scholar]
- 33.Kohrs RT, Zhao C, Sun YL, et al. Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29:384–389. doi: 10.1002/jor.21247. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi M, Zhao CF, Thoreson AR, et al. The Effect of Lubricin on the Gliding Resistance of Mouse Intrasynovial Tendon. Plos One. 2013;8 doi: 10.1371/journal.pone.0083836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taguchi M, Sun YL, Zhao CF, et al. Lubricin Surface Modification Improves Tendon Gliding After Tendon Repair in a Canine Model in Vitro. J Orthop Res. 2009;27:257–263. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeilly CM, Banes AJ, Benjamin M, et al. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- 38.Thorpe CT, Chaudhry S, Lei I, et al. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports. 2014 doi: 10.1111/sms.12333. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Cadby JA, Buehler E, Godbout C, et al. Differences between the Cell Populations from the Peritenon and the Tendon Core with Regard to Their Potential Implication in Tendon Repair. Plos One. 2014;9 doi: 10.1371/journal.pone.0092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biewener AA, Rizzo N. Elastic Energy-Storage in the Horse. Am Zool. 1989;29:A182–A182. [Google Scholar]
- 41.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol. 2007;88:241–248. doi: 10.1111/j.1365-2613.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thorpe CT, Udeze CP, Birch HL, et al. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 2012;9:3108–3117. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batson EL, Paramour RJ, Smith TJ, et al. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet J. 2003;35:314–318. doi: 10.2746/042516403776148327. [DOI] [PubMed] [Google Scholar]
- 44.Thorpe CT, Klemt C, Riley GP, et al. Helical sub-structures in energy-storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater. 2013;9:7948–7956. doi: 10.1016/j.actbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Thorpe CT, Riley GP, Birch HL, et al. Effect of fatigue loading on structure and functional behaviour of fascicles from energy-storing tendons. Acta Biomater. 2014;10:3217–3224. doi: 10.1016/j.actbio.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Thorpe CT, Udeze CP, Birch HL, et al. Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age-related tendinopathy? Eur Cells Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- 47.Patterson-Kane JC, Rich T. Achilles tendon injuries in elite athletes: lessons in pathophysiology from their equine counterparts. Ilar J. 2014;55:86–99. doi: 10.1093/ilar/ilu004. [DOI] [PubMed] [Google Scholar]
- 48.Thorpe CT, Streeter I, Pinchbeck GL, et al. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. The Journal of biological chemistry. 2010;285:15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Screen HRC, Chhaya VH, Greenwald SE, et al. The influence of swelling and matrix degradation on the microstructural integrity of tendon. Acta Biomater. 2006;2:505–513. doi: 10.1016/j.actbio.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Screen HRC, Lee DA, Bader DL, et al. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. P I Mech Eng H. 2004;218:109–119. doi: 10.1243/095441104322984004. [DOI] [PubMed] [Google Scholar]
- 51.Screen HRC, Toorani S, Shelton JC. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys. 2013;35:96–102. doi: 10.1016/j.medengphy.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Thorpe CT, Udeze CP, Birch HL, et al. Capacity for Sliding between Tendon Fascicles Decreases with Ageing in Injury Prone Equine Tendons: A Possible Mechanism for Age-Related Tendinopathy? Eur Cells Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- 53.Kjaer M, Magnusson P, Krogsgaard M, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosager S, Aagaard P, Dyhre-Poulsen P, et al. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- 55.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner K, Arnoczky SP, Caballero O, et al. The effect of stress-deprivation and cyclic loading on the TIMP/MMP ratio in tendon cells: An in vitro experimental study. Disabil Rehabil. 2008;30:1523–1529. doi: 10.1080/09638280701785395. [DOI] [PubMed] [Google Scholar]
- 57.Dakin SG, Dudhia J, Smith RKW. Science in brief: Resolving tendon inflammation. A new perspective. Equine Vet J. 2013;45:398–400. doi: 10.1111/evj.12030. [DOI] [PubMed] [Google Scholar]
- 58.Galloway MT, Lalley AL, Shearn JT. The Role of Mechanical Loading in Tendon Development, Maintenance, Injury, and Repair. J Bone Joint Surg Am. 2013;95A:1620–1628. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Screen HRC, Bader DL, Lee DA, et al. Local strain measurement within tendon. Strain. 2004;40:157–163. [Google Scholar]
- 60.Screen HRC, Lee DA, Bader DL, et al. Development of a technique to determine strains in tendons using the cell nuclei. Biorheology. 2003;40:361–368. [PubMed] [Google Scholar]
- 61.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bi YM, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 63.Pauly S, Klatte F, Strobel C, et al. Characterization of Tendon Cell Cultures of the Human Rotator Cuff. Eur Cells Mater. 2010;20:84–97. doi: 10.22203/ecm.v020a08. [DOI] [PubMed] [Google Scholar]
- 64.Dudek M, Meng QJ. Running on time: the role of circadian clocks in the musculoskeletal system. Biochem J. 2014;463:1–8. doi: 10.1042/BJ20140700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu LN, Patel MS, Karsenty G. The circadian modulation of leptin-controlled bone formation. Prog Brain Res. 2006;153:177–188. doi: 10.1016/S0079-6123(06)53010-9. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Patel SP, McCarthy JJ, et al. A non-canonical E-box within the MyoD core enhancer is necessary for circadian expression in skeletal muscle. Nucleic acids research. 2012;40:3419–3430. doi: 10.1093/nar/gkr1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gossan N, Zeef L, Hensman J, et al. The Circadian Clock in Murine Chondrocytes Regulates Genes Controlling Key Aspects of Cartilage Homeostasis. Arthritis Rheum-Us. 2013;65:2334–2345. doi: 10.1002/art.38035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeung CYC, Gossan N, Lu YH, et al. Gremlin-2 is a BMP antagonist that is regulated by the circadian clock. Sci Rep-Uk. 2014;4 doi: 10.1038/srep05183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kjaer M, Hansen M. The mystery of female connective tissue. J Appl Physiol. 2008;105:1026–1027. doi: 10.1152/japplphysiol.91008.2008. [DOI] [PubMed] [Google Scholar]
- 70.Takeuchi-Igarashi H, Kubota S, Tachibana T, et al. Matrix remodeling response of human periodontal tissue cells toward fibrosis upon nicotine exposure. Odontology. 2014 doi: 10.1007/s10266-014-0177-y. [DOI] [PubMed] [Google Scholar]
- 71.Heinemeier KM, Schjerling P, Heinemeier J, et al. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb C-14. Faseb J. 2013;27:2074–2079. doi: 10.1096/fj.12-225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 73.Riley G. Tendinopathy - from basic science to treatment. Nat Clin Pract Rheum. 2008;4:82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 74.Legerlotz K, Jones GC, Screen HRC, et al. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand J Med Sci Sports. 2013;23:31–37. doi: 10.1111/j.1600-0838.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shepherd JH, Riley GP, Screen HRC. Early stage fatigue damage occurs in bovine tendon fascicles in the absence of changes in mechanics at either the gross or micro-structural level. J Mech Behav Biomed. 2014;38:163–172. doi: 10.1016/j.jmbbm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean BJ, Franklin SL, Carr AJ. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Joint Res. 2012;1:158–166. doi: 10.1302/2046-3758.17.2000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tilley JM, Murphy RJ, Chaudhury S, et al. Effect of tear size, corticosteroids and subacromial decompression surgery on the hierarchical structural properties of torn supraspinatus tendons. Bone Joint Res. 2014;3:252–261. doi: 10.1302/2046-3758.38.2000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010;43:274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andarawis-Puri N, Sereysky JB, Jepsen KJ, et al. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech. 2012;45:59–65. doi: 10.1016/j.jbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andarawis-Puri N, Philip A, Laudier D, et al. Temporal effect of in vivo tendon fatigue loading on the apoptotic response explained in the context of number of fatigue loading cycles and initial damage parameters. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32:1097–1103. doi: 10.1002/jor.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hast MV, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. 2014;3:193–202. doi: 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]


