Abstract
Purpose
We have previously reported an approach to ovarian stimulation for the purpose of fertility preservation (FP) in women with breast cancer via embryo freezing with the concurrent use of letrozole. The aim of this study was to provide the pregnancy and FP outcomes when embryos generated with the same protocol are used.
Patients and Methods
In all, 131 women with stage ≤ 3 breast cancer underwent ovarian stimulation and received concurrent letrozole 5 mg per day before receiving adjuvant chemotherapy and cryopreserving embryos.
Results
Thirty-three of the 131 women underwent 40 attempts to transfer embryos to their own uterus (n = 18) or via the use of a gestational carrier (n = 22) at a mean age of 41.5 ± 4.3 years with a median 5.25 years after embryo cryopreservation. The overall live birth rate per embryo transfer was similar to the US national mean among infertile women of a similar age undergoing in vitro fertilization–embryo transfer (45.0 v 38.2; P = .2). Seven (38.8%) of the 18 pregnancies were twins with no higher-order pregnancies being encountered. No fetal anomalies or malformations were reported in 25 children after a mean follow-up of 40.4 ± 26.4 months. Seventeen of the 33 women attempting pregnancy had at least one child, translating into an FP rate of 51.5% per attempting woman.
Conclusion
Embryo cryopreservation after ovarian stimulation with the letrozole and follicle-stimulating hormone protocol preserves fertility in women with breast cancer and results in pregnancy rates comparable to those expected in a noncancer population undergoing in vitro fertilization.
INTRODUCTION
In 2014, the estimated number of new cases of invasive malignancies among females was approximately 810,000 in the United States, with breast cancer in the lead at a proportion of 29%.1 Approximately 15% of all cases of invasive breast cancer occur in females of reproductive age, with clustering between the ages of 30 and 40 years.1,2 In addition to this predominance among young women, advances in diagnostic and therapeutic modalities have increased the number of premenopausal women diagnosed with earlier-stage cancer. As a positive consequence, the early diagnosis and intervention have led to a continuous decline in mortality rates over the last few decades. During the most recent 10 years for which there are data (2001 to 2010), death rates for breast cancer decreased by 1.9% per year in females.3 Furthermore, the overall trend for delaying the initiation of childbearing to later in life has increased the likelihood of being childless at the time of cancer therapy.4 These historical developments created a growing population of young breast cancer survivors that is more likely to experience premature ovarian failure and the infertility consequences of chemotherapy-induced ovarian reserve damage and that will be in need of fertility preservation (FP).5
In addition to the general trend in delaying childbearing and hence being more prone to age-related infertility at the time of breast cancer diagnosis, women with estrogen receptor (ER) –positive tumors may experience further delays because of the initiation of hormonal therapy.6 Because approximately 80% of breast cancers express ERs,7 many premenopausal women have to undergo years of antihormonal therapy with tamoxifen. Because pregnancy is contraindicated during tamoxifen treatment, this delay will result in an age-related decline in ovarian reserve in addition to chemotherapy-induced losses.8,9 Fertility begins to decline significantly after age 37 and is completely lost by the ages of 45 to 46 in females. Given that recent studies recommended extension of tamoxifen use to 10 years and given that a typical breast cancer chemotherapy regimen can result in an estimated 10 years' worth of ovarian reserve loss,6,10 one can argue that nearly all premenopausal women with breast cancer, especially those with ER-positive tumors, stand to risk their fertility by the end of their treatment if no action is taken.
Oocyte and embryo cryopreservation are established FP techniques for women planning to undergo cancer treatments.11 Both techniques require ovarian stimulation for approximately 2 weeks. However, traditional ovarian stimulation protocols can result in estrogen levels that are 10 to 20 times higher than physiologic levels, which raises the concern of accelerated tumor growth in patients with estrogen-sensitive cancer.10 To protect these patients from the potential deleterious effects of increased estrogen levels, we developed an ovarian stimulation protocol that uses letrozole in combination with follicle-stimulating hormone (FSH) for the purpose of FP by embryo or oocyte cryopreservation.12 We reported previously that similar numbers of embryos were obtained with the letrozole-FSH protocol compared with conventional ovarian stimulation protocols.12 Furthermore, no significant increase in short-term recurrence risk has been observed in patients with breast cancer undergoing ovarian stimulation with letrozole protocol even after multiple cycles.13,14
Addressing the growing need for FP among women with breast cancer, these results enhanced the acceptability and utility of embryo and oocyte cryopreservation. However, without the availability of pregnancy outcomes, the assessment of the efficacy and safety of this approach in preserving fertility is not complete. It is the purpose of this study to provide the final information from the work we started more than a decade ago and report on the pregnancy and FP outcomes of those women who returned to use their previously cryopreserved embryos generated via the letrozole protocol.
PATIENTS AND METHODS
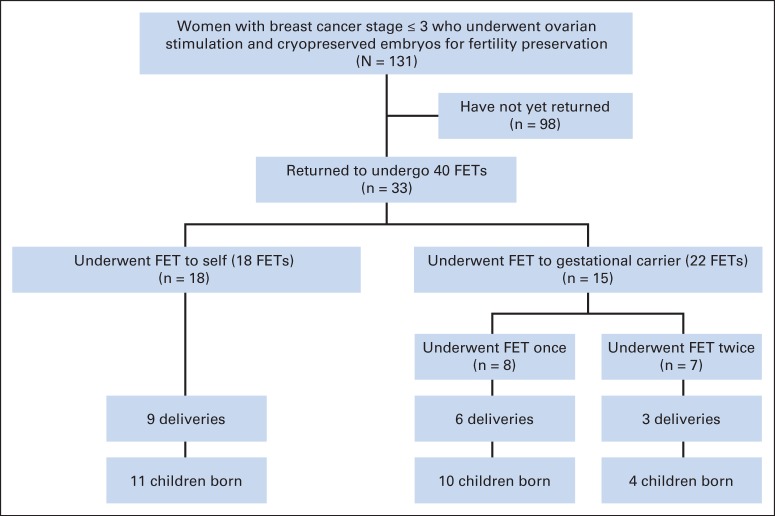
The study protocol was approved by the institutional review board. Under this protocol, 131 women age ≤ 45 years, diagnosed with stage ≤ 3 breast cancer underwent ovarian stimulation and cryopreserved their embryos for FP at the Innovation Institute for Fertility Preservation and In Vitro Fertilization. Of the 131 women, 33 returned to undergo frozen embryo transfer (FET) to self or used a gestational carrier (Fig 1). The reasons for using a gestational carrier were that these women were either undergoing treatment with tamoxifen which precluded gestation or they were concerned about the safety of pregnancy after breast cancer although no prior studies have suggested an increased risk of relapse.
Fig 1.
Study diagram. FET, frozen embryo transfer.
The details of the ovarian stimulation protocol were previously described.15 Briefly, letrozole (Novartis Oncology, East Hanover, NJ) 5 mg per day was initiated on cycle day 2 (CD2) followed by gonadotropins (follicle-stimulating hormone; Organon, West Orange, NJ, or follitropin alfa; Serono, Rockville, MD), 150 to 450 IU per day on CD4. The letrozole treatment was then continued throughout the stimulation. Oocytes were fertilized by intracytoplasmic sperm injection, and embryos were frozen at the two pronuclei stage. Cryopreservation of all embryos was undertaken with a vitrification method.
Patients or gestational carriers were prepared for embryo transfer with an artificial programmed cycle. First, pituitary gonadotropin secretion was regulated by initiating daily short-acting subcutaneous gonadotropin-releasing hormone analog injections given subcutaneously during the midluteal phase of the previous menstrual cycle. On menstruation, endometrial development was induced by administration of estrogen patches (estradiol; Bayer HealthCare Pharmaceuticals, Wayne, NJ) once every 3 days starting on CD2 of the menstrual cycle and continuing until the endometrial thickness reached more than 7 mm, and a triple line pattern was evident on ultrasound examination. Once endometrial optimum thickness was confirmed by ultrasound, progesterone 200 mg per day (Ferring, Parsippany, NJ) was administered vaginally to support the luteal phase. Embryo transfer was performed 3 to 5 days later under abdominal ultrasound guidance. Intramuscular progesterone 50 mg once per day was added for 7 days during the peri-implantation phase. Implantation rate was defined as the percentage of embryos that successfully underwent implantation among those that were transferred. Clinical pregnancy was defined as the presence of at least one gestational sac during the first ultrasound examination by the eighth week of gestation. Miscarriage was defined as the spontaneous loss of embryo before the 20th week of pregnancy. Fertility was defined as the ability to have a live birth, and FP success was defined as the restoration of that ability for at least one live birth. We defined the fertility preservation rate (FPR) as the percentage of women who were able to have at least one live birth among all who attempted an FET after FP by embryo cryopreservation.
The pregnancy rates were compared with the US national data from the Society of Assisted Reproductive Technology (SART) by using a comparable age group (age 35 to 37 years) and time period (2009) corresponding to the median year of embryo cryopreservation for study patients.
Statistical analyses were performed with SPSS 15 for Windows (SPSS, Chicago, IL). The variables were investigated by using visual (histograms, probability plots) and analytic methods (Kolmogorov-Smirnov and Shapiro-Wilks tests) to determine whether they were distributed normally. Continuous data were analyzed by t test. χ2 and Fisher's exact tests were used to compare proportions of different groups. Statistical significance was set at P < .05. Data were presented as mean ± standard deviation (SD).
RESULTS
In all, 131 women underwent 152 cycles of embryo cryopreservation at a mean age of 35.8 ± 4.1 years before initiating chemotherapy. Seventeen women underwent two cycles of embryo cryopreservation and two underwent three cycles. This resulted in the cryopreservation of a mean number of 5.9 ± 4.5 embryos.
Of the 131 women undergoing embryo cryopreservation, 33 returned to have FET at a median 5.25 years (range, 2 to 8.2 years) after oocyte retrieval. Forty FETs were performed with the embryos from these 33 women (Fig 1). Demographic characteristics of all patients with breast cancer are provided in Table 1. The mean number of total and mature oocytes retrieved, oocyte maturity rate (percentage ± SD), fertilization rate (percentage ± SD), and the mean number of embryos cryopreserved in women who returned for FET were 11.8 ± 8.9, 8.1 ± 5.2, 77.3 ± 22.6, 75.6 ± 21.5, and 6.5 ± 4.9, respectively. Post-thaw survival rate of embryos was 98 (84.4%) of 116, and 17 embryos were refrozen at a further stage after thawing.
Table 1.
Characteristics of Women With Breast Cancer Undergoing Embryo Cryopreservation for FP
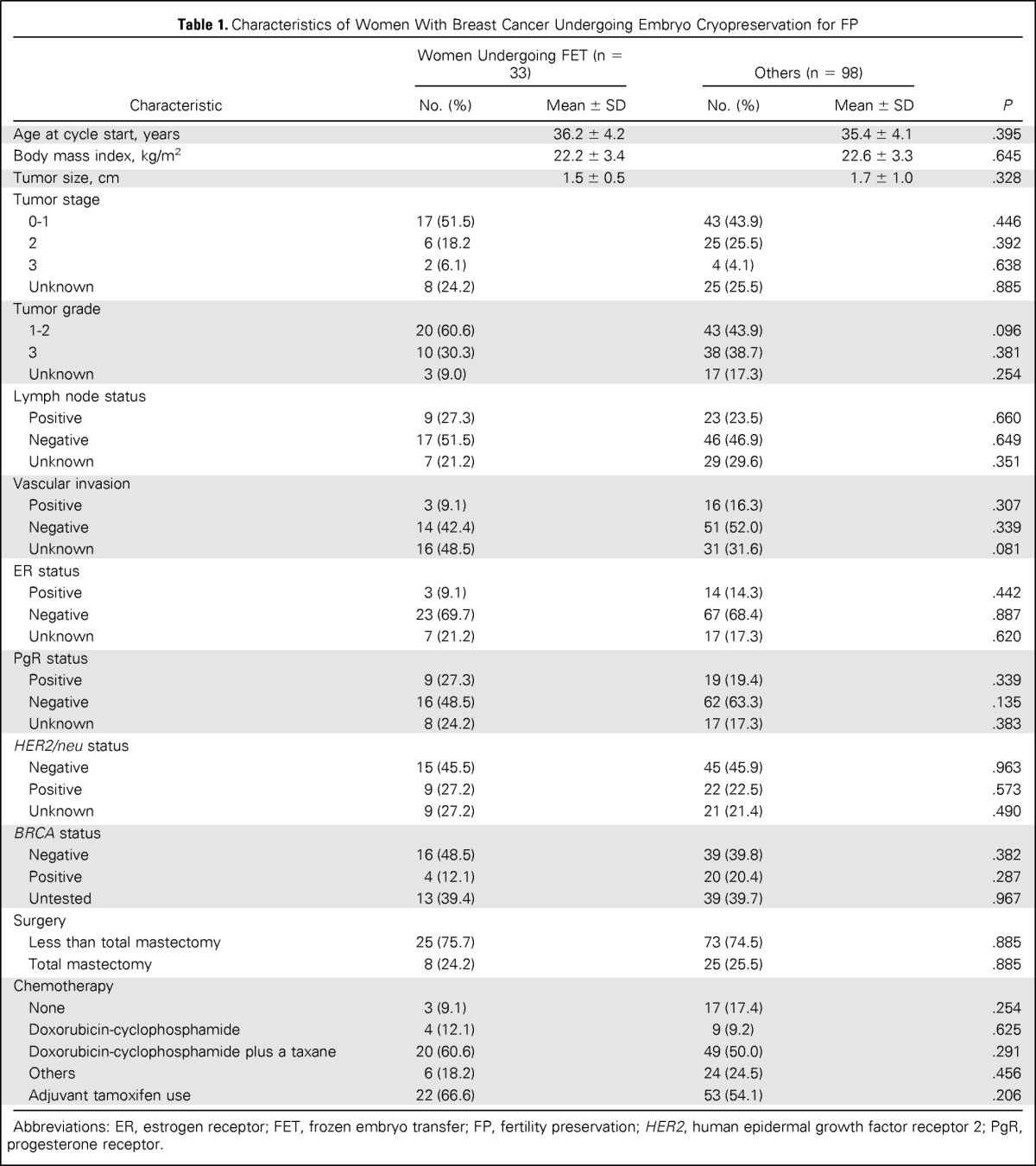
| Characteristic | Women Undergoing FET (n = 33) |
Others (n = 98) |
P | ||
|---|---|---|---|---|---|
| No. (%) | Mean ± SD | No. (%) | Mean ± SD | ||
| Age at cycle start, years | 36.2 ± 4.2 | 35.4 ± 4.1 | .395 | ||
| Body mass index, kg/m2 | 22.2 ± 3.4 | 22.6 ± 3.3 | .645 | ||
| Tumor size, cm | 1.5 ± 0.5 | 1.7 ± 1.0 | .328 | ||
| Tumor stage | |||||
| 0-1 | 17 (51.5) | 43 (43.9) | .446 | ||
| 2 | 6 (18.2 | 25 (25.5) | .392 | ||
| 3 | 2 (6.1) | 4 (4.1) | .638 | ||
| Unknown | 8 (24.2) | 25 (25.5) | .885 | ||
| Tumor grade | |||||
| 1-2 | 20 (60.6) | 43 (43.9) | .096 | ||
| 3 | 10 (30.3) | 38 (38.7) | .381 | ||
| Unknown | 3 (9.0) | 17 (17.3) | .254 | ||
| Lymph node status | |||||
| Positive | 9 (27.3) | 23 (23.5) | .660 | ||
| Negative | 17 (51.5) | 46 (46.9) | .649 | ||
| Unknown | 7 (21.2) | 29 (29.6) | .351 | ||
| Vascular invasion | |||||
| Positive | 3 (9.1) | 16 (16.3) | .307 | ||
| Negative | 14 (42.4) | 51 (52.0) | .339 | ||
| Unknown | 16 (48.5) | 31 (31.6) | .081 | ||
| ER status | |||||
| Positive | 3 (9.1) | 14 (14.3) | .442 | ||
| Negative | 23 (69.7) | 67 (68.4) | .887 | ||
| Unknown | 7 (21.2) | 17 (17.3) | .620 | ||
| PgR status | |||||
| Positive | 9 (27.3) | 19 (19.4) | .339 | ||
| Negative | 16 (48.5) | 62 (63.3) | .135 | ||
| Unknown | 8 (24.2) | 17 (17.3) | .383 | ||
| HER2/neu status | |||||
| Negative | 15 (45.5) | 45 (45.9) | .963 | ||
| Positive | 9 (27.2) | 22 (22.5) | .573 | ||
| Unknown | 9 (27.2) | 21 (21.4) | .490 | ||
| BRCA status | |||||
| Negative | 16 (48.5) | 39 (39.8) | .382 | ||
| Positive | 4 (12.1) | 20 (20.4) | .287 | ||
| Untested | 13 (39.4) | 39 (39.7) | .967 | ||
| Surgery | |||||
| Less than total mastectomy | 25 (75.7) | 73 (74.5) | .885 | ||
| Total mastectomy | 8 (24.2) | 25 (25.5) | .885 | ||
| Chemotherapy | |||||
| None | 3 (9.1) | 17 (17.4) | .254 | ||
| Doxorubicin-cyclophosphamide | 4 (12.1) | 9 (9.2) | .625 | ||
| Doxorubicin-cyclophosphamide plus a taxane | 20 (60.6) | 49 (50.0) | .291 | ||
| Others | 6 (18.2) | 24 (24.5) | .456 | ||
| Adjuvant tamoxifen use | 22 (66.6) | 53 (54.1) | .206 | ||
Abbreviations: ER, estrogen receptor; FET, frozen embryo transfer; FP, fertility preservation; HER2, human epidermal growth factor receptor 2; PgR, progesterone receptor.
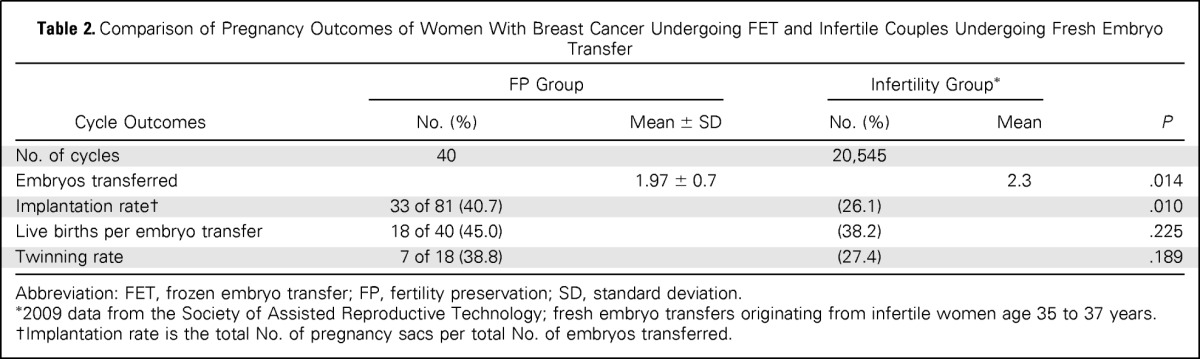
Of the 40 embryos transferred, 22 (55.0%) were transferred to a gestational carrier. The mean number of embryos transferred was 1.97 ± 0.7. The overall clinical pregnancy/FET rate was 65.0% (26 of 40), live birth/FET rate was 45.0% (18 of 40), and implantation rate was 40.7% (33 of 81). Comparison of our data with SART data (fresh embryo transfers from nondonor oocyte in vitro fertilization embryo transfer cycles in infertile couples (age 35 to 37 years) indicated similar live birth rates (Table 2). A total of 25 babies were born from 18 pregnancies of which 38.8% (seven of 18) were twins, with no higher-order pregnancies encountered. The mean gestational age was 38.0 ± 2.3 weeks (range, 34 to 41 gestational weeks), and the mean birth weight was 3,071.4 ± 711.1 g (range, 2,300 to 4,100 g). No fetal anomalies or malformations occurred. Likewise, no developmental problems were reported by parents after 40.4 ± 26.4 months (range, 6 to 84 months) follow-up. No women experienced any medical complications during pregnancies or during the postpartum period.
Table 2.
Comparison of Pregnancy Outcomes of Women With Breast Cancer Undergoing FET and Infertile Couples Undergoing Fresh Embryo Transfer
| Cycle Outcomes | FP Group |
Infertility Group* |
P | ||
|---|---|---|---|---|---|
| No. (%) | Mean ± SD | No. (%) | Mean | ||
| No. of cycles | 40 | 20,545 | |||
| Embryos transferred | 1.97 ± 0.7 | 2.3 | .014 | ||
| Implantation rate† | 33 of 81 (40.7) | (26.1) | .010 | ||
| Live births per embryo transfer | 18 of 40 (45.0) | (38.2) | .225 | ||
| Twinning rate | 7 of 18 (38.8) | (27.4) | .189 | ||
Abbreviation: FET, frozen embryo transfer; FP, fertility preservation; SD, standard deviation.
2009 data from the Society of Assisted Reproductive Technology; fresh embryo transfers originating from infertile women age 35 to 37 years.
Implantation rate is the total No. of pregnancy sacs per total No. of embryos transferred.
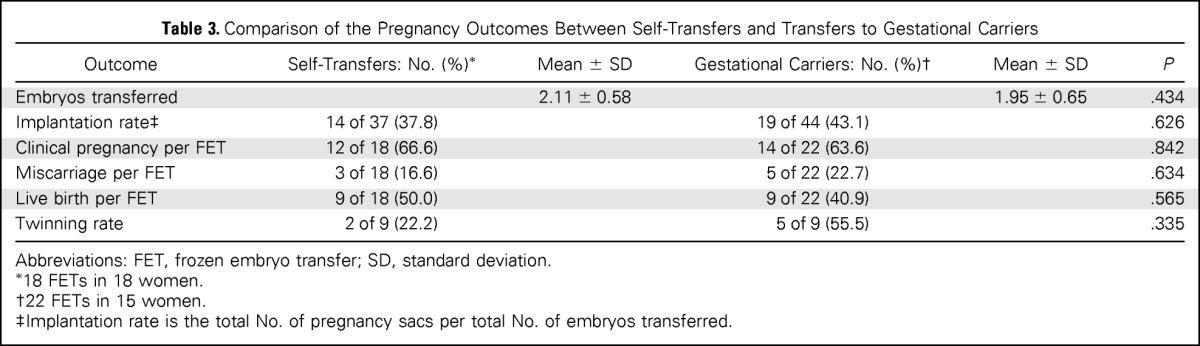
The mean age at cryopreservation was similar in patients undergoing self transfer versus using a gestational carrier (36.6 ± 4.0 v 35.8 ± 4.4 years; P = .595). The mean time from embryo cryopreservation to FET was significantly longer in patients undergoing self transfer than in those using a gestational carrier (65.3 ± 14.3 v 45.3 ± 17.4 months; P = .002). The pregnancy outcomes, clinical pregnancy, and live birth rates/FET were not significantly different between the self transfer (66.6% v 63.6%) and gestational carrier (50.0% v 40.9%) groups (Table 3).
Table 3.
Comparison of the Pregnancy Outcomes Between Self-Transfers and Transfers to Gestational Carriers
| Outcome | Self-Transfers: No. (%)* | Mean ± SD | Gestational Carriers: No. (%)† | Mean ± SD | P |
|---|---|---|---|---|---|
| Embryos transferred | 2.11 ± 0.58 | 1.95 ± 0.65 | .434 | ||
| Implantation rate‡ | 14 of 37 (37.8) | 19 of 44 (43.1) | .626 | ||
| Clinical pregnancy per FET | 12 of 18 (66.6) | 14 of 22 (63.6) | .842 | ||
| Miscarriage per FET | 3 of 18 (16.6) | 5 of 22 (22.7) | .634 | ||
| Live birth per FET | 9 of 18 (50.0) | 9 of 22 (40.9) | .565 | ||
| Twinning rate | 2 of 9 (22.2) | 5 of 9 (55.5) | .335 |
Abbreviations: FET, frozen embryo transfer; SD, standard deviation.
18 FETs in 18 women.
22 FETs in 15 women.
Implantation rate is the total No. of pregnancy sacs per total No. of embryos transferred.
Of the 33 women attempting pregnancy, 26 underwent FET once and seven underwent FET twice. This eventually resulted in 20 women having at least one pregnancy. Of the 20 women, three did not have a baby because of spontaneous abortions (one women had a single and two others had two consecutive spontaneous abortions), bringing the FPR to 51.5% among those attempting. Of note, three of the 17 women who had live births also experienced one spontaneous miscarriage each before undergoing a second FET that resulted in a live birth, and one woman had two live births.
DISCUSSION
Because of the potential estrogen sensitivity of breast cancer, established FP methods such as embryo or oocyte cryopreservation may pose additional risks to patients. To reduce the concerns relating to the increased estrogen levels during standard ovarian stimulation regimens used for embryo and oocyte cryopreservation, we developed a unique protocol using aromatase inhibition in women with estrogen-sensitive cancer.12 Although we have already shown the short-term safety13 as well as the relative efficacy16 of this approach, the most crucial information regarding the pregnancy outcomes and FP success was missing. Here we studied the pregnancy outcomes of FET with embryos generated after ovarian stimulation with the letrozole-FSH protocol for FP in patients with breast cancer since 2001. We found that FET with cryopreservation after the letrozole protocol resulted in a 65.0% overall clinical pregnancy rate, with a 45.0% live birth rate. These success rates were similar to those of infertile women undergoing embryo transfer. Furthermore, the live birth rates with FET after letrozole-FSH stimulation were comparable to those from the US national registry as reported in the SART data from a comparable time period for women age 35 to 37 years undergoing in vitro fertilization embryo transfer, despite the fact that fewer embryos were transferred in the FP group (Table 2). Frozen embryo cycles of infertility patients represent a selection of lesser quality embryos because better-quality embryos would have been transferred during the initial fresh transfer. Because such bias does not exist with up-front freeze with patients who have cancer, comparison to FET in infertility patients would have favored the FP patients. For this reason, we used a fresh transfer group for comparison. In fact, had we used the live birth rates for FET from the US general population in SART, we would have found that the FP group was superior (45% live birth rate in this study v 30.9% in SART data for the 35- to 37-year-old age group reporting on 5,519 women; P < .05). This indicates that embryo cryopreservation with the letrozole-gonadotropin protocol yields success rates at least similar to those achieved with standard protocols used in infertile populations and hence is effective in preserving fertility. In this study, of the 33 women attempting pregnancy with FET, 51.5% were able to have at least one live birth and hence were able to preserve their fertility.
Today, embryo cryopreservation is used routinely worldwide for surplus embryos after infertility treatments, but its use in the noninfertile cancer population is more recent. Although earlier reports in infertile patients suggested that FET may not be as efficient as fresh embryo transfer, current research indicates success rates similar to those seen with unfrozen embryos.17,18 In general, survival rates per thawed embryo are in the range of 75% to 90%, implantation rates are 8% to 43%, and cumulative pregnancy rates are 26% to 60%.17–21 Regardless of whether a gestational carrier was used or not, our overall results with the novel letrozole protocol were comparable to results reported with standard protocols in infertility patients.
In the early years of the introduction of aromatase inhibitors as ovarian stimulants, concerns were raised on the potential impact of the treatment on the resulting pregnancy, especially given the fact that in an animal study, letrozole was shown to be teratogenic when fetuses were exposed during organogenesis.22 However, clinical studies did not associate letrozole with birth abnormalities when it was used as an ovulation induction agent. A study assessed 911 human babies born after ovulation induction with letrozole or clomiphene (a standard oral ovulation induction agent) for 5 days from days 3 to 7 of the cycle in an infertile population. The study found no differences in the overall rates of fetal malformations or chromosomal abnormalities among newborns of mothers who conceived after letrozole (n = 514) or clomiphene citrate (n = 397) treatments.23 In a more recent study, 750 women with polycystic ovary syndrome were randomly assigned to receive letrozole or clomiphene for ovulation induction in a 1:1 ratio. Four major congenital anomalies in the letrozole group (four of 102) versus one in the clomiphene group (one of 66) were reported (P = .65).24 These are not surprising findings. Given that letrozole has a 48-hour half-life,25 and the embryo implantation would have taken place weeks after the last administration of letrozole, the drug would have been eliminated from the circulation by the time organogenesis had started. The study then suggests that letrozole does not have any detrimental impact on oocyte development when used for a short period during the early phase of a menstrual cycle. Despite the fact that in the FP setting, the cryopreserved embryos are generated in vitro and have no direct exposure to letrozole, safety data were needed for assurance that there was no detrimental effect at the oocyte level with the continuous use of letrozole during ovarian stimulation. Notwithstanding the limited sample size, we presented the first data on newborns from women with breast cancer who used their frozen embryos generated after ovarian stimulation with letrozole. We found no minor or major fetal malformations or developmental abnormalities after a mean follow-up of more than 3 years (40.4 ± 26.4 months).
In our study, the median time since the cryopreservation of embryos in the 33 women who returned for FET was 5.25 years, which suggests that more than half the women had waited until 5 years of hormonal therapy was complete. The other half who had not waited the standard 5 years used gestational carriers, which likely reflects the patients' and their physicians' ongoing concern regarding the risk of breast cancer recurrence with pregnancy after breast cancer. As a result, the self-transfer and gestational carrier–transfer populations may be different, although we did not find any differences in tumor characteristics.
Only a quarter of those who underwent ovarian stimulation with letrozole plus FSH have returned for embryo transfer in this 14-year-long study, although the characteristics of those who returned versus those who did not were similar (Table 1). Because not all women who have frozen embryos may elect to, need to, or be able to use their frozen embryos, these numbers may not significantly increase, even with much longer follow-up. It is possible that among those who did not return, some have conceived spontaneously and hence did not need to use their frozen embryos. Because this study was not designed to determine the spontaneous conception rate among the participants, we are not able to elaborate on the specifics of those who have not yet used their embryos. In the meantime, it is prudent that pregnancy outcome information becomes available to reassure women with breast cancer who consider and continue to resort to the letrozole protocol to preserve their fertility. To our knowledge, this is the first and the largest prospective study that provides much needed pregnancy outcome data from women with breast cancer undergoing FET subsequent to letrozole plus gonadotropin stimulation for FP.
In conclusion, FET with embryos generated after ovarian stimulation with the letrozole-FSH protocol in women with breast cancer resulted in outcomes comparable to those expected in the general infertility populations. Our findings contribute to the safety track record of the letrozole protocol and indicate that letrozole has no detrimental effect on the oocyte, the resulting embryos, or the offspring. Given this sustained track record, we surmise that the letrozole protocol should be used more commonly to offer the possibility of FP by established cryopreservation methods in women with breast cancer.
Footnotes
See accompanying editorial on page 2413
Supported by Grants No. R01 HD053112 and R21 HD061259 from the National Institute of Child Health and Human Development.
Presented at the 69th Annual Meeting of the American Society for Reproductive Medicine, Boston, MA, October 12-17, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00504699.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Kutluk Oktay
Financial support: Kutluk Oktay
Administrative support: Kutluk Oktay
Provision of study materials or patients: Kutluk Oktay
Collection and assembly of data: Volkan Turan, Giuliano Bedoschi, Fernanda S. Pacheco
Data analysis and interpretation: Volkan Turan, Fred Moy
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Fertility Preservation Success Subsequent to Concurrent Aromatase Inhibitor Treatment and Ovarian Stimulation in Women With Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kutluk Oktay
No relationship to disclose
Volkan Turan
No relationship to disclose
Giuliano Bedoschi
No relationship to disclose
Fernanda S. Pacheco
No relationship to disclose
Fred Moy
No relationship to disclose
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Anders CK, Johnson R, Litton J, et al. Breast cancer before age 40 years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/archive/csr/1975_2011/ [Google Scholar]
- 4.Rodriguez-Wallberg KA, Oktay K. Fertility preservation medicine: Options for young adults and children with cancer. J Pediatr Hematol Oncol. 2010;32:390–396. doi: 10.1097/MPH.0b013e3181dce339. [DOI] [PubMed] [Google Scholar]
- 5.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–1717. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Wallberg KA, Oktay K. Fertility preservation in women with breast cancer. Clin Obstet Gynecol. 2010;53:753–762. doi: 10.1097/GRF.0b013e3181f96e00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, et al. Immunohistochemistry of estrogen and progesterone receptors reconsidered: Experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 8.Sainsbury R. The development of endocrine therapy for women with breast cancer. Cancer Treat Rev. 2013;39:507–517. doi: 10.1016/j.ctrv.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–434. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 11.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: A prospective controlled study. J Clin Oncol. 2008;26:2630–2635. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 14.Turan V, Bedoschi G, Moy F, et al. Safety and feasibility of performing two consecutive ovarian stimulation cycles with the use of letrozole-gonadotropin protocol for fertility preservation in breast cancer patients. Fertil Steril. 2013;100:1681–1685. doi: 10.1016/j.fertnstert.2013.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy J, Turan V, Bedoschi G, et al. Triggering final oocyte maturation with gonadotropin-releasing hormone agonist (GnRHa) versus human chorionic gonadotropin (hCG) in breast cancer patients undergoing fertility preservation: An extended experience. J Assist Reprod Genet. 2014;31:927–932. doi: 10.1007/s10815-014-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 17.Aflatoonian A, Oskouian H, Ahmadi S, et al. Can fresh embryo transfers be replaced by cryopreserved-thawed embryo transfers in assisted reproductive cycles? A randomized controlled trial. J Assist Reprod Genet. 2010;27:357–363. doi: 10.1007/s10815-010-9412-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 19.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 20.Borini A, Cattoli M, Bulletti C, et al. Clinical efficiency of oocyte and embryo cryopreservation. Ann N Y Acad Sci. 2008;1127:49–58. doi: 10.1196/annals.1434.012. [DOI] [PubMed] [Google Scholar]
- 21.Marrs RP, Greene J, Stone BA. Potential factors affecting embryo survival and clinical outcome with cryopreserved pronuclear human embryos. Am J Obstet Gynecol. 2004;190:1766–1771. doi: 10.1016/j.ajog.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Tiboni GM. Aromatase inhibitors and teratogenesis. Fertil Steril. 2004;81:1158–1159. doi: 10.1016/j.fertnstert.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761–1765. doi: 10.1016/j.fertnstert.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119–129. doi: 10.1056/NEJMoa1313517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casper RF. Letrozole: Ovulation or superovulation? Fertil Steril. 2003;80:1335–1337. doi: 10.1016/j.fertnstert.2003.05.004. [DOI] [PubMed] [Google Scholar]