Abstract
Strategies for bone tissue regeneration have been continuously evolving for the last 25 years since the introduction of the “tissue engineering” concept. The convergence of the life, physical, and engineering sciences has brought in several advanced technologies available to tissue engineers and scientists. This resulted in the creation of a new multidisciplinary field termed as “regenerative engineering”. In this article, the role of biomaterials in bone regenerative engineering is systematically reviewed to elucidate the new design criteria for the next generation of biomaterials for bone regenerative engineering. We highlight the exemplary design of biomaterials harnessing various materials characteristics towards successful bone defect repair and regeneration. In particular, we concentrate our attention on the attempts of incorporating advanced materials science, stem cell technologies, and developmental biology into biomaterials design to engineer and develop the next generation bone grafts.
Graphical Abstract
The role of biomaterials for bone regenerative engineering has been redefined at the convergence of advanced materials science, stem cell science and developmental biology. The novel design of materials leveraging new tool box in these fields for bone regeneration are reviwed.
1. Introduction
Bone and its related diseases still remain a significant clinical challenge worldwide as they account for half of all chronic conditions in people over the age of 50[1,2]. Among these problems, large bone defects caused by trauma, fracture nonunion, bone tumor resection, spinal deformities, and infection present a severe threat to the health of this age group[3]. Unfortunately, no satisfactory solutions for bone grafts are available currently due to the unmet regenerative potential of bone defects and the limited effectiveness of treatment options. Autograft is regarded as the gold standard in clinic but it is largely limited by its availability and significant donor site morbidity[4,5]. Allografts as another alternative, also suffer from issues such as immune rejection, disease transmission and high failure rate[6]. In response to the urgent needs for novel bone defect treatment, bone regenerative engineering has offered a promising approach to effectively regenerate bone and circumvent the limitations associated with conventional treatments[7]. By combining a biocompatible scaffold, cells and morphogenetic signaling molecules together in a 3D complex system, functional bone grafts can be created in an engineering setup and utilized to treat various bone related diseases and injuries[8,9]. Illustrative applications of bone regenerative engineering towards addressing bone grafts shortages include filling of large voids caused by nonunion fractures, bridging of gaps in spinal fusion, and stabilizing of vertebral compression fractures[10–16].
While major advances have been made in tissue engineering by numerous scientists and engineers since the first use of the term “tissue engineering” in 1987[9], we have also witnessed the rise of a few other exciting fields including advanced biomaterials science, stem cell science, developmental biology, and their convergence with tissue engineering in the last decade. The integration of these fields with tissue engineering has brought the birth of a new multidisciplinary paradigm termed as “regenerative engineering”, which is defined by Laurencin as “the integration of materials science and tissue engineering with stem and developmental cell biology and regenerative medicine toward the regeneration of complex tissues, organs, or organ systems”[17]. Advances in materials science, especially the development of nanotechnology has added extremely valuable and exquisite tools to manipulate cell behaviors towards tissue formation for tissue engineers[18,19]. A great example is the application of electrospinning techniques for nanofiber fabrication, which has been later used in a variety of regenerative engineering fields such as bone, skin, ligament and neural regeneration[20,21]. The breakthrough in stem cells research including the discovery of embryonic stem cells in 1990s and induced pluripotent stem cells (iPS cells) in 2006 has opened enormous opportunities for tissue regeneration as they have almost completely addressed the issue of cell source for regenerative engineering and regenerative medicine[22–24]. Besides, the knowledge gained in developmental biology has brought us with deeper understanding of important phenomena such as limb development, which can be leveraged and incorporated into the development of new regenerative approaches[25,26]. Regenerative engineering strategies can significantly benefit from learning and establishing some of the morphogenetic events that are keys in developing or forming the tissue in the first place. Thus, we speculate that implementation of the concept of regenerative engineering in bone regeneration would catalyze the further success of bone regenerative engineering and finally translate these technologies from bench side to the patients’ bed side.
Considering the new role of biomaterials at the convergence of the life, physical, and engineering sciences, this review article focuses on the recent development on design and application of biomaterial that integrate the concept of regenerative engineering. We firstly provide a brief overview of biomaterial evolution in the last quarter of century. Then, we discuss the roles of biomaterials on different aspects of bone regeneration and how they could influence the design of biomaterials for bone regenerative engineering. Finally, we highlight the exemplary design of biomaterials harnessing various materials characteristics towards successful bone healing. In particular, we concentrate our attention on the attempts of incorporating advanced materials science, stem cell technologies, and developmental biology aspects into biomaterials design for the next generation of bone grafts.
2. Evolution of biomaterials for bone regenerative engineering
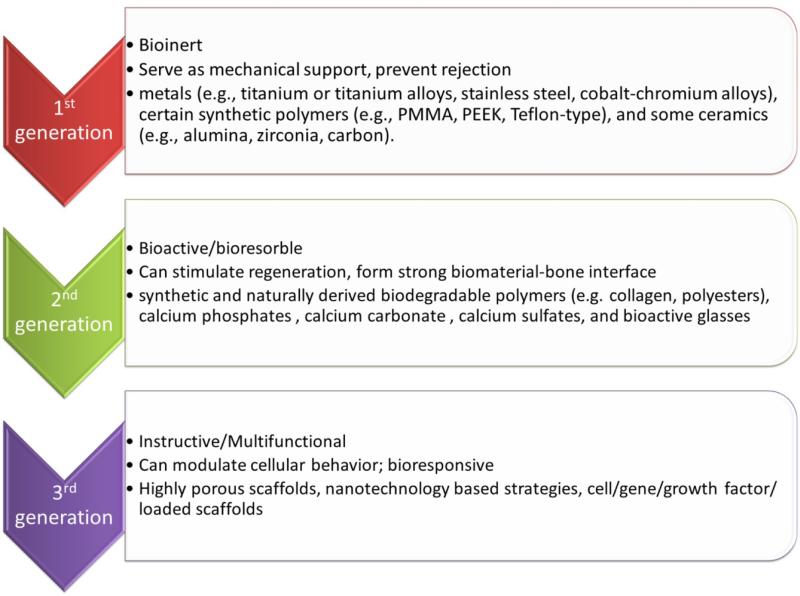
Historically, the need for basic research concerning implantable biomaterials was initiated when physicians first attempted to leave non-biologic materials imbedded in the body following surgery[27]. Numerous archeological findings show that attempts to replace missing teeth with materials like corals, ivory, metals, human and animal bones and even wood, date back to the early stages of humanity [28]. Later on, biomaterials with desirable properties such as biocompatibility, biodegradability, and osteoconductivity have been designed and developed to better serve as bone grafts[29]. Clinically used bone grafts can also be classified on the basis of their origin into biological (autografts, allografts and xenografts) or synthetic materials. A piece of bone taken from the patient's own body and implanted into another location of the same patient is termed as autograft. They possess optimal osteogenic, osteoconductive and osteoinductive properties and cause no immunogenic reaction, therefore are considered as the gold standard for bone repair. However, the key limitation with the use of autografts is donor site morbidity, in which the remaining tissue at the harvest site is damaged by removal of the graft[4,30]. On the other hand, allografts are tissues harvested from one individual and implanted into another individual of the same species. They also have their own limitations, such as potential pathogenic transmissions and host immune response[31]. Xenografts are cells, tissues and organs harvested from one species to another, which have been severely restricted by immunogenic barriers between species[32]. The limitations associated with the biological bone replacement materials led to the use of synthetic alternatives for bone repair, replacement and augmentation, resulting in the inception of a multidisciplinary field of “Biomaterials” in the early 1960s[33]. Here, we briefly review the history of biomaterials development for bone regeneration as this field has kept on dynamically evolving due to its highly multidisciplinary background and urgent needs from the aging society (Fig. 1).
Fig 1.
Evolution of biomaterials from 1st generation to 3rd generation with improving functionality
2.1 First generation biomaterials in bone regeneration[34]
In 1960s, the first generation of biomaterials was developed with an aim to “achieve a suitable combination of physical properties to match those of the replaced tissue with a minimal toxic response to the host”[35]. Generally termed as “bioinert” i.e. biologically inert, once placed in the human body, these materials exhibited minimal interaction with its surrounding tissue. Hence, they did not stimulate bone formation but resulted in formation of fibrous tissue[36]. Broadly, the first generation biomaterials can be categorized into the following types: metals (e.g., titanium or titanium alloys, stainless steel, cobalt-chromium alloys), synthetic polymers (e.g., poly methyl methacrylate, Teflon-type), and ceramics (e.g., alumina, zirconia, carbon)[37–39].
The first successful substitutive joint prosthesis developed by Charnley in late 1950s was made of stainless steel [40]. Stainless steel is resistant to corrosion due to high chromium content. However, the rather poor wear resistance of stainless steel led to the introduction of cobalt-chromium alloys[41]. These materials exhibited excellent corrosion and wear resistance. However, their elastic modulus was an order of magnitude higher (220-230 GPa) than that of the human cortical bone (20-30 GPa) [39]. In this case, the implant would take most of the load due to its high modulus resulting in stress shielding of the adjacent bone. The lack of mechanical stimuli induced bone resorption with eventual failure and loosening of the implant[42]. This could be explained by Wolff's law which states that “every change in the form and function of the bone or of their function time is followed by certain definite changes in their internal architecture, and equally definite alteration in their external conformation, in accordance with mathematical laws”[43]. Hence, when using materials with significantly high elastic modulus than the native bone, the adjacent bone experienced lower load or stress (i.e., stress shielding) and responded by decreasing bone mass, which eventually led to loosening of and thus failure of the implant[44].
Titanium and its alloys, originally used in aeronautics, generated great interest in orthopedics due to their excellent corrosion resistance, moderate elastic modulus (~110 GPa) and a low density (approx. 4700 kg/m3) [45]. Branemark introduced the concept of osseointegration for implants in 1940s, which is the formation of direct bonding between a load-bearing implant and host bone tissue without soft tissue formation [46]. He showed that titanium implants could become permanently incorporated within bone such that the implant and the bone could not be separated without fracture[47,48]. Osseointegration gradually became one of the most important requirements for bone implants[46,49]. For instance, various surface treatment strategies such as plasma-spraying, acid-etching, and anodization have been used to improve the osseointegration of titanium based implants[50]. It was found that rough surface generated by acid-etching significantly accelerated the integration of titanium implants after implantation[51]. This greatly improved the long-term behavior of implantable devices, decreasing the risk of implant loosening and failure.
Charnley introduced self-polymerizing poly methyl methacrylate (PMMA) bone cement for anchorage of femoral head prosthesis to femur shaft [40]. Due to its inert nature, though PMMA could provide an excellent primary fixation to the prosthesis, it could not promote a biological secondary fixation. Moreover, it was associated with other disadvantages such as highly exothermic polymerization reaction, tendency of residual monomer to enter the blood stream leading to fat embolism, shrinkage of the cement during polymerization, to name a few. Ultrahigh molecular weight polyethylene (UHMWPE) was another polymer used for arthroplasties due to its unique properties of high abrasion resistance, low friction, unparalleled toughness, ease of fabrication, and satisfactory biocompatibility[52]. The main problem associated with the use of these polymers is oxidative degradation caused by the combination of the irradiation used for sterilization and oxygen which leads to a decrease in wear resistance and mechanical properties[52]. The particles produced by the wear can further lead to an inflammatory reaction in the surrounding tissues [53]. Silicone based implants were first introduced by Swanson in mid 1960s for replacement of arthritic or destroyed joints [54]. They were proven to effectively reduce pain and slightly improve the range of motion in arthritic patients. Certain non-resorbable composite materials were also designed, e.g. carbon reinforced composites with polymers like polyethylene, polysulfone for improved stability and lower rigidity in comparison to metallic biomaterials[55].
2.2 Second generation biomaterials in bone regeneration
The second generation biomaterials included synthetic and naturally-derived biodegradable polymers (e.g. collagen, polyesters), calcium phosphates (synthetic or derived from natural materials such as corals, algae, bovine bone), calcium carbonate (natural or synthetic), calcium sulfates, and bioactive glasses (silica or non-silica based)[56–58]. Many biomaterials derived from nature possess excellent biocompatibility and biodegradability as they are essential components of tissues. Naturally derived polymers like collagen and hyaluronic acid can provide an innate biological informational guidance to cells leading to improved cell attachment as well as better chemotactic responses, when compared to certain synthetic polymers [59,60]. However, they suffer from some drawbacks such as immunogenic response, batch-to-batch variation due to complex purification processes, restrictions with respect to the design of devices with specific biomechanical properties and variable rate of in vivo degradation (especially in case of enzymatically degradable polymers) [61,62]. Synthetic polymers on the other hand, provide the flexibility to tailor mechanical properties and degradation kinetics to suit various applications, and can be fabricated into various shapes with desired characteristics [63]. Some of the most extensively studied synthetic biodegradable polymers include polylactide (PLA), polyglycolide (PGA), polycaprolactone (PCL), polyhydroxybutyrate (PHB), polyorthoesters, and their copolymers[64,65].
The first artificial bioactive material “Bioglass” was invented by Larry Hench in 1969. It was composed of 46.1 mol % SiO2, 24.4 mol % Na2O, 26.9 mol % CaO and 2.6 mol % P2O5, later termed 45S5 and Bioglass®. Bioglass® was the first artificial osseointegrative material designed to form direct chemical bonding with bone [66]. The concept started to spread out in the mid 1980s when the use of “bioactive materials” in a number of dental and orthopedic applications was implemented aiming to produce bioactive components that could elicit favorable biological response in the physiological environment [67]. Also, the demand of materials with specific physical, chemical, biological, biomechanical, and degradation properties led to the use of “biodegradable” materials. The concept of bioresorbable/bioabsorbable/biodegradable materials was introduced by Kulkarni et al. [68] in the 1960s. Bioresorbable materials exhibited clinical relevance via controlled chemical breakdown, therefore were extensively used as biomaterials later on. The term “bioactive material” refers to a material, which upon being placed within the human body interacts with the surrounding tissue that forms a bond between the tissues and material by eliciting a specific biological response at the material interface[67]. For instance, bioactive materials for bone healing could lead to formation of a biologically active carbonated apatite (CHAp) layer on the implant which is chemically and crystallographically comparable to the natural bone apatite [69].
The first application of calcium phosphates for bone repair was reported in 1920 by Albee and Morrison [70]. Calcium phosphate ceramics such as hydroxyapatite (HA), tricalcium phosphate (TCP) and octacalcium phosphate (OCP) differ in their chemical formula as well as the Ca/P ratio. TCP has a Ca/P ratio of 1.5 and is marked by a high dissolution rate that accelerates material resorption. Pure HA has a Ca/P ratio of 1.67 and is highly stable [71]. The biological apatites, such as bone mineral, dentine, tooth enamel possess numerous substitutions with hydrogenophosphate (HPO4)2-, and carbonate (CO32-) etc., which provides them special biological, functional and chemical features. They may further contain various trace elements such as fluoride, silicon etc.[72]. The compositional resemblance of calcium phosphate bioceramics to the bone mineral provide them superior properties for the stimulation of bone formation and bone bonding [72].
2.3 Third generation biomaterials in bone regeneration
Third generation biomaterials are designed to incorporate instructive cues into the materials to induce favorable cellular response such as improved cell survival, directed cell differentiation, and specific lineage commitment [73,74]. Some of these approaches involve the use of soluble factors (growth factors, cytokines, hormones and chemicals), insoluble factors (extracellular matrix molecules, immobilized adhesion ligands, biomaterial mechanical and structural properties) or use of external stimuli (mechanical loading, compressive stress, shear stress, cyclic stretch, use of conducting polymers)[75]. The development of materials to activate specific genes and molecular tailoring of biomaterials to elicit desired cellular responses are some of the strategies utilized for development of third generation materials[76]. Materials with appropriate physical characteristics such as high porosity and interconnectivity have been designed and engineered to facilitate material/cell interactions, nutrient/oxygen infiltration and vascularization[77–79]. For example, Nukavarapu et al., developed optimally porous and mechanically compatible scaffolds for bone regenerative engineering [80]. In a later study, it was demonstrated that theses matrices control oxygen-tension inside the pore structure so that the matrix can support osteogenic and vasculogenic cell survival even deep inside the matrix pore structure, resulting in large-area bone regeneration [81,82]. The instructive role of biomaterials can also be introduced by supplementation of osteogenic components such as bone marrow aspirate (BMA) or by addition of osteinductive components such as bone morphogenetic proteins, rhBMP-2, rhBMP-7 (e.g. INFUSE® bone graft, OP-1 implant and putty), to actively recruit progenitor cells from surrounding tissue, guide stem cell homing and enhance cellular differentiation at the defect sites[83,84].
The application of nanotechnology to the field of regenerative engineering has offered the means to control the biochemical and mechanical microenvironment for successful cell delivery and tissue regeneration [85][86–88]. Nanomaterials are materials that are composed of feature size between 1-100 nm, which is comparable to the feature size of living tissue. Webster et al have highlighted the potential superiority of nanomaterials for bone regeneration as they mimic the nanostructured hierarchal self-assembly of native bone[89,90]. The major applications of nanotechnology in bone regeneration include, a) incorporation of nanomaterials to obtain composites with superior mechanical, biological or electrical properties; b) surface modification at nano-level for improving cell adhesion and functions; c) generation of degradable alternatives (e.g. nanoceramics); d) Change in and use of nanotopographical features to improve osteoblast functions; and e) Use of nano drug delivery to promote healing and functional recovery [91–97]. An example where the excellent properties of nanomaterials were harnessed for bone tissue engineering is the use of nanocrystalline calcium phosphates which demonstrate faster degradation and enhanced bone cell functions compared to micron grain size calcium phosphate [98]. In another set of studies, Stupp et al. has successfully used self-assembled amphilphile nanofiber for bone regeneration application[99,100]. They found that the efficacy of BMP-2 was amplified by up to one order of magnitude after combining BMP-2 on supramolecular nanofiber via a heparin binding domain, indicating the great potential of nanotechnology in bone regenerative engineering [101]. As one of the three essential components of regenerative engineering, nanotechnology shall revolutionarily shift the paradigm of biomaterials for bone regeneration and holds the promise for the future generation of bone substitute grafts.
3. The role of biomaterials in regenerative engineering of bone
3.1 Osteoconduction
Osteoconductivity, the ability of biomaterials to support new bone formation on their surfaces, serves as one of the most important prerequisites of biomaterials used for bone regeneration[102,103]. Osteoconductive materials allow the migration, proliferation, differentiation, and extracellular matrix (ECM) deposition of osteoprogenitor cells within the bone defects, which are the key steps toward new bone formation[77,104]. Specifically, the formation of a thin carbonated hydroxyapatite (cHAp) layer on these materials adsorbs proteins, which facilitate bone forming cell attachment and subsequent activities related to bone matrix deposition[28]. The apposition of bone mineral initiated by the osteoconductivity of the materials leads to integration of newly formed bone tissue into surrounding bone tissue or conjunction of an implant with host bone, thus it is of critical importance in term of achieving functional bone regeneration[30].
The osteoconductivity of biomaterials during bone healing highly depends on their physicochemical characteristics. Important features of biomaterials’ osteoconductivity include appropriate chemical composition, surface property, architectural geometry, etc.[28]. Calcium phosphate (CaP) based ceramics such as hydroxyapatite (HA), tricalcium phosphate (TCP) possess excellent osteoconductivity due to their similarity to natural bone mineral[105,106]. Bioglass also represents another type of osteoconductive material that is capable of forming direct bonding with bone[107]. Besides, type I collagen is also generally considered as osteoconductive material as its composition and structure is conducive for mineral deposition through binding of noncollagenous matrix proteins, which initiate and control mineralization[99].
Osteoconductivity can also be introduced into non-biological materials such as metal, ceramics and synthetic polymers via various strategies such as coating and composite. For example, although titanium is generally considered as not osteoconductive, bone conduction was observed after the formation of a tatania layer on its surface via appropriate surface treatment[108]. Direct bone bonding was also realized by applying a hydroxyapatite coating on these metal implant surface[57,109,110]. For synthetic polymers such as poly (lactic acid)(PLA), poly(lactide-co-glycolide) (PLGA), and poly (ε-caprolactone) (PCL), introduction of osteoconductivity into these materials were realized by either forming into composite with CaP ceramics or formation of CaP coating on their surfaces[79,111–114].
3.2 Osteoinduction
The property of biomaterials to directly induce bone formation at an ectopic site is termed as osteoinductivity. This pivotal property of biomaterials has been identified since the publication of seminal work by Urist in 1965, in which they showed that consistent bone formation was observed in rabbit muscles after implantation of decalcified bone[115]. Although the exact mechanism of osteoinduction still remains largely unknown, researchers have made tremendous progress toward unveiling the role of osteoinductive materials during bone regeneration in the past few decades[116]. Biomaterials with osteoinductivity have demonstrated influence on ectopic bone formation at multiple levels: i) at tissue level, they tend to actively facilitate nutrition, oxygen, and waste exchange between the material and tissue; they also encourage vascularization within the materials, which is essential for new tissue growth[117,118]; ii) at cellular level, the formation of biological carbonated apatite layer can trigger the differentiation of stem cells/osteoprogenitor cells toward osteogenic linage[119–121]. The released calcium and phosphate ion can also serve as strong cell chemotaxis for migration and directed growth of multiple cell types at the implantation sites[122,123]; iii) at molecular level, osteoinductive materials may be able to concentrate osteogenic protein such as BMP-2 and BMP-7 due to their high affinity to these bodily present osteoinductive proteins. The enrichment of local growth factors may promote a series of cellular activities on biomaterials surface[124,125]. On the other hand, the released calcium and phosphate ions may help reach supersaturation level in the void of implants and accelerate mineralization in the context of bone formation[126,127].
To date, calcium phosphate based bioceramics are the most widely used osteoinductive materials. Osteoinduction has been demonstrated on a diverse of CaP materials including hydroxyapatite (HA)[128,129], tricalcium phosphate (TCP)[130], biphasic calcium phosphate[131], and coralline hydroxyapatite[132]. The chemical composition of these CaP materials--presence of calcium and phosphate--is the principle element of their osteoinductive property. However, other materials including poly (hydroxyethyl methacrylate) (Poly-HEMA)[133], alumina ceramic[134], Bioglass[107], and titanium[69] which do not contain CaP are also found to be osteoinductive under certain circumstances. For example, bone formation was observed in the soft tissue of young pigs using Poly-HEMA sponge[133]. Later, it was discovered that a calcification process analogous to CaP ceramics also took place on these materials prior to bone formation, which again corroborate the importance of chemical composition to osteoinductivity of materials[135].
Another essential characteristic of osteoinductive materials is porous macrostructure as bone induction was never observed on a flat surface[110]. Instead, bone formation was always detected inside the pores within the implants, where calcium and phosphate ions were trapped and precipitated after reaching supersaturation[136]. Recent reports have also shown that microstructure is closely associated with osteoinductivity of biomaterials. Significantly different levels of bone induction were observed on implants with varying roughness and porosity[137,138]. For example, only titanium implants with micropore structure after appropriate surface treatment induced bone formation, while no bone induction was found on untreated titanium[139].
3.3 Vascularization
Vascularization is a key process during bone regeneration as blood vessel formation is required for any tissue with size beyond 200 μm which is the diffusion limit of oxygen in vivo[140]. Functional bone tissue formation must be closely associated with development of a vascular system, which properly integrates with the host blood supply[141]. The newly formed vessels ensure the supply of nutrients such as glucose, oxygen to the surrounding cells, as well as the removal of metabolic byproducts, such as carbon dioxide, lactate, and urea[142,143]. Besides, vascular network also plays an important key role in recruiting progenitor cells to the defect sites to participate in the tissue regeneration process[144]. Although blood vessels tend to invade into the bone defects after injuries or trauma in response to the local hypoxia microenvironment, this spontaneous process is usually too slow and cannot match the rapid tissue healing rate, which would cause nutrient deficiencies and severe hypoxia and finally lead to failure of bone healing[145].
In light of the critical needs for accelerated establishment of a functional vascular network during bone regeneration, biomaterials capable of promoting a variety of aspects of vessel network formation have been developed and widely implemented in bone regenerative engineering. As the most heavily used biomaterials formulations, scaffolds and hydrogels can easily serve as temporal matrix to mediate progenitor cells and pericytes migration and provide mechanical support for capillary sprout[146–150]. The impact of scaffolds on tissue engineered constructs can also be realized by tuning the architecture of scaffolds during fabrication. Choi et al. found that inverse opal scaffolds with smaller pore size favored vascular network formation in vivo[151]. More recently, new technologies such as 3D printing have been introduced to fabricate more perfusable engineered tissue constructs. The seminal work by Chen et al. employed 3D printing technology to generate cylindrical networks that could be populated with endothelial cells. They demonstrated that these artificial vascular systems were able to sustain the metabolic function of primary hepatocytes[152]. The chemical composition of biomaterials also greatly affects vascularization as it directly interacts with endothelial cells during vessel formation[153,154]. While most materials used for bone regenerative engineering such as collagen, PCL, PLGA, and silk were found to be compatible with endothelial cells, some materials were identified to be proangiogenic during tissue healing[155]. For example, hydrogel made of dextran has shown to be able to remarkably promote neovascularization and accelerate skin regeneration[147]. A silicate bioceramic, Akermanite successfully induced angiogenesis during bone regeneration by providing a suitable Si ion concentration to stimulate human aortic endothelial cell proliferation and gene expression[156]. Biomaterials such as fibrin, heparan sulfate, and hydroxyapatite can also regulate vascularization via their high affinity to angiogenic cytokines such as vascular endothelial growth factor (VEGF), endothelial growth factor (EGF), and basic fibroblast growth factor (bFGF)[84,153,157,158]. These biomaterials can efficiently sequester endogenous growth factors at the defect site and improve bone formation through enhanced vascular network development[159]. Hubbell et al. has worked on the utilization of fibronectin-based hydrogel to sequester growth factors and largely amplified their efficacy. By incorporating a recombinant fragment of fibronectin into the hydrogel, greatly enhanced regenerative effects of growth factors via potent synergistic signaling was observed[160]. Another important type of materials possessing this property is heparin based glycosaminoglycans, which are abundant in extracellular matrix components secreted by a number of cell types in human body[158]. Growth factor activity can be localized within these natural ECMs. Inspired by the interaction between growth factors and ECMs, synthetic biomaterials that can non-covalently interact with growth factors have been developed by modifying materials with heparin proteoglycans binding peptides. For instance, Hudalla et al. functionalized a 2D self-assembled monolayers (SAMs) with a heparin-binding peptide HEPpep(KRTGQYKL). They found that HEPpep grafted SAMs promoted the spread of human umbilical vein endothelial cells (HUVEC) with medium supplemented with FGF-2, which indicated that HEPpep functionalized surface could sequester serum-borne heparin amplify growth factor activity[161]. Similar growth factor sequestering approach leveraging growth factor receptors has also been explored for controlled angiogenesis factor capturing and release[162,163].
3.4 Cell-biomaterial interactions
At cellular level, the impact of biomaterials on bone regeneration is mainly through the interaction between surrounding cells and biomaterials. Among these interactions, cell adhesion plays a central role in determining the cellular behaviors on the biomaterials surface[36]. Integrins, the heterodimeric receptor in the cell membrane, serve as linkers between cells and substrates through their binding to adhesive proteins adsorbed on biomaterials surface[164]. Integrin mediated cell adhesion is closely related to a series of intracellular signaling pathways, thus it is a critical determinant of subsequent cell activities including cell morphology, mobility, proliferation and differentiation[165]. Upon binding of integrin receptors on adhesive protein, integrin clusters interact with the actin cytoskeleton to form focal adhesions[166], therefore the cell morphology is dictated to integrin mediated cell adhesion. Cell morphology on substrates plays an important role in determining the fate of these cells as evidenced by many studies using a variety of cell types including chondrocytes, osteoblasts, mesenchymal stem cells, and progenitor cells[167]. Furthermore, formation of focal adhesions can also combine with growth factor receptors on cell membrane to activate multiple intracellular pathways such as mitogen-activated protein kinase/extracellular signal-regulated kinase (MARK/ERK) pathway and c-Jun NH(2)-terminal kinase (JNK) pathway, which regulate transcription factor activity and determine cell cycle progression[168,169].
In general, the majority of these interactions take place at the biomaterials surface, thus the surface characteristics such as chemical composition, hydrophilicity, and topography of the biomaterials are the key factors to control the cellular behaviors on corresponding materials[36]. More importantly, since the materials are immediately coated with a layer of proteins from the environment once implanted, thus controlling the adsorption of protein at the interface of cell/biomaterials provides a feasible way to achieve desirable cell responses[170]. A series of surface modification strategies have been developed based on this mechanism and largely expanded to integrin-adhesive molecules interactions. Many ECM macromolecules such as collagens, laminins, fibronectin, and vitronectin have been immobilized on various biomaterials surface to modulate the cellular performance on these surfaces[171]. For example, collagen and its derivatives have been extensively used in many bone substitutes such as HEALOS® and INFUSE bone graft to facilitate material-cell interactions upon implantation[13,31]. The immobilization of full protein molecules on biomaterials surface was then simplified into short peptide sequences encoding small functional domains on the ECM proteins. One of the most well studied cell adhesive peptides, arginine–glycine–aspartic acid (RGD) originally derived from fibronectin, has been widely used as adhesive motif to enhance cellular attachment[172]. By combining with bioinert substance such as poly (ethylene glycol) (PEG), RGD has been extensively incorporated in fully synthetic biological systems to offer precise control over material properties for selective cell behavior studies[173,174].
Besides adsorbed protein-mediated biomaterials-cell interactions, there are also other materials surface characteristics remarkably affect their bone forming capability by regulating cellular behaviors during bone regeneration. The influence of chemical composition on bone formation has been readily demonstrated by the application of calcium phosphate coating on various orthopedic devices and bone regenerative engineering scaffolds[175,176]. Surface energy can also contribute to new bone formation by affecting osteoblast response on materials surface. Olivares-Navarrete et al. found that increase in surface energy has led to improved osteoblast differentiation on titanium surface[177]. Topography of biomaterials is also found to be able to affect bone formation. This was first confirmed by showing osteoblast alignment on grooved titanium surface without changing their composition[178]. More recent studies have focused on micro and nano-fabrication methods to create multi-scale physical features to induce bone formation. Webster et al., have shown that nanoscale features (below 100 nm) could be identified by osteoblasts and exert different cell activities[98,179].
Another factor influencing cell-biomaterials interactions is the mechanical properties of the biomaterials. Cells sense and respond to stiffness of ECM through mechanotransduction via various mechanisms such as mechanosensitive ion channels, forced unfolding of proteins, and remodeling of focal adhesion sites[180]. In particular, the change in matrix stiffness plays an important role during stem cell fate determination and act as a critical regulator in driving cellular behavior. For instance, MSCs differentiated into neural cells on matrix with stiffness of brain whereas they appeared osteogenic when they are exposed to matrix with stiffness of bone[181].
3.5 Integration with host tissue
The integration of newly formed bone tissue with surrounding natural environment is one of the prerequisites for functional bone regeneration. During this process, biomaterials can not only serve as a scaffold for cell infiltration and tissue deposition, but also provide inductive signals to facilitate tissue connection with the corresponding host networks including vasculature and nerve system[182]. As the first step of tissue integration, scaffolds made of various biomaterials support adhesion of cells on their surface. The porous structure of scaffolds supports sufficient nutrient and oxygen diffusion, which allows the cells to migrate and populate within the scaffolds[183]. In the next stage, the infiltrated endothelial cells and pericytes start to reorganize and form into capillaries, which is crucial to maintain the viability of newly formed tissues. Biomaterials with appropriate chemical composition and microstructure are capable of supporting vascular formation and stabilization[184]. Meanwhile, large amount of tissue matrix including collagen and mineral may start to deposit by osteoblasts along the structure of the scaffolds. Finally, these newly formed ECM is bridged to the natural ECM via a remodeling process mainly mediated by osteoclasts[185]. Scaffold degradation matching the remodeling process is another key for the integration of newly formed bone with host bone tissue[186]. Overall, biomaterials play an indispensable role for bone tissue integration through their impact on multiple stages of bone formation.
A variety of strategies have been developed to enhance the integration of biomaterials into host bone tissue during bone regenerative engineering. Design of scaffold porosity and pore architecture facilitating more efficient nutrient and oxygen transport have shown to be a feasible approach to improve tissue integration[150]. Another way to promote tissue integration is to design the chemical composition of biomaterials via modification of intrinsic chemical structure or change of surface properties of materials through techniques such as grafting, coating and patterning[187,188]. Introduction of cell adhesive molecules has been widely explored and have shown promising outcomes towards improved tissue integration[189]. Besides, the incorporation of biological components into scaffolds enabling cell-mediated remodeling also represents an intriguing approach to achieve satisfactory bone integration. This approach has been demonstrated by Hubbell's group via the incorporation of a series of cell cleavable peptide sequences into hydrogels[190,191]. The results have shown that the degradation of hydrogel in response to invading cells resulted in better bone formation while also accomplishing its integration with surrounding native bone[186].
4. Design of biomaterials for bone regenerative engineering
4.1 Bioceramic composites
Due to the multifold requirements of the scaffold design for bone regenerative engineering, composite materials have been widely used to combine the advantages of two or more materials together to meet these needs[192–194]. One important type of composite materials in bone regenerative engineering is inorganic-organic composites, which combine the ductility of a polymer phase with the stiffness and strength of an inorganic components to generate advanced biomaterials with improved mechanical properties and desirable degradation profiles[195]. Besides, the addition of polymers into the composites also allows for better manipulation and control over the composite structure and uniformity[196]. Bioceramics such as hydroxyapatite, bioactive glass (e.g. Bioglass®), alumina, TiO2 and calcium phosphates have been extensively used for bone regenerative engineering since they can significantly improve the mechanical properties of these composites, as well as enhance the bioactivity of the biomaterials[197][196]. For example, addition of hydroxyapatite into biodegradable polymers such as poly (L- lactic acid) (PLA), poly(D,L-lactic-co-glycolic acid) (PLGA), and poly (ε- caprolactone) (PCL) led to the formation of composite materials possessing both excellent mechanical properties and bioactivity[198–201]. More importantly, multiple studies have shown that incorporation of nano-hydroxyapatite into porous 3D PLGA scaffolds substantially enhanced preosteoblast growth, differentiation and mineralization [79][202–204].
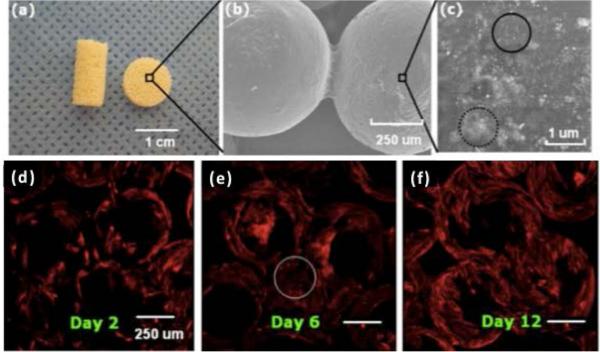
Calcium phosphate based bioceramics are the most popularly used additives used for bone regenerative engineering scaffold fabrication due their resemblance to bone mineral. They are capable of stimulating formation, precipitation, and deposition of CaP and forming a direct bond between implants and native bone[205–207]. Multiple studies by Laurencin et al. have clearly demonstrated the tremendous potential of calcium phosphate/polymer composites in the treatment of critical size bone defects. They firstly fabricated 3D composites of PLGA (50:50) and HA using a solvent leaching/particle leaching method[208]. The composite biomaterials were able to maintain porous structure with an average pore diameter of 100 μm during a 6-week degradation study. Long term osteoblast culture in vitro showed that the PLGA-HA scaffolds supported cell proliferation, differentiation, and mineral formation. Taking advantage of the degradability of PLGA and the strong mechanical properties of HA together, the composite scaffold showed great promise as a synthetic matrix for bone regeneration[209–211]. They then successfully incorporated HA into PLGA microspheres and formed the composite microsphere scaffolds via a sintering approach to generate load-bearing scaffolds with excellent mechanical properties, interconnected porosity, and favorable bioactivity[79,212,213]. HA was also combined with poly-phosphazenes, another family of biodegradable polymer with tunable physical and biological properties, to make electrospun fiber scaffolds or microspheres. The advantage of nanofiber scaffolds is due to their flexibility, excellent biocompatibility, and specific surface area for cells to grow on. For example, Bhattacharyya et al. electrospun poly[bis(ethyl alanato)phosphazene] (PNEA) as well as n-HA-PNEA composite nanofiber matrices as scaffolds for bone tissue regeneration applications. The uniform presence of n-HA crystal particles within the nanofibers was confirmed by calcium mapping[214][215]. Such poly-phosphazene nanofiber structures closely mimic ECM architecture, and exhibited excellent osteoconductivity and osteointegrativity[209,215–217]. In another study done by Nukavarapu et al., polyphosphazenes substituted with ethyl phenylalanine side-group was chosen as a candidate material for forming three-dimensional (3-D) porous composite microspheres with 100 nm sized hydroxyapatite (nHAp). The scaffolds showed compressive moduli between 46 to 81 MPa with mean pore diameters in the range of 86–145 μm. The three-dimensional polyphosphazene-nHAp composite microsphere scaffolds showed good osteoblast cell adhesion, proliferation and alkaline phosphatase expression (Fig. 2). [211] In the above-cited examples, polyphosphazene-based biomaterials were employed because of the reason that these polymers provide neutral by-products upon degradation, unlike acidic degradation products in the case of polyester based polymers. Later on, polyphosphazenes were blended with PGA, PLA and PLGA polymers and produced a series of novel biomaterials with the mitigated acidic by-products problem and tunable physical and biological properties for bone regenerative engineering[217–221]
Fig 2.
Macro, micro and nano structure of PNEPhA-20 nHAp composite microsphere scaffolds. (a) Optical image showing cylindrical (10 mm length & 4.5 mm diameter) and disk (2 mm thick & 8 mm diameter) shaped scaffolds fabricated using the dynamic solvent sintering method. Cylindrical scaffolds were used for mechanical testing, and disk shaped scaffolds for porosity and in vitro cell studies. (b) SEM showing the microstructure of the scaffolds where the adjacent microspheres are fused via the dynamic solvent sintering method. (c) High magnification scanning electron micrograph showing nano HAp particle dispersion on a microsphere surface. The circled regions show nHAp mono (solid line) and poly (dotted line) dispersion. Cytoskeletal actin distribution of primary rat osteoblast cells grown on composite microsphere matrix for (d) 2, (e) 6 and (f) 12 days. The circled region shows higher initial cell proliferation at the microsphere adjoining areas. DAPI (nuclei stain) emission is not included because of its interference with polymer PNEPhA blue emission[211]. Figures reproduced with permission
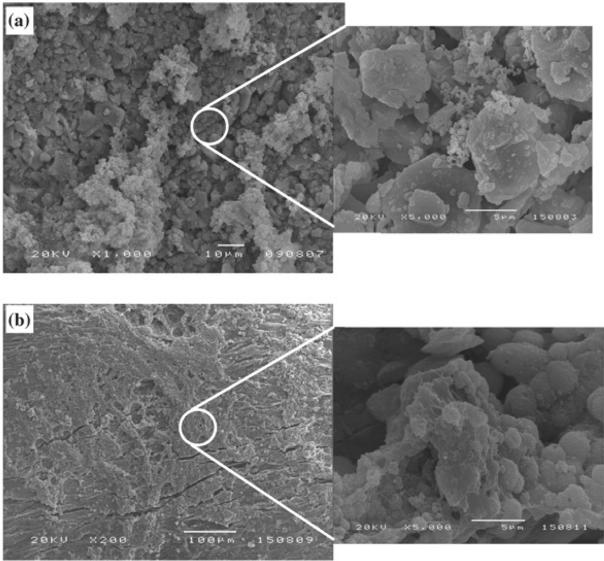
Bioactive glasses, another important class of bioceramics, have also been employed to form polymer-ceramic composites owing to the superior biocompatibility and bioactivity demonstrated since the invention of bioactive glass in 1970s by Hench[222][223]. Incorporation of Bioglass® particles into polymeric matrix can introduce both osteoconductivity and osteoinductivity to the formed composites, which has made this combination an attractive approach to improve the performance of bone grafts[224]. It was reported that ionic components such as Si+, Na+, and Ca2+ released into body fluid can react and deposit a thin layer of physiologic CaP layer, which can facilitate protein adsorption and osteoblast attachment[225,226]. These favorable properties of Bioglass composites can lead to deposition of new bone and bonding between native bone and implants[227]. In an illustrative example, Blaker et al. showed that the Bioglass® filled PLA foams accelerated the formation of carbonated hydroxyapatite on foam surface and stimulated osteoblast migration into the composite foam (Fig. 3)[228]. More importantly, incorporation of Bioglass into polymeric matrix has led to the successful launch of commercially available bone substitute grafts Vitoss® in 2008, which is one of the best-selling synthetic bone substitutes[227]. Applications of Vitoss® for bone disorder treatment include bone void fillers, treatment for surgically caused osseous defects, and spine fusion[229]. In particular, when combined with fresh bone marrow aspirates, Vitoss® can precisely mimic iliac crest bone graft which is considered as the gold standard for bone grafts[230,231]. These recent developments of Bioglass composites have shown tremendous potential of synthetic biomaterials for bone regenerative engineering.
Fig 3.
SEM micrographs showing surfaces of BioglassI-coated PDLLA foams after degradation in contact with SBF for: (a) 7 days and (b) 28 days. The micrographs reveal formation of HA crystals and development of a surface HA layer[228]. Figures reproduced with permission
Recent efforts are also towards developing composite biomaterials with human cortical bone compatible mechanical properties. The development of such advanced biomaterials is critical because bone regenerative engineering of critical-sized defects is not feasible without the availability of biodegradable and yet mechanically compatible scaffolds, screws, rods and plates. In other words, an all-biodegradable strategy for regenerative engineering of bone is only viable with the development of advanced and mechanically superior biomaterials and scaffolds [28]. To realize this, biodegradable polymer, PLGA was combined with functionalized carbon nanotubes to form a mechanically superior composite biomaterials and scaffolds. The composite scaffolds further demonstrated enhanced biomineralization ability, biocompatibility in vitro and in vivo [232]. The authors chose functionalized carbon nanotubes over the un-modified CNTs due to the fact that CNTs with hydrophilic functional groups, such as OH, COOH, NH2, and SH are water dispersible, therefore have potential to be cleared from the body upon the implant degradation[233,234].
4.2 Introducing electrical stimulus
There is a growing interest in using electrical stimulus as one of the ways to stimulate tissue repair and regeneration. In regenerative engineering, this is mainly realized by invoking conducting polymers as biomaterials or part of a biomaterial to provide the needed electrical stimulus. Conducting polymers (CPs) were first produced in the mid-1970s, and their biomedical application expanded greatly in the 1980s after they were found to be compatible with many biological tissues. So far, conducting polymers have been widely used for numerous applications such as neural probes,[235] neural prostheses [236][237] and controlled release applications.[238][239][240][241][242]. Major conductive polymer such as polypyrrole (PPy)[243][244] polyaniline (PANi) [245][243] polythiophene [246] and their derivatives[247][248][249][250] possess physicochemical properties that are desired for regenerative engineering applications including conductivity, reversible oxidation, redox stability, biocompatibility, hydrophobicity [251][252]. The primary approach to incorporate conducting polymer into biomaterials is through mixing[252][253]. This process is influenced by factors such as polaron length, chain length, charge transfer to adjacent molecules and conjugation length[254]. In one study by Hsiao et al., the conducting polymer polyaniline (PANI) was incorporated into PLGA electrospun fibers to form aligned composite nanofibers. The resultant composite nanofibers were transformed into a conductive form carrying positive charges and capable of attracting negatively charged adhesive proteins such as fibronectin and laminin, and promoting cell adhesion. [255] Although the vast majority of regenerative engineering applications using conducting polymers focused on neural tissue, cardiovascular tissue and muscle tissue, their potential in bone regenerative engineering has been demonstrated with various conducting polymers recently. Bone healing can be greatly accelerated under electrical stimuli, thus creation of conducting scaffolds, which can locally provide electrical signals is highly desirable[256]. Attempts of incorporating conducting polymers into bone repairing biomaterials have been vigorously explored and encouraging results have been obtained.
Polypyrrole (PPy) is by far the most studied conductive polymer and has numerous applications in drug delivery, nerve regeneration, biosensing, as well as coatings for neural probe, nerve guidance channel[241,257–262]. For flexibility, PPy has been deposited onto polyester and polyethylene terephthalate (PET) fabrics[263][264]. For bone regenerative engineering applications, in a study done by Sajesh et al., Chitosan/ Polypyrrole–alginate scaffold was developed. PPy scaffold seeded with MG-63 cells supported cell attachment and proliferation. In vitro mineralization of the scaffold suggested the bioactivity of the scaffold by forming the apatite layer. It is suggested that the scaffold can be employed for bone regenerative engineering by combing with the bone forming cells. Also, by combining electrical stimulation with a bioreactor system, the role of conducting substrate in bone regeneration can be studied[265]. In another study, heparin was covalently immobilized onto electrically conductive polypyrrole (PPy) film through poly(ethylene glycol) methacrylate (PEGMA) graft copolymerization and subsequent cyanuric chloride activation. The PEGMA-graft-copolymerized PPY surfaces with immobilized heparin have good bioactivity indicated by low level of protein adsorption, high ratio of albumin to fibrinogen adsorption, and low thrombus formation and PPy film retained significant electrical conductivity after surface modification. [266]
In recent years, Poly (3,4-ethylenedioxythiophene) (PEDOT) captured a considerable amount of attention owing to its good electrical, chemical and environmental stability[267] and better conductivity and thermal stability than PPy[267]. For instance, Shahini et al., successfully incorporated PEDOT into 3D gelatin/bioactive glass scaffolds to form conducting tissue scaffolds. Their results showed that increasing PEDOT in their system not only stabilized the microstructure of the scaffolds, but also enhanced cellular performance of mesenchymal stem cells via local electrical stimulation (Fig. 4). In particular, they observed that the cell viability of mesenchymal stem cells on scaffolds with more PEDOT amount was higher and these cells grown on PEDOT composite scaffolds displayed normal cell morphology visualized by both fluorescence staining and SEM[268]. Besides, PEDOT has also been extensively studied in drug delivery applications, which could be further utilized for bone regenerative engineering. For instance, PEDOT coated electrospun nanofibers were incorporated with dexamethasone. It was reported that the drugs can be released from the nanofibers in a controlled fashion via electrical stimulation, which provides a valuable tool to achieve in situ real time drug release[239]. Collectively, conducting polymers can be used to stimulate various cells and tissues in order to obtain desired effects; therefore they have tremendous potential in the field of bone regenerative engineering.
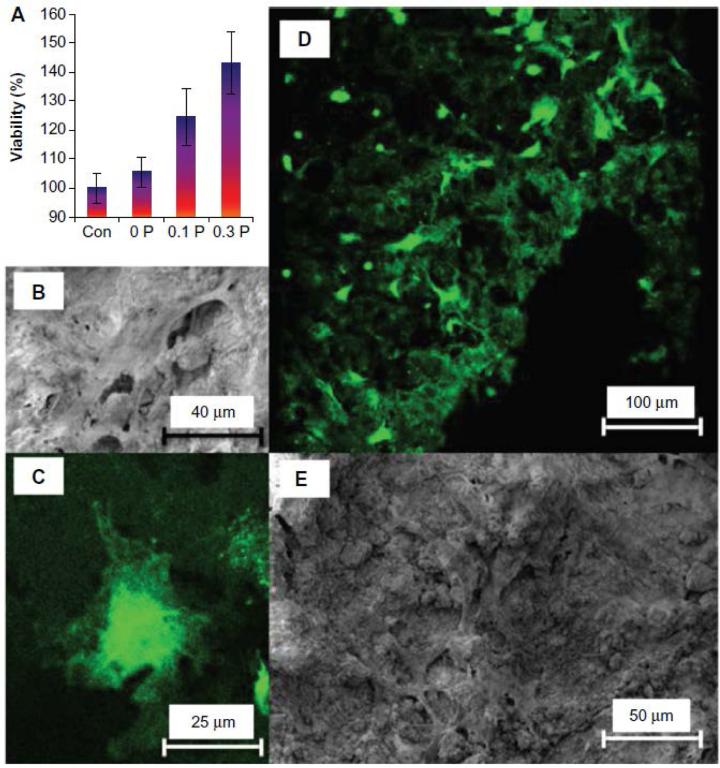
Fig 4.
Effect of incorporation of PEDOT on bone tissue engineering scaffolds: (A) Cytoplasmic content of human mesenchymal stem cells on the 0 P, 0.1 P, and 0.3 P scaffolds in comparison to tissue culture plastic (control sample) shows that the number of viable cells increases by increasing the poly(3,4-ethylenedioxythiophene) poly(4-styrene sulfonate) concentration in the composition of scaffolds. The values are mean ± standard deviation (number of samples =3). (B) Scanning electron microscopy and (C) confocal fluorescent microscopy images of a cell on the 0.3 P scaffold. (D) Scanning electron microscopy and (E) confocal fluorescent image of cell distribution on the 0.3 P scaffold. Enhanced cell attachment is observed for the conductive scaffolds[268]. Figures reproduced with permission
4.3 Harnessing mechanical signaling
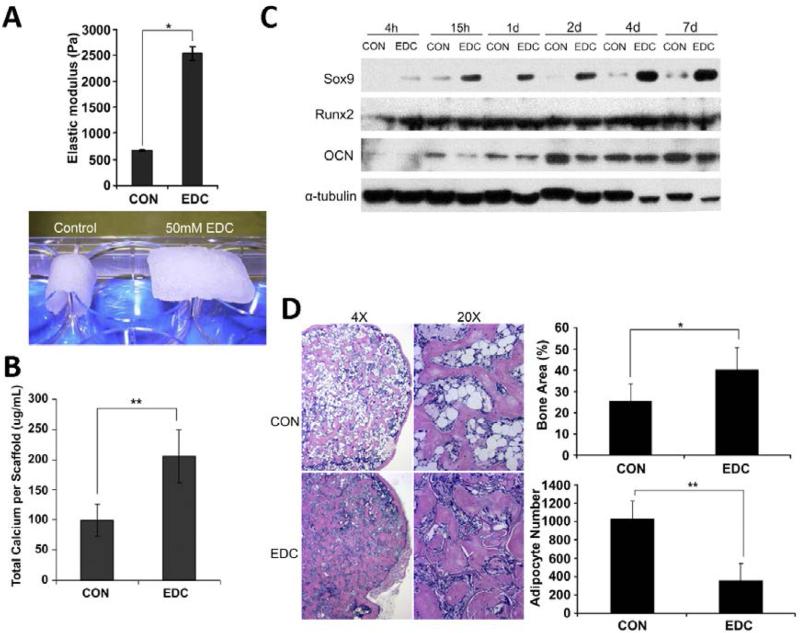
There is growing recognition that mechanical properties of biomaterials can regulate biological response, therefore trigger a powerful set of new design parameters for bone regeneration[181,269]. Thus, manipulation of matrix stiffness has become an enabling approach in exploring new biomaterials for bone regenerative engineering. Elastomeric polymer networks, such as hydrogels have been extensively explored to accommodate these applications as their stiffness can be simply controlled by changing their crosslink density. The elastic modulus of hydrogel can be modulated between 1 kPa to 500 kPa, which covers the modulus range of all types of tissues in the human body[269]. The most commonly used materials for synthetic hydrogels is polyethylene glycol (PEG) due to their tunable stiffness and precise control over cue presentation via chemical modification. In the work by Anseth et al., a facile strategy was developed to create photodegradable PEG hydrogels with tunable physical, chemical and biological properties, which provided a vibrant platform to answer fundamental questions about materials regulation of cell function[270]. They further demonstrated that cell phenotype could be directed by in situ modulation of the dynamic cell microenvironment composed of photodegradable hydrogels. The investigation further revealed that myofibroblasts were de-activated by simply tuning the elastic modulus of the cell substrates[271]. Other polymers such as poly (acrylamide) (PAAm) and alginate have also been used to study the role of stiffness in stem cell fate determination and their applications in various fields including bone regenerative engineering[272,273]. Most recently, Sun et al. demonstrated stem cell-mediated bone regeneration could be controlled by tailoring the mechanical properties of collagen scaffolds (Fig. 5-A). Their investigation showed that collagen scaffolds with distinct elasticity significantly influenced cellular performance in vitro. 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) treated scaffolds substantially increased osteogenic differentiation of cells in vitro (Fig. 5-B&C). Transplantation data in vivo showed that EDC treated group increased both chondrogenesis and trabecular bone formation via micro-computed tomography and histological analysis (Fig. 5-D). They also concluded that the enhanced bone formation in high mechanical strength scaffolds was achieved by promoting endochondral ossification[274]. Mechanical properties of biomaterials can also control osteogenic differentiation of stem cells by combining with chemical cues such as fibronectin and growth factors. Nii et al. found that adipose-derived stem cells showed strongest oteogenic differentiation on gels with intermediate stiffness (~55 kPa) and low fibronectin concentration (10 μg/mL)[275]. Besides, Tan et al. observed that combination of hydrogel stiffness and growth factor (e.g. BMP-2) had synergetic effect on cell osteogenic differentiation[276]. These examples collectively demonstrate that mechanical signaling can be used as an important approach to control bone regeneration.
Fig 5.
Influence of scaffold mechanical properties on stem cell-mediated bone regeneration: A) The elastic modulus of the scaffolds was significantly increased after EDC-treatment. EDC-treated scaffold were able to resistant deformation due to gravitational force. B) Total calciumwas increased by EDC-treatment in osteoblasts (n ¼ 3). **p < 0.01. C) Chondrogenic and osteogenic protein levels on different scaffolds were detected by Western blot analysis. The experiments were repeated at least 2 times, and the representative data are shown. D) Histologic analysis of new bone formation. EDC scaffolds significantly enhanced the bone volume fraction (BVF). Moreover, the adipocyte numbers in the EDC group were significantly lower than in the CON group (n ¼ 7 or 9). Data are expressed as means ? SD. *p < 0.01; **p < 0.001 Figures reproduced with permission
Substrate stiffness has been increasingly recognized as a key player in stem cell differentiation toward different lineages including osteogenic lineage. Huebsch et al. found that mesenchymal stem cells predominantly committed to osoteoblasts at substrate stiffness of 11-30 kPa (Fig. 6-A). Unlike 2D culture, cell fate was not correlated with morphology but manipulated by traction-mediated integrin binding and adhesive ligand reorganization[273]. Similar impact of substrate stiffness on stem cell fate was also elegantly demonstrated by Fu et al using micromolded elastomeric micropost arrays instead of hydrogels. They first observed that cell morphology was closely associated with traction force, which was controlled by the height of the microposts (Fig. 6-B). Then, strong correlation was also identified between cell traction forces and ultimate cell differentiation status (Fig. 6-C), indicating that cell function could be effectively regulated by mechanical properties of the materials[277]. The molecular mechanisms behind these observations were elucidated in a recent study by Swift et al. where they revealed via proteomics analysis that, the nucleoskeletal protein lamin-A was the pivotal regulator in response to the change of tissue elasticity. Matrix stiffness directly influenced lamin-A levels, which then contributed to lineage determination via the vitamin A/retinoic acid (RA) pathway[278]. Another important finding regarding the influence of mechanical signals on stem cell fate was the identification of stem cell mechanical memory, which is the previous mechanical environment on their fate determination. Yang et al. discovered that hMSC osteogenic differentiation was achieved by increasing the mechanical dosing on stiff TCPS (E~3 GPa) during a previous culture period. They also found that the mechanical information from the past physical environment was stored in the Yes-associated protein (YAP) and transcriptional coactivator with PDZ- binding domain (TAZ) as well as the pre-osteogenic transcription factor RUNX2 genes[279]. This study implicates that substrate mechanical stiffness can be harnessed to prime stem cells to drive cells toward the desired lineage, thus stiffness of biomaterials may serve as a promoter towards osteogenesis via the activation of YAP and other related mechanotransduction pathways.
Fig 6.
Stiffness of biomaterials influence the behavior of stem cells such as adhesion and cell fate commitment: A) mMSC grown on alginate matrices with different stiffness and immunofluorescence stained for OCN (green) and nuclear (DAPI, blue)[273] B) hMSC plated on PDMS micropost arrays with varying rigidity controlled by indicated heights and imaged with scanning electron microscope (SEM). Scale bar, 30 μm C) ALP and Lip staining on hMSCs after 14 d culture in bipotential differentiation medium on micropost arrays of indicated rigidities. Scale bar, 300 μm[277]. Figures reproduced with permission
4.4 Incorporation of Inducerons
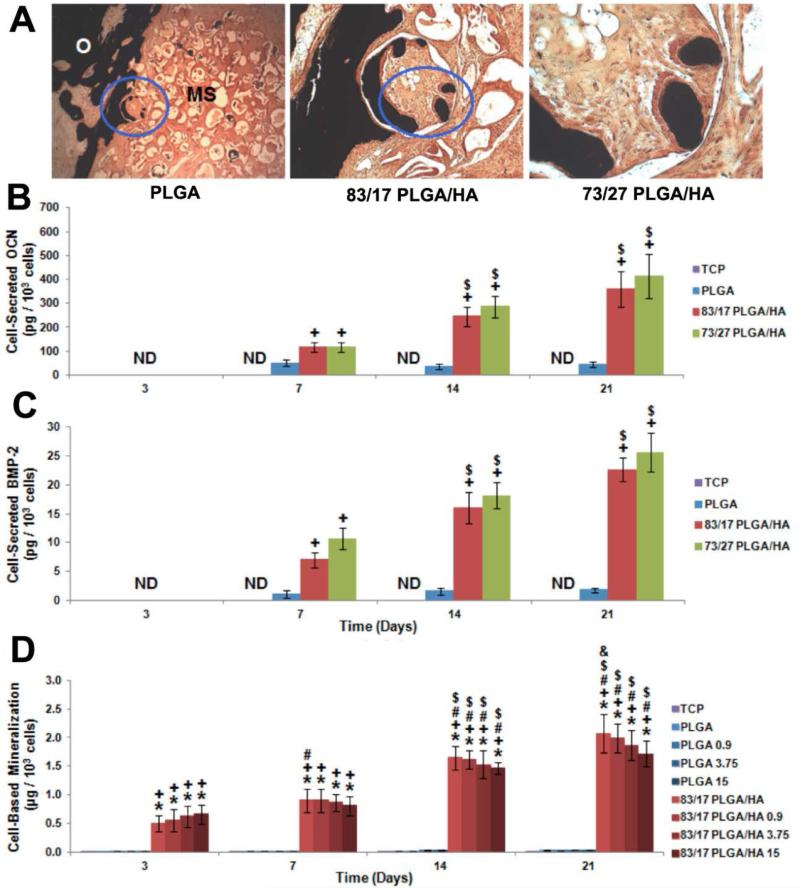
Another emerging field growing rapidly is to incorporate simple signaling molecules in biomaterials design and development for bone regeneration[280–282]. Laurencin has described these molecules as Inducerons. Unlike conventional osteoinductive factors such as BMP-2 which directly induce bone formation, these small molecules are capable of influencing cell behaviors via stimulating autocrine and paracrine secretion of related growth factors[282]. An illustrative example is calcium and phosphate ions serving as important regulators during bone metabolism[119]. While the profound impact of these ions on various aspects of bone formation such as osteoprogenitor cell homing, osteoblast migration, proliferation and mineralization has been reported. Laurencin et al., have recently demonstrated that the release of calcium and phosphate ions from CaP ceramics could directly induce the production of endogenous BMP-2. In this example, neo-bone formation was observed in 3D PLGA/HA composite scaffold where HA was incorporated into PLGA microspheres via in situ precipitation (Fig. 7-A). Enhanced osteogenic differentiation of human adipose-derived MSCs was also achieved on these composite scaffolds in vitro by showing increased osteocalcin secretion and cell-based mineralization (Fig. 7-B). Meanwhile, significant amounts of BMP-2 were detected with MSCs seeded on PLGA/HA scaffolds during 21 days of culture while no detectable BMP-2 was secreted by MSCs on PLGA scaffolds (Fig. 7-C). More interestingly, it was found that the addition of supra-physiological dosage of rhBMP-2 loaded into PLGA/HA scaffolds was unable to increase the production of cell-secreted BMP-2, which indicates that the presence of low crystalline HA in the scaffold was the key driving force of BMP-2 production by MSCs (Fig. 7-D). Since BMP-2 production by MSCs on the scaffolds was parallel to the release of calcium ion during culture period, they theorized that the gradual release of calcium and phosphate ions should serve as signaling molecule, which is termed by Laurencin as an induceron[283].
Fig 7.
Simple signaling molecules for inductive bone regenerative engineering: A) New bone formation visualized by von Kossa staining on scaffolds with different composition microspheres B) OCN expression by MSCs after seeded on various PLGA/HA scaffolds: the presence of HA in scaffolds contributed to the majority of OCN production. C) BMP-2 secreted from MSCs grown on PLGA/HA scaffolds for various time: significant BMP-2 expression was only observed in HA containing groups. D) Mineralization assessed by Alizarin Red staining after seeding MSCs on various scaffolds: cell mediated mineralization was only observed on HA containing groups[283]. Figures reproduced with permission
Indeed, a series of molecules capable of inducing relevant growth factor production have been identified in the past and can all be categorized as inducerons. For example, bisphosphonates have been used for osteoporosis treatment in clinical practice[284]. It was also found that some formulation of bisphosphonates such as zoledronate could substantially enhance BMSC osteogenic differentiation by up-regulating BMP-2 mRNA expression, which is aligned with the effect of calcium ions[285]. Redox can be regarded as another type of induceron due to their capability of regulating VEGF signaling pathways[286]. For instance, hydrogen peroxide (H2O2) is able to induce VEGF-A production at micromolar concentrations (0.1-10 μM) and increase the tube formation of endothelial cells during angiogenesis[287]. Other inducerons include nitric oxide (NO), hydrogen sulfide (H2S), which can effectively influence cell differentiation via inductive growth factor production[288,289]. Compared with the recombinant growth factors (BMP-2 or BMP-7), these inducerons are inexpensive, stable, and lack side effects (within their dosage range), Therefore they might represent a paradigm shift in the field of bone regenerative engineering.
5. Concluding remarks and future directions
As a key component of bone regenerative engineering, biomaterials play a pivotal role towards the design of ideal bone grafts. Although the approaches for bone regeneration are slowly changing as new biomaterials and novel strategies are being developed, this field can make giant steps due to an understanding of the importance of convergence with nanotechnology, stem cell science, and developmental biology, and perhaps other fields. As such, the function of biomaterials during bone regeneration has largely expanded from simply serving as templates to multifunctional systems, to actively regulate various aspects of bone regeneration. Materials used for bone regenerative engineering not only should possess basic properties including biocompatibility, osteoconductivity, and osteoinductivity, but also positively regulate their interactions with cells and integration with host tissues. We highlighted examples of novel biomaterials design, leveraging the advances in the converging fields to promote the development of next generation of biomaterials for bone regenerative engineering.
Acknowledgements
This work was supported by NIH R01 AR063698 and the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences. We would like to thank Dr. Syam Nukavarapu ()for proofreading this manuscript and his valuable comments and discussion.
References
- 1.Baroli B. J. Pharm. Sci. 2009;98:1317. doi: 10.1002/jps.21528. [DOI] [PubMed] [Google Scholar]
- 2.Brinker MR, O'Connor DP. J. Bone Joint Surg. Am. 2004;86-A:290. [PubMed] [Google Scholar]
- 3.Laurencin C, Khan Y, El-Amin SF. Expert Rev. Med. Devices. 2006;3:49. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Parikh SN. J. Postgrad. Med. 2002;48:142. [PubMed] [Google Scholar]
- 5.Santos MI, Reis RL. Macromol. Biosci. 2010;10:12. doi: 10.1002/mabi.200900107. [DOI] [PubMed] [Google Scholar]
- 6.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Orthop. Clin. North Am. 2006;37:65. doi: 10.1016/j.ocl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Crane GM, Ishaug SL, Mikos AG. Nat. Med. 1995;1:1322. doi: 10.1038/nm1295-1322. [DOI] [PubMed] [Google Scholar]
- 8.Laurencin CT, Ambrosio a M., Borden MD, Cooper J. a. Annu. Rev. Biomed. Eng. 1999;1:19. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Langer R, Vacanti JP. Science (80-. ) 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Lin H, Fang T, Li X, Dai W, Uemura T, Dong J. Biomaterials. 2010;31:1171. doi: 10.1016/j.biomaterials.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 11.Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Nat Biotechnol. 2004;22:560. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 12.Kwon B, Jenis LG. Spine J. 2005;5:224S. doi: 10.1016/j.spinee.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Burkus JK, Heim SE, Gornet MF, Zdeblick TA. J. Spinal Disord. Tech. 2003;16:113. doi: 10.1097/00024720-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Sun G, Wei D, Liu X, Chen Y, Li M, He D, Zhong J. Nanomedicine. 2013;9:829. doi: 10.1016/j.nano.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Correia J, Correia SI, Oliveira JM, Reis RL. Biotechnol. Adv. 2013;31:1514. doi: 10.1016/j.biotechadv.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Hitchon PW, Goel V, Drake J, Taggard D, Brenton M, Rogge T, Torner JC. J. Neurosurg. 2001;95:215. doi: 10.3171/spi.2001.95.2.0215. [DOI] [PubMed] [Google Scholar]
- 17.Laurencin C, Khan Y. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg M, Langer R, Jia X. J. Biomater. Sci. Polym. Ed. 2007;18:241. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes CP, Sell S. a, Boland ED, Simpson DG, Bowlin GL. Adv. Drug Deliv. Rev. 2007;59:1413. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. J. Biomed. Mater. Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 21.Deng M, Kumbar SG, Nair LS, Weikel AL, Allcock HR, Laurencin CT. Adv. Funct. Mater. 2011;21:2641. doi: 10.1002/adfm.201090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson J. a. Science (80-. ) 1998;282:1145. [Google Scholar]
- 23.Takahashi K, Yamanaka S. Cell. 2006;126:663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Science (80-. ) 2007;318:1917. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 25.Nacu E, Tanaka EM. Annu. Rev. Cell Dev. Biol. 2011;27:409. doi: 10.1146/annurev-cellbio-092910-154115. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Gomez AW, Armstrong BE, Henner A, Stankunas K. Cell Rep. 2014;6:482. doi: 10.1016/j.celrep.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall CW. J. Biomed. Mater. Res. 1971;5:1. [Google Scholar]
- 28.LeGeros RZ. Chem. Rev. 2008;108:4742. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 29.Amini A, Laurencin CT, Nukavarapu SP. Biomed. Eng. 40:363. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannoudis PV, Dinopoulos H, Tsiridis E. Injury. 2005;36(Suppl 3):S20. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Spine (Phila. Pa. 1976) 2006;31:E636. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 32.Dohmen PM, Ozaki S, Verbeken E, Yperman J, Flameng W, Konertz WF. Asian Cardiovasc. Thorac. Ann. 2002;10:25. doi: 10.1177/021849230201000107. [DOI] [PubMed] [Google Scholar]
- 33.Burny F, Donkerwolcke M, Muster D. Mater. Sci. Eng. A. 1995;199:53. [Google Scholar]
- 34.RATNER BD, HOFFMAN AS, SCHOEN FJ, LEMONS JE. Biomaterials Science: An Introduction to Materials in Medicine. Academic Press; 2004. p. 851. [Google Scholar]
- 35.Hench LL. Science. 1980;208:826. doi: 10.1126/science.6246576. [DOI] [PubMed] [Google Scholar]
- 36.Tirrell M, Kokkoli E, Biesalski M. Surf. Sci. 2002;500:61. [Google Scholar]
- 37.Hench LL. Biomaterials. 1998;19:1419. doi: 10.1016/s0142-9612(98)00133-1. [DOI] [PubMed] [Google Scholar]
- 38.Geetha M, Singh AK, Asokamani R, Gogia AK. Prog. Mater. Sci. 2009;54:397. [Google Scholar]
- 39.Gotman I. J. Endourol. 1997;11:383. doi: 10.1089/end.1997.11.383. [DOI] [PubMed] [Google Scholar]
- 40.CHARNLEY J. J. Bone Joint Surg. Br. 1960;42-B:28. doi: 10.1302/0301-620X.42B1.28. [DOI] [PubMed] [Google Scholar]
- 41.Haynes DR, Rogers SD, Hay S, Pearcy MJ, Howie DW. J. Bone Joint Surg. Am. 1993;75:825. doi: 10.2106/00004623-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Bauer TW, Schils J. Skeletal Radiol. 1999;28:483. doi: 10.1007/s002560050552. [DOI] [PubMed] [Google Scholar]
- 43.Frost HM. Angle Orthod. 1994;64:175. doi: 10.1043/0003-3219(1994)064<0175:WLABSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Ridzwan MIZ, Shuib S, Hassan AY, Shokri AA, Ibrahim MNM. J. Med. Sci. 2007;7:460. [Google Scholar]
- 45.Navarro M, Michiardi A, Castaño O, Planell JA. J. R. Soc. Interface. 2008;5:1137. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.BREINE U, JOHANSSON B, ROYLANCE PJ, ROECKERT H, YOFFEY JM. Acta Anat. (Basel) 1964;59:1. [PubMed] [Google Scholar]
- 47.Mavrogenis AF, Dimitriou R, Parvizi J, Babis GC. J Musculoskelet Neuronal Interact. 2009;9:61. [PubMed] [Google Scholar]
- 48.Branemark PI. Scand J Clin Lab Invest. 1959;11(Supp 38):1. [PubMed] [Google Scholar]
- 49.Brånemark R, Brånemark PI, Rydevik B, Myers RR. J. Rehabil. Res. Dev. 38:175. [PubMed] [Google Scholar]
- 50.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Dent. Mater. 2007;23:844. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 51.Cho S-A, Park K-T. Biomaterials. 2003;24:3611. doi: 10.1016/s0142-9612(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 52.Brach Del Prever EM, Bistolfi A, Bracco P, Costa L. J. Orthop. Traumatol. 2009;10:1. doi: 10.1007/s10195-008-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maloney WJ, Smith RL. Instr. Course Lect. 1996;45:171. [PubMed] [Google Scholar]
- 54.SWANSON A. Hand. 1969;1:38. [Google Scholar]
- 55.Sagomonyants KB, Jarman-Smith ML, Devine JN, Aronow MS, Gronowicz GA. Biomaterials. 2008;29:1563. doi: 10.1016/j.biomaterials.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Ulery BD, Nair LS, Laurencin CT. J. Polym. Sci. B. Polym. Phys. 2011;49:832. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan HP, Yang ZJ, de Bruijn JD, de Groot K, Zhang XD. Biomaterials. 2001;22:2617. doi: 10.1016/s0142-9612(00)00450-6. [DOI] [PubMed] [Google Scholar]
- 58.Xynos ID, Hukkanen MVJ, Batten JJ, Buttery LD, Hench LL, Polak JM. Calcif Tissue Int. 2000;67:321. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 59.Pérez RA, Won J-E, Knowles JC, Kim H-W. Adv. Drug Deliv. Rev. 2013;65:471. doi: 10.1016/j.addr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Moroni L, Elisseeff J. Mater. today. 2008;11:44. [Google Scholar]
- 61.Davies JE. Biomaterials. 2007;28:5058. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 62.Khan W, Challa VGS, langer R, Domb AJ. In: Focal Controlled Drug Delivery. Domb AJ, Khan W, editors. Springer; 2014. pp. 3–32. [Google Scholar]
- 63.Domb AJ, Kumar N, Ezra A, editors. Biodegradable Polymers in Clinical Use and Clinical Development. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2011. [Google Scholar]
- 64.Roohani-Esfahani SI, Lu ZF, Li JJ, Ellis-Behnke R, Kaplan DL, Zreiqat H. Acta Biomater. 2012;8:302. doi: 10.1016/j.actbio.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 65.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Chem. Rev. 1999;99:3181. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 66.Hench LL. J. Mater. Sci. Mater. Med. 2006;17:967. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 67.Shin H, Jo S, Mikos AG. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 68.Kulkarni RK, Pani KC, Neuman C, Leonard F. Arch. Surg. 1966;93:839. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 69.Neo M, Nakamura T, Ohtsuki C, Kokubo T, Yamamuro T. J. Biomed. Mater. Res. 1993;27:999. doi: 10.1002/jbm.820270805. [DOI] [PubMed] [Google Scholar]
- 70.Albee FH. Ann. Surg. 1920;71:32. doi: 10.1097/00000658-192001000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang LJ, Nancollas GH. Chem. Rev. 2008;108:4628. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrère F, van Blitterswijk CA, de Groot K. Int. J. Nanomedicine. 2006;1:317. [PMC free article] [PubMed] [Google Scholar]
- 73.Hench LL, Polak JM. Science. 2002;295:1014. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 74.Yu X, Botchwey EA, Levine EM, Pollack SR, Laurencin CT. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11203. doi: 10.1073/pnas.0402532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alsberg E, von Recum HA, Mahoney MJ. Expert Opin Biol Ther. 2006;6:847. doi: 10.1517/14712598.6.9.847. [DOI] [PubMed] [Google Scholar]
- 76.Hubbell J. a. Curr. Opin. Botechnology. 1999;10:123. doi: 10.1016/s0958-1669(99)80021-4. [DOI] [PubMed] [Google Scholar]
- 77.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 78.Cushnie EK, Khan YM, Laurencin CT. J. Biomed. Mater. Res. A. 2008;84:54. doi: 10.1002/jbm.a.31380. [DOI] [PubMed] [Google Scholar]
- 79.Lv Q, Deng M, Ulery BD, Nair LS, Laurencin CT. Clin. Orthop. Relat. Res. 2013;471:2422. doi: 10.1007/s11999-013-2859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nukavarapu SP, Amini AR. Conf. Proc. ... Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2011;2011:2464. doi: 10.1109/IEMBS.2011.6090684. [DOI] [PubMed] [Google Scholar]
- 81.Amini AR, Nukavarapu SP. Ann. Biomed. Eng. 2014;42:1261. doi: 10.1007/s10439-014-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Tissue Eng. Part A. 2012;18:1376. doi: 10.1089/ten.tea.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bongio M, van den Beucken JJJP, Leeuwenburgh SCG, Jansen JA. J. Mater. Chem. 2010;20:8747. [Google Scholar]
- 84.Yu X, Khalil A, Dang PN, Alsberg E, Murphy WL. Adv. Funct. Mater. 2014;24:3082. doi: 10.1002/adfm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Verma S, Domb AJ, Kumar N. Nanomedicine (Lond) 2011;6:157. doi: 10.2217/nnm.10.146. [DOI] [PubMed] [Google Scholar]
- 86.Webster TJ, Siegel RW, Bizios R. Biomaterials. 1999;20:1221. doi: 10.1016/s0142-9612(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, Webster TJ. Nano Today. 2009;4:66. [Google Scholar]
- 88.Balasundaram G, Sato M, Webster TJ. Biomaterials. 2006;27:2798. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Webster T. Biomaterials. 2000;21:1803. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 90.Adler AF, Leong KW. Nano Today. 2010;5:553. doi: 10.1016/j.nantod.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat. Mater. 2009;8:543. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 92.Zhang S. Nat. Biotechnol. 2003;21:1171. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 93.Sahoo NG, Pan YZ, Li L, He CB. Nanomedicine (Lond) 2013;8:639. doi: 10.2217/nnm.13.44. [DOI] [PubMed] [Google Scholar]
- 94.Zhao F, Wang J, Guo H, Liu S, He W. J. Nanomater. Article ID 893545, In press. [Google Scholar]
- 95.Palin E, Liu H, Webster TJ. Nanotechnology. 2005;16:1828. [Google Scholar]
- 96.Webster TJ, Ahn ES. Adv Biochem Eng Biotechnol. 2007;103:275. doi: 10.1007/10_021. [DOI] [PubMed] [Google Scholar]
- 97.Gu W, Wu C, Chen J, Xiao Y. Int J Nanomedicine. 2013;8:2305. doi: 10.2147/IJN.S44393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Biomaterials. 2000;21:1803. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 99.Hartgerink JD, Beniash E, Stupp SI. Science (80-. ) 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 100.Sargeant TD, Rao MS, Koh CY, Stupp SI. Biomaterials. 2008;29:1085. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee SS, Huang BJ, Kaltz SR, Sur S, Newcomb CJ, Stock SR, Shah RN, Stupp SI. Biomaterials. 2013;34:452. doi: 10.1016/j.biomaterials.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Albrektsson T, Johansson C. Eur. Spine J. 2001;10:96. doi: 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Urist MR, Lietze A, Dawson E. Clin. Orthop. Relat. Res. 1984;187:277. [PubMed] [Google Scholar]
- 104.LeGeros RZ. Clin. Orthop. Relat. Res. 2002:81. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 105.Rodrigues CVM, Serricella P, Linhares a. B. R., Guerdes RM, Borojevic R, Rossi M. a., Duarte MEL, Farina M. Biomaterials. 2003;24:4987. doi: 10.1016/s0142-9612(03)00410-1. [DOI] [PubMed] [Google Scholar]
- 106.Chen KY, Shyu PC, Dong GC, Chen YS, Kuo WW, Yao CH. Biomaterials. 2009;30:1682. doi: 10.1016/j.biomaterials.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 107.Yuan H, de Bruijn JD, Zhang X, van Blitterswijk CA, de Groot K. J Biomed Mater Res. 2001;58:270. doi: 10.1002/1097-4636(2001)58:3<270::aid-jbm1016>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 108.Yan W-Q, Nakamura T, Kawanabe K, Nishigochi S, Oka M, Kokubo T. Biomaterials. 1997;18:1185. doi: 10.1016/s0142-9612(97)00057-4. [DOI] [PubMed] [Google Scholar]
- 109.Barrere F, Layrolle P, Van Blitterswijk CA, De Groot K. J Mater Sci Mater Med. 2001;12:529. doi: 10.1023/a:1011271713758. [DOI] [PubMed] [Google Scholar]
- 110.Habibovic P, Li J, van der Valk CM, Meijer G, Layrolle P, van Blitterswijk CA, de Groot K. Biomaterials. 2005;26:23. doi: 10.1016/j.biomaterials.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 111.Charles-Harris M, Koch MA, Navarro M, Lacroix D, Engel E, Planell JA. J. Mater. Sci. Mater. Med. 2008;19:1503. doi: 10.1007/s10856-008-3390-9. [DOI] [PubMed] [Google Scholar]
- 112.Nie H, Wang C-H. J. Control. Release. 2007;120:111. doi: 10.1016/j.jconrel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 113.Charles LF, Shaw MT, Olson JR, Wei M. J. Mater. Sci. Med. 2010;21:1845. doi: 10.1007/s10856-010-4051-3. [DOI] [PubMed] [Google Scholar]
- 114.Murphy WL, Mooney DJ. J Am Chem Soc. 2002;124:1910. doi: 10.1021/ja012433n. [DOI] [PubMed] [Google Scholar]
- 115.Urist MR. Science (80-. ) 1965;150:893. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 116.Habibovic P, De Groot K. J. tissue Eng. 2007:25. doi: 10.1002/term.5. [DOI] [PubMed] [Google Scholar]
- 117.Chai YC, Carlier a, Bolander J, Roberts SJ, Geris L, Schrooten J, Van Oosterwyck H, Luyten FP. Acta Biomater. 2012;8:3876. doi: 10.1016/j.actbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 118.Li X, Liu H, Niu X, Fan Y, Feng Q, Cui F, Watari F. J. Biomed. Mater. Res. B. Appl. Biomater. 2011;97:10. doi: 10.1002/jbm.b.31773. [DOI] [PubMed] [Google Scholar]
- 119.Barradas AMC, Fernandes HAM, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen J, van Blitterswijk CA, de Boer J. Biomaterials. 2012;33:3205. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 120.Ripamonti U. Biomaterials. 2006;27:807. doi: 10.1016/j.biomaterials.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 121.Maeno S, Niki Y, Matsumoto H, Morioka H, Yatabe T, Funayama A, Toyama Y, Taguchi T, Tanaka J. Biomaterials. 2005;26:4847. doi: 10.1016/j.biomaterials.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 122.Lobovkina T, Gözen I, Erkan Y, Olofsson J, Weber SG, Orwar O. Soft Matter. 2010;6:268. [Google Scholar]
- 123.Breitwieser GE. Int. J. Biochem. Cell Biol. 2008;40:1467. doi: 10.1016/j.biocel.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Urist MR, Huo YK, Brownell AG, Hohl WM, Buyske J, Lietze A, Tempst P, Hunkapiller M, DeLange RJ. Proc. Natl. Acad. Sci. 1984;81:371. doi: 10.1073/pnas.81.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 126.Liu Y, Wu G, de Groot K. J. R. Soc. Interface. 2010 doi: 10.1098/rsif.2010.0115.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. Biomaterials. 2002;23:1921. doi: 10.1016/s0142-9612(01)00318-0. [DOI] [PubMed] [Google Scholar]
- 128.Ripamonti U. Biomaterials. 1996;17:31. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 129.Laurencin CT, Attawia MA, Lu LQ, Borden MD, Lu HH, Gorum WJ, Lieberman JR. Biomaterials. 2001;22:1271. doi: 10.1016/s0142-9612(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 130.Kondo N, Ogose A, Tokunaga K, Umezu H, Arai K, Kudo N, Hoshino M, Inoue H, Irie H, Kuroda K, Mera H, Endo N. Biomaterials. 2006;27:4419. doi: 10.1016/j.biomaterials.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 131.Le Nihouannen D, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, Layrolle P, Daculsi G. J Mater Sci Mater Med. 2008;19:667. doi: 10.1007/s10856-007-3206-3. [DOI] [PubMed] [Google Scholar]
- 132.Ripamonti U, Crooks J, Khoali L, Roden L. Biomaterials. 2009;30:1428. doi: 10.1016/j.biomaterials.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 133.Winter GD, Simpson BJ. Nature. 1969;223:88. doi: 10.1038/223088a0. [DOI] [PubMed] [Google Scholar]
- 134.Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. Biomaterials. 2005;26:3565. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 135.Li PJ, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, Degroot K. J. Biomed. Mater. Res. 1994;28:7. doi: 10.1002/jbm.820280103. [DOI] [PubMed] [Google Scholar]
- 136.Liu Y, De Groot K, Hunziker EB. Bone. 2005;36:745. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 137.Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Eur Cell Mater. 2011;21:407. doi: 10.22203/ecm.v021a31. [DOI] [PubMed] [Google Scholar]
- 138.Habibovic P, van der Valk CM, van Blitterswijk CA, De Groot K, Meijer G. J Mater Sci Mater Med. 2004;15:373. doi: 10.1023/b:jmsm.0000021104.42685.9f. [DOI] [PubMed] [Google Scholar]
- 139.Takemoto M, Fujibayashi S, Neo M, Suzuki J, Matsushita T, Kokubo T, Nakamura T. Biomaterials. 2006;27:2682. doi: 10.1016/j.biomaterials.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 140.Muschler GF, Nakamoto C, Griffith LG. J Bone Jt. Surg Am. 2004;86-A:1541. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 141.Krishnan L, Willett NJ, Guldberg RE. Ann. Biomed. Eng. 2014;42:432. doi: 10.1007/s10439-014-0969-9. [DOI] [PubMed] [Google Scholar]
- 142.Novosel EC, Kleinhans C, Kluger PJ. Adv. Drug Deliv. Rev. 2011;63:300. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 143.Sieminski AL, Gooch KJ. Biomaterials. 2000;21:2232. doi: 10.1016/s0142-9612(00)00149-6. [DOI] [PubMed] [Google Scholar]
- 144.Phelps E. a, García AJ. Curr. Opin. Biotechnol. 2010;21:704. doi: 10.1016/j.copbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J. Biotechnol. Bioeng. 2004;86:9. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 146.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 147.Sun G, Zhang X, Shen Y-I, Sebastian R, Dickinson LE, Fox-Talbot K, Reinblatt M, Steenbergen C, Harmon JW, Gerecht S. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20976. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanczler JM, Oreffo ROC. Eur. Cell. Mater. 2008;15:100. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 149.Popp JR, Laflin KE, Love BJ, Goldstein AS. J. Tissue Eng. Regen. Med. 2011;5:780. doi: 10.1002/term.376. [DOI] [PubMed] [Google Scholar]
- 150.Hollister SJ. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 151.Choi S-W, Zhang Y, Macewan MR, Xia Y. Adv. Healthc. Mater. 2013;2:145. doi: 10.1002/adhm.201200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen A. a, Galie P. a, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Nat. Mater. 2012;11:768. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, Lv Q, Nair LS, Doty SB, Laurencin CT. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11099. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Unger RE, Peters K, Huang Q, Funk A, Paul D, Kirkpatrick CJ. Biomaterials. 2005;26:3461. doi: 10.1016/j.biomaterials.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 155.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate J. a, Cuy JL, Hauch KD, Laflamme M. a, Murry CE, Ratner BD. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15211. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhai W, Lu H, Chen L, Lin X, Huang Y, Dai K, Naoki K, Chen G, Chang J. Acta Biomater. 2012;8:341. doi: 10.1016/j.actbio.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 157.Schmoekel H, Schense JC, Weber FE, Gratz KW, Gnagi D, Muller R, Hubbell JA. J Orthop Res. 2004;22:376. doi: 10.1016/S0736-0266(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 158.Hudalla GA, Murphy WL. Adv. Funct. Mater. 2011;21:1754. doi: 10.1002/adfm.201002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hubbell J. a., Chilkoti a. Science (80-. ) 2012;337:303. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 160.Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn G. a, Müller R, Livne E, Eming S. a, Hubbell J. a. Sci. Transl. Med. 2011;3:100ra89. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- 161.Hudalla GA, Koepsel JT, Murphy WL. Adv. Mater. 2011;23:5415. doi: 10.1002/adma.201103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Belair DG, Khalil AS, Miller MJ, Murphy WL. Biomacromolecules. 2014;15:2038. doi: 10.1021/bm500177c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Biomaterials. 2012;33:3475. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yamada KM, Even-ram S. Nat. Cell Biol. 2002;4:E75. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 165.Comoglio PM, Boccaccio C, Trusolino L. Curr. Opin. Cell Biol. 2003;15:565. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 166.García A. Polym. Regen. Med. 2006:171. [Google Scholar]
- 167.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Dev. Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 168.Giancotti FG. Nat. Cell Biol. 2000;2:E13. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 169.Lee YJ, Ko JS, Kim HM. Biomaterials. 2006;27:3738. doi: 10.1016/j.biomaterials.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 170.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Tissue Eng. 2005;11:1. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 171.Place ES, Evans ND, Stevens MM. Nat. Mater. 2009;8:457. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 172.Ruoslahti E. Matrix Biol. 2003;22:459. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 173.VandeVondele S, Voros J, Hubbell JA. Biotechnol Bioeng. 2003;82:784. doi: 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- 174.Nguyen EH, Zanotelli MR, Schwartz MP, Murphy WL. Biomaterials. 2014;35:2149. doi: 10.1016/j.biomaterials.2013.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu X, Wei M. Biomed Res. Int. 2013;2013:1. doi: 10.1155/2013/832790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee JS, Wagoner Johnson AJ, Murphy WL. Adv. Mater. 2010;22:5494. doi: 10.1002/adma.201002970. [DOI] [PubMed] [Google Scholar]
- 177.Olivares-Navarrete R, Raines AL, Hyzy SL, Park JH, Hutton DL, Cochran DL, Boyan BD, Schwartz Z. J. Bone Miner. Res. 2012;27:1773. doi: 10.1002/jbmr.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu X, Leng Y, Zhang X, Xu J, Qin L, Chan C-W. Biomaterials. 2005;26:1793. doi: 10.1016/j.biomaterials.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 179.Webster TJ, Ejiofor JU. Biomaterials. 2004;25:4731. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 180.Ingber D. Ann. Med. 2003;35:564. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 181.Discher DE, Mooney DJ, Zandstra PW. Science (80-. ) 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang D. -a., Williams CG, Yang F, Elisseeff JH. Adv. Funct. Mater. 2004;14:1152. [Google Scholar]
- 183.Karageorgiou V, Kaplan D. Biomaterials. 2005;26:5474. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 184.Rouwkema J, Rivron NC, van Blitterswijk C. a. Trends Biotechnol. 2008;26:434. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 185.Marie P, Debiais F, Cohen-Solal M, de Vernejoul MC. Jt. Bone Spine. 2000;67:150. [PubMed] [Google Scholar]
- 186.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stevens MM, George JH. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 188.Anselme K. Biomaterials. 2000;21:667. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 189.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 190.Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, Weber FE, Lutolf MP. Biomacromolecules. 2007;8:3000. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]
- 191.Ehrbar M, Rizzi SC, Hlushchuk R, Djonov V, Zisch AH, Hubbell JA, Weber FE, Lutolf MP. Biomaterials. 2007;28:3856. doi: 10.1016/j.biomaterials.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 192.James R, Deng M, Laurencin CT, Kumbar SG. Front. Mater. Sci. 2011;5:342. [Google Scholar]
- 193.Wang M, Bonfield W. Biomaterials. 2001;22:1311. doi: 10.1016/s0142-9612(00)00283-0. [DOI] [PubMed] [Google Scholar]
- 194.Habraken W, Wolke JGC, Jansen JA. Adv. Drug Deliv. Rev. 2007;59:234. doi: 10.1016/j.addr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 195.STEVENS M. Mater. Today. 2008;11:18. [Google Scholar]
- 196.Kim S-S, Sun Park M, Jeon O, Yong Choi C, Kim B-S. Biomaterials. 2006;27:1399. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 197.Pérez R. a, Won J-E, Knowles JC, Kim H-W. Adv. Drug Deliv. Rev. 2013;65:471. doi: 10.1016/j.addr.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 198.Deng C, Weng J, Cheng QY, Zhou SB, Lu X, Wan JX, Qu SX, Feng B, Li XH. Curr. Appl. Phys. 2007;7:679. [Google Scholar]
- 199.Durucan C, Brown PW. J. Biomed. Mater. Res. 2000;51:726. doi: 10.1002/1097-4636(20000915)51:4<726::aid-jbm22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 200.Kim HW, Knowles JC, Kim HE. Biomaterials. 2004;25:1279. doi: 10.1016/j.biomaterials.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 201.Baji A, Wong SC, Srivatsan TS, Njus GO, Mathur G. Mater. Manuf. Process. 2006;21:211. [Google Scholar]
- 202.Ngiam M, Liao S, Patil AJ, Cheng Z, Chan CK, Ramakrishna S. Bone. 2009;45:4. doi: 10.1016/j.bone.2009.03.674. [DOI] [PubMed] [Google Scholar]
- 203.Lv Q, Nair L, Laurencin CT. J. Biomed. Mater. Res. A. 2009;91:679. doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- 204.Choi S-W, Zhang Y, Thomopoulos S, Xia Y. Langmuir. 2010;26:12126. doi: 10.1021/la101519b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hamadouche M, Sedel L. J. Bone Joint Surg. Br. 2000;82:1095. doi: 10.1302/0301-620x.82b8.11744. [DOI] [PubMed] [Google Scholar]
- 206.Kokubo T, Ito S, Huang ZT, Hayashi T, Sakka S, Kitsugi T, Yamamuro T. J Biomed Mater Res. 1990;24:331. doi: 10.1002/jbm.820240306. [DOI] [PubMed] [Google Scholar]
- 207.van Blitterswijk CA, Grote JJ, de Groot K, Daems WT, Kuijpers W. J Biomed Mater Res. 1986;20:989. doi: 10.1002/jbm.820200713. [DOI] [PubMed] [Google Scholar]
- 208.Devin JE, Attawia MA, Laurencin CT. J. Biomater. Sci. Polym. Ed. 1996;7:661. doi: 10.1163/156856296x00435. [DOI] [PubMed] [Google Scholar]
- 209.Laurencin CT, Attawia MA, Elgendy HE, Herbert KM. Bone. 1996;19:93S. doi: 10.1016/s8756-3282(96)00132-9. [DOI] [PubMed] [Google Scholar]
- 210.Borden M, Attawia M, Khan Y, Laurencin CT. Biomaterials. 2002;23:551. doi: 10.1016/s0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 211.Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, Hindenlang MD, Nair LS, Allcock HR, Laurencin CT. Biomacromolecules. 2008;9:1818. doi: 10.1021/bm800031t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Khan YM, Katti DS, Laurencin CT. J. Biomed. Mater. Res. A. 2004;69:728. doi: 10.1002/jbm.a.30051. [DOI] [PubMed] [Google Scholar]
- 213.Borden M, El-Amin SF, Attawia M, Laurencin CT. Biomaterials. 2003;24:597. doi: 10.1016/s0142-9612(02)00374-5. [DOI] [PubMed] [Google Scholar]
- 214.Bhattacharyya S, Kumbar SG, Khan YM, Nair LS, Singh A, Krogman NR, Brown PW, Allcock HR, Laurencin CT. J. Biomed. Nanotechnol. 2009;5:69. doi: 10.1166/jbn.2009.032. [DOI] [PubMed] [Google Scholar]
- 215.Bhattacharyya S, Nair LS, Singh A, Krogman NR, Greish YE, Brown PW, Allcock HR, Laurencin CT. J. Biomed. Nanotechnol. 2006;2:36. doi: 10.1166/jbn.2009.032. [DOI] [PubMed] [Google Scholar]
- 216.Nair LS, Bhattacharyya S, Bender JD, Greish YE, Brown PW, Allcock HR, Laurencin CT. Biomacromolecules. 5:2212. doi: 10.1021/bm049759j. [DOI] [PubMed] [Google Scholar]
- 217.Deng M, Nair LS, Nukavarapu SP, Jiang T, Kanner WA, Li X, Kumbar SG, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. Biomaterials. 2010;31:4898. doi: 10.1016/j.biomaterials.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Krogman NR, Singh A, Allcock HR, Laurencin CT. Biomaterials. 2008;29:337. doi: 10.1016/j.biomaterials.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Brown JL, Krogman NR, Weikel AL, Allcock HR, Laurencin CT. J. Biomed. Mater. Res. Part A. 2010;92A:114. doi: 10.1002/jbm.a.32334. [DOI] [PubMed] [Google Scholar]
- 220.Krogman NR, Weikel AL, Kristhart KA, Nukavarapu SP, Deng M, Nair LS, Laurencin CT, Allcock HR. Biomaterials. 2009;30:3035. doi: 10.1016/j.biomaterials.2009.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Deng M, Nair LS, Nukavarapu SP, Kumbar SG, Jiang T, Weikel AL, Krogman NR, Allcock HR, Laurencin CT. Adv. Funct. Mater. 2010;20:2794. doi: 10.1002/adfm.201090073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hench LL, Splinter RJ, Allen WC, Greenlee TK. J. Biomed. Mater. Res. 1971;5:117. [Google Scholar]
- 223.Mohamad Yunos D, Bretcanu O, Boccaccini AR. J. Mater. Sci. 2008;43:4433. [Google Scholar]
- 224.Peter M, Binulal NS, Nair SV, Selvamurugan N, Tamura H, Jayakumar R. Chem. Eng. J. 2010;158:353. [Google Scholar]
- 225.Xynos ID, Edgar AJ, Buttery LD, Hench LL, Polak JM. Biochem. Biophys. Res. Commun. 2000;276:461. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 226.Oonishi H, Kushitani S, Yasukawa E, Iwaki H, Hench LL, Wilson J, Tsuji E, Sugihara T. Clin. Orthop. Relat. Res. 1997:316. [PubMed] [Google Scholar]
- 227.Epstein NE. spine J. 2008;8:882. doi: 10.1016/j.spinee.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 228.Blaker JJ, Gough JE, Maquet V, Notingher I, Boccaccini a R. J. Biomed. Mater. Res. A. 2003;67:1401. doi: 10.1002/jbm.a.20055. [DOI] [PubMed] [Google Scholar]
- 229.Epstein NE. Spine J. 2009;9:630. doi: 10.1016/j.spinee.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 230.Vaccaro AR. Orthopedics. 2002;25:s571. doi: 10.3928/0147-7447-20020502-05. [DOI] [PubMed] [Google Scholar]
- 231.Erbe EM, Marx JG, Clineff TD, Bellincampi LD. Eur. Spine J. 2001;10(Suppl 2):S141. doi: 10.1007/s005860100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mikael PE, Amini AR, Basu J, Josefina Arellano-Jimenez M, Laurencin CT, Sanders MM, Barry Carter C, Nukavarapu SP. Biomed. Mater. 2014;9:035001. doi: 10.1088/1748-6041/9/3/035001. [DOI] [PubMed] [Google Scholar]
- 233.Prato M, Singh R, Pantarotto D, Lacerda L, Pastorin G, Bianco A, Kostarelos K. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3357. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mikael PE, Nukavarapu SP. J. Biomater. Tissue Eng. 2011;1:76. [Google Scholar]
- 235.Cui X, Hetke JF, Wiler JA, Anderson DJ, Martin DC. Sensors Actuators A Phys. 2001;93:8. [Google Scholar]
- 236.Fattahi P, Yang G, Kim G, Abidian MR. Adv. Mater. 2014;26:1846. doi: 10.1002/adma.201304496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Layton KN, Abidian MR. 2011 5th Int. IEEE/EMBS Conf. Neural Eng. 2011:298. [Google Scholar]
- 238.Chikar J. a, Hendricks JL, Richardson-Burns SM, Raphael Y, Pfingst BE, Martin DC. Biomaterials. 2012;33:1982. doi: 10.1016/j.biomaterials.2011.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Abidian MR, Kim D-H, Martin DC. Adv. Mater. 2006;18:405. doi: 10.1002/adma.200501726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Luo X, Cui XT. Electrochem. commun. 2009;11:1956. doi: 10.1016/j.elecom.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Svirskis D, Travas-Sejdic J, Rodgers A, Garg S. J. Control. Release. 2010;146:6. doi: 10.1016/j.jconrel.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 242.Guo B, Glavas L, Albertsson A-C. Prog. Polym. Sci. 2013;38:1263. [Google Scholar]
- 243.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, Yao J, Chen F, Chen G. J. Biomed. Mater. Res. A. 2004;68:411. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 244.Ramanaviciene A, Kausaite A, Tautkus S, Ramanavicius A. J. Pharm. Pharmacol. 2007;59:311. doi: 10.1211/jpp.59.2.0017. [DOI] [PubMed] [Google Scholar]
- 245.Wang CH, Dong YQ, Sengothi K, Tan KL, Kang ET. Synth. Met. 1999;102:1313. [Google Scholar]
- 246.Zhao H, Zhu B, Sekine J, Luo S-C, Yu H. ACS Appl. Mater. Interfaces. 2012;4:680. doi: 10.1021/am2012905. [DOI] [PubMed] [Google Scholar]
- 247.Dong H, Cao X, Li CM. 2009;1:1599. doi: 10.1021/am900267e. [DOI] [PubMed] [Google Scholar]
- 248.Wang J, Shi W, Jiang H, Wu G, Ruan C, Ge D. J. Memb. Sci. 2011;373:89. [Google Scholar]
- 249.Ko S, Jang J. 2007. p. 182.
- 250.Luo S-C, Mohamed Ali E, Tansil NC, Yu H-H, Gao S, Kantchev EAB, Ying JY. Langmuir. 2008;24:8071. doi: 10.1021/la800333g. [DOI] [PubMed] [Google Scholar]
- 251.Hardy JG, Lee JY, Schmidt CE. Curr. Opin. Biotechnol. 2013;24:847. doi: 10.1016/j.copbio.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 252.Ravichandran R, Sundarrajan S, Venugopal JR. 2010.
- 253.Ghasemi-mobarakeh L, Prabhakaran MP, Morshed M. 2011.
- 254.Brédas JL, Silbey R, editors. Conjugated Polymers. Springer Netherlands; Dordrecht: 1991. [Google Scholar]
- 255.Hsiao C-W, Bai M-Y, Chang Y, Chung M-F, Lee T-Y, Wu C-T, Maiti B, Liao Z-X, Li R-K, Sung H-W. Biomaterials. 2013;34:1063. doi: 10.1016/j.biomaterials.2012.10.065. [DOI] [PubMed] [Google Scholar]
- 256.Yonemori K, Matsunaga S, Ishidou Y, Maeda S, Yoshida H. Bone. 1996;19:173. doi: 10.1016/8756-3282(96)00169-x. [DOI] [PubMed] [Google Scholar]
- 257.Balint R, Cassidy NJ, Cartmell SH. Acta Biomater. 2014;10:2341. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 258.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Baharvand H, Kiani S, Al-Deyab SS, Ramakrishna S. J. Tissue Eng. Regen. Med. 2011;5:e17. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 259.Isaksson J, Kjäll P, Nilsson D, Robinson ND, Berggren M, Richter-Dahlfors A. Nat. Mater. 2007;6:673. doi: 10.1038/nmat1963. [DOI] [PubMed] [Google Scholar]
- 260.Lee JY, Bashur C. a, Goldstein AS, Schmidt CE. Biomaterials. 2009;30:4325. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Liang D, Hsiao BS, Chu B. Adv. Drug Deliv. Rev. 2007;59:1392. doi: 10.1016/j.addr.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cosnier S, Holzinger M. 2010.
- 263.Jiang X, Marois Y, Traoré A, Tessier D, Dao LH, Guidoin R, Zhang Z. Tissue Eng. 2002;8:635. doi: 10.1089/107632702760240553. [DOI] [PubMed] [Google Scholar]
- 264.Zhang Z, Roy R, Dugré FJ, Tessier D, Dao LH. J. Biomed. Mater. Res. 2001;57:63. doi: 10.1002/1097-4636(200110)57:1<63::aid-jbm1142>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 265.Sajesh KM, Jayakumar R, Nair SV, Chennazhi KP. Int. J. Biol. Macromol. 2013;62:465. doi: 10.1016/j.ijbiomac.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 266.Li Y, Neoh KG, Kang E-T. J. Colloid Interface Sci. 2004;275:488. doi: 10.1016/j.jcis.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 267.Peramo A, Urbanchek MG, Spanninga S. a, Povlich LK, Cederna P, Martin DC. Tissue Eng. Part A. 2008;14:423. doi: 10.1089/tea.2007.0123. [DOI] [PubMed] [Google Scholar]
- 268.Shahini A, Yazdimamaghani M, Walker KJ, Eastman M. a, Hatami-Marbini H, Smith BJ, Ricci JL, Madihally SV, Vashaee D, Tayebi L. Int. J. Nanomedicine. 2014;9:167. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mitragotri S, Lahann J. Nat. Mater. 2009;8:15. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kloxin A, Kasko A, Salinas C, Anseth K. Science (80-. ) 2009;59 doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kloxin AM, Benton J. a, Anseth KS. Biomaterials. 2010;31:1. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Trappmann B, Gautrot JE, Connelly JT, Strange DGT, Li Y, Oyen ML, Stuart MAC, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WTS. Nat. Mater. 2012;11:642. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 273.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Riverafeliciano J, Mooney DJ. Nat. Mater. 2010;9:518. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sun H, Zhu F, Hu Q, Krebsbach PH. Biomaterials. 2014;35:1176. doi: 10.1016/j.biomaterials.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Nii M, Lai JH, Keeney M, Han LH, Behn A, Imanbayev G, Yang F. Acta Biomater. 2013;9:5475. doi: 10.1016/j.actbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 276.Tan S, Fang JY, Yang Z, Nimni ME, Han B. Biomaterials. 2014;35:5294. doi: 10.1016/j.biomaterials.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 277.Fu J, Wang Y, Yang M, Desai R, Yu X. Nat. 2010;7 [Google Scholar]
- 278.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Yang C, Tibbitt MW, Basta L, Anseth KS. Nat. Mater. 2014:1. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lo KW-H, Ulery BD, Kan HM, Ashe KM, Laurencin CT. J. Tissue Eng. Regen. Med. 2014;8:728. doi: 10.1002/term.1573. [DOI] [PubMed] [Google Scholar]
- 281.Laurencin CT, Ashe KM, Henry N, Kan HM, Lo KW-H. Drug Discov. Today. 2014;19:794. doi: 10.1016/j.drudis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Lo KW-H, Jiang T, Gagnon K. a, Nelson C, Laurencin CT. Trends Biotechnol. 2014;32:74. doi: 10.1016/j.tibtech.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cushnie EK, Ulery BD, Nelson SJ, Deng M, Sethuraman S, Doty SB, Lo KWH, Khan YM, Laurencin CT. PLoS One. 2014;9:e101627. doi: 10.1371/journal.pone.0101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fleisch H. Eur. Spine J. 2003;12(Suppl 2):S142. doi: 10.1007/s00586-003-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS. Biomaterials. 2005;26:6941. doi: 10.1016/j.biomaterials.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 286.Roy S, Khanna S, Sen CK. Free Radic. Biol. Med. 2008;44:180. doi: 10.1016/j.freeradbiomed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 287.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, Kuroiwa Y. Life Sci. 1998;64:249. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 288.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H336. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 289.Liu D, Wang Z, Zhan J, Zhang Q, Wang J, Zhang Q, Xian X, Luan Q, Hao A. Pharmacol. Biochem. Behav. 2014;116:55. doi: 10.1016/j.pbb.2013.11.009. [DOI] [PubMed] [Google Scholar]