Abstract
Purpose
This phase II trial evaluated the effect of neoadjuvant chemotherapy with or without second-look surgery before craniospinal irradiation on response rates and survival outcomes in children with newly diagnosed nongerminomatous germ cell tumors.
Patients and Methods
Induction chemotherapy consisted of six cycles of carboplatin/etoposide alternating with ifosfamide/etoposide. Patients demonstrating less than complete response after induction chemotherapy were encouraged to undergo second-look surgery. Patients who did not achieve complete response or partial response after chemotherapy with or without second-look surgery proceeded to high-dose chemotherapy with thiotepa and etoposide and autologous peripheral blood stem-cell rescue before craniospinal irradiation.
Results
The study included 102 patients treated between January 2004 and July 2008. Median age was 12 years, and 76% were male; 53.9% had pineal region masses, and 23.5% had suprasellar lesions. Sixty-nine percent of patients achieved complete response or partial response with neoadjuvant chemotherapy. At 5 years, event-free survival was 84% ± 4% (SE) and overall survival was 93% ± 3%. During the median follow-up of 5.1 years, 16 patients recurred or progressed, with seven deaths after relapse. No deaths were attributed to therapy-related toxicity. Relapse occurred at the site of primary disease in 10 patients, at a distant site in three patients, or both in one patient. In two patients, progression was detected by marker increase alone. Increased serum α-fetoprotein was a negative prognostic variable. Histologic subtype and increase of beta-human chorionic gonadotropin were not significantly correlated with worse outcomes.
Conclusion
Neoadjuvant chemotherapy with or without second-look surgery achieved high response rates contributing to excellent survival outcomes in children with newly diagnosed nongerminomatous germ cell tumors. This regimen should be included as a backbone for further studies.
INTRODUCTION
Primary germ cell tumors (GCTs) are relatively uncommon CNS malignancies, comprising less than 4% of brain tumors in children in Western countries.1,2 In Japan and continental Asia, CNS GCTs account for approximately 11% of pediatric brain tumors.3–6 These tumors arise predominantly in pineal and suprasellar locations, and approximately 90% of these tumors occur before age 20 years with peak incidence at age 10 to 12 years.3 Overall, CNS GCTs predominate in males at a ratio of 3:1.7,8
CNS GCTs include a heterogeneous group of histologic subtypes linked to prognosis and treatment success and divided into germinoma and nongerminomatous germ cell tumors (NGGCTs). The NGGCT group comprises several histologies, including embryonal carcinoma, endodermal sinus tumor (yolk sac tumors), choriocarcinoma, immature teratoma, teratoma with malignant transformation, and mixed tumors. Mixed tumors may include any of these components as well as germinomatous elements.7 Compared with pure germinomas, NGGCTs are less radiosensitive, and their prognosis with radiotherapy alone has been poor (5-year survival of 20% to 45%).9
The Children's Oncology Group (COG) developed a phase II study (ACNS0122; Phase II Study to Assess the Ability of Neoadjuvant Chemotherapy With or Without Second Look Surgery to Eliminate All Measurable Disease Prior to Radiotherapy for NGGCT) to assess the ability of neoadjuvant chemotherapy, with or without second-look surgery before radiotherapy, to eliminate measureable disease in children with newly diagnosed NGGCTs. The trial measured response rates after six cycles of neoadjuvant chemotherapy, evaluated event-free survival (EFS) and overall survival (OS), and correlated serum and CSF tumor markers (α-fetoprotein [AFP] and beta-human chorionic gonadotropin [β-hCG]) with radiographic response. This trial also attempted to determine whether high-dose chemotherapy (HDCT) and autologous peripheral blood stem-cell rescue (PBSCR) could improve response before craniospinal irradiation (CSI) for patients with poor response to neoadjuvant chemotherapy.
PATIENTS AND METHODS
Patients
Between January 26, 2004, and July 18, 2008, patients 3 to 24 years of age with newly diagnosed NGGCTs or high-risk germinoma were eligible for study inclusion (Fig 1). Histologic diagnoses of NGGCTs included endodermal sinus tumor (yolk sac tumor), embryonal carcinoma, choriocarcinoma, immature teratoma, teratoma with malignant transformation, and mixed germ cell tumors. Patients were eligible for study without histologic confirmation if serum and/or CSF β-hCG was more than 50 IU/L, or if AFP was more than 10 IU/L or above the institutional normal range. Any germinoma with serum or CSF β-hCG of more than 50 IU/L was defined as high risk and was eligible for study. No previous therapy was allowed other than surgical intervention and corticosteroids.
Fig 1.
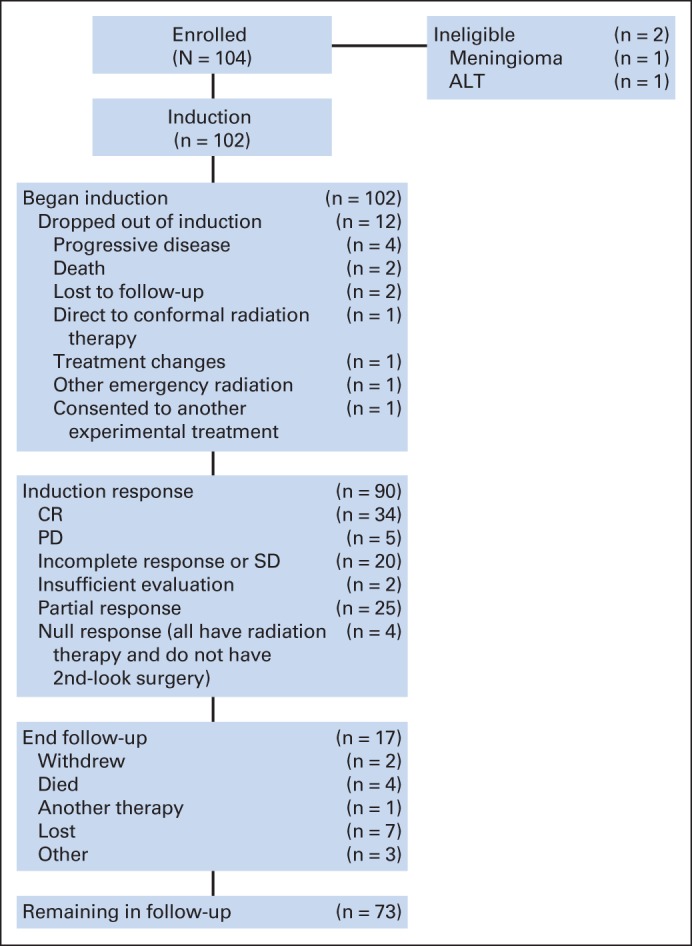
CONSORT diagram. CR, complete response; PD, progressive disease; SD, stable disease.
Tests and Examinations
Baseline magnetic resonance imaging (MRI) scans of the brain and spine with and without gadolinium were required. For patients who underwent initial surgical resection, postoperative MRI scans were required within 72 hours of surgery or after a minimum of 21 days after resection. Serum and CSF β-hCG and AFP were measured at the time of enrollment; CSF was obtained unless clinically contraindicated. Serum tumor markers were determined before each chemotherapy cycle, and CSF markers and cytology were repeated every other cycle (Appendix Table A1, online only).
Study Treatments
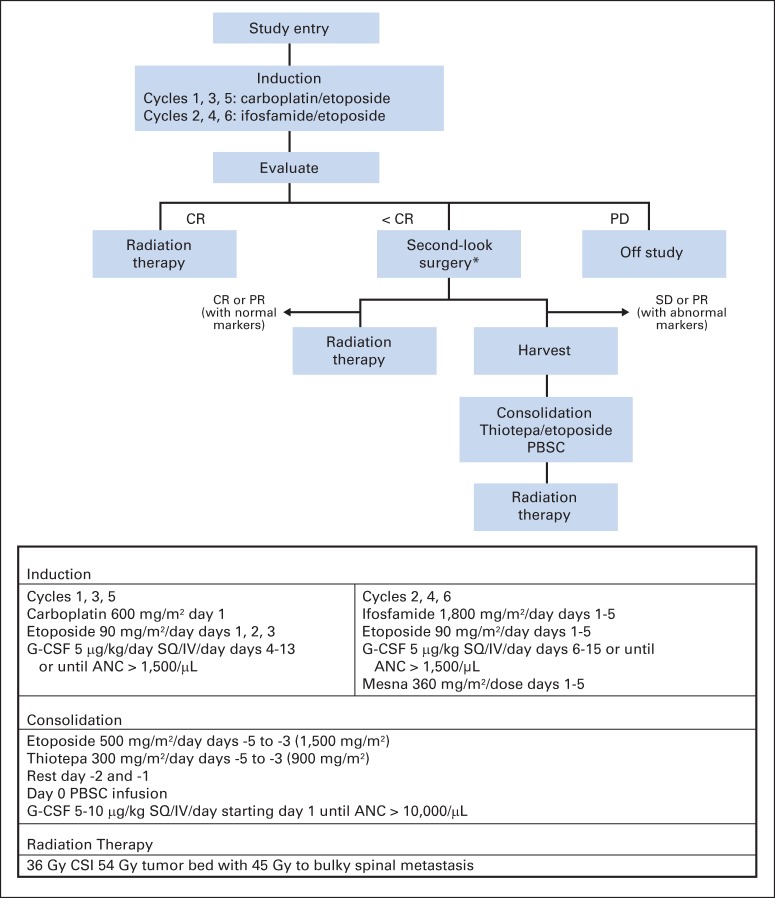
The treatment schema and dosing regimens are outlined in Figure 2. All patients began therapy within 31 days of diagnosis. Patients received six cycles of intravenous neoadjuvant chemotherapy on a 21-day cycle consisting of carboplatin 600 mg/m2 on day 1 plus etoposide 90 mg/m2 on days 1 through 3 (cycles 1, 3, and 5) alternating with ifosfamide 1,800 mg/m2 on days 1 through 5 and etoposide 90 mg/m2 on days 1 through 5 (cycles 2, 4, and 6). Granulocyte colony-stimulating factor (5 μg/kg per day) was given after each cycle. After completion of induction therapy, patients with residual tumor and/or persistently elevated tumor markers were strongly encouraged to undergo second-look surgery. Patients whose tumor met the criteria for complete response (CR) or partial response (PR), with or without second-look surgery, proceeded to CSI. For those who did not meet the criteria for CR or PR, it was recommended that they undergo consolidation therapy with thiotepa 300 mg/m2 and etoposide 500 mg/m2 on days −5 to −3, followed by PBSCR and CSI.
Fig 2.
Treatment schema and dosing. ANC, absolute neutrophil count; CSI, craniospinal irradiation; CR, complete response; G-CSF, granulocyte colony-stimulating factor; IV, intravenous; PBSC, peripheral blood stem cell; PD, progressive disease; PR, partial response; SD, stable disease; SQ, subcutaneous. (*) Strongly recommended but not required.
Radiation was administered to the craniospinal axis to 36 Gy with a boost to tumor bed sites totaling 54 Gy. For patients with disseminated disease at diagnosis, sites of bulky metastasis received a boost to 45 Gy regardless of response to induction chemotherapy.
Study End Points
The primary end point was response to induction chemotherapy, determined on the basis of MRI response, second-look surgery, and serum and CSF tumor markers. Centralized review of induction response occurred at the Quality Assurance Review Center (QARC). Tumor response was measured in three dimensions by using either T1- or T2-weighted MRI images. CR was defined as disappearance of all radiographic disease or evidence of only fibrosis or mature teratomatous elements on second-look surgery, as well as normalization of serum and CSF tumor markers. PR was defined as a more than 65% decrease in the sum of the products of the three perpendicular diameters of all target lesions on MRI scan and normalization of serum and CSF tumor markers. Stable disease (SD) was defined as neither sufficient decrease nor increase in the product of the three-dimensional (3D) measurements of a lesion to meet response or progression criteria without increase of tumor markers from baseline. Progressive disease (PD) was defined as a more than 40% increase in the products of a lesion, taking as a reference the smallest product observed since the start of treatment, the appearance of any new lesion, or an increase in tumor markers. If 3D measurements could not be obtained, then standard 2D definitions of imaging response were applied.
Secondary end points of treatment efficacy were EFS and OS. EFS was defined as the time to first disease progression, disease recurrence, death as a result of any cause, or occurrence of a second malignant neoplasm. OS was defined as the time to death as a result of any cause.
Statistical Analysis
The primary statistical analysis estimated response rate of NGGCTs to induction chemotherapy. Secondary analyses included estimating long-term EFS and OS rates and the rates of key toxicities. Nonparametric EFS and OS curves were computed by using product-limit (Kaplan-Meier) estimates, with standard errors calculated by using the Greenwood formula.
RESULTS
Patient Characteristics
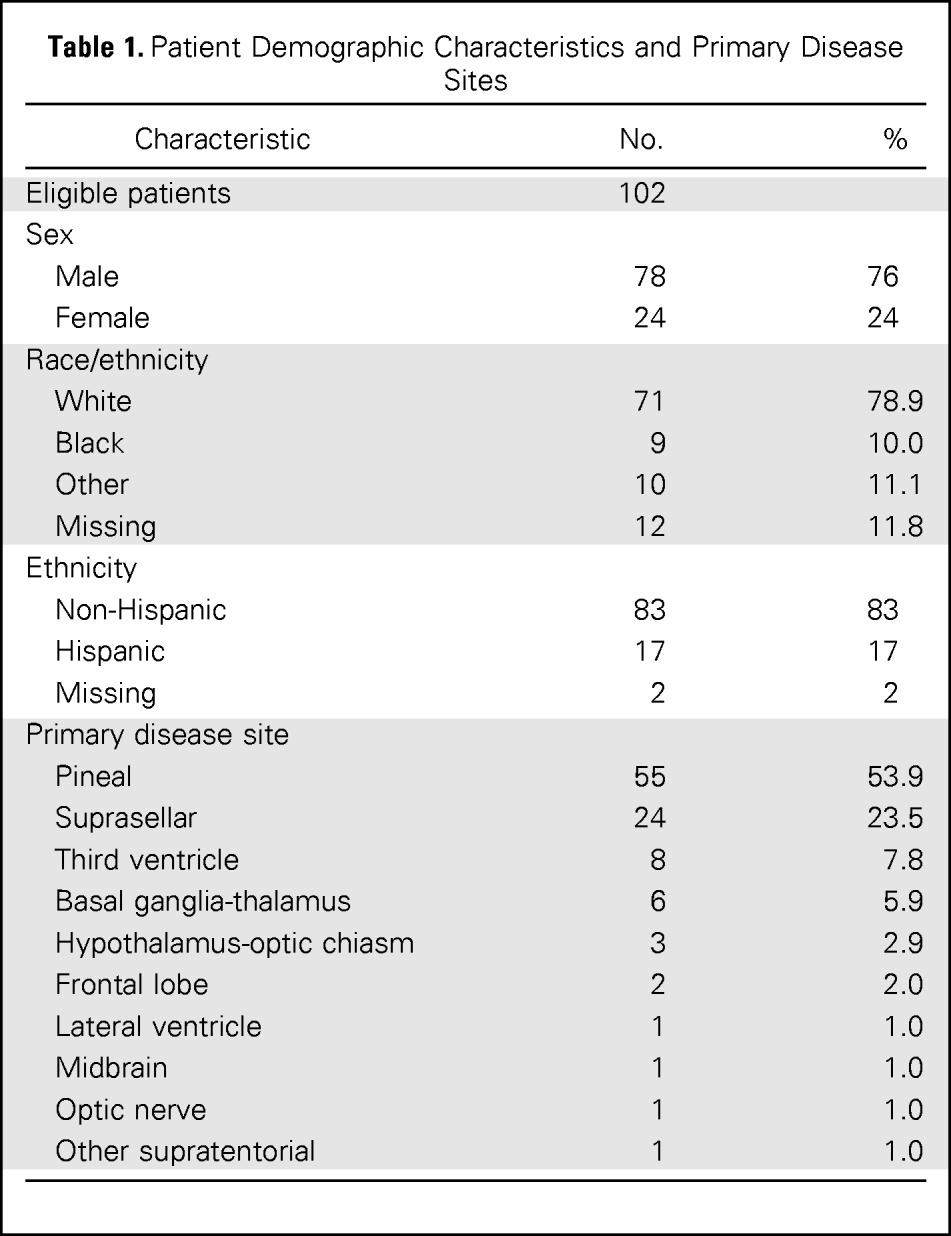
One hundred four patients were enrolled onto the study, of whom 102 were eligible. Age ranged from 3 to 23 years (median 12 years). Seventy-nine patients (76%) were male. Other patient and tumor characteristics are presented in Table 1. Primary tumor sites were pineal (53.9%) and suprasellar (23.5%). Pineal involvement was present in five (21%) of 24 females and 50 (64%) of 78 males, whereas tumor was located in the suprasellar region in 14 (58%) of 24 females and only 10 (13%) of 78 males. Eight patients presented with bifocal (pineal and suprasellar) disease, of whom six were male.
Table 1.
Patient Demographic Characteristics and Primary Disease Sites
| Characteristic | No. | % |
|---|---|---|
| Eligible patients | 102 | |
| Sex | ||
| Male | 78 | 76 |
| Female | 24 | 24 |
| Race/ethnicity | ||
| White | 71 | 78.9 |
| Black | 9 | 10.0 |
| Other | 10 | 11.1 |
| Missing | 12 | 11.8 |
| Ethnicity | ||
| Non-Hispanic | 83 | 83 |
| Hispanic | 17 | 17 |
| Missing | 2 | 2 |
| Primary disease site | ||
| Pineal | 55 | 53.9 |
| Suprasellar | 24 | 23.5 |
| Third ventricle | 8 | 7.8 |
| Basal ganglia-thalamus | 6 | 5.9 |
| Hypothalamus-optic chiasm | 3 | 2.9 |
| Frontal lobe | 2 | 2.0 |
| Lateral ventricle | 1 | 1.0 |
| Midbrain | 1 | 1.0 |
| Optic nerve | 1 | 1.0 |
| Other supratentorial | 1 | 1.0 |
Thirty-two patients (31.4%) underwent resection or biopsy at the time of diagnosis: 14 patients underwent gross total resection; one patient had a near total resection, eight had a subtotal resection, six had a partial resection, and three patients underwent biopsy only. The other 70 patients (68.6%) were enrolled based solely on the presence of increased serum and/or CSF β-hCG or AFP.
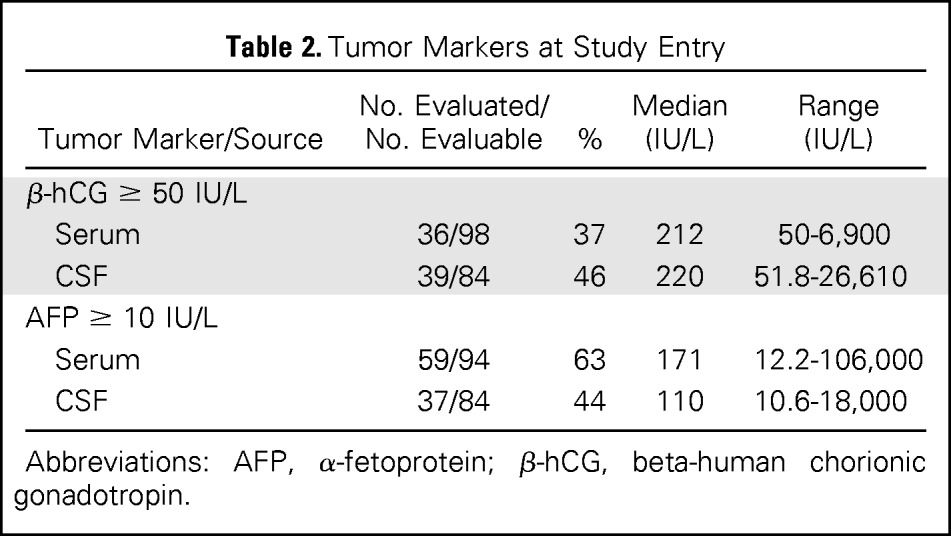
Tumor marker data are presented in Table 2. At study entry, both serum and CSF β-hCG and AFP were measured for 70.6% of patients (72). Thirty patients were missing data for at least one marker. Overall, 89% of patients (91 of 102) had at least one marker increased at baseline.
Table 2.
Tumor Markers at Study Entry
| Tumor Marker/Source | No. Evaluated/ No. Evaluable | % | Median (IU/L) | Range (IU/L) |
|---|---|---|---|---|
| β-hCG ≥ 50 IU/L | ||||
| Serum | 36/98 | 37 | 212 | 50-6,900 |
| CSF | 39/84 | 46 | 220 | 51.8-26,610 |
| AFP ≥ 10 IU/L | ||||
| Serum | 59/94 | 63 | 171 | 12.2-106,000 |
| CSF | 37/84 | 44 | 110 | 10.6-18,000 |
Abbreviations: AFP, α-fetoprotein; β-hCG, beta-human chorionic gonadotropin.
Response to Induction Chemotherapy
End-of-induction response was reviewed for 89 patients: 34 (38%) achieved CR, 25 (28%) PR, and 20 (22%) SD, whereas five patients had PD and five had insufficient (2) or missing (3) data for evaluation of response. Overall, induction produced a 69% objective response rate (CR or PR) in the evaluable patients. Of the 89 patients reviewed centrally by the QARC, four were not reviewed secondary to missing data. For the evaluable patients, QARC reported 51% (43 of 85) CR, 36% (31 of 85) PR, 5% (four of 85) SD, and 8% (seven of 85) PD rates. Overall, QARC reported an 87% response rate (CR plus PR) to induction chemotherapy (Table 3).
Table 3.
Institutional and Central Review Results
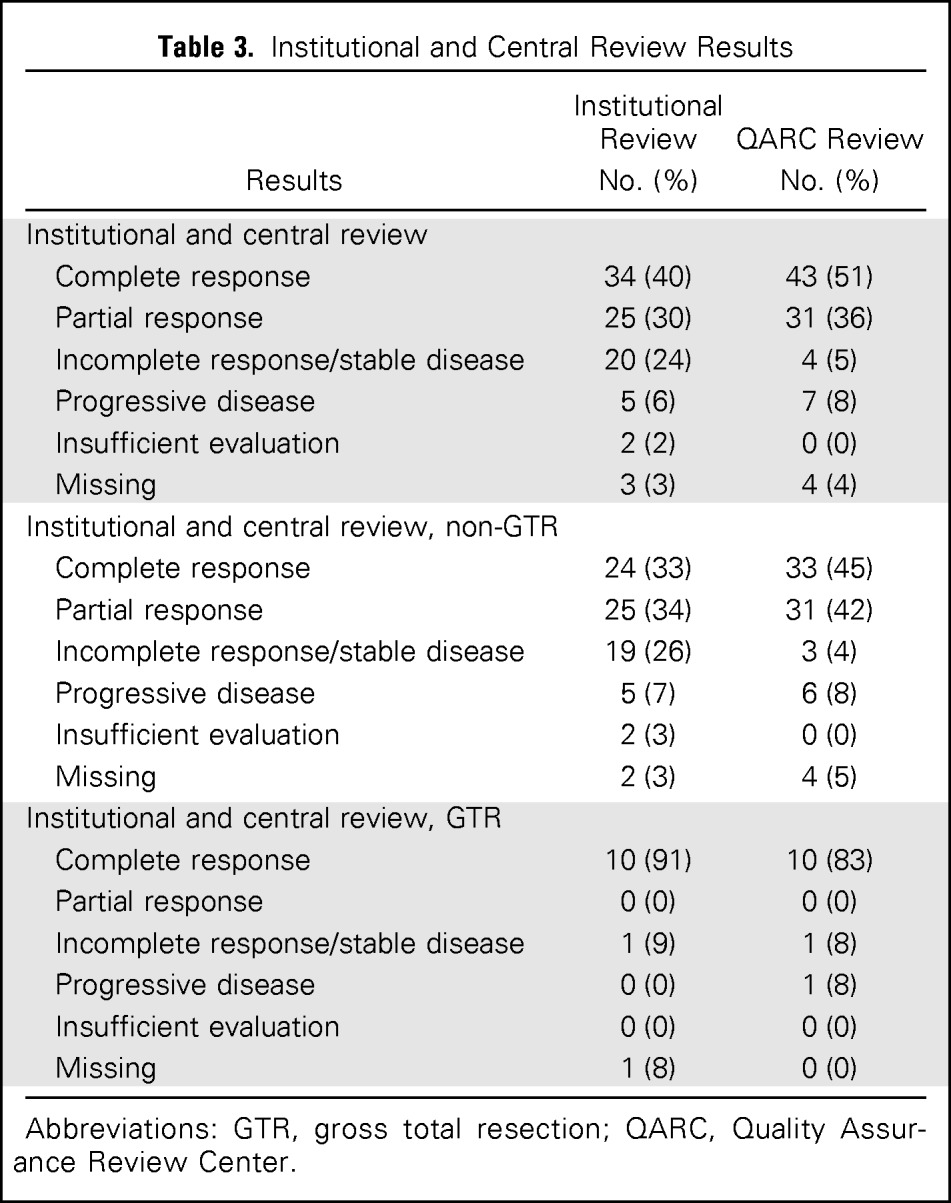
| Results | Institutional Review No. (%) | QARC Review No. (%) |
|---|---|---|
| Institutional and central review | ||
| Complete response | 34 (40) | 43 (51) |
| Partial response | 25 (30) | 31 (36) |
| Incomplete response/stable disease | 20 (24) | 4 (5) |
| Progressive disease | 5 (6) | 7 (8) |
| Insufficient evaluation | 2 (2) | 0 (0) |
| Missing | 3 (3) | 4 (4) |
| Institutional and central review, non-GTR | ||
| Complete response | 24 (33) | 33 (45) |
| Partial response | 25 (34) | 31 (42) |
| Incomplete response/stable disease | 19 (26) | 3 (4) |
| Progressive disease | 5 (7) | 6 (8) |
| Insufficient evaluation | 2 (3) | 0 (0) |
| Missing | 2 (3) | 4 (5) |
| Institutional and central review, GTR | ||
| Complete response | 10 (91) | 10 (83) |
| Partial response | 0 (0) | 0 (0) |
| Incomplete response/stable disease | 1 (9) | 1 (8) |
| Progressive disease | 0 (0) | 1 (8) |
| Insufficient evaluation | 0 (0) | 0 (0) |
| Missing | 1 (8) | 0 (0) |
Abbreviations: GTR, gross total resection; QARC, Quality Assurance Review Center.
Second-Look Surgery
Fifteen patients underwent second-look surgery after induction chemotherapy. Only two of these patients (13%) had residual NGGCTs (one embryonal carcinoma and one mixed germ cell tumor). Nine had teratomas (six mature teratomas and three malignant teratomas) and four had fibrosis/no evidence of tumor. Four of five patients who underwent second-look surgery at progression were also found to have teratoma with no other GCT elements. Subsequently, one of these four had further progression, and one was found to have increased AFP without any identifiable CNS or systemic lesion.
Response to Consolidation Therapy and PBSCR
Two patients with residual disease underwent consolidation therapy with PBSCR while on study. Both patients achieved a CR 39 to 53 months after therapy.
Survival Outcomes
For the 102 eligible patients in the study, the median follow-up without an event was 5.1 years (range, 0.06 to 8.0 years). Five-year EFS and OS were 84% ± 4% and 93% ± 3%, respectively (Fig 3A). For the 14 patients without radiographic disease at diagnosis, 5-year EFS and OS were both 100%. The 88 patients with measurable/evaluable disease at diagnosis had 5-year EFS of 81% ± 4% and OS of 92% ± 3% (Fig 3B). Histologic stratification did not reveal any significant differences in OS rate (Fig 3C).
Fig 3.
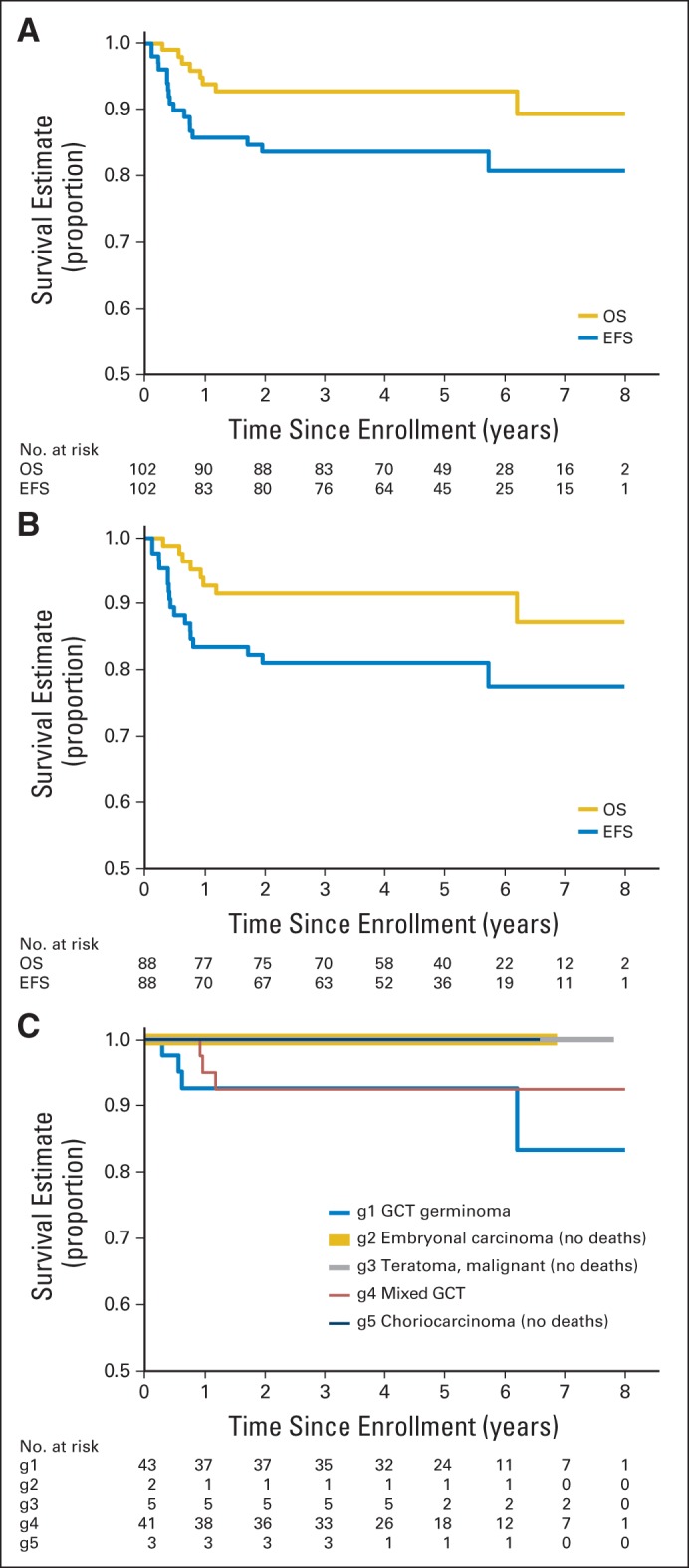
Overall survival (OS) and event-free survival (EFS). (A) OS and EFS for all eligible patients. (B) OS and EFS for eligible patients excluding those with gross total resection. (C) OS by histology (blue line, germinoma with > 50 IU/L hCG; gold, embryonal carcinoma; gray, teratoma malignant; red, mixed germ cell tumor [GCT]; black, choriocarcinoma). Of note, patients with increased markers and no histology were coded as having mixed germ cell disease. g, group.
Patients with serum or CSF β-hCG ≥ 100 IU/L had a 5-year EFS rate of 84% and OS rate of 92% (Fig 4A). There was no significant difference in survival of patients stratified by serum or CSF β-hCG ≥ 1,000 IU/L (EFS: 86%, P = .960; OS: 91%, P = .378; Fig 4B). Increased serum or CSF AFP (≥ 10 IU/L or within institutional normal rates) was associated with EFS of 78% and OS of 91%. Increased AFP approached a statistically significant negative association with EFS (P = .063; Fig 4C). Of note, patients who met eligibility for the study based solely on marker increases in serum and/or CSF were coded as mixed germ cell tumors independent of any histologic findings determined by second-look surgery.
Fig 4.
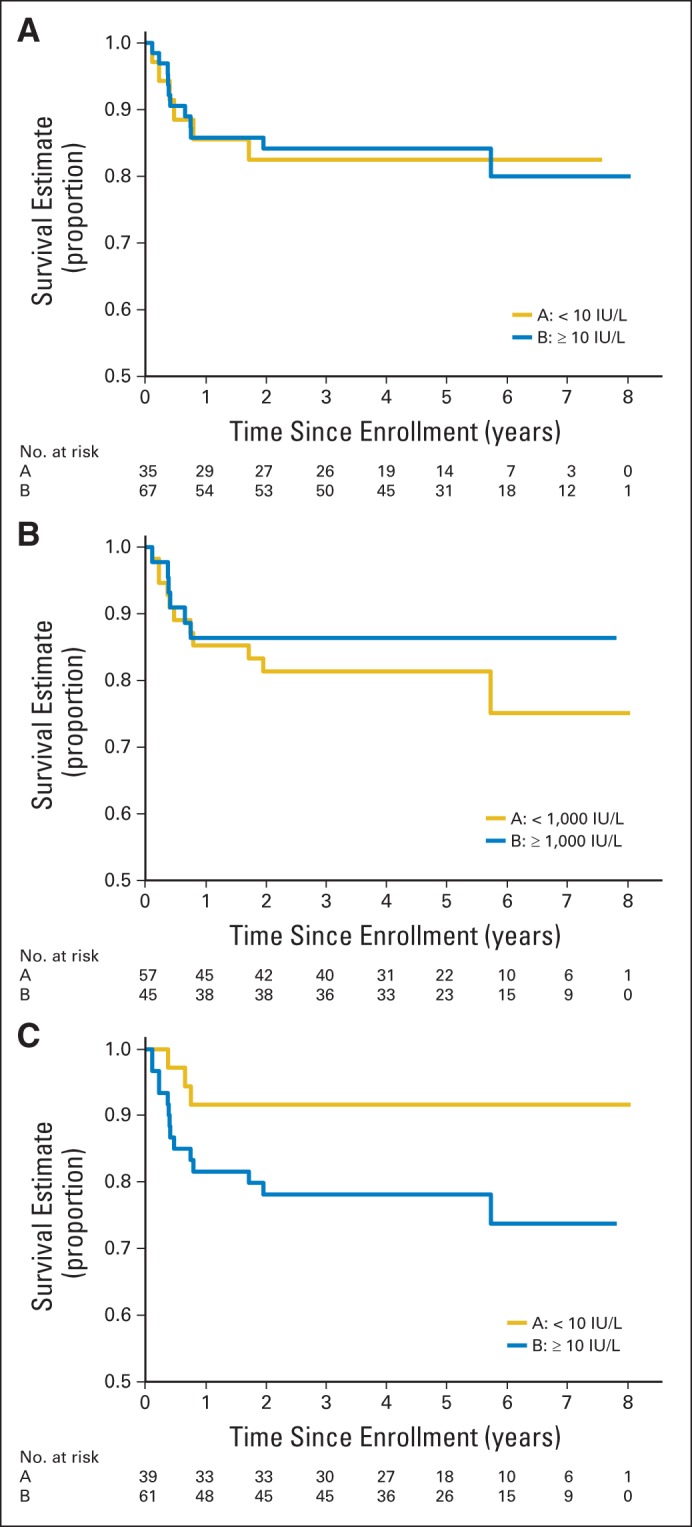
Event-free survival (EFS) stratified by serum or CSF markers. (A) EFS stratified by serum or CSF beta human chorionic gonadotropin less than 100 IU/L and ≥ 100 IU/L. (B) EFS stratified by serum or CSF beta human chorionic gonadotropin at less than 1,000 IU/L and ≥ 1,000 IU/L. (C) EFS stratified by serum or CSF α-fetoprotein at less than 10 IU/L and ≥ 10 IU/L.
The EFS was 92% and the OS was 98% at 3 years for all patients who achieved CR or PR either after induction chemotherapy or with documentation of absence of malignant elements at second-look surgery (Fig 5).
Fig 5.
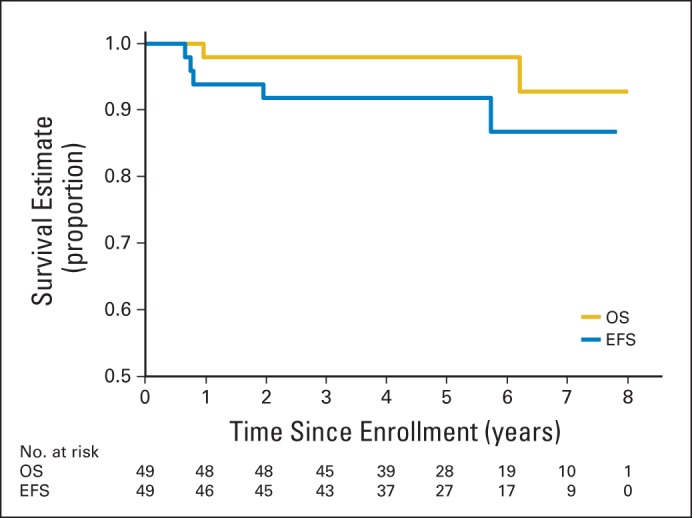
Overall survival (OS) and event-free survival (EFS) for patients with localized disease who achieved complete response or partial response with induction chemotherapy or second-look surgery.
Treatment Failures
At a median follow-up of 5.1 years, 16 of the 102 patients had recurred or progressed, of whom seven died. One patient died without relapse, for a total of 17 treatment failure events. There were 10 local failures, one combined local and distant, three distant, and two by marker increase alone without imaging evidence of progression. Four of the patients with local failure underwent second-look surgery and were found to have only teratoma/malignant teratoma (growing teratoma syndrome), and were coded as PD on the basis of protocol criteria. Of the seven deaths subsequent to relapse, four patients had embryonal carcinoma. One of these four patients had a late recurrence that was coded pathologically as undifferentiated carcinoma.
Toxicity
Toxicities were evaluated by using the Common Toxicity Criteria Evaluation (version 4). No deaths were attributed to study treatment. The most common grade 3 and 4 toxicities consisted of transient and expected anemia (range of incidence during cycles of chemotherapy administered during induction, 17.6% to 30.3%); neutropenia (29.4% to 31.5%); thrombocytopenia (11.8% to 31.5%); and electrolyte abnormalities with hyponatremia (10.1% to 8.6%), hypernatremia (4.9% to 1.2%), and hypokalemia (6.7% to 10.3%) during induction cycles of chemotherapy. Overall, less than 5% of patients developed transient febrile neutropenia during therapy.
DISCUSSION
For children and adolescents with NGGCTs, the study regimen of six cycles of neoadjuvant chemotherapy (carboplatin/etoposide alternating with ifosfamide/etoposide) was well-tolerated with significant objective response rates and improved OS following CSI (93%). Historically, the survival rates for patients with NGGCTs have been poor compared with those for patients with germinomas. NGGCTs are less radiosensitive, and CSI alone has resulted in 5-year OS rates of only 30% to 50%. In previous studies, most relapses occurred within 18 months of diagnosis.1,2,8,10 Although our median follow-up was beyond 5 years, one patient recurred at 68 months, warranting further follow-up of our cohort.
The use of chemotherapy regimens for GCT was first introduced for testicular and extragonadal tumors. The cure rates for males with disseminated GCT of the testis increased from less than 10% to more than 50% after the introduction in 1974 of the Einhorn regimen of vinblastine, bleomycin, and cisplatin.11,12 The first trial of adjuvant chemotherapy for NGGCTs was reported in 1981 by Matsutani et al1 from the University of Tokyo. Three trials performed by the International CNS GCT Study Group evaluated a chemotherapy-only approach, and although the majority of patients responded to chemotherapy, radiation was necessary to provide maximal cure rates.13–15 Robertson et al16 led a multi-institutional trial in 18 patients with NGGCTs who were treated with cisplatin and etoposide followed by irradiation. Twelve patients also underwent postradiation chemotherapy with bleomycin, vinblastine, etoposide, and carboplatin. Nine of twelve assessable patients had an objective response to induction chemotherapy (four CR and five PR). The 4-year EFS rate was 67% and the 4-year OS rate was 74%.16 In 1999, the French Society of Pediatric Oncology combined carboplatin, ifosfamide, and etoposide followed by focal radiotherapy. Induction chemotherapy normalized tumor markers in all patients, all of whom had increased markers at diagnosis. At a median follow-up of 53 months, the OS rate was 74%.17 The German Cooperative Group MAKEI (Maligue Keimzelltümoren) 89 study reported a 67% 5-year OS for patients with NGGCTs treated with two cycles of bleomycin, etoposide, and cisplatin, followed by two cycles of vinblastine, ifosfamide, and cisplatin.18,19
There is some controversy regarding the substitution of carboplatin for cisplatin for this patient population. In the treatment of systemic germ cell tumors, studies have shown superiority of cisplatin to carboplatin.20,21 Data from our trial revealed not only excellent response rates to a carboplatin-based induction regimen but also a 5-year EFS of 84% ± 4% and OS of 93% ± 3%, which are nominally superior to those of the previously reported French, German, and smaller American trials. Our regimen had no toxicity-related deaths and minimal grade 4 toxicities, supporting the notion that carboplatin can safely replace cisplatin for the treatment of CNS NGGCTs.
Our study protocol included standard-dose CSI. The French Society of Pediatric Oncology (SFOP) study reported a 74% OS rate with focal radiation therapy and used CSI only for those with metastatic or disseminated disease.17 In the study by Robertson et al,16 five patients received CSI and 13 received involved field irradiation; four of the six relapsing patients were in the involved field group and had isolated CNS relapses outside the radiation field. On the basis of these outcomes, we included CSI in our protocol, with the goal of moving more patients toward CR and achieving better survival outcomes. On the basis of this trial's preliminary data, current ongoing trials are evaluating CSI dose and volume reductions as part of their study design.
Growing teratoma syndrome (GTS) led to PD in four patients. GTS was first reported by Logothetis et al22 in 1982 in six patients with systemic, nonseminomatous germ cell tumors and has been estimated to occur in 2% to 7% of systemic GCTs.23,24 A PubMed search revealed only 10 publications involving intracranial GTS, most of which were case reports. In a review of 170 cases of intracranial germ cell tumors in South Korea, 11 patients (6.5%) developed GTS after therapy for NGGCTs.25 In our study, patients underwent second-look surgery at the end of induction in an attempt to bring patients to CR or PR. Of the 15 patients who underwent second-look surgery at the end of induction, nine had teratomas with six of the nine showing no malignant elements. Of five patients who underwent resection following radiographic PD, four were found to have GTS. On the basis of this experience, the ongoing COG trial has incorporated a definition of GTS into the evaluation process.
HDCT has been investigated in patients with relapsed CNS GCTs.26–29 One aim of this trial was to determine whether patients who did not achieve PR or CR with induction chemotherapy with or without second-look surgery would benefit from preirradiation HDCT with PBSCR. We were unable to test this hypothesis because only two patients proceeded to HDCT with PBSCR before radiation therapy. Unfortunately, data on patients with PD who underwent HDCT with PBSCR off study were not captured as part of the trial.
Previous studies have suggested that GCT histologic subtype is of prognostic importance. Matsutani et al1 reviewed 153 histologically verified intracranial GCTs that were treated uniformly and reported that patients with pure choriocarcinoma, endodermal sinus tumor, or embryonal carcinoma fared poorly with a 5-year OS of 9.3%. In comparison, the 5-year OS was 70% for those patients with germinoma, teratoma, or teratoma with malignant elements. Results from our trial did not show a significant difference in outcomes by initial histologic subtype (Fig 3C). This may reflect that patients who met eligibility criteria with increased tumor markers without histologic confirmation were coded as mixed germ cell tumors. It is possible that the high OS rate reflects a relative although undocumented paucity of the highest-risk tumor types in our study population.
Previous studies have documented the prognostic importance of disease status after chemotherapy. The Society of Paediatric Oncology (SIOP) –CNS-GCT-96 (Combination Chemotherapy and Radiation Therapy in Treating Patients With Germ Cell Tumors in the Brain) trial reported outcomes for 34 patients with residual disease after induction chemotherapy; 47% relapsed, with a PFS rate of 37% ± 10%, which was inferior to the relapse rate for patients in CR after chemotherapy.30 The Japanese Brain Tumor Study Group trial of intermediate-risk GCT found that only one of 13 patients relapsed after achieving a CR compared with progression in two of 10 patients who had less than CR.31–33 Therefore, it is imperative that treatment regimens be designed to maximize responses to chemotherapy.
In this trial, nearly 70% of patients responded to neoadjuvant chemotherapy, based on institutional review. If we include patients whose tumors were localized at diagnosis and those who underwent second-look surgery, the 5-year EFS was 92% and OS was 98% for these groups. This subgroup of patients is currently receiving reductions in radiotherapy volume and dose as part of the ongoing COG trial. Of note, the 14 patients who had a gross total resection at diagnosis had a 5-year EFS of 100%, raising the question of whether the potential morbidity of resection before chemotherapy is justified in patients with NGGCTs. A future publication will present our findings regarding tumor location correlated with the extent of resection and morbidity and whether more aggressive surgical procedures might be considered in these patients.
In conclusion, we showed that the neoadjuvant chemotherapy regimen, with or without second-look surgery, used in this study is not only feasible and well tolerated but also produces high response rates and OS rates for children and adolescents with NGGCTs.
Acknowledgment
We thank Stacey Tobin, PhD, and Jason Fangusaro, MD, for their expertise in manuscript review and Chris Williams-Hughes for her guidance, patience, and leadership in the CNS committee at the Children's Oncology Group.
Appendix
Table A1.
Required Observations
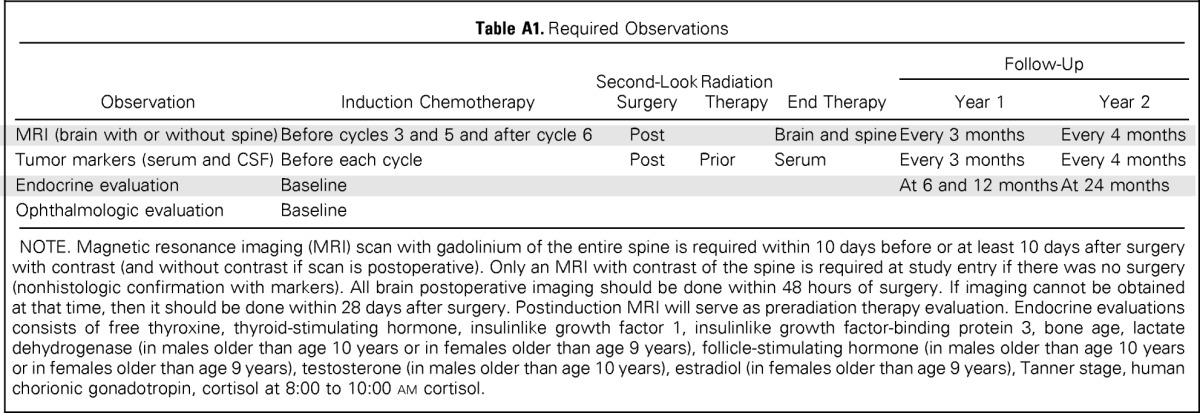
| Observation | Induction Chemotherapy | Second-Look Surgery | Radiation Therapy | End Therapy | Follow-Up |
|
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | |||||
| MRI (brain with or without spine) | Before cycles 3 and 5 and after cycle 6 | Post | Brain and spine | Every 3 months | Every 4 months | |
| Tumor markers (serum and CSF) | Before each cycle | Post | Prior | Serum | Every 3 months | Every 4 months |
| Endocrine evaluation | Baseline | At 6 and 12 months | At 24 months | |||
| Ophthalmologic evaluation | Baseline | |||||
NOTE. Magnetic resonance imaging (MRI) scan with gadolinium of the entire spine is required within 10 days before or at least 10 days after surgery with contrast (and without contrast if scan is postoperative). Only an MRI with contrast of the spine is required at study entry if there was no surgery (nonhistologic confirmation with markers). All brain postoperative imaging should be done within 48 hours of surgery. If imaging cannot be obtained at that time, then it should be done within 28 days after surgery. Postinduction MRI will serve as preradiation therapy evaluation. Endocrine evaluations consists of free thyroxine, thyroid-stimulating hormone, insulinlike growth factor 1, insulinlike growth factor-binding protein 3, bone age, lactate dehydrogenase (in males older than age 10 years or in females older than age 9 years), follicle-stimulating hormone (in males older than age 10 years or in females older than age 9 years), testosterone (in males older than age 10 years), estradiol (in females older than age 9 years), Tanner stage, human chorionic gonadotropin, cortisol at 8:00 to 10:00 am cortisol.
Footnotes
Supported by Children's Oncology Group study Grants No. CA98543 (Chair's grant), CA180886 (National Clinical Trials Network [NCTN] Operation Center grant), and CA98413 and CA180899 (NCTN Statistics and Data Center grants) from the National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Stewart Goldman, Tianni Zhou
Collection and assembly of data: Stewart Goldman, Eric Bouffet, Paul G. Fisher, Patricia L. Robertson, Paul J. Chuba, Cynthia S. Kretschmar, Tianni Zhou, Ian F. Pollack
Data analysis and interpretation: Stewart Goldman, Eric Bouffet, Jeffrey C. Allen, Patricia L. Robertson, Bernadine Donahue, Cynthia S. Kretschmar, Tianni Zhou, Allen B. Buxton, Ian F. Pollack
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial Assessing the Ability of Neoadjuvant Chemotherapy With or Without Second-Look Surgery to Eliminate Measurable Disease for Nongerminomatous Germ Cell Tumors: A Children's Oncology Group Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Stewart Goldman
No relationship to disclose
Eric Bouffet
No relationship to disclose
Paul G. Fisher
Stock or Other Ownership: Johnson & Johnson
Other Relationship: Associate Editor, The Journal of Pediatrics
Jeffrey C. Allen
No relationship to disclose
Patricia L. Robertson
No relationship to disclose
Paul J. Chuba
No relationship to disclose
Bernadine Donahue
No relationship to disclose
Cynthia S. Kretschmar
No relationship to disclose
Tianni Zhou
No relationship to disclose
Allen B. Buxton
No relationship to disclose
Ian F. Pollack
No relationship to disclose
REFERENCES
- 1.Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545–551. doi: 10.3171/jns.1991.74.4.0545. [DOI] [PubMed] [Google Scholar]
- 3.Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: A review. Oncologist. 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- 4.Allen JC, DaRosso RC, Donahue B, et al. A phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74:940–944. doi: 10.1002/1097-0142(19940801)74:3<940::aid-cncr2820740323>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol. 1999;17:933–940. doi: 10.1200/JCO.1999.17.3.933. [DOI] [PubMed] [Google Scholar]
- 6.Shibamoto Y, Abe M, Yamashita J, et al. Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys. 1988;15:285–290. doi: 10.1016/s0360-3016(98)90006-2. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Ohgaki H, Wiestler OD, et al. WHO classification of tumours of the central nervous system. In: Bosman F, Jaffe E, Lakhani S, et al., editors. WHO Health Organization Classification of Tumours (ed 4) Lyon, France: International Agency for Research on Cancer; 2007. p. 309. [Google Scholar]
- 8.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 9.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics. 1994;25:26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 10.Dearnaley DP, A'Hern RP, Whittaker S, et al. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962-1987. Int J Radiat Oncol Biol Phys. 1990;18:773–781. doi: 10.1016/0360-3016(90)90396-2. [DOI] [PubMed] [Google Scholar]
- 11.Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol. 1990;8:1777–1781. doi: 10.1200/JCO.1990.8.11.1777. [DOI] [PubMed] [Google Scholar]
- 12.Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation: A novel approach for newly diagnosed CNS germ cell tumors—Results of an international cooperative trial: The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. 1996;14:2908–2915. doi: 10.1200/JCO.1996.14.11.2908. [DOI] [PubMed] [Google Scholar]
- 14.da Silva NS, Cappellano AM, Diez B, et al. Primary chemotherapy for intracranial germ cell tumors: Results of the third international CNS germ cell tumor study. Pediatr Blood Cancer. 2010;54:377–383. doi: 10.1002/pbc.22381. [DOI] [PubMed] [Google Scholar]
- 15.Kellie SJ, Boyce H, Dunkel IJ, et al. Primary chemotherapy for intracranial nongerminomatous germ cell tumors: Results of the second international CNS germ cell study group protocol. J Clin Oncol. 2004;22:846–853. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol. 1997;32:71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 17.Baranzelli MC, Patte C, Bouffet E, et al. Nonmetastatic intracranial germinoma: The experience of the French Society of Pediatric Oncology. Cancer. 1997;80:1792–1797. [PubMed] [Google Scholar]
- 18.Calaminus G, Bamberg M, Harms D, et al. AFP/beta-HCG secreting CNS germ cell tumors: Long-term outcome with respect to initial symptoms and primary tumor resection—Results of the cooperative trial MAKEI 89. Neuropediatrics. 2005;36:71–77. doi: 10.1055/s-2005-837582. [DOI] [PubMed] [Google Scholar]
- 19.Calaminus G, Bamberg M, Jürgens H, et al. Impact of surgery, chemotherapy and irradiation on long term outcome of intracranial malignant non-germinomatous germ cell tumors: Results of the German Cooperative Trial MAKEI 89. Klin Padiatr. 2004;216:141–149. doi: 10.1055/s-2004-822626. [DOI] [PubMed] [Google Scholar]
- 20.Bokemeyer C, Köhrmann O, Tischler J, et al. A randomized trial of cisplatin, etoposide and bleomycin (PEB) versus carboplatin, etoposide and bleomycin (CEB) for patients with ‘good-risk’ metastatic non-seminomatous germ cell tumors. Ann Oncol. 1996;7:1015–1021. doi: 10.1093/oxfordjournals.annonc.a010493. [DOI] [PubMed] [Google Scholar]
- 21.Lokich J, Anderson N. Carboplatin versus cisplatin in solid tumors: An analysis of the literature. Ann Oncol. 1998;9:13–21. doi: 10.1023/a:1008215213739. [DOI] [PubMed] [Google Scholar]
- 22.Logothetis CJ, Samuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer. 1982;50:1629–1635. doi: 10.1002/1097-0142(19821015)50:8<1629::aid-cncr2820500828>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Geisler JP, Goulet R, Foster RS, et al. Growing teratoma syndrome after chemotherapy for germ cell tumors of the ovary. Obstet Gynecol. 1994;84:719–721. [PubMed] [Google Scholar]
- 24.Tonkin KS, Rustin GJ, Wignall B, et al. Successful treatment of patients in whom germ cell tumour masses enlarged on chemotherapy while their serum tumour markers decreased. Eur J Cancer Clin Oncol. 1989;25:1739–1743. doi: 10.1016/0277-5379(89)90343-x. [DOI] [PubMed] [Google Scholar]
- 25.Kim CY, Choi JW, Lee JY, et al. Intracranial growing teratoma syndrome: Clinical characteristics and treatment strategy. J Neurooncol. 2011;101:109–115. doi: 10.1007/s11060-010-0238-1. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney DH, Jr, Strother D, Camitta B, et al. High-dose melphalan and cyclophosphamide with autologous bone marrow rescue for recurrent/progressive malignant brain tumors in children: A pilot Pediatric Oncology Group study. J Clin Oncol. 1996;14:382–388. doi: 10.1200/JCO.1996.14.2.382. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Mazumdar M, Bajorin DF, et al. High-dose carboplatin, etoposide, and cyclophosphamide with autologous bone marrow transplantation in first-line therapy for patients with poor-risk germ cell tumors. J Clin Oncol. 1997;15:2546–2552. doi: 10.1200/JCO.1997.15.7.2546. [DOI] [PubMed] [Google Scholar]
- 28.Tada T, Takizawa T, Nakazato F, et al. Treatment of intracranial nongerminomatous germ-cell tumor by high-dose chemotherapy and autologous stem-cell rescue. J Neurooncol. 1999;44:71–76. doi: 10.1023/a:1006395719917. [DOI] [PubMed] [Google Scholar]
- 29.Bouffet B, Baranzelli C, Patte D, et al. High-dose etoposide and triotepa for refractory and recurrent malignant intracranial germ cell tumors; 9th International Symposium in Pediatric Neuro-Oncology; June 11-14, 2000; San Francisco, CA. [Google Scholar]
- 30.Calaminus G, Patte G. Germ cell tumors in children and adolescents. In: Argarwal B, editor. International Society of Paediatric Oncology Education Book 2005. Vancouver, BC, Canada: International Society of Paediatric Oncology; 2005. pp. 109–116. [Google Scholar]
- 31.Matsutani M. Treatment of intracranial germ cell tumors: The second phase II study of Japanese GCT Study Group. J Neurooncol. 2008;10:420. [Google Scholar]
- 32.Alapetite C, Patte C, Frappaz D. Intracranial germinoma treated with primary chemotherapy followed by focal radiation treatment: The SFOP-90 experience final analysis. 40th Congress of the International Society of Paediatric Oncology; October 2-6, 2008; Berlin, Germany. zet.al. [Google Scholar]
- 33.Calaminus G, Alapetite C, Frappaz D, et al. Outcome of localized and metastatic germinoma treated according to SIOP CNS GCT 96 protocol: Update on risk profile and outcome. J Neurooncol. 2008;10:369–517. [Google Scholar]